Abstract
Sarcoidosis affects the bone directly in only a minority of patients. Nonetheless, bone health should be considered in the management of all patients with sarcoidosis. Deficiency in vitamin D, an important contributor to bone health, has been linked to autoimmune disease incidence. Studies have shown that patients with sarcoidosis frequently have low levels of vitamin D-25 but may have normal or increased levels of vitamin D-1,25. In addition, granuloma formation has been linked to a failure of the innate immune system, which could be related to a deficiency in vitamin D, although this relationship has not been fully characterized. Furthermore, many patients with sarcoidosis are treated with corticosteroids, which are known to induce osteoporosis. Therefore, bone health may be impacted in several ways in sarcoidosis—by direct involvement with granulomas, vitamin D deficiency, or corticosteroid therapy.
Keywords: Sarcoidosis, Bone health, Vitamin D, Calcium, Hypercalcemia, Bisphosphonates, Corticosteroids, Osteoporosis
Introduction
Sarcoidosis is a heterogeneous disease characterized by noncaseating granulomas infiltrating affected organs. Lung involvement is reported in the vast majority of cases, and more than 90% of patients have a combination of lung, skin, and/or eye involvement. Less commonly, osseous lesions may infiltrate bone and cause bone resorption and bone loss. Such involvement has been reported in about 13% of patients with sarcoidosis [1]. However, this estimation relies mostly on case reports and to our knowledge has not been evaluated in epidemiologic studies [1]. Although the skeleton is rarely affected directly by the disease, bone health is an important consideration for several reasons. First, vitamin D sufficiency is an important contributor to bone health, and vitamin D deficiency has been linked to autoimmune disease. Studies have shown that patients with sarcoidosis have elevated levels of 1,25 (OH)2D (calcitriol), the active form of vitamin [2, 3•]. The cause of sarcoidosis remains unknown, but several etiologies have been suggested. In one possible scenario, granuloma formation is attributed to a defect in innate immunity that fails to overcome a mycobacterial infection. This defect has been linked in part to vitamin D [4•]. Second, many patients with sarcoidosis are treated with corticosteroids, which causes bone loss. In this article, we review the different ways in which the skeleton is affected by sarcoidosis, both directly and indirectly.
Bone Diseases in Sarcoidosis: Osseous Sarcoidosis and Sarcoid Arthritis
When sarcoid lesions are present in bone, the disease is usually systemic and progressive [5, 6]. In osseous sarcoidosis, both axial and appendicular components of the skeleton can be affected, with hands and feet most commonly showing signs and symptoms of sarcoidosis [7]. Such signs and symptoms of osseous sarcoidosis may include tenderness, swelling, stiffness, deformity, and redness near the site of bone involvement and/or associated joints [6, 8]. Case reports have also noted that some patients experience pain. For example, back pain may be the presenting symptom of granulomatous infiltration of vertebrae [9].
Osseous sarcoidosis is generally discovered by imaging techniques, including standard radiography, MRI (Fig. 1), and CT. Lytic and sclerotic lesions have been observed in cases of vertebral sarcoidosis [10, 11]. Although most patients with sarcoidosis have evidence of thoracic parenchymal sarcoidosis on chest imaging, normal chest x-rays have also been obtained from patients with vertebral involvement [9, 11]. The sensitivity of MRI may be the preferred modality for the diagnosis of osseous sarcoidosis, especially when sacroiliitis is in the differential diagnosis [11, 12]. MRI has proven useful in directing appropriate sites for bone biopsy. However, lesions discovered by MRI are not specific for sarcoidosis, and other diagnoses, such as malignancy and tuberculosis, must be excluded [13]. Laboratory tests, including erythrocyte sedimentation rate and alkaline phosphatase levels, are normal in patients with osseous sarcoidosis, making these studies of limited utility in the diagnostic work-up [9]. As in other forms of sarcoidosis, a biopsy is required to establish the diagnosis of osseous lesions. Positive emission transmission (PET) (Fig. 1) and gallium scanning may prove useful in detecting skeletal involvement.
Fig. 1.
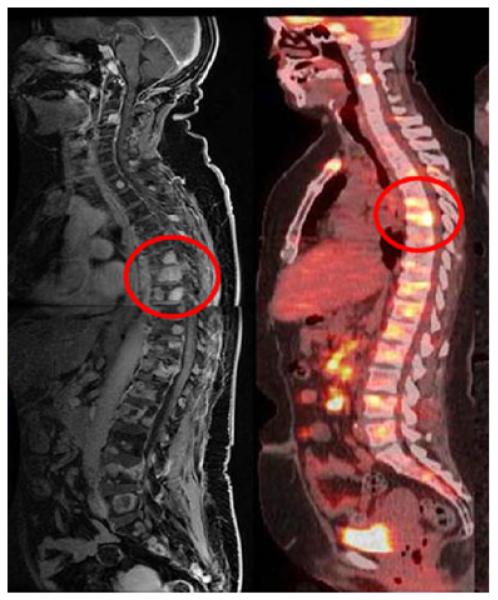
Lateral views of the MRI (left) and positron emission tomography (right) scans of the torso of a patient with sarcoidosis. Lesions are seen in multiple areas, including the thoracic spine (circled).
