Abstract
Triiodothyronine (T3) supplementation improves clinical outcomes in infants after cardiac surgery using cardiopulmonary bypass by unknown mechanisms. We utilized a translational model of infant cardiopulmonary bypass to test the hypothesis that T3 modulates pyruvate entry into the citric acid cycle (CAC), thereby providing the energy support for improved cardiac function after ischemia-reperfusion (I/R). Neonatal piglets received intracoronary [2-13Carbon(13C)]pyruvate for 40 min (8 mM) during control aerobic conditions (control) or immediately after reperfusion (I/R) from global hypothermic ischemia. A third group (I/R-Tr) received T3 (1.2 μg/kg) during reperfusion. We assessed absolute CAC intermediate levels and flux parameters into the CAC through oxidative pyruvate decarboxylation (PDC) and anaplerotic carboxylation (PC) using [2-13C]pyruvate and isotopomer analysis by gas and liquid chromatography-mass spectrometry and 13C-nuclear magnetic resonance spectroscopy. When compared with I/R, T3 (group I/R-Tr) increased cardiac power and oxygen consumption after I/R while elevating flux of both PDC and PC (∼4-fold). Although neither I/R nor I/R-Tr modified absolute CAC levels, T3 inhibited I/R-induced reductions in their molar percent enrichment. Furthermore, 13C-labeling of CAC intermediates suggests that T3 may decrease entry of unlabeled carbons at the level of oxaloacetate through anaplerosis or exchange reaction with asparate. T3 markedly enhances PC and PDC fluxes, thereby providing potential substrate for elevated cardiac function after reperfusion. This T3-induced increase in pyruvate fluxes occurs with preservation of the CAC intermediate pool. Our labeling data raise the possibility that T3 reduces reliance on amino acids for anaplerosis after reperfusion.
Keywords: metabolism, pediatrics, reperfusion
cardiopulmonary bypass disrupts thyroid hormone homeostasis and depresses circulating triiodothyronine (T3) and thyroxine levels. This disruption is particularly profound in infants undergoing surgery for congenital heart defects and persists through a substantial portion of the postoperative period (2, 21, 23). Some investigators have linked thyroid hormone levels to surgical outcome and suggested that these disruptions represent an adaptive phenomenon, which reduces metabolic demand in concordance with impaired cardiac function and output. We have recently challenged this concept by evaluating T3 repletion in infants undergoing cardiopulmonary bypass for repair of congenital heart defects with a multicenter placebo-controlled clinical trial (TRICC trial) (23). In infants less than 5 mo old, T3 repletion shortened postoperative mechanical ventilation time, reduced use of inotropic agents, and improved some indexes of cardiac function. The mechanism underlying this positive thyroid action in young infants is unknown. Thyroid hormone elicits ubiquitous effects on the heart and circulation. Elucidation of the mechanisms could reveal specific targets and further promote development of strategies to prevent cardiac dysfunction in the postoperative infant after cardiopulmonary bypass.
Basic research studies have shown that thyroid hormone modulates myocardial energy metabolism through transcriptional and posttranscriptional actions on mitochondrial function and substrate oxidation (10, 14, 25, 28). These actions may be modified by the specific metabolic and aerobic state of the heart (10, 17, 24, 29). For instance, Liu et al. (14) demonstrated that T3 induces pyruvate decarboxylation (PDC) and glucose oxidation after global ischemia-reperfusion (I/R) in isolated working rat hearts. This suggests a hypothesis that T3 modulates pyruvate entry into the citric acid cycle (CAC), thereby providing the energy support for improved cardiac function after reperfusion. Conditions of reperfusion after cardiopulmonary bypass differ substantially from those prevalent with other experimental models. In particular, the immature heart during and after cardiopulmonary bypass is also subjected to the combined influence of multiple substrates, hormones, and neurological stimuli. These hearts have also been treated with various cardioprotective strategies such as hypothermia and cardioplegia. Although T3 has been investigated with regard to clinical response under these conditions, the specific role of T3 on intermediary metabolism after I/R from cardiopulmonary bypass has not been investigated. T3 enhancement of pyruvate flux into the CAC could represent at least one mechanism for the therapeutic action observed in the TRICC trial. To test this hypothesis, we mapped pyruvate entry and contribution to CAC intermediates in a neonatal swine model, which emulates conditions of cardiopulmonary bypass and reperfusion in infants. Specifically, we gave intracoronary [2-13Carbon (13C)]pyruvate and utilized 13C-nuclear magnetic resonance spectroscopy (MRS) as well as gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) to assess pyruvate entry into the CAC through oxidative decarboxylation and anaplerotic carboxylation.
MATERIALS AND METHODS
Model.
All experiments were approved by Seattle Children's Research Institute Animal Care Committee and conform with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The surgical preparation followed our methods described previously (16). Briefly, male piglets between the ages of 10 and 18 days old and weighing between 9 and 13 kg were used. Initial sedation was achieved with an intramuscular injection of ketamine (33 mg/kg; Vedco, St. Joseph, MO) and xylazine (2 mg/kg; Vedco). Under propofol anesthesia (3 mg/kg intravenous loading followed by 3 mg·kg−1·h−1 for maintenance; Abbott Labs, Chicago, IL), the animals were intubated and ventilated with 40% oxygen. The femoral artery and vein were cannulated. Anesthesia was adjusted if continuously monitored physiologic parameters (heart rate and blood pressure) increased by greater than 10% from baseline measurements and/or the piglets responded to stimuli.
