Abstract
Intramural gradients of intracellular Ca2+ (Cai2+) Cai2+ handling, Cai2+ oscillations, and Cai2+ transient (CaT) alternans may be important in long-duration ventricular fibrillation (LDVF). However, previous studies of Cai2+ handling have been limited to recordings from the heart surface during short-duration ventricular fibrillation. To examine whether abnormalities of intramural Cai2+ handling contribute to LDVF, we measured membrane voltage (Vm) and Cai2+ during pacing and LDVF in six perfused canine hearts using five eight-fiber optrodes. Measurements were grouped into epicardial, midwall, and endocardial layers. We found that during pacing at 350-ms cycle length, CaT duration was slightly longer (by ≃10%) in endocardial layers than in epicardial layers, whereas action potential duration (APD) exhibited no difference. Rapid pacing at 150-ms cycle length caused alternans in both APD (APD-ALT) and CaT amplitude (CaA-ALT) without significant transmural differences. For 93% of optrode recordings, CaA-ALT was transmurally concordant, whereas APD-ALT was either concordant (36%) or discordant (54%), suggesting that APD-ALT was not caused by CaA-ALT. During LDVF, Vm and Cai2+ progressively desynchronized when not every action potential was followed by a CaT. Such desynchronization developed faster in the epicardium than in the other layers. In addition, CaT duration strongly increased (by ∼240% at 5 min of LDVF), whereas APD shortened (by ∼17%). CaT rises always followed Vm upstrokes during pacing and LDVF. In conclusion, the fact that Vm upstrokes always preceded CaTs indicates that spontaneous Cai2+ oscillations in the working myocardium were not likely the reason for LDVF maintenance. Strong Vm-Cai2+ desynchronization and the occurrence of long CaTs during LDVF indicate severely impaired Cai2+ handling and may potentially contribute to LDVF maintenance.
Keywords: intracellular calcium transient, transmural activation, optrode, membrane voltage
ventricular fibrillation (VF) is a major cause of sudden cardiac death and mortality in the United States (44). The majority of studies of the mechanism of VF maintenance have focused on short-duration VF (duration < 1 min) or VF in perfused hearts, whereas in patients with sudden cardiac arrest the majority of VF episodes occur out of the hospital with a relatively long response time of emergency service, when VF lasts >1 min without coronary perfusion [long-duration VF (LDVF)]. The survival of patients after LDFV is much lower than after short-duration VF, with the survival rate dropping by 7–10% for every min of LDVF (7). Improvement in patient survival requires better understanding of the mechanisms of LDVF maintenance and electrophysiological changes in the ventricular myocardium.
It is generally believed that VF is maintained by reentry, either by a single mother rotor (35) or multiple reentrant circuits. However, an electrical mapping study (26) of the porcine heart found that intramural reentry was present in early but not late VF, suggesting that mechanisms other than reentry may be involved in LDVF maintenance. A study (3) of single cells demonstrated that focal arrhythmias may be caused by spontaneous intracellular Ca2+ (Cai2+) release (SCR) that triggers a rise in membrane voltage (Vm) and arrhythmic beats. Optical mapping studies in isolated right porcine ventricle (28) and in guinea pig hearts (22) demonstrated SCRs during perfused VF and tachyarrhythmias, indicating that they could maintain VF. Cai2+ handling may be involved in VF via another mechanism, in which instability in Cai2+ cycling causes alternans in the amplitude of Cai2+ transients (CaTs) (13, 32). CaT amplitude (CaA) alternans (CaA-ALT) may promote discordant alternans of the action potential (AP) duration (APD; APD-ALT) (36), increasing spatial APD gradients and causing wave break and reentry (34).
The roles of SCRs, APD-ALT, and CaA-ALT in LDVF maintenance are not known. Cai2+ handling can be transmurally heterogeneous (6, 23, 39), and both SCRs and alternans may have preferential intramural localization (1, 24, 31). Therefore, their study necessitates intramural measurements of Vm and Cai2+. Previously, intramural Vm-Cai2+ mapping has been performed in left ventricular (LV) wedge preparations (14, 16, 24, 43), but such preparations don't support VF. We (19, 20) recently reported the use of bundles of optical fibers (optrodes) for intramural measurements of Vm during LDVF in whole hearts. The goals of the present work were 1) to develop a technique for simultaneous intramural measurements of Vm and Cai2+ using optrodes in isolated canine hearts and 2) to measure intramural Vm and Cai2+ alternans during rapid pacing and to measure spontaneous Cai2+ releases during LDVF.
METHODS
Heart preparation.
Animal use conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). The protocol was approved by the Institution Animal Care and Use Committee of the University of Alabama (Birmingham, AL). Six male beagles (∼10 kg) were anesthetized with telazol (4.4 mg/kg), xylazine (4.4 mg/kg), and antropine (0.04 mg/kg). Anesthesia was maintained with the inhalation of isoflurane (1.3–2.5%) in O2 during surgery. Heparin (500 U/kg) was given 10 min before heart extraction. To improve heart preservation, cold cardioplegic solution [which contained (in mmol/l) 110 NaCl, 16 KCl, 16 MgCl2, 1.2 CaCl2, and 10 NaHCO3] was infused into the clamped aorta before the heart was excised, and the coronary arteries were then flushed with cardioplegic solution immediately after the heart was removed. The left and right coronary arteries were cannulated using two short cannulas protruding into arteries by ∼1 mm to avoid occlusion of the left anterior descending coronary and left circumflex arteries. Hearts were perfused with Tyrode solution [containing (in mmol/l) 128.5 NaCl, 20 glucose, 4.7 KCl, 0.7 MgCl2, 0.5 NaH2PO4, 1.5 CaCl2, and 28 NaHCO3] bubbled with 95% O2-5% CO2 at a temperature of 37 ± 0.5°C. The perfusion pressure was maintained at ∼70 mmHg. Hearts were double stained with 1.25 mg of voltage-sensitive dye RH-237 (Invitrogen, Carlsbad, CA) diluted in DMSO and 2 mg of Ca2+-sensitive dye rhod-2 (Biotium, Hayward, CA) diluted in DMSO injected into a bubble trap. To prevent optical motion artifacts, heart contraction was halted with blebbistatin (20 μmol/l, Tocris Bioscience, Ellisville, MO).
Optrode recordings of intramural Vm and Cai2+.
