Abstract
Thiamin is essential for normal function of pancreatic acinar cells, and its deficiency leads to a reduction in pancreatic digestive enzymes. We have recently shown that thiamin uptake by rat pancreatic acinar cells is carrier-mediated and that both thiamin transporter (THTR)-1 and THTR-2 are expressed in these cells; little, however, is known about the relative contribution of these transporters toward total carrier-mediated thiamin uptake by these cells. We addressed this issue using a gene-specific silencing approach (siRNA) in mouse-derived pancreatic acinar 266–6 cells and Slc19a2 and Slc19a3 knockout mouse models. First we established that thiamin uptake by mouse pancreatic acinar cells is via a carrier-mediated process. We also established that these cells as well as native human pancreas express THTR-1 and THTR-2, with expression of the former (and activity of its promoter) being significantly higher than that of the latter. Using gene-specific siRNA against mouse THTR-1 and THTR-2, we observed a significant inhibition in carrier-mediated thiamin uptake by 266–6 cells in both cases. Similarly, thiamin uptake by freshly isolated primary pancreatic acinar cells of the Slc19a2 and Slc19a3 knockout mice was significantly lower than uptake by acinar cells of the respective littermates; the degree of inhibition observed in the former knockout model was greater than that of the latter. These findings demonstrate, for the first time, that both mTHTR-1 and mTHTR-2 are involved in carrier-mediated thiamin uptake by pancreatic acinar cells.
Keywords: transporter, small-interfering ribonucleic acid, thiamin transporter, pancreatic islets
vitamin b1 (thiamin) is essential for normal cellular function, growth, and development because of its role as a coenzyme, mainly thiamin pyrophosphate, in vital metabolic reactions related to energy production (1). Thiamin plays a role in reducing cellular oxidative stress via its role in bridging the energy-producing glycolytic and the pentose phosphate metabolic pathway that is critical for creating chemical reducing power in cells (2, 5). Therefore, low levels of thiamin in cells lead to impairment in energy metabolism and to oxidative stress. Other studies have shown that reduction in intracellular thiamin level also lead to apoptosis (10, 13, 26). Thiamin deficiency in humans leads to a variety of clinical abnormalities that includes neurological and cardiovascular disorders (31, 33). This deficiency occurs in a variety of conditions, including chronic alcoholism (4, 30), diabetes mellitus, celiac disease, and renal disease (15, 17, 24, 32).
The pancreas is a complex organ with important exocrine and endocrine functions. A variety of disease conditions and factors affect the health of this vital organ leading to significant morbidity and mortality. The exocrine portion of the pancreas constitutes the major portion (∼82%) of this organ and is composed of polarized acinar cells that secret digestive enzymes. Thiamin is important for this function of the exocrine pancreas, since its deficiency leads to a marked reduction in its content of digestive enzymes (25). We have recently characterized the cellular and molecular mechanisms involved in the transport of thiamin in rat pancreatic acinar cells and showed the process to be specific and carrier-mediated in nature (28). We also showed that thiamin transporter (THTR)-1 and THTR-2 (products of the Slc19a2 and Slc19a3 genes, respectively) are both expressed in rat pancreatic acinar cells, but little is known about their relative contribution toward total carrier-mediated thiamin uptake by these cells in any species. We addressed this issue in the present investigation using a gene-specific small-interfering RNA (siRNA) silencing approach and Slc19a2 and Slc19a3 knockout mouse models. Our results showed that both THTR-1 and THTR-2 systems are involved in thiamin uptake by pancreatic acinar cells with a slightly greater role for the former than the latter system.
MATERIALS AND METHODS
Materials
[3H]thiamin (specific activity 20 Ci/mM; radiochemical purity >98%) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Real-time PCR primers (Table 1) used in this study were synthesized by Sigma Genosys (Woodlands, TX). Nitrocellulose filters (0.45 μm pore size) were obtained from Fisher Scientific (Chicago, IL). All other chemicals, including molecular biology reagents, were obtained from commercial vendors and were of analytical grade.
Table 1.
Primers used for amplifying coding region of the respective genes by real-time PCR
| Gene Name | Forward and Reverse Primers (5′-3′) |
|---|---|
| mTHTR-1 | GTTCCTCACGCCCTACCTTC; GCATGAACCACGTCACAATC |
| mTHTR-2 | TCATGCAAACAGCTGAGTTCT; ACTCCGACAGTAGCTGCTCA |
| mARP0 | GCTGAACATCTCCCCCTTCTC; ATATCCTCATCTGATTCCTCC |
| hTHTR-1 | AGCCAGACCGTCTCCTTGTA; TAGAGAGGGCCCACCACAC |
| hTHTR-2 | TTCCTGGATTTACCCCACTG; GTATGTCCAAACGGGGAAGA |
| hβ-Actin | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
THTR, thiamin transporter; ARP0, acidic ribosomal phosphoprotein; m, mouse; h, human.
