Abstract
Inflammatory bowel disease (IBD) results from dysregulation of intestinal mucosal immune responses to microflora in genetically susceptible hosts. A major challenge for IBD research is to develop new strategies for treating this disease. Berberine, an alkaloid derived from plants, is an alternative medicine for treating bacterial diarrhea and intestinal parasite infections. Recent studies suggest that berberine exerts several other beneficial effects, including inducing anti-inflammatory responses. This study determined the effect of berberine on treating dextran sulfate sodium (DSS)-induced intestinal injury and colitis in mice. Berberine was administered through gavage to mice with established DSS-induced intestinal injury and colitis. Clinical parameters, intestinal integrity, proinflammatory cytokine production, and signaling pathways in colonic macrophages and epithelial cells were determined. Berberine ameliorated DSS-induced body weight loss, myeloperoxidase activity, shortening of the colon, injury, and inflammation scores. DSS-upregulated proinflammatory cytokine levels in the colon, including TNF, IFN-γ, KC, and IL-17 were reduced by berberine. Berberine decreased DSS-induced disruption of barrier function and apoptosis in the colon epithelium. Furthermore, berberine inhibited proinflammatory cytokine production in colonic macrophages and epithelial cells in DSS-treated mice and promoted apoptosis of colonic macrophages. Activation of signaling pathways involved in stimulation of proinflammatory cytokine production, including MAPK and NF-κB, in colonic macrophages and epithelial cells from DSS-treated mice was decreased by berberine. In summary, berberine promotes recovery of DSS-induced colitis and exerts inhibitory effects on proinflammatory responses in colonic macrophages and epithelial cells. Thus berberine may represent a new therapeutic approach for treating gastrointestinal inflammatory disorders.
inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn's disease, is associated with chronic, relapsing inflammation of the intestinal tract. Evidence from immunological, microbiological, and genetic studies suggests that IBD results from dysregulation of the mucosal immune system leading to excessive immunological responses to intestinal microflora, or changes in the composition of intestinal microflora and/or deranged epithelial barrier function that elicits pathological responses from the normal mucosal immune system in genetically susceptible hosts (37, 42). In IBD, the immune response is initiated by the interaction between the innate immune system, including macrophages and dendritic cells, and antigens (34). In addition, the intestinal epithelium is actively involved in innate immune responses in the intestine (3). Once the innate immune response is initiated, factors derived from innate immune cells and intestinal epithelial cells, such as increased levels of inflammatory cytokines and chemokines, including tumor necrosis factor (TNF), interleukin (IL)-1β, IL-6, and the neutrophil chemoattractant IL-8 (30), lead to exaggerated adaptive immune responses, including T and B cell-mediated responses in IBD and animal models of colitis (5). Unrestrained reactions against luminal antigens and microflora lead to devastating proinflammatory cytokine and chemokine production, which causes intestinal tissue damage. Thus innate immunity is important in the onset and regulation of the severity of IBD.
Several therapies have been targeted toward suppression of these immune regulators in IBD. However, these therapies are limited by their incomplete clinical efficacy and their side effects. For example, clinical trials showed the efficacy of anti-TNF therapy only in about half of treated patients (7). Thus a major challenge of IBD research is to develop new strategies for the treatment of this disease.
Since the use of complementary and alternative medicine has attracted increasing attention in research, berberine has recently emerged as a potential alternative medical therapy. Berberine, an isoquinoline alkaloid, is present in several plants, such as Hydrastis canadensis (goldenseal), Berberis aquifolium (Oregon grape), and Berberis vulgaris (barberry). The berberine alkaloid can be found in the roots, rhizomes, and stem bark of plants. Berberine as an herbal medicine has been used to treat bacteria-associated diarrhea, intestinal parasitic infections, and ocular trachoma infections for several decades. Several mechanisms attribute to its efficacy, including decreasing enterotoxin-induced intestinal secretion of water and electrolytes (33), bactericidal activity (2), and inhibition of protozoan growth (17).
Increasing evidence has revealed that berberine exerts various beneficial effects on several diseases. Berberine has been shown to induce vasodilation of rat mesenteric arteries through regulation of endothelium and the underlying vascular smooth muscle (20), reduce cholesterol levels in humans and hamsters by elevating LDL receptor expression (21), inhibit hepatic gluconeogenesis to improve glucose metabolism in diabetic rats (43), and reduce the permeability of the blood-brain barrier and attenuate autoimmune encephalomyelitis in mice (25). Furthermore, berberine's immunoregulatory potentials have been demonstrated. Berberine has been shown to inhibit human immunodeficiency virus (HIV) protease inhibitor-induced TNF and IL-6 production in macrophages (45) and enhance progression of Type 1 diabetes in mice and decrease Th17 and Th1 cytokine production, and Th17 and Th1 cell differentiation by regulation of mitogen-activated protein kinase (MAPK) pathways in this mouse model (8).
By using an IL-12-driven Th1 immune response-mediated colitis model, 2,6,4-trinitrobenzenesulfonic acid (TNBS)-induced colitis, berberine has been found to prevent colitis and decrease proinflammatory cytokine production in this model (18, 22, 46, 47). However, treatment studies of established colitis are lacking. In addition, in vitro studies showed that berberine inhibits lipopolysaccharide (LPS)-induced cytokine production and MAPK and NF-κB activation in macrophages (22).
The purpose of this work was to determine the effects of berberine on treating intestinal injury and inflammation and the potential mechanisms of berberine's action in colonic macrophages and epithelial cells. We studied dextran sulfate sodium (DSS)-induced colitis in mice. Colitis in DSS-treated mice is initiated by disruption of intestinal epithelial barrier function and activation of nonlymphoid cells such as macrophages and release of proinflammatory cytokines, including TNF, which causes intestinal tissue damage (12, 19). Studies have shown that mice lacking T cells, B cells, or NK cells can still develop colitis in response to DSS (4, 10). However, Th1/Th2 responses have also been reported to occur in macrophage-induced inflammation in DSS-induced colitis model (9). Here, we report that berberine promotes recovery of DSS-induced colitis, reduces proinflammatory cytokine levels, and preserves barrier function in the colon of DSS-treated mice. Furthermore, we show that berberine promotes colonic macrophage apoptosis in DSS-treated mice. In addition, berberine inhibits proinflammatory cytokine production and signaling pathways involved in cytokine production in colonic macrophages and epithelial cells in DSS-treated mice. These findings suggest that berberine may be a useful agent for treating gastrointestinal inflammatory diseases.
MATERIALS AND METHODS
Mice and treatment.
