Abstract
Despite intensive research studies, theories have yet to focus on the contribution of hypoxia to patency differences observed clinically between arterial vs. venous grafts. This study investigates the differential hypoxic response of smooth muscle cells (SMC) to hypoxia-derived endothelial cell (EC) growth factors. Initiation of SMC proliferation under hypoxia (<5% O2) occurred only after incubation with hypoxic endothelial cell-conditioned media (H-ECM). After the investigation of several possible growth factors in the H-ECM that may be responsible for SMC proliferation, the greatest difference was observed in vascular endothelial growth factor (VEGF-A) and platelet-derived growth factor homodimer B (PDGF-BB) expression. VEGF-A increased (2-fold) significantly (P < 0.05) in arterial-derived smooth muscle cells (ASMC) under hypoxia compared with venous-derived smooth muscle cells (VSMC), which showed no significant change. VSMC showed significant (P < 0.05) increase in VEGFR-2 expression under hypoxia compared with ASMC. Incubation with VEGFR-2-neutralizing antibody/PDGFR antagonist in VSMC before addition of H-ECM resulted in decreased proliferation. ASMC proliferation under hypoxia did not decrease during incubation with VEGFR-2-neutralizing antibody but did decrease upon PDGFR antagonist incubation. Current therapies focusing on treating intimal hyperplasia have negated the fact that combinational therapy might be required to combat induction of SMC proliferation. Clinically, therapy with PDGFR antagonists plus anti-VEGFR-2 may prove to be efficacious in managing SMC proliferation in venous-derived grafts.
Keywords: vascular endothelial growth factor-A, platelet-derived growth factor homodimer B, graft patency
intimal hyperplasia (IH) occurs when smooth muscle cells (SMC) migrate and proliferate from the tunica media into the tunica intima of a vessel due to hypoxia following injury (22). IH occurs in most vascular surgeries including vascular grafts and by-pass surgeries, which ultimately leads to vessel occlusion and graft failure (18, 22). Studies conducted on graft patency have shown that arterial grafts have a higher patency than venous grafts. Almost 90% occlusion occurring in venous grafts after 10 yr compared with only 50% occlusion in arterial grafts (3, 7, 18).
IH is a growing concern because the effective treatment for the prevention of IH in clinical practice still continues to elude vascular surgeons (19). This is of great concern considering that there is an overall increase in coronary arterial disease incidences in America (21). Surgical bypass of arterial occlusions using autogenous vein provides an effective treatment for many patients with advanced coronary atherosclerosis (9, 21). Conventional pharmacotherapy has limited impact on graft failure (9). Therefore, it is necessary to investigate the mechanism by which IH occurs so as to identify novel therapeutic targets that can inhibit IH.
Several growth factors have been implicated to modulate IH; however, controversy surrounds the exact initiating factors involved in SMC migration and proliferation (25). As important as determining the initiating events, the identification and characterization of key factors that are functionally important in propagation of IH are needed since these factors could be potential targets for therapeutic intervention (2, 25). Various theories state that hypoxia alone in an autocrine mechanism acts as a stimuli on SMC to initiate proliferation (8, 13, 27, 28). Another theory suggests that the combined actions of growth factors, proteolytic agents, and extracellular matrix proteins that are produced by a dysfunctional endothelium following injury or hypoxia induce proliferation and migration of resident SMC from the media into the intima (12, 22). However, most theories agree that hypoxia plays a pivotal role in SMC migration and proliferation. Studies (8, 12, 22, 27) that have been conducted to show the interaction between SMC and endothelial cell (EC) under hypoxia have been inconclusive and confusing. Moreover, these studies have not focused on how hypoxia impacts the differences observed between arterial derived SMC (ASMC) and venous-derived SMC (VSMC) proliferation (8, 13, 14).
Among various growth factors induced in EC under hypoxia vascular endothelial growth factor (VEGF-A) and platelet-derived growth factor (PDGF-BB) have been implicated most significantly in the regulation of SMC proliferation and de-differentiation (13, 22, 25). VEGFR-2 is the primary mediator of VEGF signaling and is responsible for the proliferative effects observed with VEGF (6). PDGF-BB signals via PDGFR-β, a tyrosine kinase involved in proliferative effects of PDGF-BB.
In this study, we hypothesized that the differences between venous- and arterial-derived graft patency observed clinically are due to differential responses of SMC proliferation to hypoxic EC-derived growth factors under hypoxia. We investigated the interaction of EC with SMC under hypoxia as a potential mechanism for initiation of intimal hyperplasia.
Our findings showed that VSMC vs. ASMC cells under hypoxia showed differences in growth factor profiles (VEGF-A and PDGF-BB) as well as their receptor (R) expression. These differences play a crucial role in causing differential proliferation in ASMC vs. VSMC proliferation.
METHODS
Cell Culture
Human umbilical VSMC were obtained from ScienceCell Research Laboratories (Carlsbad, CA) and maintained in SmGM-2 (Lonza, Walkersville, MD). Human ASMC and human aortic endothelial cells (AEC), obtained from Lonza, were maintained in SmGM-2 and EGM-2 (Lonza) medium, respectively. Human umbilical vein endothelial cells (VEC; kind gift from S. Ramakrishnan, University of Minnesota) were also maintained in EGM-2 media.
Reagents.
p-ERK1/2, total-ERK1/2 primary antibodies, anti-rabbit IgG, and anti-mouse IgG secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA). Hypoxia inducible factor-1-α (HIF1-α), VEGFR-2, α-tubulin, β-actin, ephrin B2, and PDGFRβ primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); anti-human VEGFR2 [phycoerythrin (PE)], anti-human VEGFR-2-neutralizing antibody, anti-human PDGF-BB-neutralizing antibody, and Eph-B4 (PE) were purchased from R&D Systems (Minneapolis, MN); and anti-rabbit secondary antibody (FITC) for ephrin-B2 was acquired from BD Biosciences. Imatinib mesilate (PDGFR antagonist) and VEGFR-2 tyrosine kinase inhibitor were purchased from Calbiochem (Rockland, MA).
Treatment.
Cells were serum starved for 24 h in 1% FBS-supplemented media (EBM-2 and SmBm) and then subjected to normoxia and hypoxia for different time end points. The 3-h treatment group was used for isolation of total mRNA in cells and 24 h for the proliferation assay, growth factor, and growth factor receptor analysis.
Normoxic conditions (21% oxygen) are defined here as normal room air in a 5% CO2, 37°C cell culture incubator. To achieve hypoxia (3–5% O2), cells were placed in a modular chamber (Billups Rothenberg, Del Mar, CA) and flushed with a mix of 0% O2, 5%CO2, and 95% N2 at 10 l/min for 15 min. Chambers remained tightly sealed and placed in a 5% CO2, 37°C incubator. This method achieves Po2 levels <35 mmHg as determined from cell culture medium analyzed using a blood gas analyzer (Rapid Lab248; Chiron Diagnostics Tarrytown, NY); the Po2 levels of culture supernatant from cells grown under normoxic conditions were 150–160 mmHg.
