Abstract
Recent studies have indicated that inhibitors of the synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE) may have direct neuroprotective actions since they reduce infarct volume after ischemia reperfusion in the brain without altering blood flow. To explore this possibility, the present study used organotypic hippocampal slice cultures subjected to oxygen-glucose deprivation (OGD) and reoxygenation to examine whether 20-HETE is released by organotypic hippocampal slices after OGD and whether it contributes to neuronal death through the generation of ROS and activation of caspase-3. The production of 20-HETE increased twofold after OGD and reoxygenation. Blockade of the synthesis of 20-HETE with N-hydroxy-N′-(4-butyl-2-methylphenol)formamidine (HET0016) or its actions with a 20-HETE antagonist, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid, reduced cell death, as measured by the release of lactate dehydrogenase and propidium iodide uptake. Administration of a 20-HETE mimetic, 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-20-HEDE), had the opposite effect and increased injury after OGD. The death of neurons after OGD was associated with an increase in the production of ROS and activation of caspase-3. These effects were attenuated by HET0016 and potentiated after the administration of 5,14-20-HEDE. These findings indicate that the production of 20-HETE by hippocampal slices is increased after OGD and that inhibitors of the synthesis or actions of 20-HETE protect neurons from ischemic cell death. The protective effect of 20-HETE inhibitors is associated with a decrease in superoxide production and activation of caspase-3.
Keywords: 20-hydroxyeicosatetraenoic acid, brain, ischemic injury, superoxide, caspase-3
20-hydroxyeicosatetraenoic acid (20-HETE) is a potent vasoconstrictor that is produced from ω-hydroxylation of arachidonic acid (AA) by cytochrome P-450 (CYP) enzymes in cerebral arteries (8, 12). It is also produced by brain tissue (39). Plasma levels of 20-HETE increase after transient cerebral ischemia (26), and inhibitors of the synthesis and/or action of 20-HETE markedly reduce infarct size after cerebral ischemia (26, 28, 31, 39). The mechanism of the neuroprotective effects of inhibitors of the synthesis and/or actions of 20-HETE remains unknown. They were initially assumed to improve cerebral perfusion. However, recent studies (28, 31) have demonstrated that inhibitors of the synthesis of 20-HETE have no effect on regional cerebral blood flow during the ischemic period and only attenuate the delayed postischemic fall in cerebral blood flow. Moreover, the previous finding that inhibitors of the synthesis of 20-HETE can reduce infarct size in the brain even when administered 4 h after reperfusion (26) suggests that these drugs may enhance the survival of neurons after ischemic injury independent of their effects on cerebral blood flow.
In this regard, there is increasing evidence that 20-HETE stimulates oxidative stress through several mechanisms, including the activation of NADPH oxidase, phosphorylation and uncoupling of nitric oxide synthase, and stimulation of ROS by mitochondria (6, 11, 20). More recently, 20-HETE has been reported to increase the susceptibility of renal epithelial cells to ischemic injury by increasing the generation of superoxide, which activates caspase-3, leading to apoptosis and cell death (23). Inhibitors of the synthesis of 20-HETE have also been reported to block apoptosis after myocardial ischemia-reperfusion injury in the rat (19) and markedly reduce infarct size in the dog (9, 10, 24). These observations have led to the current hypothesis that the beneficial effect of inhibitors of the synthesis of 20-HETE on ischemia-reperfusion injury in the brain may be due to a reduction in oxidative stress and inhibition of apoptosis. To test this hypothesis, the present study examined the effects of a selective inhibitor of the synthesis of 20-HETE, N-hydroxy-N-(4-butyl-2-methylphenyl)formamidine (HET0016) (21, 33), a chemically dissimilar 20-HETE antagonist, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (6,15-20-HEDE), and a 20-HETE mimetic, 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-20-HEDE) (42), on the responses of organotypic hippocampal slices to oxygen-glucose deprivation (OGD) followed by reoxygenation in vitro. This is a well-established model of cerebral ischemic injury that exhibits a rapid decline in ATP levels, increased release of glutamate, a rise in intracellular Ca2+ concentration, and the generation of ROS, mitochondrial dysfunction, and cell death by both necrotic and apoptotic pathways (4, 7, 25, 36, 40).
MATERIALS AND METHODS
Chemicals.
HET0016, 6,15-20-HEDE, and 5,14-20-HEDE were synthesized by J. R. Falck. Manganese III tetrakis (1-methyl-4-pyridyl)porphyrin pentachloride (MnTMPyP) was purchased from Enzo Life Sciences (Plymouth Meeting, PA). Propidium iodide (PI) and dihydroethidium (DHE) were obtained from Molecular Probes (Eugene, OR).
Organotypic hippocampal slice cultures.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. The hippocampal slice cultures were prepared according to the method of Stoppini et al. (35). Briefly, 5- to 6-day-old neonatal Sprague-Dawley rats (Harlan, Madison, WI) were decapitated, and the brains were quickly removed. The hippocampus was quickly dissected and cut into 400-μm transverse sections with a tissue slicer (Stoelting, Wood Dale, IL). Slices were placed on 0.4-μm porous membrane inserts (Millicell-CM, Millipore, Bedford, MA) in six-well culture plates containing 1 ml of culture medium containing 50% minimal essential medium (MEM), 30% HBSS, 20% heat-inactivated horse serum, 1 mmol/l l-glutamine, 30 mmol/l HEPES, and 13 mmol/l d-glucose. Hippocampal slices were incubated at 35°C and 5% CO2 for 7 days, during which time the culture medium was changed 3 times/wk before use in an experiment.
