Abstract
Cinaciguat (BAY 58–2667) is a novel nitric oxide (NO)-independent activator of soluble guanylate cyclase (sGC), which induces cGMP-generation and vasodilation in diseased vessels. We tested the hypothesis that cinaciguat might trigger protection against ischemia/reperfusion (I/R) in the heart and adult cardiomyocytes through cGMP/protein kinase G (PKG)-dependent generation of hydrogen sulfide (H2S). Adult New Zealand White rabbits were pretreated with 1 or 10 μg/kg cinaciguat (iv) or 10% DMSO (vehicle) 15 min before I/R or with 10 μg/kg cinaciguat (iv) at reperfusion. Additionally, adult male ICR mice were treated with either cinaciguat (10 μg/kg ip) or vehicle 30 min before I/R or at the onset of reperfusion (10 μg/kg iv). The PKG inhibitor KT5283 (KT; 1 mg/kg ip) or dl-propargylglycine (PAG; 50 mg/kg ip) the inhibitor of the H2S-producing enzyme cystathionine-γ-lyase (CSE) were given 10 and 30 min before cinaciguat. Cardiac function and infarct size were assessed by echocardiography and tetrazolium staining, respectively. Primary adult mouse cardiomyocytes were isolated and treated with cinaciguat before simulated ischemia/reoxygenation. Cinaciguat caused 63 and 41% reduction of infarct size when given before I/R and at reperfusion in rabbits, respectively. In mice, cinaciguat pretreatment caused a more robust 80% reduction in infarct size vs. 63% reduction when given at reperfusion and preserved cardiac function following I/R, which were blocked by KT and PAG. Cinaciguat also caused an increase in myocardial PKG activity and CSE expression. In cardiomyocytes, cinaciguat (50 nM) reduced necrosis and apoptosis and increased H2S levels, which was abrogated by KT. Cinaciguat is a novel molecule to induce H2S generation and a powerful protection against I/R injury in heart.
Keywords: BAY 58–2667, protein kinase G, infarction, cystathione-γ-lysase
during the last several years, a number of experimental and clinical studies have reported the efficacy of phosphodiesterase-5 (PDE-5) inhibitors in cardioprotection. We have demonstrated that PDE-5 inhibitors including sildenafil, vardenafil, and tadalafil protect against ischemia/reperfusion (I/R) injury in rabbits (16,23), mice (24, 20), and adult cardiomyocytes (5) subjected to simulated ischemia/reoxygenation (SI/RO). Sildenafil and vardenafil, when given at reperfusion, also reduced infarct size in rabbits (22). In addition, we demonstrated that sildenafil increased endothelial and inducible nitric oxide synthase expression in the heart (24), a novel off-target effect of the drug that could also contribute to enhancing cGMP levels through activation of soluble guanylate cyclase (sGC).
Increased oxidative stress during I/R injury can affect the heme-containing NO receptor of sGC by both decreasing its expression and potentially impairing NO-induced activation (15). As a result, cardioprotective therapies such as NO donors or other pharmacological agents that protect the heart through NO-dependent activation of sGC may not be as beneficial in the setting of I/R injury. PDE-5 inhibitors are exciting small molecules, but caution has to be exercised before their use in patients taking nitrates for angina (4). Therefore, compounds that activate NO-insensitive sGC with a profile of activity similar to organic nitrates/NO donors, but devoid of the problem of tolerance and the potential cytotoxic actions of NO, may be promising for reducing damage from I/R injury. Cinaciguat (BAY 58–2667) is one such compound that activates NO- and heme-independent sGC. This compound selectively triggers a state of sGC that is indistinguishable from the NO-insensitive oxidized/heme-free state of the enzyme (27). In the present study, we investigated the effect of cinaciguat in protection against I/R injury. Specifically, our goal was to determine the effect of cinaciguat on reduction of myocardial infarct size when given before ischemia and at the onset of reperfusion. We also determined if cinaciguat caused a reduction in necrosis and apoptosis in adult cardiomyocytes subjected to SI/RO. Since the gaseous molecule hydrogen sulfide (H2S) is involved in cardioprotection with the long acting PDE-5 inhibitor tadalafil (20) in a cGMP-dependent protein kinase (PKG)-mediated mechanism, we further hypothesized that cinaciguat might induce protection through PKG-mediated H2S generation.
MATERIALS AND METHODS
Drugs and Chemicals
KT5823 (KT), dl-propargylglycine (PAG), triphenyltetrazolium chloride, and DMSO (vehicle for cinaciguat) were purchased from Sigma-Aldrich (St. Louis, MO). Cinaciguat powder was kindly provided by Bayer Schering Pharma.
Animals
Male New Zealand white rabbits (2.6–3.2 kg) were used for the studies.
