Abstract
A subthreshold pharyngeal stimulus induces lower esophageal sphincter (LES) relaxation and inhibits progression of ongoing peristaltic contraction in the esophagus. Recent studies show that longitudinal muscle contraction of the esophagus may play a role in LES relaxation. Our goal was to determine whether a subthreshold pharyngeal stimulus induces contraction of the longitudinal muscle of the esophagus and to determine the nature of this contraction. Studies were conducted in 16 healthy subjects. High resolution manometry (HRM) recorded pressures, and high frequency intraluminal ultrasound (HFIUS) images recorded longitudinal muscle contraction at various locations in the esophagus. Subthreshold pharyngeal stimulation was induced by injection of minute amounts of water in the pharynx. A subthreshold pharyngeal stimulus induced strong contraction and caudal descent of the upper esophageal sphincter (UES) along with relaxation of the LES. HFIUS identified longitudinal muscle contraction of the proximal (3–5 cm below the UES) but not the distal esophagus. Pharyngeal stimulus, following a dry swallow, blocked the progression of dry swallow-induced peristalsis; this was also associated with UES contraction and descent along with the contraction of longitudinal muscle of the proximal esophagus. We identify a unique pattern of longitudinal muscle contraction of the proximal esophagus in response to subthreshold pharyngeal stimulus, which we propose may be responsible for relaxation of the distal esophagus and LES through the stretch sensitive activation of myenteric inhibitory motor neurons.
Keywords: upper esophageal sphincter, longitudinal muscle of esophagus, stretch sensitive relaxation, mechanosensitive motor neurons
a threshold stimulation of the pharynx induces a swallow that is associated with relaxation of the upper and lower esophageal sphincters along with a peristaltic contraction in the esophagus (primary peristalsis) (23). On the other hand, a subthreshold pharyngeal stimulus induces isolated relaxation of the lower esophageal sphincter (LES) without esophageal peristalsis (20, 25, 31), in both animal and human studies. In addition to LES relaxation, a subthreshold pharyngeal stimulus can halt the progression of peristaltic motor activity in the esophagus (30). It is felt that a threshold or suprathreshold pharyngeal stimulus excites initial inhibitory followed by excitatory vagal nerves of the esophagus and sphincters. On the other hand, a subthreshold stimulus may activate only the inhibitory motor nerve activity that results in relaxation of the LES and inhibition of any ongoing peristaltic contraction.
Recent studies show that an orally directed stretch on the esophagus activates LES relaxation in the opossum (8), mice (13), and rats (14). Swallow-related as well as transient LES relaxation is associated with movement of the LES in the oral/cranial direction (6, 22, 27), which is due to the contraction of longitudinal muscles of the esophagus. The patterns of longitudinal muscle contraction with swallow and transient LES relaxation are distinct (2). With swallow, circular and longitudinal muscles contract together and traverse together along the length of the esophagus in an aborally directed peristaltic fashion (21). On the other hand, with transient LES relaxation, a selective longitudinal muscle contraction begins above the LES and progresses in the oral direction. Irrespective of the pattern of longitudinal muscle contraction, an orally directed stretch occurs during swallow-induced as well as transient LES relaxation. Based on the above observations, we propose that under physiological circumstances stretch of the LES due to longitudinal muscle contraction activates inhibitory motor neurons of the myenteric plexus, which in turn release nitric oxide to induce LES relaxation (13). It is not clear, however, whether, similar to swallow-induced and transient LES relaxations, a subthreshold pharyngeal stimulation-induced LES relaxation is also associated with longitudinal muscle contraction of the esophagus. Therefore, the goal of our study was to identify whether longitudinal muscle contraction of the esophagus occurs in response to subthreshold pharyngeal stimulation and the characteristics of the above contraction.
METHODS
Recording equipment and technique.