Bromodeoxyuridine Cell Proliferation Assay
Bromodeoxyuridine (BrdU) ELISA from Roche Diagnostics (Madison, WI) was used to measure cell proliferation. SMC (1 × 103) were plated in a 96-well plate after serum starvation for 24 h. Cells were then placed under hypoxia for 24 h. At 24 h, BrdU (100 μM) was added for 4 h, which is taken up into the newly synthesized DNA of the replicating cells. For experiments determining the effect of conditioned EC media on SMC proliferation, 100 µl of conditioned media were added to each well of the 96-well plates before hypoxia treatment for 6, 12, 24, and 48 h and then labeled with BrdU. To determine the VEGF dose response, 5 × 103 cells (ASMC, VSMC, and VEC) were plated in a 48-well tissue culture plate and placed under hypoxia for 24 h in the presence of 0, 5, 10, and 15 ng/ml of VEGF. BrdU incorporation in these cells was used as an indicator of cell proliferation. Cells were fixed with FixDenature solution (Roche Diagnostics) and then were exposed for immunodetection with a peroxidase-conjugated anti-BrdU antibody. Absorbance was measured at a wavelength of 370 nm using a Fluostar Omega BMG Labtech (Cary, NC) microplate reader.
MTT Assay
SMC (1 × 103) were plated in 96-well plates, serum starved, and then placed under hypoxic and normoxic conditions for 24 h. The cells were then incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay; 2.5 mg/ml) for 4 h. Cells were solubilized with 100% DMSO (Sigma-Aldrich, St. Louis, MO), and absorbance at 450 nm was measured using a plate reader (Omega BMG Labtech, Cary, NC). Percent change in cell viability was determined and normalized to control (cell viability under normoxia).
In Vitro Coculture Assay
The proliferative effect of endothelial-derived hypoxia-induced growth factors on SMC was tested in a coculture model. Serum-starved SMC were seeded (30 000 cell/cm2 ) into a 12-well culture insert companion plate (Corning, Corning, NY) and allowed to adhere. Serum-starved EC (AEC and VEC) were seeded (15,000 cell/cm2) onto a 0.4-μm pore size polycarbonate membrane inserts at 15,000 cell/cm2 and were placed above the SMC. Cells were placed under hypoxia and normoxia for 24 h. BrdU incorporation was used as an indicator of SMC proliferation. Cells were labeled with BrdU after serum starvation. Data are expressed as percent change in SMC proliferation normalized to control. Proliferation of SMC under normoxia without addition of EC was used as a normalizing control for cells under normoxia and proliferation of SMC under hypoxia without addition of EC was used as a normalizing control for cells under hypoxia.
Angiogenesis Assay for Growth Factor Secretion
An angiogenesis ELISA strip II for analysis of cytokines from Signosis (Sunnyvale, CA) was used to compare levels of PDGF-BB, IGF-I, FGF-b, and transforming growth factor (TGF)-β growth factors in EC. EC (5 × 103) were plated in a 48-well plate, serum starved, and placed under normoxic or hypoxic conditions for 24 h. Supernatant (100 μl) was collected and tested for the growth factors described above according to the manufacturer's instructions. Absorbance was measured at 560 nm using a microplate reader.
Quantitative Analysis of VEGF-A and PDGF-BB Secretion
Protein levels of VEGF-A and PDGF-BB secreted in cell culture media were determined using a human PDGF-BB and VEGF-A (ELISA) kit per manufacturer's instructions. SMC and EC cells (5 × 103) were plated in a 48-well plate, serum starved, and placed under hypoxia for 24 h. Cell culture supernatants (100 μl) were collected and analyzed for secreted VEGF-A and PDGF-BB. (R&D Systems).
Quantitative RT-PCR and RT-PCR
RNA isolation and cDNA preparation.
Total RNA was extracted from cells (SMC and EC) using TRIZOL reagent from Invitrogen (Carlsbad, CA). RNA (1 µg) of each sample was reverse transcribed using an oligo d(T) primer and RNase Moloney murine leukemia virus reverse transcriptase, according to the manufacturer's protocol (Promega, Madison, WI). cDNA (100 ng) was used for real-time PCR and gel-based PCR to study the genes listed below.
Sense and antisense oligonucleotide primers for the growth factors (VEGF, PDGF-BB, FGF-2, and TGF-β) and their receptors (PDGFRβ, TGFβ-R1, and TGFβ-R2) and β-actin (internal control) were designed for RT-PCR using DNA sequence information obtained from the Genome Database (National Center for Biotechnology Information) and were synthesized at Bio-Medicine Genomic Facility at the University of Minnesota, and miR-125b primers were purchased from Qiagen (Valencia, CA).
The following specific primers were used: VEGF-A, sense: 5′-ATC ATG CGG ATC AAA CCT CA-3′ and antisense: 5′-CAA GGC CCA CAG GGA TTT TC-3′; PDGF-BB, sense: 5′-CGA GTT GGA CCT GAA CAT GA-3′ and antisense: 5′-GTC ACC GTG GCC TTC TTA AA-3′ [Osada-Oka et al. (24)]; PDGFRβ, sense: 5′-TGC TCA TCT GTG AAG GCA AG-3′ and antisense: 5′-TGG CAT TGT AGA ACT GCT CG-3′; FGF-2, sense: 5′-AGA GCG ACC CTC ACA TCA AG-3′ and antisense: 5′-ATA GCT TTC TGC CCA GGT CC-3′; TGF-β sense: 5′-TAT CGA CAT GGA GCT GGT GA-3′ and antisense: 5′-CAC GTG CTG CTC CAC TTT TA-3′; TGF-β-R-1, sense: 5′-ACA GAT GGG CTC TGC TTT GT-3′ antisense 5′-AGG GCG ATC TAA TGA AGG GT-3′; TGF-β-R-2, sense: 5′-TGC CCC AGC TGT AAT AGG AC-3′ and antisense: 5′-GGA GAA GCA GCA TCT TCC AG- 3′; and β-actin, sense 5′-GAT CAT TGC TCC TCC TGA GC-3′ and antisense 5′-CAC CTT CAC CGT TCC AGT TT-3′.
The real-time PCR analysis was performed using SyBR-Green mix (Applied Biosystems, Carlsbad, CA) on a 7500 Real Time PCR station (Applied Biosystems). Transcript levels of RNU6B were used as endogenous control for mir-125b levels. The results for real-time PCR were calculated as ratio target gene expression (experimental/ control) and were expressed as fold change. Image J software was used to quantify intensity of the bands obtained after running PCR product on a 1% agarose gel for gel-based PCR.
Western Blot Analysis
SMC and EC (1 × 106) cells were plated in a cell culture flask and placed under normoxia and hypoxia for 24 h. Cells were then lysed with 500 µl lysis buffer from Sigma according to the manufacturer's instructions. Total protein concentration of the supernatants was determined using Bio-Rad DC protein assay from Bio-Rad (Hercules, CA). Thirty micrograms of total protein were loaded for p-ERK1/2 Western blot (WB), 100 µg for HIF1-α WB, and 50 µg for VEGFR-2 and PDGFR-β WB analysis and their respective loading controls. The samples were electrophoresed in a 7% discontinuous SDS-PAGE. The resolved proteins were transferred to a PVDF membrane from Bio-Rad (Hercules, CA), which was then blocked for 1 h with 5% nonfat milk at room temperature. The membrane was incubated with (1:500) primary antibody concentration (PDGFR-β, α-tubulin, β-actin, ERK1/2, and VEGFR-2) overnight at 4°C. Membrane bound primary antibodies were detected using anti-mouse or -rabbit IgG secondary antibodies (1:1,000) conjugated with horseradish peroxidase. Immunoblots were detected with UltraQuant 6.0 Ultralum (Claremont, CA) using enhanced chemiluminescence technique (Supersignal Western HRP substrate; Thermo, Rockford, IL). Quantification of bands was performed using UltraQuant 6.0 (Claremont, CA).