Adult male outbred ICR mice were supplied by Harlan Sprague Dawley (Indianapolis, IN).
Upon their arrival, the animals were allowed to readjust to the housing environment for at least 3 days before any experiment. Standard food and water were freely accessible for the animals used in these studies. The care and use of the animals were conducted in accordance with the guidelines of and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University, and experiments adhered to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals [DHHS Publication No. (NIH) 80–23; Office of Science and Health Reports, Bethesda, MD 20205].
Rabbit studies.
experimental protocol.
Rabbits were treated with cinaciguat as follows: 1) before ischemia, rabbits were given intravenous bolus of vehicle solution (10% DMSO), 1 μg/kg, or 10 μg/kg of cinaciguat 15 min before regional ischemia. 2) Before reperfusion, a bolus dose of vehicle solution (10% DMSO iv) or 10 μg/kg cinaciguat was given 5 min before initiation of reperfusion following 25 min of ischemia.
surgical preparation for the rabbit heart.
The rabbit model of I/R protocol has been described previously (16). Briefly, a left thoracotomy was performed through the fourth intercostal space, and the pericardium was opened to expose the heart. The left anterior descending coronary artery was occluded for 30 min followed by reperfusion for 180 min.
Mouse studies.
experimental protocol.
Mice were treated as follows: 1) before ischemia, adult male ICR mice were pretreated with either cinaciguat (10 μg/kg ip) or 10% DMSO 30 min before regional ischemia. The PKG inhibitor KT (1 mg/kg ip) was given 10 min before cinaciguat or 10% DMSO (control). The cystathionine-γ-lyase (CSE) inhibitor PAG (50 mg/kg ip) was given 30 min before ischemia. 2) Before reperfusion, a bolus dose of vehicle solution (10% DMSO iv) or 10 μg/kg cinaciguat (iv) was given 5 min before initiation of reperfusion following 25 min of ischemia.
myocardial infarction protocol.
The methodology of in vivo myocardial infarction protocol has been previously described in detail (20). A left thoracotomy was performed at the fourth intercostal space, and the heart was exposed by stripping the pericardium. The left anterior descending coronary artery was identified and occluded for 30 min followed by 24 h of reperfusion. At the onset of reperfusion, the air was expelled from the chest. The animals were extubated and then received intramuscular doses of analgesia (buprenex; 0.1 mg/kg sc) and antibiotic (gentamicin; 0.7 mg/kg im). Infarct size was measured by tetrazolium staining using computer morphometry (Bioquant imaging software) as described previously (19).
echocardiography.
Echocardiography was performed using the Vevo770 imaging system (VisualSonics, Toronto, Canada) before surgery (baseline) and 24 h after reperfusion before the animal was killed. Pentobarbital (30 mg/kg ip) was used for anesthesia and the procedure was carried out as previously described (19) to measure left ventricular (LV) end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD). LV fractional shortening (FS) was calculated as (LVEDD − LVESD)/LVEDD * 100.
isolation of adult ventricular cardiomyocytes.
The ventricular cardiomyocytes were isolated using an enzymatic technique as previously reported (5, 6). After 1 h of plating, the cardiomyocytes were treated with one of the following concentrations of cinaciguat (25 nM and 50 nM) and were incubated at 37°C and 5% CO2 for 1 h. The cardiomyocytes were then subjected to SI for 40 min by replacing the cell medium with an “ischemia buffer” that contained 118 mM NaCl, 24 mM NaHCO3, 1.0 mM NaH2PO4, 2.5 mM CaCl2-2H2O, 1.2 mM MgCl2, 20 mM sodium lactate, 16 mM KCl, and 10 mM 2-deoxyglucose (pH adjusted to 6.2) as reported previously (5, 6). In addition, the cells were incubated under hypoxic conditions at 37°C by adding N2 gas into a tri-gas incubator and maintained at 1–2% O2 and 5% CO2 during the entire SI period. Reoxygenation was accomplished by replacing the ischemic buffer with normal cell medium and resuming normoxic conditions. Cell necrosis and apoptosis were assessed after 1 or 18 h of reoxygenation, respectively.
evaluation of cell viability and apoptosis.
Cell viability was assessed by trypan blue exclusion assay as reported previously (5, 6). Cardiomyocyte apoptosis was analyzed by terminal deoxynucleotidyl transferase staining, using a commercial kit (BD Biosciences) that detects nuclear DNA fragmentation via a fluorescence assay as previously reported (5, 6).
PKG activity assay.
