Abstract
Obesity is becoming increasingly more common among patients with inflammatory bowel disease. In this review, we will explore the epidemiological trends of inflammatory bowel disease, the complex interplay between the proinflammatory state of obesity and inflammatory bowel disease, outcomes of surgery for inflammatory bowel disease in obese as compared with non-obese patients, and technical concerns pertaining to restorative proctocolectomy and ileoanal pouch reservoir, stoma creation and laparoscopic surgery for inflammatory bowel disease in obese patients.
Keywords: Obesity, Crohn's disease, ulcerative colitis, inflammatory bowel disease, laparoscopy, restorative proctocolectectomy
Obesity in patients with inflammatory bowel disease (IBD) was previously felt to be a rare occurrence; however, in the last two decades it has become increasingly prevalent. Obesity poses two distinct challenges to the already complex nature of the surgical management of inflammatory bowel disease: (1) increased medical co-morbidity and higher risk of complications; and (2) increased anatomical challenges and technical complexity. Taken together, these factors have the potential to impact outcomes after surgery.
We will review the available studies for surgery specific to inflammatory bowel disease in obese as compared with non-obese patients. We will also discuss specific technical concerns pertaining to restorative proctocolectomy and ileoanal pouch reservoir, stoma creation and laparoscopic surgery for inflammatory bowel disease in obese patients. Lastly, the complex interplay between the proinflammatory state of obesity and inflammatory bowel disease will be addressed, including the possible role of obesity surgery in patients with inflammatory bowel disease.
EPIDEMIOLOGICAL TRENDS OF OBESITY IN INFLAMMATORY BOWEL DISEASE
Increasing Frequency of Obesity in Inflammatory Bowel Disease
Obesity in inflammatory bowel disease has previously been considered to be uncommon. As the prevalence of obesity has increased worldwide, several authors have reported on how this epidemic has influenced the IBD patient population. Steed et al report an observational study in Tayside, Scotland in which the authors found that 18% of their IBD population was obese (BMI > 30 kg/m2) in comparison to 23% of the population at large.1 Overall, 38% of inflammatory bowel disease patients were overweight (BMI > 25 kg/m2), which was the same proportion as in the general population. The authors found that there were significantly more obese patients with Crohn's disease than ulcerative colitis (p = 0.05). Furthermore, overweight/obese patients with ulcerative colitis were more likely to require surgery, whereas the converse was true for patients with Crohn's disease. Similar observations have been made in the pediatric population. Long et al used a large multicenter pediatric inflammatory bowel disease registry enrolling 1598 children (4–16 years old) with endoscopically confirmed Crohn's disease or ulcerative colitis, and found that the prevalence of overweight/obese status was 23.6% (20.0% in Crohn's disease and 30.1% in ulcerative colitis).2 The authors also found that history of prior surgery was associated with overweight/obese status in Crohn's disease patients (OR 1.73, CI: 1.07–2.82).
Is There a Difference Between Crohn's and Ulcerative Colitis with Regards to Obesity?
Increasing rates of obesity have been observed in patients with inflammatory bowel disease, and there is speculation that obesity itself may be a risk factor for the development of inflammatory bowel disease. A case-control study assessed obesity at the time of inflammatory bowel disease diagnosis as a risk factor for Crohn's disease versus ulcerative colitis or the general population.3 Information was obtained from a standardized questionnaire. After adjustment for known risk factors for Crohn's disease (age, smoking, family history, history of appendectomy), the authors reported a significant association between a diagnosis of Crohn's disease and obesity as compared with UC or controls (OR 3.31; CI: 1.62–6.75) in patients between 50–70 years old. Furthermore, in this subgroup, obesity had a stronger association with a diagnosis of Crohn's disease than smoking. The authors also report a ‘dose–response,’ where increasing degrees of obesity were associated with increased risk of Crohn's disease. The authors propose that there may be two separate underlying mechanisms of disease involved in the commonly recognized low BMI young-onset Crohn's disease and the older onset Crohn's disease associated with morbid obesity. They suggest that the former is a more severe form of the disease, principally but not solely presenting in adolescence and early adulthood with a strong genetic component. Obesity may predispose to a less severe form of disease, commonly but not exclusively presenting at older age.
Taken together, these reports highlight the increasing frequency of obesity in patients with inflammatory bowel disease. However further studies are required to understand the association between obesity and Crohn's disease and ulcerative colitis respectively, and to delineate these associations within the spectrum of all age groups.
