Abstract
Explanations for arrhythmia mechanisms at the cellular level are usually based on experiments in nonhuman myocytes. However, subtle electrophysiological differences between species may lead to different rhythmic or arrhythmic cellular behaviors and drug response given the nonlinear and highly interactive cellular system. Using detailed and quantitatively accurate mathematical models for human, dog, and guinea pig ventricular action potentials (APs), we simulated and compared cell electrophysiology mechanisms and response to drugs. Under basal conditions (absence of β-adrenergic stimulation), Na+/K+-ATPase changes secondary to Na+ accumulation determined AP rate dependence for human and dog but not for guinea pig where slow delayed rectifier current (IKs) was the major rate-dependent current. AP prolongation with reduction of rapid delayed rectifier current (IKr) and IKs (due to mutations or drugs) showed strong species dependence in simulations, as in experiments. For humans, AP prolongation was 80% following IKr block. It was 30% for dog and 20% for guinea pig. Under basal conditions, IKs block was of no consequence for human and dog, but for guinea pig, AP prolongation after IKs block was severe. However, with β-adrenergic stimulation, IKs played an important role in all species, particularly in AP shortening at fast rate. Quantitative comparison of AP repolarization, rate-dependence mechanisms, and drug response in human, dog, and guinea pig revealed major species differences (e.g., susceptibility to arrhythmogenic early afterdepolarizations). Extrapolation from animal to human electrophysiology and drug response requires great caution.
Keywords: cardiac action potential, delayed rectifier current, drug effects, human cardiac model
studies of basic cardiac electrophysiology and arrhythmia are usually performed with experiments in nonhuman (dog or small rodents) hearts or isolated cells. However, ion channel currents, which determine the ventricular action potential (AP), are species dependent (1, 3, 38). Moreover, so are arrhythmia mechanisms (e.g. Ref. 37) and responses to administration of drugs. The fact that species differences exist seems trivial. However, the consequences of these differences with respect to arrhythmia mechanisms and drug responses are not trivial, and because of the complex interrelatedness of bioelectrical processes (26), it is impossible to predict how findings from nonhuman species apply to human. Broadly, it is important to ask: how does ion channel function differ across species, and how do these differences affect the AP and its rate dependence and response to pharmacological intervention? Species-specific mathematical models provide a platform from which to investigate these questions. Demonstrated differences between species highlight the fact that great caution should be used when extrapolating results from nonhuman experiments to human cellular electrophysiology, arrhythmic behaviors, and response to drugs.
Materials and Methods
Mathematical models.
Previously developed and extensively validated models of the human (21), dog (7), guinea pig (10), and rabbit (28) ventricular epicardial (Epi) APs were used. Epi cells were chosen (specified if otherwise) because the transient outward K+ current (Ito), an important determinant of AP morphology and rate dependence, is most prominent in Epi cells. Model codes are available on our website (http://rudylab.wustl.edu). The Shannon-Bers rabbit model was executed using CellML repository and tools (www.cellml.org).
Simulation of β-adrenergic response to extracellular application of β-agonist isoproterenol (ISO) was according to previous work in human [(20) using original current formulations (21) with τxs1,PKA = 0.6 * τxs1 and GKs,PKA = 3.2 * GKs, in accord with experiments (36)], dog (14), and guinea pig (9). Definitions of transmural cell types were from previous work [human (21) and dog (30), using mild IKr protein heterogeneity mid-myocardium (M)/Epi = 0.91 as observed experimentally (32), and guinea pig (35)].
Definitions and pacing protocol.
The duration of the AP (APD) at 30, 50, 70, and 90% of complete repolarization (APD30–90, in ms) was determined. APD was measured from the time of maximum dVm/dt during the AP upstroke (Vm = membrane potential). For APD rate dependence, we paced to steady state at each cycle length (CL). Only the last or last two paced beats are shown.
Results
Block of delayed rectifier K+ currents, simulating effects of drugs or mutation.