Wild-type C57BL/6 mice were housed on a 12-h light and 12-h dark cycle. Eight- to 10-wk-old mice were administered 3% DSS (molecular weight 36–50 kDa, MP Biomedical, Solon, OH) in drinking water for 4 days, then mice were killed 3 days after recovery with water. Mice were also treated with 3% DSS for 7 days, followed by recovery with water for 2, 4, and 6 days before killed. Berberine chloride (Sigma-Aldrich, St. Louis, MO) was administered through gavage at 100 mg·kg body wt−1·day−1 for the 3-day recovery after 4-day DSS treatment or 2-, 4-, and 6-day recovery after 7-day DSS treatment. Mice receiving drinking water alone were used as controls (Fig. 1A and data not shown). All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee at Vanderbilt University, Nashville, TN.
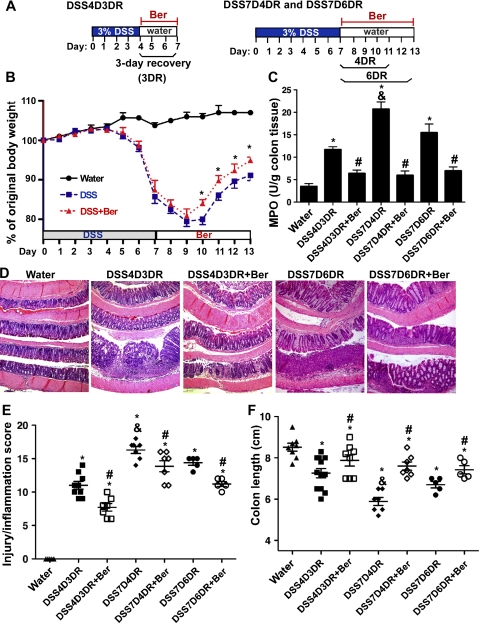
Fig. 1.
Berberine (Ber) promotes recovery of dextran sulfate sodium (DSS)-induced intestinal injury and inflammation in mice. Mice were treated with 3% DSS in drinking water for 4 days to induce colitis, then mice were provided water alone for 3 days for recovery (3DR) before euthanasia (DSS4D3DR). Mice were treated with 3% DSS for 7 days, then water for 4 and 6 days for recovery (4DR and 5DR) before euthanasia (DSS7D4DR and DSS7D6DR). Berberine solubilized in water was administered through gavage for the 3-day (DSS4D3DR+Ber), the 4-day (DSS7D4DR+ Ber), and the 6-day (DSS7D6DR+Ber) recovery period (A). Control mice received water alone. Body weights of mice were measured every day and are presented as percentage of original body weight (B). MPO activity in the colonic tissue was detected (C). Paraffin-embedded colon sections were stained with hematoxylin and eosin for light microscopic assessment of epithelial damage (D). Colon injury/inflammation scores are shown (E). The length of colon was measured when mice were euthanized (F). In B, *P < 0.05 compared with the percentage of original body weight at the same day in DSS-treated group. In C, E, and F, *P < 0.05 compared with water group, #P < 0.05 compared with DSS4D3DR, DSS7D4DR, or DSS7D6DR group. &P < 0.05 compared with DSS4D3DR or DSS7D6DR group.
MPO assay.
Colon tissues were weighed and homogenized in tissue suspension buffer containing 50 mM potassium phosphate (pH 6.0) and 5 mg/ml hexadecyltrimethylammonium bromide (Sigma-Aldrich) at a ratio of 50 mg tissue to 1 ml of buffer. The tissue suspensions were centrifuged at 12,000 rpm for 15 min, and 7 μl of tissue samples were mixed with 200 μl of the reaction buffer containing 17% o-dianisidine (Sigma-Aldrich), 5 mM potassium-phosphate, and 0.0005% H2O2 and incubated for 5 min. Absorbance was recorded at 450 nm. Pure myeloperoxidase (MPO; Calbiochem/EMD Biosciences, Darmstadt, Germany) was used to prepare a concentration curve, and tissue suspension buffer was used as negative control. The results were calculated as units per gram colon tissue.
Analysis of colon inflammation.
Paraffin-embedded tissue sections of Swiss-rolled whole colon were stained with hematoxylin and eosin for light microscopic examination to assess colon injury and inflammation. Samples from the entire colon were examined by a pathologist blinded to treatment conditions. A modified combined scoring system including degree of inflammation (scale of 0–3) and crypt damage (0–4), percentage of area involved by inflammation (0–4) and crypt damage (0–4), and depth of inflammation (0–3) was applied for assessing colitis induced by DSS. The total score is 0 (normal) to 18 (severe colitis).
Colonic cell isolation for immunophenotyping.
Colonic cells were isolated as described before (6). The colon tissues were digested in DMEM containing 1 mg/ml dispase, 0.25 mg/ml collagenase A, and 25 U/ml DNase at 37°C for 20 min with shaking. The suspension was passed through a 70-μm cell strainer. Cells were harvested by centrifugation and washed with DMEM. Viable cells were counted with trypan blue. Cells were incubated with Golgi inhibitor (BD Biosciences, San Jose, CA) in DMEM containing 10% FBS at 37°C for 4 h to block protein transport (secretion).
To label cell surface markers for testing cell types, cells were labeled with fluorescent-tagged antibody mixtures containing antibodies specific against respective cell markers for 30 min at room temperature. We used the following antibodies for detection of indicated cell types: anti-F4/80-APC (macrophages, dilution 1:200, BD Biosciences). The cells were then fixed in 0.1% paraformaldehyde/PBS at 4°C overnight and then permeabilized for labeling intracellular cytokine proteins. Cells were labeled with anti-TNF-Cy7 (dilution: 1:200, BD Biosciences) for 30 min at 4°C. Cells were analyzed by multicolor flow cytometry to determine the percentage of positive cells by use of a BD LSRII system (BD Biosciences). The results were calculated as % of cells × total immune cells/colon weight (gram) = number of cells/gram of colon weight.
Colonic macrophage isolation.
Colonic macrophages were isolated from colonic cells by use of a biotin-labeled anti-mouse F4/80 antibody (Invitrogen, Carlsbad, CA) and streptavidin magnetic beads (Invitrogen), as described in Ref. 6.
Colonic epithelial cell isolation.
Mouse colon epithelial cells were isolated as described in Ref. 35. The colon tissues were incubated with 0.5 mM dithiothreitol and 3 mM EDTA at room temperature for 1.5 h without shaking. Crypts were released from the colon by vigorous shaking. Epithelial cells were sorted using a biotin-labeled E-cadherin antibody and streptavidin magnetic beads.
Detection of cytokine protein level in the colon.
