Abstract
We hypothesized that chronic hyperglycemia has a detrimental effect on neurovascular coupling in the brain and that this may be linked to protein kinase C (PKC)-mediated phosphorylation. Therefore, in a rat model of streptozotocin-induced chronic type 1 diabetes mellitus (T1DM), and in nondiabetic (ND) controls, we monitored pial arteriole diameter changes during sciatic nerve stimulation and topical applications of the large-conductance Ca2+-operated K+ channel (BKCa) opener, NS-1619, or the K+ inward rectifier (Kir) channel agonist, K+. In the T1DM vs. ND rats, the dilatory response associated with sciatic nerve stimulation was decreased by ∼30%, whereas pial arteriolar dilations to NS-1619 and K+ were largely suppressed. These responses were completely restored by the acute topical application of a PKC antagonist, calphostin C. Moreover, the suffusion of a PKC activator, phorbol 12,13-dibutyrate, in ND rats was able to reproduce the vascular reactivity impairments found in T1DM rats. Assay of PKC activity in brain samples from T1DM vs. ND rats revealed a significant gain in activity only in specimens harvested from the pial and superficial glia limitans tissue, but not in bulk cortical gray matter. Altogether, these findings suggest that the T1DM-associated impairment of neurovascular coupling may be mechanistically linked to a readily reversible PKC-mediated depression of BKCa and Kir channel activity.
Keywords: cerebrovascular disease, pial vessels, potassium channels, protein kinase C, large-conductance calcium-operated potassium channel, inward-rectifier potassium channel
the mechanisms involved in neurovascular coupling have been extensively studied, and many factors appear to participate in this process. Among these are diffusible paracrine factors released by astrocytes and neurons, e.g., ATP and K+, as well as cyclooxygenase and cytochrome P-450 products [reviewed by Attwell et al. (3)]. Indeed, nature has provided the brain with a multiplicity of means to couple neural activity with blood supply. Such redundancy may ensure an adequate O2 and glucose supply to neurons when their energy demands increase over a wide array of physiological levels and pathophysiological conditions.
Type 1 diabetes mellitus is a widespread clinical condition (6). Cerebral complications of type 1 diabetes mellitus include microangiopathy, retinopathy, and an increased risk of Alzheimer's disease (30), stroke, mild cognitive impairment, and dementia (1). Collectively, these complications are termed diabetic encephalopathy (for review, see Ref. 25). In experimental animals with chemically induced diabetes, evidence has been accumulated indicating a profound impairment in cerebral vasodilatory function (2, 22) although none of those studies addressed neurovascular coupling. We previously reported that one possible mechanism responsible for this diabetes-related vascular impairment may be chronic activation of protein kinase C (PKC) (29). Findings from in vitro and in vivo models have indicated a vital role for inward-rectifier and large-conductance, Ca2+-operated K+ channels (Kir and BKCa, respectively) in neurovascular coupling (11, 13, 26). Thus, K+ released from astrocytic endfeet via BKCa channels is thought to interact with Kir channels on arteriolar smooth muscle cells, inducing relaxation. Furthermore, evidence suggests that Kir and BKCa channels are affected by diabetes (22) and sensitive to inhibition via PKC-mediated phosphorylation (10, 18, 33, 42, 47).
In the present study, we used a well-established rat in vivo neurovascular coupling model involving sciatic nerve stimulation-induced pial arteriolar dilation (38, 44). The following hypotheses were addressed: 1) neurovascular coupling efficiency is impaired in type 1 diabetes mellitus; 2) that impairment involves Kir and BKCa channels; and 3) PKC-mediated phosphorylation is a contributing factor to the Kir and BKCa dysfunction in type 1 diabetes mellitus rats.
MATERIALS AND METHODS
Surgical procedures and diabetes model.
Adult Sprague-Dawley female rats (4–6 mo old, 250–350 g) were used for cranial window experiments. Experimental procedures were approved by the Animal Care and Use Committee of the University of Illinois at Chicago. According to previously published reports (26, 39, 44), on the day of the experiment, rats were anesthetized with 2% isoflurane and mechanically ventilated (70% N2O-30% O2). The femoral artery and vein on the side not used for the nerve stimulation were cannulated for blood sampling, arterial pressure monitoring, and drug infusions. Rectal temperature was servocontrolled at 37°C with a heating pad. The head was stabilized in a stereotaxic frame, and a 10-mm-diameter craniotomy was performed over the skull midline. After carefully removing the dura, a glass window outfitted with three ports was mounted for pial vessel observation. The space under the cranial window was suffused, at 0.5 ml/min, with artificial cerebrospinal fluid (aCSF), bubbled with 5% CO2, 20% O2, and 75% N2. One hour before drug or sciatic nerve stimulation testing, isoflurane was replaced by fentanyl (10 μg/kg loading dose), and anesthesia was maintained with 25 μg·kg−1·h−1 fentanyl-70% N2O-30% O2. Paralysis was then induced with d-tubocurarine. Temperature, mean arterial blood pressure, and intracranial pressure were monitored and maintained within normal limits. Arterial blood samples were taken at 60-min intervals and analyzed using a blood gas/pH analyzer (Gem3000; Instrumentation Laboratories, Lexington, MA). PaO2, PaCO2, and pH were maintained within physiological ranges throughout the study.
Diabetes was induced with a single intravenous streptozotocin (STZ) injection (50 mg/kg) to 2-mo-old rats around 16–18 wk before the experiment. Animals were considered diabetics when blood glucose exceeded 350 mg/dl 3 days after STZ. Blood glucose was measured two times a week (Contour glucometer; Bayer, Mishawaka, IN) for the entire period before the study. Animals showing signs of infections or a body weight loss exceeding 20% compared with the other rats in the same batch were killed and not used for the study. No insulin was administered to the diabetic or nondiabetic rats.
Experimental protocol.
In the typical experiment, 1 h after the anesthesia switch and regular aCSF suffusion (equilibration period), the rats were subjected to one or two sciatic nerve stimulation episodes followed by NS-1619 (10 and 50 μM) or K+ (6 and 12 mM) suffusions under the cranial window for 5 min at each concentration. After a recovery period of at least 5 min, we initiated a suffusion of the activator/inhibitor. Forty minutes later, a second sciatic nerve stimulation was imposed. NS-1619 or K+-induced dilations were then reevaluated. The concentrations of paxilline (10 μM), Ba2+ (100 μM), calphostin C (CalC, 100 nM) and phorbol 12,13-dibutyrate (PdBu, 10 nM) used in these experiments were established in pilot experiments, with guidance from published findings (5, 11, 26). To ensure that vessel reactivity was still intact throughout the experiment, a CO2 challenge was performed before the animals were killed. A CO2 reactivity index (% of diameter increase per mmHg of PaCO2 increase) between one and two was considered normal. This criterion was not met on only three occasions, and experimental data from those animals were discarded. Time control experiments, covering a period of 3 h, showed a limited variability (no significant differences) in all dilatory responses, relative to initial responses. This included both diabetic and nondiabetic rats (data not shown).
