Abstract
Arteriosclerosis with aging leads to central arterial stiffening in humans, which could be a prime cause for increased cardiac afterload in the elderly. The purpose of the present study was to assess the effects of 1 yr of progressive exercise training on central aortic compliance and left ventricular afterload in sedentary healthy elderly volunteers. Ten healthy sedentary seniors and 11 Masters athletes (>65 yr) were recruited. The sedentary seniors underwent 1 yr of progressive exercise training so that at the end of the year, they were exercising ∼200 min/wk. Central aortic compliance was assessed by the Modelflow aortic age, which reflects the intrinsic structural components of aortic compliance. Cardiac afterload was assessed by effective arterial elastance (Ea) with its contributors of peripheral vascular resistance (PVR) and systemic arterial compliance (SAC). After exercise training, Ea, PVR, and SAC were improved in sedentary seniors and became comparable with those of Masters athletes although the Modelflow aortic age was not changed. Moreover, after exercise training, when stroke volume was restored with lower body negative pressure back to pretraining levels, the exercise training-induced improvements in Ea, PVR, and SAC were eliminated. Aortic stiffening with aging was not improved even after 1 yr of progressive endurance exercise training in the previously sedentary elderly, while left ventricular afterload was reduced. This reduced afterload after exercise training appeared to be attributable to cardiovascular functional modulation to an increase in stroke volume rather than to intrinsic structural changes in the arterial wall.
Keywords: aortic stiffness, cardiac afterload, aging, exercise training, biologic aortic age
arteriosclerosis with aging leads to large vessel arterial stiffening in humans, the process of which is characterized primarily by structural changes in the arterial wall such as the development of fibrosis and degeneration of the elastin matrix (20, 21). Recent epidemiological studies demonstrated that central arterial stiffness is an important, independent determinant of cardiovascular risk in subjects with hypertension (3) and diabetes mellitus (10) and in those aged over 70 yr (27, 41). Thus, although arterial stiffening with aging was once considered as an inevitable consequence of normal aging, it is now considered to be a clinically relevant process to be treated or prevented in even “healthy” seniors aged over 70 yr and probably younger.
Effective arterial elastance (Ea), which represents net arterial load imposed on the left ventricle, is determined by a resistive component (peripheral vascular resistance) and a pulsatile component (systemic arterial compliance) within a cardiac cycle (19, 40). Several studies (8, 9, 31, 32) have shown that Ea increases with advancing age in humans. Moreover, one recent community-based study showed that the age-associated increase in Ea was primarily attributable to the age-associated increase in central arterial stiffness, because pulse pressure increased with age, whereas peripheral vascular resistance and heart rate did not change (31). Thus one subclinical outcome from the arterial stiffening with aging is augmented cardiac afterload, which causes left ventricular hypertrophy and may be associated with the high prevalence of heart failure with preserved ejection fraction in the elderly population (18, 46).
Several conditions, such as diabetes, obesity, hyperlipidemia, and hypertension, have been reported to accelerate arteriosclerosis with aging by stimulating the development of collagen cross-linking in the arterial wall (24, 43). In contrast, endurance exercise training may decelerate arteriosclerosis by improving these clinical and subclinical conditions as well as having direct effects on large vessels (33, 34). For example, it has been reported that the highly trained elderly have more compliant central arteries than their age-matched controls as determined by pulse wave velocity (44) or beta-stiffness index of the common carotid artery (42), suggesting that life-long exercise may inhibit vascular stiffening with aging. Our laboratory (37) also showed that the biologic aortic age, which reflects the intrinsic structural changes in the central aortic wall without the confounding influence of ambient blood pressure, was “younger” in highly trained elderly athletes than in their sedentary peers. Moreover, several studies showed the beneficial effects of short-term (∼3 mo) endurance exercise training on central arterial compliance in healthy young and middle aged adults (4, 42), although a few studies showed no significant effects of exercise training in some specific cohorts such as hypertensive patients (11) or octogenarians (39). Therefore, endurance exercise training could be a potential strategy to treat central arterial stiffening with aging and thus reduce left ventricular afterload in healthy elderly individuals, although it has not yet been validated for this purpose.
The objective of the present study was to assess the effect of 1 yr of progressive and vigorous exercise training on the biologic aortic age and left ventricular afterload in the previously sedentary healthy elderly. We hypothesized that age-appropriate old and stiff aortas in the healthy but sedentary elderly population would become more youthful and compliant after 1 yr of exercise training and that cardiac afterload increased with arterial stiffening would be concomitantly reduced.