OGD protocol.
Cultures of hippocampal slices were washed twice with Dulbecco's PBS and then transferred into new six-well plates containing 1 ml of glucose- and serum-free deoxygenated DMEM, which was bubbled with 95% N2-5% CO2 for 1 h. Cultured hippocampal slices were incubated at 37°C in a humidified anaerobic incubator with a gas mixture containing 95% N2-5% CO2 for 90 min. Control cultures were incubated under normoxic conditions in serum-free DMEM supplemented with 4.5 mg/ml d-glucose. At the end of the OGD period, the cultured hippocampal slices were returned to normoxic conditions in serum-free DMEM containing glucose for a 2-h recovery period. The effects of the 20-HETE inhibitors or agonist on cell survival were studied after the addition of the compounds to the cell culture media 30 min before the exposure to OGD for 90 min followed by a 2-h recovery period in a medium containing glucose and oxygen. In these experiments, HET0016 (10 μM, an inhibitor of the synthesis of 20-HETE), 6,15-20-HEDE (10 μM, a 20-HETE antagonist), 5,14-20-HEDE (10 and 30 μM, a 20-HETE mimetic), or vehicle (ethanol) was added to the media of hippocampal slice cultures in a volume of 1 μl/ml media.
Lactate dehydrogenase activity assay.
Cell death was assessed by measurements of lactate dehydrogenase (LDH) release into the incubation medium. LDH activity was measured from the reduction of NAD+ after the oxidative conversion of l-lactate to pyruvate using an ACE Autoanalyzer Clinical Chemistry System (Alfa Wasserman, West Caldwell, NJ). LDH activity was normalized by the protein content of the hippocampal slices as measured by the Bradford method and expressed in units of LDH activity per milligram of tissue protein.
PI assay.
Cell damage was assessed by the uptake of PI (Molecular Probes) as previously described (14). PI (4.6 μg/ml) was added to culture medium 30 min before the end of the 2-h recovery period. PI fluorescence in the fields was visualized at ×5 magnification using a confocal laser microscope (LSM 510, Zeiss, Jena, Germany) equipped with HeNe laser with an excitation wavelength of 536 nm and an emission wavelength of 617 nm. Approximately 200- to 300-μm z-stack images of each treatment were flattened using the extended focus module of Axiovision software (Zeiss), and PI fluorescence intensities were quantified using the automatic measurement module of Axiovision software. Results are expressed as fold changes in fluorescence intensity relative to those seen in paired control hippocampal slices incubated under normoxic conditions.
Superoxide production.
The production of superoxide in hippocampal slice cultures after OGD was assessed by measuring DHE oxidation product fluorescence using a fluorescence microtiter plate reader (Biotech Synergy II, Highland Park, Winooski, VT) as previously described (6, 17). DHE (final concentration: 10 μM) was added to the culture media (4 slices/well) at the beginning of the reoxygenation period. The plate was read at 2-min intervals for 60 min using an excitation wavelength of 530 nm and an emission wavelength of 620 nm. MnTMPyP, a cell-permeant SOD mimetic, was added at a concentration of 50 μM to paired slices to confirm that the fluorescence detected was derived from superoxide generated during the incubation period. DHE fluorescence was expressed as intensity per milligram of tissue protein. At the end of the incubation period, slices were examined using a confocal laser microscope (LSM 510, Zeiss) equipped with HeNe laser at an excitation wavelength of 561 nm and an emission wavelength of 575 nm to identify the regions of brain slices in which superoxide was generated.
Measurement of 20-HETE levels.
The production of 20-HETE in the organotypic cultures of hippocampal slices was measured using liquid chromatography-tandem mass spectrometry. HET0016 (10 μM) or vehicle was added to the culture media 30 min before OGD. Slices were homogenized in a 10 mmol/l potassium phosphate buffer (pH 7.7), and 10 ng of an internal standard (20-HETE-d6; Cayman Chemicals, Ann Arbor, MI) were added to each sample. Samples were then extracted with ethyl acetate. The ethyl acetate layer was separated, dried under nitrogen, and reconstituted in 50 μl of a 50% solution of methanol in water. The metabolites of AA were separated by HPLC on a C18 column (150 × 2.1 mm, 3 μm, Betabasic, Thermo Hypersil-Keystone, Bellefonte, PA) at a flow rate of 0.2 ml/min using an isocratic elution with a 51:9:40:0.01 mixture of acetonitrile-methanol-water-acetic acid for 30 min followed by a step gradient to a 68:13:19:0.01 mixture of acetonitrile-methanol-water-acetic acid for 25 min. The effluent was ionized using a negative ion electrospray (450°C, 4500 V) with the collision-activated dissociation gas set at 7 l/min. All transitions had a scan time of 0.2 s and a unit resolution in both Q1 and Q3 sets at 0.7 ± 0.1 full width at half-maximum. The peaks eluting with a mass/charge ratio of 319 > 301 (HETEs and epoxyeicosatrienoic acids), 337 > 319 (dihydroxyeicosatetraenoic acids), 319 > 245 (20-HETE), 325 > 251 (20-HETE-d6), 351 > 271 (PGD2 and PGE2), 353 > 309 (PGF2α), 369 > 245 (PGF1α), and 369 > 195 (thromboxane A2) were monitored in the multiple reaction monitoring mode using a triple quadrapole mass spectrometer (ABI 3000, Applied Biosystems, Foster City, CA). The ratio of ion abundances in the peaks of interest versus that seen in the internal standard were determined and compared with four-point individual standard curves generated for each sample run over a range from 0.2 to 10 ng.