Video imaging and data analysis.
Intravital video microscopy was used to study pial arteriole diameter changes in response to drugs and/or sciatic nerve stimulation. Images were acquired through a CCD camera (Photometrics, Tucson, AZ) mounted on an upright stereomicroscope (Nikon, Melville, NY). We studied arterioles overlying the hindlimb somatosensory cortex (23). Starting at 20 s before sciatic nerve stimulation and continuing for 60 s postsciatic nerve stimulation, pial arterioles were monitored, and the recordings were saved for subsequent analysis. Arteriolar diameter changes were measured off line using Metamorph software (MDS Analytical Technologies, Sunnyvale, CA). The diameter values of three different pial arterioles were averaged for each time point. The peak dilation induced by sciatic nerve stimulation was calculated as the percent change relative to the baseline, where baseline was taken as the average diameter value measured over 20 s immediately preceding sciatic nerve stimulation. The dilation induced by NS-1619 or K+ was measured after 5 min of perfusion and compared with the baseline diameter.
Sciatic nerve stimulation and electrophysiological recordings.
The sciatic nerve on one side was surgically exposed and sectioned. A ring electrode was carefully secured to the nerve ending and connected to an isolated stimulator unit (Stimulator HC, ML155; AD Instruments, Sidney, Australia) controlled via Labchart 7 software (AD Instruments). The parameters used for the stimulation were as follows: 40 rectangular current pulses, 0.7 mA amplitude, 0.1 ms duration at 2 Hz (20-s train) (26, 39, 44, 45). Electrophysiological recordings of the somatosensory evoked potentials (SEPs) were obtained through stainless steel screws placed on the border of the cranial window (positive on the parietal and negative on the frontal bones) referenced to another screw placed over the cerebellum. The signal was fed to a DP-311 preamplifier (Warner Instruments, Hamden, CT), bandpass filtered (0.1–1 kHz), and digitized at 2 kHz by a PowerLab acquisition board (AD Instruments). Peak-to-peak N1P1 and N2P2 amplitudes were measured and compared before and after administration of drugs (and in controls vs. diabetics) to evaluate possible effects on SEPs. Values were obtained by averaging two to four trains of stimuli per each condition. No significant differences were detected (data not shown).
Western blot.
To examine the protein expression changes of BKCa subunits (α, β1, and β4) and Kir2.1 channels, glio-pial tissue samples from diabetic and nondiabetic rats were processed for Western immunoblot analysis. There are several reasons for using glio-pial tissue. First, we recently showed (26) that the pial arteriolar dilating response to the BKCa opener NS-1619 was primarily dependent upon BKCa channels in the superficial glia limitans. Second, the vasodilation elicited by NS-1619 was dependent upon normally functioning Kir channels, presumably on pial arteriolar smooth muscle (26). Third, pial microvessels, at least in piglets, have been shown to be adherent to the underlying astrocytic processes of the glia limitans (19). Thus, glio-pial tissue was harvested by stripping off pia mater and pial vessels from the whole cortical surface of the isolated brain with fine tip forceps. The pia mater peels off easily and carries with it the embedded pial vessels along with short stumps of penetrating vessels, as well as fragments of the glia limitans. Tissue collection was performed shortly after the rat was killed and trancardially perfused with PBS. Tissues were quickly immersed into a lysing buffer containing (in mM): 20 Tris, 2 EDTA, 2 EGTA, 1 phenylmethylsulfonyl fluoride, 1 NaVO4, 20 NaF; 1% (vol/vol) Nonidet P-40, and 2% (vol/vol) Sigma protease inhibitor cocktail. Samples were sonicated three times on ice for 10–15 s, centrifuged at 100,000 g for 1 h at 4° C, and stored in multiple small aliquots at −80° until used. Loading volumes were adjusted to obtain 10 μg of total protein in each lane. Proteins were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred on Immobilon FL (Millipore, Billerica, MA) polyvinylidene difluoride membranes. The blots were hybridized with rabbit anti-BKCa α-subunit (1:400) (Alomone Labs, Jerusalem, Israel), rabbit anti-BKCa β1-subunit (1:200) (Pierce, Rockford, IL), rabbit anti-BKCa β4-subunit (1:200) (Alomone Labs), goat anti-Kir2.1 (1:200) (clone N-18; Santa Cruz, Santa Cruz, CA), mouse anti-GFAP (1:500) (Millipore), and mouse anti-S-100 β (1:200) (Sigma, St. Louis, MO) and subsequently incubated with appropriate secondary antibodies conjugated with infrared dye (IRDye 680 or 800CW; LI-COR Biosciences, Lincoln, NE) diluted 1:5,000 in 0.5× Odyssey blocking buffer and 0.1% Tween 20. Mouse β-actin or α-tubulin were used as loading controls [mouse monoclonal, 1:2,000–4,000 (Sigma); goat antimouse, 1:10,000, IRDye 680 (Rockland Immunochemicals, Philadelphia, PA)]. Blot membranes were then scanned using the Licor Odyssey Infrared Imaging System (LI-COR Biosciences). The protein levels in each brain sample (n = 6–7 in each group) were expressed as the ratio of the optical densities of the protein of interest over the housekeeping protein, normalized to the control average. To improve reliability, the average of the values from three different blots for each brain sample was used for statistical comparison between groups.
PKC activity assay.
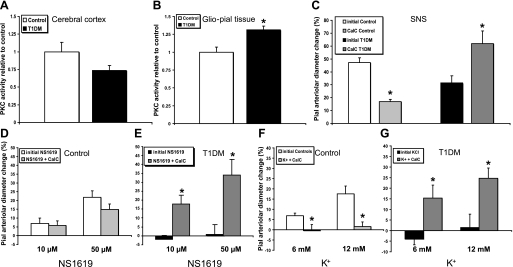
Two nonradioactive PKC assays (Promega, Madison, WI; and Assaydesigns, Farmingdale, NY) were used for this study. The results obtained were qualitatively consistent between the two assays and reproducible. Two different sets of animal brains, each including seven with diabetes and six without diabetes, were used for the analyses with the two different assays. Cerebral cortex or glio-pial tissues were prepared as described in Western blot. The assays were performed by thoroughly following the manufacturer's instructions, and the colorimetric reactions were quantified by a plate reader (SLT Spectra, Milan, Italy). Each sample was in triplicate and thawed only one time. In Fig. 4, A and B, the results from the Promega assay are shown.
Fig. 4.