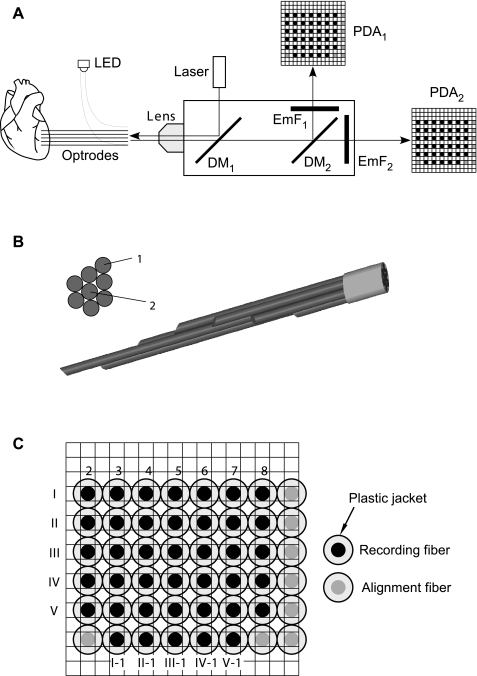
The previously described one-optrode system for intramural Vm measurements (19) was modified to allow simultaneous measurements of both Vm and Cai2+ with five optrodes. Figure 1A shows the design of the optical system for intramural Vm and Cai2+ recordings. Excitation light was provided by a 532-nm laser (Verdi V-5, Coherent, Santa Clara, CA) operated at 3-W power. Light intensity was reduced using a 50% neutral density filter. The excitation light was deflected by a dichroic mirror with transmission at >553 nm and focused on the optrode detector end by a microscope lens (×5 Fluar, Carl Zeiss, Thornwood, NY). The fluorescence delivered by optrodes from the heart was collected by the same lens, passed through the first dichroic mirror, and split by a second dichroic mirror with transmission at >610 nm. The light deflected by the second dichroic mirror was filtered by a band-pass emission filter with transmission at 560–600 nm. The light transmitted by the second dichroic mirror was passed through a long-pass emission filter with transmission at >700 nm. These two light beams carrying information about changes in Cai2+ and Vm, respectively, were converted to voltages using two precisely aligned 16 × 16 photodiode arrays (PDAs) integrated with current-to-voltage converters with 100-MOhm feedback resistors (model C4675-102, Hamamatsu).
Fig. 1.
A: schematic diagram of the optical system. LED, light-emitting diode; DM1 and DM2, first and second dichroic mirrors, respectively; EmF1, emission filter; PDA1 and PDA2, first and second photodiode arrays, respectively. B: cross-sectional and side views of the of the optrode tissue end. C: view of the detector end of the optrode array overlaid on the PDA mask. Solid circles, recording optical fibers; shaded circles: alignment fibers. Seven intramural recording fibers (fibers 2–8) from each of the five optrodes (optrodes I–V) were arranged in rows. Five epicardial recording fibers (fibers I-1 to V-1) were arranged in another row.
Electrical signals were conditioned and digitized using a custom-built 512-channel recording system, which was similar to the previously described 256-channel system (10, 19). The new system contained eight 64-channel boards with second-stage amplifiers (Innovative Technologies, Germantown, MD), which were used to subtract the background fluorescence, amplify signals using a gain of 100, and low-pass filter signals at 0.5-kHz corner frequency. These signals were digitized at a sampling rate of 1 kHz and 12-bit resolution using eight 64-channel data-acquisition cards (PCI-6071E, National Instruments, Austin, TX) installed in a 13-slot PCI expansion system (model 2123, SBS Technologies, St Paul, MN). An additional eight-channel digital card (PCI-6602, National Instruments) was used for the digital control of auxiliary equipment, including shutter opening, tissue stimulation, and change of amplifier settings. The software for digital control, data acquisition, and signal analysis was custom written using Delphi (Borland).
Variability in response times between individual recording channels may introduce errors in measurements of Vm-Cai2+ delays. To quantify the delay between the corresponding channels of two PDAs, rectangular light pulses from a light-emitting diode (LED) were simultaneously measured by two PDAs using the same settings that were used during optical Vm-Cai2+ measurements. The light pulse onset times were calculated at the 50% level of the pulse amplitude using interpolation between sampling points. The average difference in the pulse onset times between the corresponding channels of two PDAs was 0.05 ± 0.25 ms. This delay is negligible compared with the measured Vm-Cai2+ delays.
To quantify the cross-talk between Vm and Cai2+ measurements, optical recordings were performed first by staining a heart with one dye (either rhod-2 or RH-237) and then repeated after staining the heart with the second dye. When the heart was first stained with rhod-2, the signal magnitude in Vm channels was 2.5 ± 2.2% of the signal magnitude measured in the same channels after staining with RH-237. Similarly, when the heart was first stained with RH-237, the signal magnitude in Cai2+ channels was 2.7 ± 2.4% of the signal magnitude in the same channels after rhod-2 was loaded. Thus, the cross-talk between Vm and Cai2+ channels was small and comparable with the noise level.
Optrode fabrication.
Optrodes were made from silicon fibers (FT-300-UMT, Thorlabs, Newtown, NJ) with an outer diameter of 325 μm, a core diameter of 300 μm, and a numerical aperture of 0.39. The fibers were enclosed in protective plastic jackets with an outer diameter of 650 μm. On the optrode tissue end, plastic jackets were removed, and the fiber ends were polished at an ∼45° angle (19). Seven bare fibers were arranged in a hexagonal pattern, as shown in Fig. 1B, with fiber ends spaced 2 mm apart. An additional straight-cleaved fiber (fiber 1) was added to the bundle for recording from the epicardial surface. The total diameter of the fiber bundle inserted into the heart muscle varied between 325 and 975 μm from the most distal to the most proximal fiber. This is comparable with the diameter of plunge needle electrodes used previously in multiple studies. In one of these studies (21), it was shown that the insertion of 66 plunge electrodes did not significantly alter activation spread during regular rhythm and rapid pacing. In another study (33), it was shown that the insertion of 10 plunge needles at 2-mm spacing did not alter activation patterns during VF. Therefore, the insertion of five optrodes with a similar diameter at ∼1-cm spacing was not likely to affect activation spread.
