Abstract
Intestinal inflammation is characterized by epithelial disruption, leading to loss of barrier function and the recruitment of immune cells, including neutrophils. Although the mechanisms are not yet completely understood, interactions between environmental and immunological factors are thought to be critical in the initiation and progression of intestinal inflammation. In recent years, it has become apparent that the di/tripeptide transporter PepT1 may play an important role in the pathogenesis of such inflammation. In healthy individuals, PepT1 is primarily expressed in the small intestine and transports di/tripeptides for metabolic purposes. However, during chronic inflammation such as that associated with inflammatory bowel disease, PepT1 expression is upregulated in the colon, wherein the protein is normally expressed either minimally or not at all. Several recent studies have shown that PepT1 binds to and transports various bacterial di/tripeptides into colon cells, leading to activation of downstream proinflammatory responses via peptide interactions with innate immune receptors. In the present review, we examine the relationship between colonic PepT1-mediated peptide transport in the colon and activation of innate immune responses during disease. It is important to understand the mechanisms of PepT1 action during chronic intestinal inflammation to develop future therapies addressing inappropriate immune activation in the colon.
inflammatory bowel disease (IBD) is a chronic inflammatory condition that affects the gastrointestinal (GI) tract. IBD affects 1.4 million people in the United States and 2.2 million people in Europe (49, 65). Thus understanding the etiology and the relevant pathological mechanisms of IBD are of great interest in the gastroenterology field. Two clinical forms of IBD have been extensively studied: Crohn's disease (CD) and ulcerative colitis (UC). In CD, inflammation occurs anywhere throughout the GI tract but primarily affects the ileum, whereas, in UC, the colonic mucosa is principally involved (43). However, both diseases are thought to feature alterations in the immune response to GI microbiota in individuals genetically predisposed to IBD, which is characterized by intestinal epithelial barrier disruption and an influx of immune cells (64). These events increase the extent of inflammation and upregulate proinflammatory cytokine production (50). Although the precise etiology of IBD remains unknown, several key mechanisms of IBD pathogenesis have been identified in vitro using animal models and via genetic analyses of IBD-affected individuals. In the present review, we will focus on one member of the proton-coupled oligopeptide transporter (POT) family, PepT1, and the role played by this protein in intestinal inflammation. We will summarize the status of present research suggesting the existence of an important link between PepT1 transporter activity and initiation of the innate immune response in IBD.
The POT Superfamily
The POT family includes four transporter proteins belonging to the SLC15A solute carrier group: two H+-coupled oligopeptide transporters, PepT1 (SLC15A1) and PepT2 (SLC15A2), and two peptide/histidine transporters (PHTs), PHT1 (SLC15A4) and PHT2 (SLC15A3). POT members transport a vast array of di/tripeptides and also various peptide-derived drugs (29). In contrast to the PepT transporters, PHT family members transport free histidine in addition to di/tripeptides (29). The expression levels of POT family members vary among the tissue and cell types of the body; the expression patterns also vary among species. PHT1 expression has been detected in the human brain, retina, placenta (29), and immune cells (8), in addition to the human cell lines HeLa, HEK293T, and MCF-7 (47). Although the function of PHT2 is poorly understood, this protein is expressed in immune tissue including the spleen and thymus (29, 63), in addition to the lung, heart, and adrenal gland (63). PHT1 and PHT2 are only 20–26% homologous to PepT proteins at the amino acid level (37), and work on the roles played by PHT proteins in both health and disease has only recently been initiated. However, many studies have explored the roles played by PepT1 and PepT2, and an understanding of the functions of these proteins in both health and disease is emerging.
Originally, PepT2 expression was considered to be primarily confined to the kidney, so PepT2 was termed the renal isoform. However, expression is also evident in several other tissues and cell types including astrocytes and epithelial cells within the choroid plexus (79), enteric glial cells (11), lung epithelial cells (5, 35), myeloid cells (17), nasal epithelium (4), and many cell lines including HEK293T, HeLa, and MCF-7 (47). PepT1 is known as the intestinal isoform because the protein is primarily expressed in the brush border membranes of enterocytes of the small intestine. PepT1 and PepT2 share 50% amino acid sequence identity, and both proteins transport di/tripeptides but not tetrapeptides or free amino acids using the energy generated by an inwardly directed transmembrane proton gradient (7). Hydropathy plots predict that both PepT proteins contain 12 membrane-spanning domains and that both the amino- and carboxy-termini are most likely located in the cytoplasm (11, 21, 31). However, the three-dimensional structures of PepT1 and PepT2 remain unknown. Because of the high expression of PepT1 in the intestine, several studies have sought to identify the role played by the protein in diseases that affect the GI tract, including IBD.
Structure, Function, and Expression of PepT1
The cDNA encoding human PepT1 is 2,263 base pairs in length and has an open reading frame of 2,127 base pairs, encoding a 708-amino-acid protein (16). The predicted molecular weight of PepT1 is 78 kDa (48). Many site-directed mutagenesis studies have identified amino acids within PepT1 that are necessary for substrate binding and transport (52); these include the extracellular histidine residue of the second transmembrane domain (H57). When this histidine is mutated, PepT1 function is eliminated (28). Additionally, the first four amino-terminal and the seventh and ninth transmembrane domains are required for substrate binding and transporter functionality (29). Hydropathy analysis predicts the existence of a 200-amino acid hydrophilic segment between transmembrane domains 9 and 10, presumably containing seven N-linked glycosylation sites and two potential protein kinase C-dependent phosphorylation sites (37). Several studies have shown that the human intestinal cell line, Caco2-BBE, is an appropriate model for exploration of the expression and function of PepT1. Earlier work on dipeptide uptake by Caco-2 cells determined that uptake was twofold greater when the extracellular pH was pH 6.0 compared with pH 7.5 (2). Additionally, Thwaites et al. (72) found that intracellular pH decreased when the dipeptide, glycylsarcosine (Gly-Sar), was added to the medium, and, in a separate study, Brandsch et al. (2, 12) reported that intracellular acidification of Caco-2 cells upon addition of NH4Cl to the medium decreased the transport of Gly-Sar. Overall, these reports, among others, established that di/tripeptide PepT1 transport was mediated by an inwardly directed proton gradient.
