Abstract
The development of alcohol-induced fatty liver is associated with a reduction of white adipose tissue (WAT). Peroxisome proliferator-activated receptor (PPAR)-γ prominently distributes in the WAT and plays a crucial role in maintaining adiposity. The present study investigated the effects of PPAR-γ activation by rosiglitazone on lipid homeostasis at the adipose tissue-liver axis. Adult C57BL/6 male mice were pair fed liquid diet containing ethanol or isocaloric maltose dextrin for 8 wk with or without rosiglitazone supplementation to ethanol-fed mice for the last 3 wk. Ethanol exposure downregulated adipose PPAR-γ gene and reduced the WAT mass in association with induction of inflammation, which was attenuated by rosiglitazone. Ethanol exposure stimulated lipolysis but reduced fatty acid uptake capacity in association with dysregulation of lipid metabolism genes. Rosiglitazone normalized adipose gene expression and corrected ethanol-induced lipid dyshomeostasis. Ethanol exposure induced steatosis and upregulated inflammatory genes in the liver, which were attenuated by rosiglitazone. Hepatic peroxisomal fatty acid β-oxidation was suppressed by ethanol in associated with inhibition of acyl-coenzyme A oxidase 1. Rosiglitazone elevated plasma adiponectin level and normalized peroxisomal fatty acid β-oxidation rate. However, rosiglitazone did not affect ethanol-reduced very low-density lipoprotein secretion from the liver. These results demonstrated that activation of PPAR-γ by rosiglitazone reverses ethanol-induced adipose dysfunction and lipid dyshomeostasis at the WAT-liver axis, thereby abrogating alcoholic fatty liver.
Keywords: lipid storage disorder, adipose lipid metabolism, alcoholic fatty liver
long-term excess alcohol consumption causes liver damage; the earliest pathological stage is fatty liver, which is characterized by lipid droplet accumulation in the hepatocytes under microscope (13). The liver plays a crucial role in lipid metabolism and whole body energy homeostasis. Previous studies have shown that chronic alcohol consumption affects multiple lipid metabolic pathways in the liver, such as stimulating de novo lipogenesis, enhancing fatty acid uptake, and suppressing fatty acid oxidation and very low-density lipoprotein (VLDL) export (7, 24, 46, 47). All these alcohol effects on hepatic lipid metabolism favor lipid accumulation in the liver. Furthermore, extrahepatic factors such as adipokines secreted from the white adipose tissue (WAT) critically regulate hepatic lipid homeostasis (34, 36, 48). Previous studies have demonstrated that alcohol consumption causes WAT dysfunction, which impacts hepatic lipid homeostasis via an organ-organ interaction mechanism (36, 48).
WAT plays an important role in whole body energy homeostasis by acting as a major organ for lipid storage and adipokine secretion (34). Adiponectin is one of the most important adipokines and regulates hepatic lipid metabolism toward reduction of lipid content in the liver (34, 36, 48). Adiponectin signaling in the liver leads to activation of AMPK (5′-adenosine monophosphate-activated protein kinase) pathway via AdipoR1/2 (adiponectin receptor 1/2). AMPK activation negatively regulates hepatic lipid level by stimulating fatty acid oxidation and suppressing fatty acid influx and de novo lipogenesis (34). Chronic alcohol exposure has been shown to decrease plasma adiponectin level in a variety of animal models, including mice, rats, and micropigs (36, 48). Replacement with recombinant mouse adiponectin attenuated alcohol-induced steatosis and inflammation (43). Elevation of plasma adiponectin level was associated with protective effects of dietary supplementation with saturated fat, resveratrol, taurine, or rosiglitazone against alcoholic fatty liver (3, 6, 37, 45). These studies indicate that dysregulation of adipokines contributes to the pathogenesis of alcoholic fatty liver.
Adipose tissue, as an energy-buffering organ, stores triglyceride in a positive energy condition and releases fatty acids in a negative energy balance condition. Disorder in adipose fat storage function may cause excess fatty acid influx into the liver, leading to steatosis (8, 23, 42). Experimental elimination of adipose triglyceride buffering capacity by restricting adipose expansion has been shown to cause fatty liver and insulin resistance (41), whereas increasing adipose expansion ability improved diet-induced fatty liver (21). Therefore, healthy adipose tissue with appropriate expanding capacity is required for maintaining hepatic lipid homeostasis. Both clinical and animal studies have shown that alcoholic fatty liver is accompanied by reduction of adipose tissue mass (1, 2, 14, 17). Peroxisome proliferator-activated receptor (PPAR)-γ prominently distributes in the WAT and plays a crucial role in maintaining adipose expansion and adiposity (4). Previous studies have demonstrated that PPAR-γ activation attenuates alcoholic fatty liver, and stimulation of adiponectin secretion and hepatic adiponectin-SIRT1-AMPK signaling accounts for protective action of rosiglitazone (37). The present study reports that activation of PPAR-γ reversed alcohol-induced adipose tissue dysfunction and improved lipid homeostasis at the adipose tissue-liver axis.
MATERIALS AND METHODS
Animals and treatments.
Male C57BL/6 mice were purchased from Harlan (Indianapolis, IN). All the mice were treated according to experimental procedures approved by the Institutional Animal Care and Use Committee. Mice were pair fed a modified Lieber-DeCarli alcohol liquid diet containing either ethanol or isocaloric maltose dextrin as control for 8 wk. Ethanol was gradually increased from 35 to 38% of total calories in the diet. For PPAR-γ activation, rosiglitazone was added to the liquid diet at a daily intake of 10 mg/kg body wt for the last 3 wk of feeding. At the end of the feeding experiment, mice were fasted for 4 h and euthanized under Avertin (300 mg/kg body wt). Blood, liver, epididymal (eWAT), and subcutaneous (sWAT) white adipose tissue samples were collected for analysis.
Blood metabolites assay.