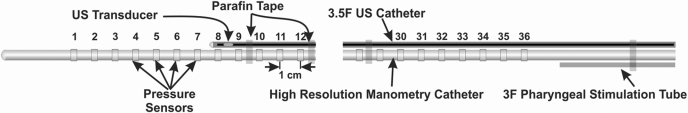
Sixteen normal healthy asymptomatic subjects (9 males and 7 females) participated in the study protocol. The ages of the subjects ranged from 18 to 55 years (median age, 32 years). The protocol for these studies was approved by the “Human Subject Protection Committee of the University of California San Diego,” and each subject signed an informed consent form before the study. A catheter assembly consisting of a high resolution manometry (HRM) catheter, a pharyngeal injection catheter, and a high frequency intraluminal ultrasound (HFIUS) catheter was used for these studies (Fig. 1). The HRM catheter was equipped with 36 solid state pressure transducers (Sierra Scientific, Los Angeles, CA). A 3 F silicon catheter with an opening, located 2 cm above the most proximal transducer on the HRM catheter, was taped to the HRM catheter. A 3.2 F, HFIUS catheter equipped with a 20 MHz transducer was also taped to the HRM catheter. The ultrasound (US) transducer could be moved within its static catheter sheath, without moving the HRM catheter, thus allowing the recording of US images at the desired location in the esophagus.
Fig. 1.
Schematic of the catheter assembly. The high resolution manometry catheter, high frequency ultrasound catheter probe, and pharyngeal injection catheter are taped together as shown. The ultrasound (US) transducer can be moved in its catheter sheath to record images of the esophagus at desired locations.
The subject's nostril was anesthetized using 2% viscous lidocaine, and the catheter assembly was placed transnasally and positioned such that the two most proximal transducers were located just above the upper end of upper esophageal sphincter (UES). The remainder of the pressure transducers spanned the entire length of the esophagus with at least two or more pressure transducers in the stomach. In the above catheter position, the pharyngeal injection port also was located 4 cm above the upper end of the UES. The catheter assembly was secured to the tip of the nose with tape and connected to the manoscan module for the HRM recordings (Sierra Scientific). Ultrasound images were recorded on a VHS tape recorder interfaced to the HP Sonos 100 machine (Hewlett-Packard, Watertown, MA).
Study protocol.
The pharynx was stimulated using small amounts of water at room temperature. Water was injected into the pharynx using a 1-ml insulin syringe, and the subject was asked to hold swallow for at least 30 s following the injection. The first injection volume was 0.1 ml, which was gradually increased by 0.1 ml until the peak effect on the LES pressure without pharyngeal swallow or primary peristalsis occurred. The above volume was tested at least three times in each subject with the US transducer located first at 2 cm above the LES, followed by 8 cm above the LES, and finally at a location 2 to 3 cm below the UES. At each of the above sites three pharyngeal stimulations at the maximal water volume used in the previous trials were performed.
Subjects were asked to perform five dry swallows to induce esophageal contractions and peristalsis. If dry swallows induced peristalsis consistently, three additional dry swallows that were immediately followed by pharyngeal stimulation (within 2 to 3 s of a swallow) were studied. The volume of water injection used during these instances was the same as the volume used before swallows.
Data analysis.