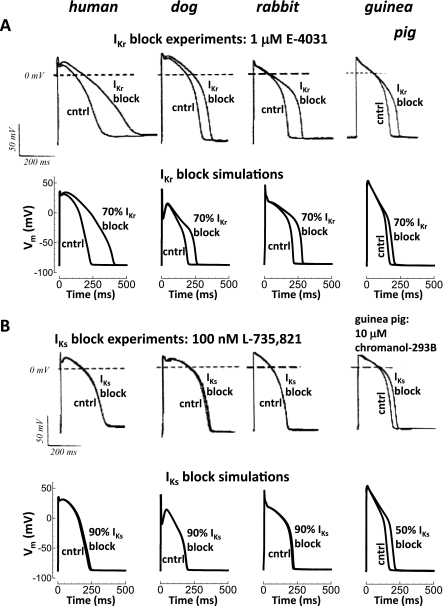
Figure 1 shows simulations and corresponding experiments (18) for the species dependence of delayed rectifier K+ current block (rapid and slow, IKr and IKs, respectively) during steady-state pacing (CL = 1,000 ms). For 70% IKr block (Fig. 1A), simulated APD90 was substantially prolonged in human (77%, 172-ms prolongation). Block consequence was comparatively small for dog (34%, 65-ms APD prolongation), rabbit (32%, 67-ms APD prolongation), and guinea pig (21%, 35-ms APD prolongation). In Fig. 1B, 90% IKs block was essentially of no consequence for human (4%, 10-ms APD prolongation), dog (1%, 2-ms APD prolongation), and rabbit (3%, 8-ms APD prolongation). By contrast, only 50% IKs block (Fig. 1, right), was required to substantially prolong APD in guinea pig (27%, 44-ms APD prolongation).
Fig. 1.
Species dependence of delayed rectifiers block. A: rapid delayed rectifier current (IKr) block. B: slow delayed rectifier current (IKs) block. Results from human, dog, rabbit, and guinea pig are shown from left to right. Shown are steady-state APs at CL = 1,000 ms for control and K+ current block. Experiments from Jost et al. (18) are shown above simulations. A dose of 1 μM E-4,031 was used to block IKr, and 100 nM L-735,821 was used to block IKs in human, dog, and rabbit. The 10 μM of chromanol-293B was used to block IKs in guinea pig (lower potency). Simulated block level was chosen to match experiments: 70% block of IKr and 90% block of IKs, except in guinea pig, where IKs block was only 50% due to application of a different drug in the experiments. Vm, membrane potential. [From Jost et al. (18), courtesy of Promenade Publishing House, Budapest, and Hungary.]
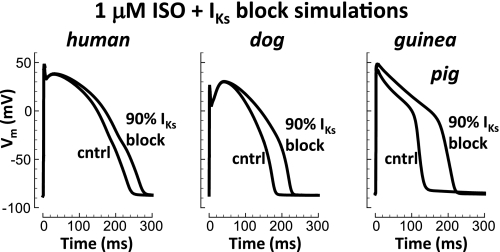
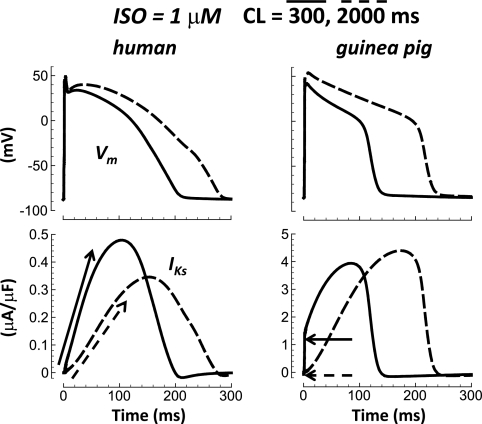
Figure 2 shows that after application of 1 μM ISO (a saturating dose), consequence of IKs block increased for human and dog. Still, block effect in human and dog remained smaller than in guinea pig (14, 26, and 61%; 33-, 45-, and 90-ms APD90 prolongation, respectively).
Fig. 2.
Species dependence of IKs block with β-adrenergic stimulation [1 μM isoproterenol (ISO) a saturating dose]. From left to right, results for human, dog, and guinea pig are shown. Pacing cycle length (CL) was 700, 500, and 300 ms for human, dog, and guinea pig (resting rates).
APD rate dependence.
