Abstract
The mechanisms mediating arterial stiffening with aging and menopause are not completely understood. We determined whether administration of tetrahydrobiopterin (BH4), a critical cofactor for endothelial nitric oxide synthase to produce nitric oxide, would increase vascular endothelial-dependent vasodilatory tone and decrease arterial stiffness in estrogen-deficient postmenopausal women. Additionally, we examined whether the beneficial effects of estrogen on vascular function were possibly related to BH4. Arterial stiffness (carotid artery compliance) and endothelial-dependent vasodilation [brachial artery flow-mediated dilation (FMD)] were measured in postmenopausal (n = 24; 57 ± 1 yr, mean ± SE) and eumenorrheic premenopausal (n = 9; 33 ± 2 yr) women before and 3 h after the oral administration of BH4. Subsequently, in postmenopausal women, vascular testing (before and after BH4) was repeated following randomization to either 2 days of transdermal estradiol or placebo. Baseline carotid artery compliance and brachial artery FMD were lower in postmenopausal than in premenopausal women (P < 0.0001). BH4 administration increased carotid artery compliance (0.61 ± 0.05 to 0.73 ± 0.04 mm2·mmHg−1·10−1 vs. baseline, P < 0.0001) and brachial artery FMD (P < 0.001) in postmenopausal women but had no effect in premenopausal women (P = 0.62). Carotid artery compliance (0.59 ± 0.05 to 0.78 ± 0.06 mm2·mmHg−1·10−1, P < 0.001) and FMD increased in postmenopausal women in response to estradiol (P = 0.02) but were not further improved with the coadministration of BH4, possibly because estrogen increased BH4 bioavailability. Carotid artery compliance and FMD increased with BH4 in the placebo group (P = 0.02). Although speculative, these results suggest that reduced vascular BH4 may be an important contributor to arterial stiffening in estrogen-deficient postmenopausal women, related in part to reduced endothelial-dependent vasodilatory tone.
Keywords: aging, menopause, sex hormones, oxidative stress, cardiovascular disease
arterial stiffening, a biomarker of vascular aging, is a major risk factor for the development of cardiovascular disease (CVD) (24, 47). In women and men, the stiffness of large elastic arteries increases with advancing age, even in the absence of clinical CVD (31, 35, 43). However, in women, the age-associated increase in arterial stiffness appears to be augmented around the menopause transition presumably because of estrogen deficiency (35, 43, 54). Indeed, estrogen-deficient postmenopausal women show greater arterial stiffening compared with age-matched premenopausal women (54), and the age-associated increase in arterial stiffening is attenuated in postmenopausal women on chronic menopausal hormone therapy (MHT) compared with non-MHT users (26, 31, 35, 42). Thus, determining the mechanisms by which aging and estrogen deficiency contribute to arterial stiffening in women is clinically important.
Tetrahydrobiopterin (BH4) is an essential cofactor for endothelial nitric oxide synthase (eNOS) to synthesize nitric oxide (NO), a potent endothelial-derived vasodilator and regulator of arterial stiffness (1, 55). Reduced BH4 bioavailability leads to the uncoupling of eNOS and favors the production of reactive oxygen species (ROS) instead of NO (25). In this regard, we previously demonstrated that oxidative stress contributes to arterial stiffening in estrogen-deficient postmenopausal women (33). BH4 supplementation improves vascular endothelial-dependent vasodilation in healthy older men (10). However, it is unknown whether BH4 modulates endothelial vasodilatory function in postmenopausal women or whether BH4 is mechanistically linked to arterial stiffening.
Accordingly, we tested the hypothesis that reduced BH4 contributes to the impairment in endothelial vasodilatory function and arterial stiffening in estrogen-deficient postmenopausal women. Additionally, because estrogen is associated with a lower production of ROS and ovariectomized rats have lower aortic content of BH4 compared with estrogen-replete rats (22, 23, 29), we examined whether the beneficial effects of estrogen on vascular function may be related to BH4.
METHODS
Study population.
We studied 33 healthy women: 24 postmenopausal (51–65 yr of age) who were estrogen-deficient and 9 premenopausal women (24–40 yr of age) who had regular menstrual cycles (21–35 days). All women were sedentary or recreationally active (not exercising regularly >2 days/wk) and had not used oral contraceptives or MHT for at least 6 mo before study entry. Participants were included if they were nonsmokers, normotensive (resting blood pressure <140/90 mmHg), nondiabetic [fasted plasma glucose concentrations <7.0 mmol/l (126 mg/dl)], had a fasted low-density lipoprotein (LDL)-cholesterol <4.1 mmol/l (160 mg/dl), a body mass index (BMI) <36 kg/m2, and were free of overt chronic diseases as assessed by medical history, physical examination, standard blood chemistries, and hematological evaluation. Participants had not taken any cardiovascular or lipid-lowering medications for at least 6 mo or aspirin, nonsteroidal anti-inflammatory medications, or vitamin supplements for at least 4 wk. Postmenopausal women with contraindications to estrogen (e.g., history of active estrogen-dependent neoplasms, thromboembolism) were excluded. All subjects gave their written informed consent to participate. All procedures were reviewed and approved by the University of Colorado Denver Colorado Multiple Institutional Review Board.
Measurements.
Subjects were studied in the supine position following an overnight fast with proper hydration (water drinking only) and abstinence from caffeine. The participants' normal dietary patterns were maintained, including sodium intake for the 2-day period immediately before any measurements. Premenopausal women were tested 7–10 days after onset of menstruation (i.e., midfollicular phase). The study took place at the University of Colorado Denver Colorado Clinical Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC).