Immunohistochemistry.
Organotypic hippocampal slices were fixed for 2 h in a 4% solution of paraformaldehyde and embedded in paraffin, and 6-μm paraffin sections were prepared and mounted on slides. Sections were deparaffinized in xylene and rehydrated with step down concentrations of ethanol. Antigens were retrieved by an incubation for 30 min at 98°C in 1 mM EDTA plus 0.05% Tween 20. Sections were incubated with a 1:100 dilution of a polyclonal goat CYP4A primary antibody (cat. no. 299230, Daiichi Pure Chemicals, Tokyo, Japan) in 1% rabbit serum in PBS overnight followed by a 1:100 dilution of an Alexa fluor 488-conjugated rabbit anti-goat secondary antibody (cat. no. A-21222, Invitrogen, Eugene, OR) for 90 min. Slides were counterstained with a 0.001% solution of Evans blue to quench endogenous fluorescence and to reveal cellular structures when viewed with a rhodamine filter and coverslipped with Permafluor mounting media (cat no. TA-30-FM, Thermo Scientific, Hudson, NH). Negative controls were performed by incubating the samples in 1% rabbit serum in PBS without primary antibody. Images were obtained using a Nikon Eclipse 55i fluorescence microscope equipped with a color camera using the FITC and rhodamine filters.
Caspase-3 activity.
Caspase-3 activity was measured in hippocampal slices using a commercially available kit (cat. no. K105-100, BioVision, Mountain View, CA) following the manufacturer's instructions. The assay is based on the detection of cleavage of the substrate DEVD-AFC, which was measured using a fluorescence microtiter plate reader (Biotech Synergy II) equipped with a 400-nm excitation filter and a 505-nm emission filter. The results were normalized to tissue protein level, and data are expressed as fold changes in intensity over that seen in the control group.
Western blot analysis.
Anti-cleaved caspase-3 antibody (cat. no. 9664) was obtained from Cell Signaling Technology (Danvers, MA). Anti-β-actin antibody (cat. no. SC-47778) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Slices were homogenized in 1× RIPA buffer (Millipore) with 2% Halt TM protease inhibitors and 1% Halt TM phosphatase inhibitors (Pierce Biotechnology, Rockford, IL). The lysates were spun down at 11,000 g for 15 min, and the protein concentrations of the supernatant were measured using the Bradford method (Bio-Rad, Hercules, CA). Aliquots of homogenate protein (70 μg) were separated on a 15% SDS-PAGE gel and transferred to a nitrocellulose membrane (Bio-Rad). The membrane was blocked in 10% milk and incubated with a primary antibody (1:1,000 for cleaved caspase-3 and 1:3,000 for β-actin) overnight at 4°C. After a subsequent wash with TBS and 0.1% Tween 20, membranes were incubated with horseradish peroxidase-coupled secondary anti-rabbit (1:3,000, Santa Cruz Biotechnology) or anti-mouse (1:5,000, Bio-Rad) antibodies at room temperature for 1 h. Protein bands were visualized with the Amersham ECL Plus Western blot detection system (GE Healthcare, Pistcataway, NJ).
Statistical analysis.
Data are presented as mean values ± SE. The significance of differences in mean values between treatment groups was assessed using one-way ANOVA followed by a Holm-Sidak post hoc test. The significance of differences in mean values of DHE fluorescence intensity between treatment groups was assessed using two-way ANOVA for repeated measures followed by a Holm-Sidak post hoc test. P values of <0.05 were considered significant.
RESULTS
Effect of 20-HETE inhibition on OGD- and reoxygenation-induced cell death.
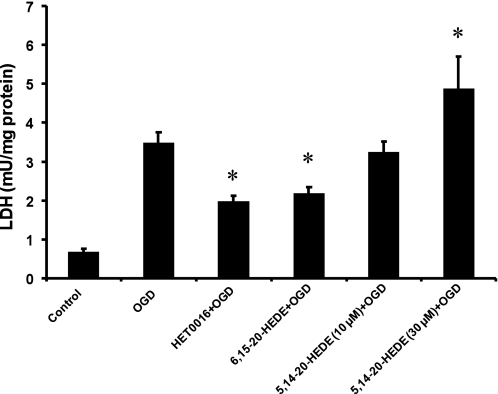
Exposure of organotypic hippocampal slices to OGD for 90 min followed by 2 h of recovery increased LDH release by approximately fivefold compared with the level seen in control slices. Pretreatment of hippocampal slices with the 20-HETE synthesis inhibitor HET0016 or the 20-HETE antagonist 6,15-20-HEDE significantly reduced LDH release by the hippocampal slices subjected to OGD by 45% and 37%, respectively. Pretreatment with the 20-HETE mimetic 5,14-20-HEDE (10 μM) had no effect on the release of LDH, whereas pretreatment of the slices with a higher concentration of the 20-HETE mimetic (30 μM) increased the release of LDH by 40% over the level seen in slices subjected to OGD alone (Fig. 1). Similar effects were observed using acute hippocampal slices prepared from adult rats, but the results were more consistent using slices from newborn animals because they survive better in culture (data not shown).
Fig. 1.