Protein kinase C (PKC) activity levels measured ex vivo, and effects of PKC inhibition, via topically applied calphostin C (CalC) on pial arteriolar reactivities. A and B: relative PKC activity levels in samples of either bulk cortical gray matter (A) or superficial glio-pial tissue (B). PKC activity was significantly increased in the glio-pial (but not cerebral cortex) tissue of diabetic (T1DM) rats (n = 7) compared with age-matched controls (n = 6, *P < 0.05). C: SNS-evoked pial arteriolar dilation in the absence and the presence of CalC (100 nM) showed a markedly different response in control (n = 5) (∼60% decrease) and T1DM (n = 6) (∼100% increase) rats. *P < 0.005 vs. respective initial responses. D and E: effects of CalC on the dose-dependent dilations of pial arterioles, in control (n = 6) (D) and T1DM (E) (n = 6, *P < 0.05 vs. initial responses) rats to suffusions of the BKCa opener NS-1619. Note that CalC completely restored the dilation in diabetic animals (E) without affecting responses in control rats (D). F and G: effects of CalC on K+-evoked, Kir-linked dilations of pial arterioles, in control (F) (n = 6, *P = 0.03 vs. initial responses) and T1DM (G) (n = 5, *P < 0.05 vs. initial responses) rats. In control rats, the complete loss of K+ reactivity in the presence of CalC (F) differed markedly from the lack of a CalC effect on BKCa-induced dilations (D).
Drugs.
NS-1619, BaCl2, paxilline, CalC, PdBu, and STZ were obtained from Sigma-Aldrich (St. Louis, MO).
Statistics.
Statistical comparisons between groups were performed using an unpaired Student's t-test, whereas the effect of treatments within the same experiment was evaluated via paired Student's t-test. Data are expressed as means ± SE. Statistical significance was set at P = 0.05; n refers to the number of animals or samples (only one sample derived from each animal).
RESULTS
Neurovascular coupling impairment in diabetic rats.
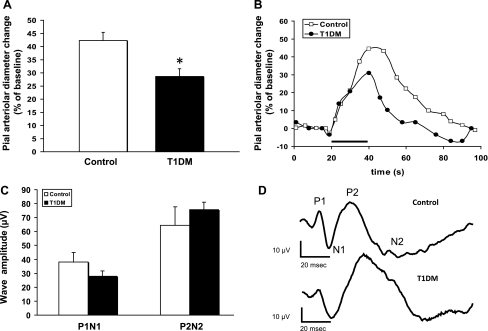
Two sets of rats were used for this study: 1) euglycemic 4- to 6-mo-old nondiabetic (ND) rats (control group) and 2) STZ-treated diabetic rats (T1DM group) (6 mo old, 4 mo post-STZ). Plasma glucose in diabetic rats was 563 ± 29 and 114 ± 19 mg/dl in controls. A well-established closed-cranial window technique was used to monitor the reactivity of pial arterioles overlying the contralateral hindlimb area of the somatosensory cortex (∼2 mm caudally to the bregma and 2–3 mm lateral to the sagittal suture). The baseline diameters of the arterioles studied were 34.8 ± 2.6 μm for nondiabetics and 32.5 ± 2.9 μm for diabetics (P > 0.05). We compared pial arteriole dilations evoked by a 20-s sciatic nerve stimulation in ND control (n = 18) and diabetic rats (T1DM, n = 17). The average peak response in the type 1 diabetes mellitus group was ∼30% lower than in the control group (Fig. 1A; P = 0.003; representative vascular response curves provided in Fig. 1B). The SEPs recorded during sciatic nerve stimulation, as reflected in the P1N1 and P2N2 amplitudes (Fig. 1C), did not show any significant differences when comparing control (n = 8) and diabetic (n = 13) rats (Fig. 1D; see also Ref. 28). Therefore, the decreased vascular response to sciatic nerve stimulation in diabetic rats was not attributable to a decreased neuronal activation.
Fig. 1.
Neurovascular coupling impairment in 4 mo diabetic [type 1 diabetes mellitus (T1DM)] rats. A: sciatic nerve stimulation (SNS)-evoked responses in T1DM rats (n = 17) show an ∼30% decrease in the peak diameter change compared with nondiabetic controls (n = 18). *P = 0.003. B: representative time courses of SNS-evoked pial arteriolar diameter changes in nondiabetic control (open circles) and T1DM (closed circles) rats. SNS duration is indicated by the black bar. C: summary of the somatosensory evoked potentials (SEPs) P1N1 and P2N2 peak-to-peak amplitudes recorded from control and T1DM rats. No significant differences were observed. D: representative SEPs from a control and a T1DM rat.
BKCa and Kir channel function in normoglycemic and diabetic rats.
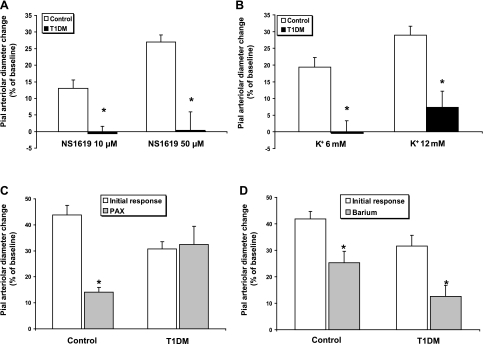
Recent publications (11, 13) and our own data (26, 40) indicate BKCa/Kir-linked communication at the glio-vascular interface. We first endeavored to confirm whether, in our 4 mo model of type 1 diabetes mellitus, vasodilations elicited by activation of BKCa and Kir channels in pial arterioles in vivo were suppressed, similar to the findings reported by Mayhan et al. (22). To that end, the space under the cranial window was suffused with the selective BKCa agonist NS-1619 (10 and 50 μM), which induced a dose-dependent dilation of pial arterioles in ND animals (n = 8) (Fig. 2A). However, NS-1619 failed to induce dilation in type 1 diabetes mellitus rats (n = 8) at both concentrations used (P < 0.001). We are aware of a possible inhibitory effect of NS-1619 on L-type Ca2+ channels, which could contribute to the dilation observed (15). However, this component, according to published data on inhibition of the NS-1619 response with paxilline, is limited to about one-third of the response induced by the highest concentration of NS-1619 (26). Therefore, the bulk of the dilation can be considered dependent on BKCa channels.
Fig. 2.
Pial arteriolar diameter changes (expressed as a percentage of the baseline diameter) elicited by SNS or suffusions of specific activators of high-conductance Ca2+-operated K+ (BKCa) and K+ inward rectifier (Kir) channels in control and T1DM rats. A: pial arteriolar diameter changes in response to the BKCa agonist NS-1619 (10 and 50 μM). *P < 0.001 for both concentrations; n = 8. B: pial arteriolar responses to the Kir agonist K+ (6 and 12 mM). *P < 0.002 for both concentrations; n = 8–9. C: effects of the BKCa specific inhibitor paxilline (PAX, 10 μM) on SNS-induced pial arteriolar dilation. In controls, paxilline blocked ∼70% of the response (n = 9, *P < 0.001); but, in T1DM rats (n = 6), it did not interfere with the vasodilation. D: effects of the Kir blocker barium (Ba2+, 100 μM) on SNS-linked vascular responses in control (40% decrease, n = 9, *P = 0.003) and T1DM (60% decrease, n = 5, *P = 0.004) rats.