After performance of a median sternotomy in all groups, a flow probe was placed around the ascending aorta to measure cardiac output (TS420; Transonic Systems, Ithaca, NY). A Millar high-fidelity micromanometer (Millar Instruments, Houston, TX) was used to measure left ventricular pressure. The left anterior descending (LAD) coronary artery was cannulated just distal to the origin of the first branch with a 24-gauge infusion catheter. Piglets that did not undergo cardiopulmonary bypass constituted the control (Cont) group. In animals undergoing cardiopulmonary bypass (groups I/R and I/R-Tr), the proximal aorta was cannulated for cardioplegia administration. The hemiazygous vein was ligated. To measure coronary venous flow, a cannula was placed into the coronary sinus that drained into the jugular vein. A Transonic flow probe (ME-4PXL; Transonic Systems) was placed around this shunt for continuous flow monitoring. Myocardial oxygen consumption was measured via this flow and oxygen content data from femoral artery and the coronary sinus. Cannulae were placed in the ascending aorta and right atrium and connected to a cardiopulmonary bypass circuit with a Stockert-Shiley roller pump and a hollow fiber oxygenator (Minimax X Plus; Medtronic, Minneapolis, MN). The circuit was primed with 10% Dextran 40-0.9% sodium chloride solution. The animal was initially maintained at a temperature at 37°C as measured by an esophageal thermo probe.
A PowerLab 16/30 recorder (AD Instruments, Colorado Springs, CO) continuously recorded data in all groups. Baseline data were recorded before pyruvate administration in Cont and before initiating cardiopulmonary bypass in groups I/R and I/R-Tr. After 10 min of stable parameters were achieved, mechanical support was initiated and, subsequently, cooling was performed to a core temperature of 30°C. At that time, the aortic snare was tightened and cardioplegia (composition in miliequivalents per liter 140 Na+, 45 K+, 3 Mg2+, 104 Cl−, 27 acetate, and 23 gluconate and adjusted to pH 7.8) was instilled antegrade into the aortic root. After 60 min of ischemia, the aortic snare was released and reperfusion was initiated. The swine were rewarmed over 10 min to 36°C. Circulatory support was gradually decreased starting 10 min after aortic snare release and discontinued 30 min after release. Defibrillation was performed if necessary.
Pyruvate and thyroid hormone infusion.
For all groups, sodium [2-13C]pyruvate (Isotec, Miamisburg, OH) was dissolved into 0.9% normal saline and infused at body temperature directly into the LAD for 40 min (n = 6–8) to achieve an estimated 8 mM final concentration, similar to our previous study with this model (16). Calculation of the pyruvate infusion rate was based upon the mean left ventricular coronary artery flow per body weight determined in preliminary neonatal pig experiments.
The [2-13C]pyruvate was given to control piglets without cardiopulmonary bypass after coronary artery cannulation and stabilization. Figure 1 provides a graphical representation of the cardiopulmonary bypass protocol as well as including the Cont protocol. In groups I/R and I/R-Tr, [2-13C]pyruvate infusion began 10 min after release of the aortic cross clamp. The infusion continued until 20 min after discontinuing cardiopulmonary bypass. Group I/R-Tr also received two intravenous boluses of T3 (Triostat; King Pharmaceuticals, Cary, NC) at a dose of 0.6 μg/kG per dose. T3 was given upon release of aortic cross clamp and at discontinuation of cardiopulmonary bypass as noted in Fig. 1.
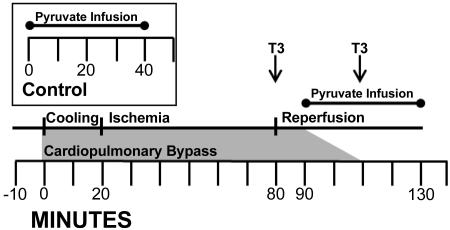
Fig. 1.
The ischemia-reperfusion (I/R) protocol. Time 0 is the start of cardiopulmonary bypass. Baseline hemodynamic and plasma data were performed before time 0. The aortic cross clamp was placed at time 20 min and released at time 80 min. Cardiopulmonary bypass weaning started at time 90 min and was completed by time 110 min. Intracoronary [2-13C]pyruvate infusion was given to groups I/R and I/R-Tr for 40 min starting 10 min after release of aortic cross clamp. In the I/R-Tr group only, 0.6 μg·kG−1·dose−1 iv triiodothyronine (T3) was given during reperfusion at the times denoted by T3 in the figure. Final hemodynamic measurements were performed near pyruvate infusion completion. Left ventricular tissue was collected at the end of the infusion. Inset: control protocol. Intracoronary [2-13C]pyruvate was infused for 40 min starting at time 0. Left ventricular tissue was collected at the end of the infusion.
Immediately upon completion of the [2-13C]pyruvate infusion, LAD perfused portions of the heart were rapidly excised, freeze clamped, weighed, and stored in liquid nitrogen for further analyses. Plasma samples were collected before cardiopulmonary bypass and just before animal euthanization to determine total T3 concentrations using a chemiluminescence assay (Roche Elecsys, Basel, Switzerland). Total left ventricular free wall weight (for oxygen consumption data) was made adding weights of the freeze-clamped myocardium to the remaining left ventricular free wall weight. Euthanasia was performed via rapid exsanguination under general anesthesia by removal of the portion of left ventricular tissue for the metabolic studies.
Metabolic analyses by 13C-MRS.
Metabolic analyses by 13C-MRS were performed on the porcine myocardium as described previously for determination of specific carbon glutamate labeling (16). Briefly, MRS spectra were obtained within a 600 MHz Inova (Varian, Palo Alto, CA) at the 13C-resonance frequency using a 45° pulse angle and 3-s recycle delay and 67,426 data points. Protons were decoupled with a Waltz decoupling scheme. Fourier transformed spectra were fitted with commercial software (NUTS; Acorn NMR, Livermore, CA), and areas for glutamate carbon 2 (C2), carbon 3 (C3), and carbon 5 (C5) appearing as singlets were determined. Peak areas were corrected for differential saturation calculated using the above pulse sequence on pig heart samples spiked with glutamate.