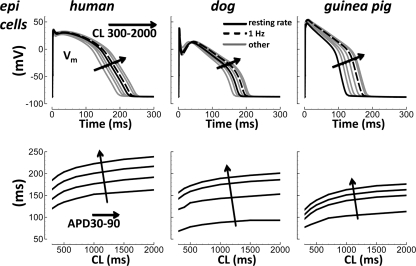
APD90 was longest for human. As shown in Fig. 3, top, at CL = 1,000 ms (dashed black), APD90 was 223 ms for human, 189 ms for dog, and 163 ms for guinea pig. Dog AP repolarization rate was the most gradual. At CL = 1,000 ms, the time difference between APD30 and APD90 (APD90 − APD30) was 70 ms for human, 101 ms for dog, and 60 ms for guinea pig (71, 94, and 40 ms at physiological resting rates for these species; Fig. 3, solid black). APD30 occurred relatively early in dog because of the prominent phase 1 notch due to Ito, which lowered the voltage of the phase 2 AP dome, resulting in more gradual repolarization. The range of APD90 over CLs from 300 to 2,000 ms was 54 ms for human, 45 ms for dog, and 59 ms for guinea pig. Note that 1-Hz pacing may be a poor choice for representing physiological AP dynamics in guinea pig, a species in which resting heart rates are much faster (compare solid and dashed black curves).
Fig. 3.
Simulation of action potential (AP) duration (APD) rate dependence in different species. Human, dog, and guinea pig results are left, middle, and right, respectively. Top: APs at different rates. CL changes are indicated by the arrows. Species specific resting rates are solid black. The 1 Hz is dashed black. Other rates are grey. Bottom: APD rate dependence. APDs at 30–90% of complete repolarization (APD30–90) are indicated by the arrows.
Intracellular sodium accumulation.
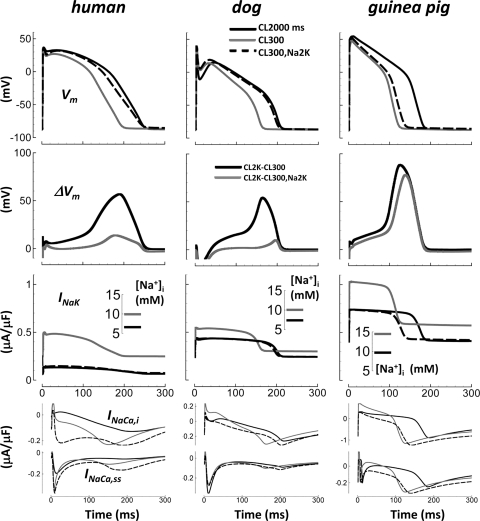
We clamped Na+ concentrations in the myoplasm and submembrane space of the models ([Na+]i and [Na+]ss, respectively) to their value at CL = 2,000 ms during the final paced beat at CL = 300 ms. In the absence of [Na+] clamp, [Na+]i is 10.0, 10.0, and 14.5 mM at CL = 300 ms and 6.6, 7.4, and 9.7 mM at CL = 2,000 ms in human, dog, and guinea pig, respectively. Figure 4 shows the AP, the difference between slow and fast rate APs (ΔVm), and the Na+/K+-ATPase current (INaK) for these simulations ([Na+]i; Fig. 4, inset). When [Na+] was clamped to the relatively small CL = 2,000 ms values (Na2K) during pacing at CL = 300 ms, APD increased relative to CL = 300 ms control in all species. With [Na+] clamp, APD90 was 26, 26, and 12% longer for human, dog, and guinea pig, respectively, relative to control at CL = 300 ms. For human and dog, ΔVm was greatly reduced by [Na+] clamp, indicating that rate-dependent [Na+] changes play an important role in rate-dependent AP changes, via electrogenic INaK. In other words, when [Na+] is clamped, slow and fast rate APs are similar. However, for guinea pig, ΔVm remained large despite [Na+] clamp, indicating a minor role for [Na+] in AP rate dependence.
Fig. 4.