At the optrode detector end, 40 jacketed fibers from 5 optrodes (solid circles) were polished and arranged in a rectangular array, as shown in Fig. 1C. Seven intramural recording fibers (fibers 2–8, solid circles) from each of the five optrodes (optrodes I–V) were arranged in rows. Five epicardial recording fibers (fibers I-1 to V-1) were arranged in another row. Additional fibers (shaded circles) were included in the array for alignment.
An important parameter of optical recordings is the tissue depth from which fluorescent signals are recorded. This parameter was previously evaluated using a Monte-Carlo model of light propagation in cardiac muscle stained with RH-237 and illuminated with a laser beam (8). For a laser beam with a diameter of 0.2 mm, the signal collection depth was ∼0.6 mm, and it increased only 16% when the beam diameter was increased to 1 mm. In each case, the diameter of the fluorescence collection volume was the same as the diameter of the laser beam. Using these data for optical fibers, it can be estimated that the depth and diameter of the fluorescence collection volume for a 300-μm fiber are ∼620 and 300 μm, respectively.
Optrode-PDA alignment.
The detector end of the optrode array and the two PDAs were mounted on individual xyz micropositioners. They were mutually aligned by sending light pulses from an LED at the alignment fibers (Fig. 1C), measuring optical signals by two PDAs, and adjusting the optrode-lens distance, the lens-PDA distances, and lateral positions of the optrode array and the PDAs until signals were maximized and the cross-talk between the recording and the neighboring nonrecording photodiodes was minimized. Because of the presence of protective plastic jackets and the presence of one nonrecording photodiode between each two recording photodiodes, it was possible to prevent any cross-talk between the recording photodiodes. Additional cross-talk tests were performed by 1) immersion of the individual optrodes into a solution of a fluorescent dye and measuring signals by all photodiodes and 2) insertion of individual optrodes into cardiac tissue stained with a fluorescent dye. All tests showed complete absence of interchannel cross-talk.
Study protocol.
The five optrodes were inserted into the anterior LV wall (Fig. 2A, inset) along a base-apex line at ∼1-cm separation. Because of the variability in the optrode insertion angle and the wall curvature, the intramural interoptrode spacing was somewhat smaller and more variable than on the epicardium. Typically, two optrodes penetrated the anterolateral papillary muscle (PM). Hearts were paced near the LV apex via a bipolar electrode at 2 × diastolic threshold strength. Preliminary experiments showed that soon after optrode insertion, APs were short and had a triangular shape, reflecting tissue damage caused by optrode insertion, and that APD increased and AP shape returned to normal morphology and stabilized 7–8 min after insertion. This is similar to the S-T segment elevation caused by insertion of plunge needle electrodes, which disappeared within 2–5 min (9, 37), reflecting complete uncoupling of damaged cells from the surviving tissue. Therefore, optical measurements were started 19 ± 7 min (minimum of 11 min) after optrode insertion. Optical signals were recorded during sinus rhythm; pacing with cycle length (CL) decreasing from 350 to 200 ms in 50-ms steps, and then from 200 ms in 10-ms steps until loss of 1:1 capture; perfused VF; and nonperfused LDVF. To limit dye photobleaching, the duration of optical recordings was limited to 1–1.5 s for sinus and paced rhythms and to 3 s for VF. Pump was stopped for 10–15 s to make recordings during sinus rhythm, pacing, or perfused VF because the pump created additional noise in optical recording. VF was induced with a 9-V battery. Perfusion was maintained for 1 min after the VF onset and then it was stopped for 5 min (LDVF). During LDVF, the oxygen in the room air may limit the degree of epicardial ischemia. Pilot experiments demonstrated that in such conditions, the activation rate (AR) on the epicardium was significantly different from that in the subepicardial tissue layers. To avoid this effect and to ensure epicardial ischemia during LDVF, the surrounding oxygen was eliminated using the method previously described for studies of ischemia in papillary muscle (18). To accomplish this, a gas mixture of N2 (95%) and CO2 (5%) was blown around the heart after coronary perfusion was halted. To ensure constant heart temperature and humidity, the N2-CO2 mixture was passed through a nebulizer containing warm water. The rate of the N2-CO2 flow and the water temperature were set so that the temperature in the perfusion chamber was maintained at 37°C. Epicardial and endocardial temperatures in the heart were monitored using two temperature probes. At the end of LDVF, the transmural temperature difference in six hearts was 1.4 ± 0.5°C. Optical recordings were made at 30 s of perfused VF, immediately after the halt of perfusion, and every minute during 5 min of LDVF. After experiments, the LV wall was cut to measure wall thickness at each optrode insertion site.
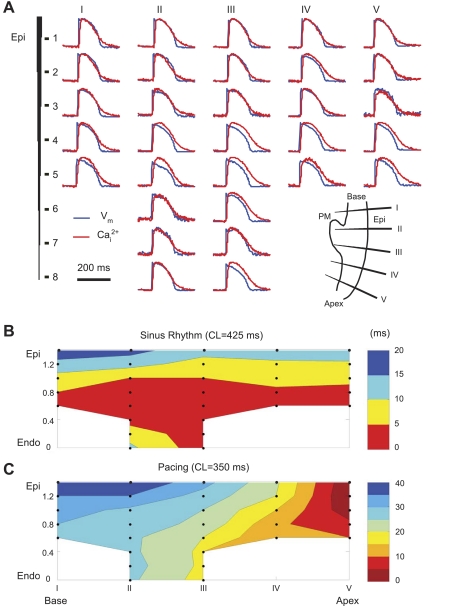
Fig. 2.
Intramural membrane voltage (Vm) and intracellular Ca2+ (Cai2+) measured during regular rhythm. A: raw traces of Vm (blue) and Cai2+ (red) from five optrodes. The inset in the bottom right corner schematically shows the optrode insertion sites. PM, papillary muscle; Epi, epicardium. B and C: approximate maps of transmural activation during sinus rhythm (B) and Epi pacing at a cycle length (CL) of 350 ms (C). The solid circles schematically depict the locations of the recording sites. Endo, endocardium.
Data analysis.