PKG activity was measured according to the manufacturer's instructions (Cyclex). Spectrophotometric absorbance was measured at 450 nm, and the results were normalized per milligrams of protein.
measurement of cGMP.
cGMP in lysates from cardiomyocytes was measured using a competitive immunoassay EIA cyclic GMP kit (Enzo Life Sciences International, Plymouth Meeting, PA) as per manufacturer's instructions. Briefly, the cell lysate is incubated with a cGMP polyclonal antibody at room temperature for 1 h, and the excess reagents are washed away. The substrate is added, and after a short incubation period, the enzyme reaction is stopped, and the yellow color intensity is measured using a microplate reader at 405 nm. The intensity of the bound yellow color is inversely proportional to the concentration of cGMP in either standards or samples. The measured optical density is used to calculate the concentration of cGMP. Protein concentration of lysate was measured spectrophotometrically at 595 nm. The results are expressed as picomoles per milligrams of protein.
measurement of CSE mRNA with real-time PCR.
Total RNA was isolated from frozen control and cinaciguat-treated whole heart tissue using RNeasy mini kit (Qiagen) as per manufacturer's protocol. The concentration of total RNA was measured using Agilent Nanodrop (Agilent Technologies).
Briefly, 5 μg of total RNA were reverse transcribed to first strand cDNA Transcriptor First Strand CDNA synthesis kit (Roche, Manheim, Germany) as per the recommended protocol from the company.
Multiplex quantitative real-time PCR primers were designed using Roche Probe Finder Software based on the mouse CSE nucleotide input sequence (NCBI Accession No. NM-145953.2). The quantitative PCR was performed using the cDNA as template and specific primers for CSE: forward primer, 5′-ATAGTCGGCTTCGTTTCCTG-3′ and reverse primer, 5′-TCGGCAGCAGAGGTAACAAT- 3′; for β-actin: forward primer, 5′GCCAACCGTGAAAAGATGAC-3′ and reverse primer, 5′-GAGGCATACAGGGACAGCAC-3′; and UPL hydrolysis probe no. 68 for CSE and cat. no. 05046190001 for β-actin.
The following parameters were used in the PCR cycle: an initial denaturation at 95°C for 10 min followed by 40 cycles of 95°C for 10 s; annealing at 60°C for 30 s and an extension at 72°C for 10 s. The Quantification of relative gene expression was analyzed using the 2−ΔΔCt method.
CSE Western blotting.
Isolated LV samples (n = 3/group) were homogenized, sonicated, and centrifuged at 10,000 g for 15 min at 4°C. Total proteins (100 μg) from each sample were separated by SDS-PAGE on 10% acrylamide gels, transferred onto a nitrocellulose membrane, and blocked with 5% nonfat dry milk in Tris-buffered saline (5, 24). Membranes were incubated overnight with mouse monoclonal antibody (dilution 1:500; cat. no. SC-3653381; Santa Cruz Biotechnology, Santa Cruz, CA) specific for CSE and goat polyclonal antibody (dilution 1:1,000; cat no. Sc-1616; Santa Cruz Biotechnology) specific for actin. The blot was then incubated for 1 h with the corresponding secondary horseradish peroxidase-conjugated antibody and developed using Western Lightning Plus-Ecl substrate (PerkinElmer). The densitometric analysis for the corresponding CSE and β-actin band was done using ImageJ software.
measurement of H2S levels.
H2S was measured as described previously (20). Briefly, snap-frozen hearts or cardiomyocyte lysates were homogenized with 100 mmol/l potassium phosphate buffer (pH 7.4). To trap H2S, 250 μl of zinc acetate (1% wt/vol) were added to the homogenate followed by 30 min incubation at 37°C. The reaction was stopped by addition 250 μl of trichloroacetic acid (10% wt/vol) to the assay mixture and incubated for 60 min at 37°C before centrifugation at 14,000 g for 10 min. The H2S concentration of the supernatants was measured using a highly specific H2S sensor connected to a single channel analyzer (Apollo 1000; WPI) and was calculated using a calibration curve of NaHS standards. Protein concentration was measured spectrophotometrically at 595 nm. The results are expressed as micromoles per milligrams of protein.
Statistics
All measurements of infarct size and risk areas are expressed as group means ± SE. Changes in echocardiography parameters and infarct size were analyzed using the random effects ANOVA for repeated-measures to determine the main effect of time, group, time-by-group interaction, and the post hoc two-sided Dunnett's test to compare two groups at a time. Statistical differences were considered significant if the P value was < 0.05. Discrete variables were presented as absolute and percent value. The χ2 test (or the Fisher's exact test when appropriate) was used to compare discrete variable in different groups. The Bonferroni correction for post hoc analysis was used when comparing two groups from three or more groups.
RESULTS
Rabbit Studies
Infarct size.