Effect of intracellular Na+ concentration ([Na+]i) clamp at fast pacing (CL = 300 ms) to slow rate values during the final paced beat (Na+ at CL = 2,000 ms, Na2K). The AP, the difference between slow and fast rate APs (ΔVm), Na+/K+ pump current (INaK), and Na+/Ca2+ exchange current (INaCa,i and INaCa,ss, myoplasm and subpace components) are shown from top to bottom, respectively. Inset: [Na+]i. Solid black, CL = 2,000 ms, no [Na+] clamp; solid gray, CL = 300 ms, no [Na+] clamp; dashed black, CL = 300 ms, [Na+] clamp.
Change in INaK is greatest for guinea pig. However, its effect on AP is the smallest because it occurs on the background of a large slow delayed rectifier K+ current, IKs (8). IKs is much larger in guinea pig than in the other species (see Fig. 1). It is the net change in the balance of currents that alters the AP. Na+/Ca2+ exchange current (INaCa) in the models is the sum of two components: INaCa,i is exchange current in the bulk myoplasmic compartment, and INaCa,ss is exchange current in the subspace compartment near the T-tubular membrane. Subspace Ca2+ concentrations are ∼10-fold larger and decay ∼10-fold faster than bulk myoplasmic Ca2+ concentrations, causing a large and early inward spike in INaCa,ss (forward mode Ca2+ extrusion). Both INaCa,i and INaCa,ss were affected by Na+ clamp (Fig. 1, bottom). However, the relatively small magnitude of INaCa prevented it from playing a major role in APD rate dependence in human and dog. Note that in guinea pig, INaCa,i and INaK are of similar magnitude. Because of this, outward current from reverse mode INaCa,i elicited by Na+ accumulation with fast pacing is large enough to meaningfully promote APD shortening in guinea pig, as previously described (8).
Na+ concentrations in guinea pig were higher than in the other species [including rabbit, see Shannon et al. (28)], raising the question of whether this is an artifact of the model. However, the rate dependence of Na+ in the guinea pig model was validated against experiments in a previous study (8). Still, the Na+ concentration is notoriously difficult to measure, and fluorescence indicators may tend to overestimate its value (23).
Mechanism of IKs rate dependence.
In guinea pig, slow deactivation of IKs results in incomplete deactivation between beats at fast pacing rate and channel accumulation in the open state. Consequently, IKs accumulates to be larger during the AP, thereby shortening the APD. In human and dog, IKs deactivates faster, which counteracts the possibility of accumulation in the open state. As shown previously [experiments (24) and simulations (29)], in these large mammals IKs channels accumulate at fast rate in closed states that are kinetically proximal to the open state [termed “available reserve” (29)] from which they can open rapidly to generate larger current during the AP repolarization phase, contributing to APD shortening. The available reserve was shown to be augmented by β-adrenergic stimulation (14).
Figure 5 shows APs at slow (CL = 2,000 ms) and fast (CL = 300 ms) rates and the corresponding IKs currents in the presence of ISO for human (left) and guinea pig (right). Guinea pig IKs exhibits an instantaneous “jump” of current upon stimulation at fast pacing (solid arrow) but not at slow pacing (dashed arrow), revealing open state accumulation. In contrast, human IKs does not display an instantaneous jump at fast rate; rather, it increases faster than at slow rate (solid arrow compared with dashed arrow) to reach a higher peak during AP repolarization, revealing the available reserve mechanism of IKs participation in APD shortening. Similar behavior and mechanism are observed in the dog [shown extensively by Heijman et al. (14)]. This mechanism plays an important role in providing a repolarization reserve to ensure proper repolarization when IKr is reduced by drug block or mutations (20, 25, 29).
Fig. 5.
Rate dependence of IKs accumulation. AP and corresponding IKs current are shown on the top and bottom, respectively. Human is left, and guinea pig is right. IKs scales are an order of magnitude different. Dashed black, CL = 2,000 ms; solid black, CL = 300 ms. Arrows show steeper IKs increase at fast rate compared with slow rate in human and open state accumulation at fast rate but not at slow rate in guinea pig. Note that there is no open state accumulation in human and that the IKs slope is rate insensitive in guinea pig.
Rate dependence of β-adrenergic effects on INaK and IKs.