All traces were normalized using respective signal amplitudes measured during regular pacing. Some APs recorded during regular pacing had a low signal-to-noise ratio, some exhibited abnormal shape due to remaining motion artifacts, and some had a triangular shape, likely due to poor local perfusion. Sites with such signals (a total of ∼8% of cases) were excluded from data analysis. AP and CaT amplitudes were calculated as signal differences before and after the upstrokes. Activation time was calculated as the time when a signal was at 50% of its upstroke amplitude. APD and CaT duration (CaD) were calculated as the time between activation and 60% level of signal recovery (APD60 and CaD60, repectively). CL was calculated as the interval between two consecutive activation times. The AR during LDVF was measured as the average inverse CL over 3 s of recording time. A Vm deflection was considered an active response if its normalized amplitude was >15% of the normal AP amplitude. APD-ALT was calculated as the APD60 difference between two consecutive APs. APD-ALT was considered present when it was >4 ms. CaA-ALT was calculated as the CaA difference divided by the largest CaA of two successive CaTs. The threshold for the presence of CaA-ALT was 10%.
Because the signal amplitude and signal-to-noise ratio decreased during LDVF, signals were digitally filtered using a Butterworth low-pass filter with a cutoff frequency of 100 Hz to reduce the high-frequency noise. Although signal quality was high during pacing, to assure consistency of signal analysis, the same filtration was used on all signals. Comparison of raw and filtered signals during regular pacing demonstrated that such filtration did not affect the overall shape of APs and CaTs, including the fastest signal portions and AP upstrokes. Activation times calculated from them were not affected by such filtration as well.
To present the intramural distributions of electrophysiological parameters and activation spread, isoline color maps were generated using the Matlab contour fill function with linear interpolation between recording sites. Interpolated data were not used in the analysis of transmural electrophysiological distributions; such statistical analysis was performed only on the original data.
For statistical analysis, parameters were grouped into three layers: epicardial, midwall, and endocardial, with each layer occupying ∼33% of the wall thickness. Transmural and longitudinal differences were analyzed using one-way ANOVA with post hoc comparisons between groups of data using the Tukey range test. All data are expressed as means ± SD. Differences were considered statistically significant if P < 0.05.
RESULTS
Intramural APs and CaTs during regular rhythm.
Figure 2A shows an example of simultaneous intramural Vm and Cai2+ measured with five optrodes during epicardial pacing (CL: 350 ms). The larger number of signals in optrodes II and III was because of the increased wall thickness at the PM. Figure 2, B and C, shows approximate (without taking into account exact optrode orientations inside the wall) transmural maps of activation during sinus rhythm and epicardial pacing. Despite the small number of recording intramural sites, both maps exhibited expected propagation patterns. During sinus rhythm, the endocardium at the LV base and apex activated nearly simultaneously, and the activation then spread to the epicardium. In the PM, the earliest activation arose from the middle and spread to the epicardium and PM tip. In all hearts (n = 6), the earliest activation either was distributed over a broad area of the endocardium, similar to that shown in Fig. 2B (n = 4), or was in the area of the PM-LV junction (n = 2). During epicardial pacing, activation spread faster on the endocardium and then bent toward the epicardium, possibly due to a sudden fiber orientation change near the PM or due to the presence of Purkinje fibers. CaT rises followed AP upstrokes with an average delay of 7.2 ± 1.6 ms (n = 6). As shown in Fig. 3A, the AP-CaT delay was longer in the endocardium than in the epicardium (8.1 ± 2.1 vs 6.3 ± 0.8 ms, P < 0.05, n = 6); in the midwall, it was not different from the other two layers.
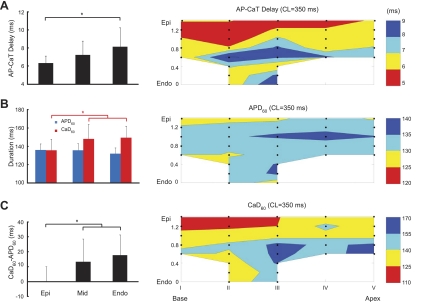
Fig. 3.
Transmural differences of action potentials (APs) and Cai2+ transients (CaTs). A: average values for the three left venticular layers [Epi, midwall (Mid), and Endo; left] and an example of a transmural map (right) of the AP-CaT distribution during regular pacing (CL: 350 ms). B: similar characteristics for AP duration (APD) at 60% repolarization (APD60) and CaT duration (CaD) between activation and 60% level of signal recovery (CaD60; B). C: CaD60-APD60 difference. *Statistical significance (P < 0.05).
Figure 3, B and C, shows examples and mean values of transmural distributions of APD60 and CaD60 during epicardial pacing. Although there were transmural APD60 variations, they were relatively small and not consistent across all hearts. In six hearts, the mean APD60 values in the three layers were not statistically different (P > 0.05). In contrast, CaD60 exhibited statistically significant transmural differences, with shorter CaD60 in the epicardium than in the midwall and endocardium (136 ± 12 vs. 148 ± 16 and 149 ± 13 ms, respectively, n = 6, P < 0.05). Even more pronounced was the transmural heterogeneity of the CaD60-APD60 difference.
Intramural AP and CaT alternans during rapid pacing.
Alternans was negligible during regular rhythm. However, a reduction of pacing CL caused both APD-ALT and CaA-ALT. CaA-ALT averaged over six hearts reached the threshold change of 10% first in the endocardium at a CL of 250 ms. Further CL reduction produced CaA-ALT in other layers. The averaged APD-ALT reached threshold (>4 ms) at a CL of 180 ms in the epicardial and endocardial layers, and it increased with decreasing CL. Figure 4A shows transmural alternans measured by one optrode during pacing at a CL of 150 ms. At this CL, the mean CaA-ALT was 47 ± 16% (n = 6). The mean APD-ALT was 5.7 ± 2.6 ms (n = 6), or ∼7.3 ± 3.4% of the mean APD, and it was not significantly different among the three muscle layers. There was a tendency for larger CaA-ALT in the endocardium and midwall than in the epicardium, although the difference was not statistically significant (P = 0.14).
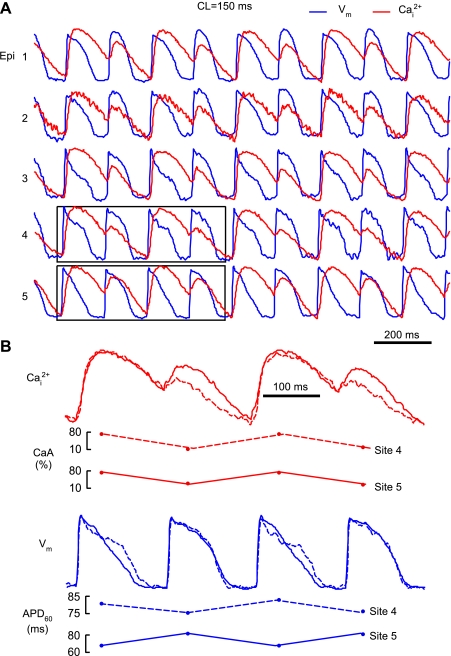
Fig. 4.
