Abstract
Pharmacological treatment of atrial fibrillation (AF) exhibits limited efficacy. Further developments require a comprehensive characterization of ionic modulators of electrophysiology in human atria. Our aim is to systematically investigate the relative importance of ionic properties in modulating excitability, refractoriness, and rotor dynamics in human atria before and after AF-related electrical remodeling (AFER). Computer simulations of single cell and tissue atrial electrophysiology were conducted using two human atrial action potential (AP) models. Changes in AP, refractory period (RP), conduction velocity (CV), and rotor dynamics caused by alterations in key properties of all atrial ionic currents were characterized before and after AFER. Results show that the investigated human atrial electrophysiological properties are primarily modulated by maximal value of Na+/K+ pump current (GNaK) as well as conductances of inward rectifier potassium current (GK1) and fast inward sodium current (GNa). GNaK plays a fundamental role through both electrogenic and homeostatic modulation of AP duration (APD), APD restitution, RP, and reentrant dominant frequency (DF). GK1 controls DF through modulation of AP, APD restitution, RP, and CV. GNa is key in determining DF through alteration of CV and RP, particularly in AFER. Changes in ionic currents have qualitatively similar effects in control and AFER, but effects are smaller in AFER. The systematic analysis conducted in this study unravels the important role of the Na+/K+ pump current in determining human atrial electrophysiology.
Keywords: atrium, action potential, reentry, antiarrhythmic agents, in silico high-throughput screening
atrial fibrillation (AF) is the most commonly diagnosed cardiac arrhythmia, with a 0.4–1% incidence in the overall population and over 8% incidence in patients over 80 years old (63). AF patients have severe prognosis of high incidence of stroke and mortality (63). AF is considered to be a reentrant arrhythmia sustained by mechanisms including both rapid ectopic activity, usually generated in pulmonary veins, reentrant circuits, and structural and/or focal changes (31, 36). Establishment and stability of reentrant circuits are favored by short refractory periods and slow conduction, enhanced by electrical remodeling [AF-related electrical remodeling (AFER)] caused by persistent AF (68).
Pharmacological management is still the main option for AF treatment (35), and it is applied by targeting modulation of excitability and refractoriness for rate or rhythm control (61). Anti-arrhythmic Class I and Class III drugs are used to decrease excitability (by inhibiting the sodium current) and prolong the refractory period (by blocking potassium currents), respectively. Multichannel action compounds such as amiodarone and dronedarone are commonly used but they exhibit limited efficacy and numerous adverse side effects (52). Therefore, major breakthroughs are urgently needed for a more effective control of AF (2, 25, 41, 52, 60), which would benefit from a systems approach to drug development (56).
A large number of studies have provided insights into the ionic basis of atrial electrophysiology (12, 13, 18, 50, 60, 65, 66). Research has focused primarily on the role of either sodium or potassium channel block in modulating atrial electrophysiology (8, 48) and has often been performed in different animal species, usually either before or after AFER (68, 74). Importantly, new agents such as vernakalant and dronedarone, and the commonly used amiodarone, all exhibit multichannel effects on a variety of sodium, potassium, and calcium currents (21), and even Na+/K+ pump activity (26, 30). However, very little is known about the relative importance of drug action on each current in modulating AF-related electrophysiology in humans.
The goal of our study is to provide a systematic characterization of the relative importance of transmembrane ionic currents in modulating key electrophysiological properties and reentrant activity in human atrial tissue. The ultimate aim is to aid in the identification of novel avenues for a more effective management of patients with AF and to aid in the interpretation of existing findings. A comprehensive in silico investigation is performed under normal and AFER atrial conditions using human atrial tissue models in an attempt to provide insight into differences in the response of atrial tissue to pharmacological interventions in short-term versus long-term AF.
METHODS
Membrane kinetics.
Human atrial electrophysiology was simulated using a recent human atrial action potential (AP) model proposed by Maleckar et al. (42) based on the original Nygren et al. model (49), and will be referred to as the MGGT model. A second AP model, developed by Courtemanche et al. (17) and referred to in the text as the CRN model, was also used to confirm model-independence of the main results obtained in the present study. Both models include formulations for the main transmembrane ionic currents in human atrial electrophysiology and detailed intracellular calcium dynamics, as well as homeostasis of the main ionic species involved in the course of the human atrial AP.
AFER was simulated by altering ionic properties as in previous studies (18, 50, 65, 66): 70% reduction in L-type calcium current conductance (GCaL); 50% reduction in transient outward current conductance (Gto); 50% reduction in ultrarapid delayed potassium current conductance (GKur); and 100% increment in inward rectifier potassium current conductance (GK1).
Single cell simulations.
Simulations using both models were conducted for 20 min of stimulation at a cycle length (CL) of 1,000 ms (74), for default control and AFER conditions, and following alterations in conductances and gate time constants of ionic currents (between −100% and +300%). The conductances altered included maximal value of the Na+/K+ pump current (GNaK), GK1, GKur, GCaL, maximal value of the Na+/Ca2+ exchanger current (GNaCa), Gto, fast inward sodium current conductance (GNa), rapid delayed rectifier potassium conductance (GKr), and slow delayed rectifier potassium current (GKs). Ionic current kinetics of INa, ICaL, Ito, IKur, IKr, and IKs were also varied in the simulations, with parameters considered including activation and inactivation time constants of all gating variables. AP duration (APD), measured at 90% repolarization (APD90), and resting membrane potential (RMP) were calculated.
Two APD restitution protocols were applied to determine the S1S2 and the dynamic restitution curves. The S1S2 restitution protocol consisted of applying 10 S1 stimuli at CL = 1,000 ms followed by a S2 extra-stimulus at varying diastolic intervals (DIs). The dynamic restitution protocol consisted of applying trains of 100 stimuli at decreasing CL from 3,000 ms to 150 ms. APD90 following the last stimulus of each protocol was plotted versus the previous DI to obtain the corresponding restitution curve. The maximal values of the slopes (Ss1s2 and Sdyn) of the S1S2 and dynamic APD90 restitution curves were computed, since they have been proposed previously as arrhythmic risk biomarkers related to rotor stability (45, 74). In all single cell simulations, stimuli were of 2-ms duration and twice diastolic threshold amplitude (threshold is 750 pA for MGGT model, and 1,000 pA for CRN model, for control conditions).
Tissue simulations.
Tissue simulations were conducted to characterize the main determinants of refractory period (RP), conduction velocity (CV), and rotor dynamics in human atrial tissue in control and AFER conditions. Electrical propagation through atrial tissue was simulated using the monodomain equation with isotropic diffusion coefficient of 1.3 cm2/s in a homogeneous tissue, similar to those used in previous studies (13, 73). Stimuli were of 2-ms duration and twice diastolic threshold amplitude (threshold is 0.025 pA/μm3, for control conditions with both the MGGT and the CRN models).
RP and CV obtained from tissue simulations were evaluated in both control and AFER for default conditions (i.e., original model parameters) and at different timings following the alteration of ionic currents properties (i.e., 1 s and 1, 3, and 5 min). In this study, a S1S2 protocol was chosen to determine the RP as in previous studies (57, 68). Thus RP was determined by applying a train of S1 stimuli (CL = 1,000 ms) at the center of a 1 × 1 cm2 tissue followed by an extra-stimulus (S2) at the same location for varying coupling intervals (CIs) (step of 1 ms). RP was defined as the shortest CI that ensured propagation following S2 (15, 57, 68). The tissue size in the simulations was large enough to avoid boundary effects on RP measurements, since these were only noticeable in a strip of 0.25 cm close to the domain boundaries (14).