PepT1 is primarily expressed in the brush border membranes of enterocytes of the small intestine. However, expression has also been detected in nasal epithelium, kidney cells, bile duct epithelial cells, and macrophages (4, 15, 16). PepT1 is most abundantly expressed in the jejunum, followed by the ileum, and little or no expression is evident in the normal colon, stomach, or esophagus (60). Recent work has shown that PepT1 associates with lipid rafts within small intestinal brush border membranes and that this association modulates peptide transport, based on the polarity of the cell type analyzed (58). Under normal physiological conditions in the small intestine, intestinal epithelial cells apically express PepT1, which aids in the transport and absorption of di/tripeptides from the diet. During chronic inflammation, however, the expression profile of PepT1 within the GI tract is altered. In patients with chronic diseases such as IBD and short bowel syndrome, PepT1 expression is upregulated such that the protein is expressed in the colon (53, 80). This enhanced expression of PepT1 may be explained in several ways. For example, upregulation of proinflammatory cytokine and hormone levels during disease may enhance PepT1 expression. Both TNF-α and IFN-γ levels are upregulated in IBD patients, and both cytokines are capable of enhancing PepT1 expression and transport activity in the human intestinal cell line Caco2-BBE (13, 76). Recently, it was shown that IFN-γ upregulated PepT1 expression and transport function in primary human intestinal monolayers (32). Furthermore, in addition to proinflammatory cytokines, the adipocyte-secreted hormone leptin has been found to increase PepT1 expression and to act on Caco2-BBE cells. Leptin-mediated upregulation of hPepT1 promoter activity was dependent on expression of the transcription factors CREB and Cdx2 (56). Interestingly, leptin is not expressed in the small or large intestines of healthy individuals but has been detected in colonic epithelial cells from inflamed tissues (68).
Pathogenic bacteria have also previously been shown to induce expression of PepT1 in the colon (59). Nguyen et al. (59) showed that in vitro, enteropathogenic Escherichia coli enhanced PepT1 expression and transport activity in the human colonic epithelial cell line HT29-Cl.19A, which does not normally express PepT1. Increased synthesis of PepT1 was dependent on activation of the Cdx2 transcription factor. In vivo, Citrobacter rodentium (C. rodentium) induced PepT1 expression in both the proximal and distal colon of mice (59). Interestingly, in cell cultures prepared from colons of transgenic mice in which PepT1 was overexpressed on the villin promoter, C. rodentium attachment was attenuated compared with the levels associated with wild-type colon cultures. In addition, KC mRNA and protein levels were downregulated when PepT1 expression increased (59). These data suggest that PepT1 may play a protective role during C. rodentium infection. In a separate study, Lactobacillus casei, a probiotic bacterium, increased PepT1 transporter activity in Caco-2 cells (57). Finally, butyrate, a short-chain fatty acid produced in the colon by bacterial fermentation of carbohydrates, enhanced PepT1 expression in Caco2-BBE cells via a pathway involving Cdx2 activity (26). Stimulation of Caco2-BBE cells with butyrate also enhanced PepT1 transporter activity. Overall, work in this area has shown that bacteria and/or bacterial products play a role in regulating PepT1 gene expression and activity.
A recently described class of noncoding RNAs, microRNAs, regulate gene expression via cleavage of mRNA complexes or via translational inhibition (62). Recently, Dalmasso et al. (27) showed that microRNA-92b specifically regulated PepT1 expression in differentiating Caco2-BBE cells. MicroRNA-92b suppressed PepT1 expression at both the mRNA and protein levels, and upon transfection with microRNA-92b, PepT1 transporter activity decreased (27). Overall, previous findings have shown that PepT1 expression is regulated by various means in the colon. When PepT1 expression becomes upregulated during chronic inflammation, PepT1-mediated transport of di/tripeptides increases, and disease may be exacerbated if the peptides internalized are pathogenic.
PepT1 Uptake of Bacterial Di/tripeptides Triggers Inflammatory Responses in Intestinal Epithelial Cells
The concentrations of bacterial di/tripeptides are much lower in the small intestine than in the colon, as might be expected from the fact that the human small intestine contains only low numbers of prokaryotes. Interestingly, PepT1 expression is normally restricted to the small intestine, where bacterial peptide concentrations are low, reflecting the low bacterial load of this tissue compared with that of the colon. Thus the profile of PepT1 expression along the normal human digestive tract is such that bacterial peptides have little access of PepT1, a condition that minimizes intracellular uptake of bacterial peptides. This normal expression pattern becomes altered in patients with IBD; thus with chronic UC or CD, PepT1 expression becomes evident in the colon. Commensal bacteria colonizing the human colon produce significant amounts of di/tripeptides, and several studies have shown that PepT1 can transport these small molecules into various cell types. Therefore, expression of PepT1 in the colon may lead to increased intracellular accumulation of prokaryotic peptides, triggering downstream proinflammatory effects. Previous work showed that PepT1 transported N-formylmethionyl-leucyl-phenylalanine (fMLP), a tripeptide produced by Escherichia coli, into both intestinal epithelial cells (54) and the human monocyte cell line KG-1 (15). PepT1-mediated transport of fMLP into Caco2-BBE cells was increased upon stimulation with IFN-γ (54).