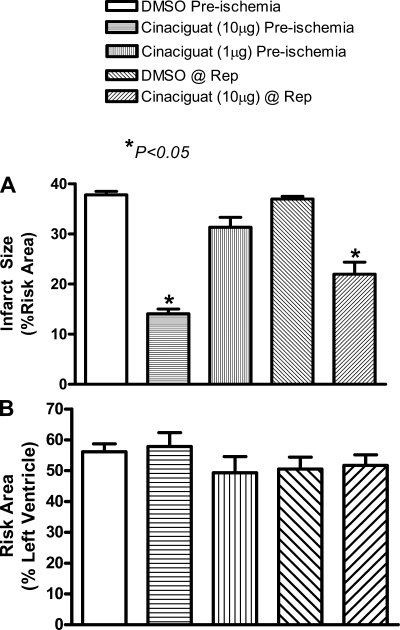
As shown in Fig. 1A, infarct size (expressed as %risk area) was reduced from 37.8 ± 0.7 in the vehicle control animals to 14.1 ± 0.9 when cinaciguat (10 μg/kg) was administered before ischemia (means ± SE; 63% reduction; P < 0.05; n = 6/group). With the lower dose of cinaciguat (1 μg/kg), infarct size was reduced marginally from 37.8 ± 0.7 in control to 31.3 ± 2.0 in the drug-treated group (P > 0.05; n = 6/group). The higher dose of cinaciguat (10 μg/kg), when administered 5 min before reperfusion, reduced infarct size from 37.0 ± 0.5% in the control to 22.0 ± 2.9% in the cinaciguat-treated rabbits (P < 0.05; n = 6/group; Fig. 1A). We did not study the effect of cinaciguat with a lower dose of 1 μg/kg at reperfusion since it failed to reduce infarct size when given before ischemia. The risk area (expressed as %LV) was not different between vehicle and cinaciguat-treated group as shown in Fig. 1B.
Fig. 1.
Infarct size in rabbits. A: myocardial infarct size [%risk area (RA)] measured following 3 h of reperfusion (Rep). The first 3 bars depict treatment with cinaciguat before ischemia, and the last 2 represent cinaciguat administration at reperfusion. Note that infarct size was significantly reduced with 10 μg cinaciguat given as a preconditioning mimetic (63% reduction) or at the time of reperfusion (41% reduction) compared with the respective controls. B: area at risk [%left ventricle (LV)] was similar in all groups.
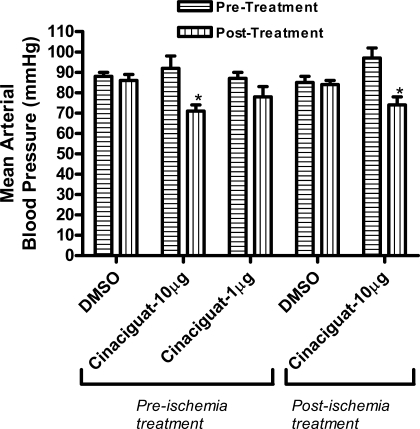
Effect of cinaciguat on mean arterial blood pressure.
Administration of cinaciguat (10 μg/kg) significantly reduced mean arterial blood pressure within 1 min in both treatment protocols, i.e., before or postischemia. However, this decline in blood pressure bounced back to normal within 5 min. Vehicle (10% DMSO) or the lower dose of cinaciguat had no effect on mean arterial pressure (Fig. 2).
Fig. 2.
Hemodynamics. Changes in mean arterial pressure (mmHg) following drug administration. Note that cinaciguat, at a dose of 10 μg, caused a significant decline in mean arterial pressure when given as a preconditioning mimetic or infused at reperfusion compared with baseline. Lower dose of cinaciguat (1 μg) or vehicle (DMSO) did not alter mean arterial pressure. *P < 0.05 vs. pretreatment.
Mouse Studies
Infarct size.
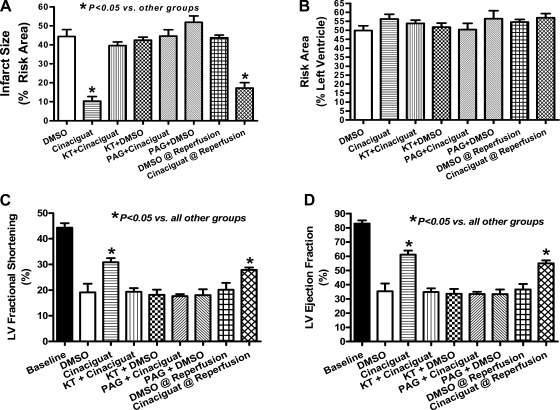
Similar to rabbits, infarct size (means ± SE) was reduced with cinaciguat compared with vehicle controls (n = 6/group). The PKG inhibitor KT blocked the infarct-limiting effect of cinaciguat. KT alone had no effect on infarct size. Similarly, the CSE inhibitor PAG also blocked the infarct-sparing effect of cinaciguat (Fig. 3A). Moreover, cinaciguat administration at the onset of reperfusion also caused a significant decrease in infarct size. There was no difference in the risk area of the various treated groups (Fig. 3B).