HRM data was analyzed with Manoview analysis software (Sierra Scientific). The following parameters were measured before and after stimulation using Manoscan's smart mouse option: UES pressure, UES descent, UES length, esophageal pressure at 5 cm above the LES, esophageal length, and LES pressure. UES pressure was taken as an average of all pressures constituting the UES high pressure zone over a 10-s period. UES descent was determined from the axial movement of the proximal border of the UES high pressure zone. UES length was the axial high pressure zone spanned by the UES. Esophageal pressure was measured at 5 cm above the LES and averaged over 10 s. Esophageal length was the distance between the distal edge of the UES and the proximal edge of the LES high pressure zones. Esophageal shortening was the difference between the esophageal lengths, before and after pharyngeal stimulation. LES pressure was taken as the 3-s nadir over a 10-s interval to capture the end-expiratory LES pressure. HFIUS images of the esophagus at 2 cm, 8 cm above the LES, and 2 to 3 cm below the UES were analyzed using the methods described previously, during the 10-s period before and 60-s period following pharyngeal stimulations. Briefly, US images were digitized using a video capture card (ACEDVio, Canopus Co, Kobe, Japan) with 640 × 480 pixel spatial resolution and 256 shades of gray using Adobe Premier 6.0 (Adobe Systems, Mountain View, CA). B-mode US image data was then converted to 16-equispaced M-mode US images. The M-mode US image passing perpendicular to the esophageal wall muscle that did not show significant echo dropout was selected for the image analysis. In each M-mode image, two edges corresponding to the inner circular and outer longitudinal muscle layers were manually drawn using Sigma Scan Pro 5 (Jandel Scientific, San Rafael, CA). The distance between these two edges was measured in millimeters and was referred to as muscle thickness.
Statistical analysis.
Data are reported as means ± SE, unless stated otherwise. Paired and unpaired Student's t-tests were used for statistical comparison as appropriate. P value <0.05 was considered statistically significant.
RESULTS
Effect of pharyngeal stimulus on the UES, esophagus and LES.
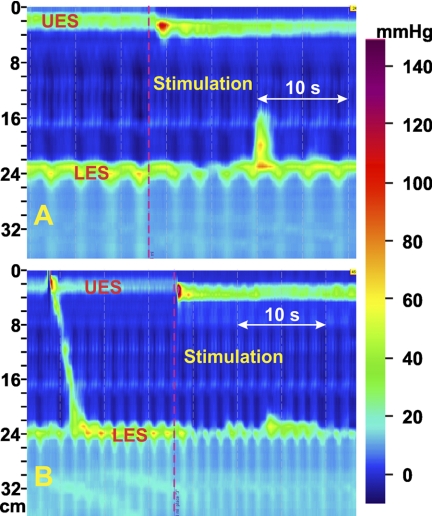
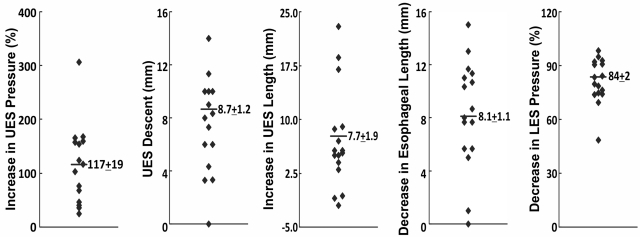
Pharyngeal injection with the water volume that produced a maximal LES relaxation without inducing a swallow or peristalsis was used for all the stimulations. Pharyngeal stimulation resulted in a strong contraction of the UES with a resultant increase in UES pressure of 122 ± 19% above the baseline value. Along with the increase in pressure, there was lengthening and descent of the UES. All of these changes in the UES occurred simultaneously (Fig. 2). Figure 3 shows absolute changes in the above parameters in each of the 16 subjects. UES pressure was increased in all, and descent was seen in all but one subject. The mean UES descent and increase in the UES length were 8.1 ± 1.1 and 7.1 ± 1.8 mm, respectively. Esophageal pressure, 5 cm above the LES, was not affected by pharyngeal stimulation. LES pressure tracings revealed a fall in the end-expiratory LES pressure without significant change in the inspiratory pressure oscillations or crural diaphragm contractions. Mean reduction in the end-expiratory LES pressure with pharyngeal stimulus was 82 ± 3%. There was no change in the craniocaudal location of the LES. As a result of UES descent and increase in UES length, esophageal length decreased significantly (mean decrease, 8.3 ± 1.0 mm).
Fig. 2.
A and B: effect of pharyngeal stimulus on the upper esophageal sphincter (UES), esophagus, and lower esophageal sphincter (LES). Shown here are 2 examples of the effects of injection of minute amounts of water in the pharynx on the esophagus. Note the increase in UES pressure along with descent of the UES. Also note the relaxation of the LES. Inspiratory pressure oscillations are not affected by pharyngeal stimulation. Esophageal length is shortened with each pharyngeal stimulus.