Additional simulations were conducted to determine ionic modulators of CV. As in Refs. 13, 14, and 24, CV was measured in one-dimensional simulations following a train of S1 stimuli (CL = 1,000 ms) at one side of a 1-cm long fiber. CV was calculated as the ratio between the distance separating two distant nodes (separated 0.1 cm) located at the center of the fiber and the time taken by the wavefront to propagate between the two nodes following the last applied stimulus (13, 14, 24, 68). This approach avoids measuring CV at locations that are too close to the domain boundaries, as well as to avoid further difficulties derived from front curvature (37). No significant differences in CV were observed using longer fibers stimulated at CL = 1,000 ms. Ionic modulators of CV were investigated at CL = 1,000 ms, and therefore the magnitudes of CV reported throughout the present study represent CV close to maximal values.
Reentrant activity was initiated in two-dimensional 5 × 5 cm2 atrial tissue by applying a cross-field stimulation protocol (27, 69). As in previous studies (22, 34, 50), the choice of the two-dimensional tissue geometry avoids confounding interpretation of the ionic mechanisms with added complexities such as structural and electrophysiological heterogeneities. A planar wavefront was initiated by a stimulus applied at the lower tissue edge, followed by another stimulus applied on a square area in the tissue bottom-left corner at a CI within the vulnerable window. Simulations were conducted using the MGGT model to assess changes in reentrant activity occurring immediately and 5 min after each ionic alteration in control and AFER. Similar simulations were not conducted using the CRN model due to the instability of the rotors in control conditions, as stated in previous studies (13). Tissue simulations were initialized using the steady-state values obtained in single cell simulations for default control and AFER conditions and following 5 min of application of the corresponding ionic current alteration. Once established, rotors remained stable for all conditions simulated using the MGGT model. Corresponding pseudo-ECGs were calculated as in Refs. 4, 28, and 53, and dominant frequency (DF) values were obtained from the pseudo-ECG power spectral density with a precision of 0.1 Hz. The trajectory of phase singularities (PS), defined as wavetips where all AP phases intersect, was calculated using the zero-normal-velocity condition (24).
Computational tools and numerical methods.
In total, 6,436 single cell and tissue simulations were run in this study. Briefly, the open source software Chaste (www.cs.ox.ac.uk/chaste) was used on grid computing facilities through use of the middleware platform Nimrod/G, accessible via web interface (1). This allowed the simultaneous launch of a large number of simulations considering different parameters sets. The monodomain equation was solved in double precision using a Galerkin Finite Element Method with linear basis functions, as described in detail by Pathmanathan et al. (51). The mesh resolution for one- and two-dimensional simulations was fixed at 250 μm, time steps were of 0.02 ms for the partial differential equation, and 0.02 ms for the coupled cell systems. To prove convergence of the numerical methods, simulations were run for mesh spatial resolutions varying from 1,000 to 100 μm, concluding that resolutions finer than 300 μm were convergent to stable RP, CV, and DF values. Single cell simulations were conducted using the forward Euler method with time step of 0.02 ms.
RESULTS
Electrophysiological properties in control and AFER.
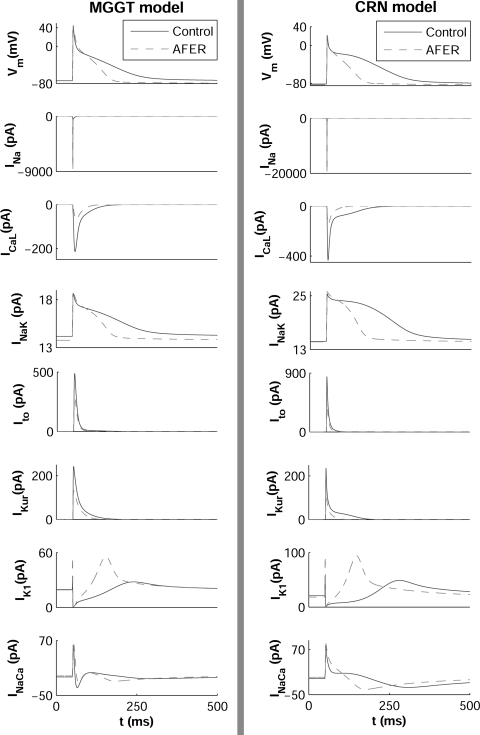
Figure 1 shows the time course of the steady-state AP and the main underlying ionic currents in control and AFER atrial cardiomyocytes, using the MGGT model (left) and the CRN model (right). In both models, AFER results in shortening of APD90 and slight RMP hyperpolarization, consistent with previous experimental and theoretical human atrial studies (6, 12, 50). Steady-state APD90 shortens from 200 ms in control to 115.8 ms in AFER in simulations using the MGGT model and from 252 ms to 115.5 ms using the CRN model.
Fig. 1.
Steady-state action potential (AP) and ionic currents under control conditions (solid line) and atrial fibrillation-related electrical remodeling (AFER) conditions (dashed line) at cycle length (CL) = 1,000 ms using a human atrial AP model proposed by Maleckar et al. (42) based on the original Nygren et al. model (49) (referred to as the MGGT model; left) and a second AP model developed by Courtemanche et al. (17) (referred to as the CRN model; right) human atrial AP models. INaK, Na+/K+ pump current; IK1, inward rectifier potassium current; INa, Na+ current; IKur, ultrarapid delayed potassium current; Ito, transient outward current; ICaL, L-type calcium current; INaCa, Na+/Ca2+ exchanger current.
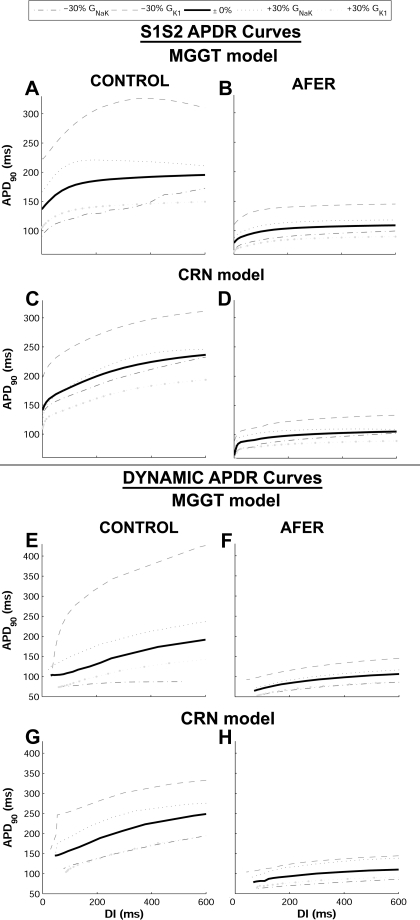
Differences in APD90 restitution properties between control and AFER were also characterized using both AP models (Fig. 2). AFER results in flattening of the maximum slope in both S1S2 and dynamic restitution curves with the MGGT model (Ss1s2 is 0.63 in control and 0.31 in AFER, whereas Sdyn is 0.37 in control and 0.23 in AFER). With the CRN model, the maximum slope of the dynamic restitution slope also flattens due to AFER, but the S1S2 restitution curve is steeper in AFER than in control (Ss1s2 is 0.44 in control and 1.3 in AFER, whereas Sdyn is 0.28 in control and 0.16 in AFER). AFER-induced flattening of the restitution curves is consistent with previous studies showing that patients with persistent AF exhibited shallower restitution slopes than those with paroxysmal AF (45). Tissue simulations also showed that AFER results in alterations in RP and CV, with qualitatively similar results in both models. AFER-induced changes in APD90 and RMP result in decrease of RP, in agreement with Ref. 70: RP decreases from 217 ms in control to 147 ms in AFER with the MGGT model and from 270 ms in control to 124 ms in AFER with the CRN model, in agreement with clinical studies on chronic AF patients (72). In contrast, AFER-induced changes in ionic currents lead to only a slight decrease in steady-state CV from 47.97 cm/s in control to 46.17 cm/s in AFER, using the MGGT model, and from 58.14 cm/s in control to 56.38 cm/s using the CRN model. This decrease in steady-state CV in AFER is in agreement with results in clinical studies (44, 47) and quantitatively consistent with those reported in previous studies of AF (13).