The rationale behind our labeling protocol was described previously (16). The [2-13C]pyruvate yields [2-13C]oxaloacetate in a carboxylation reaction. With the assumption of complete equilibration via malate dehydrogenase and fumarase, [3-13C]oxaloacetate also forms in equal proportions. On the first turn of the CAC, this yields [3-13C]glutamate or [2-13C]glutamate. On later turns, [1-13C]glutamate could be produced in limited amounts, which are below the detection level in our spectra (16). The C2 and C3 peaks represent carbon entry through pyruvate carboxylation (PC), an anaplerotic pathway. In the PDC pathway, [2-13C]pyruvate yields [1-13C]acetyl-CoA and produces [5-13C]glutamate in the first turn of the CAC; it should never label C2, C3, or C4 of glutamate. The area of the C5 peak represents carbon entry through PDC. Accordingly, the ratio of PC to total pyruvate entry (PC + PDC) into the CAC is estimated from: PC/(PC + PDC) = C2 + C3/C2 + C3 + C5. Representative spectra are shown in Fig. 2.
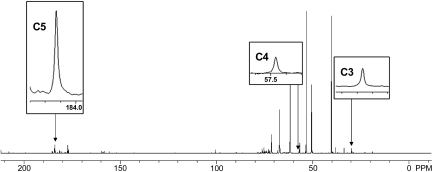
Fig. 2.
Representative proton-decoupled 13C-spectra from the porcine myocardium infused with [2-13C]pyruvate and T3. The peaks for glutamate carbons 2 (C2), 3 (C3), and 5 (C5) are inserted. No labeling was detected at C1 or C4. Peaks for C2 and C3 represent pyruvate carboxylation, whereas peak C5 represents pyruvate decarboxylation. Peak areas were corrected for differential saturation as described in materials and methods. C3 is at 29.767 ppm, C4 is at 57.467 ppm, and C5 is at 184.129 ppm. Magnification and scaling were kept constant between peak inserts for the figure.
Metabolic analyses by GC-MS.
GC-MS (Agilent 6890N gas chromatograph equipped with a HP-5 column coupled to a 5973N mass spectrometer; Agilent Technologies, Santa Clara, CA) was performed to measure the 13C-enrichment and concentrations of CAC intermediates (citrate, succinate, fumarate, and malate) and pyruvate as described previously (7, 12, 30). GC-MS data are reported as the 13C-molar percent enrichment (MPE), defined previously (7, 12, 30). Mass isotopomers of metabolites containing 1 to n 13C-labeled atoms were identified as Mi, with i = 1, 2, … n. The total and absolute MPE of individual 13C-labeled mass isotopomers (Mi) of a given metabolite was calculated as follows: total MPE = % ∑AMi /(AM + ∑AMi) and absolute MPE(Mi) = %AMi/(AM + ∑AMi), where AM and AMi represent the peak areas from ion chromatograms corrected for natural abundance, corresponding to the unlabeled (M) and 13C-labeled (Mi) mass isotopomers, respectively. A detailed description for the calculations of flux ratios has also been published previously (7, 12, 30). Flux ratios are calculated from the measured 13C-labeling pattern of tissue citrate and pyruvate. The 13C-enrichment mass isotope distribution of the acetyl moiety of citrate (ACCIT; carbons 4 + 5CIT) is calculated from citrate and of its oxaloacetate moiety as determined before and after sample treatment with citrate lyase (5, 31). PDC flux rate relative to citrate synthase (CS) was determined using the following formula: PDC/CS = M1 ACCIT/M1 intracellular pyruvate, where M1 is the 13C-enrichment in M1 isotopomers. This ratio represents flux through pyruvate decarboxylation leading to citrate synthesis arising from all tissue pyruvate sources; including glucose, lactate, and [2-13C]pyruvate. With our labeling strategy, the fractional contribution of [2-13C]pyruvate to total tissue pyruvate is defined as follows: fractional contribution (FC)[2–13C] pyruvate→pyruvate = MPE M1 intracellular pyruvate. The FC of other carbohydrates (presumably glucose and lactate) to the tissue pyruvate pool is defined as: FCother carbohydrates→pyruvate = 1 − FC [2–13C] pyruvate→ pyruvate.
Absolute flux rates were calculated using the stoichiometric relationship between oxygen consumption and citrate formation from pyruvate, glucose, and lactate based upon the assumptions and equations by Panchal et al. (18, 19), Vincent et al. (30), and Khairallah et al. (12). Consumed O2 (1 μmol) results in the formation of 0.400 and O.333 μmol of citrate from pyruvate and glucose/lactate, respectively. Absolute CAC flux includes the contribution from fatty acid sources. Based upon Panchal et al. (19), it was assumed that palmitate and oleate supply 40% and 60% of fatty acids oxidized by the heart and that 1 μmol of consumed O2 results in the formation of 0.348 and O.353 μmol of citrate from palmitate and oleate, respectively. Thus, 1 μmol of consumed O2 results in the formation of 0.351 μmol citrate from fatty acids. Absolute fluxes for each piglet were calculated using PDC/CS derived from GC-MS, FC [2–13C] pyruvate→ pyruvate, FCother carbohydratess→pyruvate, and experimentally determined myocardial oxygen consumption (MVO2) where: CAC flux = MVO2[(FC [2–13C] pyruvate→ pyruvate·PDC/CS·0.400) +(FCother carbohydrates→pyruvate·PDC/CS·0.333) + (1 − (PDC/CS)·0.351)] and PDC flux = MVO2[(FC [2–13C] pyruvate→ pyruvate·PDC/CS·0.400) + (FCother carbohydrates→pyruvate·PDC/CS·0.333)].
Measurement of steady-state coronary flow and systemic and coronary sinus O2 content for MVO2 calculations was made near the end of pyruvate infusion. PC flux was determined from relative PC-to-PDC ratio derived from MRS data where PC flux = PDC flux·(PC-to-PDC ratio).
Amino acid analysis by LC-MS.