Kir channel-mediated dilation of pial arterioles is shown in Fig. 2B. Suffusion with the Kir agonist, K+ (6 and 12 mM), dilated only ND (n = 9), but not type 1 diabetes mellitus (n = 8) arterioles (P < 0.002 for both concentrations).
Role of BKCa and Kir channels in neurovascular coupling in vivo.
We assessed the responses to sciatic nerve stimulation in the absence and presence of the BKCa specific inhibitor paxilline (10 μM) or the Kir blocker barium (Ba2+) (100 μM) in type 1 diabetes mellitus and age-matched ND controls. In control animals, the response to sciatic nerve stimulation, in the presence of paxilline, was decreased by ∼70% (n = 9, P < 0.001), relative to the initial response (Fig. 2C). In type 1 diabetes mellitus animals, in contrast to controls, the response to sciatic nerve stimulation was not affected by paxilline suffusion (n = 6, Fig. 2C). This result is in line with the lack of response to NS-1619 in diabetic rats and points to a minimal role for BKCa channels in the sciatic nerve stimulation response in diabetic rats. Thus, it could, at least in part, account for the impaired neurovascular coupling seen in type 1 diabetes mellitus rats.
The potassium ions released through BKCa channels on astrocytic endfeet are thought to activate Kir channels located on smooth muscle cells of pial and penetrating arterioles (11, 13). Thus, we topically administered the selective Kir channel inhibitor Ba2+ (100 μM), and we observed, in ND controls, a partial block of the response to sciatic nerve stimulation (by ∼40%) (n = 9, P = 0.003) (Fig. 2D). On the other hand, 100 μM Ba2+ was still able to partially block the response to sciatic nerve stimulation in diabetic animals (by ∼60%) (n = 5, P = 0.004). A comparison of the responses to sciatic nerve stimulation following Ba2+ (Fig. 2D) and paxilline (Fig. 2C) administration in ND rats did not quite achieve statistical significance (P = 0.07).
Mechanisms underlying BKCa and Kir channel dysfunction in diabetic rats.
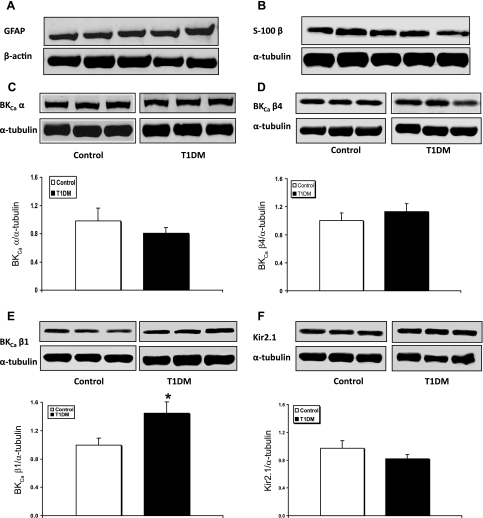
In the model proposed by Filosa and coworkers (11), based upon data obtained in brain slices, opening of BKCa channels on adjacent perivascular astrocytic endfeet releases K+ into the extracellular fluid (ECF) bathing the cerebral arterioles. Only a modest increase in ECF K+ (5–15 mM) is needed to activate Kir channels on arteriolar smooth muscle, leading to hyperpolarization and relaxation. In our model, the perivascular astrocytic component is represented by the glia limitans underlying the pial arterioles (44). Therefore, we sought to determine whether the loss of BKCa and Kir channel function in diabetic rats can be attributed to changes in protein expression of the channels. For these Western immunoblot analyses, pial surface tissue from the parietal cortex was harvested. This was done to obtain a vascular- and astrocyte-enriched sample for analysis. That was confirmed in rat pial samples, where we found a consistent presence of the glial markers GFAP and S-100 β (Fig. 3, A and B). Thus, the pial tissue samples were termed “glio-pial tissue.”
Fig. 3.
Western immunoblot protein analyses of GFAP, S-100 β, BKCa channel subunits, and Kir2.1 channels in samples obtained by stripping off pial tissue from the cerebral cortex surface in control and 4 mo diabetic (T1DM) rats. Those samples not only included pial vessels but also adherent elements of the underlying glia limitans, as revealed by the expression of astrocytic markers GFAP (A) and S-100 β (B). Also shown are representative blots and quantitative protein expression data for BKCa channel α-subunit (BKCaα) (C); BKCa channel β4-subunit (BKCaβ4) (D); BKCa channel β1-subunit (BKCaβ1) (E); and Kir2.1 channels (F) in control and T1DM rats. *P < 0.05; n = 6–7 in each group. Each graph shows the average of 3 independent blots. α-Tubulin or β-actin was used as a loading control. Ratios were normalized to the control's group average. The bands within a frame are contiguous, and splicing is shown by a white space between frames.
When comparing diabetic with ND rats, the pore-forming α-subunit of the BKCa channel did not show any significant change in protein expression in glio-pial tissue (Fig. 3C). Consistent with our findings, Dong et al. (8) reported no change in α-subunit expression in cerebrovascular tissue of type 1 diabetes mellitus mice. Because the electrophysiological properties of BKCa channels are significantly affected by the regulatory β-subunits, we also evaluated the expression of the most abundant β-subunits in the brain, namely β4 (BKCaβ4) and β1 (BKCaβ1) (14). No difference was found in the expression of the brain-specific BKCaβ4 in the glio-pial tissue (Fig. 3D) of diabetic vs. ND rats. On the other hand, it was recently shown that a decrease in BKCaβ1 expression occurs in the cerebrovascular tissue of 5 wk diabetic mice (8). In contrast to the findings in mice, our results showed that, in 4 mo diabetic rats, BKCaβ1 was significantly upregulated compared with controls (P = 0.03; Fig. 3E).
Western blot analysis of Kir2.1 expression failed to show any significant differences between diabetic and ND glio-pial tissues (Fig. 3F).
PKC activity assessment and effects of acute PKC inhibition via CalC.