Blood glucose was measured via a OneTouch Ultra2 blood glucose meter (Life Scan, Milpitas, CA). Blood ketone bodies were determined by use of a CardioCheck analyzer with PTS Panels ketone test strips (Polymer Technology Systems, Indianapolis, IN). Plasma triglyceride and cholesterol concentrations were measured with the Infinity Triglyceride Reagent and Infinity Cholesterol Reagent (Thermo Scientific, Waltham, MA), respectively. Concentrations of plasma free fatty acids (FFA) were determined with a FFA Quantification Kit (BioVision, Mountain View, CA). Insulin and adiponectin levels were determined with ELISA kits (Millipore, Billerica, MA).
Adipose tissue explants culture ex vivo.
Adipose tissue explants were obtained from the eWAT and sWAT. Adipose tissue explants were cut into small pieces (<10 mg), and placed in a culture plate with prewarmed Dulbecco's PBS containing penicillin (100 U/ml) and streptomycin (100 mg/ml) to remove connective tissue and blood vessels. Explants were weighed and transferred to 24-well plates (∼40 mg/well) in DMEM with l-glutamine (2 mM), penicillin (50 U/ml), streptomycin (50 mg/ml), and 2% fatty acid-free bovine serum albumin. Tissue culture was conducted at 37°C in a humidified atmosphere with 5% CO2 for 2 h. FFA released in the culture medium was determined by a FFA Quantification Kit (BioVision, Mountain View, CA).
Estimation of adipocyte fatty acid uptake.
Adipocytes were isolated from the eWAT as described previously (15). Fatty acid uptake by isolated adipocytes was measured by using QBT Fatty Acid Uptake Assay Kit (Molecular Devices, Sunnyvale, CA) according to the manufacturer's instructions. Briefly, 50,000 adipocytes/well were placed in a 96-well black fluorescence plate. QBT dye was added, and the plate was read once a minute for 60 min in a Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT) at 485 nm excitation and 528 nm emission wavelengths.
Measurement of adipocyte size.
Adipocyte size measurement was performed as described previously (32). Paraffin sections of the eWAT and sWAT were stained with hematoxylin and eosin. Images were taken at a final magnification of ×320 by use of Nikon NIS-Elements F3.0 software. The longest diameter of each adipocyte and its perpendicular diameter were measured. These two values were then used to calculate the mean diameter. Diameters of 200–240 adipocytes from four to five randomly selected, different optical fields from three individual mice per diet group were measured.
Estimation of liver lipid export.
Liver VLDL-triglyceride secretion was determined with the Triton WR1339 method as described (9, 17). In brief, mice fasted for 4 h were intraperitoneally injected with Triton WR1339 solution (tyloxapol; Sigma Chemical, St. Louis, MO) at 0.5 mg/g body wt. Blood samples were collected via tail vein at 0 and 90 min. Plasma samples were used for triglyceride quantification. Hepatic VLDL-triglyceride secretion rate was expressed as milligrams per gram of liver per hour.
Determination of liver injury.
To examine liver injury, plasma liver enzyme activity, and liver pathology and lipid concentrations were determined. Plasma alanine aminotransferase (ALT) activity was colorimetrically measured using an Infinity ALT Reagent (Thermo Scientific, Waltham, MA). Liver tissue paraffin sections were prepared and stained with hematoxylin and eosin. Lipid quantitative assays were conducted by measuring the concentrations of triglyceride, cholesterol, and FFA in the liver tissues as described previously (17).
Measurement of liver peroxisomal β-oxidation.
Peroxisomal β-oxidation activity was measured as described previously (27). Briefly, 30 mg liver was homogenized in nine volumes of cold 0.25 M sucrose with silicon beads (Next Advance, Averill, NY). The homogenates were centrifuged at 600 g for 10 min. The supernatants were assayed for peroxisomal β-oxidation in the presence of potassium cyanide (KCN) after addition of 10% (wt/vol) of Triton X-100 to reach to 1.0% final concentration. The rate of nicotinamideadenine dinucleotide-positive (NAD+) reduction is directly related to the fatty acid oxidation rate. The NAD+ reduction was measured spectrophotometrically at 340 nm for 5 min by addition of 0.01 mM palmitoyl-CoA to the assay mixture containing 47 mM Tris·HCl (pH 8.0), 0.2 mM NAD+, 1 mM dithiothreitol, 0.0075% (wt/vol) bovine serum albumin, 0.01% (wt/vol) Triton X-100, 0.1 mM coenzyme A, 0.01 mM flavin adenine dinucleotide, and 1 mM KCN.
qRT-PCR analysis.
Liver and adipose tissues were homogenized and total RNA was isolated. Total RNA 1 μg was reverse transcribed with TaqMan Reverse Transcription Reagents (Life Technologies, Carlsbad, CA). The gene expression of related mRNA was measured in triplicate by the comparative cycle threshold method using 7500 real-time PCR system (Applied Biosystems, Carlsbad, CA). The primer sets for real-time PCR were purchased from IDT (Integrated DNA Technologies, Coralville, IA). The primer sequences are shown in Table 1. The data were normalized to β-actin expression and presented as relatively changes, setting the values of control mice as one.
Table 1.