After transport of fMLP by PepT1 was described, other di/tripeptides commonly found in the colon were evaluated as PepT1 substrates. Our laboratory and others reported that other small bacterial peptides were transported by PepT1; these included muramyl dipeptide (MDP) (75) and L-Ala-γ-D-Glu-meso-diaminopimelic acid (Tri-DAP) (24). MDP is a motif found in peptidoglycans within the cell walls of Gram-negative and -positive bacteria, whereas Tri-DAP is a degradation product of peptidoglycan, primarily from Gram-negative bacteria. Upon cellular uptake by intestinal epithelial cells via PepT1 action, bacterial di/tripeptides in the cytosol activate inflammatory signal transduction pathways and trigger downstream effector functions.
After it was established that PepT1 transports small bacterial peptides, several studies explored the downstream consequences of transport of these di/tripeptides in intestinal epithelial cells. Uptake of fMLP by colonic epithelial cells was shown to increase expression of major histocompatibility (MHC) class I molecules (53). Interestingly, previous studies in mice had shown that MHC class I molecules, including H-2M3a, present fMLP at the cell surface (19). These results suggest that transport of fMLP may lead to increased antigenic presentation of bacterial products by colonic epithelial cells. Therefore, induction of PepT1 expression in the colon may cause colonic epithelial cells to become more sensitive to the presence of bacterial peptides and to activate inflammatory pathways. Additionally, treatment of Caco2-BBE cells with fMLP increased the activation of NF-κB and activator protein-1 transcription factors (14). MDP and Tri-DAP also initiated NF-κB activity in Caco2-BBE cells, as was also true of fMLP in intestinal epithelia (24, 75). Additionally, Tri-DAP mediated MAPK pathway activation and upregulation of IL-8 production in Caco2-BBE cells (24). MDP-stimulated Caco2-BBE cells exhibited upregulation of IL-8 and monocyte chemoattractant protein (MCP)-1 (75). Interestingly, mucosal IL-8 and MCP-1, chemoattractants for neutrophils and monocytes, respectively, are also upregulated in colonic IBD regions (6). In a separate contrasting study, primary murine macrophages transported MDP in a PepT1-independent manner, suggesting that PepT1 transport may be cell and/or species specific (51). Overall, the data suggest that PepT1-mediated transport of bacterial peptides by intestinal epithelial cells results in downstream activation of inflammatory pathways, leading to migration of activated immune cells toward regions with higher bacterial loads, including the colon (Fig. 1). Earlier work showed that PepT1 could transport bacterial di/tripeptides into cells to initiate inflammatory functions; however, the mechanism underlying activation of downstream transcription factors remains under investigation. Recent data from our laboratory and others suggest that PepT1-mediated transport of bacterial di/tripeptides leads to interactions between bacterial di/tripeptides and intracellular innate immune receptors, initiating a proinflammatory response.
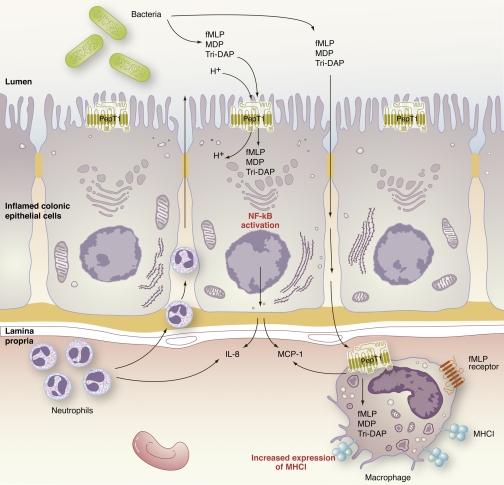
Fig. 1.
Model for PepT1 interaction with bacterial peptides. PepT1 becomes upregulated in cases of chronic inflammation, such as inflammatory bowel disease (IBD). Because of upregulation of PepT1 in the colon, bacterial di/tripeptides in the lumen are transported by PepT1 into intestinal epithelial cells. Known bacterial ligands of PepT1 include N-formylmethionyl-leucyl-phenylalanine (fMLP), muramyl dipeptide (MDP), and L-Ala-γ-D-Glu-meso-diaminopimelic acid (Tri-DAP). Upon intracellular accumulation of bacterial di/tripeptides, the NF-κB pathway is activated and downstream proinflammatory cytokine and chemokine production increases. Disruption of barrier function is associated with IBD; therefore transport of fMLP, MDP, and Tri-DAP most likely occurs via the paracellular pathway, as well as through PepT1-mediated transport. Once in the lamina propria, fMLP and other bacterial di/tripeptides may be taken up by macrophages. Upon PepT1-mediated transport of fMLP, macrophages upregulate major histocompatibility class I molecules. The increased production of cytokines/chemokines most likely contributes to increased cellular infiltration into areas of inflammation. MCP, monocyte chemoattractant protein.