Effects of N-hydroxy-N-(4-butyl-2-methylphenyl)formamidine (HET0016), 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (6,15-20-HEDE), and 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-20-HEDE) on oxygen-glucose deprivation (OGD)- and reoxygenation-induced lactate dehydrogenase (LDH) release. HET0016 (10 μM), 6,15-20-HEDE (10 μM), 5,14-20-HEDE (10 and 30 μM), or vehicle (ethanol) were added to the media of organotypic hippocampal slices 30 min before the slices were subjected to OGD and reoxygenation and were present throughout the experiment. LDH release was measured at the end of the 2-h reoxygenation period in the incubation media. Results are mean values ± SE of at least 5 experiments/group. *P < 0.05 vs. the OGD group pretreated with vehicle.
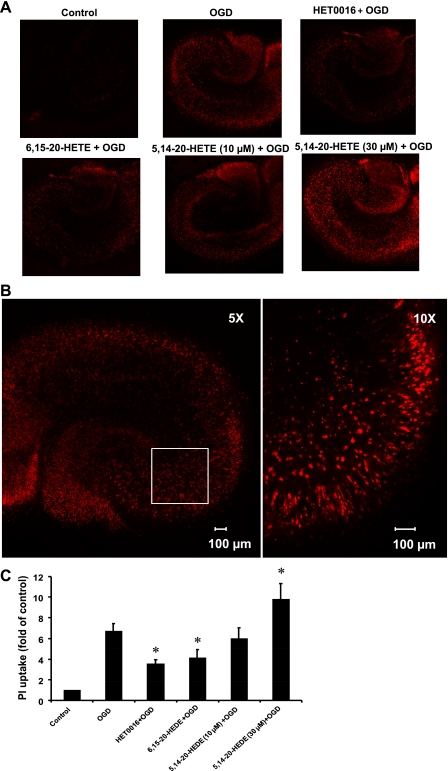
The effect of 20-HETE inhibition on the uptake of PI by hippocampal slices subjected to OGD is shown in Fig. 2. Pretreatment of the hippocampal slices with the 20-HETE synthesis inhibitor HET0016 or with the 20-HETE antagonist 6,15-20-HEDE reduced the uptake of PI after OGD by 43% and 38%, respectively, compared with the levels seen in the vehicle-treated slices subjected to OGD. On the other hand, administration of the 20-HETE analog 5,14-20-HEDE (30 μM) increased PI uptake by 36%, whereas a lower concentration (10 μM) had no effect compared with the vehicle-treated slices subjected to OGD (Fig. 2C). Representative images of PI uptake in hippocampal slices after OGD that were treated with vehicle, HET0016, and the 20-HETE agonist and antagonist are shown in Fig. 2A. Exposure of the slices to OGD for 90 min followed by 2 h of reoxygenation induced damage of neurons in the CA1, CA3, and dentate gyrus regions of the hippocampus (Fig. 2, A and B) and increased PI staining sixfold (Fig. 2C). The degree of PI staining after OGD was significantly reduced in the slices pretreated with HET0016 or the 20-HETE antagonist 6,15–20-HETE and increased in the slices pretreated with 30 μM of the 20-HETE mimetic 5,14-20-HEDE (Fig. 2C).
Fig. 2.
Effects of HET0016, 6,15-20-HEDE, and 5,14-20-HEDE on OGD- and reoxygenation-induced increases in propidium iodide (PI) uptake. Hippocampal slices were pretreated with HET0016 (10 μM), 6,15-20-HEDE (10 μM), 5,14-20-HEDE (10 and 30 μM), or vehicle for 30 min before OGD and throughout the experiment. Slices were exposed to 90 min of OGD followed by 2 h of reoxygenation. PI uptake was analyzed at the end of the reoxygenation period by confocal microscopy using a ×5 objective (flattened images of 200- to 300-μm z-stacks). A: representative PI fluorescence images indicating neuronal cell damage in the hippocampal slices. B: ×10 magnified image of a region (box) of the same hippocampal slice showing a close-up view of the cellular damage. C: quantification of cell death using total PI fluorescence. Results are expressed as fold increases over control. Values in C are means ± SE of 4 experiments/group. *P < 0.05 vs. the OGD group.
Effect of HET0016 on 20-HETE levels in hippocampal slices.
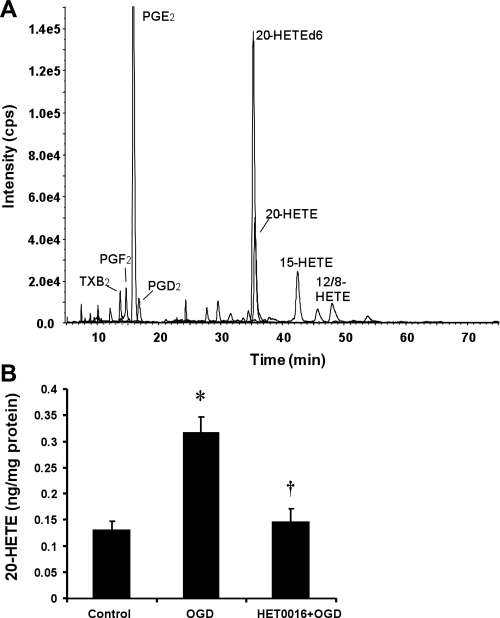
Hippocampal slices incubated under control normoxic conditions produced primarily PGE2, thromboxane, and 12-, 15-, and 20-HETE (Fig. 3A). Exposure to OGD followed by reoxygenation increased the production of 20-HETE in the hippocampal slices by about twice of that seen in control hippocampal slices (Fig. 3B). Pretreatment with HET0016 (10 μM) reduced 20-HETE levels in slices exposed to OGD to the levels seen in the control group (Fig. 3B).