Cell Culture and Uptake Studies
Mouse-derived pancreatic acinar 266–6 cells were obtained from American Type Tissue Collection (Rockville, MD) and cultured in DMEM growth medium containing 10% FBS and antibiotic cocktail. Cells were used between passages of 2 to 10. Uptake was measured at 37°C (unless otherwise stated) in cells suspended in Krebs-Ringer (K-R) buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES; pH adjusted as indicated). Labeled (and unlabeled) thiamin was added to the incubation medium at the onset of incubation, and uptake was examined during the initial linear period of uptake (7 min). The reaction was terminated by the addition of 2 ml of ice-cold K-R buffer followed by immediate aspiration. Cells were then rinsed two times with ice-cold K-R buffer, digested with 1 ml of 1 N NaOH, neutralized with 10 N HCl, and then measured for radioactive content using a scintillation counter. Digested samples (100 μl each) were taken for determining protein concentration (Bio-Rad Dc protein assay kit).
Slc19a2 and Slc19a3 Knockout Mice and Their Littermates and Transgenic Mice Carrying the Human SLC19A2 and SLC19A3 Promoters
The Slc19a3 knockout (THTR-2-deficient) mice (C57Bl6 background) were generated and characterized by us (20). Founders of the Slc19a2 knockout mice (THTR-1-deficient) were kindly provided by Dr. Bruce D. Gelb (Mount Sinai School of Medicine, New York, NY) (14). Transgenic mice carrying the SLC19A2 (2,214-bp) or SLC19A3 (2,016-bp) promoters fused to the firefly luciferase reporter gene were used in this investigation and have been well characterized previously (12, 21). All animal studies performed in this investigation were reviewed and approved by the Long Beach Veterans Affairs Medical Center Subcommittee on Animal Studies and by the University of California Irvine Institutional Animal Care and Use Committee.
Isolation of Mouse Primary Pancreatic Acinar Cells
Mouse pancreatic primary acinar cells were isolated from the pancreas of adult mice using a Worthington collagenase type IV (Lakewood, NJ) digestion method as described previously (3, 7, 11, 22, 28). Isolated pancreatic acinar cells were suspended in K-R buffer, and uptake was performed within 1 h of isolation (22, 28). The Trypan blue exclusion method was used to test viability of the isolated acinar cells and shown to be >90%.
Isolation of Mouse Primary Pancreatic Islet Cells
Mouse pancreatic islets [which contain a high proportion (>90%) of β-cells; see Ref. 8] were isolated on the day of experiment by stationary digestion (collagenase type IV) and the Ficoll gradient method as described previously (6, 11). Isolated islet cells were suspended in K-R buffer. Viability of the mouse pancreatic islet cells (determined by Trypan blue exclusion) was ∼80%.
Real-Time PCR Analysis
Two micrograms of total RNA isolated from cultured mouse pancreatic acinar 266–6 cells, primary mouse pancreatic acinar cells, and human pancreatic total RNA samples (Biochain, Hayward, CA) were treated with DNase I (Invitrogen, Carlsbad, CA). The DNase I-treated samples were subjected to cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). To amplify the coding region of mouse (m) THTR-1, mTHTR-2, human (h) THTR-1, hTHTR-2, human β-actin, and acidic ribosomal phosphoprotein, we used the gene-specific primers (Table 1) for real-time PCR study. Real-time PCR conditions were the same as described previously (11, 28), and, for quantification, the relative relationship method was followed as described previously (9).
Western Blot Analysis
Western blot analysis was performed as previously described using a membranous fraction (60 μg) of mouse primary pancreatic acinar cells (22, 28). Briefly, acinar cells were resuspended in homogenizing buffer [300 mM mannitol, 12 mM Tris/HEPES, and a cocktail of protease inhibitors (Boehringer Manheim), pH 7.4] and homogenized using a mechanical homogenizer. The homogenate was centrifuged at 500 g for 5 min. The supernatant was centrifuged at 20,000 g for 20 min, and then the pellet was resuspended in vesicle buffer (100 mM mannitol, 100 mM KCl, 1 mM MgSO4, and 20 mM Tris/HEPES, pH 7.4) after centrifugation (20,000 g for 10 min) (29). The isolated membranous fractions were resolved onto a premade 4–12% Bis-Tris minigel (Invitrogen) (22, 28). After electrophoresis, proteins were electroblotted onto an immobilon polyvinylidene difluoride membrane (Fisher Scientific) and then blocked overnight with Odyssey blocking solution (Li-COR Bioscience, Lincoln, NE) at 4°C. Along with blocking buffer, the membranes were also incubated overnight either with mouse THTR-1 or THTR-2 specific (1:500 dilution) polyclonal antibody (raised in rabbit) along with β-actin (1:3,000 dilution) monoclonal antibody (raised in mouse). The mTHTR-1, mTHTR-2, and mouse β-actin immunoreactive bands were detected as described previously (11, 20).