METHODS
Subject Population
Ten healthy sedentary (regular endurance exercise: <30 min/session and <3 times/wk) adults older than 65 yr of age were recruited for the exercise intervention. Also, 11 Masters athletes older than 65 yr of age were recruited to compare 1 yr of progressive and vigorous exercise training to life-long constant and vigorous exercise training. The detailed recruitment criteria and exercise history of these Masters athletes were reported previously (1). In brief, Masters athletes were consistent age-group place winners at regional and national endurance events and had participated in regular competitions for 23 ± 8 yr with a weekly running mileage of 32 ± 10 miles or equivalent swimming or cycling (1). Left ventricular pressure-volume relations and Doppler indexes for left ventricular diastolic function in these subjects before and after training were previously reported (1, 12, 30). All subjects were rigorously screened for the presence of hypertension, obstructive coronary artery disease, or structural heart disease by use of 24-h blood pressure recordings, baseline and exercise ECG, and echocardiogram. Subjects were excluded if one of following was present: 1) mean daytime blood pressure greater than 140/90 mmHg; 2) ECG changes suggestive of ischemic heart disease, left bundle branch block, atrial flutter/fibrillation, or atrioventricular block greater than first degree; 3) baseline or exercise induced wall motion abnormality, valvular heart disease other than mild valvular insufficiency, or right or left ventricular hypertrophy; 4) untreated thyroid disorder; 5) chronic lung disease; 6) regular cigarette smoking within the previous 10 yr; 7) body mass index of 30 or greater; and 8) cardiovascular medication.
The experimental procedures were explained to all subjects with informed consent obtained as approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas.
Measurements
All experiments were performed in the morning ≥2 h after a light breakfast in a quiet environmentally controlled laboratory with an ambient temperature of 22–25°C. The subjects were asked to refrain from heavy exercise and caffeinated or alcoholic beverages for ≥24 h before the tests. All measurements were performed in the supine position after confirmation of hemodynamic stability.
Cardiac output/stroke volume.
Cardiac output was measured every 5 min with the foreign gas (C2H2) rebreathing method (14), and stroke volume was calculated from cardiac output and each coincident heart rate from three-lead ECG. An average of two to three measurements was used for data analysis.
Blood pressure.
A continuous finger arterial pressure waveform was recorded with Photoplethysmography (Portapres). Intermittent brachial cuff blood pressures were also obtained simultaneously with electrosphygmomanometry (Suntech) during cardiac output measurements.
One-Year Exercise Training and Stroke Volume Restoration
All subjects underwent progressive and vigorous exercise training in accordance with a training program prescribed individually using heart rate monitoring and training zones derived from exercise testing for each subject over 1 yr (12, 28). All of the exercise training was supervised regularly by exercise physiologists. Subjects gradually increased their exercise frequency, duration and intensity and finally achieved weekly exercise training equivalent to ∼200 min of 75% maximal exercise at the end of 1 yr training (28). All measurements of physiological variables were repeated after the exercise training.
To eliminate the effects of increased stroke volume due to cardiac physiological remodeling on arterial function measurements, lower body negative pressures of −15 and −30 mmHg (LBNP-15 and LBNP-30) were applied to restore stroke volume down to the level of preexercise training after the exercise intervention.
Data Analysis
Modelflow aortic age.
The Modelflow aortic age was analyzed as described previously (37). First, Modelflow stroke volumes were generated from a given central blood pressure waveform reconstructed from the finger blood pressure waveform (Beatscope 1.1a; FMS) by using input ages to the Modelflow system from 20 to 90 yr (17, 45). Then, the linear regression analysis between the input age and generated Modelflow stroke volumes was constructed. The Modelflow aortic age was then determined by an inverse function of this linear regression equation using stroke volume measured with the C2H2 rebreathing method as the input variable.
This index was well validated by our previous study showing high age specificity in sedentary healthy populations and high reproducibility during hemodynamic changes by LBNP (37); the coefficient of variation was three times smaller for the Modelflow aortic age (21%) than for static systemic arterial stiffness (61%), central pulse wave velocity (61%), or common carotid artery beta stiffness index (58%). The typical error as a coefficient of variation for the Modelflow aortic age was <7% during LBNP-15 and LBNP-30, which was two times smaller than for static systemic arterial stiffness (>13%).