Arterial stiffness.
Carotid artery compliance was determined using high-resolution ultrasound imaging as previously described (50). To determine whether the effects of the interventions were selective for the carotid arteries, arterial compliance of the brachial artery, a medium-sized muscular artery, was also measured. Carotid and brachial images were analyzed for arterial distention using Vascular Analysis Tools version 5.5. All images were coded by number, blinded to group assignment, and analyzed by the same individual.
Brachial artery blood pressure.
Peripheral arterial blood pressure was measured in triplicate in the seated and the supine positions with a semiautomated device (Dinamap; Johnson & Johnson) over the brachial artery, as previously described (50).
Vascular endothelial-dependent vasodilation.
Ultrasound measurements of brachial artery flow-mediated dilation (FMD) were performed according to the method originally described by Celermajer et al. (3) and as previously described (9). Brachial artery FMD was determined using duplex ultrasonography (GE Vivid I) using a multifrequency linear-array transducer as previously described (3, 9). Briefly, a pediatric cuff was placed on the upper forearm, and brachial artery images were acquired ∼3–6 cm above the antecubital fossa. The ultrasound probe was clamped to ensure the location of the same arterial segment with the serial measurements and to avoid any involuntary movement. After obtaining concurrent measures of baseline brachial artery diameter and blood flow velocity, reactive hyperemia was produced by inflating the cuff up to 250 mmHg of pressure for 5 min followed by rapid deflation. After the release of the arterial occlusion, Doppler blood flow velocity was acquired, and B-mode ultrasound brachial artery diameter images were measured continuously for 2 min. Brachial artery diameter and blood flow velocity were analyzed using a commercially available software package and analyzed by the same individual (Vascular Analysis Tools 5.5.1; Medical Imaging Applications, Iowa City, IA). The initial 10 velocity waveform envelopes after cuff deflation were averaged to obtain the time-averaged peak velocity. Brachial artery peak hyperemic shear rate was calculated as the peak mean blood velocity divided by occlusion diameter. All procedures conformed strictly with recently published guidelines for assessing FMD in human subjects (5).
Body composition, metabolic risk factors, sex hormones, and oxidative stress markers.
Total fat mass and fat-free mass were determined using dual energy x-ray absorptiometry (DPX-IQ; Lunar). Minimal waist and hip circumferences were measured according to previously published guidelines, and the waist-to-hip ratio was calculated (27).
Fasting plasma concentrations of insulin and total (Roche Diagnostic Systems, Indianapolis, IN)- and high-density lipoprotein (Diagnostic Chemicals, Oxford, CT) cholesterol were determined using enzymatic/colorimetric methods, and LDL-cholesterol was determined using the Friedewald equation (12). Serum concentration of estradiol was measured using chemiluminescence, and plasma endothelin-1 was measured using an enzyme-linked immunoassay (14). Oxidized LDL, an indirect measure of oxidative stress, was determined with enzyme-linked immunosorbent plate assays (Alpco Diagnostics, Windham, NH). Total antioxidant status (TAS), a measure of the overall antioxidant defenses, was determined on serum samples using the Randox Laboratories (Oceanside, CA) enzymatic kit, and glutathione peroxidase, an antioxidant found in the cytoplasm and mitochondria, was measured using the RANDOX RANSEL glutathione peroxidase assay (Randox Laboratories). All assays were performed by the University of Colorado Denver CCTSI CTRC core laboratory.
Experimental design.
To determine whether BH4 deficiency contributes to reduced endothelial vasodilatory function and arterial stiffening in estrogen-deficient postmenopausal women, measurements were obtained in premenopausal and postmenopausal women before and 3 h after oral BH4 [(6R)-5,6,7,8-tetrahydro-l-biopterin dihydrochloride; Schirks Laboratories] dosing (10 mg/kg body wt). Three hours was chosen because that is the time when BH4 reaches its maximal plasma concentrations (11). To examine whether the improvements in vascular function with estrogen could be related to BH4, postmenopausal women were randomly assigned to either transdermal placebo or estradiol (0.05 mg/day, Climara; Bayer HealthCare Pharmaceuticals) for 2 days, and then vascular measurements were repeated before and 3 h after BH4.
Statistical analysis.
Unpaired t-tests were used to assess group differences in subject characteristics, humoral vasoactive factors, oxidative stress markers, and baseline brachial artery EDD and carotid arterial compliance between premenopausal and postmenopausal women. Paired t-tests were used to determine the effects of oral BH4 and transdermal estradiol or placebo on vasodilatory function. Secondary analyses were performed using two-group t-tests on the changes in carotid artery compliance and brachial artery FMD with BH4 and transdermal estradiol or placebo. ANCOVA was used to adjust for potential confounders. Exploratory analyses were performed using Pearson product-moment correlations to test for the presence of significant linear bivariate relations between variables of interest. Data analysis was performed with SPSS software, version 18.0.
RESULTS
Subject characteristics.
Postmenopausal women were on average 8.7 ± 1.2 yr postmenopause. BMI, systolic blood pressure, fasted glucose, total and LDL cholesterol were higher, and estradiol was lower in postmenopausal compared with premenopausal women (all P < 0.05). Plasma endothelin-1 tended to be higher in postmenopausal women (P = 0.08). There were no other significant group differences in body composition, fasted insulin, oxidized LDL, or antioxidant concentrations (Table 1).
Table 1.