Assessment of Luciferase Activity in 266–6 Cells, Primary Pancreatic Acinar Cells, and Islet Cells Isolated From Transgenic Mice Carrying SLC19A2 and SLC19A3 Promoters
The full-length human SLC19A2 (−2,250 to −36) and SLC19A3 (−1,957 to +59) promoters fused to the luciferase reporter gene used in this study were previously generated and characterized by us (12, 21). Pancreatic acinar 266–6 cells were cotransfected in 12-well plates at 50–80% confluency with 2 μg of each promoter construct and 100 ng of the transfection control plasmid containing Renilla luciferase-thymidine kinase (Promega, Madison, WI). Transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Two days later, Renilla-normalized firefly luciferase activity was determined by the Dual Luciferase Assay System (Promega) as described before (12, 21).
Transgenic mice carrying the SLC19A2 (2,214-bp) or SLC19A3 (2,016-bp) promoters fused to the firefly luciferase reporter gene were used in this investigation and have been well characterized previously (12, 21). For transgene assays, primary pancreatic acinar cells and islets were isolated as described earlier (11, 28), homogenized in ice-cold passive lysis buffer (Promega), and centrifuged to pellet debris (25,000 g, 10 min). Supernatants were taken for measurement of the firefly luciferase activity using a luciferase assay system as described above. Luciferase activity was normalized to total protein concentration for each sample.
Statistical Analysis
Uptake data are presented as means ± SE from multiple experiments and are expressed in ficomoles per milligram protein per unit time or as a percentage relative to simultaneously performed controls. The Student's t-test was used for statistical analysis. A P value of <0.05 was considered as statistically significant. Carrier-mediated uptake of [3H]thiamin was determined by subtracting uptake in the presence of a high pharmacological concentration of unlabeled thiamin (1 mM) from uptake in its absence. Protein, mRNA, and promoter assays were performed on at least three different occasions using at least three different preparations.
RESULTS
General Characteristics of the Thiamin Uptake by Mouse Pancreatic Acinar Cells
Physiological aspects.
In these studies, we first examined certain physiological aspects of the thiamin uptake process of pancreatic acinar cell of our animal model, the mouse, using mouse-derived pancreatic acinar 266–6 cells and freshly isolated primary mouse pancreatic acinar cells. Our results showed that adding unlabeled thiamin (1 mM) to the incubation medium of cultured 266–6 cells causes significant inhibition (P < 0.01) in the initial rate (7 min; data not shown) of [3H]thiamin (25 nM) uptake (43.12 ± 1.30 and 11.86 ± 2.20 fmol·mg protein−1·7 min−1 for control and unlabeled thiamin, respectively). On the other hand, adding the unrelated organic cation tetraethylammonium (TEA) did not significantly affect [3H]thiamin uptake (32.75 ± 4.52 and 29.97 ± 4.50 fmol·mg protein−1·7 min−1 for control and in the presence of TEA, respectively). The presence of the membrane transport inhibitor amiloride (1 mM) [a known inhibitor of carrier-mediated thiamin uptake by a variety of cellular systems (11, 23, 27)] in the incubation medium also significantly (P < 0.05) inhibited [3H]thiamin (25 nM) uptake (17.34 ± 1.90 and 11.87 ± 1.53 fmol·mg protein−1·7 min−1 for control and in the presence of amiloride, respectively). Finally, carrier-mediated [3H]thiamin (25 nM) uptake by 266–6 cells was found to be significantly (P < 0.05) higher at pH 7.4 compared with pH 5.5 (31.02 ± 4.4 and 17.2 ± 3.56 fmol·mg protein−1·7 min−1, respectively). These findings suggest the involvement of a specific, amiloride- and pH- sensitive carrier-mediated process for thiamin uptake by 266–6 cells.