APD and CaT amplitude (CaA) alternans during rapid pacing (CL: 150 ms). A: examples of APD and CaA alternans from one optrode. B: superimposed Cai2+ and Vm traces from two intramural sites together with corresponding values of CaA and APD.
With regard to spatial alternans concordance, close examination of the Vm-Cai2+ traces showed that CaA-ALTs were in phase at all intramural sites. Because APD-ALTs were relatively small, it was harder to analyze their concordance. However, the available data indicated that they were often discordant. This is shown in Fig. 4B, which shows overlaid traces from intramural sites 4 and 5. Whereas CaA alternated in phase, APD at these locations alternated out of phase. Similar results were obtained in the other hearts. A total of 30 optrode recordings were made in 6 hearts. In 28 of 30 recordings, CaA-ALTs were transmurally concordant. Of the 28 recordings with concordant CaA-ALTs, 15 recordings (54%) exhibited discordant APD-ALTs. Similar results were obtained when APD-ALT was analyzed using APD at 80% (APD80) or 90% repolarization (APD90) instead of APD60 (data not shown). At some sites, the character of APD-ALT could change from discordant to concordant when using APD90 instead of APD60 or APD80, but discordant APD90-ALTs were recorded at other locations. Overall, the number of optrode recordings exhibiting discordant APD90-ALT and concordant CaA-ALT was 14, which was similar to the number of recordings exhibiting discordant APD60-ALT and concordant CaA-ALT.
The CaD60 distribution showed no significant transmural differences during rapid pacing (data not shown), which was different from regular rhythm. Similar to regular rhythm, however, there were no statistically significant transmural APD60 differences.
Intramural Vm and Cai2+ during VF.
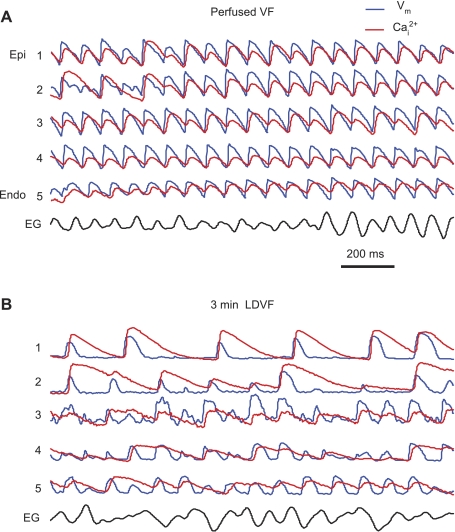
Figure 5A shows recordings from one optrode during perfused VF. At this stage, APs followed each other closely, without diastolic intervals. Almost every AP was followed by a CaT. For most beats, APs and CaTs were transmurally synchronous and alternating. Similar to rapid pacing, CaA-ALT during VF was more pronounced than APD-ALT. APD-ALT were larger in the epicardium (9.9 ± 5.1 ms) compared with the endocardium (6.5 ± 2.8 ms, n = 6, P < 0.05). Similarly, CaA-ALT was larger at the epicardium and midwall (82 ± 20% and 74 ± 23%, respectively) than at the endocardium (50 ± 20%, n = 6, P < 0.05). This was reversed from rapid pacing, where CaA-ALT was larger in the endocardium-midwall than in the epicardium.
Fig. 5.
Intramural Vm and Cai2+ during ventricular fibrillation (VF). A: recordings from one optrode located near the apex during early perfused VF. EG, epicardial electrogram. B: Vm and Cai2+ recorded from the same optrode at 3 min of long-duration VF (LDVF).
After the stop of perfusion, Vm and Cai2+ dynamics changed rapidly. At ∼2 min of LDVF, the AR became transmurally nonuniform, with faster rates in the endocardium than in the epicardium. Such transmural AR heterogeneity became prominent at 3 min of LDVF (Fig. 5B). In addition, APs and CaTs became highly irregular. The synchronization between Vm and Cai2+ became partially lost, with not every AP being followed by a CaT. However, the loss of synchronization was not complete: CaTs still followed APs with every CaT upstroke being preceded by an AP upstroke. CaTs became progressively longer, especially in the epicardium, whereas APs became shorter everywhere.
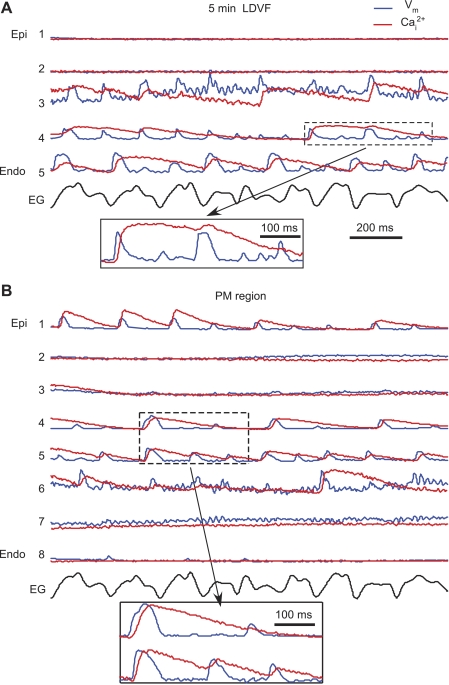
At ∼5 min of LDVF (Fig. 6), activations disappeared from the majority of epicardial sites, but they were still present in the midwall and endocardium. The remaining activations were highly irregular, with short APs and very long CaTs. This is illustrated by the enlarged portions of Vm and Cai2+ traces in Fig. 6, insets. The average CaD60 at this stage was 212 ± 44 ms (n = 6), which was ∼240% larger than at VF onset (62 ± 7 ms), with individual values sometimes exceeding 400 ms, whereas APD60 was 44 ± 12 ms (decrease of 17% from 53 ± 4 ms).
Fig. 6.
Intramural Vm and Cai2+ recordings during late LDVF. A: Vm and Cai2+ traces from an apical optrode at 5 min of LDVF. B: recordings from an optrode located at the PM region. Insets show selected traces with long CaTs and short APDs.