Ea and other indexes.
Ea (0.9 * systolic blood pressure/stroke volume; Ref. 19), systemic arterial compliance (stroke volume/pulse pressure; Ref. 7), peripheral vascular resistance (80 * mean blood pressure/cardiac output) were calculated using cardiac output and stroke volume from the C2H2 rebreathing method and coincident arm cuff blood pressure.
Statistical Analysis
Data are presented as means ± SD. Statistical probability was assessed with Student's paired t-test to test the difference between values before and after exercise training and with Student's unpaired t-test to test the difference between groups. One-way repeated ANOVA was used to test the effect of LBNP, and Student Newman post hoc test was applied when P values were <0.05. The reproducibility of indexes was assessed as the typical error, which is calculated by the SD of difference scores divided by and expressed as a percentage of the grand mean (13).
RESULTS
Subject Characteristics
While body mass index was higher, age was slightly older, and peak oxygen uptake was lower in the sedentary elderly (Table 1). Body mass was decreased in the sedentary elderly after 1 yr of progressive endurance exercise training while peak oxygen uptake was augmented (Table 1). These results were reported in previous studies from our laboratory (12, 28, 36).
Table 1.
Subject characteristics
| Pretraining | Posttraining | Masters Athletes | |
|---|---|---|---|
| Male/female | 6/4 | — | 5/6 |
| Age, yr | 71 ± 3† | — | 68 ± 3 |
| Height, cm | 171 ± 9 | — | 170 ± 12 |
| Weight, kg | 74 ± 10 | 70 ± 10* | 65 ± 14 |
| BMI, kg/m2 | 26.0 ± 1.8† | 24.7 ± 1.9*† | 22.1 ± 1.9 |
| BSA, m2 | 1.86 ± 0.19 | 1.81 ± 0.19* | 1.74 ± 0.25 |
| V̇o2max, ml·min−1·kg−1 | 22.4 ± 3.6† | 26.7 ± 4.4*† | 38.2 ± 6.2 |
Values are means ± SD.
BMI, body mass index; BSA, body surface area; V̇o2max, maximal oxygen consumption.
P < 0.05 pre- vs. posttraining.
P < 0.05 vs. Masters athletes.
Physiological Variables
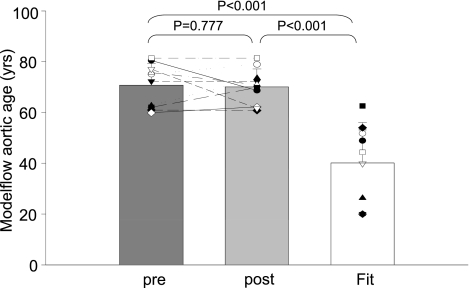
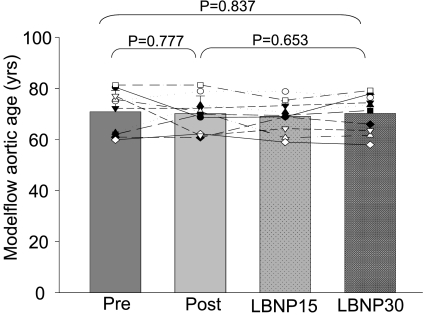
The Modelflow aortic ages of the sedentary elderly were comparable with their chronological age (Modelflow aortic ages: 71 ± 8 yr, chronological age: 71 ± 3 yr; P = 0.965; changes in mean: −0.4%; typical error as a coefficient of variation: 7.4%) and older than those of Masters athletes who showed younger Modelflow aortic ages than their chronological age (Modelflow aortic ages: 40 ± 16 yr; chronological age: 68 ± 3 yr; P < 0.001; changes in mean: −45.5%; typical error as a coefficient of variation: 35.8%; Fig. 1) as previously reported (37).
Fig. 1.
Modelflow aortic age before and after exercise training in the previously sedentary elderly (pre and post) and Masters athletes (Fit).