Fig. 3.
Effect of HET0016 on 20-15-hydroxyeicosatetraenoic acid (20-HETE) levels in hippocampal slice cultures subjected to OGD followed by reoxygenation. Hippocampal slices were treated with HET0016 (10 μM) or vehicle for 30 min before OGD and throughout the experiment. Free levels of 20-HETE in hippocampal slices were determined by liquid chromotography-tandem mass spectroscopy (LC-MS/MS) 15 min after reoxygenation. A: representative LC-MS/MS chromatogram. TXB2, thromboxane B2. B: bar graph showing the levels of 20-HETE under control conditions, after OGD followed by reoxygenation, and after OGD in slices pretreated with HET0016 (10 μM). Values are means ± SE of 4 experiments/group. *P < 0.05 vs. the control group; †P < 0.05 vs. the OGD group.
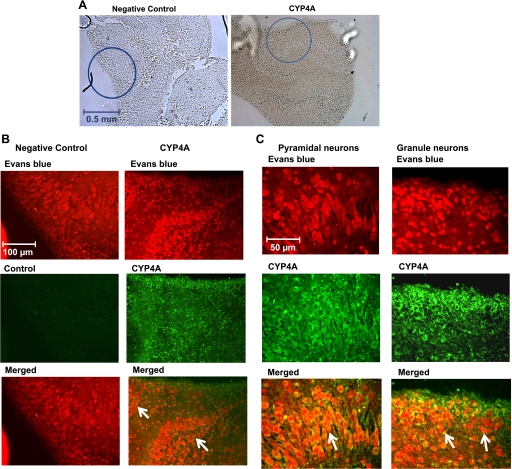
Immunohistochemical staining was also performed for CYP4A protein to better identify the cells that express these enzymes in the hippocampal slices. Representative sections are shown in Fig. 4, A–C. CYP4A protein was widely expressed in cell bodies of both granule and pyramidal neurons throughout the hippocampal region.
Fig. 4.
Immunohistochemistry for cytochrome P-450 (CYP)4A protein in organotypic hippocampal slices. Hippocampal sections were stained with CYP4A antibody followed by an Alexa fluor 488 secondary antibody (green). Sections were counterstained with 0.001% Evans blue to suppress autofluorescence and to allow cellular structures to be viewed using a rhodamine filter. Hippocampal sections incubated without primary antibody served as negative controls. A: low-magnification images (×5 objective: approximately ×50) of the hippocampal tissue showing the regions (circles) from where the images of immunohistochemical staining for CYP4A and negative control were taken. B: intermediate-magnification images (approximately ×250) of the hippocampal region showing that the CYP4A enzyme is widely expressed in the cell bodies of many neurons (merged images, indicated by arrows). C: high-magnification images (approximately ×500) showing CYP4A-positive neuronal cells in the hippocampal region showing that CYP4A protein is expressed in the cell bodies of both pyramidal and granule neurons (merged images, indicated by arrows).
Effects on the generation of ROS in hippocampal slices.
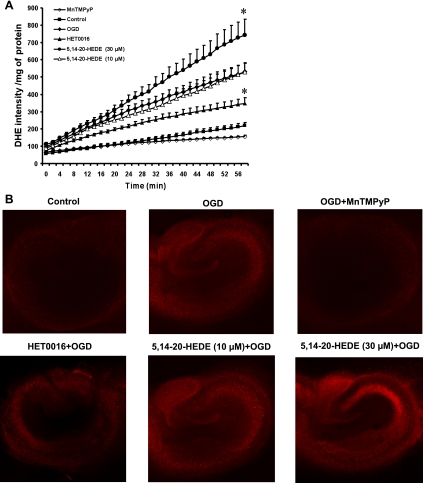
Organotypic cultures of hippocampal slices subjected to OGD exhibited a 2.5-fold increase in DHE fluorescence compared with the levels seen in the control slices incubated under normoxic conditions. Addition of the antioxidant MnTMPyP (50 μM) markedly reduced the generation of superoxide, as revealed by a decrease in DHE fluorescence in cultured hippocampal slices subjected to OGD alone (Fig. 5A). Furthermore, the increase in fluorescence intensity was reduced by 35% in slices pretreated with HET0016 (10 μM). In contrast, the rate of rise of DHE fluorescence increased by 40% in slices pretreated with the 20-HETE mimetic 5,14-20-HEDE (30 μM). Pretreatment with a lower concentration of 5,14-20-HEDE (10 μM) had no effect on DHE staining compared with the OGD group (Fig. 5A). Representative images showing the pattern of DHE staining after OGD and treatment with the 20-HETE inhibitor and mimetic are shown in Fig. 5B. Exposure to OGD increased the staining of cell bodies throughout the hippocampus. The degree of DHE staining was markedly reduced by the administration of the SOD mimetic MnTMPyP and was significantly lower in slices pretreated with HET0016. Pretreatment of the slices with 30 μM of the 20-HETE mimetic 5,14-20-HEDE enhanced DHE staining, especially in cell bodies in the CA3 region.
Fig. 5.