OBESITY AND INFLAMMATORY BOWEL DISEASE ACTIVITY
Chronic obesity has been shown to be associated with immune dysregulation resulting in a low-grade proinflammatory state.4,5 This appears to be mediated by known cytokines such as interleukin-6 or tumor necrosis factor-α, as well as more recently discovered adipokines such as leptin, adiponectin and resistin, or neuropeptides, such as substance P. These molecules are all either produced within adipocytes or within macrophages and lymphocytes that infiltrate the mesenteric fat.5,6 Patients with active inflammatory bowel disease, as well as those with morbid obesity, have elevated serum levels of interleukin-6 and tumor necrosis factor-α.5 The degree of expression of these cytokines has been shown to correlate with adipocyte mass.7 Furthermore, these cytokines are overexpressed in the mesenteric fat of patients with active inflammatory bowel disease.5,8
In addition to known cytokine overexpression, several inflammatory mediators, termed “adipokines”, found in adipose tissue and known to be implicated in obesity are also overexpressed in the mesenteric fat of patients with inflammatory bowel disease. Leptin, the best studied such adipokine, is a protein produced by adipocytes in proportion to fat mass and is involved in control of appetite and metabolism. It has been shown to be overexpressed in the mesenteric fat and bowel wall of patients with active Crohn's disease.5 Resistin is another such molecule that is mainly produced within macrophages and is postulated to be involved in the proinflammatory profile in obesity; in addition, its level of expression was found to correlate with the level of C-reactive protein in patients with Crohn's disease and ulcerative colitis.5,9 Adiponectin is also produced in adipocytes and has been shown to have an antidiabetic and antiatherogenic profile; however its role in inflammatory bowel disease is still unknown.5
Another class of molecules implicated in obesity and inflammatory bowel disease, are neuropeptides such as substance-P.4 Substance P has been postulated to play a proinflammatory role in both obesity and inflammatory bowel disease. This neuropeptide appears to have direct effects on fat tissue expansion. This in turn then creates a proinflammtory environment and is thought to be involved in the ‘creeping fat’ frequently seen in Crohn's bowel mesentery.4
The clinical implication of the molecular interactions between inflammatory bowel disease and obesity is yet to be fully understood. To date, there are only two reports in the literature addressing IBD clinical disease activity and obesity. Blain et al10 report that obese patients (BMI >30 kg/m2) with Crohn's disease (3% of their 2065 patients) have a different disease course compared with patients with Crohn's disease who have normal weight. They report that obese patients with Crohn's are significantly older at diagnosis (32 versus 28 years), have a higher incidence of perianal disease (35 versus 24%), more disease relapse (OR 1.50) and more frequent hospitalizations (OR 2.35). Hass et al conducted a retrospective case-control study to assess severity of Crohn's disease in obese (BMI >25 kg/m2) and nonobese patients (BMI <25 kg/m2). The authors used time to first surgery for a complication of Crohn's disease as a marker for disease severity. Of 148 patients, 32.4% were obese. The two groups were similar in overall number of surgeries, medical therapy and disease distribution; however the obese patients had a significantly older age at diagnosis (35.0 versus 22.5 years). Time to surgery in obese patients (24 months) was shorter than non-obese (72 months) although this did not reach statistical significance. A subgroup analysis comparing obese patients (BMI >25 kg/m2) and those with patients who are underweight (BMI <18.5 kg/m2) demonstrated significantly shorter time to first surgery in obese versus underweight patients (24 versus 252 months).
OBESITY SURGERY IN THE INFLAMMATORY DISEASE PATIENT
There is little evidence in the literature regarding the outcomes of obesity surgery in patients with inflammatory bowel disease and the possible concomitant risks and complications.
As inflammatory bowel disease and obesity may share a common systemic chronic inflammatory response manifested by increased inflammatory mediators, a plausible advantage of obesity surgery would be decreased inflammatory bowel disease activity. The number of patients undergoing bariatric surgery has increased dramatically in the last decade,11 with the Roux-en-Y gastric bypass being the most favored bariatric procedure in the United States.12 Recent publications suggest that obesity may induce a low-grade systematic inflammatory response.4,5,13 The inflammatory markers implicated in obesity have been shown to be associated with many co-morbid conditions including diabetes, hypertension, thromboembolic disease, infections and cancer; and are similar to many disease mediators identified in inflammatory bowel disease. Furthermore, it has been shown that the success of weight loss surgery in treating the complications associated with obesity is probably related to the reduction of these inflammatory mediators.14
The impact of obesity surgery on inflammatory bowel disease activity is limited to several case reports in the literature. Lascano et al report an interesting case8 of a 39 year old morbidly obese male (BMI 57 kg/m2) with a longstanding history of ulcerative colitis and hypertension. The patient's disease was controlled with oral mesalamine, mesalamine enemas and azathioprine, however he required varying doses of prednisone for worsening colitis which compounded his already longstanding morbid obesity. A laparoscopic Roux-en-Y gastric bypass was performed with successful rapid weight loss. The patient concomitantly had considerable symptomatic relief of his ulcerative colitis, with a reduction from 3–6 bowel movements per day to 1–2 bowel movements per day, resolution of frequent tenesmus and of pyoderma gangrenosum. Accordingly, his medication requirements were tapered by decreasing his azathioprine dose, cessation of mesalamine and tapering of prednisone. Although his endoscopic findings were not altered at 2 years, having achieved an 80% excess weight loss, his colitis was under significantly better clinical control.