Fig. 3.
Infarct size and LV function in mice. A: myocardial infarct size (%RA) measured 24 h postmyocardial infarction (MI). Note that infarct size was significantly reduced with cinaciguat (80% reduction compared with control with pretreatment and 63% reduction when given at the onset of reperfusion). Preconditioning with cinaciguat was blocked by KT5283 (KT) and dl-propargylglycine (PAG). KT and PAG alone had no effect on infarct size. B: area-at-risk (%LV) was similar in all groups. C: LV fractional shortening assessed at 24 h post-MI. MI caused a 57% decline in fractional shortening from baseline, which was partially preserved with cinaciguat (30% decline from baseline; P < 0.05 vs. control). PAG and KT abolished the preservation in fractional shortening observed with cinaciguat. D: ejection fraction was also preserved with cinaciguat, and PAG and KT abrogated this preservation in function.
Echocardiography.
Figure 3, C and D, shows fractional shortening and ejection fraction (EF) at baseline and 24 h following reperfusion in experimental groups pretreated with DMSO, cinaciguat, cinaciguat + PAG, and DMSO + PAG, as well as mice treated with cinaciguat or DMSO at the onset of reperfusion. None of the groups presented with significant LV dilatation at 24 h (not shown); however, cinaciguat preserved FS (31 ± 1.6%) and EF (61 ± 2.8%) compared with DMSO controls (FS: 19 ± 3.3 and EF: 35 ± 5.5%; P < 0.05). Baseline FS and EF were 44 ± 1.7 and 83 ± 2.2%, respectively, and KT or PAG administration following cinaciguat abrogated the preservation of LV function as demonstrated by a marked decline in FS (19 ± 1.1 and 18 ± 1.0%, respectively) and EF (32 ± 2.2 and 34 ± 1.5%, respectively; P < 0.05 vs. cinaciguat; Fig. 3, C and D). Moreover, administration of cinaciguat at the onset of reperfusion also preserved fractional shortening (29 ± 1%) and EF (55 ± 1%) compared with DMSO-treated mice at reperfusion (FS: 21 ± 2.8 and EF: 34 ± 2.2%, respectively; P < 0.05 vs. cinaciguat at reperfusion; Fig. 3, C and D).
Cell viability and apoptosis.
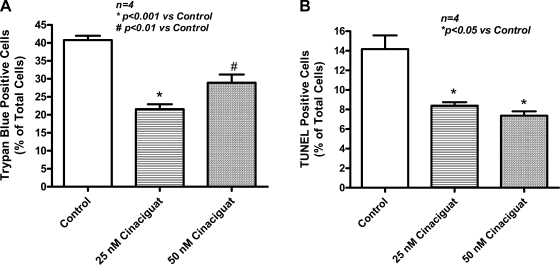
Cardiomyocytes were treated with 25 or 50 nM of cinaciguat. After SI (40 min)/RO (1 h), the percentage of necrotic (trypan blue-positive) cardiomyocytes decreased to 21.6 ± 1.3 (25 nM) and 28.9 ± 2.3 (50 nM) compared with untreated cells (40.8 ± 1.2; n = 4 each group; P < 0.01; Fig. 4A).
Fig. 4.
Necrosis and apoptosis in vitro. A: necrosis assessed by trypan blue staining following simulated ischemia/reoxygenation (SI/RO) in primary adult mouse cardiomyocytes showing decrease with cinaciguat at concentrations of 25 nM (47% reduction) and 50 nM (29% reduction) compared with control. B: apoptosis assessed by terminal deoxynucleotidyl (TUNEL) transferase assay. Cinaciguat decreased apoptosis following SI/RO in cardiomyocytes at concentrations of 25 nM (41% reduction) and 50 nM (48% reduction) compared with control.
Apoptosis was detectable after 40 min of SI and 18 h of RO. Cinaciguat treatment reduced the terminal deoxynucleotidyl transferase-positive cells to 8.4 ± 0.4 and 7.4 ± 0.4 with 25 and 50 nM concentrations, respectively, compared with 14.2 ± 1.4 in the untreated cells (n = 4; P < 0.05; Fig. 4B).
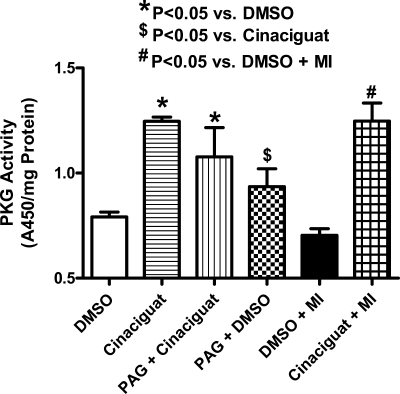
PKG activity and cGMP levels.