Effect of HET0016 and 5,14-20-HEDE on superoxide production by hippocampal slices subjected to OGD and reoxygenation. Hippocampal slices were pretreated with vehicle, HET0016 (10 μM), and 5,14-20-HEDE (10 and 30 μM) 30 min before OGD and throughout the experiment. At the beginning of the reoxygenation period, slices were placed in fresh HBSS containing 10 μM dihydroethidium (DHE), and DHE fluorescence was measured using a fluorescence microtiter plate reader. Manganese III tetrakis (1-methyl-4-pyridyl)porphyrin pentachloride (MnTMPyP; 50 μM) was added to the slices exposed to OGD at the beginning of the reoxygenation period. A: quantitative comparison of DHE fluorescence intensity measured during the different experimental conditions as indicated in the graph. Values are means ± SE from 7 experiments/group. *P < 0.05 vs. the OGD group. B: representative images showing increased DHE fluorescence after exposure to OGD and treatment with the 20-HETE analog 5,14-20-HEDE or decreased levels after treatment with HET0016 or MnTMPyP.
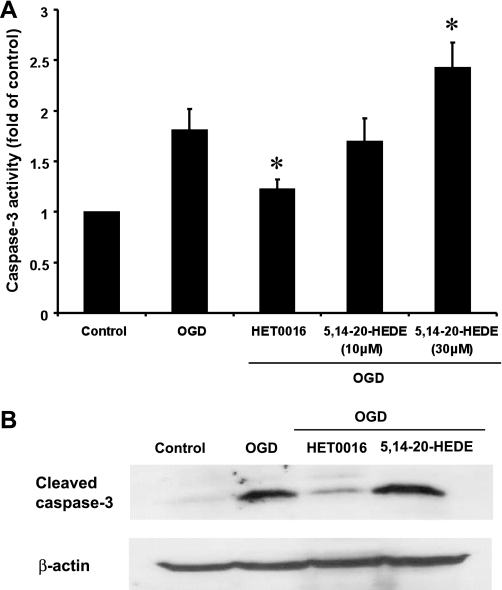
Effects of inhibition of the synthesis of 20-HETE on caspase-3 activity.
Exposure of hippocampal slices to OGD for 90 min followed by 2 h of reoxygenation increased caspase-3 activity relative to that seen in control slices incubated under normoxic conditions. Blockade of the synthesis of 20-HETE with HET0016 (10 μM) attenuated the increase in capase-3 activity in slices subjected to OGD by 35%. In contrast, administration of the 20-HETE mimetic 5,14-20-HEDE (30 μM) had the opposite effect and increased caspase-3 activity by 30%, whereas a lower concentration of 5,14-20-HEDE (10 μM) had no effect (Fig. 6A). Results of the Western blot analysis for cleaved caspase-3 are shown in Fig. 6B. The levels of cleaved capase-3 protein increased fivefold after OGD. Administration of HET0016 (10 μM) markedly reduced activated caspase-3 levels after OGD, whereas pretreatment of the slices with 5,14-20-HEDE (30 μM) had no additional effect relative to the levels seen after exposure to OGD alone.
Fig. 6.
Effects of HET0016 and 5,14-20-HEDE on caspase-3 activation in hippocampal slice cultures subjected to OGD followed by reoxygenation. Hippocampal slices were pretreated with vehicle, HET0016 (10 μM), or 5,14-20-HEDE (10 and 30 μM) 30 min before OGD and throughout the experiment. Slices were subjected to 90 min of OGD followed by 2 h of reoxygenation. A: comparison of caspase-3 activity measured using a fluorometric assay in the various groups. Values are means ± SE from 5 experiments/group. *P < 0.05 vs. the OGD group. B: representative Western blot showing caspase-3 protein levels in hippocampal slices under control conditions, after exposure to OGD and reoxygenation, or after pretreatment with the 20-HETE synthesis inhibitor HET0016 (10 μM) or the 20-HETE mimetic 5,14-20-HEDE (30 μM). β-Actin was used as a protein loading control.
DISCUSSION
Recent studies have indicated that inhibitors of the synthesis and/or action of 20-HETE markedly reduce infarct size after transient occlusion of the middle cerebral artery in the rat and primate (26, 28, 31, 39) but have no effect on cerebral perfusion during the ischemic period (28, 31). Moreover, inhibitors of 20-HETE synthesis are effective in reducing infarct size in the brain even when administered up to 4 h after reperfusion (26). The protective effect of inhibition of the synthesis of 20-HETE in vivo was associated with reduced levels of 20-HETE in cerebral tissue (28, 31, 39). The present study thus examined whether 20-HETE is synthesized and released in the brain after ischemic stress and whether it contributes to neuronal death through increased generation of ROS and activation of caspase-3-mediated apoptotic pathways. To explore this hypothesis, the present study used organotypic hippocampal slices subjected to OGD followed by reoxygenation as an in vitro model of ischemic injury to neurons (4, 7, 25, 36, 40).
The results of the present study indicate that the production of 20-HETE by hippocampal slices is increased after OGD. This is consistent with the view that Ca2+ levels increase after the depletion of ATP in cerebral ischemic tissue and that Ca2+ stimulates phospholipase A2 to release AA from membrane phospholipids, which is the rate-limiting step for the formation of 20-HETE. Indeed, previous studies have shown that the levels of AA and other fatty acids increase markedly in cerebrospinal fluid after ischemia-reperfusion injury (27, 32) and the activity of phospholipase A2 increases in neurons of hippocampal slices after an exposure to OGD and reoxygention (1). The results of the present study further demonstrate that blockade of the synthesis of 20-HETE with HET0016 and administration of 6,15-20-HEDE, a competitive inhibitor of the actions of 20-HETE (42), reduce neuronal cell death after OGD, as measured by LDH release and PI uptake. Conversely, pretreatment of the tissue with the 20-HETE mimetic 5,14-20-HEDE increases injury after OGD.