Subject characteristics of pre- and postmenopausal women
| Variable | Premenopausal | Postmenopausal |
|---|---|---|
| n | 9 | 24 |
| Age, yr | 33 ± 2 | 57 ± 1* |
| Body mass, kg | 63.1 ± 3.4 | 67.1 ± 2.1 |
| BMI | 22.7 ± 1.3 | 26.0 ± 0.9* |
| Body fat, % | 31 ± 3 | 37 ± 2 |
| Waist circumference, cm | 77 ± 5 | 83 ± 3 |
| WHR | 0.81 ± 0.03 | 0.83 ± 0.02 |
| Systolic BP, mmHg | 103 ± 5 | 120 ± 3* |
| Diastolic BP, mmHg | 67 ± 4 | 73 ± 2 |
| Total cholesterol, mmol/l | 4.3 ± 0.3 | 5.0 ± 0.2* |
| LDL-cholesterol, mmol/l | 2.4 ± 0.2 | 2.9 ± 0.1* |
| HDL-cholesterol, mmol/l | 1.5 ± 0.1 | 1.6 ± 0.1 |
| Fasting insulin, pmol/l | 52 ± 13 | 77 ± 8 |
| Fasting glucose, mmol/l | 4.5 ± 0.1 | 4.9 ± 0.1* |
| Estradiol, pmol/l | 286 ± 86 | 122 ± 53* |
| Endothelin-1, pg/ml | 5.8 ± 0.5 | 6.6 ± 0.3 |
| Oxidized LDL, U/l | 48.1 ± 5.4 | 53.9 ± 3.1 |
| TAS, mmol/l | 1.35 ± 0.05 | 1.40 ± 0.03 |
| Glutathione peroxidase, U/l | 6,173 ± 395 | 6,906 ± 228 |
Values are means ± SE; n, no. of subjects. BMI, body mass index; WHR, waist-to-hip ratio; BP, blood pressure; LDL, low-density liproprotein; HDL, high-density lipoprotein; TAS, total antioxidant status.
P < 0.05 vs. premenopausal.
BH4 administration in premenopausal and postmenopausal women.
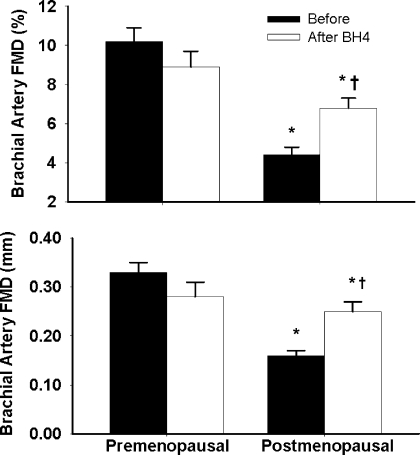
Baseline carotid artery compliance (i.e., before BH4 administration) was lower (P < 0.0001) in the postmenopausal women when compared with premenopausal women (Fig. 1). The reduced baseline carotid artery compliance in the postmenopausal women was associated with a lower baseline brachial artery FMD (before BH4 administration, P < 0.0001 vs. premenopausal women; Fig. 2). Carotid artery compliance was strongly correlated with brachial artery FMD (r=0.66; P < 0.0001) in the pooled women, as well as within the postmenopausal women (r=0.47, P = 0.02). Baseline brachial artery compliance was not different between pre- and postmenopausal women (0.10 ± 0.02 vs. 0.10 ± 0.01 mm2·mmHg−1·10−1, respectively, P = 0.98), nor was it correlated with carotid artery compliance (r=−0.08, P = 0.64) or brachial artery FMD (r=−0.13, P = 0.49).
Fig. 1.
Carotid artery compliance before and after oral tetrahydrobiopterin (BH4) administration in premenopausal and postmenopausal women. Data are reported as means ± SE. *P < 0.0001 vs. premenopausal women. †P < 0.001 vs. baseline of the same group.
Fig. 2.
Brachial artery flow-mediated dilation (FMD) before and after oral BH4 administration in premenopausal and postmenopausal women. Data are reported as means ± SE. *P < 0.0001 vs. premenopausal women. †P < 0.001 vs. baseline of the same group.
BH4 administration increased carotid artery compliance by 24 ± 5% in the postmenopausal women (vs. baseline, P < 0.001) but had no effect in the premenopausal women (P = 0.62; Fig. 1) or on brachial artery compliance of either group (P = 0.52, data not shown). The increase in carotid artery compliance corresponded with a 64 ± 11% increase in brachial artery FMD with BH4 administration in the postmenopausal women (P < 0.001; Fig. 2) but did not have a significant effect in the premenopausal women (P = 0.10). Moreover, in the postmenopausal women, the changes in carotid artery compliance and in FMD from baseline in response to BH4 administration were correlated with each other (r=0.54, P = 0.01). There was no change in carotid or brachial artery lumen diameter, brachial artery peak hyperemic shear rates, arterial blood pressure, or heart rate with BH4 administration (Table 2).
Table 2.
Carotid and brachial artery parameters and hemodynamics in premenopausal and postmenopausal women
| Variable | Premenopausal | Postmenopausal |
|---|---|---|
| Carotid diameter, mm | ||
| Baseline | 5.78 ± 0.15 | 5.95 ± 0.09 |
| After BH4 | 5.73 ± 0.15 | 5.93 ± 0.09 |
| Carotid distention, mm | ||
| Baseline | 0.58 ± 0.03 | 0.33 ± 0.02* |
| After BH4 | 0.59 ± 0.03 | 0.39 ± 0.02† |
| Supine systolic BP, mmHg | ||
| Baseline | 104 ± 5 | 123 ± 3* |
| After BH4 | 101 ± 5 | 123 ± 3 |
| Diastolic BP, mmHg | ||
| Baseline | 63 ± 3 | 71 ± 2* |
| After BH4 | 61 ± 3 | 72 ± 3 |
| Pulse pressure, mmHg | ||
| Baseline | 40 ± 4 | 52 ± 2* |
| After BH4 | 41 ± 3 | 51 ± 2 |
| Brachial diameter, mm | ||
| Baseline | 3.3 ± 0.2 | 3.8 ± 0.1* |
| After BH4 | 3.1 ± 0.2 | 3.7 ± 0.1 |
| Peak shear rate§, s−1 | ||
| Baseline | 135 ± 11 | 125 ± 7 |
| After BH4 | 132 ± 9 | 114 ± 6 |
Data are means ± SE. BH4, tetrahydrobiopterin.