Vitamin D in Sarcoidosis
The role of vitamin D in bone health is well-established. It plays a regulatory role in calcium and skeletal homeostasis and ensures intestinal absorption of calcium [14]. Vitamin D also functions in the immune system by assisting in innate immunity and in the destruction of foreign antigens [15]. More recently appreciated roles of vitamin D include negatively regulating angiogenesis and promoting cellular differentiation [15].
Vitamin D deficiency may cause symptomatic osteomalacia, including pain, fatigue, and weakness [16, 17]. It has been estimated that about 36% of individuals in the United States have insufficient levels of vitamin D [14]. Although no standard definition of vitamin D deficiency exists, some reports suggest that levels of the inactive form of vitamin D, Vit D-25, below 25 ng/mL are indicative of a deficiency [14, 15]. The inactive form of vitamin D is the end product of the metabolism of synthetic vitamin D2 and vitamin D3, which is ingested from food or produced by the skin in response to UV light In this inactive form, Vit D-25 is the major circulating form of the vitamin and the form of vitamin D that is measured to evaluate adequacy of vitamin D intake [14].
In patients with sarcoidosis, vitamin D abnormalities are common, as are the symptoms associated with vitamin D deficiency. A recent report evaluated the levels of vitamin D in a sarcoidosis population [3•]. The investigators found that 49% of patients had Vit D-25 levels below 10 ng/mL, indicating a severe vitamin depletion. Furthermore, only one patient had an adequate level of serum Vit D-25. Although most of the patient population was Vit D-25 deficient, 71% of patients had normal values of the active form of Vit D-1,25. Therefore, patients with sarcoidosis tend to have normal levels of active—but not inactive—vitamin D.
Also contributing to the complex association between vitamin D and sarcoidosis is the fact that sarcoidosis preferentially affects African Americans. Normal vitamin D levels in people with darker skin colors are typically lower than those of people with lighter skin colors [15]. In addition, African Americans tend to have higher peak bone masses [18], which may indicate that they need less vitamin D to maintain bone health than people of ancestries associated with lighter skin colors. The significance of this difference, especially as it relates to sarcoidosis, is uncertain but important to acknowledge.
One reason for the level of Vit D-25 in patients with sarcoidosis is that calcium homeostasis may be altered and hypercalcemia may be present. It has been known for some time that sarcoidosis granulomas can autonomously increase conversion of Vit D-25 to Vit D-1,25 [19], and that hypercalcemia has been associated with high levels of Vit D-1,25 [20]. The Vit D-1,25 is released by the granulomas, and the level of Vit D-1,25 is positively correlated with disease activity [21, 22•].
Conversion of Vit D-25 to Vit D-1,25 normally occurs in the kidney proximal tubule cells. Vit D-1,25 is the active form of vitamin D and maintains calcium homeostasis and balance between serum calcium and parathyroid hormone (PTH) levels. PTH is released from the parathyroid gland in response to reductions in circulating serum calcium. Measurement of serum PTH in patients with sarcoidosis can distinguish the cause of hypercalcemia, including primary hyperparathyroidism, sarcoidosis and other granulomatous diseases, and malignancy. Elevated serum PTH together with high calcium levels is diagnostic of primary hyperparathyroidism [3•]. PTH also increases the conversion of Vit D-25 to Vit D-1,25. The active form of vitamin D increases bone resorption and intestinal calcium absorption and decreases renal tubule calcium reabsorption [3•]. The biological effects of high Vit D-1,25 levels are mediated by binding to the vitamin D receptor (VDR), which is widely distributed and found in many tissues [15].
In addition to its role in calcium metabolism and bone health, vitamin D and the VDR also have actions on the immune system. Known effects include a vitamin D contribution to the immunoregulation of antigen-presenting cells. When elevated, Vit D-1,25 downregulates dendritic cells, leading to an attenuated immune response. However, severe vitamin D deficiency is thought to sensitize the antigen-presenting capacity of dendritic cells [23]. Overactivity of dendritic cells could contribute to the onset of autoimmune diseases such as systemic lupus erythematosus (SLE) [23], as inappropriate overactivity of these cells could direct the immune response toward endogenous self molecules. A subset of dendritic cells is the major producer of interferon (IFN)-α, and a large body of work has implicated this cytokine in the development of autoimmunity [24-26]. Of note, patients with sarcoidosis, like those with SLE and other autoimmune diseases, tend to have low levels of vitamin D. Therefore, some investigators believe that supplementing patients with vitamin D may improve the clinical status of SLE [23]. However, data to support this claim are lacking. The role of vitamin D replacement in sarcoidosis is also unclear.