Flow Cytometry
SMC (1 × 106) were plated in a tissue culture flask and placed under normoxia and hypoxia for 24 h. Cells were trypsinized using trypsin (0.25%) and centrifuged at 1,200 rpm for 5 min. Pelleted cells were washed twice with staining buffer (100 µl PBS + 0.5% BSA + 0.02% sodium azide) and incubated at 4°C for 30 min with 1 µg anti-human VEGFR-2 (PE). To check the purity of the SMC, we verified the appropriate phenotypic marker: anti-human Eph-B4 (PE) (R&D Systems; 1 µg) and anti-human ephrin B2 (primary antibody) with anti-rabbit FITC conjugated (secondary antibody; 1:1,000) for VSMC and ASMC, respectively, were used. Appropriate isotype control antibody was included in all the experiments. After being washed, stained cells were resuspended in staining buffer (250 µl) and acquired (10,000 events) with a FACSCanto Flow cytometer (BD Biosciences, NJ). Acquired data were analyzed using FlowJo software (Tree star, Ashland, Oregon).
Statistics
Data are expressed as means ± SD. Each experiment was performed in triplicate. Interassay and interarray variation was accounted for all experiments. Statistical computer package STATVIEW (SAS Institute, Cary, NC) was used for the ANOVA, and post hoc Bonferroni tests were performed to know the effects of each variable and to reveal the statistical significance. The confidence level of the study was proposed to be 95%; hence, P < 0.05 has been considered significant.
RESULTS
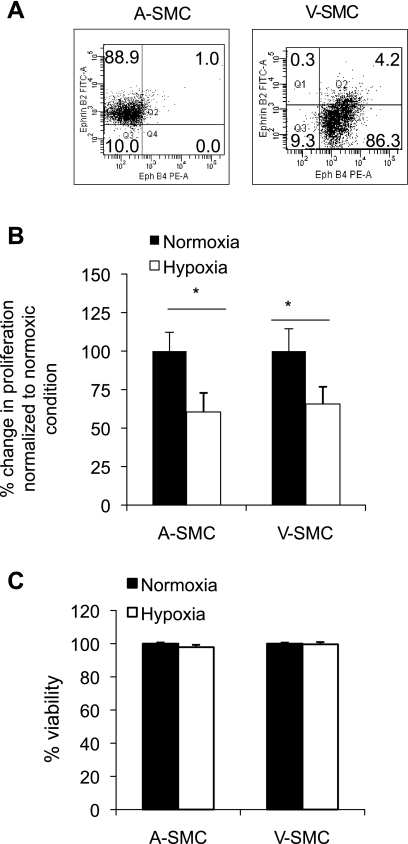
To confirm the arterial and venous phenotype of the SMC, surface expression of ephrin B2 (an arterial cell marker) and eph-B4 (a venous cell marker) was determined. ASMC were found to exclusively express ephrin B2 (88.9%) while VSMC expressed eph-B4 (86.3%; Fig. 1A).
Fig. 1.
Hypoxia reduces SMC proliferation. A: venous-derived smooth muscle cells (VSMC) and arterial-derived smooth muscle cells (ASMC) were incubated under hypoxia for 24 h. To confirm the phenotype of VSMC and ASMC, Eph-B4 [phycoerythrin (PE)], and ephrin-B2 (FITC) surface protein expression levels were determined using flow cytometric analysis before hypoxia treatment. Numbers in the quadrant represents percentage Eph-B4 and ephrin-B2 surface expression on ASMC and VSMC. Data shown represent ≥3 independent cell preparations. ASMC and VSMC were exposed to hypoxia (30 mmHg Po2) for 24 h. B: bromodeoxyuridine (BrdU) incorporation was used as an indicator of SMC proliferation. C: cell viability under hypoxia was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are expressed as %change in SMC proliferation/viability normalized to control (under normoxia). Data were considered significant at *P < 0.05 and n = 3.
To determine the different proliferative abilities of ASMC compared with VSMC, cells were placed under hypoxia for 6, 12, 24, and 48 h. Cells placed under hypoxia for 24 h showed maximum proliferation. Therefore, for this study we used 24 h as the time point for studying the mechanism associated with differential proliferation in SMC. Hypoxic treatment of VSMC and ASMC resulted in a significant reduction in BrdU incorporation compared with cells under normoxic conditions (Fig. 1B). To test whether the reduction in cell proliferation was due to a decrease in cell viability, MTT cell viability assay was performed. The assay confirmed that there was no difference in viability under hypoxia (Fig. 1C). SMC proliferation decreased (P < 0.05) under hypoxia in both ASMC and VSMC. Since hypoxia alone does not initiate SMC proliferation (Fig. 1B), next we investigated if SMC proliferation leading to IH in vivo acts through a paracrine mechanism.
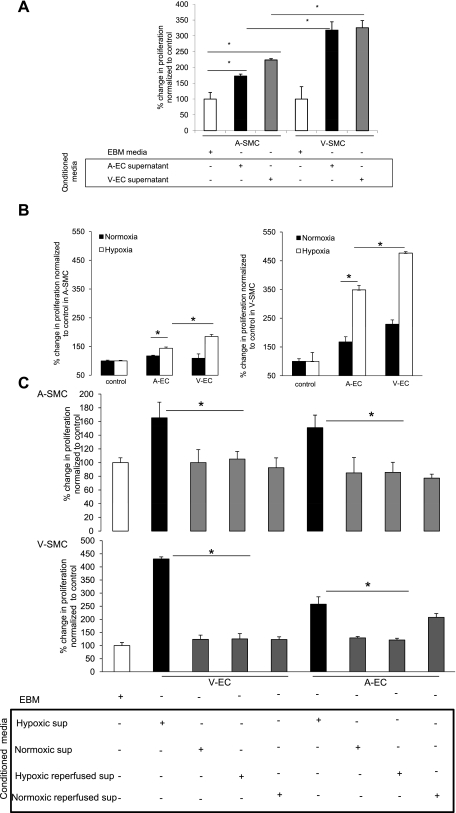
To test the paracrine mechanism, conditioned media from hypoxic EC (AEC and VEC) were incubated with ASMC and VSMC and subjected to hypoxia for 24 h. Results indicated a significant (P < 0.05) increase in both ASMC and VSMC proliferation under hypoxia when incubated with hypoxic EC-conditioned media (Fig. 2A). We concluded that SMC proliferation under hypoxia occurs via a paracrine mechanism and is initiated by hypoxic conditioned media. To simulate an in vivo model, SMC were seeded along with EC (AEC and VEC) and placed under hypoxia and normoxia for 24 h. Results showed an induction in SMC proliferation upon coculture with EC and incubation under hypoxia. VEC showed greater proliferation in SMC compared with AEC (Fig. 2B).
Fig. 2.