Diabetes has been associated with an increased activity and/or expression of several PKC isoforms in the brain (29, 31) and retina (12), as well as in other tissues (for review, see Ref. 7). Moreover, there is evidence that, at least in the rat basilar artery, PKC is able to modulate Kir channel function in vivo (5). To better characterize the pattern of PKC activity in brain tissue, we assessed specific PKC activity in both cerebral cortex and glio-pial tissue samples using two nonradioisotopic assays. The results from the two assays were in agreement. The PKC activity measured in cerebral cortex samples, surprisingly, trended toward a decrease in diabetic animals vs. controls (n = 6 in each group, P = 0.10; Fig. 4A), whereas, in the glio-pial tissue (Fig. 4B), PKC activity was significantly increased by ∼30% (P = 0.002) in diabetic (n = 7) relative to control (n = 6) rats.
Next, we examined whether the PKC inhibitor CalC was able to rescue the depressed vascular responses observed in diabetic rats. Pial diameter changes, relative to baseline, after 30 min suffusion of CalC (100 nM) under the cranial window were not significantly different when comparing nondiabetic and diabetic rats (−2 ± 3 and −3 ± 6%, respectively; P > 0.05).
After acute suffusion of CalC, sciatic nerve stimulation-induced dilation completely recovered in the diabetic group (n = 6, P = 0.005; Fig. 4C). On the other hand, in control animals, the pial arteriolar response to sciatic nerve stimulation in the presence of CalC was actually reduced (by 61%, P = 0.002, n = 5; Fig. 4C). In contrast to this finding, a recent report showed no effect of PKC inhibition (via topically applied chelerythrine) on whisker stimulation-induced increases in cerebral blood flow, measured by laser Doppler flowmetry (35). However, the substantial methodologic differences between the present study and that of Straub et al. (35) complicate comparisons.
The suppressed vasodilating response to NS-1619 was completely restored by CalC in diabetic animals (n = 6, P = 0.02 and P = 0.005 at 10 and 50 μM, respectively; Fig. 4E). In contrast, NS-1619-induced dilations were not significantly affected by CalC in ND control animals (Fig. 4D), hinting that BKCa channels are not phosphorylated by PKC under baseline conditions. On the other hand, similar to pial arteriolar responses to the BKCa channel opener NS-1619, the lost K+ responses in diabetic rats were restored to normal levels by acute PKC inhibition with CalC (n = 5, P = 0.05 for 6 mM, P < 0.001 for 12 mM; Fig. 4G). These findings, therefore, suggest that the normalization of sciatic nerve stimulation responses in diabetic animals in the presence of CalC is the result of the restoration of the vascular dilations to NS-1619 and K+. Unexpectedly, ND rats showed a significant inhibition of K+ responses after the PKC inhibitor suffusion (n = 5, P = 0.03 for both concentrations; Fig. 4F). In contrast, CalC has been reported to potentiate the response to K+ in the basilar artery of ND rats (5). That divergence may be ascribed to the different size of the arteries studied (basilar vs. pial) and/or the different anesthetics used (pentobarbital sodium vs. fentanyl/N2O).
Effects of acute PKC activation, via PdBu, in nondiabetic rats.
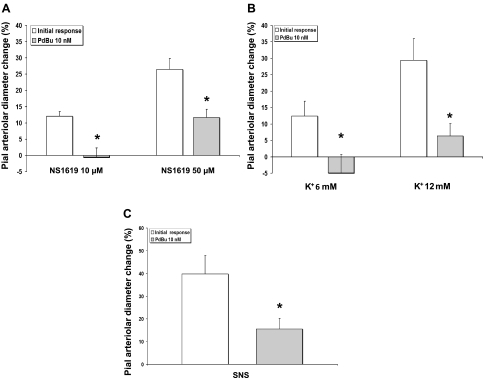
In these experiments, we suffused PdBu to reproduce, in ND rats, the effects of chronic hyperglycemia, namely activation of PKC. Pial arteriole diameters decreased by 10.6 ± 5.6%, compared with the baseline, after 30 min suffusion of 10 nM PdBu (n = 10, P < 0.05). The response to NS-1619 was significantly inhibited in the presence of PdBu (n = 5, P < 0.01 for 10 μM, P = 0.02 for 50 μM; Fig. 5A). Also, the Kir-mediated response to K+ was inhibited by PdBu (n = 5, P < 0.01 for 6 and 12 mM; Fig. 5B). Perhaps linked to the diminished BKCa and Kir function suggested by the attenuated pial arteriolar responses to NS-1619 and K+, we found, in the presence of PdBu, an ∼60% reduction in sciatic nerve stimulation-evoked pial arteriolar dilation (n = 6, P < 0.001; Fig. 5C). Interestingly, in these ND rats, topical application of both the PKC activator (Fig. 5C) and inhibitor (Fig. 4C) achieved the same degree of sciatic nerve stimulation inhibition (∼60%).
Fig. 5.
A–C: effects of PKC activation, via topically applied phorbol 12,13-dibutyrate (PdBu, 10 nM), on pial arteriolar responses in experiments involving only nondiabetic control animals. PdBu markedly inhibited dilations induced by the BKCa agonist NS-1619 (A), the Kir agonist K+ (B), and SNS (C) (n = 5 for all, *P < 0.05 vs. initial responses).
To rule out the possibility of nonspecific effects of PdBu on vessel reactivity, we examined pial arteriolar responses to increased PaCO2 in the absence and presence of PdBu in control rats. The CO2 reactivity index (% of pial arteriolar dilation/mmHg Pco2 increase in the arterial blood) was unchanged after PdBu suffusion (1.7 ± 0.1 vs. 1.6 ± 0.3 in ND and diabetics, respectively; n = 4, P > 0.05).
DISCUSSION
Evidence from reports published to date support an important role for BKCa and Kir channels in the communication between astrocytes and arterioles that represents an integral component of the process of neurovascular coupling (11, 13, 26, 40). Additional findings point to a repression of BKCa and Kir channel function in diabetic, chronically hyperglycemic rats (22). The present findings confirmed that the K+-channel dysfunction in diabetic rats extends to the level of pial arterioles but also includes sciatic nerve stimulation-evoked pial arteriolar dilations. Another key finding in the present study relates to the hypothesis that the losses in vasodilating function were due to PKC activation and could be restored by acute inhibition of PKC-mediated phosphorylation (see Ref. 29). There were two principal findings related to this hypothesis. First, the loss in pial arteriolar reactivity to sciatic nerve stimulation, NS-1619, and K+ in diabetic rats was readily restored in the presence of acute PKC inhibition. Second, PKC activation in normoglycemic animals was able to mimic the diabetic condition in terms of loss of cerebrovascular reactivity to sciatic nerve stimulation, BKCa activation, and Kir activation.