High levels of Vit D-1,25 have been found within granulomas. Specifically, IFN-γ, which regulates the production of the enzyme 1α-hydroxylase that catalyzes the conversion of vitamin D from its inactive to active form, is locally produced at sites of granulomatous inflammation [27]. Macrophages that make up sarcoid granulomas also have been reported to produce Vit D-1,25. Furthermore, tumor necrosis factor (TNF)-α and IFN-γ, which are present at sites of inflammation, may increase the production of Vit D-1,25 [28]. Other cytokines, such as IFN-α, are also produced by dendritic cells at sites of inflammation. Conversely, IFN-α expression is downregulated by vitamin D, which may serve to decrease inflammatory reactions in patients with autoimmune disease and in healthy individuals [23]. Of note, in our clinic at the University of Chicago, we have found that expression levels of TNF-α and IFN-α correlate with disease manifestations in patients with sarcoidosis and vary by patient-reported ancestry (unpublished data).
Increased expression of the VDR can occur following infection [29]. Macrophages express Toll-like receptors (TLRs), which are activated by an infection. As a result of the activation of TLRs, VDR expression is upregulated [29]. In turn, the VDRs are activated by Vit D-1,25, which induces macrophages to synthesize and secrete the bactericidal peptide cathelicidin. In doing so, infections such as Mycobacterium tuberculosis are abated, helping the presence of vitamin D [29]. In the same study, patients with increased susceptibility to M. tuberculosis had low levels of vitamin D, confirming the link between innate immunity and levels of vitamin D [29]. On the basis of these actions, some have raised the possibility that vitamin D replacement may not be helpful in treating sarcoidosis [4•]. Furthermore, supplementing vitamin D in patients with significant hypercalcemia or hypercalciuria could be dangerous.
The relationship between vitamin D, sarcoidosis, and cathelicidin production has not been characterized. However, as mentioned previously, some investigators have suggested that the formation of granulomas may result from a defect in innate immunity that fails to overcome a mycobacterial infection. Laboratory researchers are currently creating murine models that express mycobacterial proteins related to the granulomas found in patients with sarcoidosis [30]. Findings from these studies may shed light on the effect of sarcoidosis on the role of vitamin D in innate immunity.
Bone Health in Sarcoidosis: Managing Disease-induced and Steroid-induced Osteoporosis
Given the effect of sarcoidosis on calcium and vitamin D metabolism, it is not surprising that bone mineral density (BMD) in patients with sarcoidosis also may be altered by the disease. In particular, untreated patients with sarcoidosis tend to have low BMD [9].
The status of bone health in patients with sarcoidosis is complicated by the use of corticosteroids in treating the active disease, which may cause increased bone resorption, decreased bone formation, and net bone loss in response to corticosteroid use [31]. The chronic course of some forms of sarcoidosis results in the long-term use of glucocorticoids such as prednisone. In patients who remain on corticosteroids for extended periods of time, osteoporosis develops in up to 70% of cases, resulting in significant pain and disability [31]. Bone loss begins within the first 3 months of corticosteroid therapy but progressively diminishes over time [31]. With time, there is accelerated bone loss and a corresponding propensity of vertebral and nonvertebral fractures, even with use of low levels of corticosteroids [31].
In sarcoidosis specifically, one study compared the bone loss of patients with the loss reported in patients with rheumatoid arthritis and asthma and concluded that patients with sarcoidosis who receive corticosteroids have a higher frequency of bone loss [32]. These results were confirmed by a different study of patients with sarcoidosis, which showed that patients who were treated with corticosteroids experienced higher rates of bone loss than those patients not treated with corticosteroids [33]. Bone loss seemed to be accelerated in the prednisone-treated sarcoidosis population when compared with the untreated patients. However, to our knowledge, no studies have examined the outcome of osteoporosis in patients with sarcoidosis by estimating fractures and disability sustained in the development of this comorbidity. As such, further studies on this topic are warranted.
Proposed Algorithm to Maintain Bone Health in Sarcoidosis
A general consensus for treatment of osseous sarcoidosis has not been reached. In some patients with asymptomatic disease, treatment may not be necessary. For example, symptoms of acute sarcoid arthritis may spontaneously resolve after a few weeks without recurrence. In patients with osseous sarcoidosis and sarcoid arthritis, NSAIDs may be used but often may be replaced by corticosteroids due to lack of efficacy, particularly for patients experiencing pain caused by their disease. Generally, corticosteroids are reserved for patients with significant disease activity. We have previously proposed a treatment algorithm for sarcoid arthritis [34].