P < 0.05 vs. premenopausal.
P < 0.05 vs. baseline of the same group;
n = 20 postmenopausal and 9 premenopausal women.
Administration of estradiol with and without BH4 in previously estrogen-deficient postmenopausal women.
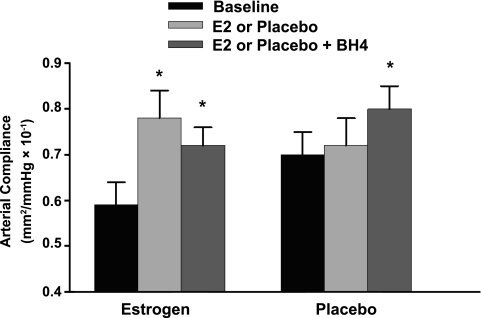
There were no differences in characteristics of the postmenopausal women randomized to transdermal estradiol or placebo (Table 3), with the exception that baseline brachial artery FMD was significantly lower in the estradiol group compared with the placebo group (P = 0.01; Fig. 4). Carotid artery compliance and brachial artery FMD increased in response to transdermal estradiol [P < 0.001 (Fig. 3) and P = 0.02 (Fig. 4), respectively] but did not change with placebo (P = 0.20 and 0.54, respectively). Brachial artery compliance remained unchanged in both groups (P = 0.26 and 0.78, respectively, data not shown). The change in carotid artery compliance in response to transdermal estradiol was strongly correlated with the change in FMD (r=0.64, P = 0.03). With the exception of an increase in serum estradiol concentration and decrease in fasted insulin concentration and systolic blood pressure with transdermal estradiol (both P < 0.05; Tables 4 and 5), there were no changes in any other humoral factor or hemodynamics with estradiol treatment. The changes in fasted insulin and systolic blood pressure and the time past menopause were not correlated with the change in carotid artery compliance or brachial artery FMD. Moreover, the change in carotid artery compliance and brachial artery FMD with estradiol was still significant (P = 0.001 and P = 0.04, respectively) after adjustment for these potential confounders. Oral BH4 had no effect on carotid artery compliance (P = 0.10; Fig. 3) or brachial artery FMD in postmenopausal women after treatment with transdermal estradiol (P = 0.91; Fig. 4). In contrast, oral BH4 increased carotid compliance (P = 0.006; Fig. 3) and brachial artery FMD (P = 0.02; Fig. 4) in placebo-treated postmenopausal women.
Table 3.
Subject characteristics for the postmenopausal women who participated in the estradiol and placebo intervention study
| Variable | Estradiol | Placebo |
|---|---|---|
| n | 12 | 10 |
| Age, yr | 57 ± 1 | 58 ± 1 |
| Menopause duration, yr | 9.2 ± 1.7 | 8.4 ± 1.7 |
| Prior HT use, % | 50 | 60 |
| Body mass, kg | 69.6 ± 3.1 | 64.6 ± 3.1 |
| BMI | 26.5 ± 1.3 | 25.5 ± 1.3 |
| Body fat, % | 39 ± 2 | 35 ± 2 |
| Waist circumference, cm | 83 ± 6 | 83 ± 6 |
| WHR | 0.85 ± 0.03 | 0.81 ± 0.03 |
| Systolic BP, mmHg | 122 ± 4 | 118 ± 4 |
| Diastolic BP, mmHg | 75 ± 3 | 71 ± 3 |
| Total cholesterol, mmol/l | 4.9 ± 0.2 | 5.1 ± 0.2 |
| LDL-cholesterol, mmol/l | 2.8 ± 0.2 | 2.9 ± 0.2 |
| HDL-cholesterol, mmol/l | 1.6 ± 0.1 | 1.7 ± 0.1 |
| Fasting insulin, pmol/l | 82 ± 12 | 72 ± 12 |
| Fasting glucose, mmol/l | 4.9 ± 0.1 | 4.9 ± 0.1 |
Data are means ± SE; n, no. of subjects. HT, hormone therapy.
Fig. 4.
Brachial artery FMD at baseline and following transdermal placebo or estradiol, and after oral BH4 administration in postmenopausal women. *P < 0.001 vs. placebo women. †P < 0.001 vs. baseline of the same group.
Fig. 3.
Carotid artery compliance at baseline and following transdermal placebo or estradiol (E2) and after oral BH4 administration in postmenopausal women. Data are reported as means ± SE. *P < 0.001 vs. placebo women.
Table 4.