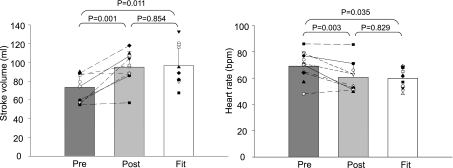
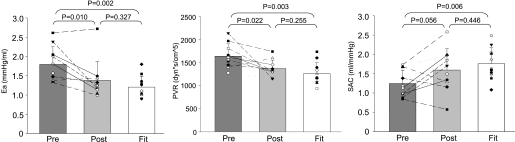
Unexpectedly, the Modelflow aortic ages did not become “younger” after exercise training in the previously sedentary elderly and thus remained age appropriate and older than in Masters athletes (P = 0.777; changes in mean: −0.9%; typical error as a coefficient of variation: 8.6%; Fig. 1). In contrast, almost all the other physiological indexes in the sedentary elderly were improved towards being comparable with those of Masters athletes after exercise training including heart rate, stroke volume, blood pressures, Ea, peripheral vascular resistance, and systemic arterial compliance (Fig. 2 and 3 and Table 2); pulse pressure increased after exercise training, resulting in a greater difference of pulse pressure between sedentary elderly and Masters athletes, although it did not reach conventional levels of statistical certainty (Table 2).
Fig. 2.
Stroke volume (left) and heart rate (right) before and after exercise training in the previously sedentary elderly (pre and post) and Masters athletes (Fit).
Fig. 3.
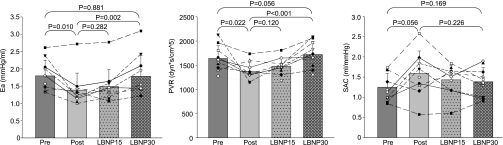
Effective arterial elastance (Ea; left), peripheral vascular resistance (PVR; middle), and systemic arterial compliance (SAC; right) before and after exercise training in the previously sedentary elderly (pre and post), and Masters athletes (Fit).
Table 2.
Baseline blood pressures
| Pretraining | Posttraining | Masters Athletes | |
|---|---|---|---|
| SBP, mmHg | 140 ± 10 | 139 ± 21 | 125 ± 20 |
| DBP, mmHg | 80 ± 7† | 73 ± 4* | 69 ± 10 |
| MBP, mmHg | 100 ± 8† | 95 ± 8* | 88 ± 13 |
| PP, mmHg | 60 ± 6 | 65 ± 21 | 56 ± 12 |
Values are means ± SD. SBP, DBP, MBP, and PP; systolic, diastolic, mean, and pulse blood pressure, respectively.
P < 0.05 pre- vs. posttraining.
P < 0.05 vs. Masters athletes.
Physiological Variables During LBNP
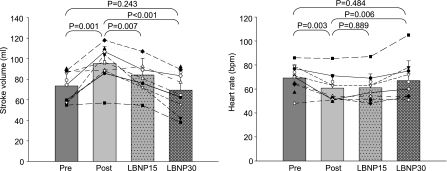
LBNP was applied in the sedentary elderly after 1 yr of exercise training to restore stroke volume to the level of pretraining. As expected, stroke volume decreased back down to the level of pretraining at LBNP-30, which was accompanied by an increase in heart rate back up to the level of pretraining (Fig. 4). Concomitantly, systemic arterial compliance decreased, and peripheral vascular resistance and effective arterial elastance increased during LBNP, all of which consequently became comparable with those of preexercise training at LBNP-30 (Fig. 5). In contrast, Modelflow aortic age did not change during LBNP and remained age-appropriate throughout LBNP (Fig. 6). Blood pressures did not change during LBNP except that pulse pressure decreased at LBNP-30 compared with baseline (Table 3).
Fig. 4.
Stroke volume (left) and heart rate (right) before and after exercise training in the previously sedentary elderly (pre and post) and during LBNP-15 and LBNP-30 after exercise training.
Fig. 5.
Ea (left), PVR (middle), and SAC (left) before and after exercise training in the previously sedentary elderly (pre and post) and during LBNP-15 and LBNP-30 after exercise training.
Fig. 6.
Modelflow aortic age before and after exercise training in the previously sedentary elderly (pre and post) and during lower body negative pressure of −15 and −30 mmHg after exercise training (LBNP-15 and LBNP-30).
Table 3.
Blood pressures during LBNP after exercise
| Baseline | LBNP-15 | LBNP-30 | |
|---|---|---|---|
| SBP, mmHg | 139 ± 21 | 132 ± 17 | 126 ± 11 |
| DBP, mmHg | 73 ± 4 | 71 ± 7 | 76 ± 8 |
| MBP, mmHg | 95 ± 8 | 91 ± 10 | 92 ± 8 |
| PP, mmHg | 65 ± 21 | 61 ± 13 | 50 ± 9* |
Values are means ± SD. LBNP-15 and LBNP-30, lower body negative pressures of −15 and −30 mmHg, respectively.