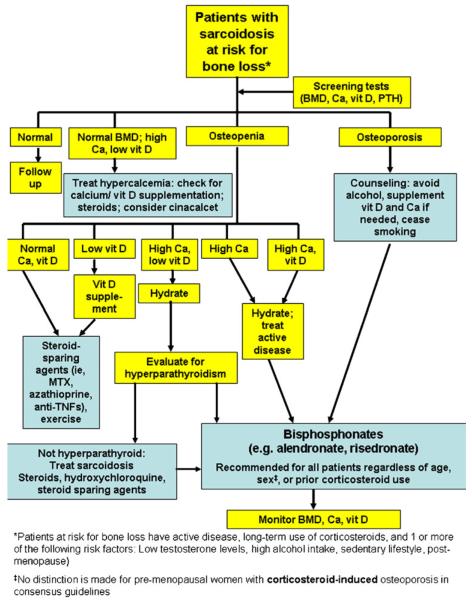
We propose an algorithm for the management of patients who require consideration for bone health issues, including vitamin D deficiency and/or osteoporosis (Fig. 2). The first step is to be sure that the hypercalcemic patient is not taking supplemental vitamin D or calcium. In general, corticosteroid-based regimens are considered the standard of care in the treatment of sarcoidosis. Therefore, baseline BMD measurement is of high value when obtained before onset of treatment (Fig. 2). Calcium supplements and/or vitamin D should be considered with caution in patients with hypercalcemia, hypercalciuria, high levels of PTH, and kidney stones.
Fig. 2.
Proposed algorithm for management of bone health issues in sarcoidosis patients. BMD bone mineral density, Ca calcium, MTX methotrexate, PTH parathyroid hormone, TNF tumor necrosis factor, vit D vitamin D
Corticosteroids are the most reliable and rapid treatment of sarcoidosis and also correct hypercalcemia using relatively low doses. For some patients, methylprednisolone has been selected over other corticosteroid preparations because of studies showing that steroid resistance is mediated by TLRs in SLE patients. SLE patients tend to require much higher doses of corticosteroids than patients with other autoimmune diseases [35•]. However, other than the role of TLRs in this disease, the reason for this requirement is not clear and warrants further investigation.
Hydroxychloroquine is also used in treating sarcoid patients, particularly those who may require long-term therapy. Other second-line agents, including methotrexate and azathioprine, and third-line treatments such as anti-TNF are often used in patients with chronic sarcoidosis whose disease cannot be controlled by hydroxychloroquine and low-dose corticosteroids. Of note, anti-TNF agents have been used to treat patients with refractory sarcoidosis, with variable success. [36-44] Garg and colleagues [39] reported success with infliximab, which improved clinical symptoms and radiologic lesions in a patient with vertebral sarcoidosis refractory to conventional treatments. Similarly, Agrawal et al. [40] reported efficacy of infliximab in treating sarcoid sacroiliitis.
In addition to their role in treating the underlying sarcoidosis, anti-TNF agents may be useful in treating hypercalcemia and improving bone strength. Anti-TNF therapy improves bone density in rheumatoid arthritis, but whether such therapy improves bone density in patients with sarcoidosis and osteoporosis/osteopenia has not been directly assessed. However, reports have emerged of TNF inhibitors decreasing levels of calcium in patients with sarcoidosis [45]. Although the cause of this decrease remains incompletely characterized, a link between TNF and hypercalcemia has been suggested for years [46, 47], and the role of TNF in sarcoidosis also has been established [48].
In general, agents other than those used to treat sarcoidosis itself are prescribed to manage other conditions associated with the disease. For example, ketoconazole has been used successfully to treat hypercalcemia and hypercalciuria, potentially through its proposed mechanism of inhibiting the conversion of Vit D-25 to Vit D-1,25 [49] However, this proposed mechanism has not been validated. Sarcoidosis-associated hypercalcemia refractory to standard therapy is sometimes treated with cinacalcet. Although cinacalcet is an agent typically used to treat hyperparathyroidism, we have noticed improvement with this agent in our patients with sarcoidosis complicated by hypercalcemia (unpublished data). We believe that its utility may result from its regulation of PTH levels, which have been found to be abnormal in patients with sarcoidosis.
Other than hypercalcemia, nephrolithiasis is also strongly associated with chronic sarcoidosis [50]. Patients with a history of nephrolithiasis should have their 24-hour urine calcium monitored, and if the value is higher than 250 mg, then treatment to reduce, including drugs such as hydroxychloroquine, should be considered even if the patient has no symptoms. In addition, we recommend long-term preventive therapy, including avoidance of exogenous vitamin D, calcium supplementation, and increased fluid intake. One could also consider prophylactic agents such as sodium bicarbonate or allopurinol in refractory cases.