Primer sets used in quantitative RT-PCR analysis
| Gene | Full Name | Forward/Reverse (5′-3′) |
|---|---|---|
| PPAR-γ | Peroxisome proliferator-activated receptor | TCGCTGATGCACTGCCTATG/GAGAGGTCCACAGAGCTGATT |
| Hsl | Hormone sensitive lipase | GCCGGTGACGCTGAAAGTGGT/CGCGCAGATGGGAGCAAGAGGT |
| Atgl | Adipose triglyceride lipase | GAGCCCCGGGGTGGAACAAGAT/AAAAGGTGGTGGGCAGGAGTAAGG |
| CD36/FAT | Fatty acid translocase | ATGGGCTGTGATCGGAACTG/GTCTTCCCAATAAGCATGTCTCC |
| Fatp1 | Fatty acid transporter 1 | CGCTTTCTGCGTATCGTCTG/GATGCACGGGATCGTGTCT |
| Fabp4 | Fatty acid binding protein 4 | AAGGTGAAGAGCATCATAACCCT/TCACGCCTTTCATAACACATTCC |
| Acs | Acyl-CoA synthetase | TGCCAGAGCTGATTGACATTC/GGCATACCAGAAGGTGGTGAG |
| Lpl | Lipoprotein lipase | GGGAGTTTGGCTCCAGAGTTT/TGTGTCTTCAGGGGTCCTTAG |
| Vldl-r | Very low-density lipoprotein receptor | GGCAGCAGGCAATCGAATG/GGGCTCGTCACTCCAGTCT |
| Fasn | Fatty acid synthase | GGAGGTGGTGATAGCCGGTAT/TGGGTAATCCATAGAGCCCAG |
| Dgat1 | Diacylglycerol O-acyltransferase 1 | TCCGTCCAGGGTGGTAGTG/TGAACAAAGAATCTTGCAGACGA |
| Srebp1 | Sterol regulatory element binding protein 1 | GCAGCCACCATCTAGCCTG/CAGCAGTGAGTCTGCCTTGAT |
| TNF-α | Tumor necrosis factor α | CTTCTGTCTACTGAACTTCGGG/CAGGCTTGTCACTCGAATTTTG |
| IL-6 | Interleukin-6 | TAGTCCTTCCTACCCCAATTTCC/TTGGTCCTTAGCCACTCCTTC |
| MCP-1 | Monocyte chemoattractant protein-1 | GTCCCTGTCATGCTTCTGG/GCTCTCCAGCCTACTCATTG |
| KC | Chemokine ligand 1 | AACCGAAGTCATAGCCACAC/CAGACGGTGCCATCAGAG |
| Cidec | Cell death-inducing DNA fragmentation factor A-like effector C | TGGCAAAAGATACCATGTTCATG/GCTTCTGGGAAAGGGCTAGCT |
| Adipsin | Complement factor D | CATGCTCGGCCCTACATGG/CACAGAGTCGTCATCCGTCAC |
| ACOX1 | Acyl-coenzyme A oxidase 1 | TCCAGACTTCCAACATGAGGA/CTGGGCGTAGGTGCCAATTA |
| Mttp | Microsomal triglyceride transfer protein | CTCTTGGCAGTGCTTTTTCTCT/GAGCTTGTATAGCCGCTCATT |
| Apob | Apolipoprotein B100 | TTGGCAAACTGCATAGCATCC/TCAAATTGGGACTCTCCTTTAGC |
| β-Actin | GGCTGTATTCCCCTCCATCG/CCAGTTGGTAACAATGCCATGT |
Immunoblot analysis.
Liver proteins were extracted by RIPA buffer (8.1 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 7.4) containing protease inhibitors. Protein samples were separated by 10% SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane. The membrane was probed with polyclonal antibody against acyl-coenzyme A oxidase 1 (ACOX1, Proteintech, Chicago, IL). Following incubation with horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G (Santa Cruz Biotechnology, Santa Cruz, CA), proteins were visualized by an Enhanced Chemiluminescence detection system (GE Healthcare, Piscataway, NJ) and quantified by densitometry analysis.
Statistical analysis.
Data are expressed as means ± SD. Statistical analysis was determined by ANOVA followed by Newman-Keuls multiple comparison.
RESULTS
Effects of rosiglitazone supplementation on blood parameters and PPAR-γ gene expression in WAT and liver of ethanol-fed mice.
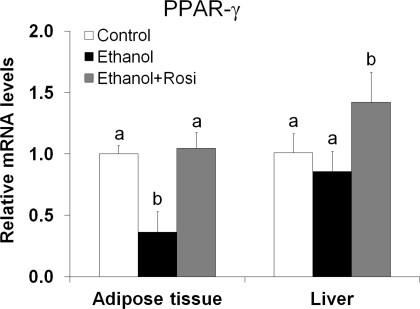
As shown in Table 2, mice after 8 wk of ethanol feeding showed a significantly lower body weight, but a higher liver weight, leading to a significant increase in the liver-to-body weight ratio. Rosiglitazone treatment for the last 3 wk attenuated ethanol-induced lower body weight. Ethanol exposure reduced plasma cholesterol and FFA levels but did not affect other parameters including glucose, insulin, and adiponectin. Rosiglitazone attenuated ethanol's effect on ALT, plasma cholesterol, and ketone bodies and increased plasma adiponectin level by threefold. PPAR-γ mRNA level in the adipose tissue was significantly decreased by ethanol feeding, which was normalized by rosiglitazone supplementation. Hepatic PPAR-γ gene expression was not affected by ethanol feeding but upregulated by rosiglitazone supplementation (Fig. 1).
Table 2.
Effects of rosiglitazone on body weight, liver weight, and some blood parameters
| Control | Ethanol | Ethanol+Rosi | |
|---|---|---|---|
| Body weight, g | 30.6 ± 1.4a | 27.5 ± 1.5b | 29.0 ± 2.3ab |
| Liver weight, g | 1.211 ± 0.067a | 1.330 ± 0.052b | 1.315 ± 0.083b |
| Blood parameters | |||
| Alanine aminotransferase, U/l | 20.7 ± 2.7a | 45.7 ± 5.6b | 26.1 ± 1.6c |
| Glucose, mg/dl | 265 ± 45a | 221 ± 19ab | 185 ± 33b |
| Insulin, ng/ml | 0.619 ± 0.163a | 0.462 ± 0.184ab | 0.264 ± 0.016b |
| Triglycerides, mg/ml | 0.723 ± 0.112a | 0.770 ± 0.213ab | 0.500 ± 0.225b |
| Cholesterol, mg/ml | 0.936 ± 0.206a | 0.598 ± 0.112b | 0.720 ± 0.128ab |
| Free fatty acid, μM | 480.6 ± 95.6a | 251.1 ± 122.8b | 334.6 ± 146.0b |
| Ketone body, mg/dl | 4.8 ± 1.4a | 7.1 ± 1.7b | 5.7 ± 0.9ab |
| Adiponectin, μg/ml | 7.793 ± 1.310a\ | 8.146 ± 1.747a | 24.419 ± 5.828b* |
Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone (Rosi) was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. Data are expressed as means ± SD; n = 6–8. Means without a common letter differ at P < 0.05;
P < 0.001.