Fig. 2.
AP duration (APD) restitution (APDR) curves obtained using the S1S2 (top, A–D) and dynamic (bottom, E–H) protocols, in control (left) and AFER conditions (right) with the MGGT and the CRN models for default values and following ±30% alterations in Na+/K+ pump current conductance (GNaK) and conductance of inward rectifier potassium current (GK1).
Relative importance of ionic currents.
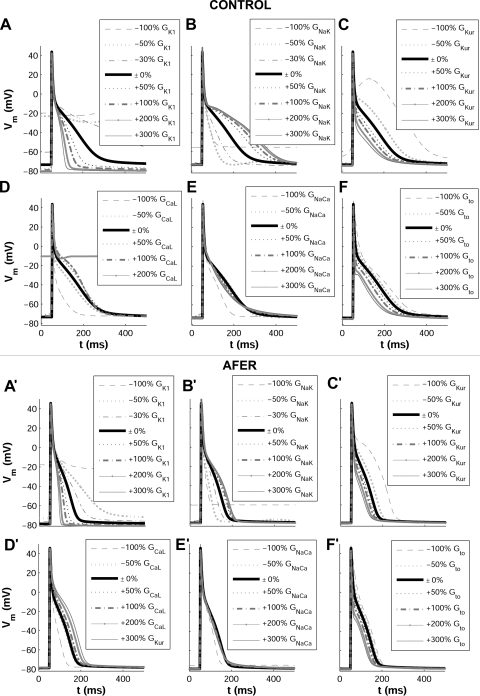
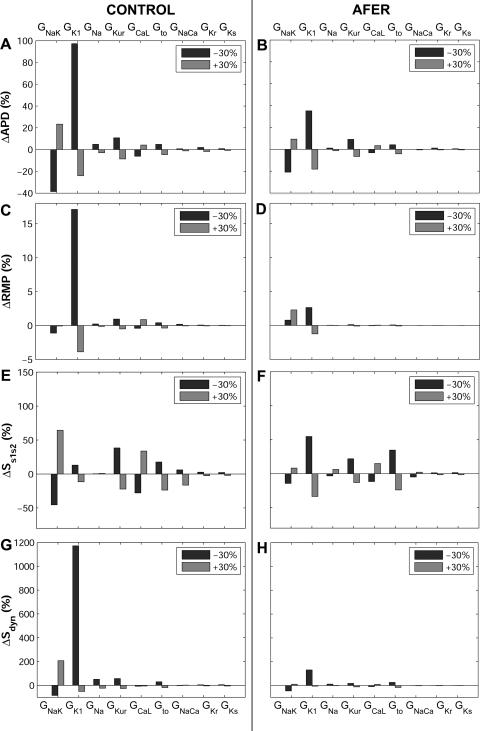
Computer simulations were conducted as described in methods to investigate the relative importance of ionic current conductances and kinetics in modulating human atrial electrophysiology in control and AFER. Figure 3 shows changes in steady-state AP induced by alterations in ionic conductances for control (left) and AFER (right) using the MGGT model. Alterations in time constants were also evaluated but results showed only small effects, i.e., <10%, in the investigated human atrial electrophysiological properties (APD90, RMP, Ss1s2, Sdyn, RP, CV) both in control and AFER. In Fig. 4, changes in steady-state APD90, RMP, Ss1s2, and Sdyn with respect to the original control (left) and AFER (right) models, i.e., default values for all parameters in both models, are further quantified using the MGGT model. Only the simulation results exhibiting the largest changes are included in the figure. Similar simulation results are depicted in Fig. 5 for the CRN model, to support the model independence of the main results of the present study.
Fig. 3.
Steady-state human atrial AP in control (top) and AFER (bottom) for default and following alterations in A and A': GK1; B and B': GNaK; C and C': ultrarapid delayed potassium current conductance (GKur); D and D': L-type calcium current conductance (GCaL); E and E': Na+/Ca2+ exchanger current conductance (GNaCa); F and F': transient outward current conductance (Gto), using the MGGT model.
Fig. 4.
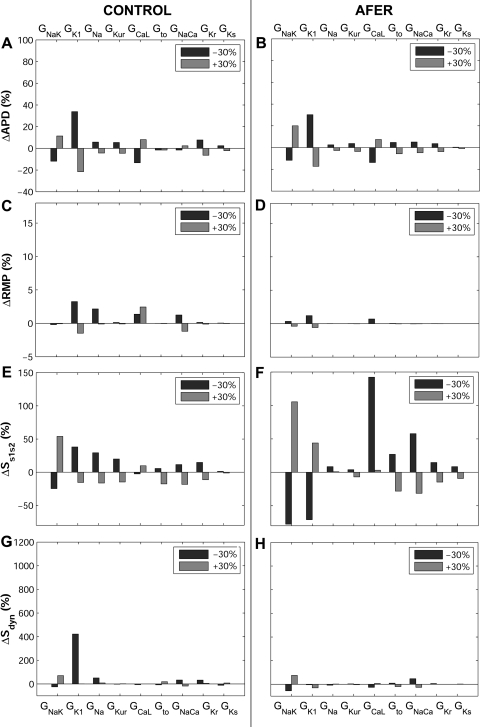
Percent changes in steady-state action potential duration at 90% repolarization (ΔAPD90; A and B), resting membrane potential (ΔRMP; C and D), Ss1s2 (ΔSs1s2; E and F), and Sdyn (ΔSdyn; G and H) with respect to default conditions produced by ±30% alterations in ionic conductances for control (left) and AFER (right) using the MGGT model.
Fig. 5.
Percent changes in steady-state APD90 (ΔAPD90; A and B), RMP (ΔRMP; C and D), and Ss1s2 (ΔSs1s2; E and F) and Sdyn (ΔSdyn; G and H) (maximal values of the slopes of the S1S2 and dynamic APD90) with respect to default conditions produced by ±30% alterations in ionic conductances for control (left) and AFER (right) with the CRN model.
Both in control and AFER, alterations in GK1 and GNaK result in the largest AP changes (Figs. 3 and 4 for the MGGT model and Fig. 5 for the CRN model). GK1 inhibition results in significant APD90 prolongation and RMP depolarization (Fig. 3A and 3A' and Fig. 4, A–D, for the MGGT model; Fig. 5, A–D, for the CRN model). This is caused by decrease in outward current and is in agreement with previous studies (20, 38). Interestingly, GNaK inhibition results in APD90 shortening and RMP elevation (Fig. 3B and 3B' and Fig. 4, A–D, for the MGGT model; Fig. 5, A–D, for the CRN model), in agreement with experiments conducted in atrial tissue from patients treated with digoxin (55). As shown in Fig. 4, A–D, for the MGGT model and Fig. 5, A–D, for the CRN model, the relative relevance of other conductances, such as GNa, GKur, GCaL and Gto, on steady-state APD90 is considerably lower than that of GK1 and GNaK, both in control and AFER [with the exception of the effect of GCaL in AFER using the CRN model, which is possibly explained by the larger magnitude of ICaL in this model (Fig. 1)]. Simulations were repeated at a shorter CL value (CL = 500 ms) with the two models. Results confirm that GNaK and GK1 are the properties exerting the maximum influence in modulating APD90 and RMP in human atrial cells for both stimulation rates.