One of the targets of β-adrenergic stimulation is INaK. Specifically, phosphorylation of phospholemman (PLM) by PKA relieves inhibition on INaK. This was modeled as an increase in Na+ binding affinity of the pump (14). Thus it is important to ask whether PKA effects on PLM are needed to preserve APD rate dependence in the presence of β-adrenergic stimulation in human and dog. This was tested by measuring the percent APD90 shortening at fast rate with 1 μM ISO vs. 1 μM ISO without PLM as a PKA target. In dog, APD shortening was by 26% whether or not PLM was a PKA target [analysis derived from Heijman et al. (14); Fig. 3A]. In human, it was 27% with and 21% without PLM as a PKA target (using CLs of 300 and 2,000 ms, 1 μM ISO). These results indicate that in the presence of β-adrenergic stimulation, PKA effects on INaK helped preserve APD rate dependence in human but were not important in dog.
The role of IKs in determining APD rate dependence rises in prominence with β-adrenergic stimulation. Experimental evidence and computer simulations suggest that fast pacing causes channel accumulation in closed states near the open state (available reserve, described above) and that β-adrenergic stimulation alters IKs gating to further promote this accumulation (31, 36). Therefore, we sought to determine the degree to which IKs accumulation determines APD rate dependence once β-adrenergic stimulation is applied. In the presence of 1 μM ISO, we reset IKs gates to their slow rate (CL = 2,000 ms) values at the start of the last paced beat at fast rate (CL = 300 ms), eliminating the available reserve accumulation at fast rate. Under these conditions, the percent APD90 shortening at fast rate was only 4% for dog, compared with 26% in the presence of IKs available reserve accumulation. For human, it was 21%, compared with 27%. We conclude that β-adrenergic stimulation affects mechanisms of APD rate dependence in a species-dependent fashion. In human, PLM phosphorylation and IKs available reserve accumulation are equally important factors, while in dog, IKs accumulation in available reserve states is more important.
Transmural cell types.
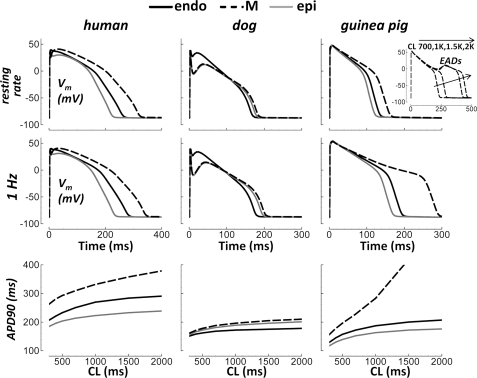
Species dependence of APs and rate dependence of APD are shown for different transmural cell types in Fig. 6. Simulations show that transmural dispersion (difference between maximum APD90 and minimum APD90) is least for dog and greatest for guinea pig, especially at slow pacing rates. In dog, the absence of large Ito in endocardium cells shortened APD relative to Epi, as seen experimentally (19). This was not true in the other species, where Epi APD was shortest. In dog, the Ito influence is not direct; rather it is caused by an indirect effect on the voltage dependence of ICaL during its plateau phase (7, 15). The ever increasing M-cell APD in guinea pig at slow rates was due to loss of IKs accumulation associated with slow pacing (29), on top of reduced conductance (expression) of IKs in M cells. In fact, early afterdepolarizations (EADs) developed at slow pacing rates in guinea pig M cells (Fig. 4, inset); note that these rates are much slower than the physiological range for guinea pig.
Fig. 6.
Species dependence of transmural cell types. Columns show simulation results for human, dog, and guinea pig from left to right. Top and middle rows: results for physiological (species specific) resting heart rate and 1 Hz, respectively. Right inset: guinea pig M cell APs at slow rates (arrows indicate CL), which demonstrate early afterdepolarizations (EADs). Bottom row: APD rate dependence (APD90 vs. CL). Endocardium (Endo), mid-myocardium (M), and epicardium (Epi) results are solid black, dashed black, and gray, respectively.
EADs.