Circulating humoral factors before and after the interventions of estradiol or placebo
| Variable | Estradiol | Placebo |
|---|---|---|
| Estradiol, pmol/l | ||
| Baseline | 66 ± 8 | 65 ± 9 |
| Follow up | 297 ± 25* | 62 ± 28 |
| Glucose, mmol/l | ||
| Baseline | 5.1 ± 0.1 | 4.9 ± 0.1 |
| Follow up | 5.0 ± 0.1 | 4.8 ± 0.1 |
| Insulin, pmol/l | ||
| Baseline | 91 ± 10 | 74 ± 11 |
| Follow up | 79 ± 10* | 69 ± 11 |
| Oxidized LDL, U/l | ||
| Baseline | 53.3 ± 4.7 | 58.8 ± 4.7 |
| Follow up | 56.2 ± 4.1 | 57.2 ± 4.1 |
| TAS, mmol/l | ||
| Baseline | 1.44 ± 0.04 | 1.36 ± 0.04 |
| Follow up | 1.40 ± 0.04 | 1.41 ± 0.04 |
| Endothelin-1, pg/ml | ||
| Baseline | 6.5 ± 0.4 | 6.8 ± 0.4 |
| Follow up | 6.2 ± 0.3 | 7.4 ± 0.3 |
| Glutathione peroxidase, U/l | ||
| Baseline | 7,081 ± 364 | 6,600 ± 364 |
| Follow up | 6,691 ± 353 | 6,139 ± 353 |
All data are means ± SE.
P < 0.05 vs. baseline of the same group.
Table 5.
Carotid and brachial artery parameters and hemodynamics after estradiol and placebo treatment
| Variable | Estrogen | Placebo |
|---|---|---|
| Carotid diameter, mm | ||
| Baseline | 6.03 ± 0.13 | 5.92 ± 0.14 |
| Follow up | 5.96 ± 0.12 | 5.94 ± 0.13 |
| Follow up + BH4 | 5.88 ± 0.13 | 5.88 ± 0.14 |
| Carotid distension, mm | ||
| Baseline | 0.33 ± 0.02 | 0.35 ± 0.03 |
| Follow up | 0.39 ± 0.02† | 0.35 ± 0.0.2 |
| Follow up + BH4 | 0.38 ± 0.02 | 0.41 ± 0.02† |
| Supine systolic BP, mmHg | ||
| Baseline | 128 ± 5 | 113 ± 5 |
| Follow up | 120 ± 5† | 115 ± 5 |
| Follow up + BH4 | 122 ± 5 | 114 ± 5 |
| Diastolic BP, mmHg | ||
| Baseline | 75 ± 2 | 65 ± 3 |
| Follow up | 72 ± 3 | 68 ± 3 |
| Follow up + BH4 | 72 ± 2 | 65 ± 4 |
| Pulse pressure, mmHg | ||
| Baseline | 53 ± 3 | 48 ± 4 |
| Follow up | 48 ± 3† | 48 ± 4 |
| Follow up + BH4 | 50 ± 3 | 48 ± 3 |
| Brachial diameter, mm | ||
| Baseline | 3.9 ± 0.13 | 3.5 ± 0.2 |
| Follow up | 3.8 ± 0.1 | 3.5 ± 0.2 |
| Follow up + BH4 | 3.8 ± 0.2 | 3.5 ± 0.2 |
| Peak shear rate§, s−1 | ||
| Baseline | 113 ± 11 | 143 ± 12 |
| Follow up | 108 ± 12 | 125 ± 12 |
| Follow up + BH4 | 109 ± 13 | 127 ± 14 |
All data are means ± SE.
P < 0.05 vs. baseline of the same group;
n = 9 estradiol and 8 placebo.
DISCUSSION
The findings of the present study provide novel insight into the mechanisms contributing to large artery stiffening in estrogen-deficient postmenopausal women. First, administration of BH4, a critical cofactor for eNOS functioning and NO production, increased carotid artery compliance in estrogen-deficient postmenopausal, but not premenopausal, women. Second, the increase in carotid artery compliance with BH4 was significantly related to the increase in endothelial-dependent vasodilation with BH4 administration. Third, while short-term transdermal estradiol increased carotid artery compliance and endothelial-dependent vasodilation in previously estrogen-deficient postmenopausal women, coadministration of BH4 with estradiol does not augment vascular function further. Collectively, these findings suggest that reduced BH4 bioavailability contributes to large artery stiffening in estrogen-deficient postmenopausal women in part through reduced endothelial vasodilatory function. Finally, our results also suggest that the beneficial effects of estrogen on arterial stiffening in previously estrogen-deficient postmenopausal women may be mediated by improved endothelial vasodilatory tone, secondary to increasing BH4 bioavailability.
Arterial stiffening in estrogen-deficient postmenopausal women: BH4 and reduced endothelial-dependent vasodilatory tone.
In the present study, baseline carotid artery compliance was ∼50% lower in healthy estrogen-deficient postmenopausal women compared with premenopausal women, whereas brachial artery compliance was not different between the groups, consistent with our and other's previous observations on central and peripheral arterial stiffness in older adults (2, 49, 51). The differential effects of arterial stiffening between the two arterial segments can be explained by differences in geometry and mechanical properties of elastic and muscular arteries and their distinct roles in hemodynamic regulation (37, 49). Arterial compliance is primarily determined by the intrinsic viscoelastic properties of the arterial wall (37). Compared with muscular arteries (i.e., brachial), which have a greater ratio of collagen to elastin content and, thus, are more rigid, the central arteries (i.e., aorta and carotid) are more elastic in nature, which allows them to “buffer” the pulsatile systolic output through elastic recoil, thereby sustaining continuous blood flow to the periphery.