Fig. 3.
Summary of the effect of pharyngeal stimulus on UES, esophageal, and LES pressure. These data were obtained from 16 subjects and show the individual data obtained in each subject and the mean values for each parameter. Note the increase in UES pressure, increase in UES length, and descent of the UES. Also note the change in esophageal length during pharyngeal stimulation. There was significant relaxation of the LES but no change in the craniocaudal location of the LES during pharyngeal stimulation.
Effect of pharyngeal stimulus on esophageal longitudinal muscle contraction.
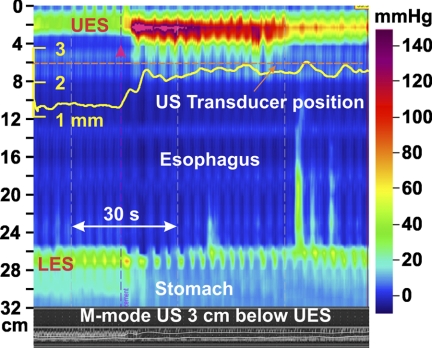
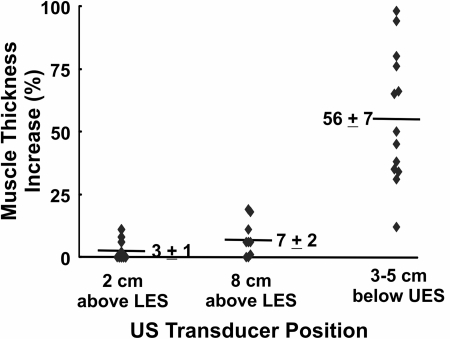
Adequate images at 2 to 3 cm below the UES were obtained in 13 subjects. M-mode images show an increase in the muscle thickness with pharyngeal stimulation as seen in Fig. 4. Except for one subject, a significant increase in the muscle thickness was observed in all subjects (it is possible that the US transducer was located somewhat distal to the contraction site in this subject). The increase in muscle thickness in 12 subjects ranged from 30% to 100%, with a mean increase of 56%. The duration of muscle thickness increase ranged from 4 to 60 s (median, 10 s). US images of the esophagus were assessed at 2 and 8 cm above the LES in nine subjects, and there was no significant change in the esophageal muscle thickness noticed at either of the two esophageal locations (Fig. 5).
Fig. 4.
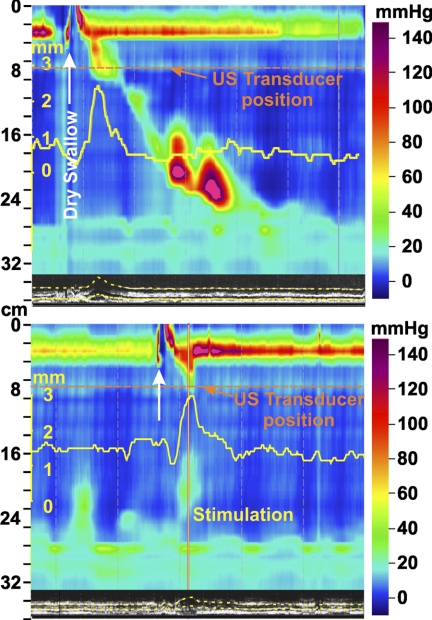
Effect of pharyngeal stimulus on the esophageal pressures and longitudinal muscle contraction in the proximal esophagus. Note the effect of pharyngeal stimulus (red arrowhead) on changes in muscle thickness that were recorded 3 cm below the UES. Changes in muscle thickness can be seen in the B-mode US image at the bottom of the high resolution manometry plot. Muscle thickness (yellow) was also quantitated (see methods for details) and the graph superimposed on the high resolution manometry plot.