Similar findings were observed with freshly isolated mouse primary pancreatic acinar cells where unlabeled thiamin (1 mM) caused a significant (P < 0.01) inhibition in [3H]thiamin (0.25 μM) uptake (37.26 ± 6.7 and 12.84 ± 1.99 fmol·mg protein−1·7 min−1 for control and in the presence of unlabeled thiamin, respectively), whereas TEA was without effect (55.57 ± 4.67 and 51.22 ± 7.74 fmol·mg protein−1·7 min−1 for control and in the presence of TEA, respectively). Also, amiloride (1 mM) led to a significant (P < 0.01) inhibition in [3H]thiamin uptake by primary mouse acinar cells (21.72 ± 2.51 and 12.28 ± 0.93 fmol·mg protein−1·7 min−1 for control and in the presence of amiloride, respectively). Finally, uptake of [3H]thiamin by primary acinar cells was significantly (P < 0.01) higher at pH 7.4 compared with pH 5.5 (30.83 ± 3.28 and 9.19 ± 0.82 fmol·mg protein−1·7 min−1, respectively). These findings suggest the involvement of a specific, amiloride- and pH-sensitive carrier-mediated process for thiamin uptake by primary pancreatic acinar cells. The findings are similar to those observed with the mouse-derived pancreatic acinar 266–6 cells and demonstrate the suitability of the latter cell type as an in vitro model system to use for further characterizing the finer details of the thiamin uptake process of pancreatic acinar cells.
Molecular aspects of thiamin uptake by mouse pancreatic acinar cells.
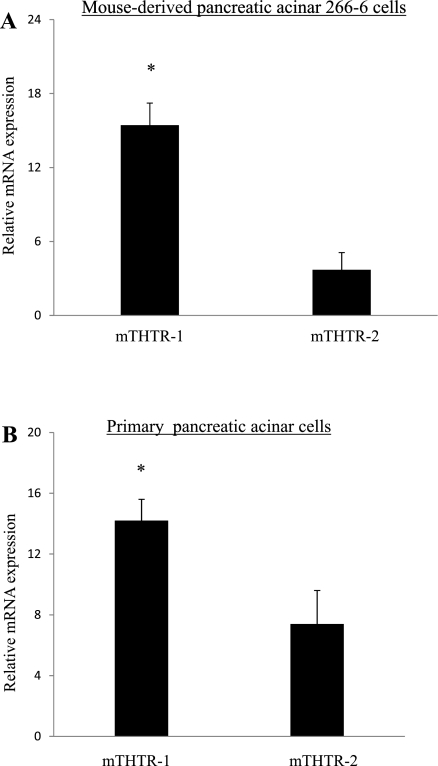
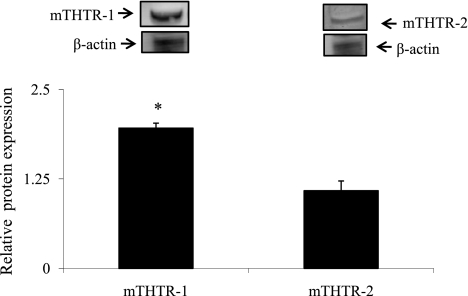
In these investigations, we examined (by mean of real-time PCR) expression of mTHTR-1 and mTHTR-2 in the 266–6 cells. The results showed that both transporters are expressed in these cells, with expression of mTHTR-1 being significantly (P < 0.01) higher than that of mTHTR-2 (Fig. 1A). Similarly, mRNA of both mTHTR-1 and mTHTR-2 are expressed in mouse primary acinar cells, with expression of the former being higher than that of the latter (Fig. 1B). We also determined the level of expression of mTHTR-1 and mTHTR-2 proteins in primary mouse acinar cells by Western blot analysis and observed a significantly (P < 0.01) higher level of expression of mTHTR-1 protein than mTHTR-2 protein (Fig. 2). Finally, we examined relative activity of the full-length promoter of SLC19A2 (the gene that encodes hTHTR-1) compared with activity of the full-length promoter of SLC19A3 (which encodes hTHTR-2) after transfection into 266–6 cells and observed a significantly (P < 0.01) higher activity for the SLC19A2 promoter compared with the SLC19A3 promoter (162 ± 30.5 and 6.7 ± 1.9 arbitrary units for SLC19A2 and SLC19A3, respectively).
Fig. 1.
Relative expression of mouse (m) thiamin transporter (THTR)-1 and mTHTR-2 mRNA in mouse-derived pancreatic acinar 266–6 cells and primary mouse pancreatic acinar cells. A and B: total RNA was isolated from pancreatic acinar 266–6 cells, and mouse primary pancreatic acinar cells were subjected to real-time PCR using mTHTR-1 and mTHTR-2 gene-specific primers (Table 1); samples were normalized relative to acidic ribosomal phosphoprotein expression. Data are means ± SE of three separate sample preparations or from at least five mice. *P < 0.01.
Fig. 2.
Expression of mTHTR-1 and mTHTR-2 at the protein levels in mouse primary pancreatic acinar cells. Western blot analyses were carried out using whole cell proteins (60 μg) isolated from mouse primary pancreatic acinar cells and were resolved on 4–12% SDS-PAGE and electroblotted onto an immobilon polyvinylidene difluoride membrane as described in materials and methods. Blots were incubated with rabbit polyclonal mTHTR-1 and mTHTR-2 antibodies. Samples were normalized relative to β-actin protein expression. Data are means ± SE from three different samples from three different mice. *P < 0.01.