The freshly excised colon was rinsed in PBS and colon tissues were homogenized in cell Lytic MT Mammalian Tissue Lysis/Extraction buffer (Sigma-Aldrich). Thirteen cytokines (IFN-γ, IL-1β, IL-4, IL-6, IL-10, IL-12 p40, TNF, IL-12 p70, IL-13, KC, MCP-1, MIP-1α, MIP-1β) were measured in tissue lysates by using a MILLIPLEX MAP Mouse Cytokine/Chemokine Panel kit according to the manufacturer's instruction (Millipore, Billerica, MA). Protein concentration was measured by using Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Concentration of each cytokine was presented as picograms per milligram colon protein.
Real-time PCR analysis.
Total RNA was isolated from homogenized colon tissue by use of an RNA isolation kit (Qiagen, Valencia, CA) and was treated with RNase-free DNase. Reverse transcription was performed with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). For real-time PCR reactions, 25-μl reactions were set up by addition of 1.25 μl of primer mix (containing 5 μM of reverse and forward primers), 5 μl of diluted cDNA template, and 12.5 μl of TaqMan Gene Expression Master Mix. Real-time PCR was performed with the 7300 Real Time PCR System (Applied Biosystems). The data were analyzed by use of Sequence Detection System V1.4.0 software. The relative abundance of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used to normalize levels of the mRNAs of interest. All cDNA samples were analyzed in triplicate. All primers are from Applied Biosystems: TNF (Mm00443259), IFN-γ (Mm99999071), KC (Mm00433859), and IL-17 (Mm00439618).
Peritoneal macrophage isolation and macrophage culture.
The mouse RAW 264.7 macrophage cell line was cultured in DMEM medium containing 10% FBS, 1% glutamine, 100,000 IU/l penicillin, and 100 mg/l streptomycin at 37°C with 5% CO2 and 95% air.
Mice were intraperitoneally injected with 500 μl of 3.5% Bio-Gel 100 (Bio-Rad Laboratories). Macrophages were isolated by peritoneal lavage 5 days after intraperitoneal injection. For mice treated with DSS with or without berberine and water control mice, no intraperitoneal injection of Bio-Gel 100 was performed. Cells were plated in cell culture medium as for RAW 264.7 cells and washed 3 h after plating to remove unattached cells. Peritoneal macrophages were cultured for 2–3 days before treatment.
ELISA.
RAW 264.7 macrophages and peritoneal macrophages were treated with LPS (1 μg/ml) in the presence or absence of berberine (50 μM) for 24 h. Culture media were collected to determine the levels of TNF by using Mouse TNF ELISA Ready-set-go Kits (eBioscience) according to the manufacturer's instructions. The protein concentrations of the cellular lysates were determined. The TNF level is presented as picograms of TNF in the cell culture supernatants per microgram cellular protein.
Apoptosis assay.
Macrophages isolated from the mouse colon and cultured RAW 264.7 macrophages and peritoneal macrophages were double stained with annexin V-FITC and PI (Calbiochem/EMD Biosciences) according to the respective manufacturer's instructions. The percentage of cells positive for annexin V and PI was determined by flow cytometry.
Apoptosis in colon tissue sections was detected by using the ApopTag In Situ Oligo Ligation (ISOL) Kit (Intergen, Purchase, NY) according to the manufacturer's guidelines and by Western blot analysis of colonic epithelial cells using anti-active caspase-3 antibody (Cell Signaling Technology, Beverly, MA) (44). Apoptotic cells were determined by counting the absolute number of positive-stained cells in at least 300 colonic crypts.
Immunohistochemistry.
Paraffin-embedded colon sections were deparaffinized. After unmasking antigens, colon sections were blocked with 10% goat serum and stained with an anti-zonula occludens-1 (ZO-1) antibody (Invitrogen) overnight at 4°C and FITC-labeled secondary antibody for 1 h at room temperature. Sections were then mounted with mounting medium containing 4,6-diamidino-2-phenylindole (DAPI) for nuclear counterstaining and observed by fluorescence microscopy. FITC and DAPI images were taken from the same field.
Preparation of cellular lysates and Western blot analysis.
Colonic macrophages and epithelial cells were solubilized using cell lysis buffer containing 1% Triton X-100, 10 mM Tris (pH 7.4), 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, and protein inhibitor cocktail (1:100) and incubated for 30 min on ice. The lysates were centrifuged, and the protein concentration of supernatants were determined by use of a DC protein assay (Bio-Rad Laboratories). The lysates were mixed with Laemmli sample buffer.
Proteins were separated by SDS-PAGE for Western blot analysis by use of anti-IκB, anti-phospho-p38, anti-phospho-stress-activated protein kinase/c-Jun aminoterminal kinase (SAPK/JNK) (Cell Signaling Technology), anti-phospho-extracellular signal-regulated kinase (ERK)1/ERK2/MAP kinase (Promega, Madison, WI), and anti-β-actin (Sigma-Aldrich) antibodies.
Statistical analysis.
Statistical significance for multiple comparisons in each study was determined by one-way ANOVA followed by Newman-Keuls analysis using Prism 5.0 (GraphPad Software). A P value <0.05 was defined as statistically significant. All data presented are representative of at least five repeat experiments and are presented as means ± SE.
RESULTS
Berberine promotes recovery of DSS-induced colitis in mice.
The DSS mouse model of acute colitis is well-characterized by increased epithelial injury and production of inflammatory cytokines (14, 28). To evaluate the treatment effect of berberine on DSS-induced colon epithelial injury and colitis, we determined the ability of berberine to ameliorate established colitis. Mice were treated with DSS for 4 days to induce colitis and then berberine was administered via gavage for the following 3-day recovery (Fig. 1A). To induce severe colitis, mice were treated with DSS for 7 days, and berberine was given to mice for the following 4- and 6-day recovery periods (Fig. 1A). Mice began to lose body weight after 5 days of DSS treatment, and body weight loss continued 2 days after the end of DSS treatment (day 8 and day 9). Mice began to regain body weight from day 10 (3 days after ending DSS treatment) in both DSS alone and DSS with berberine-treated mice (Fig. 1B). Berberine treatment significantly improved body weight recovery from day 10 to day 13, as we observed that mice reached 91.13 ± 3.0% of their original body weight at day 13 in DSS-treated mice, but 94.92 ± 2.0% in the DSS with berberine-cotreated group (P < 0.05, Fig. 1B). Mice treated with DSS for 4 days and followed by 3-day recovery did not show any body weight changes (data not shown).