There are several reports in the literature addressing the outcome of Crohn's disease activity and obesity surgery. Moun et al15 presented a case of a 40 year old woman with ileocolic Crohn's disease medically controlled with infliximab for four years, who had a BMI of 45 kg/m2, type II diabetes and hypertension. The patient underwent a Roux-en-Y gastric bypass, and 8 weeks post-operatively she had gradually increasing abdominal pain and diarrhea. Endoscopy revealed active ileocolic Crohn's disease. She underwent high dose infliximab therapy, which controlled her symptoms. Eight months post-operatively, she was still in disease remission, her BMI was 32 kg/m2 and the hypertension and diabetes resolved. Ahn et al11 also propose a deleterious consequence of obesity surgery related to Crohn's disease. They report a case series of three morbidly patients without any prior history of gastrointestinal disease, symptoms or family history of inflammatory bowel disease. These patients underwent a Roux-en-Y gastric bypass and developed newly diagnosed Crohn's disease within 11–60 months of surgery. They were all young adults (28–46 years old) who presented with watery diarrhea, nocturnal diarrhea accompanied by abdominal cramps and more than expected weight loss. After common complications of gastric bypass were ruled out, they underwent endoscopic evaluation that revealed ulcers with histological features pathognomonic for Crohn's disease. All patients responded to medical management and are well with at least 1 year follow-up.
These case reports highlight that the impact of obesity surgery in patients with Crohn's disease and ulcerative colitis is not entirely clear. While the case presented by Lascano et al seems to demonstrate some degree of improved disease activity after obesity surgery in ulcerative colitis, this promising observation needs further validation. Furthermore, altered small bowel anatomy following gastric bypass may impact construction and reach of the ileoanal pouch reservoir. The malabsorptive nature of Roux-en-Y gastric bypass anatomy may also impact long-term pouch function. There are no reports to date of outcomes of ileoanal pouch reservoirs constructed in patients with remote gastric bypass.
With regards to Crohn's disease, the same inflammatory markers that are increased in obesity are elevated in Crohn's disease, thus there may be a hypothetical benefit to weight loss manifested by improved Crohn's disease severity. However, the anatomic alterations of gastric bypass rendering a malabsorptive state, may potentially negatively influence patients with known Crohn's disease. These case reports raise the possibility that there may be a relationship between the alterations imposed on small bowel mucosa after Roux-en-Y gastric bypass and new or worsening Crohn's disease. The worsened acute inflammatory state observed in these reported patients may be due to activation of the immune response through interaction between microbial products and receptors in the host or by alterations of intestinal epithelial cell metabolism by bacteria.15 It is well known that bacterial overgrowth can develop as a consequence of gastric bypass, and this may promote a bacterial milieu in genetically predisposed patients that triggers intestinal inflammation resulting in activation of Crohn's disease. As previously reported, there is an increased risk of complications in patients with Crohn's disease when they undergo non-Crohn's related gastrointestinal surgery,16 particularly small bowel surgery. There is a potential risk of flare-up in patients with small bowel Crohn's disease in the operated segment of small bowel after Roux-en-Y gastric bypass, with an increased risk of stricture, abscesses and fistulas.
RESTORATIVE PROCTOCOLECTOMY AND ILEOANAL POUCH RESERVOIR IN THE OBESE PATIENT
Restorative proctocolectomy and ileoanal pouch reservoir has become the preferred surgical management for mucosal ulcerative colitis. Until recently, obesity was considered a relative contraindication for the procedure, along with advanced age, weak sphincter tone and perineal disease.17,18,19 However, as more obese patients are referred for surgical intervention, surgeons have gained experience with ileal pouch procedures in this population.