Relative expression of mTHTR-1 and mTHTR-2 in pancreatic acinar cells compared with pancreatic islet cells.
In these studies, we aimed at determining and comparing the relative level of expression of mTHTR-1 and mTHTR-2 in native pancreatic acinar cells compared with islet cells of the same animals, since these two cell types have different metabolic needs and function. For this we, used wild-type and transgenic mice carrying the SLC19A2 and SLC19A3 promoters that have been generated and characterized previously in our laboratory (12, 21). Our results showed a significantly (P < 0.05 for both) lower level of expression of the endogenous mTHTR-1 and mTHTR-2 mRNA in pancreatic acinar cells compared with islet cells (Fig. 3A). Similarly, the pattern of activity of the SLC19A2 and SLC19A3 promoters was found to be significantly (P < 0.01 for both) lower in pancreatic acinar cells compared with islet cells in both transgenic mouse models (Fig. 3B).
Fig. 3.
Relative activity of the SLC19A2 and SLC19A3 promoters and mTHTR-1 and mTHTR-2 mRNA expression levels in primary pancreatic acinar and islet cells. A: total RNA was isolated from mouse primary pancreatic acinar cells and islet cells from the same animal and was subjected to real-time PCR as described in the legend for Fig. 1. Data are means ± SE from three different samples from at least three different mice. **P < 0.05. B: primary pancreatic acinar and islet cells were simultaneously isolated (11) from transgenic mice carrying full-length SLC19A2 and SLC19A3 5′-regulatory region as described in materials and methods. Firefly luciferase (LU) activity in pancreatic acinar cells and islet cells was determined in transgenic mice carrying SLC19A2 and SLC19A3 5′-regulatory region fused to firefly luciferase reporter gene. Data are means ± SE of 6–8 separate determinations from multiple mice. *P < 0.01.
Relative expression of hTHTR-1 and hTHTR-2 in human pancreatic acinar cells.
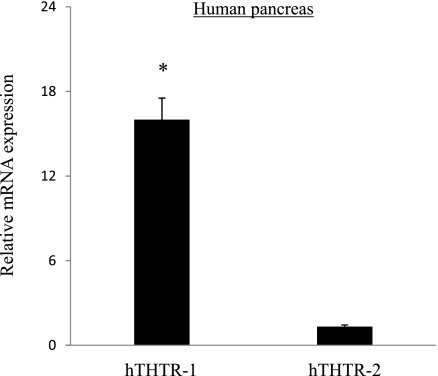
To extend our studies to the human situation, we determined the level of expression of hTHTR-1 and hTHTR-2 in native human pancreas. We reasoned that, since acinar cells represent the majority of pancreatic mass [between 80 and 85% of total pancreatic cells (28)], such an estimation would reflect the level of hTHTR-1 and hTHTR-2 in human pancreatic acinar cells. Estimation was performed by mean of real-time PCR on normal human (adult male) total RNA samples obtained from a commercial source (Biochain). The results showed that both hTHTR-1 and hTHTR-2 are expressed in human pancreas and that expression of the former is significantly higher (P < 0.01) than that of the latter (Fig. 4).
Fig. 4.
Expression of human (h) THTR-1 and hTHTR-2 mRNA in human pancreas. Real-time PCR was performed as described in materials and methods using hTHTR-1 and -2 primers and normalized using human β-actin gene. Data are means ± SE from different human RNA samples. *P < 0.01.
Relative Contribution of mTHTR-1 and mTHTR-2 Toward Carrier-Mediated Thiamin Uptake by Pancreatic Acinar Cells
Studies utilizing gene-specific siRNA and cultured 266–6 cells.
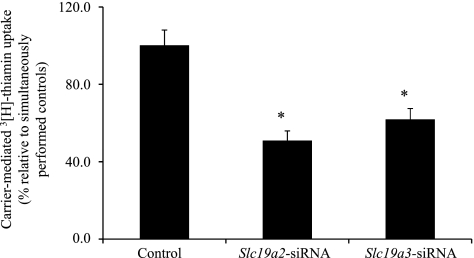
In this in vitro approach, we used gene-specific siRNA to knockdown mTHTR-1 and mTHTR-2 of the 266–6 cells and then examined the effect of that knockdown on carrier-mediated thiamin (25 nM) uptake. First we established that the use of gene-specific siRNA leads to a reduction in the level of expression of the targeted gene. Thus, cells treated with Slc19a2 siRNA showed a significant (P < 0.01) reduction in the Slc19a2 mRNA level (100 ± 7.4 and 68.8 ± 5.1 for control and Slc19a2 siRNA-treated cells, respectively). Similarly, cells treated with Slc19a3 siRNA showed a significant (P < 0.01) reduction in Slc19a3 mRNA (100 ± 3.2 and 63.0 ± 12.9 for control and Slc19a3 siRNA-treated cells, respectively). Using these cells, we then examined the effect of knocking down mTHTR-1 and mTHTR-2 on carrier-mediated thiamin uptake and observed a significant (P < 0.01 for both) inhibition in both cases (Fig. 5).