Fig. 1.
Rosiglitazone (Rosi) normalized decreased peroxisome proliferator-activated receptor-γ (PPAR-γ) gene expression in the adipose tissue of ethanol-fed mice. Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. PPAR-γ gene expression in the epididymal white adipose tissue (eWAT) and liver was analyzed by quantitative RT-PCR (qRT-PCR). Data are expressed as means ± SD (n = 4). Means without a common letter differ at P < 0.05.
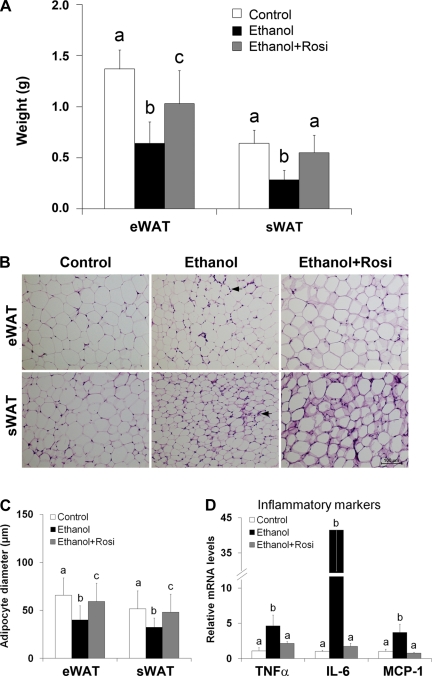
Rosiglitazone attenuated ethanol-reduced WAT mass and adipocyte size as well as WAT inflammation.
Ethanol feeding reduced both eWAT and sWAT masses, which were attenuated by rosiglitazone supplementation (Fig. 2A). Light microscopy revealed that ethanol feeding induced adipose delipidation as indicated by smaller adipocyte size in both eWAT and sWAT (Fig. 2, B and C). Measurement of adipocyte diameter showed that ethanol feeding significantly reduced the adipocyte size (Fig. 2C). Rosiglitazone supplementation to ethanol-fed mice attenuated not only ethanol-induced reduction of adipocyte size but also infiltration of inflammatory cells. The expression of inflammatory genes including TNF-α (tumor necrosis factor-α), IL-6 (interleukin-6), and MCP-1 (monocyte chemoattractant protein-1) was markedly increased by ethanol feeding, which were attenuated by rosiglitazone supplementation (Fig. 2D).
Fig. 2.
Rosiglitazone attenuated ethanol-induced adipose mass reduction, histopathological alterations, and inflammation. Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. A: white adipose tissue (WAT) weight. sWAT, subcutaneous WAT. B: WAT histopathology. Hematoxylin and eosin staining. Arrows indicate inflammatory cell infiltration. C: adipocyte diameter. D: qRT-PCR analysis of inflammatory markers in WAT. Data are expressed as means ± SD; n = 6–8 in A; n = 200–240 in C; n = 4 in D. Means without a common letter differ at P < 0.05. In C, means of epididymal adipocyte diameter differ at P < 0.001. In D, means of IL-6 differ at P < 0.001; means of TNF-α and MCP-1 differ at P < 0.01.
Rosiglitazone corrected ethanol-induced adipose dysfunction via modulating gene expression.
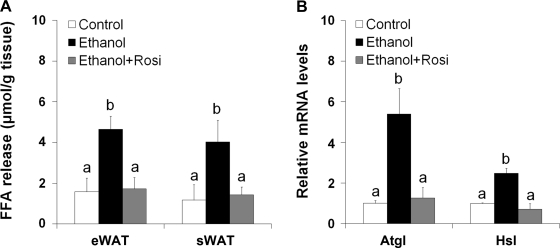
To determine the mechanism of how rosiglitazone attenuated ethanol-induced adipose delipidation, we evaluated adipose function and gene expression. Adipose tissue explant culture demonstrated that ethanol feeding stimulated lipolysis as indicated by more than twofold increase in FFA release from both eWAT and sWAT (Fig. 3A). Rosiglitazone completely inhibited ethanol-increased FFA release from both eWAT and sWAT. In accordance, the mRNA levels of the major adipose lipases adipose triglyceride lipase (Atgl) and hormone-sensitive lipase (Hsl) were upregulated by fourfold and twofold, respectively, by ethanol feeding. Rosiglitazone supplementation to ethanol-fed mice normalized the mRNA levels of Atgl and Hsl (Fig. 3B).
Fig. 3.
Rosiglitazone abrogated ethanol-stimulated adipose lipolysis and the relative gene expression. Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. A: WAT lipolysis. Lipolysis was estimated by measuring free fatty acid (FFA) release from WAT. Epididymal WAT tissue explants were cultured for 2 h and fatty acids released to the media were measured with a FFA quantification kit. B: qRT-PCR analysis of gene expression related to lipolysis. Data are expressed as means ± SD; n = 6–8 in A; n = 4 in B. Means without a common letter differ at P < 0.01.