Importantly, we also propose that clinicians provide preventive care or otherwise manage corticosteroid-induced osteoporosis. First and foremost, guidelines published by the American College of Rheumatology (ACR) recommend that patients receiving corticosteroids should undergo BMD testing [31]. It is important to note that steroid therapy may cause fractures in the absence of measurable bone loss. As such, using the World Health Organization Fracture Assessment Risk (FRAX) tool to calculate a patient’s risk of fracture is another important measure in the preventive care of patients with sarcoidosis [51]. Of note, FRAX may underestimate the fracture risk of patients receiving corticosteroids because it does not account for corticosteroid dose or duration. In our experience, fracture risk will be heavily influenced by the dose and length of corticosteroid treatment in patients with sarcoidosis. For patients with hypercalcemia, corticosteroids (eg, prednisone) are highly effective in normalizing serum calcium levels. If low bone mass is also present, then patients should be carefully evaluated for other causes of low bone mass. If all tests are normal, then low bone mass can be attributed to corticosteroid use. Because corticosteroid use may cause fractures in the presence of normal bone density, and because the rate of fractures in corticosteroid-treated patients is approximately 35%, one should be aggressive in the presence or absence of low bone mass if corticosteroids are being used [52]. A final supportive measure for these patients is vitamin D supplementation. The ACR guidelines suggest counseling patients to take vitamin D and calcium supplements to improve their bone health [31]. In the context of sarcoidosis, however, guidelines are lacking. Currently, clinicians rely in part on data from studies published regarding the relationship between vitamin D, calcium, and sarcoidosis. Mostly, clinicians rely on their own experience and preferences with respect to supplementation. Therefore, additional research on the benefits and potential dangers of vitamin D and calcium supplementation is necessary in sarcoidosis.
Despite the benefits of vitamin D supplementation, there are also some drawbacks associated with high levels of the vitamin, and, as noted above, some patients will have high levels of Vit D-1,25. In our opinion, high-dose vitamin D may be associated with sarcoidosis flares. As mentioned previously, we rely on this experience to dictate our supplementation approach. Namely, we do not recommend supplementing vitamin D for all patients, although research to support this decision is lacking. Vitamin D supplements may also cause hypercalcemia by providing increased Vit D-25 substrate for the conversion to Vit D-1,25. Furthermore, our unpublished findings indicate that vitamin D supplements in the presence of normal Vit D-1,25 and low Vit D-25 may provoke significant flares. The reason flares may result from supplementing vitamin D in the context of low Vit D-25 is not known. The obvious reason may be that low Vit D-25 is a cause and/or consequence of inflammation, which may induce flares. However, we have not tested this hypothesis. Due to our general lack of understanding of the relationship between vitamin D and sarcoidosis, we suggest a cautious approach. Measurement of serum calcium, Vit D-25, and Vit D-1,25 should be performed before starting vitamin D supplementation. From our own experience, we do not prescribe vitamin D supplementation in patients with low Vit D-25 and normal Vit D-1,25, as we have observed patients developing symptomatic hypercalcemia when supplementation has been given to some of them. There is also the potential of worsening the underlying sarcoidosis, but this has not been directly examined in clinical studies. For patients with sarcoidosis at risk of developing osteoporosis due to prolonged intake of corticosteroids, bisphosphonates can prevent or reverse bone loss. Bisphosphonates are commonly used in the treatment of osteoporosis and in corticosteroid-induced osteoporosis. The ACR recommends using them in low-risk patients receiving corticosteroid doses of 7.5 mg/d or higher, as well as in all medium- and high-risk patients receiving corticosteroids [31]. Of the available bisphosphonates, alendronate and risedronate effectively reduce bone loss and thereby may diminish fracture risk. Head-to-head comparisons have further revealed that teriparatide and zoledronic acid are more efficacious than alendronate and risedronate, respectively [31]. However, such comparisons between agents used to treat osteoporosis are imperfect because they largely rely on BMD, which is not an absolute surrogate for true fracture risk.
In sarcoidosis, studies have demonstrated that bisphosphonates can successfully prevent osteoporosis in corticosteroid-treated patients. In one prospective study, treatment with alendronate was associated with significantly less bone loss compared with placebo [9]. In rare cases, the use of bisphosphonates may be complicated by the fact that bronchoconstriction and interstitial pneumonia have been reported with these agents [53, 54]. Interstitial and granulomatous nephritis also have been observed in patients treated with pamidronate and alendronate [55-57]. Furthermore, bisphosphonates have been reported to induce the production and secretion of cytokines from macrophages and to contribute to immunologic responses (eg, uveitis) [58]. Although these findings are provocative, the evidence for the relationship between bisphosphonates is limited to case studies and individual patients in larger studies. To our knowledge, studies directly examining the pulmonary effects of bisphosphonates, including whether this relationship is causal, have not been conducted. Nonetheless, due to the common involvement of the lung in sarcoidosis and the dysregulation of immunologic pathways in this disease, we suggest that bisphosphonate use may be contraindicated in some patients. Despite these concerns, we do recommend bisphosphonates to prevent osteoporosis in patients with osteopenia and to treat osteoporosis in patients who have already developed it. However, in our algorithm, we also recommend monitoring all patients for any potential drug-related adverse effects (Fig. 2).