DSS induces neutrophil infiltration in the colon leading to increasing colonic MPO activity, which is therefore an inflammatory marker for colitis. Berberine treatment reduced MPO activity in the colon by 43.03 ± 15.97% (P < 0.01) in mice with 4-day DSS treatment followed by 3-day recovery and by 71.27 ± 12.51% (P < 0.01) and 43.01 ± 10.03% (P < 0.01) in mice with 7-day DSS treatment followed by 4- and 6-day recovery, respectively (Fig. 1C). In both treatment protocols, DSS-treated mice demonstrated injury and acute colitis with massive colon ulceration, crypt damage, and severe inflammation. These abnormalities were reduced by cotreatment with berberine (Fig. 1D). In established DSS-induced colitis, the score in DSS 4-day treatment with 3-day recovery group was 11 ± 1.73, and the scores in DSS 7-day treatment with 4-day and 6-day recovery were 16.29 ± 1.9 and 14.4 ± 0.89, respectively. Berberine treatment significantly decreased injury and inflammation score in the 4-day DSS treatment with 3-day recovery group (7.7 ± 1.5, P < 0.05) and the in 7-day DSS treatment with 4-day and 6-day recovery groups (13.86 ± 219 and 11.2 ± 0.84, P < 0.05, respectively) (Fig. 1E). In addition, berberine treatment significantly reduced DSS-induced colon shortening in these three groups (Fig. 1E). These data suggest that berberine exerts therapeutic effects on both mild (4-day DSS treatment with 3-day recovery) and severe (7-day DSS treatment with 4-day recovery) colitis associated with intestinal epithelial cell injury.
Berberine reduces production of proinflammatory cytokines in the colon of DSS-treated mice.
Increased proinflammatory cytokine production is a hallmark of DSS-induced colitis (12, 13). Therefore, we tested the effects of berberine on inflammatory cytokine production in the DSS-induced colitis model. We used Luminex assays to detect cytokine protein levels in the colon and real-time PCR analysis of RNA isolated from the colon to detect cytokine mRNA levels.
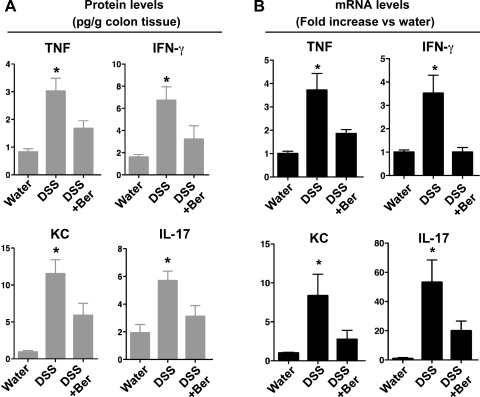
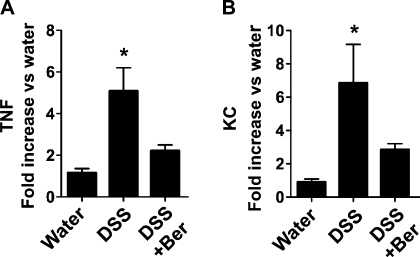
Results from Luminex assays showed that DSS treatment increased colon levels of TNF, IL-6, KC, IFN-γ, IL-17, IL-10, IL-1β, MCP-1, and MIP-1α in mice. However, there was no detectable IL-4, IL-12 (p40), IL-12 (p70), IL-13, and MIP-1β production in mice treated with DSS for 4 days and recovered for 3 days with water. Berberine treatment downregulated DSS-induced production of TNF (P < 0.05), IFN-γ (P < 0.05), KC (P < 0.05) and IL-17 (P < 0.05) (Fig. 2A), but not IL-1β, IL-6, IL-10, MCP-1, MIP-1α, and MIP-1β production (data not shown). Real-time PCR analysis of TNF, KC, IFN-γ, and IL-17 mRNA levels in mice showed similar effects of berberine, with inhibition of expression of these cytokines. Treatment with berberine led to downregulation of DSS-induced mRNA levels of TNF (P < 0.05), IFN-γ (P < 0.05), KC (P < 0.05), and IL-17 (P < 0.05) (Fig. 2B).
Fig. 2.
Berberine suppresses DSS-induced proinflammatory cytokine production in the mouse colon. Mice were treated with 3% DSS in drinking water for 4 days to induce colitis, then mice were provided with water (DSS) alone or gavaged with berberine (DSS+Ber) for 3 days before euthanasia. Control mice received water alone. Colon tissues were collected for Luminex assay to detect cytokine protein levels (A). mRNA was isolated from the colon for real-time PCR analysis of indicated cytokine mRNA expression levels. The cytokine mRNA expression level in the water group was set as 100%, and mRNA expression levels in treated mice were compared with the water group (B). *P < 0.05 compared with water group and DSS+Ber group; n = at least 5 mice in each group.
Berberine inhibits proinflammatory responses and promotes apoptosis in colonic macrophages in DSS-treated mice.
DSS-induced colitis can be dependent on or independent of adaptive immune response because DSS induces colitis in mice lacking T cells, B cells, and NK cells (4, 10), although adaptive immune responses have been shown to contribute to DSS-induced colitis (9). One possible consequence of DSS-mediated disruption of epithelial barrier function is that mucosal macrophages become activated by substances in the intestinal lumen, which leads to release of proinflammatory cytokines, such as TNF (19). Therefore, we studied the role of berberine in regulation of macrophage function in DSS-induced colitis.
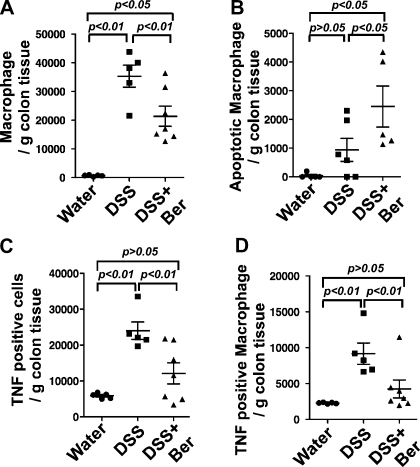
Colonic cells were isolated to detect the F4/80-positive macrophage population by flow cytometry. Berberine treatment decreased macrophage infiltration into the colon in DSS-treated mice (P < 0.05, Fig. 3A). Furthermore, colonic macrophages were isolated and apoptosis was detected by annexin V staining and flow cytometry. Interestingly, the number of apoptotic macrophages increased in these mice (P < 0.05) (Fig. 3B). Apoptosis in macrophages in the colon tissues was also detected by double immunofluorescence staining of F4/80 and active caspase-3. Consistent with the data from the annexin V staining, the number of active caspase-3 positive macrophages in the colon of mice with DSS and berberine cotreatment was higher than that in mice with DSS treatment only (data not shown).
Fig. 3.
Berberine increases apoptosis and reduces TNF production in colonic macrophages from DSS-treated mice. Mice were treated as shown in Fig. 2. The colon tissues were weighed. Colonic cells were isolated and the total cell numbers were recorded. Colonic cells were stained with antibodies to a macrophage marker, F4/80, and TNF. Percentage of positively stained cells was determined by flow cytometry analysis. Total cell number of macrophages (A), TNF-positive cells (C), and macrophages with TNF-positive staining (D) were calculated as percentage of indicated cells × total colonic cells/g colon weight. Colonic macrophages were isolated and cell number was recorded. Apoptosis was detected by annexin V staining and flow cytometry (B). Colonic apoptotic macrophages were calculated as percentage of apoptotic cells × total colonic macrophages/gram colon weight.