We also analyzed the enrichment of specific amino acids from perchloric acid extracts using mass spectrometry in the form of butyl ester. We used a short reverse phase LC-MS method with an Agilent 1100 HPLC with Zorbax SB C18 column (2.1 × 100 mm, 80A pores, and 3.6 μm particles; Agilent Technologies) coupled to an Esquire LC ion trap mass spectrometer operating in the positive ionization mode. Total MPE for alanine, aspartate, and glutamate were calculated as described above.
Statistical analysis.
Reported values are means ± SE in figures, text, and tables. Data were analyzed with repeated-measures ANOVA within groups and single factor ANOVA for multiple comparisons (StatView 4.5; Abacus Concepts, Berkeley, CA). Paired t-tests were used to compare MPEs between alanine, lactate, and pyruvate within the same piglet. Criterion for significance was P < 0.05 for all comparisons.
RESULTS
Thyroid hormone levels.
Similar to patterns in infants and children, cardiopulmonary bypass depressed circulating total T3 (P < 0.05 via paired t-test; n = 6) in the I/R group. T3 supplementation markedly elevated total T3 levels (P < 0.05; n = 6). Baseline total T3 levels (in ng/dl) were 114 ± 7 in I/R and 89 ± 9 in I/R-Tr. After cardiopulmonary bypass, total T3 levels were 78 ± 8 in I/R and 592 ± 35 in I/R-Tr. The reported levels represent peaks, since they were obtained shortly after the second bolus. They are comparable with those obtained in clinical trials showing no adverse effect in infants and children (23). Of note, total T3 levels with and without supplementation have also been shown to parallel changes in free T3 (22).
Cardiac function and myocardial oxygen consumption.
Hemodynamic indexes recorded throughout the experiment included cardiac power, cardiac output, and left ventricular pressure first derivative maximum (dP/dtmax). Absolute baseline hemodynamic values (before the start of cardiopulmonary bypass) are displayed in Table 1. Baseline cardiac functional parameters were similar between I/R and I/R-Tr. As expected, baseline Cont values were similar to I/R and I/R-Tr (data not shown). Hemodynamic parameters and MVO2 relative to baseline are reported at the end of the [2-13C]pyruvate infusion (Fig. 3) for the I/R groups. Stresses induced by cardiopulmonary bypass and reperfusion cause hemodilution and volume loading as well as cytokine and catecholamine surges, which tend to elevate values of cardiac function relative to baseline (13, 1). Strict comparisons with baseline values mask injury due to I/R because the hearts are now subjected to catecholamine stimulation. Accordingly, we used baseline as a reference for each individual experiment, although direct comparisons between function during baseline and postreperfusion would not provide accurate assessment of true change in function. However, comparisons were made between treatment groups after I/R. T3 supplementation (I/R-Tr group; Fig. 3) increased cardiac power and dP/dtmax relative to baseline compared with the I/R group. Cardiac power is generally accepted as a work index incorporating both volume and pressure work by the left ventricle. T3 also increased MVO2 in parallel to changes in power, showing that there was no reduction in cardiac energy efficiency.
Table 1.
Baseline hemodynamics
| I/R | I/R-Tr | |
|---|---|---|
| Cardiac output, l/min | 5.3 ± 0.4 | 5.4 ± 0.4 |
| Mean power, W | 232 ± 32 | 233 ± 28 |
| Maximum first derivative of left ventricular pressure, mmHg/s | 2,161 ± 524 | 1,756 ± 193 |
| Myocardial oxygen consumption, μmol·min−1·g−1 | 3.4 ± 0.2 | 2.9 ± 0.2 |
Values are means ± SE; n = 6 per group. I/R, ischemia-reperfusion group; I/R-Tr, group that received triiodothyronine (1.2 μg/kg) during reperfusion.
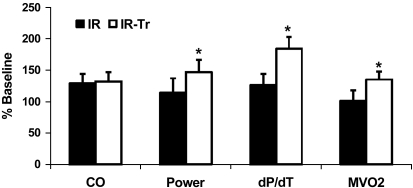
Fig. 3.
Hemodynamics in a piglet cardiopulmonary bypass model after I/R as a percentage of baseline values. Measurements are reported at pyruvate infusion completion. T3 supplementation improves some functional parameters after I/R. Values are means ± SE. *P < 0.05 vs. I/R by t-test; n = 6 per group. CO, cardiac output; dP/dt, left ventricular pressure first derivative maximum; MVO2, myocardial oxygen consumption.
Metabolic analysis pyruvate metabolism.
The Cont group was included in this analysis to represent baseline metabolic conditions without I/R. [2-13C]pyruvate was given in our experiments to provide supraphysiologic serum levels in the LAD coronary artery (16). Pyruvate can also be derived from other sources such as glucose, lactate, glycogen, and alanine. In the Cont group, the majority of the myocardial tissue pyruvate pool originated from [2-13C]pyruvate (64.7% ± 5.4%; n = 8). Cardiopulmonary bypass and I/R significantly lowered (P < 0.05) 13C-labeling of the tissue pyruvate pool in both I/R (32.2% ± 10.3%; n = 6) and I/R-Tr (30.5% ± 8.7%; n = 6) compared with Cont. Although there was no difference in pyruvate enrichment between I/R and I/R-Tr, the lower values suggest that in these conditions there is either decreased pyruvate uptake and/or enhanced flux to pyruvate or isotopic equilibration via unlabeled sources (presumably via glycolysis, alanine transamination, and/or lactate dehydrogenase). As noted above, pyruvate can potentially undergo reduction to lactate (as part of anaerobic metabolism) or transamination to form the amino acid alanine. These two reactions, catalyzed by lactate dehydrogenase and alanine aminotransferase, respectively, are bidirectional. The measured MPE values for all these metabolites suggest rapid interconversion. However, although tissue pyruvate MPE was similar to that of alanine in both I/R and I/R-Tr groups, when comparing values within individual samples, it was significantly higher than that of lactate (Fig. 4, paired t-test; P < 0.05). Because T3 increased PDC and PC flux (as will be discussed below) while establishing equilibrium between pyruvate and alanine, the lack of demonstrated equilibration between pyruvate and lactate MPE suggests that T3 supplementation did not increase the fraction of pyruvate converted to lactate via anaerobic glycolysis. However, because of the bidirectional nature of these reactions, our experiments were not designed to determine the actual net flux of pyruvate to lactate.