Fig. 5.
Effect of gene-specific Slc19a2 and Slc19a3 small-interfering RNA (siRNA) on carrier-mediated thiamin uptake in 266–6 cells. Carrier-mediated [3H]thiamin uptake was examined in control (negative) and Slc19a2 and Slc19a3 gene-specific siRNA-treated 266–6 cells. Uptake data are means ± SE of at least three separate experiments. *P < 0.01.
Studies utilizing freshly isolated primary pancreatic acinar cells from SLC19A2 and SLC19A3 knockout mouse models.
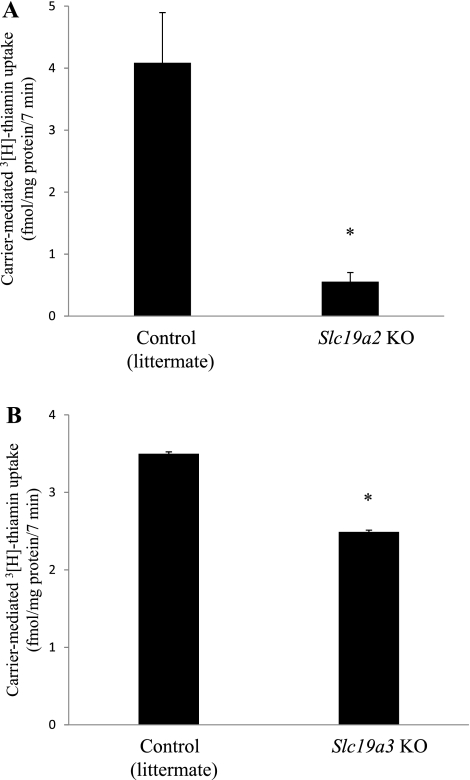
In these studies, we used Slc19a2 knockout mice (14) and Slc19a3 knockout mice (20) to determine the relative contribution of mTHTR-1 and mTHTR-2 toward carrier-mediated thiamin uptake by primary pancreatic acinar cells. Data were compared with thiamin uptake by pancreatic acinar cells of their respective littermates. The results showed a significant (P < 0.01 for both) inhibition in carrier-mediated [3H]thiamin (25 μM) uptake by pancreatic acinar cells of both knockout mouse models compared with uptake by their respective littermates (Fig. 6). However, the inhibition observed in thiamin uptake by pancreatic acinar cells of the Slc19a2 knockout mouse model was markedly (P < 0.01) greater than the inhibition observed in the Slc19a3 knockout mouse model (Fig. 6, A and B). These findings are similar to those seen with the gene-specific siRNA knockdown approach described above and provide physiological validation to the in vitro findings.
Fig. 6.
Uptake of [3H]thiamin by primary pancreatic acinar cells of Slc19a2 and Slc19a3 knockout mice. Primary pancreatic acinar cells were isolated from control (littermates) and Slc19a2 and Slc19a3 knockout mice as described in materials and methods. Carrier-mediated [3H]thiamin (0.25 μM) uptake was then determined as described earlier. Data are means ± SE from at least three different sets of samples from multiple mice. *P < 0.01.