Technical Issues Related to Restorative Proctocolectomy and Ileoanal Pouch Reservoir in the Obese Population
Obesity imposes specific technical challenges for the construction of an ileoanal pouch reservoir. First, acquiring enough length to facilitate reach of the ileal pouch to the pelvic floor may be precluded by the fatty, foreshortened nature of the mesentery in an obese patient. In addition to the routinely performed transverse incisions of the peritoneal layers of the small bowel mesentery or ligation of the ileocolic artery (Figs. 1A, B) or branches of the superior mesenteric artery, McMurrick and Dozois suggest that there are several maneuvers that can be performed in tall or obese patients when the surgeon encounters difficulty in gaining sufficient length for the ileal pouch to reach.19 The middle colic artery and its right branch can be preserved during proctocolectomy. The colon is then removed by division of the tissue between the marginal artery and the mesenteric wall of the right colon so that the mesentery of the right colon with its marginal artery proximal to this point is preserved. With this maneuver, both the ileocolic and distal superior mesenteric arteries can be ligated (after verification of adequate flow in the marginal arcade by application of bulldog clamps on the vessels to be ligated), allowing for a significant amount of extra mesenteric length to attain a tension-free anastomosis.
Figure 1.
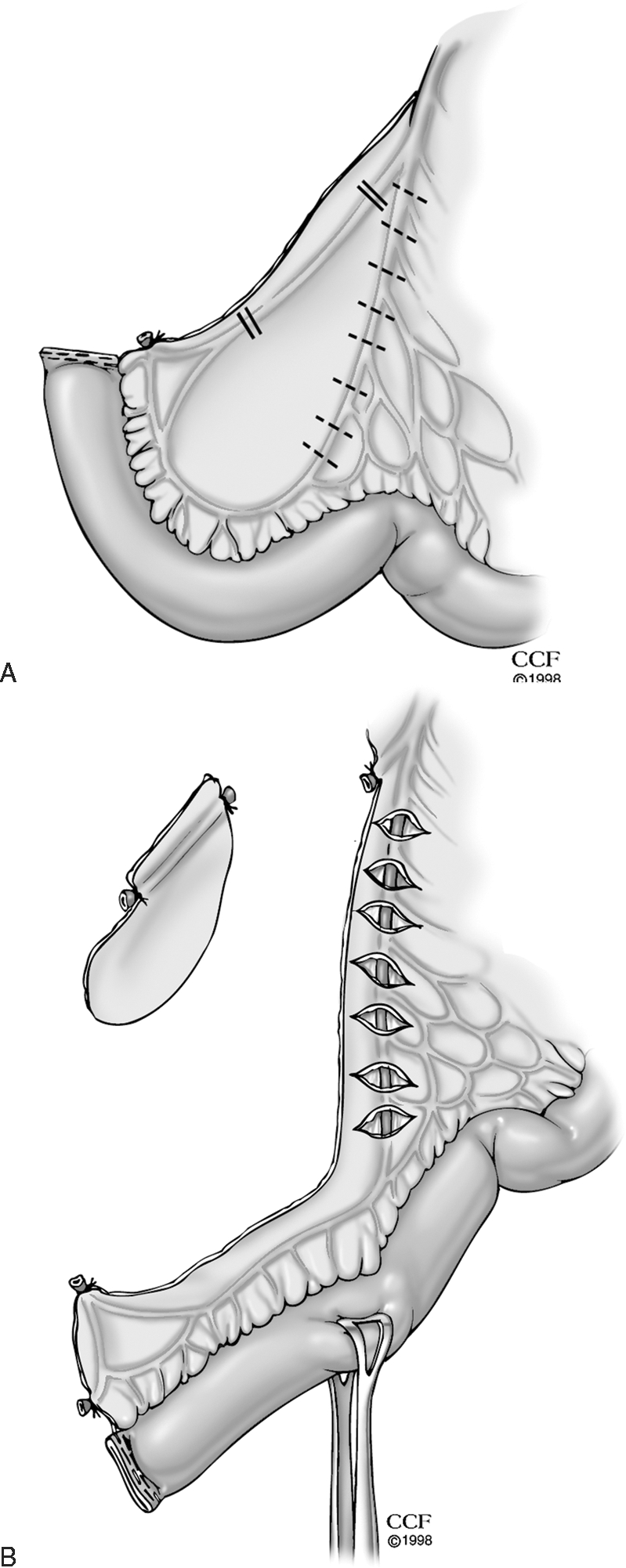
(A) Transverse incisions of the peritoneal layers of the small bowel mesentery to gain length when constructing an ileoanal pouch reservoir. (B) Ligation of the ileocolic artery to increase reach of small bowel when fashioning an ileoanal pouch reservoir. (Both reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2011. All Rights Reserved.)