Fig. 5.
Effect of pharyngeal stimulus on changes in esophageal muscle thickness. Muscle thickness was measured as the average value 10 s before and after the pharyngeal stimulus and plotted here as a percent increase. Each data point represents the mean value from 3 observations in each subject. Note the increase in muscle thickness at 2 to 3 cm below the UES location and not at 2 and 8 cm above the LES, suggesting longitudinal muscle contraction of the proximal esophagus in response to pharyngeal stimulus.
Effect of pharyngeal stimulus on the progression of swallow-induced contraction and peristalsis.
In five subjects, we studied the effect of pharyngeal stimulation on the progression of dry swallow-induced esophageal contraction. In each of these subjects, dry swallow induced a peristaltic contraction along the whole length of the esophagus. Pharyngeal stimulation within 2 to 3 s of the dry swallow halted the progression of peristaltic contraction in all instances (Fig. 6). Similar to pharyngeal stimulus in the absence of swallows, there was a strong contraction of the UES with a resultant increase in UES pressure. Along with the increase in UES pressure there were increases in the UES length and UES descent. Changes in the UES pressure, UES length, and UES descent with pharyngeal stimulation were not different in the absence or presence of swallow (UES length, P = 0.22; and UES descent, P = 0.64). The M-mode image of the esophageal wall with the transducer located 2 to 3 cm below the UES revealed a small decrease, followed by an increase in the muscle thickness with each swallow. The mean duration of muscle thickness increase (4.7 ± 0.3 s) was similar to the duration of the pressure wave at 2 to 3 cm below the UES. Pharyngeal stimulation following a swallow increased the duration of increased muscle thickness (mean, 7.5 ± 0.7 s; P < 0.05).
Fig. 6.
Effect of pharyngeal stimulus on the progression of dry swallow induced peristalsis in the esophagus. On the left is an esophageal response to swallow (white arrow) showing peristaltic relaxation of the UES and esophageal peristaltic contraction. Also note the increase in esophageal muscle thickness (yellow) with peristaltic contraction. With pharyngeal stimulation (red line at bottom) following a swallow event, there is UES contraction and esophageal contraction seen in the proximal but not the distal esophagus. Progression of peristaltic contraction is inhibited by the pharyngeal stimulus. The ultrasound image shows increased duration of muscle thickness (longitudinal muscle contraction) in response to pharyngeal stimulus.
DISCUSSION
In summary, our data show that a subthreshold pharyngeal stimulus induces the following: 1) an intense contraction of the UES, caudal descent of the UES, decrease in the esophageal length, no change in the distal esophageal pressure, and prominent LES relaxation; 2) US image analysis reveals a novel longitudinal muscle contraction pattern with subthreshold pharyngeal stimulus; it is localized to the most proximal esophagus; and 3) a subthreshold pharyngeal stimulus prevents the progression of peristaltic contraction in the esophagus, which is temporally associated with longitudinal muscle contraction of the proximal esophagus.
Effects of subthreshold pharyngeal stimulation on the UES pressure (26), inhibition of peristaltic progression in the esophagus (30), and LES (20, 31) in humans and animal (15) studies have been described in detail previously. Our studies confirm all of the above findings. Effects of pharyngeal stimulus on the UES and LES pressure in the above referenced studies were performed using a Dent sleeve sensor (5), which cannot record axial or vertical movements of the UES and LES. On the other hand, HRM technique can record craniocadual movements of the high pressure zones fairly accurately. We recently described the accuracy of HRM technique in detecting small (2–10 mm) movements of the high pressure zones on the catheter (19). The current study, for the first time, describes caudal movement of the UES with a subthreshold pharyngeal stimulus. What causes this caudal UES movement? It appears as if the UES is pulled down by a force, acting from below the UES, i.e., longitudinal muscle contraction of the esophagus. US image analysis reveals longitudinal muscle contraction localized to the proximal esophagus, i.e., 2 to 3 cm below the UES. We cannot be sure of the vertical extent of esophageal longitudinal muscle, but it appears to be limited to a short segment, i.e., 3–5 cm of the esophagus below the UES. Our initial suspicion, based on the location of contraction in association with transient lower esophageal sphincter (TLESR) (2), was that contraction of the longitudinal muscle should be localized to the distal esophagus, which was not the case. Clearly, no contraction was seen at 2 and 8 cm above the LES. Absence of proximal movement of the LES during subthreshold pharyngeal stimulation also argues against distal esophageal longitudinal muscle contraction, which is in contrast with the prominent distal esophageal contraction and LES lift seen in association with TLESR (22).