Whereas, in most cases, ionic alterations result in similar effects in control and AFER, some differences are identified. First, changes caused by ionic alterations are overall smaller in AFER than in control (Figs. 4 and 5, using the MGGT and the CRN model, respectively). Second, the effect of a 30% inhibition of GNaK implies opposite changes in RMP in control (slight hyperpolarization) and AFER (depolarization; Figs. 4 and 5, C and D). Larger degrees of GNaK inhibition (>50%), however, lead to RMP depolarization both in control and AFER (Fig. 3B and 3B'). GNaK upregulation results in insignificant changes in RMP in control, but in larger RMP depolarization in AFER. Simulations using the two human atrial AP models were also conducted to investigate the ionic mechanisms modulating S1S2 and dynamic APD restitution properties (Fig. 2; Fig. 4, E–H, for the MGGT model; and Fig. 5, E–H, for the CRN model). Simulations using the two models highlight GK1 and GNaK as the main modulators of APD restitution properties (Ss1s2 and Sdyn), in control and AFER. Results obtained with the two models were qualitatively similar but differences in the sensitivity of restitution properties to changes in some ionic currents were observed. First, the sensitivity of Sdyn to changes in GNaK and, particularly, GK1 (ΔSdyn) is important in both models, but larger in the MGGT than in the CRN model (Figs. 4G and 5G). Furthermore, Ss1s2 sensitivity to changes in ionic currents (ΔSs1s2) in AFER is significantly larger in simulations using the CRN than the MGGT model (Figs. 4F and 5F). Finally, GCaL inhibition results in a large increase in Ss1s2 with the CRN model, possibly explained by the larger magnitude of ICaL in this model, as mentioned above (Fig. 1).
Temporal adaptation of atrial electrophysiology to alterations in ionic currents.
Simulations shown in the previous section highlight the importance of GNaK and GK1 relative to other currents in modulating AP steady-state and restitution properties using two human atrial models. An analysis of the temporal evolution of electrophysiological properties following ionic current alterations shows that atrial electrophysiological properties do not adapt instantaneously. Both the time required to complete the adaptation and the temporal evolution of the analyzed properties strongly depend on the specific ionic current being altered. Figure 6 illustrates the temporal evolution of human atrial AP properties (APD90 and RMP) following ±30% changes in ionic currents in control (Fig. 6, A and C) and AFER (Fig. 6, B and D) using the MGGT model. The implications of the ionic changes in modulating the temporal evolution of RP and CV were also investigated, as shown in Fig. 6, E–H. In these simulations, alterations in GNaK, GK1, and GNa were considered, since GNaK and GK1 are identified as the main modulators of repolarization properties in our study and GNa is known to have an important effect on CV (33). Alterations in ionic conductances other than GNaK, GK1, and GNa lead to fast adaptation and small changes in the investigated properties and are therefore not shown.
Fig. 6.
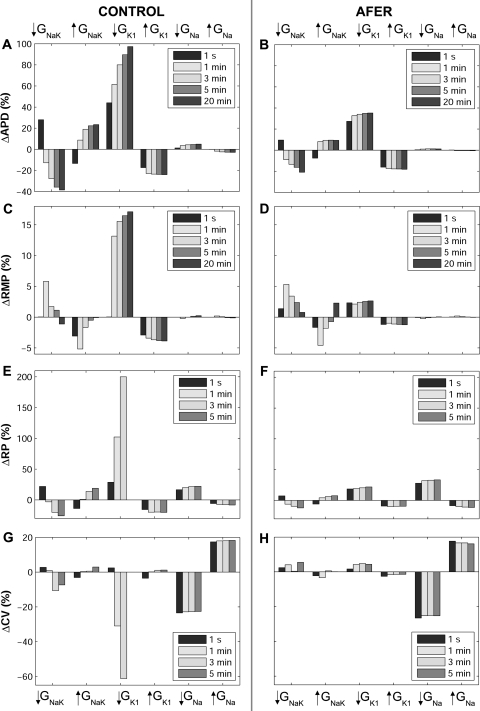
Temporal adaptation of percent changes in APD90 (ΔAPD90; A and B) and RMP (ΔRMP; C and D), for single cell simulations; RP (ΔRP; E and F), for two-dimensional tissue simulations; and CV (ΔCV; G and H), for one-dimensional fiber simulations, with respect to default conditions during ±30% alterations in GNaK, GK1, and GNa in control (left) and AFER (right) using the MGGT model. *Note: following 5 min of GK1 30% block repolarization fails.
As shown in Fig. 6, RP and CV values cannot be obtained following 5 min of IK1 blockade due to repolarization failure and subsequent propagation failure (Fig. 6, E and G). This is caused by the gradual accumulation of electrotonic effects associated to the propagation of the triangular atrial AP. In this setting, the electrotonic current results in a net inward current opposing the outward potassium currents during the repolarization phase of the AP, which eventually results in repolarization failure. This is observed using the two models, although with a smaller degree of IK1 blockade in the MGGT than in the CRN model, due to the smaller contribution of IK1 to repolarization reserve in the CRN model. RP and CV results computed with the CRN model are qualitatively similar to those obtained with the MGGT model, but the relative importance of GNa in modulating RP in AFER is slightly decreased (not shown).
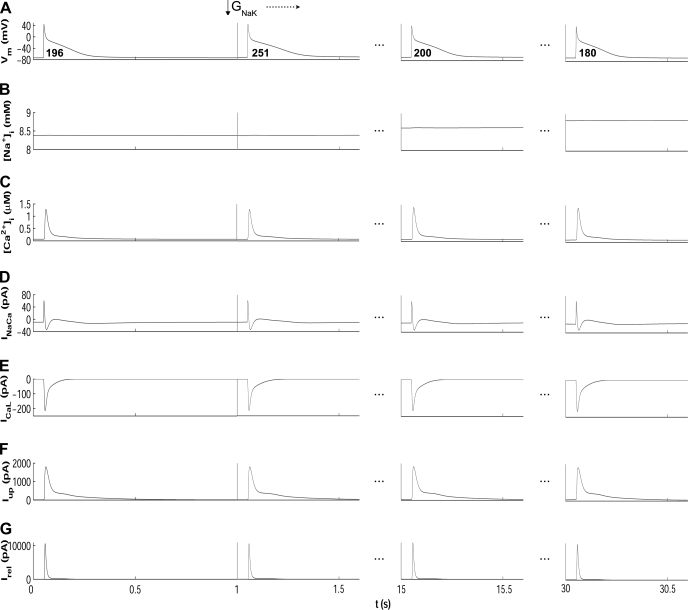
Results in Fig. 6 show that in most cases, alterations in ionic conductances result in monotonic changes in electrophysiological properties. Changes in GK1 and GNa lead to monophasic alterations in APD90, RP, and CV. However, as shown in Fig. 6, GNaK block results in biphasic changes in APD90 and RP, with an initial prolongation followed by a progressive decrease. Increase in GNaK leads to an initial decrease in APD90 and RP followed by a progressive increase on those properties. The biphasic effect of altering GNaK is also observed in RMP and CV (Fig. 6). This transient behavior following GNaK alterations also occurs for CL = 500 ms and using the CRN model (not shown). The APD90 and RP shortenings caused by decrease in GNaK can appear counterintuitive because inhibition of an outward current would be expected to prolong APD90 as stated in Ref. 10 and as shown shortly after administration of ouabain (71). However, in agreement with our results, strong blockade of Na+/K+ pump using strophantidin has been shown to result in initial APD90 lengthening followed by a progressive shortening (23, 29, 40). Figure 7 illustrates the ionic mechanisms involved in the biphasic AP changes induced by GNaK inhibition. GNaK block results in an initial APD90 lengthening caused by a decrease in the Na+/K+ pump outward current. However, sustained GNaK block results in intracellular Na+ (Fig. 7B) and, to a lesser extent, diastolic and systolic Ca2+ accumulation (Fig. 7C), which favors the outward component of the Na+/Ca2+ exchanger (Fig. 7D), slightly prolongs the open state of the calcium-dependent inactivation gating variable of ICaL (Fig. 7E), and thus results in APD90 shortening (Fig. 7A). Slight changes are therefore observed in ICaL current (Fig. 7E), whereas the amplitude of the currents between sarcoplasmic reticulum (SR) and intracellular space, such as the SR calcium uptake current (Iup; Fig. 7F) and the SR calcium release current (Irel; Fig. 7G), is slightly increased, entailing the slight Ca2+ accumulation shown in Fig. 7C (systolic intracellular Ca2+ increases 0.1 μM, whereas diastolic Ca2+ increases 0.03 μM).