PepT1 Interactions with Innate Immune Receptors
In addition to maintaining efficient physical and biological barriers, the intestinal epithelium plays an active role in modulating the innate and adaptive immune systems. Sensing the presence of a pathogen is the first step toward mounting an effective immune response, required for the elimination of invading pathogens and the subsequent development of protective immunity. The innate immune system includes receptors that can sense pathogen-associated molecular patterns. One group of receptors is the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family. This family includes more than 20 related members present in the cytosol, where these receptors recognize ligands (41). NOD receptors contain amino-terminal domains termed caspase-recruitment domains, a NOD, and several carboxy-terminal leucine-rich repeats (10). Interestingly, ligands specific for NOD1 and NOD2 are also transported by PepT1 in certain cell types. NOD1 is activated by peptides that contain a diaminophilic acid, including the PepT1 substrate, Tri-DAP (46). NOD2 recognizes muramyl dipeptides, including the PepT1 substrate MDP (34, 41). Upon binding of ligands to NOD receptors, NOD interacts with receptor-interacting serine/threonine-protein kinase 2, which phosphorylates the “inhibitor of NF-κB kinase”, resulting in downstream NF-κB activation. Previous studies identified mutations in the NOD2 gene in patients with CD (39, 61). These polymorphisms are present in 8–17% of Caucasian patients with CD (9), and homozygous individuals have up to a 20-fold increased risk of developing CD (but not UC) (22). Many studies have shown a link between PepT1 transport of bacterial peptides into intestinal epithelial cells and enhancement of inflammatory effector functions. However, the mechanism by which bacterial di/tripeptides activate inflammatory pathways remains unclear. Several recent relevant studies have focused on the roles played by POT family members, including PepT1, and the ability to transport bacterial peptides intracellularly, leading to interactions with innate immune receptors such as NOD receptors.
Dalmasso et al. (25) recently described an in vivo link between hPepT1 transport and NOD2 interaction. Transgenic mice expressing hPepT1 specifically on intestinal epithelial cells (transcription of the encoding gene was driven by the villin promoter), or systemically (genetic transcription was driven by the β-actin promoter), showed exacerbation of colitis compared with that of wild-type littermates. hPepT1 transgenic mice experienced greater weight loss, enhanced neutrophil infiltration, and more pronounced upregulation of proinflammatory cytokine mRNA levels upon dextran sodium sulfate (DSS) treatment, an established murine model of colitis. Exacerbated colitis in β-actin-hPepT1 transgenic mice was dependent on the presence of bacteria in the colon and NOD2 expression (25). These data suggest that PepT1 transporter activity plays an important role in increasing the intracellular concentration of bacterial peptides that subsequently interact with NOD2 to mediate downstream inflammatory functions. Future studies should address the in vivo roles played by PepT1-mediated transport and innate immune receptors including NOD receptors and intracellular Toll-like receptors (TLRs) in intestinal inflammation. Furthermore, it will be necessary to generate PepT1-deficient mouse strains for use in models of inducible colitis. Such strains will permit exploration of how endogenous colonic levels of PepT1 affect the severity of colitis. It may be hypothesized that, in the absence of PepT1, colitis would be attenuated because of a decrease in the level of transport of bacterial peptides.
Interestingly, PepT1 is not the only POT family member that is known to play a role in the intracellular transport of ligands that activate innate immunity. In vitro knockdown of PHT1 in HEK293T cells decreased NF-κB activity upon stimulation with the NOD1 ligand Tri-DAP (47). In vivo, PHT1-deficient mice experienced less severe DSS-induced colitis than did controls and were impaired in terms of NOD1-dependent cytokine production (66). In a separate study, it was shown that a mutation in SLC15A4 (creating the so-called “feeble mouse” strain), which encodes PHT1, caused a decrease in IFN type I production triggered by TLR7- and TLR9-dependent signaling pathways in plasmacytoid dendritic cells (8). PepT2 also acted as an MDP transporter in human myeloid cells, where PepT2 expression was associated with phagosomes (17). Finally, γ-iE-DAP, a peptide derived via breakdown of the cell wall of Gram-negative bacteria, was recognized by NOD1 in lung epithelial cells after PepT2-specific transport in association with receptor interacting protein-2 action. PepT2/NOD1-mediated signaling led to increased NF-κB activity and a rise in the production of proinflammatory cytokines (70). Interestingly, preliminary clinical studies have suggested that PHT1 mRNA levels are upregulated in patients with UC and CD (47). These data, while preliminary in nature, suggest that there may be a mechanistic role for PHT1, in addition to PepT1, in intestinal inflammation. Overall, work to date has demonstrated the importance of PepT1 and other POT family members in the transport of bacterial peptides into cells, initiating inflammatory responses via recognition of such peptides by innate immune receptors. Therefore, determination of the expression levels of PHT1 and PHT2 in the colon during colitis may be important to enhance our understanding of the roles played by di/tripeptide transport in the inflammatory response occurring during IBD. Although PepT1 action has been implicated in both cellular and animal models of intestinal inflammation, the genetic linkage of PepT1 to human disease has only recently been discovered.
A PepT1 Polymorphism is Associated with IBD
PepT1 expression is upregulated in patients suffering from IBD and short bowel syndrome (53, 80). However, until recently, little evidence was available that directly associated PepT1 polymorphisms with human intestinal disease. After the discovery of several genes, including NOD2, that mediated susceptibility toward IBD (33, 39, 61), and after the accumulation of evidence implicating PepT1-mediated transport of NOD ligands and other bacterial peptides in IBD, Zucchelli et al. (81) examined 12 hPepT1 polymorphisms for associations with IBD. The data demonstrate that a functional hPepT1 single nucleotide polymorphism (SNP) (rs2297322) was linked to IBD in Swedish and Finnish cohorts not carrying NOD2 mutations known to be important in IBD development. Interestingly, in Swedish cohorts, rs2297322 was associated with an increased risk of IBD; however, in Finnish cohorts the same SNP was shown to be protective against IBD (81). Significantly, these preliminary findings suggest that this PepT1 mutation may play a role in IBD pathology in some populations; however, more genome-wide association studies will be required to determine which populations are at risk or protected in connection to this hPepT1 SNP. Preliminary in vitro functional analyses showed that mutated hPepT1 cotransfected into HEK293 epithelial cells, in addition to NF-κB luciferase reporter and NOD2 constructs, resulted in more luciferase activity upon stimulation with MDP (81). These results could be attributable to enhanced intracellular transport of MDP in the presence of mutated PepT1; however, future studies are needed to confirm this hypothesis. Additionally, future studies are required to explore how the mutation affects the expression and function of PepT1 during IBD and how this specific SNP confers protection or susceptibility to IBD in various populations.