What is the significance/relevance of longitudinal muscle contraction of the proximal esophagus in reference to the LES relaxation and inhibition of peristaltic progression? Contraction of esophageal longitudinal muscle exerts a mechanical stretch on the segment of esophagus caudal to the site of contraction, resulting in the elongation of the esophageal segment (7, 24). Our studies show that an axial mechanical stretch in the cranial direction causes LES relaxation (13). Therefore, it is possible that longitudinal muscle contraction of the proximal esophagus is the cause of LES relaxation. One could argue that we did not observe cranial movement of the LES with pharyngeal stimulus; how then could we attribute LES relaxation to longitudinal muscle contraction? Inhibitory motor neurons that mediate LES relaxation are located several centimeters above the LES (3); it may be that longitudinal muscle contraction of the proximal esophagus causes deformation and activation of these neurons to induce LES relaxation. There are a few peculiar aspects of the effects of subthreshold pharyngeal stimulation on LES pressure that have been described previously and were also observed in the current study as well. Unlike TLESR, subthreshold pharyngeal stimulus induced LES relaxation is not usually complete and not associated with inhibition of the crural diaphragm (20). Our earlier studies show a direct relationship between the degree of LES relaxation and the degree of cranial pull on the LES. Furthermore, LES inhibition appears to be more sensitive than the crural diaphragm inhibition in response to axial stretch (16, 17). It is interesting that the longitudinal muscle contraction localized to the proximal esophagus will exert a more mild axial stretch on the LES as compared with the longitudinal muscle contraction of the distal esophagus [seen in association with the TLESR(2)] and hence a less prominent LES relaxation and lack of crural diaphragm inhibition with subthreshold pharyngeal stimulus.
Unlike LES, the body of the esophagus does not have resting tone, thus making it difficult to demonstrate inhibition of the esophagus. By creating an artificial high pressure zone with a distended balloon, Sifrim et al. (28, 29) demonstrated inhibition of the esophagus in response to a swallow. By using two balloons and creating two artificial esophageal high pressure zones they found that the onset of inhibition occurs simultaneously but the duration of inhibition was longer in the distal as compared with proximal esophagus. Using HFIUS imaging, we recently found that similar to the swallow-induced contraction wave, the inhibition wave also traverses in a peristaltic fashion along the length of the esophagus (1). The wave of inhibition is localized caudal to the site of contraction wave. With multiple swallows at closely spaced intervals, only the last swallows result in peristaltic contraction (deglutitive inhibition) (18). It is suggested that each successive swallow inhibits the previous swallow-induced contraction by the initial inhibitory impulse generated by the swallow (9–11). Similar to a swallow, a subthreshold pharyngeal stimulus also inhibits the progression of a peristaltic contraction wave elicited by a swallow (30). One possibility is that in response to subthreshold pharyngeal stimulus the impulse originates in the dorso-motor complex of the vagus nerve communicates with the inhibitory motor neurons located in the myenteric plexus of the esophagus and LES (4, 12). Another possibility is that the vagus only carries excitatory nerve fibers to the esophageal muscles; those fibers that activate longitudinal muscle contraction of the proximal esophagus are actually responsible for inhibition of the esophagus and the LES distal to it, through a stretch sensitive excitation of the inhibitory motor neurons located in the myenteric plexus (Fig. 7 schematic). Further studies are required to determine whether vagus nerve fibers actually synapse with the inhibitory motor neurons of the esophagus, which to the best of our knowledge has never been proved.