Hypoxic endothelial cell (EC)-conditioned media (H-ECM) induces SMC proliferation under hypoxia. A: H-ECM (100 μl) derived from arterial EC (AEC) and venous (VEC) was incubated with ASMC and VSMC and subjected to hypoxia for 24 h. BrdU incorporation was used as an indicator of SMC proliferation under hypoxia. Data are expressed as %change in SMC proliferation normalized to control (EBM-2). B: AEC and VEC were cocultured with ASMC and VSMC in an in vitro cell culture system and placed under normoxia and hypoxia for 24 h. BrdU incorporation was used as an indicator of SMC proliferation. Cells were labeled with BrdU after serum starvation. Data are expressed as %change in SMC proliferation normalized to control. Proliferation of SMC under normoxia without addition of endothelial cells was used as a normalizing control for cells under normoxia and proliferation of SMC under hypoxia without addition of endothelial cells was used as a normalizing control for cells under hypoxia. C: conditioned media from reperfused EC reverse SMC proliferation induction: VEC and AEC were placed under hypoxia for 24 h, and conditioned media were collected. Media in the same cells were replenished, the cells were then placed under normoxia for a further 24 h, and their conditioned media were collected (reperfusion). Data represent ASMC and VSMC proliferation under hypoxia with hypoxic EC-conditioned media and reperfused EC media using BrdU as an indicator of proliferation. Data represent %change in proliferation of SMC upon addition of conditioned media normalized to control. Basal smooth muscle cell proliferation after serum starvation and incubation with EBM-2 media under hypoxia was used as a normalizing control. Data were considered significant at *P < 0.05 and n = 6.
To further test that hypoxic EC-conditioned media were the initiator in SMC proliferation, VEC and AEC were placed under hypoxia for 24 h and conditioned media were collected. The media in the same cells were replenished, and cells were then placed under normoxia for a further 24 h, and their conditioned media were collected (reperfusion). EBM-conditioned EC media under normoxia and reperfused media under normoxia were used as controls. The results showed significant (P < 0.05) reversibility in SMC proliferation that had been initiated by hypoxic EC-conditioned media (Fig. 2C). This result further confirmed that it was indeed the hypoxic EC-conditioned media that were initiating SMC proliferation.
Hypoxia Induces Significant VEGF mRNA and Protein Expression in ASMC, AEC, and VEC but not in VSMC
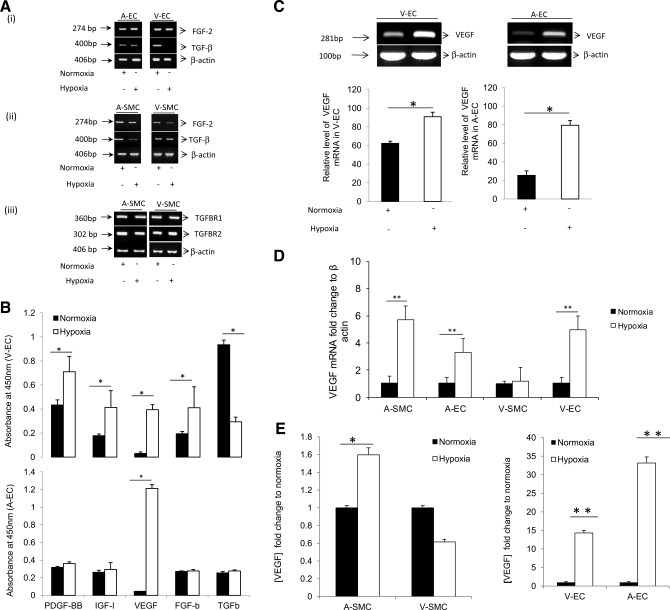
To investigate the growth factors present in the hypoxic EC-conditioned media that induced SMC proliferation, ASMC and VSMC and EC were placed under hypoxia for 3 h. EC mRNA was quantified for FGF-2 and TGF-β expression levels, and SMC mRNA was quantified for FGF-2, TGF-β, TGFβ-R1, and TGFβ-R2 expression levels under both normoxic as well as hypoxic conditions (Fig. 3A). An angiogenesis ELISA was used to quantify growth factors PDGF-BB, IGF-I, VEGF-A, FGF-b, and TGF-β protein levels (Fig. 3B). To establish the growth factors of interest we looked at which growth factors were increasing the most significantly between VEC and AEC. Although there was a significant change in IGF, FGF-b, and TGF-β levels, these changes were not common to both EC types and the fold changes were not as dramatic compared with changes in VEGF-A mRNA and protein levels. Analysis of the different growth factors showed that VEGF-A protein expression levels had the greatest induction (15-fold) under hypoxia. Therefore, to further investigate VEGF-A protein levels in SMC and EC under hypoxia, a VEGF-A ELISA was used. Results showed a significant increase (P < 0.001) in VEGF-A mRNA levels in AEC (4-fold), VEC (5-fold), and ASMC (6-fold) under hypoxia (Fig. 3, C and D). There was also a significant (P < 0.001) increase in VEGF-A protein levels in ASMC (1.6-fold), AEC (35-fold), and VEC (15-fold) under hypoxia (Fig. 3E). In contrast, VSMC showed nonsignificant changes in VEGF-A mRNA and protein expression under hypoxia (Fig. 3, D and E, respectively).
Fig. 3.
Hypoxia induces vascular endothelial growth factor (VEGF-A) expression in ASMC, AEC, and VEC but not in VSMC. AEC, VEC, ASMC, and VSMC were placed under hypoxia (3 h) and mRNA isolated. Samples were analyzed for growth factor levels and their respective receptors. A, i: FGF-2 and transforming growth factor-β mRNA expression in AEC and VEC. A, ii and iii: FGF-2, transforming growth factor (TGF)-β, TGF-β-R1, and TGF-β-R2 mRNA expression in ASMC and VSMC. B: culture supernatant from AEC and VEC was collected and an angiogenesis ELISA (Signosis, Sunnyvale, CA) was used to analyze the effect of hypoxia on growth factor protein levels platelet-derived growth factor homodimer B (PDGF-BB), IGF-I, VEGF, FGF-b, and TGF-β. VEGF mRNA was analyzed using RT-PCR (C) in AEC and VEC and quantitative (q)RT-PCR (D) in AEC, VEC, ASMC, and VSMC after 3-h exposure to hypoxia. E: VEGF protein levels for ASMC, VSMC, VEC, and AEC were determined using VEGF ELISA after 24 h under hypoxic conditions. Data was considered significant at *P < 0.05, **P < 0.001 and n = 3.