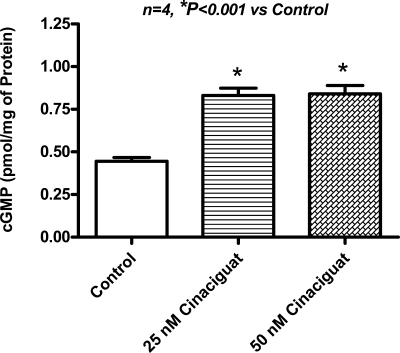
A robust increase in myocardial PKG activity was observed at 1 h after treatment with cinaciguat (n = 3; P < 0.01; Fig. 5) and at 1 h after the onset of reperfusion or 2.5 h after treatment (n = 3; P < 0.01; Fig. 5). Interestingly, PAG did not abrogate the increase in PKG activity observed with cinaciguat (Fig. 5). Moreover, cinaciguat increased cGMP levels in cardiomyocytes (n = 4; P < 0.001; Fig. 6).
Fig. 5.
cGMP-dependent protein kinase activity. Myocardial PKG activity 1 h following treatment with cinaciguat or 10% DMSO or 1 h after reperfusion in cinaciguat or DMSO-treated mice. Note that cinaciguat increased PKG activity compared with DMSO (P < 0.05) in both scenarios. Moreover, PAG did not abrogate the increase in PKG activity observed with cinaciguat.
Fig. 6.
cGMP concentration. Cardiomyocyte cGMP concentration increased robustly with 25 and 50 nM cinaciguat (2-fold increase) compared with control.
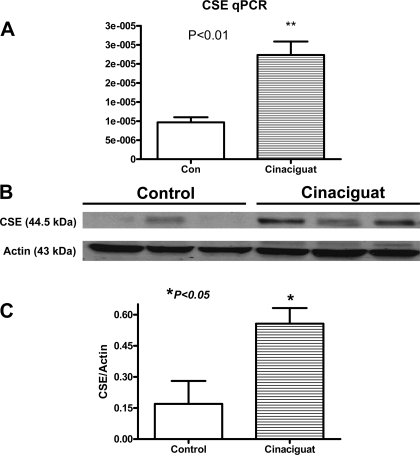
CSE mRNA and protein expression.
Real-time PCR data revealed a significant 2.8-fold increase in CSE mRNA at 1 h following treatment with cinaciguat (Fig. 7A). This was confirmed with Western blotting, which also demonstrated a 3.2-fold increase (Fig. 7, B and C).
Fig. 7.
Myocardial cystathionine-γ-lyase (CSE) mRNA and protein expression. A: cinaciguat caused a significant 2.8-fold increase in CSE mRNA compared with 10% DMSO control (n = 3 per group; P < 0.01). B: cinaciguat also caused a significant induction in CSE protein expression as evidenced by Western blot analysis. C: densitometric analysis illustrating the increase in CSE protein expression with cinaciguat vs. control (n = 3 per group; P < 0.05).
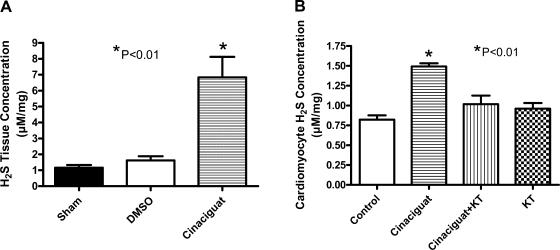
H2S production.
In the intact heart, cinaciguat increased H2S tissue levels (μM/mg protein) at 1 h posttreatment to 6.8 ± 1.3 compared with 1.2 ± 0.2 in untreated hearts and 1.6 ± 0.3 in DMSO-treated hearts (n = 3; P < 0.01; Fig. 8A). In cardiomyocytes, 50 nM cinaciguat also increased H2S generation from 0.9 ± 0.1 in untreated cells to 1.5 ± 0.1 (n = 4; P < 0.01; Fig. 8B), which was inhibited by KT (1.0 ± 0.1).
Fig. 8.
H2S concentration in whole heart and cardiomyocytes. H2S tissue levels assessed in hearts harvested 1 h after treatment with cinaciguat (A) or cardiomyocytes at 1 h after incubation with cinaciguat with or without KT (B). cinaciguat caused an increase in H2S compared with sham and control, respectively (P < 0.01). KT abrogated the increase in H2S caused by cinaciguat in cardiomyocytes (P < 0.01). KT alone had no effect on H2S concentration.