Fig. 7.
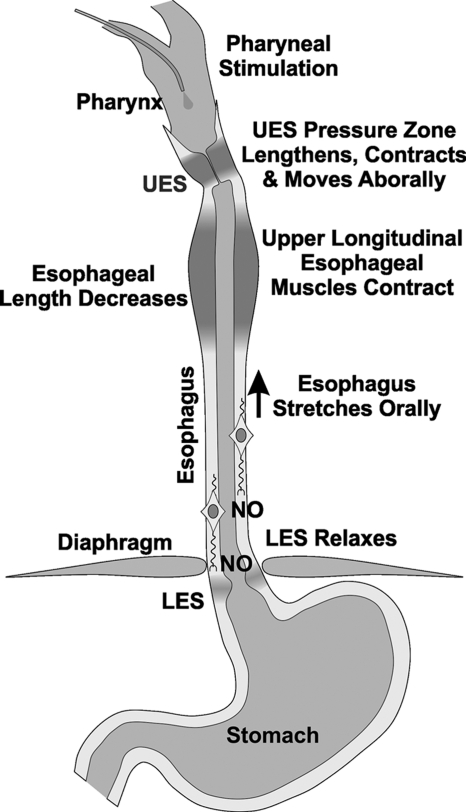
Schematic of the effects of subthreshold pharyngeal stimulus on the esophagus and LES. A subthreshold pharyngeal stimulus induces contraction of the longitudinal muscle of the proximal esophagus, which we propose activates stretch sensitive inhibitory motor neurons of the esophagus and LES to release nitric oxide (NO) to induce relaxation of the esophagus and lower esophageal sphincter.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant NIH-RO1-DK060733.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.L. performed experiments; E.L. and V.B. analyzed data; V.B. and R.K.M. conception and design of research; V.B. and R.K.M. interpreted results of experiments; V.B. prepared figures; V.B. and R.K.M. edited and revised manuscript; V.B. and R.K.M. approved final version of manuscript.
REFERENCES
- 1. Abrahao L, Jr, Bhargava V, Babaei A, Ho A, Mittal RK. Swallow induces a peristaltic wave of distension that marches in front of the peristaltic wave of contraction. Neurogastroenterol Motil 23: 201–207, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Babaei A, Bhargava V, Korsapati H, Zheng WH, Mittal RK. A unique longitudinal muscle contraction pattern associated with transient lower esophageal sphincter relaxation. Gastroenterology 134: 1322–1331, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Brookes SJ, Chen BN, Hodgson WM, Costa M. Characterization of excitatory and inhibitory motor neurons to the guinea pig lower esophageal sphincter. Gastroenterology 111: 108–117, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol 284: G357–G366, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Dent J. A new technique for continuous sphincter pressure measurement. Gastroenterology 71: 263–267, 1976 [PubMed] [Google Scholar]
- 6. Dodds WJ. 1976 Walter B. Cannon lecture: current concepts of esophageal motor function: clinical implications for radiology. AJR Am J Roentgenol 128: 549–561, 1977 [DOI] [PubMed] [Google Scholar]
- 7. Dodds WJ, Stewart ET, Hodges D, Zboralske FF. Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest 52: 1–13, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dogan I, Bhargava V, Liu J, Mittal RK. Axial stretch: a novel mechanism of the lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 292: G329–G334, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Gidda JS, Cobb BW, Goyal RK. Modulation of esophageal peristalsis by vagal efferent stimulation in opossum. J Clin Invest 68: 1411–1419, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gidda JS, Goyal RK. Regional gradient of initial inhibition and refractoriness in esophageal smooth muscle. Gastroenterology 89: 843–851, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Gidda JS, Goyal RK. Swallow-evoked action potentials in vagal preganglionic efferents. J Neurophysiol 52: 1169–1180, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Jean A. Electrophysiologic characterization of the swallowing pattern generator in the brainstem. Nature Publishing, 2006 [Google Scholar]