HIF1-α Stabilization Under Hypoxia Is Greater in VSMC Compared with ASMC
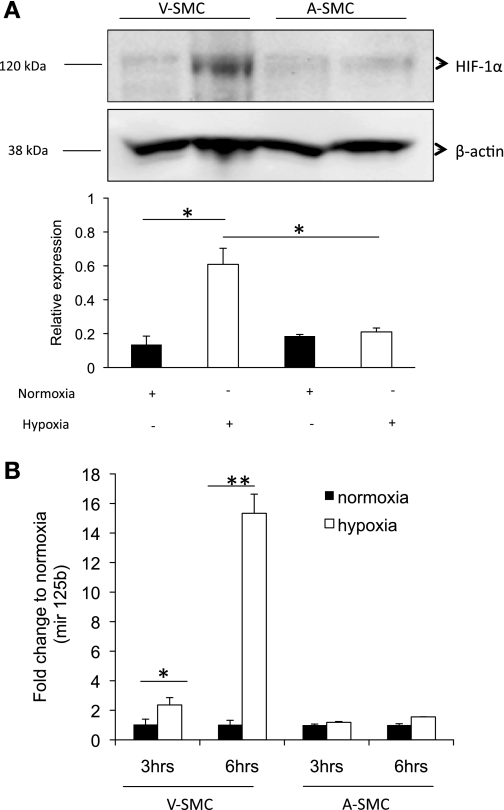
To investigate whether the lack of VEGF-A increase in VSMC was due to lack of HIF1-α stabilization under hypoxia, we looked at HIF1-α levels. HIF1-α is stabilized under hypoxia and translocates to the nucleus where it is responsible for transcription of hypoxia regulated genes like VEGF-A. VSMC and ASMC were placed under hypoxia and HIF1-α levels determined using WB. The results (Fig. 4A) showed greater HIF1-α stabilization (3-fold) in VSMC under hypoxia compared with ASMC. Based on this result, we concluded that the lack of VEGF-A expression in VSMC under hypoxia was not due to lack of HIF1-α stabilization. A possible explanation could be due to an increase in microRNAs that target VEGF-A mRNA. A micro-RNA array was used to screen for microRNAs that are regulated under hypoxia in ASMC and VSMC. Of particular interest mir-125b, 29a, and 29b showed VEGF-A as a target gene (unpublished data). Under hypoxia, VSMC showed 15-fold increase in mir-125b expression at 6 h compared with ASMC, which did not show a significant change (Fig. 4B). Based on these data, we hypothesized that upregulation of mir-125b in VSMC might be a possible reason why VSMC do not express significant amounts of VEGF-A under hypoxia.
Fig. 4.
Hypoxia inducible factor-1-α (HIF1-α) is differentially expressed in SMC under hypoxia. A: VSMC and ASMC were incubated under hypoxia for 24 h. HIF-1α protein levels expression was analyzed using Western blot. B: mir-125b levels in ASMC and VSMC under hypoxia was determined by qRT-PCR (3 and 6 h). Significance was determined at *P < 0.05, **P < 0.001 and n = 3.
Based on our results in Fig. 3, we concluded that there may be other growth factors in the hypoxic EC culture supernatant that were initiating SMC proliferation especially in ASMC since they expressed VEGF-A under hypoxia but yet failed to proliferate. To investigate the lack of ASMC proliferation under hypoxia even in the presence of significant VEGF-A amounts, we investigated VEGFR-2 expression levels.
VEGFR-2 Is Differentially Expressed Under Hypoxia in SMC
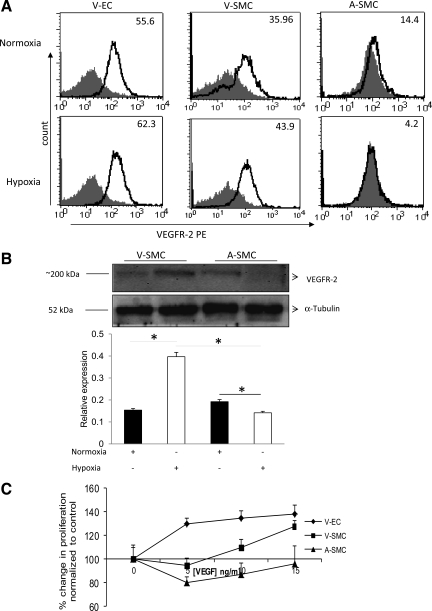
VEGFR-2 is implicated in the proliferative abilities of VEGF-A (6). To investigate if the inability of ASMC to proliferate under hypoxia in the presence of VEGF-A was due to lack of receptor expression, we looked at VEGFR-2 levels on these cells under hypoxia using VEGFR-2 antibody. Fluorescence activated cell sorter was used to determine receptor expression on these cells under hypoxic conditions. The gray-filled histogram in Fig. 5 represents staining with the isotype-matched control, and the bold line represents staining with VEGFR-2 PE antibody. Numbers on the histogram indicates the percentage of VEGFR-2-positive cells. Under hypoxia, VEGFR-2 expression is 62.3% in VEC, 43.9% in VSMC, and 4.2% in ASMC (Fig. 5A). This observation was further confirmed by WB analysis. The results from the WB showed twofold higher VEGFR-2 expression levels in VSMC compared with ASMC under hypoxia (Fig. 5B). From this result, we concluded that ASMC lacked significant VEGFR-2 expression under hypoxia.
Fig. 5.
VEGFR-2 is differentially expressed under hypoxia in SMC. VEC, VSMC, and ASMC were incubated under hypoxia for 24 h. VEGFR-2 protein expression levels were determined using Flow cytometric analysis (A) of VEGFR-2 expression on ASMC and VSMC under hypoxic and normoxic condition. Gray-filled histogram represents staining with the isotype-matched control and the bold line represents staining with VEGFR-2 PE antibody. Numbers on the histogram indicate the percentage of VEGFR-2-positive cells. B: Western blot. C: VEGF dose response on VEC, VSMC, and ASMC proliferation was determined using the following VEGF concentrations (0, 5, 10, and 15 ng/ml) for 24 h under hypoxia. In these experiments (A–C), VEC was used as a positive control. Significance for differences in VEGFR-2 expression levels was determined at *P < 0.05; n = 4.
VSMC Proliferation but not ASMC Is Partially Mediated Through VEGFR2 Under Hypoxia
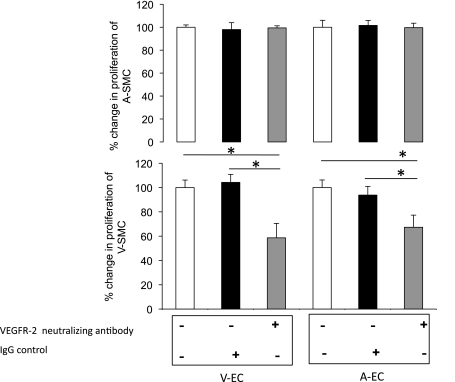
The role of VEGF-A in SMC proliferation under hypoxia was determined by a VEGF-A (0, 5, 10, and 15 ng/ml) dose-response experiment in VEC, ASMC, and VSMC. Results showed that VEGF-A induced VEC proliferation under hypoxia (Fig. 5C). VEGF only induced VSMC proliferation at higher doses (10 and 15 ng/ml; Fig. 5C). VEGF treatment did not have an effect on ASMC proliferation. To further validate the role VEGF-A plays in inducing SMC proliferation under hypoxia, we used VEGFR-2-neutralizing antibody. Neutralization of VEGFR-2 did not result in any proliferative changes for the ASMC but caused a significant decrease (P < 0.05) in VSMC proliferation upon addition of hypoxic EC-conditioned media under hypoxia (Fig. 6). Based on these data, we concluded that VEGF-A was not the main cause of ASMC proliferation but contributed to VSMC proliferation. Our data however (Fig. 7A) showed a greater than fivefold induction in PDGF-BB expression under hypoxia. We next determined PDGF-BB levels in EC under hypoxia.