Fig. 7.
Time course of AP (A), [Na+]i (B), [Ca2+]i (C), INaCa (D), ICaL (E), Iup (F), and Irel (G) for default conditions (left) and following 15 and 30 s after 30% GNaK block using the MGGT model. APD90 values are shown in bold for each AP.
Role of ionic currents in modulating reentrant activity.
The impact of the mechanisms unraveled above in the modulation of rotor dynamics in atrial tissue is investigated in this section, as explained in methods. Simulations show that DF increases from 3.52 Hz in control to 7.2 Hz in AFER, in agreement with previous clinical and theoretical studies (9, 32, 46, 50). Figure 8 describes changes in DF values and rotor dynamics with respect to default conditions obtained following 10 s and 5 min of alterations in GNaK, GK1, and GNa in both control (Fig. 8, A and C) and AFER (Fig. 8, B and D), using the MGGT model. Consistent with the results described in previous sections, simulations of rotor dynamics show that 1) GNaK, GK1, and GNa alterations are the interventions exerting the strongest influence on rotor dynamics; 2) GNaK, GK1, or GNa inhibitions have larger effects on reentrant dynamics than their corresponding upregulations; 3) alterations in the rest of ionic conductances entail negligible effects on DF; and 4) the main difference found between control and AFER is an enhanced relevance of GNa changes in AFER and a decreased relevance of GK1.
Fig. 8.
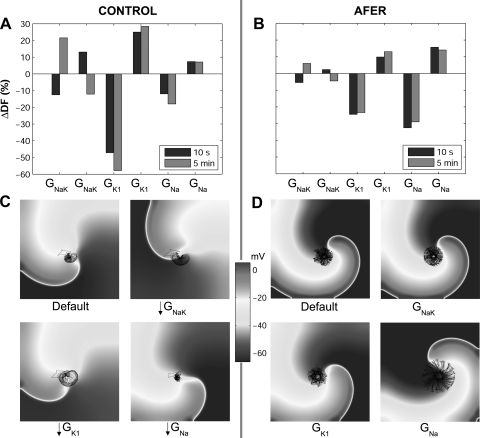
Percent changes in dominant frequency (ΔDF) with respect to default conditions produced by ±30% alterations in GNaK, GK1, and GNa in control (A) and AFER (B) using the MGGT model. DF values are shown for reentry initiated at 10 s (blue bars) and 5 min (red bars) following changes in ionic parameters. Snapshots of reentry 10 s after the change in ionic parameters under default conditions and 30% block of GNaK, GK1, and GNa in control (C) and AFER (D). Black lines on snapshots represent the corresponding phase singularities (PS) trajectories.
Simulations show that GNaK, GK1, or GNa block leads to decrease in DF values in both control and AFER immediately after the block. In the case of GK1 and GNa block, the change in DF is monophasic, resulting in lower values following 5 min of block. The most significant changes occur in two cases. First, GK1 block in control leads to an immediate decrease in DF with respect to default conditions (Fig. 8A). Second, GNa block in AFER results in a decrease in DF (Fig. 8B). These effects are explained by the relative importance of GK1 and GNa in modulating RP and CV in control and AFER, respectively, as highlighted in Fig. 6, E–H. As expected from the results shown in the previous section, alterations in GNaK result in biphasic temporal changes in DF. Decrease in GNaK initially decreases DF, but then leads to an increase following 5 min block. Increase in GNaK has the opposite effect.
Figure 8, C and D, further illustrates alterations in reentrant dynamics following GNaK, GK1, and GNa inhibition. The surface area traced out by the PS over its trajectory under default control conditions is 0.36 cm2. Figure 8C shows enlargement of the PS meandering in control after 30% block of GNaK or GK1, whereas 30% GNa block leads to a slightly smaller area of PS trajectory. In AFER, the PS meandering surface is increased to 1.02 cm2, as shown in Fig. 8D, in good agreement with a previous study (66). The PS surface is only significantly altered when GNa is blocked, leading to a threefold increase in PS trajectory surface (3.01 cm2), as compared with control. This is consistent with the higher sensitivity of RP and CV to GNa changes found in AFER and in good concordance with previous studies (15).
DISCUSSION
A systematic investigation was conducted to identify the relative importance of ionic current conductances and kinetics in modulating refractoriness, CV, and rotor dynamics in human atrial tissue in the presence and absence of AFER. Throughout the present study, simulation results are compared with previous experimental and theoretical studies to reconstruct the complex mosaic of the ionic basis of AF-related mechanisms in the human in a systematic framework. Simulations using two human atrial AP models were conducted to support the model independence of the main findings of the study. The simulation results obtained with the two models were overall qualitatively similar, but quantitative differences were observed, which could possibly be due to variability in human atrial electrophysiological measurements or differences in model construction. Our analysis aids in the interpretation of existing controversies in our understanding of the ionic modulators of AF and helps in the identification of novel aspects of human atrial electrophysiology. Importantly, our results identify GNaK as a key modulator of human atrial steady-state and restitution properties as well as rotor dynamics and confirm the importance of GK1 and GNa in human atrial electrophysiology in both control and AFER. Other current properties including GKur and GCaL play only a secondary role, which is in most cases negligible under the conditions investigated. Importantly, the ionic basis of human atrial dynamics are similar in control and AFER, but the sensitivity of electrophysiological properties to changes in ionic currents is in most cases smaller in AFER than in control. This could explain, at least in part, the lower efficacy of pharmacological treatment in patients with long-term versus short-term AF. Furthermore, our results reveal the great importance of GK1 in control and GNa in AFER in modulating rotor dynamics.
Role of INaK.
Our simulation results highlight the importance of GNaK as an essential determinant of human atrial electrophysiology, both in control and AFER. Alterations in GNaK result in important changes in APD90, APD restitution, and RP, and therefore in DF. Our results also show biphasic adaptation of APD90, RP, and DF after changes in GNaK. These findings are supported by previous data showing that the pump current is a strong modulator of APD90 and RMP in patients with and without chronic AF (55, 72). The importance of the Na+/K+ pump as one of the key modulators of repolarization and rotor dynamics in human atria should be taken into consideration in the evaluation and development of pharmacological treatments for AF. Previous investigations into AF-related mechanisms have, however, preferentially focused on sodium and potassium currents (19), and systematically ignoring the effect of other currents and in particular the Na+/K+ pump (48). Similarly, screening of drug compounds usually targets sodium, calcium, and potassium channels. It is nevertheless important to note that some anti-AF drugs such as amiodarone interfere with Na+/K+ pump regulation (26). Amiodarone is known to be more efficient in maintaining sinus rhythm than some of their derivatives, such as dronedarone, which do not affect the activity of the pump (26, 52). According to our results, the effects of amiodarone on Na+/K+ pump regulation could be important in explaining the antiarrhythmic action of the drug. Furthermore, digitalis (i.e., GNaK blocker) is also moderately successful in the treatment of AF patients with heart failure. The positive effects of digitalis in AF patients have been explained through the modulation of sodium and calcium concentrations (43). Indeed, the Na+/K+ pump is essential to the maintenance of cell homeostasis but, as highlighted in our study, it is also a critical determinant of atrial refractoriness. Our results suggest that the antiarrhythmic properties of drugs such as amiodarone and digitalis used for AF treatment could at least in part be due to the modulation of important electrophysiological properties, such as APD restitution, by partial blocking of the Na+/K+ pump.