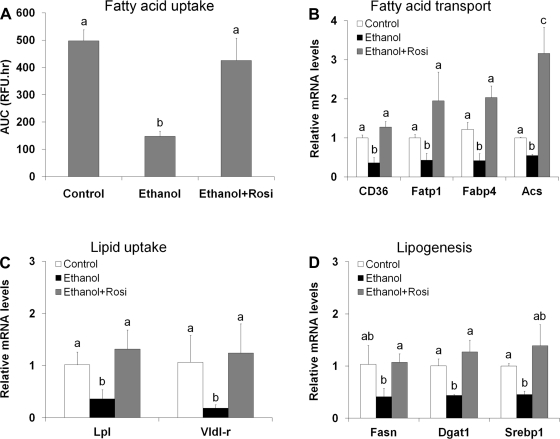
Fatty acid transport and fatty acid and triglyceride synthesis are important functions for adipose fat storage. Functional analysis with exogenous fluorescence-labeled fatty acids demonstrated that ethanol feeding for 8 wk significantly inhibited the adipocyte capacity in fatty acid uptake, which was normalized by rosiglitazone supplementation (Fig. 4A). Quantitative RT-PCR (qRT-PCR) analysis showed that ethanol feeding downregulated all the genes related to fatty acid transport including CD36 (fatty acid translocase), Fatp1 (fatty acid transporter protein 1), Fabp4 (fatty acid binding protein 4), and Acs (acetyl-CoA synthetase) (Fig. 4B). Ethanol feeding also downregulated genes related to VLDL uptake, such as Lpl (lipoprotein lipase) and Vldl-r (Fig. 4C), and to lipogenesis, such as Fasn (fatty acid synthase), Dgat1 (diacylglycerol acyltransferase 1), and Srebp1c (sterol regulatory element binding protein 1c) (Fig. 4D). All these repressive effects of ethanol on adipose gene expression were reversed by rosiglitazone supplementation.
Fig. 4.
Rosiglitazone normalized ethanol-suppressed adipose fatty acid uptake and related gene expression. Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. A: adipose fatty acid uptake. Adipocytes were isolated from epididymal adipose tissue, and fatty acid uptake ability was measured by using a QBT fatty acid uptake assay kit. B–D: qRT-PCR analysis of epididymal WAT genes related to fatty acid transport (B), VLDL uptake (C), and lipogenesis (D). Data are expressed as means ± SD; n = 6–8 in A; n = 4 in B–D. Means without a common letter differ at P < 0.05. In A, means differ at P < 0.01; In B, means of CD36 and Fabp4 differ at P < 0.01. AUC, area under curve.
Rosiglitazone reversed ethanol-induced fat accumulation in the liver in association with enhanced peroxisomal β-oxidation.
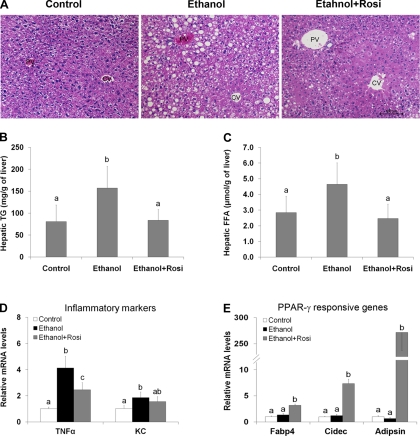
Histopathological examination revealed that chronic ethanol feeding causes severe fat accumulation (micro and macro vacuoles) in the cytoplasm of hepatocytes (Fig. 5A). Quantitative measurements of hepatic lipid showed ethanol feeding increased the liver triglyceride concentration by approximately twofold compared with pair-fed controls. Rosiglitazone supplementation reduced hepatic triglyceride concentration to the control level (Fig. 5B). Hepatic level of FFA was also significantly elevated in ethanol-fed mice, which was normalized by rosiglitazone supplementation (Fig. 5C). Rosiglitazone also attenuated ethanol-increased gene expression of inflammatory markers, TNF-α (tumor necrosis factor-α), and KC (keratinocyte-derived chemokine), in the liver (Fig. 5D). PPAR-γ direct target genes, including Fabp4 (fatty acid binding protein 4/aP2), Cidec (cell death-inducing DNA fragmentation factor-α-like effector C/fat-specific protein 27) and adipsin (complement factor D), were remarkably upregulated in the liver by rosiglitazone supplementation, whereas these genes were not affected by ethanol feeding (Fig. 5E).
Fig. 5.
Rosiglitazone reversed ethanol-induced lipid accumulation in the liver. Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. A: liver histopathology. Hematoxylin and eosin staining. CV, central vein; PV, portal vein. B: hepatic triglyceride (TG) content. C: Hepatic FFA content. D: gene expression of inflammatory cytokines TNF-α and KC in the liver by qRT-PCR. E: expression of PPAR-γ responsive genes in the liver by qRT-PCR. Data are expressed as means ± SD; n = 6–8 in A–C; n = 4 in D and E. Means without a common letter differ at P < 0.05. In D, means of TNF-α gene expression differ at P < 0.01; means of KC gene expression differ at P < 0.05. In E, means without a common letter differ at P < 0.001.
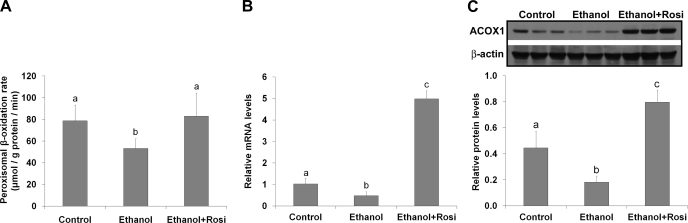
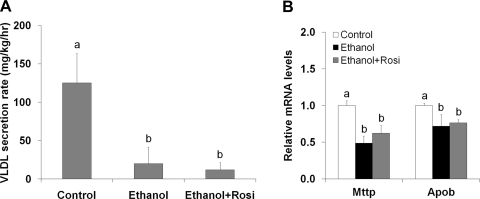
To determine how rosiglitazone improved ethanol-induced hepatic lipid dyshomeostasis, peroxisomal β-oxidation and VLDL secretion were measured. Ethanol feeding reduced peroxisomal β-oxidation rate (Fig. 6A) in association with reduction of peroxisomal Acox1 mRNA (Fig. 6B) and protein levels (Fig. 6C). Rosiglitazone supplementation normalized peroxisomal β-oxidation rate in association with fivefold increase in Acox1 mRNA level and threefold increase in Acox1 protein level. Ethanol-fed mice showed a dramatic decrease in VLDL secretion rate, which was not affected by rosiglitazone (Fig. 7A). Accordingly, the mRNA levels of Mttp (microsomal triglyceride transfer protein) and Apob (apolipoprotein B100) were reduced by ethanol feeding regardless rosiglitazone supplementation (Fig. 7B).