Fig. 6.
VSMC proliferation but not ASMC is partially mediated through VEGFR-2 under hypoxia. ASMC and VSMC proliferation after addition of AEC and VEC derived H-ECM with preincubation of VEGFR-2-neutralizing antibody. Data is expressed as %change in SMC proliferation normalized to control (EBM-2). Significance was determined at *P < 0.05; n = 6.
Fig. 7.
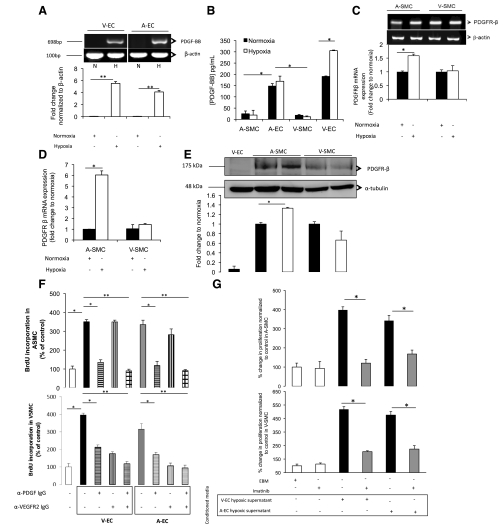
PDGF-BB mRNA and protein levels are highly induced under hypoxia in EC. A: PDGF-BB mRNA levels in VEC (left) and AEC (right) were analyzed as described in Fig. 3C. Unregulated β-actin used as loading control is identical to Fig. 3C. N and H, normoxia and hypoxia, respectively. B: PDGF-BB protein levels in AEC, VEC, ASMC, and VSMC under hypoxia using ELISA. Cells (ASMC and VSMC) were exposed to hypoxia for 6 h. PDGFR-β mRNA and β-actin for ASMC and VSMC were analyzed using qRT-PCR (C) and real-time (D) PCR. E: PDGFR-β protein expression in SMC using Western blot. F: ASMC and VSMC proliferation after addition of (AEC and VEC) derived H-ECM with preincubation of PDGF-BB and VEGFR-2-neutralizing antibody. BrdU incorporation using an ELISA was used as an indicator of SMC proliferation. G: ASMC and VSMC proliferation after addition of (AEC and VEC) derived H-ECM with preincubation of PDGFR antagonist (Imatinib mesilate, 1 uM). *P < 0.05 and **P < 0.001 n = 3.
PDGF-BB Initiates ASMC Proliferation Under Hypoxia
To determine other possible growth factors that were in the hypoxic EC culture media that were causing SMC proliferation, especially ASMC proliferation, we measured PDGF-BB levels in ASMC, VSMC, VEC, and AEC. Our results showed that there was a significant (P < 0.001) three- to sixfold increase in PDGF-BB mRNA levels in AEC and VEC under hypoxia (Fig. 7A). There was an increase in PDGF-BB protein levels under hypoxia in VEC (2-fold) and although the increase in AEC was not significant, basal level expressions of PDGF-BB were still high (7-fold higher) when compared with PDGF-BB levels in SMC under hypoxia (Fig. 7B).
We next investigated PDGFR-β mRNA and protein expression under hypoxia. ASMC expressed greater PDGFR-β mRNA and protein levels under hypoxia compared with VSMC (Fig. 7, C–E), respectively. To confirm if PDGF-BB contributed to ASMC proliferation, we preincubated the hypoxic EC-conditioned media with PDGF-BB-neutralizing antibody before incubation with SMC under hypoxia. There was a significant decrease (P < 0.001) in ASMC and VSMC proliferation in the presence of PDGF-BB-neutralizing antibody (Fig. 7F). The differential role VEGF-A and PDGF-BB were playing in SMC proliferation was further tested by preincubation of hypoxic EC-conditioned media with PDGF-BB-neutralizing antibody and preincubation of the SMC with VEGFR-2-neutralizing antibody. Results showed a decrease to basal levels in both ASMC and VSMC under hypoxia upon incubation with both VEGFR-2- and PDGF-BB-neutralizing antibodies. To further confirm the role of PDGF-BB in SMC proliferation is induced by paracrine factors, we used imatinib mesilate (PDGFR antagonist). Imatinib mesilate (1 μM) was preincubated with SMC before hypoxic EC-conditioned media were cultured with hypoxic SMC. Results showed a decrease in both ASMC and VSMC proliferation (Fig. 7G).
VSMC Exhibit Greater Extracellular Signal-Regulated Kinase 1/2 Activation Than ASMC in the Presence of VEGF and PDGF-BB Under Hypoxia
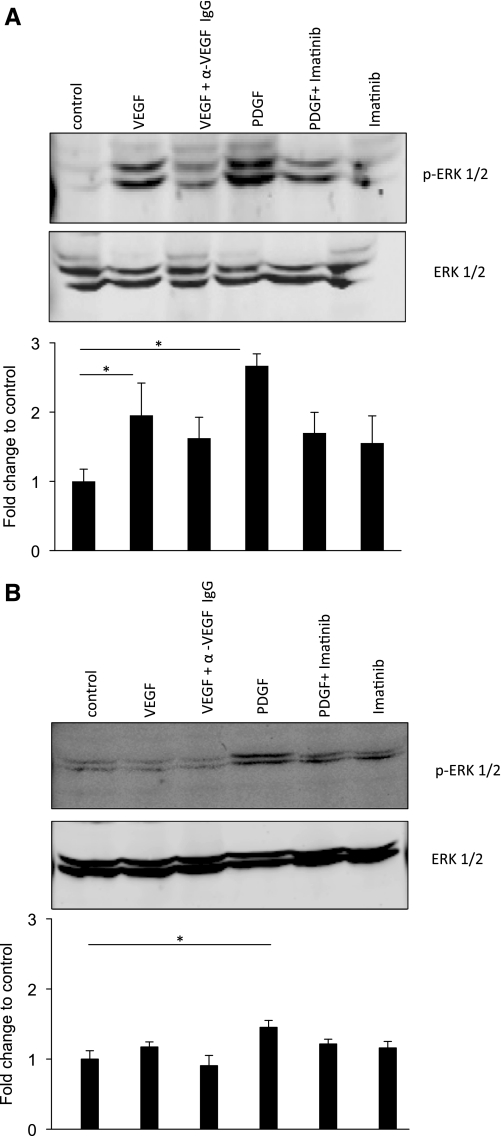
Extracellular signal-regulated kinase (ERK) 1/2 is a key regulator in mediating downstream signaling in growth factor-induced proliferation (10). To delineate the molecular mechanism involved in induction of SMC proliferation by hypoxic conditioned media, VSMC and ASMC were incubated with PDGF-BB and VEGF under hypoxia, and ERK1/2 phosphorylation was determined using WB. VSMC exhibited significantly (P < 0.05) greater ERK1/2 phosphorylation under hypoxia upon addition of VEGF (2 fold) and PDGF-BB (3-fold; Fig. 8A). ASMC show significant (P < 0.05) ERK1/2 phosphorylation upon addition of PDGF-BB under hypoxia and an insignificant change in ERK1/2 phosphorylation upon addition of VEGF (Fig. 8B). Under hypoxia, the ASMC increase in ERK1/2 phosphorylation was relatively lower compared with the response of VSMC under hypoxia (Fig. 8A). The data showed that hypoxia alone does not initiate SMC proliferation in an autocrine manner. SMC proliferation under hypoxia occurs via a paracrine mechanism and is initiated by hypoxic EC-derived growth factors (PDGF-BB and VEGF-A) in VSMC. PDGF-BB plays a more dominant role in causing ASMC proliferation. VEGF-A did not directly initiate proliferation in ASMC due to lack of VEGFR-2 expression. These observations are further supported by results showing greater ERK1/2 activation in VSMC compared with ASMC under hypoxia upon incubation with growth factors PDGF-BB and VEGF.