Somewhat different transmural distributions of activation were recorded in the PM region. As shown in Fig. 6B, activation was preserved inside the PM, whereas it was slow or lost at the PM apex and epicardial region of the LV. A similar pattern of PM activation was observed in all six hearts.
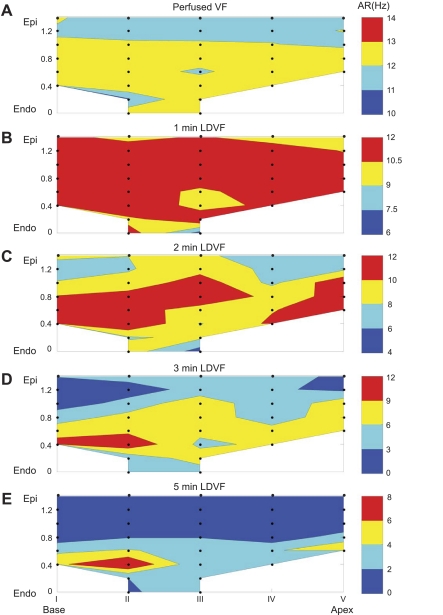
Figure 7 shows transmural maps of the AR at different stages of VF. The data were averaged over six hearts, with only those sites included that had data in at least three hearts. Similar to previous reports (20, 38), AR decreased over time at all intramural sites. There was no transmural difference in AR during early VF (Fig. 7, A and B), and the range of AR changes was relatively small. With LDVF progression (Fig. 7C), AR decreased faster in the epicardium than in the endocardium, and the range of transmural AR variation increased. After 3 min of LDVF (Fig. 7, D and E), the fastest ARs were observed in the endocardium near the PM insertion into the LV wall, suggesting that this region was the source of activation during late VF.
Fig. 7.
Transmural maps of the activation rate (AR) during VF averaged over six hearts. A: perfused VF. B–E: different stages of LDVF.
At all VF stages, CaTs always followed AP upstrokes, with an average AP-CaT delay of 10.0 ± 0.8 ms (n = 6), which was longer than during regular rhythm (P < 0.05). Thus, no incidences of SCRs were detected.
DISCUSSION
In this work, we developed a method for the intramural mapping of Vm and Cai2+ in whole hearts. This method was used to measure Vm and Cai2+ during regular rhythm, rapid pacing, and LDVF in the LV wall of intact canine hearts. The main findings were as follows: 1) during regular pacing, CaD and AP-CaT delay were larger in the endocardium than in the epicardium, whereas APD exhibited no transmural differences; 2) during rapid pacing, CaA-ALTs appeared at longer CLs than APD-ALT and were mostly concordant, whereas APD-ALTs were either concordant or discordant, indicating that CaA-ALTs were not the cause of APD-ALT; 3) AP upstrokes always preceded CaTs during VF, suggesting that SCRs are not likely responsible for LDVF maintenance; and 4) Vm and Cai2+ became desynchronized and CaTs became very long during late LDVF, indicating impaired Cai2+ handling, which may contribute to LDVF maintenance.
Transmural heterogeneity of Cai2+ transients.
Transmural differences in CaTs reflect heterogeneity of Cai2+ handling, which may contribute to the generation of arrhythmias (24). Previously, transmural heterogeneity of CaTs was measured in single myocytes (6, 39) and LV wedge preparations (23). The applicability of intramural measurements using single cells and wedge preparations to whole hearts is still debated (29, 31). Using the optrode technique in whole hearts alleviates some of these concerns. We found transmural differences in several characteristics of CaTs in canine hearts. One such parameter was the delay between Vm and Cai2+ upstrokes, which was ∼28% larger in the endocardium than in the epicardium. This corresponds to results obtained in isolated myocytes (6) showing a longer time to peak of CaT in the endocardium compared with the epicardium. The molecular mechanism for the transmural gradient of AP-CaT delay is not clear. The L-type Ca2+ current in canine hearts has been reported to be either not different (25) or larger (40) in the endocardium compared with the epicardium, indicating that this current was not responsible for the transmural AP-CaT delay difference.
Another transmurally heterogeneous parameter measured in the present work was the duration of CaT, which was ∼10% longer in the endocardium than in the epicardium. The CaD heterogeneity was independent of APD, which exhibited no statistically significant transmural differences. Even more pronounced was the heterogeneity of the CaD-APD difference (Fig. 3D). The longer endocardial CaD than epicardial CaD is consistent with Cai2+ measurements in single canine myocytes (6, 39) and wedge preparations (23). It corresponds to the transmural distribution of sarco(endo)plasmic Ca2+-ATPase 2a expression, which has been shown to be smaller in the subendocardium than in the subepicardium (23). However, it should be noted that measurements of CaD values and their differences may not be quantitatively accurate due to a nonlinear rhod-2 response (see discussion).
Transmural APD and CaA alternans.
Acceleration of heart rhythm often causes APD-ALTs, which may become spatially discordant, producing APD gradients and forming a dynamic substrate for conduction block and reentry (30, 42). The ionic mechanisms of APD-ALTs are not precisely known. Typically, APD-ALT is accompanied by CaT alternans, which suggests a causative relationship between them. Experiments in isolated voltage-clamped myocytes showed that CaA-ALTs can occur with constant APD, indicating that CaA-ALT doesn't require APD-ALT (4). Voltage clamp is not feasible in whole hearts, but simultaneous Vm-Cai2+ mapping in guinea pig hearts showed that the CL thresholds for the onset of APD-ALT and CaA-ALT and the patterns of their spatial discordance were nearly the same (32), which led to the conclusion that CaA-ALT was the cause of APD-ALT.
In the present study, CaA-ALT appeared at longer CL than APD-ALT, and, in relative terms, CaA-ALTs were much larger than APD-ALTs. Also, transmural patterns of APD-ALT and CaA-ALT concordance often did not match. During rapid pacing, CaA-ALTs during individual beats were most often transmurally concordant, whereas APD-ALTs could be either concordant or discordant. It is not clear how CaA-ALTs were synchronized across the LV wall, whereas APD-ALT were not, but these differences illustrate a certain degree of independence between APD-ALT and CaA-ALT, suggesting that additional mechanisms may be involved in the generation of APD-ALT besides alternation of Cai2+ handling. An alternative explanation might be related to the fact that the coupling between Cai2+ and Vm can be either positive or negative, so that small CaT can cause either short or long APD (36). Although it is not very likely that different Cai2+-Vm coupling mechanisms operate simultaneously at closely adjacent sites, such a possibility can't be excluded.