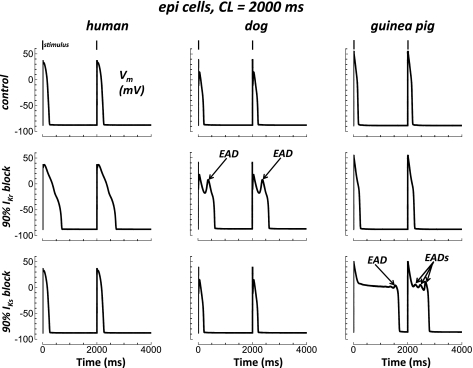
EADs may form with slow pacing (CL = 2,000 ms) and block of repolarizing K+ currents [e.g., experiments (2, 13)]. In Fig. 7, simulations under these conditions caused EAD formation with 90% IKr block in dog but not human or guinea pig. EADs developed in guinea pig with 90% IKs block. However, the same degree of IKs block did not generate EADs in human or dog. These results demonstrate that the development of EADs is species dependent.
Fig. 7.
Species dependence of EADs. Epi cells were paced at CL = 2,000 ms (final 2 beats shown). Columns show results for human, dog, and guinea pig from left to right. Rows show control, 90% IKr block, and 90% IKs block from top to bottom. Stimuli are indicated along the top. EADs are labeled with arrows.
DISCUSSION
Different mammalian species show different mechanisms of response to pacing and channel block. Quantitative species comparison revealed the following: 1) IKr reduction prolonged human AP substantially more than APs of dog and guinea pig. 2) Under basal conditions (absence of β-adrenergic stimulation), IKs reduction had almost no effect on human and dog APs, but it severely prolonged the guinea pig AP. 3) In the presence of β-adrenergic stimulation, IKs reduction affected all species to substantial degree. 4) Rate-dependent INaK changes play a major role in APD rate dependence in human and dog. The role of INaK is secondary to [Na+] accumulation at fast rates. 5) For guinea pig, IKs dominates APD rate dependence via its open-state accumulation at fast rate, while in human and dog under β-adrenergic stimulation, accumulation of IKs in available reserve closed states is important for proper AP repolarization and APD shortening at fast rate. 6) EAD formation due to delayed rectifier K+ current block at slow pacing rates is species dependent.
We did not include mouse or rat APs in this comparison, although there are models available that reproduce a wide array of physiological and drug block effects in these species [e.g., mouse(4) and rat (22)]. This choice was based on the fact that AP morphology, physiological heart rate, and rate dependence in these smaller mammalian species are set apart due to lack of plateau phase and a different cellular ion-channel profile. However, mouse and rat are comparable to the other species with respect to whole organ and physiome related investigations (e.g., conduction velocity and its restitution), which we cannot address here. Also not addressed is the important issue of differences in arrhythmic reentry across species. The ratios of AP wavelength to heart substrate area are widely different among species, affecting arrhythmia formation and sustenance.
The finding that APD shortening at fast rates was caused primarily by INaK increase secondary to intracellular [Na+] accumulation in human and in dog, but not guinea pig, has been reported previously for human (12, 21), dog (7), and guinea pig (8) using other protocols. Modeling studies are not always in agreement. For example, the Iyer-Winslow human ventricular model places a greater emphasis on the role of INaCa in rate dependence of APD (16). The ten Tusscher-Panfilov human ventricular model (33) [and updated version (34)] emphasizes IKs (under basal conditions). The direct and quantitative comparison between species presented here yields novel insights.
We found that in human β-adrenergic stimulation elevated the role of IKs available reserve accumulation in determining APD rate dependence to match that of PKA effects on INaK via PLM phosphorylation. By contrast, in dog with β-adrenergic stimulation, IKs accumulation in available reserve states was more important than PLM phosphorylation. This subtle, but significant, finding has not been shown previously. Such a result would be difficult to measure experimentally because of practical methodological limitations. One possible implication of this finding is that in human heart failure, depressed QT rate dependence observed in vivo (6) may be caused by reduced β-adrenergic capacity (11) and a consequent failure to remove PLM inhibition of INaK, coupled with weak IKs accumulation in available reserve. Importantly, the PLM effect on APD may be human specific.
AP morphology and its rate dependence are determined by a time-dependent balance between inward and outward currents. For guinea pig, rate-dependent changes in INaK secondary to [Na+] changes were large, indeed larger than for human or dog. However, as Faber and Rudy showed (8), there are even larger rate-dependent changes in guinea pig IKs. In contrast to dog and human, this makes INaK only a minor participant in rate dependence of the guinea pig AP (8).