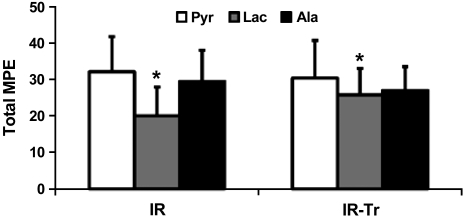
Fig. 4.
Total 13C-molar percent enrichment (MPE) levels of pyruvate (Pyr) along with its exchanging metabolite partners lactate (Lac) and alanine (Ala). In both groups, the lactate MPE did not reach equivalence with its exchanging metabolite pyruvate, whereas the pyruvate and alanine MPEs were equivalent. *P < 0.05 via paired t-test comparing Lac and Pyr MPEs within the same animal for both I/R and I/R-Tr. No other significant differences were identified. Values are means ± SE; n = 6 per group.
It is noteworthy that under our experimental conditions, recycling of labeled pyruvate through hepatic gluconeogenesis and subsequent metabolism of the labeled glucose in the heart appears unlikely. Indeed, using GC-MS, we did not detect any enrichment in M2 isotopomers for tissue lactate or pyruvate, which could occur with recycling via gluconeogenesis. Additionally, proton MRS of multiple myocardial samples in this study showed no splitting of the glucose peak, which would occur if myocardial tissue glucose was labeled with 13C (data not shown).
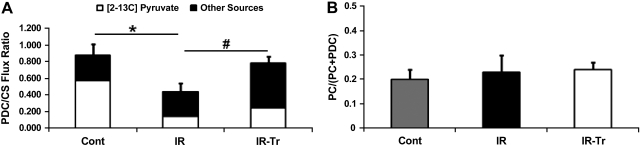
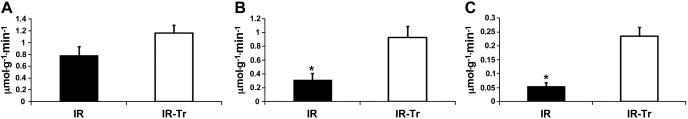
GC-MS was utilized to assess the PDC-to-CS flux ratio. The PDC-to-CS flux ratio (Fig. 5A) reflects contribution from all sources to pyruvate including [2-13C]pyruvate as well as unlabeled glucose and lactate (denoted as other sources in Fig. 5A). The PDC-to-CS flux ratio was significantly greater in Cont compared with I/R (P < 0.05), but not compared with I/R-Tr. Likewise, T3 supplementation increased the PDC-to-CS flux ratio over group I/R. CAC and PDC flux were calculated as described above for I/R and I/R-Tr and shown in Fig. 6, A and B, respectively. Although we did not observe an increase in CAC flux, T3 substantially increased (near 4-fold) the PDC flux in I/R-Tr compared with I/R (P < 0.05). Our values for this flux are similar to previous reports in the more mature pig (18, 19). It is noteworthy that the Cont group did not have a coronary sinus shunt in place; therefore, we could not measure MVO2 or calculate PDC flux.
Fig. 5.
Relative pyruvate entry into the citric acid cycle (CAC) through either pyruvate decarboxylation (PDC) or pyruvate carboxylation (PC). A: PDC-to-citrate synthase (CS) flux ratio as determined by gas chromatography-mass spectrometry (GC-MS). Included are the relative contributions of [2-13C]pyruvate vs. other pyruvate sources (presumable glucose and lactate) to the PDC-to-CS flux ratio. Note that error bars represent SE of the overall PDC-to-CS flux ratio. B: ratio of PC to total pyruvate entry into the CAC as determined by positional glutamate 13C-labeling via magnetic resonance spectroscopy (MRS). T3 increased PDC flux relative to CS flux (a surrogate for overall CAC flux) after cardiopulmonary bypass while maintaining the ratio of PC to total pyruvate CAC entry. *P < 0.05 I/R vs. control (Cont); #P < 0.05 I/R-Tr vs. I/R. No other significant differences were identified. Values are means ± SE; n = 6 per group.
Fig. 6.
Flux rates for the CAC, PDC, and PC in groups I/R and I/R-Tr after I/R from cardiopulmonary bypass. A: CAC flux. B: PDC flux. C: PC flux. T3 supplementation increased PDC and PC flux. Values are means ± SE. *P < 0.05 I/R vs. I/R-Tr; n = 6 per group.
Our MRS methodology also enabled measurement of PC relative to total pyruvate flux into the CAC, as well as calculation of absolute PC flux. The positional labeling of glutamate carbons, assessed via MRS, allowed for the determination of the ratio of [2-13C]pyruvate entering the CAC through PC relative to total labeled pyruvate CAC entry (i.e., PC/PC + PDC ratio; please refer to materials and methods for details). We confirmed glutamate 13C-labeling with LC-MS. Cardiopulmonary bypass significantly reduced the total glutamate MPE (Cont, 40.0 ± 12.7, n = 5; I/R, 14.7 ± 1.4, n = 6; I/R-Tr, 14.3 ± 1.4, n = 6; P < 0.05 for Cont vs. I/R and Cont vs. I/R-Tr); however, these values were all in the range where we had shown previously adequate MRS signal (16). MRS demonstrated that the percentage of PC relative to total pyruvate entering the CAC was similar across all groups, on average around 20% (Fig. 5B). Absolute PC flux was significantly increased in the I/R-Tr group compared with I/R (Fig. 6C) by about fourfold. These values were also similar to previous reports in older pigs (18, 19).
CAC intermediates.