Fig. 6.
Rosiglitazone stimulated peroxisomal ACOX-1 expression and fatty acid β-oxidation. Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. A: peroxisomal fatty acid β-oxidation rate. B: qRT-PCR analysis of peroxisomal ACOX-1 gene expression. C: immunoblot analysis of peroxisomal ACOX-1 protein levels. Data are expressed as means ± SD; n = 6–8 in A; n = 4 in B; n = 3 in C. Means without a common letter differ at P < 0.05.
Fig. 7.
Rosiglitazone did not improve ethanol-impaired VLDL secretion and expression of related genes. Mice were fed ethanol liquid diet for 8 wk, and rosiglitazone was supplemented in the liquid diet at 10 mg·kg body wt−1·day−1 for the last 3 wk. A: VLDL-triglyceride secretion rate was measured by the Triton WR1339 method. B: qRT-PCR analysis of hepatic gene expression of Mttp and Apob. Data are expressed as means ± SD; n = 6–8 in A; n = 4 in B. Means without a common letter differ at P < 0.05. In A, means differ at P < 0.01; In B, means of Mttp gene expression differ at P < 0.01.
DISCUSSION
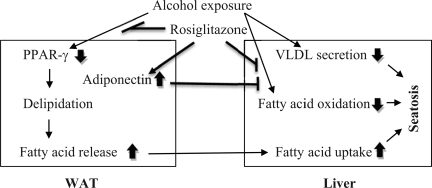
Alcohol consumption has been shown to affect WAT mass and function in both human and animal studies. Clinical studies demonstrated that alcoholics have a significantly lower body weight and lower fat mass, but higher hepatic lipid content, compared with normal controls (1, 2). Animal studies also showed that chronic alcohol feeding reduces WAT mass in association with a less body weight gain (14, 17). Although the role of WAT reduction in the pathogenesis of alcoholic fatty liver has not been fully defined, our recently study demonstrated that adipose tissue triglycerides labeled with deuterium before ethanol feeding were accumulated in the liver after ethanol feeding (50). These data suggest that ethanol feeding causes excess adipose lipolysis, leading to triglyceride reverse transport. Therefore, adipose tissue dysfunction may play an important role in the development of alcoholic steatosis. PPAR-γ is the key transcription factor determining adipose expansion ability and adiponectin secretion. As outlined in Fig. 8, the present study demonstrated that activation of PPAR-γ by rosiglitazone attenuates ethanol-reduced WAT mass, thereby reducing fatty acid overflux to the liver. Rosiglitazone also stimulated hepatic peroxisomal fatty acid oxidation through stimulating adipose adiponectin production. These data suggest that activation of PPAR-γ by rosiglitazone attenuates alcoholic fatty liver through improving lipid homeostasis at the WAT-liver axis.
Fig. 8.
Proposed effects of PPAR-γ activation by rosiglitazone on lipid homeostasis at the adipose tissue-liver axis in ethanol-fed mice.
Adipose lipid homeostasis is basically dependent on two major functions: triglyceride/fatty acid uptake and lipolysis/fatty acid release (25). Measurement of in vivo triglyceride turnover demonstrated that chronic ethanol feeding stimulates triglyceride turnover in epididymal WAT due to 2.3-fold increase in triglyceride degradation (14). Although β-adrenergic receptor plays a crucial role in activating adipose lipolysis pathway, chronic ethanol feeding even suppressed β-adrenergic receptor-stimulated lipolysis (14, 15). Moreover, insulin-mediated negative regulation of lipolysis was impaired in ethanol-fed rats (14). The present study showed that chronic ethanol feeding not only stimulated adipose lipolysis but also suppressed fatty acid uptake. Activation of PPAR-γ by rosiglitazone normalized ethanol-induced imbalance between lipid uptake and release in association with normalization of ethanol-downregulated adipose genes related to lipid metabolism. Atgl and Hsl are major triglyceride hydrolases responsible for adipose lipolysis, and rosiglitazone normalized ethanol upregulated expression of Atgl and Hsl. On the other hand, chronic ethanol exposure downregulated all the genes related to VLDL uptake, fatty acid transport, and lipogenesis, which was normalized by rosiglitazone. These data demonstrated that chronic ethanol exposure disturbs all the major lipid metabolic pathways in WAT, and PPAR-γ inactivation is likely a major molecular mechanism underlying the ethanol effects on adipose lipid metabolism.
PPAR-γ has been shown to transactivate Atgl in adipocytes (18, 29), but the present study generated a controversy data on PPAR-γ and Atgl gene expression in WAT. Ethanol feeding decreased PPAR-γ but increased Atgl gene expression in the WAT, and PPAR-γ activation by rosiglitazone attenuated ethanol-upregulated Atgl gene. These results suggest that Atgl gene may be regulated by multiple factors. Insulin has been shown to negatively regulate Atgl gene, and increased adipose Atgl mRNA was associated with insulin deficiency or insulin resistance (20, 22). Previous study has been shown that chronic ethanol exposure causes insulin resistance in mouse models of alcoholism (14). Our recent study also demonstrated that chronic ethanol feeding to mice upregulate insulin signaling inhibitors in the WAT (50). In addition, other factors such as glucocorticoids, cytokines, and nutrition status have been suggested to modulate adipose Atgl gene expression (39, 49). Previous studies demonstrated that Atgl gene expression is induced by glucocorticoid, TNF-α, or fasting. Our recent study showed that chronic alcohol feeding increases plasma corticosterone but reduces plasma glucose, which mimics the fasting condition. It is worth exploring further the precise mechanism of how PPAR-γ modulates lipolytic genes in ethanol-induced excess adipose lipolysis in future study.