The importance of GNaK in modulating atrial electrophysiology is consistent in both human atrial models used in this study and also in agreement with our previous theoretical studies using human and rabbit ventricular AP models (11, 16, 54, 58, 59). In all the models tested, GNaK was consistently found to be one of the most important modulators of APD90, restitution properties, and rate adaptation dynamics. The importance of GNaK therefore appears to be model independent and consistent in atrial and ventricular models, even if the specific formulation of INaK in the models is different, as in the MGGT and CRN models. Experimental evidence also shows that the expression of some subunits of the Na+/K+ pump is 30–50% lower in human atria than in ventricles (43). This could explain the higher sensitivity of atrial electrophysiology to a particular dose of drugs affecting GNaK (67). Experimental confirmation of the findings of the present study on the importance of GNaK in AF-related dynamics could explain the outcome of current pharmacological therapies and guide the development of new avenues for a more effective treatment of patients with AF.
Role of IK1.
As in our study, previous studies have highlighted the importance of IK1 in human atrial electrophysiology (38, 50), and therefore IK1 is considered a potential antiarrhythmic target. Furthermore, activation of inward rectifier potassium channels has been shown to increase DF in human atria (3), in good agreement with our results. Our simulations corroborate GK1 as the most important modulator of electrophysiology and reentrant dynamics both in control and AFER atrial tissue. The antiarrhythmic effects of IK1 blockade are based on APD90 and RP lengthening and therefore DF reduction.
Role of INa.
Our simulations also confirm the strong influence of GNa in the modulation of atrial rotor dynamics through alteration of CV and, to a minor extent, RP. GNa alterations have a stronger effect following AFER than in control. Sodium channel blockers such as flecainide, propafenone, or pilsicainide are widely used for the treatment of short-term AF (8, 15, 39, 50). A decrease in DF has been suggested as one of their mechanisms of action (33), which is in good agreement with our results (Fig. 8). The identification of Class I compounds with different modes of action in atria and ventricles minimizing side effects, such as ranolazine, could be a promising path for AF treatment, in particular following AFER (8, 48).
Role of other ionic currents.
In addition to GNaK, GK1, and GNa, conductances such as GKur, GCaL, and Gto also contribute to the regulation of atrial electrophysiology, particularly in control, before AFER causes their reduction (Fig. 2). Much attention has been paid to GKur because of its preferential expression in atrial tissue. Promising results have shown that drugs blocking IKur such as AVE0118 prolong atrial refractoriness with no effects on QT-interval in animal studies (5), but resulting in an increase in the peak intracellular calcium transient (29). In agreement with these results, our simulations show that GKur modulates APD90, and thus RP, Ss1s2, and Sdyn. However, GKur effects are reduced in AFER due to its lower conductance, and this could indicate potentially smaller efficacy of GKur block in the treatment of long-term AF (Fig. 3). GCaL is important in determining APD90, Ss1s2, and RP, which is consistent with previous studies showing its influence on PS tip meandering during reentry (50). Our results could also explain the ability of verapamil, a calcium channel blocker, to attenuate the RP shortening caused by AF remodeling (64). Gto, GKr, and GKs have slight effects on atrial AP (Figs. 3, 4, and 5), and their alterations are delicate due to potential increase in ventricular arrhythmic risk. The IKr blockers sotalol and dofetilide are used for AF prevention due to their stronger effect on RP at slow rather than rapid heart rates (19, 21). This is also reported in our simulations, which show larger increments in APD90, and thus RP, when Gto, GKr, and GKs are blocked in control than following AFER.
Table 1 summarizes the ionic currents investigated in our simulation study, the experimental and clinical drug studies used for comparison with our results, and the atrial electrophysiological properties found to be modulated by each of the investigated currents.
Table 1.
Ionic currents investigated in this study, drugs targeting them, and modulation of atrial electrophysiological properties
| Ionic Current and Drug | References | Important Effects |
|---|---|---|
| INaK | ||
| Amiodarone | 26, 30 | APD90, Ss1s2, Sdyn, RP, DF |
| Strophantidin | 40 | |
| Digitalis | 43 | |
| Digoxin | 55 | |
| Ouabain | 72 | |
| IK1 | ||
| Adenosine | 3 | APD90, RMP, Ss1s2, Sdyn, RP, CV, DF |
| INa | ||
| Ranolazine | 8 | RP (AFER), CV, DF |
| Flecainide | 8 | |
| Propafenone | 8 | |
| Lidocaine | 15 | |
| Pilsicainide | 33 | |
| Tetrodotoxin | 39 | |
| IKur, Ito | ||
| AVE118 | 5 | APD90, RP, Ss1, s2, Sdyn |
| ICaL | ||
| Verapamil | 64 | APD90, RP, Ss1s2 |
| IKr, IKs | ||
| Sotalol | 19 | - |
| Dofetilide | 19 |
INaK, Na+/K+ pump current; IK1, inward rectifier potassium current; INa, Na+ current; IKur, ultrarapid delayed potassium current; Ito, transient outward current; ICaL, L-type calcium current; IKr, rapid delayed rectifier potassium current; IKs, slow delayed rectifier potassium current; APD90, action potential duration at 90% repolarization; Ss1s2 and Sdyn, maximal values of the slopes of the S1S2 and dynamic action potential duration at 90% repolarization; RP, refractory period; RMP, resting membrane potential; CV, conduction velocity; DF, dominant frequency; AFER, atrial fibrillation-related electrical remodeling.
Limitations.
Our study focuses on unraveling the relative importance of ionic currents in modulating human atrial electrophysiology. The relevance of analyzing the importance of intracellular calcium dynamics was not investigated but it might be important as shown in previous studies (13, 29). Specific conditions are used to simulate control and AFER, but these are likely to exhibit significant intersubject variability (18, 65, 66).
Our study focused on the importance of ionic mechanisms in modulating electrophysiological properties related to rotor dynamics, and, therefore, other important aspects such as ectopic activity, structural changes during AF, tissue anisotropy, and heterogeneities caused by vagal stimulation are not investigated (15, 20, 39, 44). Even though their effect on AF-related reentry could be important as shown in previous studies (15), they would have a confounding effect on the results interpretation and should be the focus of further investigations. Tissue simulations are restricted to two-dimensional and thus the three-dimensional atrial geometry is not considered. Previous studies, however, show that sodium blockers effects are qualitatively similar in two- and three-dimensional models (13, 48). The role of atrial anatomy and heterogeneity in modulating pharmacological anti-AF interventions should be explored in further studies, building on insights provided by this study.
Finally, a few recent studies have focused on the comparison of different AP models aiming at targeting similar cell type and mammalian species and have highlighted differences in dynamics because of differences in the model equations and parameters (7, 11, 12, 13, 58, 59). The results of this study should be interpreted with caution since there could be different combinations of changes in the model parameters leading to similar results (62). These limitations are common to other computational studies in cardiac electrophysiology. We have, however, conducted simulations using two different human atria action potential models varying model parameters to address some of these limitations.
Conclusion
In this study, we characterize the relative importance of human atrial ionic current properties in modulating AP, refractoriness, CV, and rotor dynamics in human atrial tissue before and after AFER. Our study uncovers GNaK as a key modulator of atrial refractoriness and rotor dynamics in human and confirms GK1 and GNa as important determinants of AF-related electrophysiological properties. The importance of GNaK in modulating atrial electrophysiology could have important implications for the interpretation of the efficacy of anti-AF drugs that interfere with GNaK, such as amiodarone and digitalis. Overall, similar results are obtained before and after AFER but with smaller effects of ionic current changes on atrial electrophysiology in AFER. This could explain the reported lower efficacy of pharmacological interventions targeting ionic currents in the treatment of long-term versus short-term AF patients. The in-depth characterization of the ionic basis of atrial electrophysiology and AF-related mechanisms provided here could guide in the identification of more effective therapies against AF.