P < 0.05 vs. baseline.
DISCUSSION
The major findings from the present study were as follows: 1) contrary to our hypothesis, biologic aortic age (Modelflow aortic age) failed to become more youthful even after 1 yr of progressive and vigorous exercise training, despite a reduction in Ea to a level comparable with that of Masters athletes; and 2) the reduced Ea after exercise training appeared to be secondary to an increase in stroke volume since when stroke volume was restored back to the pretraining level by LBNP, the Ea was also restored to baseline levels, while the Modelflow aortic age consistently remained age appropriate.
Biologic Aortic Age
Arteriosclerosis with aging is characterized by structural changes in the arterial wall such as structural protein composition and collagen cross-linking (20, 21). In addition to these mechanical factors, there are at least two functional factors that could affect the clinical assessment of arterial compliance: smooth muscle tone and ambient blood pressure (2, 16, 26). Since the amount of vascular smooth muscle is relatively small in the central large arteries, the effects of smooth muscle tone on the central arterial compliance is also small (38), while it becomes a major determinant for arterial compliance in the periphery, particularly in arterioles where the majority of peripheral vascular resistance is produced. The ambient blood pressure indirectly affects operational arterial compliance due to the curvilinear relationship of the entire arterial compliance curve (22, 23). In contrast, arterioles are little affected by ambient blood pressure although they are a key determinant of systemic mean blood pressure by controlling peripheral vascular resistance.
Conceptually, the Modelflow aortic age is a pressure independent index and reflects only central aortic compliance, where vascular smooth muscle is scarce. Therefore, this index for biologic aortic age is most likely to represent structural changes of the central arterial walls with little influence by functional factors (37). The present finding that the Modelflow aortic age did not change even after 1 yr of endurance exercise training suggests that structural changes in the arterial wall such as degeneration of elastin and development of fibrosis with aging are not reversible by endurance exercise training alone in the previously sedentary healthy elderly. This finding is consistent with previous studies (11, 39) using those individuals who are likely to have severely advanced arteriosclerosis in their arteries such as hypertensives or the very elderly. For example, there was no improvement in the augmentation index of central arterial pressure waveforms, which is influenced by the large arterial stiffness, in octogenarians (>78 yr old) after 9 mo of exercise training (39). Moreover, patients with isolated systolic hypertension (64 yr old) did not show any changes in central arterial stiffness indexes including pulse wave velocity, aortic impedance, or systemic arterial compliance after 2 mo of endurance exercise training (11). Our findings extend and provide supportive evidence to previous findings by showing that even “healthy” elderly individuals in the seventh or eight decade of life cannot improve central arterial stiffening with aging even after intensive exercise training for 1 yr by using an index that specifically reflects structural changes in the central arterial wall. This fact is clinically important since central arterial stiffness is known to be an independent risk factor for cardiovascular diseases in those aged over 70 yr (27, 41).
In contrast to short duration exercise training, the finding that Masters athletes have younger aortas than their peers implies that life-long exercise training has beneficial effects on central arterial compliance, consistent with previous studies using central pulse wave velocity (44) and beta-stiffness index of the common carotid artery (42). It is possible that while accumulation of extracellular cross-linked collagen or degeneration of the elastin matrix can be prevented by life-long exercise training, once established, it is hard to reverse them by exercise training alone.
Our results from the Modelflow aortic age contradicted several previous studies that generated our original hypothesis by showing beneficial effects of endurance exercise training on central arterial compliance after even shorter duration of exercise training than that of the present study (4, 42). The discrepancy could be explained by the fact that indexes for arterial compliance used in these previous studies involved local common carotid arterial stiffness or systemic arterial compliance, which are affected by both structural and functional components of arterial compliance. Indeed, the present study showed that systemic arterial compliance estimated by stroke volume divided by pulse pressure decreased after exercise training, consistent with these previous studies (4, 42). Moreover, the previous studies applied endurance exercise training to younger populations (∼50 yr old) than those of the present study (>65 yr old). Therefore, we speculate that endurance exercise training would be most effective at improving the functional component of arterial compliance and could possibly affect structural components when arteriosclerosis is minimally developed.