DISCUSSION
The pancreas contains a high level of thiamin (16), and thiamin deficiency leads to impairment in both its exocrine and endocrine functions (18, 19, 25). With regard to the exocrine function, a reduction in the level of digestive enzymes has been reported as a result of thiamin deficiency. Pancreatic acinar cells, like all other mammalian cells, cannot synthesize thiamin and thus must obtain the vitamin from the surrounding environment. Little was known about the mechanisms involved in thiamin uptake by pancreatic acinar cells until recently where studies from our laboratory using pancreatic acinar cells of rat origin have shown the involvement of a specific carrier-mediated process in thiamin uptake by these cells (28). That study also showed that both THTR-1 and THTR-2 are expressed in these cells. Little, however, is known so far about the relative contribution of these systems toward total carrier-mediated thiamin uptake. We addressed these issues in the present investigation using the following two approaches: a gene-silencing approach with the use of gene-specific siRNA and a gene knockout approach using Slc19a2 and Slc19a3 knockout mouse models. The pancreatic acinar 266–6 cells were used as a model with the first approach. First we established that mouse pancreatic acinar cells take up thiamin via a specific carrier-mediated process. This was accomplished by demonstrating that [3H]thiamin uptake by both mouse-derived cultured 266–6 pancreatic acinar cells and by primary pancreatic acinar cells is significantly inhibited by unlabeled thiamin but not by the unrelated organic cation TEA. We also showed that both of these cell types express mTHTR-1 and mTHTR-2 and that expression of the former is markedly higher than that of the latter. The same was found when we used native human pancreatic RNA samples in that both of these thiamin transporters were expressed with expression of hTHTR-1 higher than that of hTHTR-2. In line with these findings was the observation that activity of the SLC19A2 promoter is higher than that of the SLC19A3 promoter in 266–6 cells. In a separate study, we were interested in gaining insight into the level of expression of the respective thiamin transporters in pancreatic acinar cells compared with pancreatic islets (β-cells) of the same animals, since these cells have different metabolic needs and function. For this, we used wild-type and transgenic mice carrying the SLC19A2 and SLC19A3 promoters (12, 21). The results showed a lower level of mRNA expression of endogenous mTHTR-1 and mTHTR-2 in pancreatic acinar compared with islet cells of the same animal. Similarly, activities of the SLC19A2 and SLC19A3 promoters in transgenic mice carrying these promoters were lower in pancreatic acinar cells compared with pancreatic islet cells of the same animal. Based on these data, it is reasonable to speculate that the higher level of expression of the thiamin transporters in pancreatic islets compared with acinar cells is a reflection of their higher metabolic demand for thiamin.
Following the establishment of the involvement of a carrier-mediated process in thiamin uptake by mouse pancreatic acinar cells, and expression of both mTHTR-1 and mTHTR-2 in these cells, we examine the relative contribution of these two transport systems toward total thiamin carrier-mediated uptake. Our results with the siRNA gene-silencing approach in 266–6 cells showed that knocking down mTHTR-1 and mTHTR-2 led to a significant inhibition in carrier-mediated thiamin uptake. Similarly, thiamin uptake by freshly isolated pancreatic acinar cells from Slc19a2 and Slc19a3 knockout mice was, in both cases, found to be significantly lower than uptake by pancreatic acinar cells of the respective littermate controls. The degree of inhibition in thiamin uptake upon knocking out the Slc19a2 gene was more than that observed upon knocking out the Slc19a3 gene. No such clear difference in the level of inhibition in thiamin uptake was observed with the siRNA knockdown approach, which may be because of the nature of the two approaches (i.e., in vitro knockdown of mRNA vs. genetic knockout of the transporter). Nevertheless, data of both approaches clearly point to the involvement of both mTHTR-1 and mTHTR-2 in thiamin uptake by pancreatic acinar cells.
In summary, findings of these investigations demonstrate that thiamin uptake by mouse pancreatic acinar cells is carrier-mediated in nature, that both mTHTR-1 and mTHTR-2 are expressed in these cells, and that both systems contribute toward carrier-mediated uptake of the vitamin.
GRANTS
This study was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK-56061 and AA-18071).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Berdanier CD. Advanced Nutrition: Micronutrients. New York, NY: CRC, 1998 [Google Scholar]
- 2. Calingasan NY, Gandy SE, Baker H, Sheu KF, Smith JD, Lamb BT, Gearhart JD, Buxbaum JD, Harper C, Selkoe DJ, Price DL, Sisodia SS, Gibson GE. Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am J Pathol 149: 1063–1071, 1996 [PMC free article] [PubMed] [Google Scholar]
- 3. Capdevila A, Decha-Umphai W, Song KH, Borchardt RT, Wagner C. Pancreatic exocrine secretion is blocked by inhibitors of methylation. Arch Biochem Biophys 345: 47–55, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholic. I. Aetiological role of aneurin and other B-complex vitamins. Br Med J 2: 1290–1292, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frederikse PH, Farnsworth P, Zigler JS., Jr Thiamine deficiency in vivo produces fiber cell degeneration in mouse lenses. Biochem Biophys Res Commun 258: 703–707, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation 40: 437–438, 1985 [DOI] [PubMed] [Google Scholar]