The mechanism(s) by which 20-HETE contributes to ischemia-reperfusion injury in the brain is unknown. Recent studies (6, 20) have indicated that 20-HETE increases the formation of superoxide in endothelial cells by uncoupling nitric oxide synthase and through the activation of NADPH oxidase, perhaps secondary to the marked rise in intracellular Ca2+ levels. Elevated production of free radicals has been previously shown to promote neuronal cell damage after cerebral ischemia-reperfusion injury by promoting the oxidation of cellular lipids, proteins, and DNA (5). Increased generation of ROS is also associated with increased necrosis and apoptosis of neurons (13, 18, 29, 37). Several reports have shown that the administration of SOD or catalase mimetics to reduce ROS levels has neuroprotective effects in both in vivo (2, 34) and in vitro models of cerebral ischemia (44, 45). The results of our present study demonstrate that the production of superoxide increases in hippocampal slices exposed to OGD and reoxygenation and that HET0016 partially blocks the increase in the formation of superoxide. The 20-HETE mimetic 5,14-20-HEDE had the opposite effect: to increase the production of superoxide. These findings suggest that elevations in the synthesis and release of 20-HETE by cultured hippocampal slices exposed to OGD contributes to the ischemic injury of the neurons by increasing the formation of ROS.
Previous studies (22, 38) have indicated that an increase in ROS production after ischemia and reperfusion activates a cascade of events leading to apoptosis of neurons and increases infarction after ischemia-reperfusion injury. Indeed, mice deficient in caspase-3 are resistant to ischemia-reperfusion injury in vivo (16), and upregulation of the antiapoptotic molecules that interact with this cascade attenuates ischemic injury to neurons in vivo and in vitro (15, 43). Caspase inhibitors protect against OGD-induced cell death in organotypic hippocampal slices (30) and reduce infarct size after ischemia-reperfusion injury in the brain (41). The results of the present study demonstrate that neuronal cell death in cultures of hippocampal slices exposed to OGD is associated with the activation of caspase-3 and that this effect is attenuated by pretreatment of the tissue with an inhibitor of the synthesis of 20-HETE. Moreover, administration of the 20-HETE mimetic 5,14-20-HEDE activates caspase-3 and augments the degree of apoptosis and neuronal injury after OGD and reoxygenation. These results suggest that increased synthesis of 20-HETE may mediate the proapoptotic effects in hippocampal slices subjected to OGD and reoxygenation partially by increasing the production of superoxide. Our data are consistent with those of a previous study (23) demonstrating that upregulation of the synthesis of 20-HETE significantly exacerbates cellular damage in renal cells subjected to ischemia-reperfusion injury and that the cell death is mediated by the activation of caspase-3 and was partially dependent on elevated 20-HETE-stimulated generation of free radicals. The present findings are also consistent with previous reports showing that administration of an inhibitor of the synthesis of 20-HETE has an antiapoptotic effect and reduces ischemia-reperfusion injury in the heart (19, 24) and that 20-HETE stimulates caspase-3 activity in rat cardiomyocytes (3).
In summary, the present study indicates that the production of 20-HETE by hippocampal slices is increased after OGD and that inhibitors of the synthesis of 20-HETE or actions of 20-HETE protect neurons in hippocampal slices from ischemic cell death. The protective effect of 20-HETE inhibitors appears to be associated with decreased production of superoxide and activation of caspase-3. Overall, these results indicate that inhibitors of the synthesis and/or actions of 20-HETE may have therapeutic potential as neuroprotective agents after ischemia-reperfusion injury in the brain by reducing 20-HETE-induced activation of the ROS system.
GRANTS
This work was funded in part by National Institutes of Health Grants HL-36279, HL-29587, HL-59996, and 1-UL-1-RR-031973.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.R., N.Z.G., J.R.F., D.R.H., and R.J.R. conception and design of research; M.R., S.N.K., D.G., and M.A.F. performed experiments; M.R., S.N.K., and N.Z.G. analyzed data; M.R., N.Z.G., and R.J.R. interpreted results of experiments; M.R., S.N.K., and D.G. prepared figures; M.R. drafted manuscript; M.R., S.N.K., D.G., D.R.H., and R.J.R. edited and revised manuscript; M.R. and R.J.R. approved final version of manuscript.