Ea
Ea reflects left ventricular afterload produced by the entire arterial tree (19, 40). This index is not a measure of a specific arterial property but an integrative index that incorporates the principal elements of arterial load, including peripheral vascular resistance, systemic arterial compliance, characteristic impedance, and systolic and diastolic time intervals (19, 40). One recent community-based epidemiological study (31) has shown that Ea linearly increases as the population ages primarily due to arterial stiffening with aging with a slightly higher rate in females. Interestingly, however, without an improvement of the biologic aortic age, Ea decreased and became comparable with that of Masters athletes after exercise training in the present study.
Physiological studies showed that Ea is proportional to heart rate times peripheral vascular resistance and the reciprocal of systemic arterial compliance (systemic arterial stiffness); a decrease in either peripheral vascular resistance times heart rate or systemic arterial stiffness independently reduces Ea and vice versa (6, 35). Therefore, it is very likely that reductions in peripheral vascular resistance and heart rate as well as increases in systemic arterial compliance observed after exercise training contributed to the reduction of Ea in the present study. Since systemic arterial compliance and peripheral vascular resistance by their nature are determined by both structural and functional components of arteries, while the Modelflow aortic age reflects only the structural component of the aortic wall, the discrepancy between the Modelflow aortic age vs. systemic arterial compliance or peripheral vascular resistance may be explained by the fact that only functional adaptations of arteries occurred after 1 yr of exercise training without structural changes in the aortic wall.
We also found a significant increase in stroke volume after 1 yr of exercise training. The increase in stroke volume can decrease sympathetic activity (25), which reduces heart rate via the baroreflex as well as reducing peripheral vascular resistance and systemic arterial compliance by lowering smooth muscle tone (2, 16). Moreover, it is possible that vascular endothelial nitric oxide production was more pronounced due to an increase in stroke volume and blood flow after exercise training, which also lowers smooth muscle tone (15, 29). Therefore, it is quite likely that an increase in stroke volume played a critical role in reducing Ea after exercise training in the present study.
To determine the direct effect of stroke volume on Ea, we applied lower body negative pressure to those who completed one yr exercise training. The increased stroke volume was restored back to that of pretraining at LBNP-30, whereas heart rate, peripheral vascular resistance and systemic arterial compliance all were also restored back to those preinterventions. Consequently, the reduction in Ea disappeared while the Modelflow aortic age was consistently the same during LBNP. These findings support our assumption that an increase in stroke volume played a major role in mediating the reduction in Ea after exercise training.
Alternatively, sympathetic withdrawal caused directly by endurance exercise training may lead to lower heart rate and peripheral resistance, and thus lower Ea, which, in turn, could increase stroke volume due to an optimized ventricular-arterial coupling (5). However, since reduced stroke volume with LBNP induces sympathetic activation, we believe that in either case, the primary purpose of the LBNP application, that is, to simulate a hemodynamic condition similar to that of preintervention, was reliably achieved. Therefore, whatever the actual underlying mechanisms, our results indicate that under similar hemodynamic conditions, there was no reduction in Ea after exercise training.
Limitations
One limitation of the present study is that the number of sedentary participants was relatively small, primarily because this study was performed as a part of comprehensive cardiovascular physiological study involving a very long period of controlled training (1, 12, 28, 30, 36). The observation that a difference was not observed in the Modelflow aortic age even after 1 yr of exercise training could be potentially explained by the small number of subjects. Of physiological importance, however, power analysis showed that the sample size in the present study (n = 10; SD, 8.14; 95% confidence interval, −5.070 to 6.570) was sufficient to detect a true difference in the mean change of the Modelflow aortic age of −8.093 or 8.093 with a power of 0.8. This minimal detectable difference is approximately four times smaller than the difference between the Modelflow aortic ages in sedentary subjects (71 ± 8) and in Masters athletes (40 ± 16), thus giving us adequate power to detect ∼25% of the difference between Masters athletes and sedentary subjects. Therefore, given that Ea, systemic arterial compliance, and total vascular resistance became comparable with those of Masters athletes after exercise training, it is possible but unlikely that a physiologically meaningful difference in the Modelflow aortic age was missed due to a type II error.
The other limitation of this study is the fact that the primary outcomes and interpretation relied on arterial compliance (estimated form systemic arterial compliance) and the Modelflow aortic age that we developed. The study would have been strengthened by the incorporation of other commonly used indexes for arterial stiffness such as beta-stiffness index, pulse wave velocity, or aortic impedance.