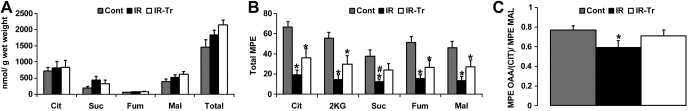
Marked reductions in CAC intermediate levels can potentially limit metabolic flux (15). Accordingly, we determined absolute CAC levels as well as their 13C-enrichment (MPE) to ascertain whether a relationship existed between absolute CAC levels and these fluxes. We included the Cont group in this analysis to establish whether our experimental conditions reduced absolute CAC levels. Conditions of I/R did not alter absolute tissue levels of the CAC intermediates (citrate, malate, succinate, and fumarate, Fig. 7A). Thus absolute CAC levels after reperfusion do not appear to be linked to the noted differences in pyruvate flux or MVO2 between groups. The conditions of cardiopulmonary bypass and I/R did significantly lower the total MPE of these intermediates (Fig. 7B) with the exception of succinate in I/R-Tr (P = 0.079 vs. Cont). T3 supplementation increased succinate MPE (P < 0.05 I/R-Tr vs. I/R) with a trend toward increased enrichment for the remaining CAC intermediates (P = 0.055–0.083 I/R-Tr vs. I/R).
Fig. 7.
Concentrations of CAC intermediates and their 13C-MPE. A: absolute CAC intermediate concentrations. B: total 13C-MPE of CAC intermediates. C: ratio of the MPE of oxaloacetate moiety of citrate [OAA(Cit)] to the MPE of malate (Mal). I/R did not affect absolute CAC intermediate concentration, but it reduced their corresponding 13C-MPEs, potentially indicating increased utilization of unlabeled sources to maintain levels. T3 supplementation partially abrogated this response. The OAA(Cit)-to-Mal MPE ratios suggest greater dilution due to anaplerosis and/or exchange reactions with aspartate only in group I/R. Values are means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. I/R-Tr. Note that P = 0.055–0.083 I/R vs. I/R-Tr for total MPE in Cit, 2KG, Mal, and fumarate (Fum) in B. Suc, succinate; total, summation of Cit, Mal, Suc, and Fum. Values are means ± SE; n = 6 for I/R and I/R-Tr and n = 8 for Cont.
Although most of the labeling of the CAC intermediates result from the metabolism of [2-13C]pyruvate to citrate through PDC and to a lesser extent from PC, the entry of unlabeled carbons into the CAC via anaplerosis at sites other than PC can dilute the labeled intermediate pool and reduce the observed MPE. Although semiquantitative, analyses of relative MPEs between intermediates can provide some assessment of anaplerotic or exchange reactions with amino acids, specifically glutamate and aspartate. The 2-ketoglutarate-to-citrate MPE ratio did not differ significantly among the three experimental groups (data not shown). This suggests that tracer dilution, resulting from exchange reactions with glutamate, did not vary among our conditions. However, using the MPE value of the oxaloacetate moiety of citrate as a proxy of tissue oxaloacetate, we found that when compared with Cont, the oxaloacetate-to-malate MPE ratio was significantly lower in the I/R group (Fig. 7C), suggesting a greater dilution due to anaplerosis and/or exchange reactions with aspartate. Interestingly, this was abrogated with T3 supplementation. Total MPE values for tissue aspartate did not significantly differ among all three experimental groups (Cont = 23 ± 6, I/R = 13 ± 6, I/R-Tr = 17 ± 3; n = 4–6).
DISCUSSION
We conducted experiments in a pig model emulating clinical conditions during cardiopulmonary bypass with I/R in infants. Although controlled trials show that T3 supplementation improves postischemic cardiac function and important clinical endpoint parameters, the role of T3 modulation of cardiac energy metabolism in influencing recovery after cardiopulmonary bypass surgery has remained undefined (23). In the current study, we found that T3 supplementation promotes a marked (4-fold) increase in flux through PDC and PC into the CAC, while elevating cardiac function during steady-state after cardiopulmonary bypass circuit weaning. Thus we provide a potential bioenergetic basis for the T3 associated clinical response noted in human infants.
Although these data cannot prove a direct link between these fluxes and cardiac function, prior investigations have shown improved postischemic cardiac function associated with pyruvate decarboxylation when using pyruvate as a primary fuel source, as opposed to lactate or glucose (32). Unlike our study, those experiments were performed exclusively in isolated perfused rabbit hearts, lacking the robust substrate supply available to hearts in vivo. Additionally, our previous work showed that pyruvate supplementation after neonatal cardiopulmonary bypass stimulates carbon entry into the CAC through two pathways involving either PDC or PC (16). The first pathway represents oxidative entry of acetyl-CoA into the CAC. The latter pathway is anaplerotic occurring through either pyruvate carboxylase contributing four carbons to form oxaloacetate or malic enzyme forming malate with either reaction replenishing the CAC intermediate pool (26). Stimulation of both pathways during early reperfusion was also associated with improved cardiac function (16).
Several investigators have tested previously the hypothesis that provision of anaplerotic substrates, including pyruvate, enhances replenishment of the CAC intermediate pool after reperfusion and rapidly restores oxidative capacity. These metabolite concentrations are fairly low compared with the rate of CAC flux during normal aerobic metabolism (6). Therefore, depletion of this intermediate pool during I/R could theoretically limit oxidative capacity. Okere and coauthors (15) showed that I/R caused by LAD constriction did not reduce CAC intermediate concentrations. Furthermore, they found that differential modulation of CAC intermediate levels, using medium chain free fatty acid infusions, did not affect cardiac function after reperfusion (15). Without measuring any flux rates, the authors suggested their findings showed that CAC impairment or modulation was not related to any functional abnormality after I/R. Our study confirmed that steady-state absolute CAC intermediate concentrations are not affected by I/R.