In the present report, similar to recent findings from our laboratory (26), selective blockade of BKCa and Kir channels in ND rats, using paxilline (10 μM) and Ba2+ (100 μM), respectively, yielded significant reductions in sciatic nerve stimulation-evoked pial arteriolar responses. However, in the current study, diabetic rats, despite a nearly complete loss of pial arteriolar reactivity to the BKCa and Kir channel openers, NS-1619 and 6–12 mM K+, displayed only a 30% reduction (instead of 60%) in the pial arteriolar dilations to sciatic nerve stimulation. This implies the occurrence of compensatory adaptations in neurovascular coupling-related mechanisms in chronically diabetic animals. Also, in accord with the complete loss of BKCa function in diabetic rats, paxilline had no effect on the portion of the sciatic nerve stimulation-induced dilation that survived the diabetic insult. On the other hand, that residual sciatic nerve stimulation response was found to be quite sensitive to the presence of 100 μM Ba2+. Although speculative, one possible scenario relates to a Ba2+ action not involving Kir channels, but another target instead. Potential candidates include tandem-pore K+ channels, such as TWIK-2 (also known as K2P6.1). This channel is expressed in rat cerebral arteries, mediates dilation, and is sensitive to BaCl2 inhibition [IC50 ∼80 μM (20, 21)]. At the 100 μM dose utilized in the present investigation, one would expect this channel to be affected by Ba2+. Furthermore, TWIK-2 currents in oocytes were reported to be elevated by exposure to the PKC activator phorbol 12-myristate,13-acetate (4). If the TWIK-2 in cerebral arteries (e.g., pial arterioles) were similarly affected by increased PKC activity, this could explain the retention of pial arteriolar Ba2+ sensitivity in T1DM animals where arteriolar Kir function is essentially lost. However, the above remains speculative, and additional experimentation is needed.
Analysis of protein abundance in superficial cerebral cortex samples [composed of pial vascular tissue and elements of the glia limitans (labeled “glio-pial” tissue)] failed to yield any indications that the diminished BKCa channel- and Kir channel-related vasodilating functions in diabetic rats were related to downregulation of channel expression. For example, no changes in the Kir isoform (Kir2.1) found in cerebrovascular smooth muscle (and crucial for KCl reactivity) were detected (32, 34, 46). In BKCa channels, no significant changes in the abundance of the pore-forming α-subunits or regulatory β4-subunits were seen. However, in diabetic vs. control rats, we did detect a significantly higher β1-subunit expression. Our α-subunit findings are similar to those recently found in cerebral artery smooth muscle in a rat model of type 2 diabetes (41). On the other hand, the same authors reported a decrease in smooth muscle β1 expression. At this time, we cannot offer any explanation for such a disparity between the two studies, other than sampling differences or factors related to type 1 vs. type 2 diabetes mellitus. Moreover, there is no current information that would permit us to link our finding of an increased β1 expression to a loss of pial arteriolar reactivity to the BKCa opener NS-1619. Perhaps β1 upregulation might be eventually shown to represent one of the putative compensatory mechanisms, resulting in a gain of function.
In lieu of any definitive evidence linking changes in the expression of BKCa or Kir channels to repression of channel-associated vasodilating responses, we focused our attention on PKC-mediated phosphorylations of channel components. These experiments consisted of three different approaches. First, we directly measured PKC activity in cortical gray matter and glio-pial tissue and compared control and diabetic rats. Analysis of PKC activity in glio-pial tissue revealed significantly higher levels in diabetic vs. ND rats. No differences were seen in cortical gray matter. Second, rats were acutely treated with a general PKC inhibitor, CalC. In these experiments, we found that acute CalC topical exposure of diabetic rat pial arterioles was accompanied by a normalization of pial arteriolar dilations elicited by NS-1619, K+, and sciatic nerve stimulation. Interestingly, in control rats, CalC exposure was associated with a substantial repression of K+ and sciatic nerve stimulation-induced (but not NS-1619-induced) dilations. For the third approach, we examined pial arteriolar dilating responses to sciatic nerve stimulation and topically applied BKCa and Kir-channel openers in the presence of the potent PKC activator PdBu. The expected increase in PKC activity induced by PdBu mimicked the diabetic condition, insofar as we found a loss of function of BKCa and Kir channels, along with a significant reduction in the sciatic nerve stimulation-evoked response. The PdBu findings, therefore, provided some support for PKC playing an important role in the diabetes-related suppression of neurovascular coupling.
The effects of both PKC activators and inhibitors on the biophysical properties of isolated Kir and BKCa channels have been described in the literature and generally support our findings on PKC influence on sciatic nerve stimulation-, BKCa channel-, and Kir channel-associated vasodilating function. Kir channel currents have been shown to be inhibited by phorbol 12-myristate,13-acetate, a PKC agonist, and increased by CalC in cerebral arteriole smooth muscle cells (43). Similarly, BKCa channel currents were inhibited by PKC agonists in smooth muscle cells of coronary arteries (24). However, the data regarding the effect of PKC on BKCa channel function are conflicting, and with differences related to specific PKC isoforms, especially in the brain (17). That complexity may also explain why CalC had no effect on NS-1619-mediated dilation in nondiabetic animals. Unfortunately, no data are currently available, to our knowledge, regarding the effects of PKC modulation of BKCa channels in astrocytes. Interestingly, in a model of type 2 diabetes mellitus, whole cell currents of BKCa channels were significantly decreased in cerebral artery smooth muscle cells, compared with control, and the sensitivities of BKCa channels to voltage, paxilline, and NS-1619 were all diminished in diabetic rats (41).
Moreover, PKC activation has been strongly linked to the inhibition of BKCa and Kir channels in smooth muscle mediated by several vasoconstrictors (18, 24, 27, 36, 47). That effect may be a function of a repression of PKA-related smooth muscle relaxation (47). Indeed, recent findings from our laboratory have shown that PKA inhibition markedly attenuates pial arteriolar dilations during sciatic nerve stimulation and in the presence of topically applied NS-1619 and K+ (26). Clearly, the involvement of PKC in the regulation of neurovascular coupling, in general, and in chronically hyperglycemic animals, in particular, needs more study. Furthermore, such investigations should include examination of the roles of other kinases as well as the different PKC isozymes, since the PKC inhibitor and activator used in the present study were not isoform-specific.
There is evidence suggesting a role for reactive oxygen species (ROS) and PKC activation in cerebrovascular dysfunction in a model of normoglycemia/hyperinsulinemia (9). Even though there are notable differences between the two experimental models, a common link may be represented by the free fatty acid-induced ROS generation with subsequent PKC activation (16). However, this link has yet to be proved in in vivo experiments and remains speculative at this time. Likewise, we cannot extend our present results to type 2 diabetes mellitus, even though it is possible that similar pathophysiological changes may occur as well. For instance, it has been shown that ROS may directly impair BKCa channel function (37).