Targeting PepT1 for Treatment of Intestinal Inflammation
In addition to transporting peptides that fulfill dietary needs, PepT1 has also been shown to transport several types of peptide-derived drugs including antibiotics, inhibitors of angiotensin-converting enzyme, and anticancer and antiviral drugs (2, 3, 71). Because PepT1 is expressed in the small intestine and is induced in the colon during inflammation, PepT1 is a potential target for treatment of both IBD and other diseases of the GI tract, including colon cancer. A particular advantage afforded by targeting PepT1 is the fact that drugs can be given orally, which simplifies drug administration. However, barriers compromising efficient drug delivery exist along the GI tract; these include physical barriers such as membranes and metabolic constraints such as enzymes and low pH.
Several studies have shown that anti-inflammatory PepT1 ligands may be useful in future treatment of intestinal inflammation. Lys-Pro-Val (KPV), a tripeptide from the COOH terminus of α-melanocyte-stimulating hormone, possesses anti-inflammatory properties (38, 42). Dalmasso et al. (23) showed that KPV was transported by PepT1 in vitro (23), inhibited NF-κB activation, and attenuated proinflammatory cytokine production by both Caco2-BBE and Jurkat cells that were stimulated with proinflammatory cytokines (23). In vivo, KPV reduced the level of intestinal inflammation in mice treated with DSS or 2,4,6-trinitrobenzene sulfonic acid, which are both well-established models of inducible colitis. Attenuation of colitis was detected histologically, and proinflammatory cytokine mRNA expression levels were also decreased, suggesting that inflammation was reduced by treatment with KPV (23). In a separate study, nanoparticles were used to target KPV to the colon. In this study, KPV nanoparticles were encapsulated in a polysaccharide gel that collapsed and released the drug load primarily in the colon (45). Oral administration of KPV-loaded nanoparticles attenuated colitis in mice treated with DSS. Animals receiving KPV throughout DSS treatment had less neutrophil activity and lower proinflammatory cytokine levels than did controls (45).
Colorectal cancer (CRC) is one of the most common types of malignancies (77), and the known link between chronic intestinal inflammation and CRC development has given rise to the term “colitis-associated cancer” (CAC) (53, 78). Development of CAC in patients suffering from UC is one of the best clinically characterized examples of an association between intestinal inflammation and carcinogenesis (30, 74). Several studies have shown that the PepT1 substrate and antitumor drug, bestatin, decreases cell proliferation and tumor growth. Previous work found that bestatin effectively inhibited the growth of colon adenocarcinoma 26 (colon 26) and myeloid leukemia C1498 in vivo (1).
In the past several years, much effort has been devoted to modifying drugs that are not natural substrates of intestinal transporters, so that the drugs may be transported by proteins including PepT1. The work involves attaching modifying compounds to potential drugs to cause binding and subsequent transport by PepT1. These efforts are important to minimize adverse drug effects and to decrease drug dosage. For example, prodrugs that penetrate colon walls only poorly and drugs targeting colorectal cancer have been adapted for targeted delivery via addition of specific carrier transporters to improve oral bioavailability (36, 69). One recent study focused on floxuridine, a drug commonly used to treat colon carcinoma and CRC (44, 73). Addition of dipeptide moieties increased prodrug affinity for PepT1 and enhanced the ability of prodrugs to permeate Caco-2 cell monolayers. Additionally, the modified prodrugs were more resistant to breakage of an important glycosidic bond than the parent floxuridine (73).
Finally, another line of research suggests that modification of gut flora composition may help alleviate IBD severity (67). Until recently, PepT1 expression had not been assessed in the course of such studies. However, recent work in mice showed that colonic PepT1 expression was increased, accompanied by enhanced cephalexin transport, when IL10−/− animals developed colitis (18). Also, in the same study, probiotic treatment was shown to downregulate expression of PepT1 in the colon. When IL-10−/− mice, which spontaneously develop colitis, were given Lactobacillus plantarum (L. plantarum), PepT1 expression and activity were reduced (18). Additionally, colitis was attenuated in IL-10−/− mice treated with L. plantarum compared with vehicle-treated IL-10−/− animals. In a separate study by the same group, L. plantarum attenuated the upregulation of adhesion molecules such as ICAM-1 and MAdCAM-1 in IL-10−/− mice (20). However, any relationship between attenuation of adhesion molecule expression and PepT1 downregulation upon treatment with L. plantarum remains undefined. Overall, these studies show that future potential therapies for IBD and CAC may target specific regions of inflammation via the oral route, using natural ligands of PepT1, chemically modified prodrugs that are transported by PepT1, or probiotics that downregulate PepT1 expression in the colon.