DISCUSSION
Several studies from our laboratory have demonstrated that NO-cGMP-PKG pathway triggered by PDE-5 inhibitors lead to cardioprotection in a number of experimental conditions, including I/R injury (12, 13, 16, 20, 21, 24), doxorubicin-induced cardiomyopathy (8), and myocardial infarction induced heart failure (19). In the present study, we examined the efficacy of cinaciguat, a NO-independent activator of sGC, in exerting cardioprotection. Our results show that preconditioning with cinaciguat reduced myocardial infarct size following I/R injury in rabbits and mice. The drug also reduced infarct size when given at reperfusion in rabbits and mice. It appears that cinaciguat might have a protective effect at reperfusion through an antioxidant mechanism considering that sudden reintroduction of molecular oxygen can cause massive production of oxygen radicals including peroxynitrite in the presence of NO (14). Moreover, the oxygen free radicals, especially superoxide, may directly scavenge NO upon its release and further contribute to its suboptimal bioavailability under stressful circumstances, thus hampering activation of PKG (17). Our results demonstrate that the cardioprotective effect of cinaciguat was also associated with increased PKG activity after 1 h of treatment in the absence of I/R and at 1 h into reperfusion post ischemia (Fig. 5). Moreover, the PKG inhibitor KT5823 abolished the protective effect of cinaciguat. Interestingly, PAG did not abrogate the increase in PKG activity observed with cinaciguat, which supports our initial hypothesis that cinaciguat-induced H2S production is downstream of PKG signaling.
These results are consistent with previous studies reporting pharmacologic postconditioning with cinaciguat involving activation of PKG and opening of mitochondrial KATP channels in rabbit and rat hearts (11). Our results also show that cinaciguat protects adult primary cardiomyocytes against SI/RO injury, which was associated with a dose-dependent increase in cGMP (Fig. 6). It has been reported that cinaciguat in concentrations as low as 1 nM was able to activate sGC thus leading to an increase in cGMP in rat cardiomyocytes (26). This finding is especially important and argues against cardioprotection solely due to hemodynamic changes that were observed in our rabbit studies, although the impact of a decline in mean arterial pressure on cardioprotection cannot be completely ruled out in our in vivo model. Treatment with 25 or 50 nM of cinaciguat before SI/RO decreased the number of necrotic and apoptotic cardiomyocytes.
Another major novel finding of this study is that cinaciguat-induced cardioprotection was dependent on the generation of H2S. This is because cinaciguat-induced reduction of infarct size and preservation of cardiac function were blocked by PAG the inhibitor of H2S-producing enzyme CSE. Moreover, cinaciguat caused enhanced generation of endogenous H2S in the heart following I/R injury in mice (Fig. 8A). These data are consistent with our recent work (20) showing PKG-dependent increase in H2S levels with the long acting PDE-5 inhibitor tadalafil. H2S is produced in the body by two other enzymes, namely cystathionine β-synthase and 3-mercaptopyruvate sulfurtransferase, in addition to CSE (18). Based on the complete reversal of cinaciguat-induced cardioprotection with PAG, it is plausible that CSE might be the enzyme responsible for H2S generation in cardiomyocytes. The role of cystathionine β-synthase or 3-mercaptopyruvate sulfurtransferase in H2S generation may be negligible or nonexistent, particularly with cinaciguat treatment in the present study. Exactly how PKG activation is associated with increased H2S generation by cinaciguat is not clear from the present study, although it may be related to PKG-dependent enhancement of CSE expression/activity in the heart. There is evidence that specificity protein 1 (SP-1; also known as Sp1 transcription factor) plays an important role in the basal transcriptional activity of CSE enzyme (10). PKG can phosphorylate Sp1 on serine residue(s), which results in transcriptional activation of Sp1 in human SW480 colon cancer cells (3) with a consequent increase in CSE activity and possibly generation of H2S. It is not known whether PKG-dependent transcriptional activation of Sp1 also occurs in the heart. Our findings clearly demonstrate a significant increase in myocardial CSE mRNA and protein following treatment with cinaciguat (Fig. 7), which was paralleled with an increase in myocardial tissue levels of H2S (Fig. 8A). Furthermore, our results also show that cinaciguat treatment significantly increased H2S generation in cardiomyocytes (Fig. 8B), which was inhibited by KT, further implicating the role of PKG in promoting H2S generation following treatment with cinaciguat.
More recently, it has been shown that H2S provides cardioprotection through protein kinase C and preservation of mitochondrial function (1, 7). Moreover, H2S has been shown to increase phosphorylation of Akt and nuclear localization of 2 transcription factors, nuclear respiratory factor 1 and nuclear factor-E2-related factor (Nrf2), that are involved in increasing the levels of endogenous antioxidants, attenuating apoptosis, and increasing mitochondrial biogenesis (2, 18).