REFERENCES
- 1. Arai K, Ikegaya Y, Nakatani Y, Kudo I, Nishiyama N, Matsuki N. Phospholipase A2 mediates ischemic injury in the hippocampus: a regional difference of neuronal vulnerability. Eur J Neurosci 13: 2319–2323, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther 284: 215–221, 1998 [PubMed] [Google Scholar]
- 3. Bao Y, Wang X, Li W, Huo D, Shen X, Han Y, Tan J, Zeng Q, Sun C. 20-Hydroxyeicosatetraenoic acid induces apoptosis in neonatal rat cardiomyocytes through mitochondrial-dependent pathways. J Cardiovasc Pharmacol 57: 294–301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonde C, Noraberg J, Noer H, Zimmer J. Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygen-glucose deprivation of hippocampal slice cultures. Neuroscience 136: 779–794, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21: 2–14, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol 294: H1018–H1026, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Cho S, Liu D, Fairman D, Li P, Jenkins L, McGonigle P, Wood A. Spatiotemporal evidence of apoptosis-mediated ischemic injury in organotypic hippocampal slice cultures. Neurochem Int 45: 117–127, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol 507: 771–781, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gross ER, Nithipatikom K, Hsu AK, Peart JN, Falck JR, Campbell WB, Gross GJ. Cytochrome P450 omega-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. J Mol Cell Cardiol 37: 1245–1249, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res 68: 18–25, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther 321: 18–27, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol Heart Circ Physiol 266: H2098–H2107, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci 17: 5089–5100, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laake JH, Haug FM, Wieloch T, Ottersen OP. A simple in vitro model of ischemia based on hippocampal slice cultures and propidium iodide fluorescence. Brain Res Brain Res Protoc 4: 173–184, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Lawrence MS, Ho DY, Sun GH, Steinberg GK, Sapolsky RM. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci 16: 486–496, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA, Moskowitz MA. Caspase activation and neuroprotection in caspase-3-deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci USA 99: 15188–15193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang HL, Hilton G, Mortensen J, Regner K, Johnson CP, Nilakantan V. MnTMPyP, a cell-permeant SOD mimetic, reduces oxidative stress and apoptosis following renal ischemia-reperfusion. Am J Physiol Renal Physiol 296: F266–F276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res 3: 327–337, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lv X, Wan J, Yang J, Cheng H, Li Y, Ao Y, Peng R. Cytochrome P450 omega-hydroxylase inhibition reduces cardiomyocyte apoptosis via activation of ERK1/2 signaling in rat myocardial ischemia-reperfusion. Eur J Pharmacol 596: 118–126, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 294: L902–L911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morita-Fujimura Y, Fujimura M, Yoshimoto T, Chan PH. Superoxide during reperfusion contributes to caspase-8 expression and apoptosis after transient focal stroke. Stroke 32: 2356–2361, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Nilakantan V, Maenpaa C, Jia G, Roman RJ, Park F. 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol Renal Physiol 294: F562–F570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nithipatikom K, Gross ER, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, Gross GJ. Inhibition of cytochrome P450 omega-hydroxylase: a novel endogenous cardioprotective pathway. Circ Res 95: e65–e71, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord 4: 435–452, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, Hachiuma K, Minagawa T, Susumu T, Yoshida S, Nakaike S, Okuyama S, Harder DR, Roman RJ. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke 37: 1307–1313, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Pilitsis JG, Coplin WM, O'Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW. Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neurosci Lett 349: 136–138, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Poloyac SM, Zhang Y, Bies RR, Kochanek PM, Graham SH. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab 26: 1551–1561, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Ratan RR, Murphy TH, Baraban JM. Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem 62: 376–379, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Ray AM, Owen DE, Evans ML, Davis JB, Benham CD. Caspase inhibitors are functionally neuroprotective against oxygen glucose deprivation induced CA1 death in rat organotypic hippocampal slices. Brain Res 867: 62–69, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Renic M, Klaus JA, Omura T, Kawashima N, Onishi M, Miyata N, Koehler RC, Harder DR, Roman RJ. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 29: 629–639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saluja I, Song D, O'Regan MH, Phillis JW. Role of phospholipase A2 in the release of free fatty acids during ischemia-reperfusion in the rat cerebral cortex. Neurosci Lett 233: 97–100, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Seki T, Wang MH, Miyata N, Laniado-Schwartzman M. Cytochrome P450 4A isoform inhibitory profile of N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET0016), a selective inhibitor of 20-HETE synthesis. Biol Pharm Bull 28: 1651–1654, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Sharma SS, Gupta S. Neuroprotective effect of MnTMPyP, a superoxide dismutase/catalase mimetic in global cerebral ischemia is mediated through reduction of oxidative stress and DNA fragmentation. Eur J Pharmacol 561: 72–79, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Meth 37: 173–182, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Strasser U, Fischer G. Quantitative measurement of neuronal degeneration in organotypic hippocampal cultures after combined oxygen/glucose deprivation. J Neurosci Meth 57: 177–186, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal 5: 597–607, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci 22: 209–217, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka Y, Omura T, Fukasawa M, Horiuchi N, Miyata N, Minagawa T, Yoshida S, Nakaike S. Continuous inhibition of 20-HETE synthesis by TS-011 improves neurological and functional outcomes after transient focal cerebral ischemia in rats. Neurosci Res 59: 475–480, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol 35: 67–86, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Yang W, Guastella J, Huang JC, Wang Y, Zhang L, Xue D, Tran M, Woodward R, Kasibhatla S, Tseng B, Drewe J, Cai SX. MX1013, a dipeptide caspase inhibitor with potent in vivo antiapoptotic activity. Br J Pharmacol 140: 402–412, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu M, Alonso-Galicia M, Sun CW, Roman RJ, Ono N, Hirano H, Ishimoto T, Reddy YK, Katipally KR, Reddy KM, Gopal VR, Yu J, Takhi M, Falck JR. 20-hydroxyeicosatetraenoic acid (20-HETE): structural determinants for renal vasoconstriction. Bioorg Med Chem 11: 2803–2821, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem 85: 1026–1036, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Zhou M, Baudry M. EUK-207, a superoxide dismutase/catalase mimetic, is neuroprotective against oxygen/glucose deprivation-induced neuronal death in cultured hippocampal slices. Brain Res 1247: 28–37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou M, Dominguez R, Baudry M. Superoxide dismutase/catalase mimetics but not MAP kinase inhibitors are neuroprotective against oxygen/glucose deprivation-induced neuronal death in hippocampus. J Neurochem 103: 2212–2223, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]