Conclusions
Continuing research has led to the development of new concepts that seek to explain the roles played by di/tripeptide transporters in intestinal disease. It is now widely accepted that PepT1 protein expression is upregulated in the colon when chronic inflammation (such as IBD) develops, and, in recent years, it has been established that PepT1 can transport bacterial peptides into cells. This internalization leads to interactions between bacterial peptides and innate immune receptors (including NOD), in turn triggering proinflammatory events (Fig. 2). Future work will determine whether PepT1-mediated transport of bacterial products leads to activation of other innate receptors such as the intracellular TLRs or other NLR proteins. Furthermore, it will be important to determine whether other POT members are involved in intestinal inflammation, as it is now known that other POT family members can transport bacterial di/tripeptides. It is essential to determine whether PepT1 can be targeted to alleviate symptoms of IBD. Colonic PepT1 is expressed at minimal levels or not at all in healthy individuals; therefore treatments that effectively downregulate PepT1 expression may attenuate the inflammation found in patients with IBD. Additionally, treatments that exploit PepT1 transporter activity will likely increase the effectiveness of particular drugs by enhancing bioavailability after oral administration. Finally, several studies have shown that PepT1 is expressed by cancer cells (40, 55). Therefore, future work should investigate the role played by PepT1 in cancer and the therapeutic potential of PepT1-mediated drug transport in both CAC and other types of cancers where PepT1 expression is present.
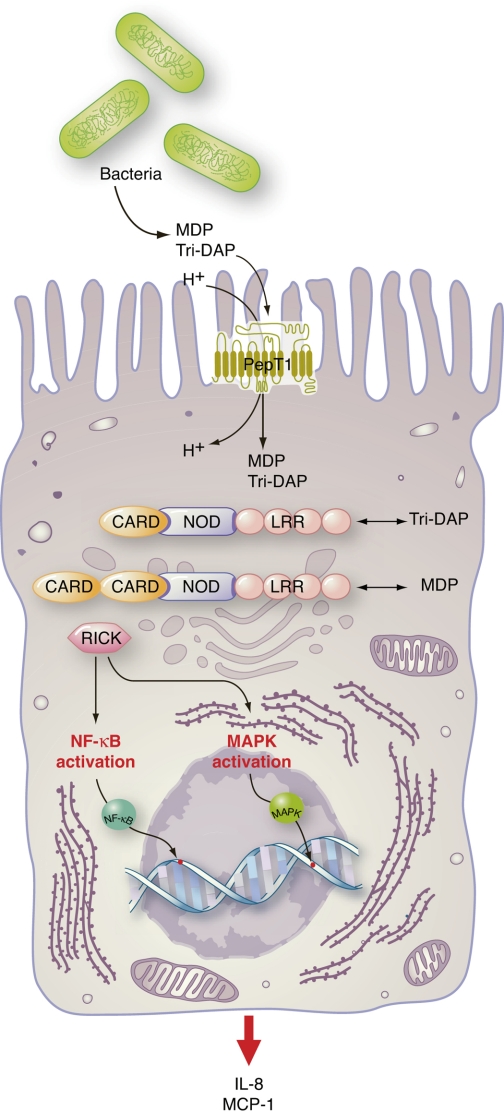
Fig. 2.
The PepT1-nucleotide-binding oligomerization domain (NOD) pathway. Upon intracellular accumulation of bacterial di/tripeptides in intestinal epithelial cells via PepT1-mediated transport, bacterial di/tripeptides interact with the NOD1/2 receptors. Associations of NOD1/2 receptors with receptor-interacting serine/threonine-protein kinase (RICK) trigger downstream activation of NF-κB and MAPK pathways leading to proinflammatory cytokine/chemokine production. Tri-DAP was previously shown to directly interact with the leucine-rich repeat (LRR) region of NOD1 to mediate its effects, whereas MDP interacts with NOD2. Because of the increase in proinflammatory cytokine/chemokine production, increased infiltration of neutrophils into inflammatory regions occurs. CARD, caspase-recruitment domains.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants, R56-DK-061941 (to D. Merlin). D. Merlin is a recipient of a Senior Research Award from the Crohn's and Colitis Foundation of America.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.A.I. prepared figures; S.A.I. drafted manuscript; S.A.I., S.A., M.A.C., H.L., Y.Y., and D.M. edited and revised manuscript; S.A.I., S.A., M.A.C., H.L., Y.Y., and D.M. approved final version of manuscript.
REFERENCES
- 1. Abe F, Shibuya K, Uchida M, Takahashi K, Horinishi H, Matsuda A, Ishizuka M, Takeuchi T, Umezawa H. Effect of bestatin on syngeneic tumors in mice. Gann 75: 89–94, 1984 [PubMed] [Google Scholar]
- 2. Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology 113: 332–340, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol 285: G779–G788, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Agu R, Cowley E, Shao D, Macdonald C, Kirkpatrick D, Renton K, Massoud E. Proton-coupled oligopeptide transporter (POT) family expression in human nasal epithelium and their drug transport potential. Mol Pharm 8: 664–672, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Bahadduri PM, D'Souza VM, Pinsonneault JK, Sadee W, Bao S, Knoell DL, Swaan PW. Functional characterization of the peptide transporter PEPT2 in primary cultures of human upper airway epithelium. Am J Respir Cell Mol Biol 32: 319–325, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol 199: 28–35, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Biegel A, Knutter I, Hartrodt B, Gebauer S, Theis S, Luckner P, Kottra G, Rastetter M, Zebisch K, Thondorf I, Daniel H, Neubert K, Brandsch M. The renal type H+/peptide symporter PEPT2: structure-affinity relationships. Amino Acids 31: 137–156, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA 107: 19973–19978, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology 124: 521–536, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3: 521–533, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Brandsch M, Knutter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol 60: 543–585, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Expression and protein kinase C-dependent regulation of peptide/H+ co-transport system in the Caco-2 human colon carcinoma cell line. Biochem J 299: 253–260, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buyse M, Charrier L, Sitaraman S, Gewirtz A, Merlin D. Interferon-gamma increases hPepT1-mediated uptake of di-tripeptides including the bacterial tripeptide fMLP in polarized intestinal epithelia. Am J Pathol 163: 1969–1977, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buyse M, Tsocas A, Walker F, Merlin D, Bado A. PepT1-mediated fMLP transport induces intestinal inflammation in vivo. Am J Physiol Cell Physiol 283: C1795–C1800, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Charrier L, Driss A, Yan Y, Nduati V, Klapproth JM, Sitaraman SV, Merlin D. hPepT1 mediates bacterial tripeptide fMLP uptake in human monocytes. Lab Invest 86: 490–503, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Charrier L, Merlin D. The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest 86: 538–546, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Charriere GM, Ip WE, Dejardin S, Boyer L, Sokolovska A, Cappillino MP, Cherayil BJ, Podolsky DK, Kobayashi KS, Silverman N, Lacy-Hulbert A, Stuart LM. Identification of Drosophila Yin and PEPT2 as evolutionarily conserved phagosome-associated