While the “J” configuration is the most commonly used pouch reconstruction, an “S” configuration may be attempted when there is inadequate length of small bowel mesentery as the end-to-end anastomosis with the distal spout of the “S” may allow for more length. When the ileum will not reach the pelvic floor despite these maneuvers, it may be necessary to perform an abdominal colectomy with Hartman's pouch and end ileostomy. With time, the mesentery of the ileum often elongates, thereby allowing adequate reach.
Performing a low anastomosis in an obese patient with a narrow pelvis can also be extremely difficult. Preoperative weight loss, when possible, will make the construction of deep pelvic anastomoses easier.20 Counseling the morbidly obese patient to seek evaluation for obesity surgery prior to ileoanal pouch reconstruction may be advantageous. To date, however, the outcomes of such a sequence of surgeries has not been studied.
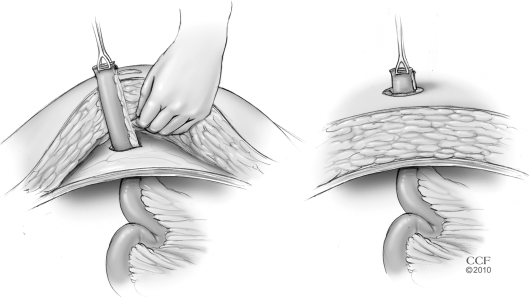
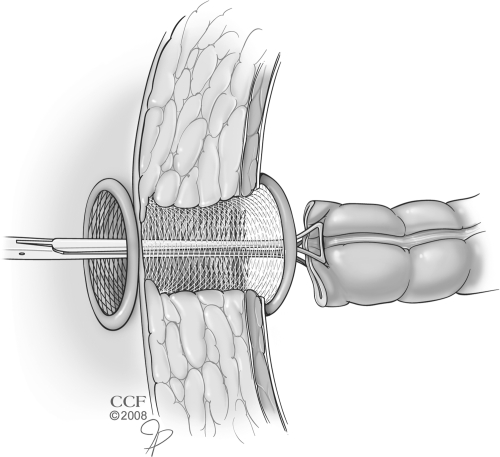
Construction of a loop ileostomy in obese patients can also be difficult. While a single-stage procedure without diversion can be safely performed in select patients, the risk of pelvic sepsis mandates a loop ileostomy in the majority of patients. A large abdominal wall in an obese patient may cause the ileostomy to be under excessive tension, cause retraction of the stoma, or force the surgeon to choose a site in the ileum which may be unacceptably proximal. If such issues are encountered, there are several described alternatives. A loop stoma through the inferior edge of the midline incision has been described for obese patients undergoing restorative proctocolectomy and ileoanal pouch reservoir in whom a standard location was not feasible.17 This is certainly not ideal. This is associated with a high risk of wound infection and the position may preclude visualization for stoma management by the patient. Another described technique is to perform a subcutaneous lipectomy to facilitate fashioning an ileostomy.21 This is done by removing the subcutaneous fat and affixing the fascia to the skin at the planned site of ileostomy. Alternatively, the loop ileostomy may be brought through the abdominal wall in two stages: 1) a subcutaneous tissue flap is raised so that the bowel may be brought out through an aperture in the fascia, and 2) the bowel can be brought out through an aligned aperture in the subcutaneous tissue (Fig. 2). Another maneuver that may be used to facilitate exteriorization of a diverting loop ileostomy in morbidly obese patients is the use of a small self-expanding wound protector such as an Alexis wound protector (Applied Medical, Rancho Santa Margarita, CA) (Fig. 3).
Figure 2.
Exteriorizing the bowel for an ileostomy in two steps: through the fascia followed by the subcutaneous tissues. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2011. All Rights Reserved.)
Figure 3.
Use of a wound protector as a conduit for exteriorizing the bowel for a stoma. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2011. All Rights Reserved.)
Even if a stoma can be safely and adequately constructed in a morbidly obese individual, complications in the postoperative period are not uncommon.22 Obese patients and patients with inflammatory bowel disease are reported to have a higher rate of stomal complications22 including high output volume, prolapse, necrosis, skin irritation and retraction.23 Fortunately, in patients presenting for restorative proctocolectomy and ileoanal pouch reservoir, ileostomies are intended to be temporary and in the great majority of patients the stoma is reversed within several months. Preoperative patient counseling and the availability of an ostomy nurse are key in improving patient outcomes.