The mechanisms that contribute to large elastic arterial stiffening in estrogen-deficient postmenopausal women are not completely understood but likely involve structural changes within the arterial wall, including increased collagen and reduced and fragmented elastin content (21, 36) and increased intimal-medial thickness (IMT) (32, 40). However, increased IMT does not appear to restrict compliance unless it exceeds a threshold of thickness representing atherosclerotic manifestations (40). The arterial wall is also composed of vascular smooth muscle cells that vasodilate and vasoconstrict in response to a number of circulating and local vasoactive mediators, including those released by the vascular endothelium. Indeed, endothelial-derived substances, including those that cause vasodilation (e.g., NO) and vasoconstriction (e.g., endothelin-1) of vascular smooth muscle cells, have been shown to modulate arterial stiffness (46, 55). As such, functional changes in endothelial regulation of vascular smooth muscle cell tone may also be involved. In this regard, aging and estrogen deficiency are associated with a reduced vasodilatory capacity of the vascular endothelium of which the loss of endothelial-derived NO bioavailability is a cardinal feature (4, 13, 48, 52). Consistent with this, we found that brachial artery FMD, a measure of systemic endothelial-dependent vasodilation mediated predominantly by NO (5, 8), was ∼50% lower in estrogen-deficient postmenopausal women compared with premenopausal women. Moreover, brachial artery FMD was strongly correlated with carotid artery compliance both in the pooled women and within the postmenopausal women, supporting the idea that large elastic arterial stiffening in estrogen-deficient postmenopausal women is mediated, in part, by an elevated state of vascular smooth muscle cell vasoconstrictor tone, possibly related to reduced NO bioavailability. In contrast, brachial artery FMD was not correlated with brachial artery compliance. This finding may be related to the fact that muscular arteries have a rich sympathetic innervation, and sympathetic activation and NO competitively impact brachial artery mechanics, possibly as a regulatory control feature to modify flow transit time through the vascular bed and back to the heart (41).
We previously reported that administration of supraphysiological concentrations of the potent antioxidant ascorbic acid increases carotid artery compliance in estrogen-deficient postmenopausal women, suggesting that oxidative stress contributes mechanistically to the large elastic artery stiffening in estrogen-deficient postmenopausal women (33, 34). We speculated that the mechanism by which ascorbic acid improved carotid artery compliance was likely mediated, in part, through protecting NO scavenging by ROS, which would in turn increase NO bioavailability and the tonic state of vascular smooth muscle cell vasorelaxation (19, 38). One of the sources of increased ROS production in the arterial wall has been attributed to “eNOS uncoupling,” a phenomenon that occurs when there are inadequate levels of BH4, a critical cofactor for eNOS enzymatic function to produce NO from the interaction with l-arginine (1, 53). BH4 can be limited when there is decreased de novo synthesis from guanosine 5′-triphosphate (GTP) and/or via reduced regeneration of BH4 by dihydrofolate reductase (7). Additionally, BH4 can be rapidly oxidized by peroxynitrite and other ROS to its inactive form dihydrobiopterin, creating a vicious cycle of vascular oxidative stress (1, 53). In the present study, we found that acute supplementation of BH4 increased brachial artery FMD and carotid artery compliance in estrogen-deficient postmenopausal women but not in premenopausal women. Moreover, the improvements in carotid artery compliance and brachial artery FMD in response to BH4 were strongly correlated with each other, suggesting that reduced BH4 bioavailability may contribute to large elastic arterial stiffening in estrogen-deficient postmenopausal women through reduced endothelial vasodilatory tone. These results extend our previous findings by providing novel insight into a potential mechanism by which oxidative stress contributes to the large elastic artery stiffening in estrogen-deficient postmenopausal women. Interestingly, the magnitude of the improvement in carotid artery compliance with BH4 (24%) was similar to what we reported previously with acute ascorbic acid (∼26%) (33). Because ascorbic acid is capable of stabilizing eNOS by recycling BH3 to BH4 (20), it is possible that part of the improvement in carotid artery compliance that we previously reported (33) may be through recycling intracellular BH4 and preventing eNOS uncoupling, in addition to its antioxidant properties.
Vascular effects of estrogen in postmenopausal women: possible role of BH4.
In the present study, estrogen supplementation, but not placebo, increased carotid artery compliance (reduced arterial stiffness) in postmenopausal women, consistent with previous investigations demonstrating reduced arterial stiffening with chronic MHT (26, 31, 35, 42). The results of the present study provide preliminary insight into the potential mechanisms by which estrogen may modulate arterial stiffening in postmenopausal women. Estrogen preserves vascular endothelial vasodilatory function in part by enhancing NO release and by protecting NO from inactivation by ROS (29). Animal studies demonstrate that estrogen supplementation prevents the decrease in NO release with ovariectomy, in part by restoring BH4 bioavailability and decreasing the overproduction of superoxide anion (22, 23). Thus, we reasoned that the modulatory influence of estrogen on arterial stiffness may be through replenishing vascular BH4 and increasing vasodilatory tone. In support of this, in the present study, the increase in carotid artery compliance in response to estrogen was associated with a marked increase in endothelial-dependent vasodilation. Moreover, while oral BH4 improved brachial artery FMD and carotid artery compliance in women randomized to placebo, there was no further improvement in brachial artery FMD and carotid artery compliance following oral BH4 in women randomized to estrogen. Thus, these findings suggest that the favorable influence of estrogen on arterial stiffening in postmenopausal women may be related in part to increased vasodilatory tone, possibly mediated by increased vascular BH4 and NO bioavailability.