Conclusions
In summary, optimal therapies for sarcoidosis with osteoporosis, vitamin D deficiency, and abnormal calcium metabolism have not been defined due to a lack of clinical trials. Treatment needs to be individualized to support bone health in patients with osteopenia/osteoporosis, prevent fracture risk, and maintain a normal vitamin D and calcium homeostasis.
Footnotes
Disclosure Dr. Favus has served as a consultant for CVS/Caremark and Amgen. Drs. Sweiss, Lower, Korsten, Niewold, and Baughman reported no potential conflicts of interest relevant to this article.
Contributor Information
Nadera J. Sweiss, Sections of Rheumatology and Pulmonary Medicine, University of Chicago Medical Center, 5841 South Maryland Avenue, MC0930, Room N005B, Chicago, IL 60637, USA
Elyse E. Lower, Division of Pulmonary and Critical Care, University of Cincinnati Medical Center, 1001 Holmes, Eden Avenue, Cincinnati, OH 45219, USA, elower@ohcmail.com
Peter Korsten, Department of Nephrology and Rheumatology, Robert-Koch-Str. 40, 37075 Goettingen, Germany, peter.korsten@med.uni-goettingen.de.
Timothy B. Niewold, Section of Pulmonary Medicine, University of Chicago Medical Center, 5841 South Maryland Avenue, MC0930, Chicago, IL 60637, USA, tniewold@medicine.bsd.uchicago.edu
Murray J. Favus, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, 5841 South Maryland Avenue, MC 01027, Chicago, IL 60637, USA, mfavus@medicine.bsd.uchicago.edu
Robert P. Baughman, Division of Pulmonary and Critical Care, University of Cincinnati Medical Center, 1001 Holmes, Eden Avenue, Cincinnati, OH 45219, USA, baughmrp@ucmail.uc.edu
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321–30. doi: 10.1097/00002281-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Rizzato G. Clinical impact of bone and calcium metabolism changes in sarcoidosis. Thorax. 1998;53:425–9. doi: 10.1136/thx.53.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31:474–84. doi: 10.1055/s-0030-1262215. This is a great review about the nuances of managing calcium and vitamin D levels in sarcoidosis. Because it specifically focuses on sarcoidosis, it may serve as a roadmap to the supportive care that is required by these patients.
- 4•.Richmond BW, Drake WP. Vitamin D, innate immunity, and sarcoidosis granulomatous inflammation: insights from mycobacterial research. Curr Opin Pulm Med. 2010;16:461–4. doi: 10.1097/MCP.0b013e32833af7e8. This intriguing and potentially controversial study has raised the question of whether vitamin D supplementation may not be helpful in treating sarcoidosis.
- 5.Chatham W. Rheumatic manifestations of systemic disease: sarcoidosis. Curr Opin Rheumatol. 2010;22:85–90. doi: 10.1097/BOR.0b013e328333ba74. [DOI] [PubMed] [Google Scholar]
- 6.Ugwonali OF, Parisien M, Nickerson KG, Scully B, Ristic S, et al. Osseous sarcoidosis of the hand: pathologic analysis and review of the literature. J Hand Surg Am. 2005;30:854–8. doi: 10.1016/j.jhsa.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Shorr AF, Murphy FT, Kelly WF, Kaplan KJ, Gilliland WR, et al. Osseous Sarcoidosis Clinical, Radiographic, and Therapeutic Observations. J Clin Rheumatol. 1998;4:186–92. doi: 10.1097/00124743-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Ly JQ. An unusual cause of knee pain: a rare case of osseous sarcoid (2003:5b) Eur Radiol. 2003;13:2055–7. doi: 10.1007/s00330-003-1962-0. [DOI] [PubMed] [Google Scholar]
- 9.Rua-Figueroa I, Gantes MA, Erausquin C, Mhaidli H, Montesdeoca A. Vertebral sarcoidosis: clinical and imaging findings. Semin Arthritis Rheum. 2002;31:346–52. doi: 10.1053/sarh.2002.31553. [DOI] [PubMed] [Google Scholar]
- 10.Poyanli A, Poyanli O, Sencer S, Akan K, Sayrak H, et al. Vertebral sarcoidosis: imaging findings. Eur Radiol. 2000;10:92–4. doi: 10.1007/s003300050011. [DOI] [PubMed] [Google Scholar]
- 11.Binicier O, Sari I, Sen G, Onen F, Akkoc N, et al. Axial sarcoidosis mimicking radiographic sacroiliitis. Rheumatol Int. 2009;29:343–5. doi: 10.1007/s00296-008-0677-6. [DOI] [PubMed] [Google Scholar]