GRANTS
This study was financially supported by European Commission preDiCT Grant DG-INFSO-224381; a United Kingdom Medical Research Council Career Development award (to B. Rodríguez); a Royal Society Visiting Fellowship and International Joint Project (to E. Pueyo and B. Rodríguez); fellowships from Ministerio de Ciencia e Innovación and from Caja de Ahorros de la Inmaculada, Spain (to E. Pueyo); grants TEC2010-19410, TEC2010-21703-C03-02 from Ministerio de Ciencia e Innovación, Spain, and PI 144/2009 from Gobierno de Aragón, Spain (to E. Pueyo and P. Laguna); fellowship BES-2008-002522 from Ministerio de Ciencia e Innovación, Spain (to C. Sánchez); and Grupo Consolidado GTC ref:T30, Gobierno de Aragón.
DISCLOSURES
Mark Davies, Jonathan Swinton, and Ingemar Jacobson are salaried employees of Astrazeneca.
AUTHOR CONTRIBUTIONS
Author contributions: C.S., E.P., and B.R. conception and design of research; C.S., A.C., and A.B.-O. performed experiments; C.S., A.C., A.B.-O., E.P., and B.R. analyzed data; C.S., A.C., A.B.-O., M.R.D., J.S., I.J., P.L., E.P., and B.R. interpreted results of experiments; C.S., A.B.-O., E.P., and B.R. prepared figures; C.S., A.C., E.P., and B.R. drafted manuscript; C.S., A.C., A.B.-O., M.R.D., J.S., I.J., P.L., E.P., and B.R. edited and revised manuscript; C.S., A.C., A.B.-O., M.R.D., I.J., P.L., E.P., and B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was technically possible thanks to help provided by Blair Bethwaite and the Nimrod developers team. We thank the reviewers for the useful comments, which have significantly improved the quality and clarity of the manuscript.
REFERENCES
- 1. Abramson D, Bernabeu MO, Bethwaite B, Burrage K, Corrias A, Enticott C, Garic S, Gavaghan D, Peachey T, Pitt-Francis J, Pueyo E, Rodríguez B, Sher A, Tan J. High-throughput cardiac science on the grid. Phil Trans R Soc A 368: 3907–3923, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Ambrosi CM, Ripplinger CM, Efimov IR, Fedorov W. Termination of sustained atrial flutter and fibrillation using low-voltage multiple-shock therapy. Heart Rhythm 8: 101–108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastín JP, Torrecilla EG, Sánchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation 114: 2434–2442, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Baher A, Qu Z, Hayatdavoudi A, Lamp ST, Yang MJ, Xie F, Turner S, Garfinkel A, Weiss JN. Short-term cardiac memory and mother rotor fibrillation. Am J Physiol Heart Circ Physiol 292: H180–H189, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Blaauw Y, Gögelein H, Tieleman RG, Van Hunnik A, Schotten U, Allessie MA. “Early” class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodelled atria of the goat. Circulation 110: 1717–1724, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Boutjdir M, Le Heuzey JY, Lavergne T, Chauvaud S, Guize L, Carpentier A, Peronneau P. Inhomogeneity of cellular refractoriness in human atrium: factor of arrhythmia? Pacing Clin Electrophysiol 9: 1095–1100, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Bueno-Orovio A, Cherry EM, Fenton FH. Minimal model for human ventricular action potentials in tissue. J Theor Biol 253: 544–560, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation 116: 1449–1457, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cabrera JA, Sanchez-Quintana D, Ho SY, Medina A, Anderson RH. The architecture of the atrial musculature between the orifice of the inferior caval vein and the tricuspid valve: the anatomy of the isthmus. J Cardiovasc Electrophysiol 9: 1186–1195, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Carmeliet E. Action potential duration, rate of stimulation, and intracellular sodium. J Cardiovasc Electrophysiol 17: S2–S7, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Carro J, Rodriguez JF, Laguna P, Pueyo E. A human ventricular cell model for investigation of cardiac arrhythmias under hyperkalemic conditions. Philos Transact A Math Phys Eng Sci 369: 4205–4232, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Cherry EM, Evans SJ. Dynamics of human atrial cell models: restitution, memory, and intracellular calcium dynamics in single cells. Prog Biophys Mol Biol 98: 24–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cherry EM, Evans SJ. Properties of two human atrial cell models in tissue: restitution, memory, propagation, and reentry. J Theor Biol 254: 674–690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cherry EM, Fenton FH. Effects of boundaries and geometry on the spatial distribution of action potential duration in cardiac tissue. J Theor Biol 285: 164–176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Comtois P, Sakabe M, Vigmond EJ, Muñoz M, Texier A, Shiroshita-Takeshita A, Nattel S. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol 295: H1489–H1504, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Corrias A, Giles W, Rodriguez B. Ionic mechanisms of electrophysiological properties and repolarization abnormalities in rabbit Purkinje fibres. Am J Physiol Heart Circ Physiol 300: H1806–H1813, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol Heart Circ Physiol 275: H301–H321, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res 42: 477–489, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Derakhchan K, Villemaire C, Talajic M, Nattel S. The class III antiarrhythmic drugs dofetilide and sotalol prevent AF induction by atrial premature complexes at doses that fail to terminate AF. Cardiovasc Res 50: 75–84, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Dobrev D, Graf E, Wettwer E, Himmel HM, Hála O, Doerfel C, Christ T, Schüler S, Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifier potassium current (IK,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced IK,ACh and muscarinic receptor-mediated shortening of action potentials. Circulation 104: 2551–2557, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs 69: 757–774, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Engelman ZJ, Trew ML, Smaill BH. Structural heterogeneity alone is a sufficient substrate for dynamic instability and altered restitution. Circ Arrhythm Electrophysiol 3: 195–203, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Faber GM, Rudy Y. Action potential and contractility changes in [Na+]i overloaded cardiac myocytes: a simulation study. Biophys J 78: 2392–2404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fenton F, Karma A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: filament instability and fibrillation. Chaos 8: 20–47, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Fenton FH, Luther S, Cherry EM, Otani NF, Krinsky V, Pumir A, Bodenschatz E, Gilmour RF., Jr Termination of atrial fibrillation using pulsed low-energy far-field stimulation. Circulation 120: 467–476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forini F, Nicolini G, Balzan S, Ratto GM, Murzi B, Vanini V, Iervasi G. Amiodarone inhibits the 3–5-3'-triiodothyronine-dependent increase of Na/K adenosine triphosphatase activity and concentration in human atrial myocardial tissue. Thyroid 14: 493–499, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Frazier DW, Wolf PD, Wharton JM, Tang ASL, Smith WM, Ideker RE. Stimulus-induced critical point: mechanism of electrical induction of reentry in normal canine myocardium. J Clin Invest 83: 1039–1052, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gima K, Rudy Y. Ionic current basis of electrocardiographic waveforms: a model study. Circ Res 90: 889–896, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 109: 1055–1066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gray DF, Mihailidou AS, Hansen PS, Buhagiar KA, Bewick NL, Rasmussen HH, Whalley DW. Amiodarone inhibits the Na+-K+ pump in rabbit cardiac myocytes after acute and chronic treatment. J Pharmacology Exp Therap 284: 75–82, 1998 [PubMed] [Google Scholar]