- 13. Jiang Y, Bhargava V, Mittal RK. Mechanism of stretch-activated excitatory and inhibitory responses in the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 297: G397–G405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang Y, Sandler B, Bhargava V, Mittal RK. Antireflux action of Nissen fundoplication and stretch-sensitive mechanism of lower esophageal sphincter relaxation. Gastroenterology 140: 442–449, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting, and swallowing. Am J Physiol Gastrointest Liver Physiol 283: G529–G536, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Pehlivanov N, Mittal RK. Baclofen blocks LES relaxation and crural diaphragm inhibition by esophageal and gastric distension in cats. Am J Physiol Gastrointest Liver Physiol 283: G1276–G1281, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Puckett JL, Takeda T, Jung HY, Mittal RK. Crural diaphragm inhibition during esophageal distension correlates with contraction of the esophageal longitudinal muscle in cats. Am J Physiol Gastrointest Liver Physiol 288: G927–G932, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Meyer GW, Gerhardt DC, Castell DO. Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol Gastrointest Liver Physiol 241: G129–G136, 1981 [DOI] [PubMed] [Google Scholar]
- 19. Mittal R, Karstens A, Leslie E, Bhargava V. Longitudinal muscle contraction of the esophagus and common cavity pressure during incomplete transient lower esophageal sphincter relaxation. Gastroenterology 140, Suppl: S623–S624, 2011. [Google Scholar]
- 20. Mittal RK, Chiareli C, Liu J, Shaker R. Characteristics of lower esophageal sphincter relaxation induced by pharyngeal stimulation with minute amounts of water. Gastroenterology 111: 378–384, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Mittal RK, Padda B, Bhalla V, Bhargava V, Liu J. Synchrony between circular and longitudinal muscle contractions during peristalsis in normal subjects. Am J Physiol Gastrointest Liver Physiol 290: G431–G438, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Pandolfino JE, Zhang QG, Ghosh SK, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology 131: 1725–1733, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Paterson WG, Rattan S, Goyal RK. Experimental induction of isolated lower esophageal sphincter relaxation in anesthetized opossums. J Clin Invest 77: 1187–1193, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology 112: 1147–1154, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Pouderoux P, Verdier E, Kahrilas PJ. Patterns of esophageal inhibition during swallowing, pharyngeal stimulation, and transient LES relaxation. Am J Physiol Gastrointest Liver Physiol 284: G242–G247, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Shaker R, Ren J, Xie P, Lang IM, Bardan E, Sui Z. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol Gastrointest Liver Physiol 273: G854–G858, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Shi G, Pandolfino JE, Joehl RJ, Brasseur JG, Kahrilas PJ. Distinct patterns of oesophageal shortening during primary peristalsis, secondary peristalsis and transient lower oesophageal sphincter relaxation. Neurogastroenterol Motil 14: 505–512, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Sifrim D, Janssens J, Vantrappen G. Failing deglutitive inhibition in primary esophageal motility disorders. Gastroenterology 106: 875–882, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Sifrim D, Janssens J, Vantrappen G. A wave of inhibition precedes primary peristaltic contractions in the human esophagus. Gastroenterology 103: 876–882, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Trifan A, Ren J, Arndorfer R, Hofmann C, Bardan E, Shaker R. Inhibition of progressing primary esophageal peristalsis by pharyngeal water stimulation in humans. Gastroenterology 110: 419–423, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Trifan A, Shaker R, Ren J, Mittal RK, Saeian K, Dua K, Kusano M. Inhibition of resting lower esophageal sphincter pressure by pharyngeal water stimulation in humans. Gastroenterology 108: 441–446, 1995 [DOI] [PubMed] [Google Scholar]