- 7. Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest 100: 1853–1862, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hellman B. Studies in obese-hyperglycemic mice. Ann NY Acad Sci 131: 541–558, 1965 [DOI] [PubMed] [Google Scholar]
- 9. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Matsushima K, MacManus JP, Hakim AM. Apoptosis is restricted to the thalamus in thiamine-deficient rats. NeuroReport 8: 867–870, 1997 [PubMed] [Google Scholar]
- 11. Mee L, Nabokina SM, Sekar VT, Subramanian VS, Maedler K, Said HM. Pancreatic β-cells and islets take up thiamin by a regulated carrier-mediated process: studies using mice and human pancreatic preparations. Am J Physiol Gastrointest Liver Physiol 297: G197–G206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Oishi K, Barchi M, Au AC, Gelb BD, Diaz GA. Male infertility due to germ cell apoptosis in mice lacking the thiamin carrier, Tht1. A new insight into the critical role of thiamin in spermatogenesis. Dev Biol 266: 299–309, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Oishi K, Hofmann S, Diaz GA, Brown T, Manwani D, Ng L, Young R, Vlassara H, Ioannou YA, Forrest D, Gelb BD. Targeted disruption of Slc19a2, the gene encoding the high-affinity thiamin transporter Thtr-1, causes diabetes mellitus, sensorineural deafness and megaloblastosis in mice. Hum Mol Genet 11: 2951–2960, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Pietrzak I, Baczyk K, Mlynarczyk M, Kaczmarek M. Content of thiamin in plasma and erythrocytes in patients with end stage renal disease. Przegl Lek 53: 423–426, 1996 [PubMed] [Google Scholar]
- 16. Prasannan KG, Sundaresan R, Venkatesan D. Thiamine deficency and protein secretion by pancreatic slices in vitro. Experientia 33: 169–170, 1977 [DOI] [PubMed] [Google Scholar]
- 17. Raskin NH, Fishman RA. Neurologic disorders in renal failure. N Engl J Med 294: 204–210, 1976 [DOI] [PubMed] [Google Scholar]
- 18. Rathanaswami P, Pourany A, Sundaresan R. Effects of thiamine deficiency on the secretion of insulin and the metabolism of glucose in isolated rat pancreatic islets. Biochem Int 25: 577–583, 1991 [PubMed] [Google Scholar]
- 19. Rathanaswami P, Sundaresan R. Effects of thiamine deficiency on the biosynthesis of insulin in rats. Biochem Int 24: 1057–1062, 1991 [PubMed] [Google Scholar]
- 20. Reidling JC, Lambrecht N, Kassir M, Said HM. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 138: 1802–1809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reidling JC, Said HM. In vitro and in vivo characterization of the minimal promoter region of the human thiamin transporter SLC19A2. Am J Physiol Cell Physiol 285: C633–C641, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Said HM, Mee L, Sekar VT, Ashokkumar B, Pandol SJ. Mechanism and regulation of folate uptake by pancreatic acinar cells: effect of chronic alcohol consumption. Am J Physiol Gastrointest Liver Physiol 298: G985–G993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Said HM, Ortiz A, Subramanian VS, Neufeld EJ, Moyer MP, Dudeja PK. Mechanism of thiamine uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM460. Am J Physiol Gastrointest Liver Physiol 281: G144–G150, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Saito N, Kimura M, Kuchiba A, Itokawa Y. Blood thiamine levels in outpatients with diabetes mellitus. J Nutr Sci Vitaminol (Tokyo) 33: 421–430, 1987 [DOI] [PubMed] [Google Scholar]
- 25. Singh M. Effect of thiamin deficiency on pancreatic acinar cell function. Am J Clin Nutr 36: 500–504, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Stagg AR, Fleming JC, Baker MA, Sakamoto M, Cohen N, Neufeld EJ. Defective high-affinity thiamine transporter leads to cell death in thiamine-responsive megaloblastic anemia syndrome fibroblasts. J Clin Invest 103: 723–729, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subramanian VS, Mohammed ZM, Molina A, Marchant JS, Vaziri ND, Said HM. Vitamin B1 (thiamine) uptake by human retinal pigment epithelial (ARPE-19) cells: mechanism and regulation. J Physiol 582: 73–85, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subramanya SB, Subramanian VS, Sekar VT, Said HM. Thiamin uptake by pancreatic acinar cells: effect of chronic alcohol feeding/exposure. Am J Physiol Gastrointest Liver Physiol 301: G896–G904, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sweet DH, Miller DS, Pritchard JB. Basolateral localization of organic cation transporter 2 in intact renal proximal tubules. Am J Physiol Renal Physiol 279: F826–F834, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Tallaksen CM, Bohmer T, Bell H. Blood and serum thiamin and thiamin phosphate esters concentrations in patients with alcohol dependence syndrome before and after thiamin treatment. Alcohol Clin Exp Res 16: 320–325, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Tanphaichitr V. Modern Nutrition in Health and Disease, edited by Shils ME, Olsen JA, Shike M. New York: Lea and Febiger, 1994, p. 359–375 [Google Scholar]
- 32. Thomson AD. The absorption of radioactive sulphur-labelled thiamine hydrochloride in control subjects and in patients with intestinal malabsorption. Clin Sci 31: 167–179, 1966 [PubMed] [Google Scholar]
- 33. Victor MG. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition. Philadelphia, PA: Davis, 1989 [Google Scholar]