Fig. 8.
PDGF-BB and VEGF induce greater ERK1/2 activation in VSMC compared with ASMC under hypoxia. VSMC (A) and ASMC (B) were incubated with PDGF-BB (10 ng/ml) and VEGF-A (10 ng/ml) under hypoxia for 24 h. VEGF-A-neutralizing antibody (α VEGF IgG) and Imatinib (PDGFR antagonist) were used as a control. ERK1/2 phosphorylation was determined using Western blot analysis. Significance was determined at *P < 0.05. Bar graph represents the means ± SD of 3 different cell preparations.
DISCUSSION
Despite intensive research for more than two decades, failure of venous-derived grafts continues to be a major clinical problem for which there is no effective preventative strategy (18). Various theories explaining why graft stenosis is more prevalent in the venous-derived than the arterial-derived graft have focused on the handling and preparation of the graft, surgical trauma, and altered hemodynamics in the arterial circulation (3, 31). The biggest limitation in these studies is that the role of hypoxia as an insult in cell modulation is neglected, although extensive research has shown that hypoxia is a common insult in most of the vascular pathologies that inevitably lead to intimal hyperplasia (4, 16, 32). The present study is one of the few reports that identify how hypoxia contributes to the underlying mechanism behind the differences observed clinically in ASMC vs. VSMC proliferation. We provide evidence showing how hypoxia-induced EC-derived growth factors modulate and interact with VSMC and ASMC as well as their respective receptors under hypoxia to initiate their proliferation.
Our study supports the hypothesis that hypoxia induces SMC proliferation via a paracrine mechanism. We further established that VSMC proliferation under hypoxia is initiated predominantly by VEGF-A and PDGF-BB via VEGFR-2 and PDGFR-β, respectively. In contrast, proliferation in ASMC under hypoxia is initiated only by PDGF-BB through a PDGFR-β-dependant mechanism. This observation was further supported by greater activation of ERK1/2 (a kinase that is known as a key regulator in cell proliferation) activation in VSMC compared with ASMC; upon addition of PDGF-BB and VEGF growth factors under hypoxia. Activation in ERK1/2 decreased upon incubation with VEGF-A-neutralizing antibody and PDGFR-β antagonist before the addition of VEGF-A and PDGF-BB, respectively, indicating that both VEGF-A and PDGFR-β played a role in VSMC proliferation under hypoxia.
Critical issues that still need to be addressed include the potential of incorporating PDGF-BB and VEGF-A inhibitors into therapeutic strategies (14, 15, 17). Although many strategies have been developed to inhibit SMC proliferation and reduce IH, most of the drugs tested so far have not been successful. (17). Therapies using anti-VEGFR-2 methods have not produced optimal outcomes in reducing IH (15).Therefore, in-depth understanding of the mechanisms involved in IH is necessary and can help adapt therapy towards specific procedures depending on the origin of graft used to reduce SMC proliferation.
Consistent with the literature, we (5, 12, 23) demonstrated induction of VEGF mRNA and protein levels in EC and ASMC under hypoxia. Interestingly, Pancholi and Earle (26) showed an ∼1.5-fold increase in VEGF-A mRNA which is consistent with our observations for VEGF-A mRNA levels in VSMC; however, our data showed an insignificant decrease in VEGF-A protein levels under hypoxia (12). A possible explanation for this observation was that HIF1-α stabilization is inhibited in VSMC compared with ASMC, but our results showed us that VSMC showed a threefold increase in HIF1-α stabilization compared with HIF1-α stabilization in ASMC. A hypothesis generated from our data suggested that VSMC express microRNA 125b that degrades and prevents translation of VEGF-A mRNA to protein under hypoxia. When comparing the differences in VEGF-A induction under hypoxia in arterial- vs. venous-derived cells our results clearly show that the arterial derived EC and SMC have significantly higher amounts of VEGF-A produced; 35-fold in AEC and 1.5-fold in ASMC compared with ∼15-fold induction for VEC and an insignificant 0.8-fold change in VSMC. We found it surprising that even in the presence of VEGF-A, ASMC did not proliferate in an autocrine manner. Studies conducted by Ferrara et al. (12) also demonstrated that ASMC were producers of VEGF. We also observed that these cells did not seem to proliferate in an autocrine manner in the presence of VEGF (12). We went on to further demonstrate that reduced proliferation in ASMC despite presence of VEGF is due to a significantly lower expression of VEGFR-2 , the receptor responsible for the proliferative effects of VEGF-A (29). As expected, incubation with VEGFR-2-neutralizing antibody before addition of hypoxic endothelial-conditioned media did not have a significant effect on ASMC proliferation.
VSMC showed VEGFR-2 expression under hypoxia and were responsive to VEGF-A induced proliferation, contrasting ASMC response. VEGFR-2 antagonist therapies might be better suitable for treatment of VSMC proliferation since VSMC express threefold greater VEGFR-2 under hypoxia.
PDGF-BB is reported to stimulate the proliferation of vascular SMC and to be involved in vascular modeling through a HIF-1-dependent mechanism (1, 24, 28). Our findings suggest the involvement of PDGF-BB in the proliferation of ASMC. EC produce 100-fold more PDGF-BB when compared with SMC upon hypoxic insult (24). VSMC proliferation that occurs in IH through PDGF-BB is mediated by the PDGF-β receptor (30). Our data showed PDGFR-β expression in both VSMC and ASMC under hypoxia with increased receptor expression under hypoxia in ASMC. An important finding in this present study was that ASMC under hypoxia expressed significantly higher (3-fold) PDGFR-β compared with VSMC. Based on this result, we concluded that SMC proliferation under hypoxia is initiated by PDGF-BB via a paracrine mechanism. Of particular interest was the contribution of PDGF-BB in ASMC proliferation, because only upon incubation with PDGF-BB-neutralizing antibody, proliferation decreases significantly. This supported the idea that PDGF-BB is the major player in induction of ASMC proliferation under hypoxia. Studies looking at differential effects of imatinib on PDGF-induced proliferation and PDGF receptor signaling in human ASMC and VSMC concluded that imatinib was efficacious towards inhibiting arterial SMC proliferation, supporting our findings that PDGF-BB plays a significant role in ASMC proliferation (20).
A limitation in our in vitro culture model is that our analysis is based on venous endothelial and SMC derived from the umbilical vein. It is possible that SMC behavior may vary in different venous-derived beds. Future studies will need to address these issues and confirm the same trend in endothelial and SMC derived from the saphenous vein.