Although caution needs to be used in the interpretation of the present study's findings, there is reason to speculate that estrogen increases the bioavailability of BH4 in postmenopausal women. Both GTP cyclohydrolase I (GTPCH I), the rate-limiting enzyme for de novo BH4 synthesis, and BH4 are reduced in mesenteric arteries isolated from aged mice compared with younger mice, and estrogen has been shown to counteract the downregulation of GTPCH I in bovine aortic endothelial cells incubated with high glucose (30, 56). Additionally, because estrogen has direct antioxidant effects in vitro and in vivo, estrogen may maintain and/or replenish BH4 by preventing its oxidation by ROS (18, 23, 45). In the present study, plasma markers of oxidative stress (oxidized LDL) and antioxidants (TAS, glutathione peroxidase) did not change with estrogen supplementation. However, these indirect systemic plasma biomarkers lack sensitivity and may not accurately reflect the amount of oxidative stress and antioxidants in the vasculature. Additionally, it is possible that estrogen modulated other sources of ROS and/or endogenous antioxidants that we did not measure. Thus, it is plausible that estrogen could replenish BH4 by increasing de novo BH4 synthesis via stimulating or increasing GTPCH I, increasing the regeneration of BH4, and/or decreasing ROS and the subsequent oxidation of BH4.
Experimental considerations and limitations.
We recognize that mechanisms other than BH4 could contribute to the decrease in arterial stiffness with estrogen supplementation, including the modulation of vasoconstrictor hormones and local factors. In the present study, plasma endothelin-1 concentrations tended to be higher in postmenopausal compared with premenopausal women but did not change with estrogen supplementation. However, plasma concentrations do not necessarily reflect differences at the local vascular level or vascular responsiveness to these factors. As such, we cannot rule out the possibility that these or other vasoactive factors were modulated with estrogen supplementation. Structural changes within the arterial wall (i.e., elastin-collagen composition) are thought to occur over a period of years, making it unlikely that 2 days of estrogen supplementation decreased arterial stiffness by such mechanism (39). Estrogen may have also decreased arterial stiffness in the postmenopausal women in part via its antioxidant effects that do not modulate BH4.
The fact that carotid artery compliance and brachial artery FMD were not restored to premenopausal levels with either BH4 or estrogen indicates that other mechanisms other than BH4 deficiency contribute to arterial stiffening and reduced endothelial vasodilatory function in postmenopausal women. Possible mechanisms include other vasoconstrictor hormones and local factors discussed above, as well as extracellular matrix-linked structural alterations within the arterial wall that cannot be modified with short-term BH4 or estrogen. Additionally, it is possible that the small effect of BH4 on carotid artery compliance, and to a lesser extent brachial artery FMD, was the result of a suboptimal dose of BH4 and that a higher dose of BH4 may have resulted in a greater improvement in arterial compliance. The dose of BH4 used in the present study (10 mg/kg of body wt) has been shown to increase plasma biopterin levels by ∼50-fold (11) and restore brachial artery FMD in sedentary older men (10), as well as in hypercholesterolemic men and women (6). In contrast to the study conducted in older men, brachial artery FMD was not restored with BH4 administration in our postmenopausal women even though a similar dose of BH4 was used (10). Thus, it is plausible that a higher dose of BH4 may be necessary to restore endothelial vasodilatory function in postmenopausal women or that other mechanisms are contributing to the reduced brachial artery FMD in postmenopausal women. It is also possible that there may be a ceiling effect for how much brachial artery FMD can be improved with any intervention in postmenopausal women.
There are several limitations associated with the present study. First, we did not measure plasma or vascular BH4 levels. Thus, we cannot demonstrate that there were group differences at baseline or that oral BH4 and estrogen supplementation replenished vascular BH4 in postmenopausal women. Second, we used brachial artery FMD to mechanistically examine whether increased NO bioavailability mediated the improvements in carotid artery compliance with BH4 and estrogen instead of directly measuring NO and vasodilatory tone in the carotid artery. Thus, because of the different vascular districts, we cannot infer a causal relationship or rule out other mechanisms possibly involved in the endothelial-dependent vasodilatory improvement with BH4 or estrogen (e.g., prostacyclin). Additionally, it is possible that the improvement in brachial artery FMD was related to a more compliant brachial artery. However, brachial artery compliance did not change with BH4 or estrogen administration, making it unlikely that the improvement in FMD with BH4 and estrogen was related to such mechanism, but rather likely related to increased NO bioavailability. The lack of change in brachial artery compliance with the interventions was not surprising given that baseline brachial artery compliance was not different between the groups. Nonetheless, it is possible that any improvements in brachial compliance could have been offset by competitive neurogenic input (41). Third, we did not measure endothelial-independent vasodilation; thus, it is possible that estrogen and BH4 could have increased vasodilatory function through actions on vascular smooth muscle cells. However, previous studies have demonstrated no improvement in endothelial-independent vasodilation with BH4 administration (10, 15–17, 28, 44). Finally, we did not conduct a randomized placebo controlled study with oral BH4 as previously done (6, 10), and, thus, improvements in vascular function in the estrogen-deficient postmenopausal women may have been because of a “placebo effect.” However, oral BH4 improved vascular function in the groups of women that we hypothesized would have reduced BH4 (i.e., estrogen-deficient postmenopausal and not premenopausal women at baseline; and in postmenopausal women following placebo treatment and not estrogen treatment), making it unlikely that our findings are related to a placebo effect.
In conclusion, our findings suggest that reduced vascular BH4 contributes to arterial stiffening in estrogen-deficient postmenopausal women, related in part to reduced endothelial vasodilatory function. Moreover, BH4 maintenance may be an underlying mechanism by which estrogen benefits endothelial-dependent vasodilation and arterial stiffness; however, further study is needed in postmenopausal women for a definitive causal link. These findings provide new mechanistic insight into the processes that mediate the biological changes in arterial stiffening in estrogen-deficient postmenopausal women that may help guide future sex-specific therapies for the prevention of CVD.