Outcomes of Restorative Proctocolectomy and Ileoanal Pouch Reservoir in Obesity
There are several studies in the literature addressing the short and long-term outcomes of restorative proctocolectomy in morbidly obese patients. Efron et al24 evaluated outcomes in a case-matched study of 31 obese (>30 kg/m2) and 31 non-obese (<30 kg/m2) patients undergoing proctocolectomy and ileoanal pouch reservoir. The authors report that despite matching for disease severity and ASA classification, obese patients required significantly longer operating times (229 versus 196 minute, p = 0.02) and had a trend toward increased post-operative complications (32.0% versus 9.7, p = 0.058), including significantly increased risk of pelvic sepsis in obese patients as compared with non-obese patients (16% versus 0%, p < 0.05). Of note, 16% (5/31) of obese patients and 10% (3/31) of non-obese patients underwent a single-stage procedure (no diverting loop ileostomy). Three of the 5 patients who suffered pelvic septic complications were performed without a diverting stoma. One patient in the obese group died from cardiac arrest during the post-operative period following closure of ileostomy. With regards to long-term outcomes, of the five obese patients with pelvic sepsis, 60% (3 patients) underwent successful pouch salvage, one underwent pouch excision and one remained diverted for pouch-anal fistula.
Long-term functional results were obtained in 24 obese and 22 non-obese patients at a mean follow-up of 50.5 months. There was no statistically significant difference between the groups in total number of bowel movements over a 24-hour period, number of nocturnal evacuations, pad usage or fecal incontinence scores. This study demonstrates that restorative proctocolectomy and ileoanal pouch reservoir is feasible in obese individuals, but it also highlights the higher immediate complications and risk of pelvic sepsis in this group. The higher than expected rate of pelvic sepsis and the below-average pouch salvage rate of 60% in obese individuals, (as compared with previously reported 90% salvage rate in non-obese individuals),25 suggests that surgeons should strongly consider a two-stage procedure in obese patients.
Similar observations were made for obese patients without inflammatory bowel disease who underwent rectal resections. Benoist et al found that anastomotic leak and post-operative hemorrhage after rectal resection were significantly more frequent in obese patients.26 Thus, as in the previous case-matched study, the authors concluded that routine fecal diversion for colorectal anastomoses of any level is advisable in obese patients.
Restorative proctocolectomy with ileoanal pouch reservoir in obese patients is a technically challenging operation, however in experienced hands the long-term results are equivalent to non-obese patients undergoing the same operation. Although creation of a diverting stoma can be difficult, we believe that the increased risk of immediate post-operative complications and pelvic sepsis in obese patients mandates proceeding with a two-stage operation, except in very select cases. Furthermore, sexual, fertility and urinary outcomes in obese patients undergoing restorative proctocolectomy and ileoanal pouch reservoir have not been reported. These may be impacted by a more difficult dissection in the morbidly obese patient, raising the possibility that a three stage procedure, with intensive intentional weight loss after a total abdominal colectomy may be indicated.
LAPAROSCOPIC SURGERY FOR INFLAMMATORY BOWEL DISEASE IN THE OBESE POPULATION
Laparoscopic bowel resection has been associated with several benefits including decreased postoperative stress response, pulmonary complications, duration of hospital stay, hernia and incidence of small bowel obstruction.27 Numerous comparative studies and several meta-analyses have assessed whether these benefits are observed in laparoscopic ileocolic resection for Crohn's disease28,29 and laparoscopic total proctocolectomy with ileoanal pouch reservoir for ulcerative colitis patients.30,31 The short-term benefits of laparoscopy including shorter hospitalization, faster return of bowel function, decreased pain and improved cosmesis were observed after laparoscopic ileocolic resection; however only improved cosmesis was identified as a benefit of laparoscopic total proctocolectomy and ileoanal pouch anastomosis. These studies included obese patients; however no subgroup analysis was performed for this population.