- 31. Haisaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quinikou G, Garrigue S, Le Moroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339: 659–666, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Harada A, Sasaki K, Fukushima T, Ikeshita M, Asano T, Yamauchi S, Tanaka S, Shoji T. Atrial activation during chronic atrial fibrillation in patients with isolated mitral valve disease. Ann Thorac Surg 61: 104–111, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Horiuchi D, Iwasa A, Sasaki K, Owada S, Kimura M, Sasaki S, Okumura K. Effect of pilsicainide on dominant frequency in the right and left atria and pulmonary veins during atrial fibrillation: association with its atrial fibrillation terminating effect. Eur J Pharmacol 608: 54–61, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Hou L, Deo M, Furspan P, Pandit SV, Mironov S, Aeurbach DS, Gong Q, Zhou Z, Berenfeld O, Jalife J. A major role for hERG in determining frequency of reentry in neonatal rat ventricular myocyte monolayer. Circ Res 107: 1503–1511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaïs P, Packer DL. Ablation vs. drug use for atrial fibrillation. Eur Heart J Supplements 9: G26–G34, 2007 [Google Scholar]
- 36. Jalife J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol 14: 776–780, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Keener JP. An eikonal-curvature equation for action potential propagation in myocardium. J Math Biol 29: 629–651, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Kharche S, Garrat CJ, Boyett MR, Inada S, Holden AV, Hancox JC, Zhang H. Atrial proarrhythmia due to increased inward rectifier current (IK1) arising from KCNJ2 mutation—a simulation study. Prog Biophys Mol Biol 98: 186–197, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, Vigmond EF, Leon J, Nattel S, Jalife J. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circ Res 96: e35–e47, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Levi A. The effect of strophantidin on action potential, calcium current and contraction in isolated guinea-pig ventricular myocytes. J Physiol 442: 1–23, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luther S, Fenton FH, Korneich BG, Squires A, Bittihn P, Hornung D, Zabel M, Flanders J, Gladuli A, Campoy L, Cherry EM, Luther G, Hasenfuss G, Krinsky VI, Pumir A, Gilmour RF, Jr, Bodenschatz E. Low-energy control of electrical turbulence in the heart. Nature 475: 235–239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am J Physiol Heart Circ Physiol 297: H1398–H1410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDonough AA, Velotta JB, Schwinger RHG, Philipson KD, Farley RA. The cardiac sodium pump: structure and function. Basic Res Cardiol 97: 19–24, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Miyamoto K, Tsuchiya T, Narita S, Yamaguchi T, Nagamoto Y, Ando SI, Hayashida K, Tanioka Y, Takahashi N. Bipolar electrogram amplitudes in the left atrium are related to local conduction velocity in patients with atrial fibrillation. Europace 11: 1597–1605, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Narayan SM, Kazi D, Krummen DE, Rappel WJ. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation. J Am Coll Cardiol 52: 1222–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nattel S. New ideas about atrial fibrillation 50 years on. Nature 415: 219–226, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Nattel S, Li D, Yue L. Basic mechanisms of atrial fibrillation: very new insights into very old ideas. Annu Rev Physiol 62: 51–77, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Nattel S, Singh BN. Evolution, mechanisms, and classification of antiarrhythmic drugs: focus on class III actions. Am J Cardiol 84: 11–19, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Nygren A, Fiset C, Firek L, Clark JW, Linblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ Res 82: 63–81, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Pandit SV, Berenfeld O, Anumonwo JMB, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J 88: 3806–3821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pathmanathan P, Bernabeu MO, Bordas R, Cooper J, Garny A, Pitt-Francis JM, Whiteley JP, Gavaghan DJ. A numerical guide to the solution of the bidomain equations of cardiac electrophysiology. Prog Biophys Mol Biol 102: 136–155, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM, Kong DF. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol 54: 1089–1095, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Plonsey R, Rudy Y. Electrocardiogram sources in a 2-dimensional anisotropic activation model. Med Biol Eng Comput 18: 87–94, 1980 [DOI] [PubMed] [Google Scholar]
- 54. Pueyo E, Husti Z, Hornyik T, Baczkó I, Laguna P, Varró A, Rodríguez B. Mechanisms of ventricular rate adaptation as a predictor of arrhythmic risk. Am J Physiol Heart Circ Physiol Heart Circ Physiol 298: H1577–H1587, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Rasmussen HH, Okita GT, Hartz RS, ten Eick RE. Inhibition of electrogenic Na+-pumping in isolated atrial tissue from patients treated with digoxin. J Pharmacol Exp Ther 252: 60–64, 1990 [PubMed] [Google Scholar]
- 56. Rodriguez B, Burrage K, Gavaghan D, Grau V, Kohl P, Noble D. The systems biology approach to drug development: application to toxicity assessment of drug toxicity. Nature Clinical Pharmacology and Therapeutics 88: 130–134, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Rodriguez B, Tice BM, Eason JC, Aguel F, Trayanova N. Cardiac vulnerability to electric shocks during phase 1A of acute global ischemia. Heart Rhythm 1: 695–703, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Romero L, Carbonell B, Trenor B, Rodriguez B, Saiz J, Ferrero JM. Systematic characterization of the ionic basis of rabbit cellular electrophysiology using two ventricular models. Prog Biophys Mol Biol 107: 60–73, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Romero L, Pueyo E, Fink M, Rodriguez B. Impact of biological variability on human ventricular cellular electrophysiology. Am J Physiol Heart Circ Physiol 297: H1436–H1445, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Schumacher SM, Martens JR. Ion channel trafficking: a new therapeutic horizon for atrial fibrillation. Heart Rhythm 7: 1309–1315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schweizer PA, Becker R, Katus HA, Thomas D. Dronedarone: current evidence for its safety and efficacy in the management of atrial fibrillation. Drug Design, Development and Therapy 5: 27–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Syed ZF, Vigmond E, Leon LJ. Automated modeling of cardiac electrical activity. Proc IEEE EMBS: 17–21, 2003 [Google Scholar]
- 63. The Office of Health Economics Estimating the direct costs of atrial fibrillation to the N.H.S. in the constituent countries of the U.K., and at S.H.A. level in England, 2008. London: 2009 [Google Scholar]
- 64. Tieleman RG, De Langen C, Van Gelder IC, De Kam PJ, Grandjean J, Bel KJ, Wijffels MCEF, Allessie MA, Crijns HJGM. Verapamil reduces tachycardia-induced electrical remodeling of the atria. Circulation 95: 1945–1953, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 85: 428–436, 1999 [DOI] [PubMed] [Google Scholar]
- 66. Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward potassium current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res 80: 772–781, 1997 [DOI] [PubMed] [Google Scholar]
- 67. Wang J, Schwinger RH, Frank K, Müller-Ehmsen J, Martin-Vasallo P, Pressley TA, Xiang A, Erdmann E, McDonough AA. Regional expression of sodium pump subunits isoforms and Na+-Ca++ exchanger in the human heart. J Clin Invest, 98: 1650–1658, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wijffels MCEF, Kirchhof CJHJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation 92: 1954–1968, 1995 [DOI] [PubMed] [Google Scholar]
- 69. Winfree AT. Various ways to make phase singularities by electric shock. J Cardiovasc Electrophysiol 11: 286–289, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Workman AJ, Kane AK, Rankin AC. Cellular bases for human atrial fibrillation. Heart Rhythm 5: S1–S6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Workman AJ, Kane AK, Rankin AC. Characterisation of the Na,K pump current in atrial cells from patients with and without chronic atrial fibrillation. Cardiovasc Res 59: 593–602, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Workman AJ, Kane AK, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res 52: 226–235, 2001 [DOI] [PubMed] [Google Scholar]
- 73. Xie F, Qu Z, Garfinkel A, Weiss JN. Electrical refractory period restitution and spiral wave reentry in simulated cardiac tissue. Am J Physiol Heart Circ Physiol 283: H448–H460, 2002 [DOI] [PubMed] [Google Scholar]
- 74. Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 81: 512–525, 1997 [DOI] [PubMed] [Google Scholar]