GRANTS
This study was supported by the National Institutes of Health Awards AG-027678 and Colorado Clinical Translational Sciences Institute (CCTSI) RR-025780, a University of Colorado Denver (UCD) Center for Women's Health Research Junior Faculty Development Award, and a UCD Women's Health Research Pilot Project Grant.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
K. Moreau: conceived and designed research, acquired and managed the data, analyzed and interpreted the data, performed statistical analyses, handled funding and supervision and drafted the manuscript; A. Meditz: provided medical oversight, made critical revision of the manuscript for important intellectual content; K. Deane: provided medical oversight and made critical revision of the manuscript for important intellectual content; and W. Kohrt: data management, interpreted data, and made critical revision of the manuscript for important intellectual content.
ACKNOWLEDGMENTS
We thank Lauren Tobin and Chelsea Bergman for technical assistance.
REFERENCES
- 1. Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bjarnegård N, Länne T. Arterial properties along the upper arm in humans: age-related effects and the consequence of anatomical location. J Appl Physiol 108: 34–38, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Cosentino F, Hurlimann D, delli Gatti C, Chenevard R, Blau N, Alp NJ, Channon KM, Eto M, Lerch P, Enseleit F, Ruschitzka F, Volpe M, Luscher TF, Noll G. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolemia. Heart 94: 487–492, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem 284: 28128–28136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001 [PubMed] [Google Scholar]
- 9. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, Thony B, Blau N. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab 81: 45–51, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Friedewald WT, Levey RI, Frederickson DS. Estimation of the concentration of LDL-C in plasma without the use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 13. Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94: 3513–3520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goddard J, Webb DJ. Plasma endothelin concentrations in hypertension. J Cardiovasc Pharmacol 35: S25–S31, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 86: E36–41, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43: 1435–1438, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, Chayama K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens 15: 326–332, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Keaney JF, Jr, Shwaery GT, Xu A, Nicolosi RJ, Loscalzo J, Foxall TL, Vita JA. 17Beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation 89: 2251–2259, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43: 562–571, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I. Aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lam KK, Ho ST, Yen MH. Tetrahydrobiopterin improves vascular endothelial function in ovariectomized rats. J Biomed Sci 9: 119–125, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Lam KK, Lee YM, Hsiao G, Chen SY, Yen MH. Estrogen therapy replenishes vascular tetrahydrobiopterin and reduces oxidative stress in ovariectomized rats. Menopause 13: 294–302, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Liang YL, Teede H, Shiel LM, Thomas A, Craven R, Sachithanandan N, McNeil JJ, Cameron JD, Dart A, McGrath BP. Effects of oestrogen and progesterone on age-related changes in arteries of postmenopausal women [published erratum appears in Clin Exp Pharmacol Physiol 24:646, 1997]. Clin Exp Pharmacol Physiol 24: 457–459, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics, 1988 [Google Scholar]
- 28. Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Luscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol 35: 173–178, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol 74: 337–343, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Miyazaki-Akita A, Hayashi T, Ding QF, Shiraishi H, Nomura T, Hattori Y, Iguchi A. 17beta-Estradiol antagonizes the down-regulation of endothelial nitric-oxide synthase and GTP cyclohydrolase I by high glucose: relevance to postmenopausal diabetic cardiovascular disease. J Pharmacol Exp Ther 320: 591–598, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57: 861–868, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Moreau KL, Donato AJ, Seals DR, Dinenno FA, Blackett SD, Hoetzer GL, Desouza CA, Tanaka H. Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol 283: H1409–H1417, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause 13: 951–958, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Nagai Y, Earley CJ, Kemper MK, Bacal CS, Metter EJ. Influence of age and postmenopausal estrogen replacement therapy on carotid arterial stiffness in women. Cardiovasc Res 41: 307–311, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, Kingwell BA. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 46: 1129–1134, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clincial Principles (4th ed.). London, UK: Arnold, 1998 [Google Scholar]
- 38. O'Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res 88: 12–21, 2001 [DOI] [PubMed] [Google Scholar]
- 39. O'Rourke MF, Avolio AP, Lauren PD, Young J. Age-related changes of elastic lamellae in the human thoracic aorta (Abstract). J Am Coll Cardiol 9: 53A, 1987. 3794111 [Google Scholar]
- 40. Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol 23: 157–164, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Salzer DA, Medeiros PJ, Craen R, Shoemaker JK. Neurogenic-nitric oxide interactions affecting brachial artery mechanics in humans: roles of vessel distensibility vs. diameter Am J Physiol Regul Integr Comp Physiol 295: R1181–R1187, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Scuteri A, Lakatta EG, Bos AJ, Fleg JL. Effect of estrogen and progestin replacement on arterial stiffness indices in postmenopausal women. Aging (Milano) 13: 122–130, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 37: 1374–1380, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest 99: 41–46, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, Yamazaki I, Yoshizumi M, Eto M, Ouchi Y. Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation 103: 724–729, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Sugawara J, Komine H, Hayashi K, Yoshizawa M, Yokoi T, Otsuki T, Shimojo N, Miyauchi T, Maeda S, Tanaka H. Effect of systemic nitric oxide synthase inhibition on arterial stiffness in humans. Hypertens Res 30: 411–415, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18: 127–132, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 51. van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HAS, Van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension 35: 637–642, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101: 2258–2263, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44: 381–386, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Westendorp IC, Bots ML, Grobbee DE, Reneman RS, Hoeks AP, Van Popele NM, Hofman A, Witteman JC. Menopausal status and distensibility of the common carotid artery. Arterioscler Thromb Vasc Biol 19: 713–717, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation 105: 213–217, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]