muramyl dipeptide transporters. J Biol Chem 285: 20147–20154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen HQ, Yang J, Zhang M, Zhou YK, Shen TY, Chu ZX, Hang XM, Jiang YQ, Qin HL. Lactobacillus plantarum ameliorates colonic epithelial barrier dysfunction by modulating the apical junctional complex and PepT1 in IL-10 knockout mice. Am J Physiol Gastrointest Liver Physiol 299: G1287–G1297, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Chiu NM, Chun T, Fay M, Mandal M, Wang CR. The majority of H2-M3 is retained intracellularly in a peptide-receptive state and traffics to the cell surface in the presence of N-formylated peptides. J Exp Med 190: 423–434, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu ZX, Chen HQ, Ma YL, Zhou YK, Zhang M, Zhang P, Qin HL. Lactobacillus plantarum prevents the upregulation of adhesion molecule expression in an experimental colitis model. Dig Dis Sci 55: 2505–2513, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Covitz KM, Amidon GL, Sadee W. Membrane topology of the human dipeptide transporter, hPEPT1, determined by epitope insertions. Biochemistry 37: 15214–15221, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, Schreiber S, Lewis CM, Mathew CG. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 122: 867–874, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Dalmasso G, Charrier-Hisamuddin L, Nguyen HT, Yan Y, Sitaraman S, Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology 134: 166–178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dalmasso G, Nguyen HT, Charrier-Hisamuddin L, Yan Y, Laroui H, Demoulin B, Sitaraman SV, Merlin D. PepT1 mediates transport of the proinflammatory bacterial tripeptide L-Ala-γ-D-Glu-meso-DAP in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 299: G687–G696, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dalmasso G, Nguyen HT, Ingersoll SA, Ayyadurai S, Laroui H, Charania MA, Yan Y, Sitaraman SV, Merlin D. The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology 141: 1334–1345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalmasso G, Nguyen HT, Yan Y, Charrier-Hisamuddin L, Sitaraman SV, Merlin D. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLos One 3: e2476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Obertone TS, Sitaraman SV, Merlin D. MicroRNA-92b regulates expression of the oligopeptide transporter PepT1 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 300: G52–G59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66: 361–384, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflügers Arch 447: 610–618, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol 6: 297–305, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 368: 563–566, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Foster DR, Landowski CP, Zheng X, Amidon GL, Welage LS. Interferon-gamma increases expression of the di/tri-peptide transporter, h-PEPT1, and dipeptide transport in cultured human intestinal monolayers. Pharmacol Res 59: 215–220, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Gaya DR, Russell RK, Nimmo ER, Satsangi J. New genes in inflammatory bowel disease: lessons for complex diseases? Lancet 367: 1271–1284, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 8869–8872, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Granillo OM, Brahmajothi MV, Li S, Whorton AR, Mason SN, McMahon TJ, Auten RL. Pulmonary alveolar epithelial uptake of S-nitrosothiols is regulated by L-type amino acid transporter. Am J Physiol Lung Cell Mol Physiol 295: L38–L43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han HK, Oh DM, Amidon GL. Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter. Pharm Res 15: 1382–1386, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Herrera-Ruiz D, Knipp GT. Current perspectives on established and putative mammalian oligopeptide transporters. J Pharm Sci 92: 691–714, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Hiltz ME, Lipton JM. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide α-MSH. FASEB J 3: 2282–2284, 1989 [PubMed] [Google Scholar]
- 39. Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411: 599–603, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Inoue M, Terada T, Okuda M, Inui K. Regulation of human peptide transporter 1 (PEPT1) in gastric cancer cells by anticancer drugs. Cancer Lett 230: 72–80, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity 27: 549–559, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Kelly JM, Moir AJ, Carlson K, Yang Y, MacNeil S, Haycock JW. Immobilized alpha-melanocyte stimulating hormone 10–13 (GKPV) inhibits tumor necrosis factor-alpha stimulated NF-kappaB activity. Peptides 27: 431–437, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 474: 307–317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Landowski CP, Vig BS, Song X, Amidon GL. Targeted delivery to PEPT1-overexpressing cells: acidic, basic, and secondary floxuridine amino acid ester prodrugs. Mol Cancer Ther 4: 659–667, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Laroui H, Dalmasso G, Nguyen HT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology 138: 843–853; e841–e842, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Laroui H, Yan Y, Narui Y, Ingersoll SA, Ayyadurai S, Charania MA, Zhou F, Wang B, Salaita K, Sitaraman SV, Merlin D. L-Ala-gamma-D-Glu-meso-diaminopimelic Acid (DAP) interacts directly with leucine-rich region domain of nucleotide-binding oligomerization domain 1, increasing phosphorylation activity of receptor-interacting serine/threonine-protein kinase 2 and its interaction with nucleotide-binding oligomerization domain 1. J Biol Chem 286: 31003–31013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem 284: 23818–23829, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. Human intestinal H+/peptide cotransporter Cloning, functional expression, and chromosomal localization. J Biol Chem 270: 6456–6463, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 126: 1504–1517, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298–306, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Marina-Garcia N, Franchi L, Kim YG, Hu Y, Smith DE, Boons GJ, Nunez G. Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation. J Immunol 182: 4321–4327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meredith D, Price RA. Molecular modeling of PepT1–towards a structure. J Membr Biol 213: 79–88, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Merlin D, Si-Tahar M, Sitaraman SV, Eastburn K, Williams I, Liu X, Hediger MA, Madara JL. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 120: 1666–1679, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Merlin D, Steel A, Gewirtz AT, Si-Tahar M, Hediger MA, Madara JL. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest 102: 2011–2018, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mitsuoka K, Kato Y, Miyoshi S, Murakami Y, Hiraiwa M, Kubo Y, Nishimura S, Tsuji A. Inhibition of oligopeptide transporter suppress growth of human pancreatic cancer cells. Eur J Pharm Sci 40: 202–208, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D. Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem 282: 1359–1373, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Neudeck BL, Loeb JM, Faith NG. Lactobacillus casei alters hPEPT1-mediated glycylsarcosine uptake in Caco-2 cells. J Nutr 134: 1120–1123, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Nguyen HT, Charrier-Hisamuddin L, Dalmasso G, Hiol A, Sitaraman S, Merlin D. Association of PepT1 with lipid rafts differently modulates its transport activity in polarized and nonpolarized cells. Am J Physiol Gastrointest Liver Physiol 293: G1155–G1165, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Nguyen HT, Dalmasso G, Powell KR, Yan Y, Bhatt S, Kalman D, Sitaraman SV, Merlin D. Pathogenic bacteria induce colonic PepT1 expression: an implication in host defense response. Gastroenterology 137: 1435–1447; e1431–1432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ogihara H, Saito H, Shin BC, Terado T, Takenoshita S, Nagamachi Y, Inui K, Takata K. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem Biophys Res Commun 220: 848–852, 1996 [DOI] [PubMed] [Google Scholar]
- 61. Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411: 603–606, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Pekow JR, Kwon JH. MicroRNAs in inflammatory bowel disease. Inflamm Bowel Dis 18: 187–193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sakata K, Yamashita T, Maeda M, Moriyama Y, Shimada S, Tohyama M. Cloning of a lymphatic peptide/histidine transporter. Biochem J 356: 53–60, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity 34: 293–302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol 11: 9–20, 2011 [DOI] [PubMed] [Google Scholar]
- 66. Sasawatari S, Okamura T, Kasumi E, Tanaka-Furuyama K, Yanobu-Takanashi R, Shirasawa S, Kato N, Toyama-Sorimachi N. The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice. Gastroenterology 140: 1513–1525, 2011 [DOI] [PubMed] [Google Scholar]
- 67. Shanahan F. Physiological basis for novel drug therapies used to treat the inflammatory bowel diseases. I. Pathophysiological basis and prospects for probiotic therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 288: G417–G421, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J 18: 696–698, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Song X, Lorenzi PL, Landowski CP, Vig BS, Hilfinger JM, Amidon GL. Amino acid ester prodrugs of the anticancer agent gemcitabine: synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol Pharm 2: 157–167, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Swaan PW, Bensman T, Bahadduri PM, Hall MW, Sarkar A, Bao S, Khantwal CM, Ekins S, Knoell DL. Bacterial peptide recognition and immune activation facilitated by human peptide transporter PEPT2. Am J Respir Cell Mol Biol 39: 536–542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thwaites DT, Anderson CM. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol 92: 603–619, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thwaites DT, Brown CD, Hirst BH, Simmons NL. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H(+)-coupled carriers at both apical and basal membranes. J Biol Chem 268: 7640–7642, 1993 [PubMed] [Google Scholar]
- 73. Tsume Y, Hilfinger JM, Amidon GL. Enhanced cancer cell growth inhibition by dipeptide prodrugs of floxuridine: increased transporter affinity and metabolic stability. Mol Pharm 5: 717–727, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 140: 1807–1816, 2011 [DOI] [PubMed] [Google Scholar]
- 75. Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127: 1401–1409, 2004 [DOI] [PubMed] [Google Scholar]
- 76. Vavricka SR, Musch MW, Fujiya M, Kles K, Chang L, Eloranta JJ, Kullak-Ublick GA, Drabik K, Merlin D, Chang EB. Tumor necrosis factor-alpha and interferon-gamma increase PepT1 expression and activity in the human colon carcinoma cell line Caco-2/bbe and in mouse intestine. Pflügers Arch 452: 71–80, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst 95: 1276–1299, 2003 [DOI] [PubMed] [Google Scholar]
- 78. Wojtal KA, Eloranta JJ, Hruz P, Gutmann H, Drewe J, Staumann A, Beglinger C, Fried M, Kullak-Ublick GA, Vavricka SR. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dipos 37: 1871–1877, 2009 [DOI] [PubMed] [Google Scholar]
- 79. Xiang J, Hu Y, Smith DE, Keep RF. PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes. Brain Res 1122: 18–23, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ziegler TR, Fernandez-Estivariz C, Gu LH, Bazargan N, Umeakunne K, Wallace TM, Diaz EE, Rosado KE, Pascal RR, Galloway JR, Wilcox JN, Leader LM. Distribution of the H+/peptide transporter PepT1 in human intestine: up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am J Clin Nutr 75: 922–930, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Satsangi J, Kontula K, Lofberg R, Kere J, D'Amato M. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis 15: 1562–1569, 2009 [DOI] [PubMed] [Google Scholar]