Although absolute CAC intermediate levels were unchanged, I/R reduced their corresponding 13C-MPEs, and this was partially abrogated by T3 supplementation. Labeling of CAC intermediates arises predominantly from the metabolism of 13C-labeled pyruvate to citrate through primarily decarboxylation to acetyl-CoA and to lesser extent carboxylation to oxaloacetate and/or malate, followed by subsequent metabolism of citrate into the CAC. Hence, the observed differences in CAC intermediate labeling concur with the measured differences in PDC and PC fluxes between groups. However, additional tracer dilution at the level of specific CAC intermediates, namely 2-ketoglutarate and malate/oxaloacetate, could also occur through anaplerotic and/or exchange reactions with the amino acids, glutamate, and aspartate, respectively. Because we are working with an in vivo model, we could not perform experiments to assess all potential anaplerotic substrate sources. However, the measured 13C-dilution between some tissue CAC intermediates suggest that T3 supplementation may impact unlabeled carbon entry through anaplerosis or exchange reactions with aspartate. Specifically, our data show that the MPE of the oxaloacetate moiety of citrate (a proxy for tissue oxaloacetate) relative to the MPE for malate is lower after I/R compared with controls only in piglets that did not receive T3 (group I/R). The oxaloacetate-to-malate MPE ratio depends on the rate of randomization of 1) labeled carbons between oxaloacetate and malate relative to that of 2) unlabeled carbons between oxaloacetate and aspartate, catalyzed by malate dehydrogenase and transaminases, respectively. We reasoned that under our conditions, the lower oxaloacetate moiety of citrate-to-malate MPE ratio in the I/R group compared with Cont is more likely due to increased entry of unlabeled carbons through anaplerosis or due to exchange reactions between oxaloacetate and aspartate. Interestingly, our data suggest that T3 supplementation abrogates this phenomenon, thus raising the possibility that T3 may reduce aspartate entry into the CAC. Of note, however, this cannot be proved without performing additional experiments that would assess directly the labeling of oxaloacetate from aspartate.
Limitations and speculations.
Based on our prior MRS studies, we used supraphysiologic doses of pyruvate (8 mM) to maximize MRS signal to noise and technically improve quality of data. Doses near 8 mM dose also have been used to improve cardiac function in patients with congestive heart failure (9). The 8 mM dose only resulted in ∼30% labeling of the intracellular pyruvate pool after I/R; therefore, the myocardium was not excessively loaded with pyruvate. Although activation of pyruvate decarboxylase does occur at lower doses (32), we were still able to further stimulate pyruvate flux into the CAC with T3 supplementation.
Although our studies revealed T3 relationships with cardiac function and metabolic flux after reperfusion in a clinically relevant translational model, we can only speculate regarding the molecular mechanisms underlying T3 effect in this model. The rapid action by T3 in these experiments eliminates transcriptional mechanisms as a cause. T3, though not thyroxine, exerts nongenomic actions including generation of second messengers at the cell membrane and within the cytosol (4, 20), which directly stimulate both the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways (3). Both of these pathways are known to affect changes in pyruvate dehydrogenase activity (11). Our data are consistent with those obtained in the substrate-restricted working rat heart, which showed T3 enhancement of postischemic cardiac function related to T3 promotion of PDC flux without an increase in glycolytic lactate production (14). The increased PDC flux observed in rat heart experiments after T3 occurred in association with elevations in PDC activity and reduced enzyme phosphorylation state. This finding suggests further complexity involving regulation of these pathways (14). Since acetyl-CoA positively regulates PC (8), we speculate that elevated PDC flux increases acetyl-CoA levels and stimulates PC. Other unknown mechanisms may account for this finding that requires further investigation.
Our studies were performed in immature swine heart, which demonstrates relatively high capacity for shuttling NADH+ into the mitochondria and maintaining glycolytic capacity (27). So conceivably, the pyruvate and T3 effects may not have the same impact in mature myocardium.
Summary.
Our data show that T3 supplementation after cardiopulmonary bypass stimulates pyruvate entry to the CAC through two distinct pathways: PDC and PC. After reperfusion, activation of these pathways maintains CAC intermediate concentrations and elevates oxidative flux in concert with improved cardiac function. Based on our results, future studies are warranted to test the impact of T3 on amino acid metabolism. Our current studies are novel in that these findings occur in a model closely emulating infant cardiopulmonary bypass with clinical correlates.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants R01-HL-60666 (to M. Portman) and K08-HL-092333 and T32-HL-07828 (to A. K. Olson) and a Thompson Family Research grant (to A. K. Olson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.K.O., X.-H.N., C.D.R., and M.A.P. conception and design of research; A.K.O., B.B., X.-H.N., N.I., and M.A.P. performed experiments; A.K.O., B.B., X.-H.N., N.I., C.D.R., and M.A.P. analyzed data; A.K.O., X.-H.N., C.D.R., and M.A.P. interpreted results of experiments; A.K.O. prepared figures; A.K.O. and M.A.P. drafted manuscript; A.K.O., C.D.R., and M.A.P. edited and revised manuscript; A.K.O., B.B., X.-H.N., N.I., C.D.R., and M.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
A portion of the research was performed using Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
REFERENCES