To test the ability of macrophages to produce TNF, the intracellular TNF protein level in colonic macrophages was determined. Berberine treatment decreased the total number of TNF-positively stained colonic cells (Fig. 3C). Berberine also decreased TNF-positive macrophages (P < 0.05) in DSS-treated mice (Fig. 3D).
These data suggest that berberine regulates macrophage function in DSS-induced colitis through increasing apoptosis and decreasing proinflammatory cytokine production, which may serve as mechanisms for downregulation of intestinal inflammation.
Berberine decreases DSS-induced peritoneal macrophage response toward LPS-stimulated TNF production.
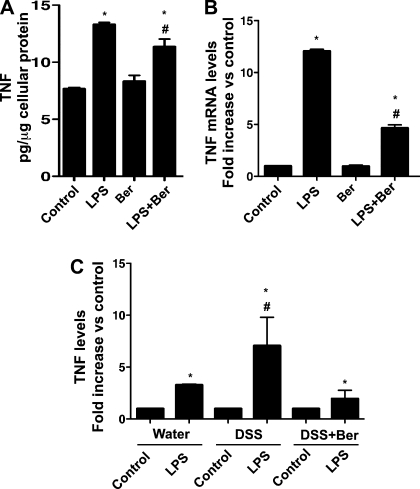
Bacterial endotoxin LPS is one of the most potent proinflammatory agonists for monocytes and macrophages. Excessive and continuing cytokine production in response to LPS or superantigens is a hallmark of the systemic inflammatory response (40). TNF is one of the cytokines produced by macrophages upon LPS stimulation. We therefore tested whether berberine inhibits LPS-induced TNF production at the protein level by ELISA and at the mRNA level by real-time PCR in vitro. Berberine inhibited LPS-induced upregulation of TNF protein level by 15.01 ± 5.8% (P < 0.05) (Fig. 4A). Similarly, berberine inhibited LPS-induced upregulation of TNF mRNA by 61.52 ± 4.54% (P < 0.05) in RAW 264.7 cells (Fig. 4B). Interestingly, we observed that, compared with unstimulated peritoneal macrophages, LPS stimulated 3.27 ± 0.09-fold and 1.95 ± 0.82-fold increase of TNF production in peritoneal macrophages isolated from water vehicle control mice and DSS with berberine-cotreated mice, respectively. However, LPS stimulated 7.0 ± 2.75-fold increase of TNF production in peritoneal macrophages isolated from DSS-treated mice (Fig. 4C). Thus berberine decreased the response of peritoneal macrophages isolated from DSS-treated mice in the case of LPS-stimulated TNF production (P < 0.05).
Fig. 4.
Berberine suppresses LPS-stimulated TNF production in macrophages in vitro. RAW 264.7 macrophages and macrophages isolated from the peritoneal cavity of mice treated with DSS as shown in Fig. 2 were treated with LPS (1 μg/ml) for 24 h in the presence or absence of 50 μM berberine. The concentration of TNF in the cell culture supernatant was determined by ELISA (A and C). The protein concentrations of the cellular lysates were determined (A and C). The TNF level is presented as pg of TNF in the cell culture supernatants/μg cellular protein (A). TNF levels in control (LPS untreated) samples from water, DSS, and DSS+Ber mice were set as 100%. TNF levels in LPS-treated samples were compared with the untreated group (C). RNA was isolated from the cells for real-time PCR analysis of TNF mRNA expression levels. The TNF mRNA expression level in control was set as 100%. mRNA expression levels in LPS-treated samples were compared with the control group (B). Data in A and B are from 3 separate experiments; n = 5 mice in each group in C. In A and B, *P < 0.05 compared with the control group, #P < 0.05 compared with the LPS group. In C, *P < 0.05 compared with control samples of the same treatment mouse group, #P < 0.05 compared with LPS-treated samples from water and DSS+Ber mice. Data represent at least 3 independent experiments.
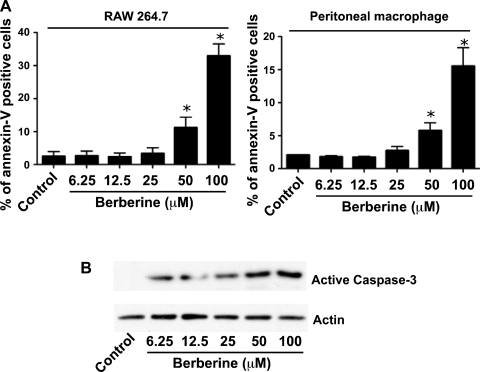
Berberine promotes macrophage apoptosis in vitro.
To determine the potential role of berberine in regulating the fate of macrophages, RAW 264.7 cells and peritoneal macrophages were treated with berberine. Apoptosis was detected by annexin V and propidium iodide (PI) staining. Berberine induced apoptosis in a concentration-dependent manner in RAW 264.7 cells and peritoneal macrophages (Fig. 5A). Furthermore, we investigated the mechanism of berberine-induced apoptosis. Total cellular lysates were collected for detecting caspase-3 activation. Berberine stimulated caspase-3 activation in RAW 264.7 cells in a concentration-dependent manner (Fig. 5B). These data indicate that berberine-induced macrophage apoptosis may contribute to decreased cytokine production and ameliorate DSS-induced colitis.
Fig. 5.
Berberine stimulates apoptosis in macrophages in vitro. RAW 264.7 cells and peritoneal macrophages were treated with the indicated concentrations of berberine for 18 h. Apoptosis was detected by annexin V and PI staining (A). Total cellular lysates were collected for detecting caspase-3 activation by Western blot analysis (B). Data represent at least 3 independent experiments. *P < 0.05 compared with the control group.
Berberine regulates colonic epithelial cell function in DSS-treated mice.
The intestinal epithelium is actively involved in innate immune responses in the intestine. Therefore, colonic epithelial cells were isolated from mice for real-time PCR analysis of TNF and KC mRNA levels. TNF and KC mRNA levels were increased in colonic epithelial cells from DSS-treated mice, and these levels were significantly decreased by berberine cotreatment (P < 0.05, Fig. 6).
Fig. 6.
Berberine decreases TNF (A) and KC (B) production in colonic epithelial cells from DSS-treated mice. Mice were treated as shown in Fig. 2. Colon tissues were collected for isolation of colonic epithelial cells. mRNA was isolated from the colonic epithelial cells for real-time PCR analysis of the indicated cytokine mRNA expression levels. The cytokine mRNA expression level in water group was set as 100%, and mRNA expression levels in treated mice were compared with the water group. *P < 0.05 compared with water group and DSS+Ber group; n = at least 5 mice in each group.