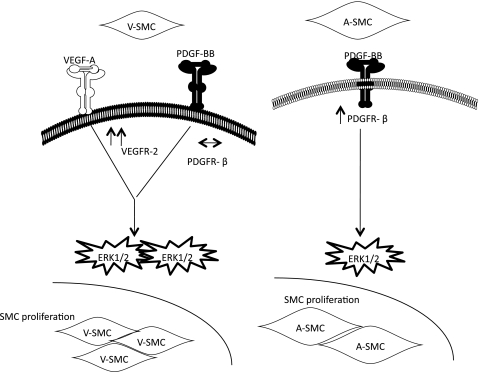
In summary, we have demonstrated that proliferation of SMC under hypoxia is a paracrine event initiated by hypoxia-derived EC growth factors. VSMC were shown to express VEGFR-2 while ASMC had significantly lower expressions under hypoxia. On the other hand the ASMC expressed higher PDGFR-β which increased under hypoxia. Our working model is illustrated in Fig. 9. Clinically, therapy with PDGFR antagonists plus anti-VEGFR-2 may prove to be efficacious in managing SMC proliferation in venous-derived grafts. Therapy with PDGFR antagonist would be more effective in managing SMC proliferation in arterial-derived grafts. These different therapies can be adapted towards the type of graft used for optimal results. However, further studies are warranted to validate the findings in an in vivo system.
Fig. 9.
Working model showing differential effects of hypoxia-derived EC growth factors on proliferation of ASMC and VSMC.
GRANTS
This study was supported by National Institute on Drug Abuse Grants RO1-DA-12104, RO1-DA-022935, KO2-DA-015349, and P50-DA-11806 (to S, Roy) and National Heart, Lung, and Blood Institute Grant RO1-HL-076316 (to S. Santili) and by funds from the Minneapolis Veteran's Affair's Medical Center (R. Barke).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C., S.M.S., and S.R. conception and design of research; A.C. and R.D. performed experiments; A.C., S.M.S., and S.R. analyzed data; A.C. and S.R. interpreted results of experiments; A.C. and R.D. prepared figures; A.C. drafted manuscript; A.C., R.D., R.C., R.B., and S.R. edited and revised manuscript; A.C., S.M.S., and S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Subhas Das for help in the preparation of this manuscript and technical support concerning RT-PCR analysis.
REFERENCES
- 1. Abedi H, Zachary I. Signalling mechanisms in the regulation of vascular cell migration. Cardiovasc Res 30: 544–556, 1995 [PubMed] [Google Scholar]
- 2. Abraham D, Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther 9, Suppl 2: S2, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Achouh P, Boutekadjirt R, Toledano D, Hammoudi N, Pagny JY, Goube P, Isselmou KO, Lancelin B, Fouquet R, Acar C. Long-term (5- to 20-year) patency of the radial artery for coronary bypass grafting. J Thorac Cardiovasc Surg 140: 73–9, 79.e1–2, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol 19: 870–876, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation 90: 649–652, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9: 777–794, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng YF, Ou HY, Tsang LL, Yu CY, Huang TL, Chen TY, Concejero A, Wang CC, Wang SH, Lin TS, Liu YW, Yang CH, Yong CC, Chiu KW, Jawan B, Eng HL, Chen CL. Vascular stents in the management of portal venous complications in living donor liver transplantation. Am J Transplant 10: 1276–1283, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Cogo A, Napolitano G, Michoud MC, Barbon DR, Ward M, Martin JG. Effects of hypoxia on rat airway smooth muscle cell proliferation. J Appl Physiol 94: 1403–1409, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Conte MS. Molecular engineering of vein bypass grafts. J Vasc Surg 45, Suppl A: A74–81, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ, Sun RH, Wu Y, Han M. Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRbeta-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res 20: 1252–1262 [DOI] [PubMed] [Google Scholar]
- 11. Faller DV. Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol 26: 74–84, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Ferrara N, Winer J, Burton T. Aortic smooth muscle cells express and secrete vascular endothelial growth factor. Growth Factors 5: 141–148, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Growcott EJ, Banner KH, Wharton J. Hypoxia amplifies the proliferative capacity of distal human pulmonary artery smooth-muscle cells. Chest 128: 600S–601S, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Hacker TA, Griffin MO, Guttormsen B, Stoker S, Wolff MR. Platelet-derived growth factor receptor antagonist STI571 (imatinib mesylate) inhibits human vascular smooth muscle proliferation and migration in vitro but not in vivo. J Invasive Cardiol 19: 269–274, 2007 [PubMed] [Google Scholar]
- 15. Helfrich I, Scheffrahn I, Bartling S, Weis J, vonFelbert V, Middleton M, Kato M, Ergun S, Schadendorf D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med 207: 491–503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hulten LM, Levin M. The role of hypoxia in atherosclerosis. Curr Opin Lipidol 20: 409–414, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 168: 2036–2053, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee ES, Bauer GE, Caldwell MP, Santilli SM. Association of artery wall hypoxia and cellular proliferation at a vascular anastomosis. J Surg Res 91: 32–37, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Lee ES, Caldwell MP, Tretinyak AS, Santilli SM. Supplemental oxygen controls cellular proliferation and anastomotic intimal hyperplasia at a vascular graft-to-artery anastomosis in the rabbit. J Vasc Surg 33: 608–613, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Li L, Blumenthal DK, Masaki T, Terry CM, Cheung AK. Differential effects of imatinib on PDGF-induced proliferation and PDGF receptor signaling in human arterial and venous smooth muscle cells. J Cell Biochem 99: 1553–1563, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Murray CJ, Kulkarni SC, Ezzati M. Understanding the coronary heart disease versus total cardiovascular mortality paradox: a method to enhance the comparability of cardiovascular death statistics in the United States. Circulation 113: 2071–2081, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Muto A, Fitzgerald TN, Pimiento JM, Maloney SP, Teso D, Paszkowiak JJ, Westvik TS, Kudo FA, Nishibe T, Dardik A. Smooth muscle cell signal transduction: implications of vascular biology for vascular surgeons. J Vasc Surg 45, Suppl A: A15–A24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Namiki A, Brogi E, Kearney M, Kim EA, Wu T, Couffinhal T, Varticovski L, Isner JM. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem 270: 31189–31195, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. VEGF-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb 15: 26–33, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 30: 364–372, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Pancholi S, Earle KA. Pattern of angiogenic cytokine release from human vascular smooth muscle cells programmed by amino acid deprivation. Cytokine 12: 1322–1325, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Ray JB, Arab S, Deng Y, Liu P, Penn L, Courtman DW, Ward ME. Oxygen regulation of arterial smooth muscle cell proliferation and survival. Am J Physiol Heart Circ Physiol 294: H839–H852, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor-1α promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290: H2528–H2534, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 312: 549–560, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Sirois MG, Plante GE, Farmer P, Sirois P. Platelet activating factor modulates peptidoleukotriene effects on the permeability of bovine aortic endothelial cell to albumin. Agents Actions 39 Spec No: C17–20, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Turner NA, Ho S, Warburton P, O′Regan DJ, Porter KE. Smooth muscle cells cultured from human saphenous vein exhibit increased proliferation, invasion, and mitogen-activated protein kinase activation in vitro compared with paired internal mammary artery cells. J Vasc Surg 45: 1022–1028, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, Webster MW. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 34: 291–299, 2001 [DOI] [PubMed] [Google Scholar]