Early in the development of laparoscopy, contraindications included patients with higher body mass index and inflammatory bowel disease.32,33 As colorectal surgeons became more proficient with minimally invasive techniques, however, the indications for laparoscopy were broadened. Studies conducted in the 1990s, addressing feasibility and outcomes of laparoscopic colectomy for all indications in obese patients, found that obesity was associated with higher conversion rates and increased post-operative complications.34,35,36,37 However, more recent reports addressing the outcomes of laparoscopic colorectal surgery in general27,38,39,40,41 and specifically for diverticular disease42 and colorectal cancer,43 report more mixed results. In terms of short-term outcomes, laparoscopic colectomy is associated with shorter length of hospitalization38,39 for laparoscopic as compared with open colorectal surgery, despite morbid obesity. Regarding operating room time, conversion rates and post-operative complications, some studies report significantly worsened outcomes in obese and morbidly obese patients,27 whereas others report no difference.40,41,42
Canedo et al assessed the impact of these two concomitant factors on short-term outcomes of laparoscopic colorectal surgery in 213 patients with inflammatory bowel disease over a seven-year period.44 They compared outcomes of Crohn's and ulcerative colitis patents with normal BMI (<25 kg/m2) undergoing laparoscopic colorectal resections with the outcomes of overweight and obese patients' (BMI >25 kg/m2). The two groups had similar indications for surgery (primarily failure of medical management and obstruction), type of resection (predominately, ileocolic resection 56% and total colectomy 40%) and use of steroids and biologic immunosuppressive agents (75%). The authors found that the patients with a normal BMI and overweight/obese individuals had similar mean operating time (200 minute and 207 minute, respectively), rate of intraoperative complications and conversion rates (18% and 22%, respectively). Furthermore, they report similar post-operative morbidity rates (21% normal BMI and 22% overweight/obese individuals) in obese and non-obese patients, including anastomotic leaks and pelvic abscesses. Need for re-operation was also not associated with higher BMI. Thus, the authors demonstrate that laparoscopic resections for inflammatory bowel disease are feasible and have equivalent post-operative outcomes to non-obese patients with inflammatory bowel disease, with no added risk of conversion or increased operating room time. As this is the only available comparative study reporting outcomes of laparoscopic surgery in obese patients with inflammatory bowel disease further prospective validation is needed.
Technical Issues Related to Laparoscopic Surgery for Inflammatory Bowel Disease in the Obese Population
Laparoscopic surgery in the obese patient is particularly challenging because of the increased amount of adipose tissue, in addition to the presence of inflammatory bowel disease and concomitant steroid and biological immunosuppressive medications which can render the tissues fragile and the mesentery friable and foreshortened. Wexner et al recommend the use of monopolar energy or harmonic devices for any laparoscopic dissection in these patients, as manipulation of the fatty mesentery is challenging and obtaining hemostasis is often difficult.45 Regarding ligation of the mesenteric vessels during laparoscopic colectomy for IBD in obese patients, ligation may be performed intracorporeally if a thin, less diseased area closer to the root of the mesentery can be identified. Alternatively, if the mesentery is too friable, extracorporeal vessel ligation may be necessary.45
CONCLUSION
Both obesity and inflammatory bowel disease heighten the complexity of surgery and post-operative care. The increasing frequency of obesity in IBD patients warrants an initiative to better understand the disease implications, challenges of management and outcome of interventions. Lessons learned from restorative proctocolectomy and ileoanal pouch reconstruction demonstrate the feasibility of this operation, however with an increased risk of anastomotic complications in this group. Likewise, although previously reported to be difficult and associated with increased morbidity, with increasing experience, laparoscopic colorectal resections for inflammatory bowel disease have recently been shown to be feasible and equivalent to surgery in nonobese patients. Weight loss prior to colorectal surgery for inflammatory bowel disease has multiple plausible benefits, including technical ease and better controlled IBD disease activity. The role of obesity surgery prior to surgery for inflammatory bowel disease needs further investigation.
References
- 1.Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts. 2009;2(6):370–372. doi: 10.1159/000262276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long M D, Crandall W V, Leibowitz I H, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2010;epub:1–7. doi: 10.1002/ibd.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendall M A, Gunasekera A V, John B J, Kumar D. Is obesity a risk factor for Crohn's disease? Dig Dis Sci. 2011;56(3):837–844. doi: 10.1007/s10620-010-1541-6. [DOI] [PubMed] [Google Scholar]