Besides rapid pacing, APD-ALT and CaA-ALT were also observed during perfused VF. In this case, both APD-ALTs and CaA-ALTs averaged over multiple beats were larger in the epicardium than in the endocardium. This was different from rapid pacing, where alternans tended to be larger in the endocardium than in the epicardium, although this difference did not reach statistical significance. The difference in alternans distribution between rapid pacing and perfused VF could be due to faster AR during VF. It indicates that results obtained during rapid pacing should be applied to VF with caution.
Dynamics of intramural Vm and Cai2+ during LDVF.
Changes of Vm during the first 5 min of LDVF were qualitatively similar to previous Vm measurements in the LV of canine hearts during no-perfusion LDVF (20, 38), with APD decreasing and CL and diastolic interval increasing over time. As VF progressed, AR became transmurally heterogeneous, with faster activations in the endocardium and middle of the PM than in the epicardium. The fastest AR was frequently observed near the PM insertion into the LV, suggesting that a VF source was located there. This corresponds with a previous report (17) showing that the PM can anchor reentry. However, this does not exclude the possibility that a VF source was located in other parts of the heart. It has recently been shown that during late LDVF, the septum can activate at a rate similar to the LV endocardium, suggesting that it may also be a source of VF (38).
Intramural recordings showed that Cai2+ and Vm were synchronized during perfused VF and the early stage of nonperfused VF when, despite a rapid excitation rate, nearly every AP was followed by a CaT. With progression of the nonperfused LDVF, Vm-Cai2+ synchronization became partially lost. One striking feature of desynchronization, which appeared at 2–3 min of LDVF, was the high irregularity of CaTs (Fig. 5B) when not every AP was followed by a CaT. The timing of this change corresponds to beginning of phase 4 of LDVF previously described based on multiparameter analysis of epicardial activation patterns recorded during 10 min of LDVF (15). Such desynchronization appeared first and developed faster in the epicardium and then in the endocardium. At the end of LDVF, APs and CaTs disappeared almost completely from the epicardium, whereas they were still preserved in the endocardium and inside PM.
In addition to strong Vm-Cai2+ desynchronization, CaTs became prolonged, whereas APs shortened, during LDVF. Very long CaTs indicate severely impaired Cai2+ reuptake during LDVF, which was likely due to a combination of tissue ischemia (5) and a high excitation rate. When CaTs were long, one or several APs could arise with Cai2+ being still at a high level (Fig. 6, A and B). Elevated Cai2+ combined with repolarized Vm could cause activation of the Na+/Ca2+ exchanger in its forward mode generating depolarizing current and extra beats. Such arrhythmogenic mechanism has previously been described as “late phase 3 early afterdeplorization” to explain atrial arrhythmias in conditions of short APD and long CaD (2). A similar mechanism has been proposed to explain the VF reinitiation after defibrillation in rabbit hearts with heart failure in which post-VF extra beats occurred during late phase 3 or phase 4 of shortened APs when Cai2+ was still highly elevated (27). Based on the similarity of the APD-CaD relationship (short APD and very long CaD) developed in the course of LDVF, it can be speculated that a similar Cai2+-dependent arrhythmogenic mechanism may cause the generation of abnormal APs contributing to LDVF maintenance. Verification of this hypothesis requires further investigation.
Lack of SCR.
Optical mapping in the isolated porcine ventricle demonstrated that Cai2+ rises preceding Vm upstrokes (SCRs) could occur during perfused VF (28). In contrast, we found that CaTs always followed AP upstrokes during both perfused and nonperfused VF, with the AP-CaT delay being longer compared with normal rhythm. In addition, arrhythmic APs were taking off when Cai2+ was decreasing, which excludes the role of slow elevation of Cai2+ as the cause of APs. Although it can't be excluded that SCRs took place in other parts of the heart, such as the specialized conduction system, their total absence from intramural recordings indicates that despite a large degree of desynchronization between Vm and Cai2+ developed during LDVF, the AP still triggers CaT and that VF maintenance was not supported by SCR. These results are in agreement with another study (41) in which CaTs were found to follow APs during VF in porcine hearts.
Limitations.
Flow to the heart was first normal and then stopped completely at the beginning of VF in this study, which may not be the exact representation of the early VF in patients in whom a partial blood flow may remain during early VF. Recordings of intramural Vm/Cai2+ were restricted to the anterior LV working myocardium with limited spatial resolution. This doesn't exclude the possibility that SCR and arrhythmogenic Cai2+ alternans could develop on the millimeter scale between the recording sites, in other parts of the heart, or in Purkinje fibers. It has previously been shown in neonatal rat cell cultures that the high-affinity dye rhod-2, due to its nonlinear response, may overestimate CaD, which was about twofold larger than APD in cell cultures (11). This was less likely the case in present measurements in which CaD was comparable with APD. However, it can't be excluded that absolute measurements of CaD or its differences were affected by the nonlinear rhod-2 response. This effect is less likely to affect measurements of AP-CaT delays since this was not the case in cell culture measurements, in which dyes with linear and nonlinear responses produced similar AP-CaT delay values (11). The finding of the increased mismatch between CaD and APD during LDVF can't also be attributed to nonlinear rhod-2 properties because the rhod-2 response is likely to become more linear during LDVF when CaT amplitude is decreased. Qualitative effects such as the concordant or discordant character of CaT-ALT should be even less likely influenced by this effect. Another potential limitation of this study is related to the use of electrical-mechanical uncoupler blebbistatin, which has been found not to affect Vm and Cai2+ during regular rhythm (12) but could potentially alter VF characteristics due to changes in the tissue energy requirements.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-067748 and HL-85370.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.K., R.E.I., and V.G.F. conception and design of research; W.K. performed experiments; W.K. and V.G.F. analyzed data; W.K., R.E.I., and V.G.F. interpreted results of experiments; W.K. prepared figures; W.K. drafted manuscript; W.K., R.E.I., and V.G.F. edited and revised manuscript; W.K., R.E.I., and V.G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the Southern Research Institute for providing experimental resources and Frank Vance for technical support.