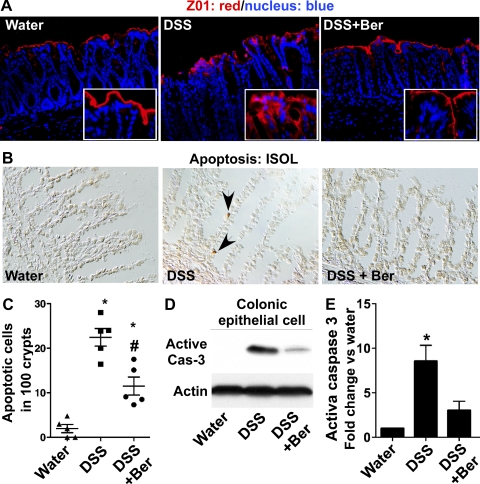
The intestinal epithelial monolayer forms a physiological barrier against pathogenic microbes and detrimental substances in the intestinal lumen. In vitro studies have suggested that berberine attenuates proinflammatory cytokine-induced disruption of intestinal epithelial tight junctional complex (1, 15, 23). As a marker of tight junction structure, we determined the distribution of a tight junctional protein, ZO-1, using immunostaining. DSS-induced redistribution of this protein from apical tight junctional complexes to the cytoplasmic compartment of colon epithelial cells was prevented by berberine treatment (Fig. 7A). In addition, DSS-induced intestinal epithelial cell apoptosis also contributes to the disruption of intestinal epithelial integrity. We found that berberine decreased DSS-induced intestinal epithelial cell apoptosis detected by ISOL staining (Fig. 7, B and C) and Western blot analysis of caspase-3 activation (Fig. 7, C and D). Thus these protective roles of berberine in the intestinal epithelium may mediate its treatment effects on DSS-induced colitis.
Fig. 7.
Berberine preserves intestinal barrier function in DSS-treated mice. Mice were treated as shown in Fig. 2. Paraffin-embedded colon tissues were used to determine zonula occludens-1 (ZO-1) distribution by immunohistochemistry using an anti-ZO-1 antibody and FITC-labeled secondary antibody and visualized by fluorescence microcopy (red staining). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; blue staining) (A). Apoptosis was detected using ApopTag In Situ Oligo Ligation (ISOL) staining. Apoptotic nuclei labeled with peroxidase were visualized using DIC microscopy (B). Arrows indicate ISOL-labeled apoptotic nuclei (brown in B). The number of apoptotic nuclei per 100 crypts is shown (C). Colonic epithelial cells were isolated from mice. Total cellular lysates were prepared for detecting caspase-3 (casp-3) activation by Western blot analysis (D). Blotting for actin was used as a protein loading control. Each lane represents epithelial cells from 1 mouse. The relative density of the activated caspase-3 band on Western blot was compared with the actin band in each group. The density ratio in the water group was set as 100%, and ratios of DSS and DSS+Ber were compared with that of the water group. Fold change of ratios is shown in E; n = at least 5 mice in each group. In C and E, *P < 0.05 compared with water groups.
Berberine downregulates signaling pathways that mediate proinflammatory cytokine production in colonic macrophages and epithelial cells.
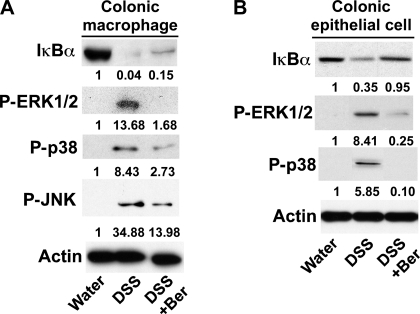
Proinflammatory cytokine production is regulated by signaling pathways, such as MAPK (ERK1/2, p38, and JNK) (11) and NF-κB activation (39). Therefore, we tested the effects of berberine on these signaling pathways in macrophages and epithelial cells in the DSS-induced colitis model. DSS treatment increased ERK1/2, p38, and JNK activation in colonic macrophages, which were inhibited by berberine treatment (Fig. 8A). Phosphorylation of IκB leads to IκB ubiquitination and degradation, which permits NF-κB release for nuclear translocation and transcriptional activation for proinflammatory cytokine production. We found that berberine inhibited DSS-stimulated IκB degradation in colonic macrophages (Fig. 8A). In addition, signaling pathways activated by DSS treatment in colonic epithelial cells, including IκB degradation and ERK1/2 and p38 activation, were decreased by berberine cotreatment (Fig. 8B). No significant JNK activation in DSS-treated colon epithelial cells was found (data not shown).
Fig. 8.
Berberine suppresses signaling pathways which mediate proinflammatory cytokine production in colonic macrophages and epithelial cells. Colonic macrophages (A) and epithelial cells (B) were isolated from mice treated as shown in Fig. 2. Each lane represents the combination of same number of macrophages pooled from 5 mice (A) or epithelial cells from 1 mouse (B) in each treatment group. Total cellular lysates were prepared for detecting the activation state of the indicated signals by Western blot analysis. The actin blot was used as protein loading control. The relative density of the signaling band on Western blot was compared with the actin band in each group. The ratio in the water group was set as 100%, and ratios of DSS and DSS+Ber were compared with that of the water group. Average fold changes are shown under the blot; n = at least 5 mice in each group.
These data suggest that regulation of signaling pathways involved in proinflammatory cytokine production may be a mechanism by which berberine inhibits proinflammatory responses in colonic macrophages and epithelial cells.
DISCUSSION
Although berberine has been used as an herbal medicine for treating diarrhea and bacterial and parasitic infections and has shown various beneficial effects on several diseases in animal models, including preventing TNBS-induced colitis (18, 22, 46, 47), reducing cholesterol levels (21), improving glucose metabolism in diabetic rats (43), attenuating autoimmune encephalomyelitis (25), and enhancing progression of Type 1 diabetes (8), in vivo studies to investigate berberine's mechanisms of action in disease treatment are needed for its clinical application. The purpose of this work was to provide insights into understanding the treatment effects and mechanisms of berberine in intestinal inflammation in an animal model of colitis. We showed that berberine exerted treatment effects on DSS-induced colonic injury and inflammation and ameliorated proinflammatory cytokine production and preserved barrier function in the mouse colon. Furthermore, berberine promoted macrophage apoptosis and inhibited proinflammatory cytokine production and NF-κB and MAPK activation in colonic macrophages and epithelial cells in DSS-treated mice. These findings may lead to the development of berberine for the treatment of intestinal inflammatory diseases, including IBD.