- 1. Anand KJ, Hansen DD, Hickey PR. Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology 73: 661–670, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Bettendorf M, Schmidt KG, Tiefenbacher U, Grulich-Henn J, Heinrich UE, Schonberg DK. Transient secondary hypothyroidism in children after cardiac surgery. Pediatr Res 41: 375–379, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Bhargava M, Lei J, Ingbar DH. Nongenomic actions of L-thyroxine and 3,5,3′-triiodo-L-thyronine. Focus on “l-thyroxine vs. 3,5,3′-triiodo-l-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase.” Am J Physiol Cell Physiol 296: C977–C979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol 19: 102–112, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Comte B, Vincent G, Bouchard B, Des Rosiers C. Probing the origin of acetyl-CoA and oxaloacetate entering the citric acid cycle from the 13C labeling of citrate released by perfused rat hearts. J Biol Chem 272: 26117–26124, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res 90: 210–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Des Rosiers C, Lloyd S, Comte B, Chatham JC. A critical perspective of the use of 13C-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab Eng 6: 44–58, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Fahien LA, Davis JW, Laboy J. Interactions between pyruvate carboxylase and other mitochondrial enzymes. J Biol Chem 268: 17935–17942, 1993 [PubMed] [Google Scholar]
- 9. Hermann HP, Pieske B, Schwarzmuller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet 353: 1321–1323, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Hyyti OM, Ning XH, Buroker NE, Ge M, Portman MA. Thyroid hormone controls myocardial substrate metabolism through nuclear receptor-mediated and rapid posttranscriptional mechanisms. Am J Physiol Endocrinol Metab 290: E372–E379, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Johnson SA, Denton RM. Insulin stimulation of pyruvate dehydrogenase in adipocytes involves two distinct signalling pathways. Biochem J 369: 351–356, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khairallah M, Labarthe F, Bouchard B, Danialou G, Petrof BJ, Des Rosiers C. Profiling substrate fluxes in the isolated working mouse heart using 13C-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am J Physiol Heart Circ Physiol 286: H1461–H1470, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg 81: S2347–S2354, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Liu Q, Clanachan AS, Lopaschuk GD. Acute effects of triiodothyronine on glucose and fatty acid metabolism during reperfusion of ischemic rat hearts. Am J Physiol Endocrinol Metab 275: E392–E399, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Okere IC, McElfresh TA, Brunengraber DZ, Martini W, Sterk JP, Huang H, Chandler MP, Brunengraber H, Stanley WC. Differential effects of heptanoate and hexanoate on myocardial citric acid cycle intermediates following ischemia-reperfusion. J Appl Physiol 100: 76–82, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Olson AK, Hyyti OM, Cohen GA, Ning XH, Sadilek M, Isern N, Portman MA. Superior cardiac function via anaplerotic pyruvate in the immature swine heart after cardiopulmonary bypass and reperfusion. Am J Physiol Heart Circ Physiol 295: H2315–H2320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orfali KA, Fryer LG, Holness MJ, Sugden MC. Interactive effects of insulin and triiodothyronine on pyruvate dehydrogenase kinase activity in cardiac myocytes. J Mol Cell Cardiol 27: 901–908, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Panchal AR, Comte B, Huang H, Dudar B, Roth B, Chandler M, Des Rosiers C, Brunengraber H, Stanley WC. Acute hibernation decreases myocardial pyruvate carboxylation and citrate release. Am J Physiol Heart Circ Physiol 281: H1613–H1620, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Panchal AR, Comte B, Huang H, Kerwin T, Darvish A, des Rosiers C, Brunengraber H, Stanley WC. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am J Physiol Heart Circ Physiol 279: H2390–H2398, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Pantos C, Mourouzis I, Saranteas T, Brozou V, Galanopoulos G, Kostopanagiotou G, Cokkinos DV. Acute T3 treatment protects the heart against ischemia-reperfusion injury via TRalpha1 receptor. Mol Cell Biochem 353: 235–241, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Plumpton K, Justo R, Haas N. Amiodarone for post-operative junctional ectopic tachycardia. Cardiol Young 15: 13–18, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Portman MA, Fearneyhough C, Ning XH, Duncan BW, Rosenthal GL, Lupinetti FM. Triiodothyronine repletion in infants during cardiopulmonary bypass for congenital heart disease. J Thorac Cardiovasc Surg 120: 604–608, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Portman MA, Slee A, Olson AK, Cohen G, Karl T, Tong E, Hastings L, Patel H, Reinhartz O, Mott AR, Mainwaring R, Linam J, Danzi S. Triiodothyronine Supplementation in Infants and Children Undergoing Cardiopulmonary Bypass (TRICC): a multicenter placebo-controlled randomized trial: age analysis. Circulation 122: S224–S233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Priestman DA, Donald E, Holness MJ, Sugden MC. Different mechanisms underlie the long-term regulation of pyruvate dehydrogenase kinase (PDHK) by tri-iodothyronine in heart and liver. FEBS Lett 419: 55–57, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Ralphe JC, Bedell K, Segar JL, Scholz TD. Correlation between myocardial malate/aspartate shuttle activity and EAAT1 protein expression in hyper- and hypothyroidism. Am J Physiol Heart Circ Physiol 288: H2521–H2526, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Russell RR, 3rd, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol Heart Circ Physiol 261: H1756–H1762, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Scholz TD, Koppenhafer SL. Reducing equivalent shuttles in developing porcine myocardium: enhanced capacity in the newborn heart. Pediatr Res 38: 221–227, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Scholz TD, TenEyck CJ, Schutte BC. Thyroid hormone regulation of the NADH shuttles in liver and cardiac mitochondria. J Mol Cell Cardiol 32: 1–10, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Sugden MC, Langdown ML, Harris RA, Holness MJ. Expression and regulation of pyruvate dehydrogenase kinase isoforms in the developing rat heart and in adulthood: role of thyroid hormone status and lipid supply. Biochem J 352: 731–738, 2000 [PMC free article] [PubMed] [Google Scholar]
- 30. Vincent G, Bouchard B, Khairallah M, Des Rosiers C. Differential modulation of citrate synthesis and release by fatty acids in perfused working rat hearts. Am J Physiol Heart Circ Physiol 286: H257–H266, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Vincent G, Khairallah M, Bouchard B, Des Rosiers C. Metabolic phenotyping of the diseased rat heart using 13C-substrates and ex vivo perfusion in the working mode. Mol Cell Biochem 242: 89–99, 2003 [PubMed] [Google Scholar]
- 32. White LT, O′Donnell JM, Griffin J, Lewandowski ED. Cytosolic redox state mediates postischemic response to pyruvate dehydrogenase stimulation. Am J Physiol Heart Circ Physiol 277: H626–H634, 1999 [DOI] [PubMed] [Google Scholar]