REFERENCES
- 1.Akar FG, Yan GX, Antzelevitch C, Rosenbaum DS. Unique topographical distribution of M cells underlies reentrant mechanism of torsade de pointes in the long-QT syndrome. Circulation 105: 1247–1253, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation 107: 2355–2360, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Capogrossi MC, Houser SR, Bahinski A, Lakatta EG. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circ Res 61: 498–503, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J 77: 2930–2941, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol 294: H1–H10, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cordeiro JM, Greene L, Heilmann C, Antzelevitch D, Antzelevitch C. Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am J Physiol Heart Circ Physiol 286: H1471–H1479, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cummins RO. From concept to standard-of-care? Review of the clinical experience with automated external defibrillators. Ann Emerg Med 18: 1269–1275, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Ding L, Splinter R, Knisley SB. Quantifying spatial localization of optical mapping using Monte Carlo simulations. IEEE Trans Biomed Eng 48: 1098–1107, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Durrer D, Van Der Tweel LH. Excitation of the left ventricular wall of the dog and goat. Ann NY Acad Sci 65: 779–803, 1957 [DOI] [PubMed] [Google Scholar]
- 10.Fast VG. Recording action potentials using voltage-sensitive dyes. In: Practical Methods in Cardiovascular Research, edited by Dhein S, Mohr FW, Delmar M. Berlin: Springer-Verlag, 2005, p. 233–255 [Google Scholar]
- 11.Fast VG. Simultaneous optical imaging of membrane potential and intracellular calcium. J Electrocardiol 38: 107–112, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hirayama Y, Saitoh H, Atarashi H, Hayakawa H. Electrical and mechanical alternans in canine myocardium in vivo. Dependence on intracellular calcium cycling. Circulation 88: 2894–2902, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol 297: H1235–H1242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Rogers JM, Killingsworth CR, Singh KP, Smith WM, Ideker RE. Evolution of activation patterns during long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol 286: H1193–H1200, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Katra RP, Laurita KR. Cellular mechanism of calcium-mediated triggered activity in the heart. Circ Res 96: 535–542, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kim YH, Xie F, Yashima M, Wu TJ, Valderrabano M, Lee MH, Ohara T, Voroshilovsky O, Doshi RN, Fishbein MC, Qu Z, Garfinkel A, Weiss JN, Karagueuzian HS, Chen PS. Role of papillary muscle in the generation and maintenance of reentry during ventricular tachycardia and fibrillation in isolated swine right ventricle. Circulation 100: 1450–1459, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kleber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res 61: 271–279, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Kong W, Fakhari N, Sharifov OF, Ideker RE, Smith WM, Fast VG. Optical measurements of intramural action potentials in isolated porcine hearts using optrodes. Heart Rhythm 4: 1430–1436, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong W, Ideker RE, Fast VG. Transmural optical measurements of Vm dynamics during long-duration ventricular fibrillation in canine hearts. Heart Rhythm 6: 796–802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovoor P, Campbell C, Wallace E, Byth K, Dewsnap B, Eipper V, Uther J, Ross D. Effects of simultaneous insertion of 66 plunge needle electrodes on myocardial activation, function, and structure. PACE 26: 1979–1985, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Lakireddy V, Bub G, Baweja P, Syed A, Boutjdir M, El-Sherif N. The kinetics of spontaneous calcium oscillations and arrhythmogenesis in the in vivo heart during ischemia/reperfusion. Heart Rhythm 3: 58–66, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res 92: 668–675, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Laurita KR, Katra RP. Delayed after depolarization-mediated triggered activity associated with slow calcium sequestration near the endocardium. J Cardiovasc Electrophysiol 16: 418–424, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol 283: H1031–H1041, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Li L, Jin Q, Huang J, Cheng KA, Ideker RE. Intramural foci during long duration fibrillation in the pig ventricle. Circ Res 102: 1256–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa M, Morita N, Tang L, Karagueuzian HS, Weiss JN, Lin SF, Chen PS. Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm 6: 784–792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omichi C, Lamp ST, Lin SF, Yang J, Baher A, Zhou S, Attin M, Lee MH, Karagueuzian HS, Kogan B, Qu Z, Garfinkel A, Chen PS, Weiss JN. Intracellular Ca dynamics in ventricular fibrillation. Am J Physiol Heart Circ Physiol 286: H1836–H1844, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Opthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart?: repolarization gradients in the intact heart. Circ Arrhythm Electrophysiol 2: 89–96, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99: 1385–1394, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Patel C, Burke JF, Patel H, Gupta P, Kowey PR, Antzelevitch C, Yan GX. Is there a significant transmural gradient in repolarization time in the intact heart? Cellular basis of the T wave: a century of controversy. Circ Arrhythm Electrophysiol 2: 80–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 94: 1083–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Rogers JM, Melnick SB, Huang J. Fiberglass needle electrodes for transmural cardiac mapping. IEEE Trans Biomed Eng 49: 1639–1641, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum DS. T wave alternans: a mechanism of arrhythmogenesis comes of age after 100 years. J Cardiovasc Electrophysiol 12: 207–209, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Samie FH, Berenfeld O, Anumonwo J, Mironov SF, Udassi S, Beaumont J, Taffet S, Pertsov AM, Jalife J. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res 89: 1216–1223, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Sato D, Shiferaw Y, Garfinkel A, Weiss JN, Qu Z, Karma A. Spatially discordant alternans in cardiac tissue: role of calcium cycling. Circ Res 99: 520–527, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Spach MS, Huang SN, Ayers CR. Electrical and anatomic study of the Purkinje system of the canine heart. Am Heart J 65: 664–673, 1963 [DOI] [PubMed] [Google Scholar]
- 38.Venable PW, Taylor TG, Shibayama J, Warren M, Zaitsev AV. Complex structure of electrophysiological gradients emerging during long-duration ventricular fibrillation in the canine heart. Am J Physiol Heart Circ Physiol 299: H1405–H1418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol 39: 419–428, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Wang HS, Cohen IS. Calcium channel heterogeneity in canine left ventricular myocytes. J Physiol 547: 825–833, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren M, Huizar JF, Shvedko AG, Zaitsev AV. Spatiotemporal relationship between intracellular Ca2+ dynamics and wave fragmentation during ventricular fibrillation in isolated blood-perfused pig hearts. Circ Res 101: e90–e101, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res 98: 1244–1253, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm 6: 251–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation 104: 2158–2163, 2001 [DOI] [PubMed] [Google Scholar]