Adipose tissue as an endocrine organ secretes adipokines, and adiponectin is one of the major adipokines that critically regulate lipid metabolism in the liver (34). Several reports have shown that ethanol feeding reduces the plasma adiponectin level in mice, rats, or micropigs (36, 43, 48). In contrast, some studies demonstrated that ethanol exposure increases the plasma adiponectin level in mice or patients with alcoholic hepatitis (5, 11, 44). Despite the controversial results, administration mouse recombinant adiponectin has been shown to attenuate alcohol-induced steatosis and inflammation (43). Elevation of plasma adiponectin level was also associated with protective effects of dietary supplementation with saturated fat, resveratrol, or rosiglitazone against alcoholic steatosis (3, 37, 45). Elevated plasma adiponectin by rosiglitazone supplementation or administration of recombinant adiponectin led to inhibition of the lipogenesis enzyme, acetyl CoA carboxylase, and stimulation of mitochondrial fatty acid β-oxidation and/or upregulation of Cpt1 (carnitine palmitoyltransferase 1) and medium chain acetyl CoA dehydrogenase (37, 43). Activation of the hepatic SIRT1-AMPK pathway has been suggested as a major mechanism of how adiponectin modulates ethanol-induced disorders in hepatic lipid metabolism (37). The present study demonstrated that rosiglitazone supplementation elevated plasma adiponectin by threefold and normalized ethanol-impaired peroxisomal fatty acid β-oxidation in the liver. Notably, both the mRNA and protein levels of Acox-1, the major peroxisomal fatty acid β-oxidation enzyme, were reduced by chronic ethanol exposure. Our previous study also found that chronic ethanol feeding suppressed the activity of catalase, a peroxisome marker enzyme (17). These data indicate that ethanol has an inhibitory effect on peroxisomal function. Rosiglitazone supplementation enhanced Acox-1 gene and protein expression to a level even higher than in the pair-fed mice. Because proliferation of the peroxisome is regulated by PPAR (26), these data suggest that rosiglitazone may enhance fatty acid β-oxidation by stimulating peroxisome proliferation.
The liver exports lipid in the form of VLDL, and previous studies have repeatedly reported that ethanol consumption reduces VLDL secretion rate as well as the content of cholesterol and triglyceride in VLDL (10, 12, 19, 38). Normalization of VLDL secretion was associated with attenuation of alcoholic steatosis by zinc, PPAR-α agonist, or betaine (17, 19, 22). Although the present study demonstrated dramatic decrease in VLDL secretion rate in ethanol-feed mice, rosiglitazone supplementation did not improve ethanol's effect on VLDL secretion. In accordance, rosiglitazone did not affect ethanol-suppressed gene expression of Mttp and Apob, which play a critical role in VLDL assembly and secretion. Recent studies suggest that adiponectin impacts VLDL metabolism. Human plasma adiponectin level was negatively correlated with plasma VLDL-Apob level and positively correlated with VLDL-Apob catabolism, whereas no correlation was found between plasma adiponectin and VLDL-Apob secretion (33). An animal study demonstrated that adiponectin overexpression increases VLDL-triglyceride catabolism via upregulating muscle Lpl and Vldl-r (35). In accordance with the previous reports, the present study demonstrated that elevation of plasma adiponectin by rosiglitazone was associated with a decrease in plasma triglyceride level. However, elevation of plasma adiponectin was not associated with alteration in hepatic VLDL secretion rate. These data suggest that adiponectin does not play a critical role in regulation of hepatic VLDL assembly and secretion.
Proinflammatory cytokines play an important role in the pathogenesis of ethanol-induced organ damage (40). Although upregulation of proinflammatory cytokine expression in the liver has been well documented in alcohol-induced liver damage, chronic ethanol exposure also upregulated TNF-α, IL-6, and MCP-1 in the WAT (16, 28). Attenuation of adipose IL-6 and MCP-1 was associated with the prevention by taurine supplementation of ethanol-reduced plasma adiponectin (6). The present study demonstrated that rosiglitazone supplementation attenuated ethanol-upregulated proinflammatory cytokine gene expression in both the liver and WAT. Although restoration of PPAR-γ gene expression was associated rosiglitazone supplementation in the present study, taurine supplementation has been shown to attenuate ethanol-induced proinflammatory cytokine gene expression without affecting PPAR-γ. Therefore, further investigations are needed to define whether the inhibitory effects of rosiglitazone on proinflammatory cytokines are dependent of PPAR-γ activation. Furthermore, the present study demonstrated that PPAR-γ activation by rosiglitazone not only modulated WAT genes, but also upregulated PPAR-γ target genes in the liver, in particular, Cidec. Cidec, the human homolog of FSP27, is a fat-specific gene and belongs to the CIDE family. Recent studies demonstrated that Cidec upregulation is associated with hepatic PPAR-γ activation and the development of fatty liver (30). A mechanistic study showed that forced expression of FSP27 in the liver inhibits mitochondrial fatty acid β-oxidation (31). Thus Cidec upregulation by rosiglitazone may have inhibitory effect on mitochondrial fatty acid β-oxidation. However, rosiglitazone supplementation enhanced mitochondrial fatty acid β-oxidation via the adiponectin-SIRT1-AMPK pathway (37). Because rosiglitazone supplementation elevated plasma adiponectin level by threefold in the present study, we assume that the inhibitory effect of Cidec on hepatic mitochondrial fatty acid oxidation is eliminated by adiponectin.
In conclusion, the present study demonstrated that activation of PPAR-γ by rosiglitazone supplementation reverses chronic ethanol feeding-induced disorders in WAT lipid metabolism. Restoration of WAT mass by rosiglitazone was associated with inhibition of ethanol-stimulated adipose lipolysis and normalization of fatty acid uptake. Rosiglitazone supplementation reversed alcoholic fatty liver in association with elevation of plasma adiponectin and normalization of ethanol-decreased peroxisomal fatty acid β-oxidation. Rosiglitazone supplementation also attenuated proinflammatory cytokine expression at the WAT-liver axis. These data suggest that inactivation of adipose PPAR-γ is an important mechanism underlying ethanol-induced lipid dyshomeostasis at the adipose tissue-liver axis.