Human and dog repolarization is due mainly to IKr, which is not rate dependent (17). The same is true for rabbit in both experiments and in simulations using the Shannon-Bers rabbit model (Fig. 1A). In these species, rate dependent IKs is small relative to guinea pig without β-adrenergic stimulation (Fig. 1B). Interestingly, rate-dependent increase of IKs at fast rates employs different mechanisms in human and dog compared with guinea pig. In guinea pig, there is open state accumulation that increases (the already large) current from the start of the AP. In human and dog, accumulation occurs in available reserve closed states from which transitions to the open state cause a gradual increase in current during the AP, reaching a peak during phase 3 repolarization, when even a small current can shift the delicate balance of currents in the repolarization direction and influence repolarization effectively. As shown by Silva and Rudy (29), with this strategy that conserves current during the early AP to maximize it late, IKs can provide the necessary repolarization reserve to compensate for reduction in IKr due to mutations or drugs. Importantly, IKs is regulated by the β-adrenergic pathway and plays a critical role in proper repolarization during exercise and emotional stress (20, 36). Mechanistically, β-adrenergic stimulation increases IKs by augmenting the available reserve (14).
IKr and IKs are critical for AP repolarization in the ventricle. Mutations that reduce these currents cause the majority of cases of inherited long QT syndrome (27), leading to lethal arrhythmia. Acquired long QT syndrome, caused by block of IKr by any of a variety of pharmacological agents [including clinically useful noncardiac drugs terfenadine, fexofenadine, risperidone, sertindole, erythromycin, and cisapride (5)], can also lead to fatal arrhythmias (25). The dangers of proarrhythmic effect and promiscuity of IKr block are so great that the Food and Drug Administration requires IKr block testing to prove drug safety. However, these safety experiments are generally not performed in human myocytes. Inherited IKs loss is the single most common cause of long QT syndrome (27). Delayed rectifier K+ current differences between species exist. Moreover, the role played by IKr and IKs is species dependent. Simulations predict that arrhythmogenic EAD formation with block of these currents was not uniform across species. It is in this context that these issues need to be addressed quantitatively and caution used when extrapolating results of drug effects tested in nonhuman species to safety and efficacy in human clinical application.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-049054-19 and R01-HL-R01033343-27 (to Y. Rudy) and American Heart Association Predoctoral Fellowship 0815539G (to T. J. O'Hara). Y. Rudy is the Fred Saigh Distinguished Professor at Washington University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.O. and Y.R. conception and design of research; T.O. performed experiments; T.O. analyzed data; T.O. and Y.R. interpreted results of experiments; T.O. prepared figures; T.O. drafted manuscript; T.O. and Y.R. edited and revised manuscript; T.O. and Y.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the members of the laboratory of Y. Rudy for helpful discussion.
REFERENCES
- 1. Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V, 3rd, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol 286: H602–H609, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110: 904–910, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blechschmidt S, Haufe V, Benndorf K, Zimmer T. Voltage-gated Na+ channel transcript patterns in the mammalian heart are species-dependent. Prog Biophys Mol Biol 98: 309–318, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bondarenko VE, Rasmusson RL. Transmural heterogeneity of repolarization and Ca2+ handling in a model of mouse ventricular tissue. Am J Physiol Heart Circ Physiol 299: H454–H469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown AM. Drugs, hERG and sudden death. Cell Calcium 35: 543–547, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Davey PP, Barlow C, Hart G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin Sci (Lond) 98: 603–610, 2000 [PubMed] [Google Scholar]