Our data suggest that berberine exerts inhibitory effects not only on mild colitis (4-day DSS-induced colitis), but also on severe colitis (7-day DSS-induced colitis). However, it should be noted that a sufficient time of treatment is needed for berberine to fully exert its effects. When mice were treated with berberine for the 2-day recovery after 7-day DSS treatment (data not shown), berberine significantly decreased the MPO level and reduced DSS-induced colon shortening in (data not shown and Fig. 1D), but the injury score was not decreased by berberine treatment in this group (data not shown). These data indicate that 2-day berberine administering may be short for colitis treatment, consistent with the fact that the response to current treatments in humans with IBD takes considerable time.
The dosage that exerts the therapeutic effect is important in determining berberine's potential clinical application. We have performed experiments to test the treatment effects of varying dosages of berberine, namely 50, 100, and 150 mg·kg body wt−1·day−1 administered to mice after colitis was induced by DSS. We found that berberine at 50 mg·kg body wt−1·day−1 did not result in significant amelioration of DSS-induced colitis, and berberine at 150 mg·kg body wt−1·day−1 showed similar effects on colitis compared with that at 100 mg·kg body wt−1·day−1 (data not shown). Therefore, we chose to treat mice with berberine at 100 mg·kg body wt−1·day−1 for this study. In China, the dosage of berberine for treating diarrhea in patients is 900–1,200 mg/day for adults and 20 mg·kg body wt−1·day−1 for children. The dosage used in this work is higher than the current dosage for treating diarrhea. However, it is difficult to determine the dosage of drugs based on results from animal studies. For example, absorption rate may be different in humans from that in animals, and the higher basal metabolic rate of mice vs. humans may also play a role in the dose requirement.
It has been reported that colitis in DSS-treated mice is initiated by disruption of intestinal epithelial barrier function, and then by crypt damage and ulceration, which trigger increased cellular responses to pathogenic microbes and detrimental substances in the intestinal lumen. One possible consequence of this change is increased proinflammatory cytokine production (12, 19). Based on the cytokine profiles that are regulated by berberine, it is possible that berberine ameliorates DSS-induced colitis through regulation of innate immunity, such as regulation of macrophage and epithelial cell-derived cytokine production, including TNF and KC. Our in vitro studies further confirmed that berberine inhibited decreased DSS-induced sensitivity of macrophages toward LPS-stimulation of TNF production. Regulation of macrophage cytokine production by berberine has also been reported upon HIV protease inhibitor stimulation in vitro (45). Deceasing TNF production in intestinal epithelial cells by berberine has also been reported in the TNBS model (22).
We also found that berberine downregulates DSS-increased IFN-γ and IL-17 production in the mouse colon. Although mice lacking T cells, B cells, and NK cells can still develop colitis in response to DSS (4, 10), Th1/Th2 responses have been reported to modulate macrophage-induced inflammation in DSS-induced colitis model (9). Berberine has been reported to decrease Th17 and Th1 cytokine production and Th1 and Th17 cell differentiation in a Type 1 diabetic mouse model (8). In addition, in TNBS-induced colitis, which is mediated by IL-12-driven Th1 immune responses, including increased TNF and IFN-γ production (38), berberine has been shown to prevent colitis and inhibit iNOS, COX-2, IL-1β, IL-6, and TNF (22). Thus berberine may directly function on Th1 and Th17 lymphocytes during inflammation.
We found that berberine decreases DSS-induced peritoneal macrophage response toward LPS-stimulated TNF production. There are two possibilities by which peritoneal macrophages are affected by oral berberine treatment in mice. First, peritoneal macrophages may include macrophages that migrate from the colon lamina propria, and, secondly, berberine may enter the peritoneal fluid, interact with macrophages, and directly exert its effects on peritoneal macrophages after it is absorbed by the intestinal epithelium when mice are orally treated with berberine.
In addition to regulation of cytokine production by macrophages, we showed that berberine regulated macrophage function by stimulating apoptosis through caspase-3 activation in DSS-induced colitis and in vitro. Decreased macrophage number should contribute to lowering levels of proinflammatory cytokines produced by macrophages in the DSS model. Previous studies showed that depletion of macrophages and dendritic cells ameliorated colitis in an IL-10 deficiency-induced mouse colitis model (41). However, macrophages have been shown to promote repair of damaged mucosal tissue by producing immunosuppressive factors (31), and a report showed that depletion of macrophage/dendritic cells led to increased DSS-induced colitis in mice (32). Thus this evidence indicates that macrophages may serve dual functions and the consequence of responses occurring in macrophages depends on the balance of effects on the different stages of diseases. Results from our study suggest that decreased macrophage number by berberine treatment was associated with promoting recovery of established colitis and reducing proinflammatory cytokine production in the DSS model of colitis.
We report here that berberine downregulates signaling pathways, including MAPK and NF-κB activation, in colonic macrophages and epithelial cells in response to DSS treatment in vivo. In addition, as reported before (22), we found that berberine inhibited these signaling pathways induced by LPS in macrophages, and by TNF in a colon epithelial cell line in vitro (data not shown). MAPK and NF-κB activation have been well characterized to enhance proinflammatory cytokine production. Thus berberine-regulated signaling may contribute to its anti-inflammatory responses. Although the mechanism of inhibition of LPS-stimulated NF-κB activation in macrophages by berberine is unclear, berberine has been reported to suppress TNF- and carcinogen-stimulated NF-κB activation via inhibiting IκBα kinase activity in lung cancer cells (29). Our findings that berberine inhibited MAPK action in macrophages are consistent with results from other reports. Berberine-induced AMP-activated protein kinase activation and subsequent MAPK activation can suppress proinflammatory responses, such as proinflammatory cytokine production upon LPS, free fatty acid, and hydrogen peroxide stimulation in macrophages and adipocytes (16) and in microglial cells (24). In addition, other signaling pathways, such as endoplasmic reticulum (ER) stress signaling, have been reported to mediate berberine inhibition of TNF and IL-6 production by macrophages upon HIV protease inhibitor stimulation (45).
The role of NF-κB activation in intestinal epithelial cells in colitis is controversial. IKK-β deletion in enterocytes and macrophages reduces the development of inflammation-associated colon cancer (14). However, ablation of IKK-γ in intestinal epithelial cells leads to chronic colitis in mice (27). Complete deletion of the p65 subunit of NF-κB in colonic epithelial cells accelerates DSS-induced colitis in mice (36). It should be noted that, in both conditions, apoptosis of colonic epithelial cell is increased, which mediates exacerbated colitis. Our data show that berberine decreased colonic epithelial cell apoptosis in DSS-treated mice. We hypothesize two factors involved in reducing DSS-induced epithelial apoptosis by berberine. First, berberine decreases proinflammatory cytokine production; thus there is less apoptotic stimulation for epithelial cells. Second, berberine not only inhibits antiapoptotic NF-κB but also other proapoptotic signals, such as p38 in the colon epithelium. In addition, our unpublished data showed that berberine induces apoptosis in colon tumor cells, but not normal epithelial cells, although berberine inhibits NF-κB in both cell lines.