- 12.Lisle D, Mitchell K, Crouch M, Windsor M. Sarcoidosis of the thoracic and lumbar spine: imaging findings with an emphasis on magnetic resonance imaging. Australas Radiol. 2004;48:404–7. doi: 10.1111/j.0004-8461.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 13.Mangino D, Stover DE. Sarcoidosis presenting as metastatic bony disease. A case report and review of the literature on vertebral body sarcoidosis. Respiration. 2004;71:292–4. doi: 10.1159/000077430. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 15.Stechschulte SA, Kirsner RS, Federman DG. Vitamin D: bone and beyond, rationale and recommendations for supplementation. Am J Med. 2009;122:793–802. doi: 10.1016/j.amjmed.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Sharma OP. Vitamin D, calcium, and sarcoidosis. Chest. 1996;109:535–9. doi: 10.1378/chest.109.2.535. [DOI] [PubMed] [Google Scholar]
- 17.Sharma OP. Vitamin D and sarcoidosis. Curr Opin Pulm Med. 2010;16:487–8. doi: 10.1097/MCP.0b013e32833cbc81. [DOI] [PubMed] [Google Scholar]
- 18.Nam HS, Shin MH, Zmuda JM, Leung PC, Barrett-Connor E, et al. Race/ethnic differences in bone mineral densities in older men. Osteoporos Int. 2010;21:2115–23. doi: 10.1007/s00198-010-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason RS, Frankel T, Chan YL, Lissner D, Posen S. Vitamin D conversion by sarcoid lymph node homogenate. Ann Intern Med. 1984;100:59–61. doi: 10.7326/0003-4819-100-1-59. [DOI] [PubMed] [Google Scholar]
- 20.Adams JS, Diz MM, Sharma OP. Effective reduction in the serum 1, 25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia with short-course chloroquine therapy. Ann Intern Med. 1989;111:437–8. doi: 10.7326/0003-4819-111-5-437. [DOI] [PubMed] [Google Scholar]
- 21.Hamada K, Nagai S, Tsutsumi T, Izumi T. Ionized calcium and 1, 25-dihydroxyvitamin D concentration in serum of patients with sarcoidosis. Eur Respir J. 1998;11:1015–20. doi: 10.1183/09031936.98.11051015. [DOI] [PubMed] [Google Scholar]
- 22•.Kavathia D, Buckley JD, Rao D, Rybicki B, Burke R. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med. 2010;104:564–70. doi: 10.1016/j.rmed.2009.12.004. This study has defined a relationship between elevated Vit D-1,25 and chronic sarcoidosis, thus adding further evidence of the role of vitamin D in this autoimmune disorder.
- 23.Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS ONE. 2010;5:e9193. doi: 10.1371/journal.pone.0009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niewold TB, Clark DN, Salloum R, Poole BD. Interferon alpha in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:948364. doi: 10.1155/2010/948364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kariuki SN, Franek BS, Kumar AA, Arrington J, Mikolaitis RA, et al. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R151. doi: 10.1186/ar3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha, 25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1987;84:3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, et al. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 29.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 30.Swaisgood CM, Oswald-Richter K, Moeller SD, Klemenc JM, Ruple LM, et al. Development of a sarcoidosis murine lunggranuloma model using mycobacterium sodA. Am J Respir Cell Mol Biol. 2010;26:26. doi: 10.1165/rcmb.2009-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res Hoboken. 2010;62:1515–26. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 32.Montemurro L, Fraioli P, Riboldi A, Delpiano S, Zanni D, et al. Bone loss in prednisone treated sarcoidosis: a two-year follow-up. Ann Ital Med Int. 1990;5:164–8. [PubMed] [Google Scholar]
- 33.Rizzato G, Tosi G, Mella C, Montemurro L, Zanni D, et al. Prednisone-induced bone loss in sarcoidosis: a risk especially frequent in postmenopausal women. Sarcoidosis. 1988;5:93–8. [PubMed] [Google Scholar]
- 34.Sweiss NJ, Patterson K, Sawaqed R, Jabbar U, Korsten P, et al. Rheumatologic manifestations of sarcoidosis. Semin Respir Crit Care Med. 2010;31:463–73. doi: 10.1055/s-0030-1262214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–41. doi: 10.1038/nature09102. This thought-provoking and somewhat controversial report has provided a potential explanation for the differential efficacy of corticosteroids in treatment of SLE.