Conclusion
Structural aortic stiffening with aging was not substantially improved in previously sedentary healthy seniors even after 1 yr of progressive and vigorous endurance exercise training when compared with life-long vigorous exercise training by Masters athletes. In contrast, left ventricular afterload was substantially reduced after exercise training. This improvement of the left ventricular afterload appeared to be attributable to cardiovascular functional modulation secondary to an increase in stroke volume rather than to substantial intrinsic structural changes in the arterial wall.
GRANTS
This study was supported by the National Institute on Aging Grant AG-17479-05 and National Space Biomedical Research Institute Postdoctoral Fellowship Grant PR01101 and Career Development Award EO00007 through National Aeronautics and Space Administration NCC 9-58.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.S. and B.D.L. conception and design of research; S.S. and B.D.L. performed experiments; S.S. analyzed data; S.S. and B.D.L. interpreted results of experiments; S.S. prepared figures; S.S. and B.D.L. drafted manuscript; S.S. and B.D.L. edited and revised manuscript; S.S. and B.D.L. approved final version of manuscript.
REFERENCES
- 1.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol Heart Circ Physiol 267: H1368–H1376, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39: 10–15, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol Heart Circ Physiol 266: H693–H701, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol 105: 1342–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chemla D, Antony I, Lecarpentier Y, Nitenberg A. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 285: H614–H620, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol Heart Circ Physiol 274: H500–H505, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 32: 1221–1227, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Solal A, Caviezel B, Laperche T, Gourgon R. Effects of aging on left ventricular-arterial coupling in man: assessment by means of arterial effective and left ventricular elastances. J Hum Hypertens 10: 111–116, 1996 [PubMed] [Google Scholar]
- 10.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 106: 2085–2090, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension 38: 222–226, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation 122: 1797–1805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 30: 1–15, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Joannides R, Richard V, Moore N, Godin M, Thuillez C. Influence of sympathetic tone on mechanical properties of muscular arteries in humans. Am J Physiol Heart Circ Physiol 268: H794–H801, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Karamanoglu M, Feneley MP. On-line synthesis of the human ascending aortic pressure pulse from the finger pulse. Hypertension 30: 1416–1424, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107: 714–720, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation 86: 513–521, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Langewouters GJ, Wesseling KH, Goedhard WJ. The pressure dependent dynamic elasticity of 35 thoracic and 16 abdominal human aortas in vitro described by a five component model. J Biomech 18: 613–620, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Langewouters GJ, Wesseling KH, Goedhard WJ. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech 17: 425–435, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Legedz L, Bricca G, Lantelme P, Rial MO, Champomier P, Vincent M, Milon H. Insulin resistance and plasma triglyceride level are differently related to cardiac hypertrophy and arterial stiffening in hypertensive subjects. Vasc Health Risk Manag 2: 485–490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol 538: 331–340, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol 34: 665–671, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol 21: 2046–2050, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol 99: 1041–1049, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99: 1629–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Saba PS, Roman MJ, Ganau A, Pini R, Jones EC, Pickering TG, Devereux RB. Relationship of effective arterial elastance to demographic and arterial characteristics in normotensive and hypertensive adults. J Hypertens 13: 971–977, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Seals DR. Habitual exercise and the age-associated decline in large artery compliance. Exerc Sport Sci Rev 31: 68–72, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol 105: 1323–1332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol 282: H1041–H1046, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Shibata S, Hastings JL, Prasad A, Fu Q, Okazaki K, Palmer MD, Zhang R, Levine BD. “Dynamic” Starling mechanism: effects of ageing and physical fitness on ventricular-arterial coupling. J Physiol 586: 1951–1962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata S, Levine BD. Biological aortic age derived from the arterial pressure waveform. J Appl Physiol 110: 981–987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonesson B, Vernersson E, Hansen F, Lanne T. Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta Physiol (Oxf) 159: 139–145, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Spina RJ, Meyer TE, Peterson LR, Villareal DT, Rinder MR, Ehsani AA. Absence of left ventricular and arterial adaptations to exercise in octogenarians. J Appl Physiol 97: 1654–1659, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Heart Circ Physiol 245: H773–H780, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Tomiyama H, Hashimoto H, Hirayama Y, Yambe M, Yamada J, Koji Y, Shiina K, Yamamoto Y, Yamashina A. Synergistic acceleration of arterial stiffening in the presence of raised blood pressure and raised plasma glucose. Hypertension 47: 180–188, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350: 1953–1959, 2004 [DOI] [PubMed] [Google Scholar]