- 7. Decker KF, Heijman J, Silva JR, Hund TJ, Rudy Y. Properties and ionic mechanisms of action potential adaptation, restitution, and accommodation in canine epicardium. Am J Physiol Heart Circ Physiol 296: H1017–H1026, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J 78: 2392–2404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faber GM, Rudy Y. Calsequestrin mutation and catecholaminergic polymorphic ventricular tachycardia: a simulation study of cellular mechanism. Cardiovasc Res 75: 79–88, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faber GM, Silva J, Livshitz L, Rudy Y. Kinetic properties of the cardiac L-type Ca2+ channel and its role in myocyte electrophysiology: a theoretical investigation. Biophys J 92: 1522–1543, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: beta-adrenergic receptors–alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med 2: 475–483, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. J Mol Cell Cardiol 48: 112–121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo D, Liu Q, Liu T, Elliott G, Gingras M, Kowey PR, Yan GX. Electrophysiological properties of HBI-3000: a new antiarrhythmic agent with multiple-channel blocking properties in human ventricular myocytes. J Cardiovasc Pharmacol 57: 79–85, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Heijman J, Volders PG, Westra RL, Rudy Y. Local control of beta-adrenergic stimulation: effects on ventricular myocyte electrophysiology and Ca(2+)-transient. J Mol Cell Cardiol 50: 863–871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation 110: 3168–3174, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophys J 87: 1507–1525, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jost N, Acsai K, Horvath B, Banyasz T, Baczko I, Bitay M, Bogats G, Nanasi PP. Contribution of I Kr and I K1 to ventricular repolarization in canine and human myocytes: is there any influence of action potential duration? Basic Res Cardiol 104: 33–41, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Jost N, Virag L, Belati B, Lengyel C, Nemeth M, Bitay M, Bogats G, Varro A, Papp JG. A kesoi egyeniranyito kaliumaram gyors (IKr) es lassu komponensenek (IKs) osszehasonlito vizsgalata egeszseges emberi, kutya, nyul es tengerimalac kamrai szivizomsejteken. Cardiologia Hungarica 34: 103–113, 2004 [Google Scholar]
- 19. Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res 72: 671–687, 1993 [DOI] [PubMed] [Google Scholar]
- 20. O'Hara T, Rudy Y. Arrhythmia formation in subclinical (“silent”) long QT syndrome requires multiple insults: quantitative mechanistic atudy using the KCNQ1 mutation Q357R as example. Heart Rhythm 2011. September 25 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol 7: e1002061, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pandit SV, Clark RB, Giles WR, Demir SS. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys J 81: 3029–3051, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pieske B, Maier LS, Piacentino V, 3rd, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation 106: 447–453, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Rocchetti M, Besana A, Gurrola GB, Possani LD, Zaza A. Rate dependency of delayed rectifier currents during the guinea-pig ventricular action potential. J Physiol 534: 721–732, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Rudy Y, Silva JR. Computational biology in the study of cardiac ion channels and cell electrophysiology. Q Rev Biophys 39: 57–116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, Mosca F, Nespoli L, Rimini A, Rosati E, Salice P, Spazzolini C. Prevalence of the congenital long-QT syndrome. Circulation 120: 1761–1767, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J 87: 3351–3371, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silva J, Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation 112: 1384–1391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silva JR, Pan H, Wu D, Nekouzadeh A, Decker KF, Cui J, Baker NA, Sept D, Rudy Y. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc Natl Acad Sci USA 106: 11102–11106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stengl M, Volders PG, Thomsen MB, Spatjens RL, Sipido KR, Vos MA. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol 551: 777–786, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szabo G, Szentandrassy N, Biro T, Toth BI, Czifra G, Magyar J, Banyasz T, Varro A, Kovacs L, Nanasi PP. Asymmetrical distribution of ion channels in canine and human left-ventricular wall: epicardium vs. midmyocardium. Pflügers Arch 450: 307–316, 2005 [DOI] [PubMed] [Google Scholar]
- 33. ten Tusscher KH, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol 286: H1573–H1589, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Ten Tusscher KH, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol 291: H1088–H1100, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: a simulation study. Circulation 99: 2466–2474, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, Beekman JD, Sipido KR, Vos MA. Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation 107: 2753–2760, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Wang ZG, Pelletier LC, Talajic M, Nattel S. Effects of flecainide and quinidine on human atrial action potentials. Role of rate-dependence and comparison with guinea pig, rabbit, and dog tissues. Circulation 82: 274–283, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Zicha S, Moss I, Allen B, Varro A, Papp J, Dumaine R, Antzelevich C, Nattel S. Molecular basis of species-specific expression of repolarizing K+ currents in the heart. Am J Physiol Heart Circ Physiol 285: H1641–H1649, 2003 [DOI] [PubMed] [Google Scholar]