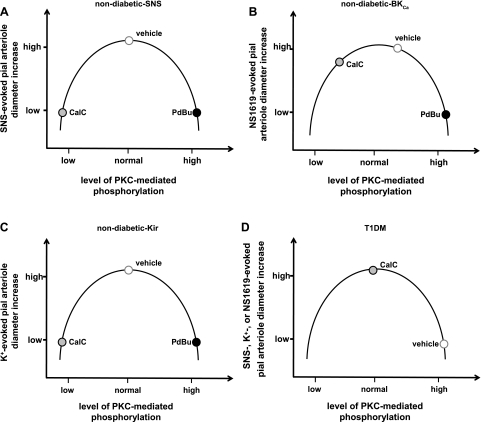
On the other hand, the fact that acute inhibition of PKC (via CalC) blocked the responses to K+ and sciatic nerve stimulation in ND control rats is more difficult to interpret. The data seem to imply the existence of a “biphasic” influence of PKC, one that might be described by an inverted U-shaped response curve, where the magnitude of the diameter change in the presence of a vasodilating stimulus would be determined by the initial level of PKC-mediated phosphorylation. To illustrate this concept, we constructed Fig. 6. For example, at a high level of baseline PKC-mediated phosphorylation (beyond the peak of the inverted U-curve), as might be found in diabetic rats (see Fig. 6D), addition of CalC would lower the level of phosphorylation, raising the magnitude of the pial arteriolar response to the vasodilator (e.g., sciatic nerve stimulation, NS-1619, or K+). If the level of phosphorylation were at the peak of the inverted U, indicative of a moderate, perhaps physiological, phosphorylation state (Fig. 6, A–C), then creating a low-phosphorylation state, via addition of CalC, could lead to a scenario whereby the response to the vasodilator would be a modest or no diameter increase. That example might be represented by the arteriolar responses to sciatic nerve stimulation and K+ in ND rats (Fig. 6, A and C). In those animals, both CalC-induced reductions and PdBu-induced increases in PKC activity were accompanied by severely attenuated vasodilating responses. Even the NS-1619 responses in ND rats (which showed little sensitivity to CalC, but were very sensitive to PdBu) would be consistent with the inverted U model if one places the normal response, in the presence of vehicle, just to the right of the peak (Fig. 6B).
Fig. 6.
A–D: illustrations representing the postulated responses of pial arterioles to SNS, NS-1619, and K+ in control animals in the presence of PdBu-associated increases or CalC-induced decreases in the levels of PKC-mediated phosphorylation. For all 3 vasodilators, with the starting level of phosphorylation (vehicle) lying near the peak of the inverted U curve, PdBu-associated enhancement of phosphorylation will be accompanied by a repressed vasodilation (as the data in Fig. 5 indicate). Going in the other direction (CalC-associated diminished phosphorylation), vascular reactivity may also be reduced. That could apply to SNS- and K+-evoked responses (see Fig. 4, C and F). If one modestly shifts the level of baseline PKC-mediated phosphorylation to the right of the peak, then, during a CalC-associated leftward shift, one might observe no change in response, similar to what we observed for the BKCa opener NS-1619 (Fig. 4D). In diabetic (T1DM) rats (D), note that the baseline (vehicle) PKC-linked phosphorylation levels are depicted as being elevated, based upon the increased PKC activity measured in the glio-pial tissue (shown in Fig. 4B). In that case, a decrease in PKC-mediated phosphorylation levels (leftward shift along the curve) improved responses to all dilating stimuli.
Among the other possible causes that could account for decreased reactivity of the diabetic cerebral vessels, certainly there is the endothelial dysfunction. However, we have demonstrated (44) that, in our model, endothelium does not participate in the response to sciatic nerve stimulation. Another possible cause for diminished neurovascular coupling response in the diabetic brain could be an impaired neuronal function, as reflected in diminished sciatic nerve stimulation-evoked neuronal responses. This possibility was ruled out by the absence of significant differences in sciatic nerve stimulation-evoked potential amplitudes when comparing diabetic and nondiabetic rats in the present and earlier studies (28).
In conclusion, the dysregulation of cerebral perfusion accompanying chronic hyperglycemia, in rat models of type 1 diabetes mellitus, is well-documented. The above pathophysiological process has been shown to include loss of BKCa and Kir channel-mediated vasodilating functions. In accord with the vital roles these channels play in the important process of neurovascular coupling, we observed a significant decrease in the pial arteriolar dilations evoked by somatosensory activation, via sciatic nerve stimulation, in STZ-treated diabetic rats. The present results also suggested that the repressed neurovascular coupling was likely linked to PKC-mediated changes in BKCa and Kir channel activity, since normal dilating responses of pial arterioles to sciatic nerve stimulation and applications of K+-channel openers were readily restored by acute PKC inhibition. It is possible that similar PKC-mediated phosphorylation changes may occur also in type 2 diabetes mellitus. Further studies, however, must be designed to address this specific issue.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-088259 to D. A. Pelligrino, JDRF postdoctoral fellowship 3–2008-462 to F. Vetri, and American Heart Association Grant 0635337N to H. L. Xu.
DISCLOSURES
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Author contributions: F.V. and D.A.P. conception and design of research; F.V. and H.X. performed experiments; F.V. and H.X. analyzed data; F.V., H.X., C.P., and D.A.P. interpreted results of experiments; F.V. prepared figures; F.V. drafted manuscript; F.V. and D.A.P. edited and revised manuscript; F.V., H.X., C.P., and D.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Susan Anderson, Rafael Chavez, and Fulong Tan for outstanding technical support.