- 36.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 37.Callejas-Rubio JL, Ortego-Centeno N, Lopez-Perez L, Benticuaga MN. Treatment of therapy-resistant sarcoidosis with adalimumab. Clin Rheumatol. 2006;25:596–7. doi: 10.1007/s10067-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 38.Sweiss NJ, Barnathan ES, Lo K, Judson MA, Baughman R. C-reactive protein predicts response to infliximab in patients with chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:49–56. [PubMed] [Google Scholar]
- 39.Garg S, Garg K, Altaf M, Magaldi JA. Refractory vertebral sarcoidosis responding to infliximab. J Clin Rheumatol. 2008;14:238–40. doi: 10.1097/RHU.0b013e318181b45a. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal S, Bhagat S, Dasgupta B. Sarcoid sacroiliitis: successful treatment with infliximab. Ann Rheum Dis. 2009;68:283. doi: 10.1136/ard.2007.087155. [DOI] [PubMed] [Google Scholar]
- 41.Moravan M, Segal BM. Treatment of CNS sarcoidosis with infliximab and mycophenolate mofetil. Neurology. 2009;72:337–40. doi: 10.1212/01.wnl.0000341278.26993.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossman MD, Newman LS, Baughman RP, Teirstein A, Weinberger SE, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:201–8. [PubMed] [Google Scholar]
- 43.Ulbricht KU, Stoll M, Bierwirth J, Witte T, Schmidt RE. Successful tumor necrosis factor alpha blockade treatment in therapy-resistant sarcoidosis. Arthritis Rheum. 2003;48:3542–3. doi: 10.1002/art.11357. [DOI] [PubMed] [Google Scholar]
- 44.Sweiss NJ, Noth IN, Baughman RP, Curran J, Naureckas E, et al. 52-Week Trial Results of Adalimumab as Novel Therapy for Refractory, Progressive Pulmonary Sarcoidosis; American Thoracic Society 2010 International Conference; New Orleans. 2010; LA Abstract A2370. [Google Scholar]
- 45.Menon Y, Cucurull E, Reisin E, Espinoza LR. Interferon-alpha-associated sarcoidosis responsive to infliximab therapy. Am J Med Sci. 2004;328:173–5. doi: 10.1097/00000441-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Ishibashi K, Kodama M, Hanada S, Arima T. Tumor necrosis factor-beta and hypercalcemia. Leuk Lymphoma. 1992;7:409–17. doi: 10.3109/10428199209049796. [DOI] [PubMed] [Google Scholar]
- 47.Mundy GR. Incidence and pathophysiology of hypercalcemia. Calcif Tissue Int. 1990;46(Suppl):S3–10. doi: 10.1007/BF02553287. [DOI] [PubMed] [Google Scholar]
- 48.Bachwich PR, Lynch JP, 3rd, Larrick J, Spengler M, Kunkel SL. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol. 1986;125:421–5. [PMC free article] [PubMed] [Google Scholar]
- 49.Adams JS, Sharma OP, Diz MM, Endres DB. Ketoconazole decreases the serum 1, 25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia. J Clin Endocrinol Metab. 1990;70:1090–5. doi: 10.1210/jcem-70-4-1090. [DOI] [PubMed] [Google Scholar]
- 50.Rizzato G, Colombo P. Nephrolithiasis as a presenting feature of chronic sarcoidosis: a prospective study. Sarcoidosis Vasc Diffuse Lung Dis. 1996;13:167–72. [PubMed] [Google Scholar]
- 51.Dawson-Hughes B. A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab. 2008;93:2463–5. doi: 10.1210/jc.2008-0926. [DOI] [PubMed] [Google Scholar]
- 52.Teitelbaum SL, Seton MP, Saag KG. Should bisphosphonates be used for long-term treatment of glucocorticoid-induced osteoporosis? Arthritis Rheum. 2010;8:8. doi: 10.1002/art.30135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arai T, Inoue Y, Hayashi S, Yamamoto S, Sakatani M. Risedronate induced BOOP complicated with sarcoidosis. Thorax. 2005;60:613–4. doi: 10.1136/thx.2005.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rolla G, Bucca C, Brussino L. Bisphosphonate-induced broncho-constriction in aspirin-sensitive asthma. Lancet. 1994;343:426–7. doi: 10.1016/s0140-6736(94)91267-x. [DOI] [PubMed] [Google Scholar]
- 55.Pena de la Vega L, Fervenza FC, Lager D, Habermann T, Leung N. Acute granulomatous interstitial nephritis secondary to bisphosphonate alendronate sodium. Ren Fail. 2005;27:485–9. doi: 10.1081/jdi-65397. [DOI] [PubMed] [Google Scholar]
- 56.Buysschaert M, Cosyns JP, Barreto L, Jadoul M. Pamidronate-induced tubulointerstitial nephritis with Fanconi syndrome in a patient with primary hyperparathyroidism. Nephrol Dial Transplant. 2003;18:826–9. doi: 10.1093/ndt/gfg044. [DOI] [PubMed] [Google Scholar]
- 57.Smetana S, Michlin A, Rosenman E, Biro A, Boaz M, et al. Pamidronate-induced nephrotoxic tubular necrosis—a case report. Clin Nephrol. 2004;61:63–7. doi: 10.5414/cnp61063. [DOI] [PubMed] [Google Scholar]
- 58.Thiebaud D, Sauty A, Burckhardt P, Leuenberger P, Sitzler L, et al. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997;61:386–92. doi: 10.1007/s002239900353. [DOI] [PubMed] [Google Scholar]