In summary, we have provided conclusive evidence that the NO-independent sGC activator cinaciguat exerts cardioprotective effects following I/R injury in rabbits, mice and adult cardiomyocytes. We have also shown that cinaciguat induces this protective effect through cGMP-PKG-dependent generation of H2S in the heart and cardiomyocytes. In this respect, cinaciguat may be an interesting novel drug to generate therapeutic levels of H2S in the diseased cardiovascular system. The pharmacokinetic and pharmacodynamic properties of cinaciguat as well as its tolerability and safety have recently been assessed also by phase-I clinical trials (9). A phase-IIb clinical study for the indication of acute decompensated heart failure is currently underway (25). We therefore believe that cinaciguat may be an important molecule for the management of I/R injury or possibly heart failure in patients with coronary artery disease.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-51045, HL-79424, and HL-93685 (to R. C. Kukreja), Bayer Schering Pharma (to R. C. Kukreja), and National Scientist Development Grant 10SDG3770011 from the American Heart Association (to F. N. Salloum).
DISCLOSURES
Rakesh C. Kukreja received cinaciguat and partial funding for preliminary experiments from Bayer Schering Pharma for the completion of this study. All other authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: F.N.S., J.-P.S., and R.C.K. conception and design of research; F.N.S., A.D., A.S., N.N.H., V.Q.C., and R.A.O. performed experiments; F.N.S., A.D., A.S., N.N.H., V.Q.C., and R.A.O. analyzed data; F.N.S., A.D., A.S., N.N.H., V.Q.C., R.A.O., J.-P.S., and R.C.K. interpreted results of experiments; F.N.S., A.D., A.S., N.N.H., V.Q.C., and R.A.O. prepared Figs.; F.N.S. drafted manuscript; F.N.S., J.-P.S., and R.C.K. edited and revised manuscript; F.N.S. and R.C.K. approved final version of manuscript.
REFERENCES
- 1.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cen B, Deguchi A, Weinstein IB. Activation of protein kinase G Increases the expression of p21CIP1, p27KIP1, and histidine triad protein 1 through Sp1. Cancer Res 68: 5355–5362, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Razzoli E, Forti G, Maggi M. The use of phosphodiesterase 5 inhibitors with concomitant medications. J Endocrinol Invest 31: 799–808, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. Biol Chem 280: 12944–12955, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283: 29572–29585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher PW, Salloum F, Das A, Hyder S, Kukreja RC. Phosphodiesterase 5A inhibition using sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in chronic model of doxorubicin-induced cardiotoxicity. Circulation 111: 1601–1610, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Frey R, Muck W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase activator cinaciguat (BAY 58–2667) in healthy male volunteers. J Clin Pharmacol 48: 1400–1410, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, Kimura H. Murine cystathionine-γ-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J 381: 113–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krieg T, Liu Y, Rütz T, Methner C, Yang XM, Dost T, Felix SB, Stasch JP, Cohen MV, Downey JM. BAY 58-2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically postconditions rabbit and rat hearts. Eur Heart J 30: 1607–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kukreja RC, Ockaili R, Salloum F, Yin C, Hawkins J, Das A, Xi L. Cardioprotection with phosphodiesterase-5 inhibition–a novel preconditioning strategy. J Mol Cell Cardiol 36: 165–173, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, Fisher PW, Wittkamp M, Hawkins J, Chou E, Kukreja AK, Wang X, Marwaha VR, Xi L. Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol 42: 219–232, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kukreja RC, Hess ML. The oxygen free radical system: from equations through membrane protein interaction to myocardial injury and protection. Cardiovasc Res 26: 641–655, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, Karas M, Stasch JP, Schmidt HH, Müller-Esterl W. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 105: 33–41, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol 283: H1263–H1269, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Paolocci N, Biondi R, Bettini M, Lee CI, Berlowitz CO, Rossi R, Xia Y, Ambrosio G, L′Abbate A, Kass DA, Zweier JL. Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J Mol Cell Cardiol 33: 671–679, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Predmore BL, Lefer DJ. Development of hydrogen sulfide-based therapeutics for cardiovascular disease. J Cardiovasc Transl Res 3: 487–498, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Salloum FN, Abbate A, Das A, Houser J, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294: H1398–H1406, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase G-dependent generation of hydrogen sulfide. Circulation 120: S31–S36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salloum FN, Das A, Thomas CS, Yin C, Kukreja RC. Adenosine A(1) receptor mediates delayed cardioprotective effect of sildenafil in mouse. J Mol Cell Cardiol 43: 545–551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, Kukreja RC. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol 42: 453–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial KATP channels in rabbits. J Mol Cell Cardiol 40: 405–411, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92: 595–597, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Hand Exp Pharmacol 191: 309–339, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schröder H, Stahl E, Steinke W, Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136: 773–783, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TYHSAK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]