REFERENCES
- 1. Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav Brain Res 198: 224–230, 2009. . [DOI] [PubMed] [Google Scholar]
- 2. Arrick DM, Sharpe GM, Sun H, Mayhan WG. Diabetes-induced cerebrovascular dysfunction: role of poly(ADP-ribose) polymerase. Microvasc Res 73: 1–6, 2007. . [DOI] [PubMed] [Google Scholar]
- 3. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chavez RA, Gray AT, Zhao BB, Kindler CH, Mazurek MJ, Mehta Y, Forsayeth JR, Yost CS. TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family. J Biol Chem 274: 7887–7892, 1999. . [DOI] [PubMed] [Google Scholar]
- 5. Chrissobolis S, Sobey CG. Inhibitory effects of protein kinase C on inwardly rectifying K+− and ATP-sensitive K+ channel-mediated responses of the basilar artery. Stroke 33: 1692–1697, 2002. . [DOI] [PubMed] [Google Scholar]
- 6. Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. J Am Med Assoc 297: 2716–2724, 2007. . [DOI] [PubMed] [Google Scholar]
- 7. Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res 55: 498–510, 2007. . [DOI] [PubMed] [Google Scholar]
- 8. Dong L, Zheng YM, Van Riper D, Rathore R, Liu QH, Singer HA, Wang YX. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. J Cereb Blood Flow Metab 28: 377–386, 2008. . [DOI] [PubMed] [Google Scholar]
- 9. Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes 53: 1352–1359, 2004. . [DOI] [PubMed] [Google Scholar]
- 10. Fakler B, Brandle U, Glowatzki E, Zenner HP, Ruppersberg JP. Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron 13: 1413–1420, 1994. . [DOI] [PubMed] [Google Scholar]
- 11. Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006. . [DOI] [PubMed] [Google Scholar]
- 12. Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 106: 1319–1331, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 107: 3811–3816, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ha TS, Heo MS, Park CS. Functional effects of auxiliary beta4-subunit on rat large-conductance Ca(2+)-activated K(+) channel. Biophys J 86: 2871–2882, 2004. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol 117: 119–129, 1996. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945, 2000. . [DOI] [PubMed] [Google Scholar]
- 17. Kim JY, Park CS. Potentiation of large-conductance calcium-activated potassium [BK(Ca)] channels by a specific isoform of protein kinase C. Biochem Biophys Res Commun 365: 459–465, 2008. . [DOI] [PubMed] [Google Scholar]
- 18. Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res 44: 65–81, 2008. . [DOI] [PubMed] [Google Scholar]
- 19. Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol 291: H2897–H2904, 2006. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lloyd EE, Crossland RF, Phillips SC, Marrelli SP, Reddy AK, Taffet GE, Hartley CJ, Bryan RM., Jr Disruption of K(2P)6.1 produces vascular dysfunction and hypertension in mice. Hypertension 58: 672–678, 2011. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lloyd EE, Marrelli SP, Namiranian K, Bryan RM., Jr Characterization of TWIK-2, a two-pore domain K+ channel, cloned from the rat middle cerebral artery. Exp Biol Med (Maywood) 234: 1493–1502, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayhan WG, Mayhan JF, Sun H, Patel KP. In vivo properties of potassium channels in cerebral blood vessels during diabetes mellitus. Microcirculation 11: 605–613, 2004. . [DOI] [PubMed] [Google Scholar]
- 23. Meno JR, Nguyen TS, Jensen EM, Alexander West G, Groysman L, Kung DK, Ngai AC, Britz GW, Winn HR. Effect of caffeine on cerebral blood flow response to somatosensory stimulation. J Cereb Blood Flow Metab 25: 775–784, 2005. . [DOI] [PubMed] [Google Scholar]
- 24. Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca(2+)-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun 190: 263–269, 1993. . [DOI] [PubMed] [Google Scholar]
- 25. Northam EA, Rankins D, Cameron FJ. Therapy insight: the impact of type 1 diabetes on brain development and function. Nat Clin Pract Neurol 2: 78–86, 2006. . [DOI] [PubMed] [Google Scholar]
- 26. Paisansathan C, Xu H, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol 299: H2009–H2017, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park WS, Han J, Kim N, Youm JB, Joo H, Kim HK, Ko JH, Earm YE. Endothelin-1 inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase C. J Cardiovasc Pharmacol 46: 681–689, 2005. . [DOI] [PubMed] [Google Scholar]
- 28. Pelligrino DA, Albrecht RF. Chronic hyperglycemic diabetes in the rat is associated with a selective impairment of cerebral vasodilatory responses. J Cereb Blood Flow Metab 11: 667–677, 1991. . [DOI] [PubMed] [Google Scholar]
- 29. Pelligrino DA, Koenig HM, Wang Q, Albrecht RF. Protein kinase C suppresses receptor-mediated pial arteriolar relaxation in the diabetic rat. Neuroreport 5: 417–420, 1994. . [DOI] [PubMed] [Google Scholar]
- 30. Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's Disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 67: 505–512, 2010. . [DOI] [PubMed] [Google Scholar]
- 31. Ramakrishnan R, Sheeladevi R, Namasivayam A. Regulation of protein kinases and coregulatory interplay of S-100beta and serotonin transporter on serotonin levels in diabetic rat brain. J Neurosci Res 87: 246–259, 2009. . [DOI] [PubMed] [Google Scholar]
- 32. Ren Y, Xu X, Wang X. Altered mRNA expression of ATP-sensitive and inward rectifier potassium channel subunits in streptozotocin-induced diabetic rat heart and aorta. J Pharmacol Sci 93: 478–483, 2003. . [DOI] [PubMed] [Google Scholar]
- 33. Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22: 505–512, 2001. . [DOI] [PubMed] [Google Scholar]
- 34. Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol 586: 1147–1160, 2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Straub SV, Girouard H, Doetsch PE, Hannah RM, Wilkerson MK, Nelson MT. Regulation of intracerebral arteriolar tone by Kv channels: effects of glucose and PKC. Am J Physiol Cell Physiol 297: C788–C796, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taguchi K, Kaneko K, Kubo T. Protein kinase C modulates Ca2+-activated K+ channels in cultured rat mesenteric artery smooth muscle cells. Biol Pharm Bull 23: 1450–1454, 2000. . [DOI] [PubMed] [Google Scholar]
- 37. Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol 11: 171–178, 2004. . [DOI] [PubMed] [Google Scholar]
- 38. Vetri F, Menicucci D, Lapi D, Gemignani A, Colantuoni A. Pial arteriolar vasomotion changes during cortical activation in rats. Neuroimage 38: 25–33, 2007. . [DOI] [PubMed] [Google Scholar]
- 39. Vetri F, Xu H, Mao L, Paisansathan C, Pelligrino DA. ATP hydrolysis pathways and their contributions to pial arteriolar dilation in rats. Am J Physiol Heart Circ Physiol 301: H1369–H1377, 2011. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vetri F, Xu HL, Mao L, Pelligrino DA. Diabetes-related changes in neurovascular coupling in rats are gender-specific. Soc Neurosci Meet 2008, Washington, DC: . [Google Scholar]
- 41.Wang Y, Zhang HT, Su XL, Deng XL, Yuan BX, Zhang W, Wang XF, Yang YB. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovasc Res 7: 75–84, 2010. . [DOI] [PubMed] [Google Scholar]
- 42. Widmer HA, Rowe IC, Shipston MJ. Conditional protein phosphorylation regulates BK channel activity in rat cerebellar Purkinje neurons. J Physiol 552: 379–391, 2003. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu BN, Luykenaar KD, Brayden JE, Giles WR, Corteling RL, Wiehler WB, Welsh DG. Hyposmotic challenge inhibits inward rectifying K+ channels in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 292: H1085–H1094, 2007. . [DOI] [PubMed] [Google Scholar]
- 44. Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol 294: H622–H632, 2008. . [DOI] [PubMed] [Google Scholar]
- 45. Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol 92: 647–651, 2007. . [DOI] [PubMed] [Google Scholar]
- 46. Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir22 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res 87: 160–166, 2000. . [DOI] [PubMed] [Google Scholar]
- 47. Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci USA 107: 8005–8010, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]