GRANTS
This research was supported in part by the National Institutes of Health (R01AA018844, R01AA020212, P01AA017103, P30AA019360, R01AA015970, R01AA018869, R37AA010762, RC2AA019385), and the Veterans Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Marion McClain for review of this manuscript. C. J. McClain is a Distinguished University Scholar of the University of Louisville.
REFERENCES
- 1. Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcohol Clin Exp Res 21: 962–967, 1997 [PubMed] [Google Scholar]
- 2. Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med 244: 387–395, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 295: G833–G842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anghel SI, Wahli W. Fat poetry: a kingdom for PPAR gamma. Cell Res 17: 486–511, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Buechler C, Schäffler A, Johann M, Neumeier M, Köhl P, Weiss T, Wodarz N, Kiefer P, Hellerbrand C. Elevated adiponectin serum levels in patients with chronic alcohol abuse rapidly decline during alcohol withdrawal. J Gastroenterol Hepatol 24: 558–563, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology 49: 1554–1562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol 34: 35–38, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep 10: 306–315, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Elzinga BM, Havinga R, Baller JF, Wolters H, Bloks V, Mensenkamp AR, Kuipers F, Verkade HJ. The role of transhepatic bile salt flux in the control of hepatic secretion of triacylglycerol-rich lipoproteins in vivo in rodents. Biochim Biophys Acta 1573: 9–20, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278: 27997–28004, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Fromenty B, Vadrot N, Massart J, Turlin B, Barri-Ova N, Lettéron P, Fautrel A, Robin MA. Chronic ethanol consumption lessens the gain of body weight, liver triglycerides, and diabetes in obese ob/ob mice. J Pharmacol Exp Ther 331: 23–34, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Guzman M, Castro J. Zonal heterogeneity of the effects of chronic alcohol feeding on hepatic fatty acid metabolism. Hepatology 12: 1098–1105, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Hall P. Pathological spectrum of alcoholic liver disease. In: Alcoholic Liver Disease (2nd ed.), edited by Hall P. London: Arnold, 1995, p. 41–88 [Google Scholar]
- 14. Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem 282: 28465–28473, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Kang L, Nagy LE. Chronic ethanol feeding suppresses beta-adrenergic receptor-stimulated lipolysis in adipocytes isolated from epididymal fat. Endocrinology 147: 4330–4338, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res 31: 1581–1588, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology 50: 1241–1250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kershaw EE, Schupp M, Guan HP, Gardner NP, Lazar MA, Flier JS. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am J Physiol Endocrinol Metab 293: E1736–E1745, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kharbanda KK, Todero SL, Ward BW, Cannella JJ, 3rd, Tuma DJ. Betaine administration corrects ethanol-induced defective VLDL secretion. Mol Cell Biochem 327: 75–78, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Kim JY, Tillison K, Lee JH, Rearick DA, Smas CM. The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-α in 3T3-L1 adipocytes and is a target for transactivation by PPARγ. Am J Physiol Endocrinol Metab 291: E115–E127, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Isoproterenol, TNFalpha, and insulin downregulate adipose triglyceride lipase in 3T3-L1 adipocytes. Mol Cell Endocrinol 240: 43–49, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab 34: 317–327, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Lakshman MR. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol 34: 45–48, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab 30: 294–309, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Latruffe N, Vamecq J. Peroxisome proliferators and peroxisome proliferator activated receptors (PPARs) as regulators of lipid metabolism. Biochimie 79: 81–94, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Lazarow PB. Assay of peroxisomal beta-oxidation of fatty acids. Methods Enzymol 72: 315–319, 1981 [DOI] [PubMed] [Google Scholar]
- 28. Lin HZ, Yang SQ, Zeldin G, Diehl AM. Chronic ethanol consumption induces the production of tumor necrosis factor-alpha and related cytokines in liver and adipose tissue. Alcohol Clin Exp Res 22: 231S–237S, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Liu LF, Purushotham A, Wendel AA, Koba K, DeIuliis J, Lee K, Belury MA. Regulation of adipose triglyceride lipase by rosiglitazone. Diabetes Obes Metab 11: 131–142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsusue K. A physiological role for fat specific protein 27/cell death-inducing DFF45-like effector C in adipose and liver. Biol Pharm Bull 33: 346–350, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab 7: 302–311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monteiro R, Soares R, Guerreiro S, Pestana D, Calhau C, Azevedo I. Red wine increases adipose tissue aromatase expression and regulates body weight and adipocyte size. Nutrition 25: 699–705, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Ng TW, Watts GF, Farvid MS, Chan DC, Barrett PH. Adipocytokines and VLDL metabolism: independent regulatory effects of adiponectin, insulin resistance, and fat compartments on VLDL apolipoprotein B-100 kinetics? Diabetes 54: 795–802, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Poulos SP, Hausman DB, Hausman GJ. The development and endocrine functions of adipose tissue. Mol Cell Endocrinol 323: 20–34, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes 57: 1824–1833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life 60: 790–797, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 298: G364–G374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Venkatesan S, Ward RJ, Peters TJ. Effect of chronic alcohol feeding on the hepatic secretion of very-low-density lipoproteins. Biochim Biophys Acta 960: 61–66, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 279: 47066–47075, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol 16: 1304–1313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang MY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci USA 105: 6139–6144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver — the link between adipocytes and hepatocytes. Digestion 83: 124–133, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu J, Lai KK, Verlinsky A, Lugea A, French SW, Cooper MP, Ji C, Tsukamoto H. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol 55: 673–682, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol 34: 39–43, 2004 [DOI] [PubMed] [Google Scholar]
- 47. You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol 287: G1–G6, 2004 [DOI] [PubMed] [Google Scholar]
- 48. You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 234: 850–859, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 50: 3–21, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin Y, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. In Press [DOI] [PMC free article] [PubMed] [Google Scholar]