We found that berberine prevented DSS-disruption of intestinal integrity by preserving apical tight junctional complexes and decreasing apoptosis. This effect may be due to less detrimental effects of proinflammatory cytokines, since these levels were decreased in berberine-treated mice. However, it has been reported that berberine prevented proinflammatory cytokine-disruption of barrier function in HT-29 cells and Caco2 cells (1, 15, 23) by modulation of tight junctional proteins. The pathways mediating berberine's action may involve Akt and NF-κB signaling. There is no increase of apoptosis with berberine cotreatment, compared with TNF alone (1). Therefore, the intestinal epithelial monolayer may be one of the targets for berberine's direct action during inflammation.
Other mechanisms underlying berberine's anti-inflammatory effects have also been reported, such as antimicrobial effects. Berberine has been shown to inhibit enterobacterial growth in TNBS-induced colitis (22). The microbial community in DSS-treated mice is changed (26). Therefore, it is possible that berberine regulates the intestinal microflora to exert its anti-inflammatory effects. Since there may be other unknown mechanisms underlying berberine's inhibitory effects on DSS-induced intestinal inflammation, decreased proinflammatory cytokine levels in berberine-treated mice found in this study may represent evidence that parallels reduced inflammation.
In summary, we have shown in vivo evidence that berberine exerts treatment effects on DSS-induced colitis in mice, and the potential mechanisms may involve regulation of innate immune responses through modulation of signaling pathways in colonic macrophages and epithelial cells. Since berberine has been widely used for treating diarrhea without significant side effects on patients, it has the potential to be developed into a drug for intestinal inflammatory diseases.
GRANTS
This work was supported by National Institutes of Health Grants R01DK081134 (F. Yang), R01DK56008 (D. B. Polk), R01AT004821 and R01DK053620 (K. T. Wilson), R01DK58587, R01CA77955, and P01116087 (R. M. Peek Jr.), P30DK058404 (Vanderbilt University Digestive Disease Research Center), Department of Veterans Affairs (K. T. Wilson) and National Nature Science Foundation of China 81070283 (B. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address for Y. Shi: School of Life Sciences, Xiamen University, Xiamen, P. R. China.
Present address for H. Cao: Cancer Center Xiamen University, Xiamen, P. R. China.
REFERENCES
- 1. Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFα-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFκB signaling. J Cell Sci 123: 4145–4155, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol 15: 1067–1076, 1969 [DOI] [PubMed] [Google Scholar]
- 3. Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8: 411–420, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res 45: 181–191, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Blumberg RS. Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis 27: 455–464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaturvedi R, Asim M, Lewis ND, Algood HM, Cover TL, Kim PY, Wilson KT. l-arginine availability regulates inducible nitric oxide synthase-dependent host defense against Helicobacter pylori. Infect Immun 75: 4305–4315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark M, Colombel JF, Feagan BC, Fedorak RN, Hanauer SB, Kamm MA, Mayer L, Regueiro C, Rutgeerts P, Sandborn WJ, Sands BE, Schreiber S, Targan S, Travis S, Vermeire S. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology 133: 312–339, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem 284: 28420–28429, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114: 385–391, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107: 1643–1652, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol 20: 55–72, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 62: 240–248, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Garside P. Cytokines in experimental colitis. Clin Exp Immunol 118: 337–339, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKb links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118: 285–296, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Gu L, Li N, Gong J, Li Q, Zhu W, Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis 203: 1602–1612, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab 296: E955–E964, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Kaneda Y, Torii M, Tanaka T, Aikawa M. In vitro effects of berberine sulphate on the growth and structure of Entamoeba histolytica, Giardia lamblia and Trichomonas vaginalis. Ann Trop Med Parasitol 85: 417–425, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Kawashima K, Nomura A, Makino T, Saito K, Kano Y. Pharmacological properties of traditional medicine (XXIX): effect of Hange-shashin-to and the combinations of its herbal constituents on rat experimental colitis. Biol Pharm Bull 27: 1599–1603, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim 48: 137–143, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, Ho K, Huang Y. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol 399: 187–196, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 10: 1344–1351, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Lee IA, Hyun YJ, Kim DH. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur J Pharmacol 648: 162–170, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Li N, Gu L, Qu L, Gong J, Li Q, Zhu W, Li J. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. Eur J Pharm Sci 40: 1–8, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem 110: 697–705, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Ma X, Jiang Y, Wu A, Chen X, Pi R, Liu M, Liu Y. Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One 5: e13489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 17: 917–926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446: 557–561, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98: 694–702, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-κB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res 68: 5370–5379, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Podolsky DK. Inflammatory bowel disease. N Engl J Med 347: 417–429, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA 102: 99–104, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol 80: 802–815, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Sack RB, Froehlich JL. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect Immun 35: 471–475, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol 11: 9–20, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Singh K, Chaturvedi R, Barry DP, Coburn LA, Asim M, Lewis ND, Piazuelo MB, Washington MK, Vitek MP, Wilson KT. The apolipoprotein E-mimetic peptide COG112 inhibits NF-kappaB signaling, proinflammatory cytokine expression, and disease activity in murine models of colitis. J Biol Chem 286: 3839–3850, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol 180: 2588–2599, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest 117: 514–521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 20: 495–549, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol 17: 1–14, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol 13: 437–457, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Watanabe N, Ikuta K, Okazaki K, Nakase H, Tabata Y, Matsuura M, Tamaki H, Kawanami C, Honjo T, Chiba T. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis Sci 48: 408–414, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y, Yang D, Liang H, Ye J, Weng J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One 6: e16556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132: 562–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zha W, Liang G, Xiao J, Studer EJ, Hylemon PB, Pandak WM, Jr, Wang G, Li X, Zhou H. Berberine inhibits HIV protease inhibitor-induced inflammatory response by modulating ER stress signaling pathways in murine macrophages. PLoS One 5: e9069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang M, Long Y, Sun Y, Wang Y, Li Q, Wu H, Guo Z, Li Y, Niu Y, Li C, Liu L, Mei Q. Evidence for the complementary and synergistic effects of the three-alkaloid combination regimen containing berberine, hypaconitine and skimmianine on the ulcerative colitis rats induced by trinitrobenzene-sulfonic acid. Eur J Pharmacol 651: 187–196, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharmacol Exp Ther 294: 822–829, 2000 [PubMed] [Google Scholar]