- 4.Karagiannides I, Pothoulakis C. Substance P, obesity, and gut inflammation. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):47–52. doi: 10.1097/MED.0b013e328321306c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertin B, Desreumaux P, Dubuquoy L. Obesity, visceral fat and Crohn's disease. Curr Opin Clin Nutr Metab Care. 2010;13(5):574–580. doi: 10.1097/MCO.0b013e32833cf0f4. [DOI] [PubMed] [Google Scholar]
- 6.Karmiris K, Koutroubakis I E, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis E A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(2):100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 7.Das U N. Is obesity an inflammatory condition? Nutrition. 2001;17(11-12):953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 8.Lascano C A, Soto F, Carrodeguas L, Szomstein S, Rosenthal R J, Wexner S D. Management of ulcerative colitis in the morbidly obese patient: is bariatric surgery indicated? Obes Surg. 2006;16(6):783–786. doi: 10.1381/096089206777346718. [DOI] [PubMed] [Google Scholar]
- 9.Filková M, Haluzík M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin Immunol. 2009;133(2):157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre J P, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21(1):51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]
- 11.Ahn L B, Huang C S, Forse R A, Hess D T, Andrews C, Farraye F A. Crohn's disease after gastric bypass surgery for morbid obesity: is there an association? Inflamm Bowel Dis. 2005;11(6):622–624. doi: 10.1097/01.mib.0000165113.33557.3a. [DOI] [PubMed] [Google Scholar]
- 12.Tice J A, Karliner L, Walsh J, Petersen A J, Feldman M D. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121(10):885–893. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Cottam D R, Mattar S G, Barinas-Mitchell E, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14(5):589–600. doi: 10.1381/096089204323093345. [DOI] [PubMed] [Google Scholar]
- 14.Cottam D R, Schaefer P A, Shaftan G W, Velcu L, Angus L D. Effect of surgically-induced weight loss on leukocyte indicators of chronic inflammation in morbid obesity. Obes Surg. 2002;12(3):335–342. doi: 10.1381/096089202321088101. [DOI] [PubMed] [Google Scholar]
- 15.Moum B, Jahnsen J. [Obesity surgery in inflammatory bowel disease] Tidsskr Nor Laegeforen. 2010;130(6):638–639. doi: 10.4045/tidsskr.09.1281. [DOI] [PubMed] [Google Scholar]
- 16.Hass D J, Brensinger C M, Lewis J D, Lichtenstein G R. The impact of increased body mass index on the clinical course of Crohn's disease. Clin Gastroenterol Hepatol. 2006;4(4):482–488. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Church J M. The ileal pouch—anal anastomosis in the challenging patient: stretching the limits. Aus and NZ J Surg. 1995;65:104–106. doi: 10.1111/j.1445-2197.1995.tb07271.x. [DOI] [PubMed] [Google Scholar]
- 18.Becker J M. Ileal pouch-anal anastomosis: current status and controversies. Surgery. 1993;113(6):599–602. [PubMed] [Google Scholar]
- 19.Murrick P J, Dozois R R. Chronic ulcerative colitis: surgical options. In: Fazio V W, Church J M, Delaney C P, editors. Current Therapy in Colon and Rectal Surgery. 2nd ed. Philadelphia: Mosby; 2005. pp. 211–217. [Google Scholar]
- 20.Dietz D W, Bailey R H. Postoperative complications. In: Wolf B G, Fleshman J W, Beck D E, Pemberton J H, Wexner S D, editors. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer; 2007. pp. 141–155. [Google Scholar]
- 21.Meguid M M, McIvor A, Xenos L. Creation of a neoabdominal wall to facilitate emergency placement of a terminal ileostomy in a morbidly obese patient. Am J Surg. 1997;173(4):298–300. doi: 10.1016/s0002-9610(96)00393-5. [DOI] [PubMed] [Google Scholar]
- 22.Leenen L P, Kuypers J H. Some factors influencing the outcome of stoma surgery. Dis Colon Rectum. 1989;32(6):500–504. doi: 10.1007/BF02554506. [DOI] [PubMed] [Google Scholar]
- 23.Duchesne J C, Wang Y Z, Weintraub S L, Boyle M, Hunt J P. Stoma complications: a multivariate analysis. Am Surg. 2002;68(11):961–966. discussion 966. [PubMed] [Google Scholar]
- 24.Efron J E, Uriburu J P, Wexner S D, et al. Restorative proctocolectomy with ileal pouch anal anastomosis in obese patients. Obes Surg. 2001;11(3):246–251. doi: 10.1381/096089201321336520. [DOI] [PubMed] [Google Scholar]
- 25.Marcello P W, Roberts P L, Schoetz D J, Jr, Coller J A, Murray J J, Veidenheimer M C. Long-term results of the ileoanal pouch procedure. Arch Surg. 1993;128(5):500–503. discussion 503–504. doi: 10.1001/archsurg.1993.01420170030003. [DOI] [PubMed] [Google Scholar]
- 26.Benoist S, Panis Y, Alves A, Valleur P. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179(4):275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 27.Senagore A J, Delaney C P, Madboulay K, Brady K M, Fazio V W. Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg. 2003;7(5):712. doi: 10.1016/s1091-255x(02)00124-5. [DOI] [PubMed] [Google Scholar]
- 28.Milsom J W, Hammerhofer K A, Böhm B, Marcello P, Elson P, Fazio V W. Prospective, randomized trial comparing laparoscopic vs. conventional surgery for refractory ileocolic Crohn's disease. Dis Colon Rectum. 2001;44(1):1–8. discussion 8–9. doi: 10.1007/BF02234810. [DOI] [PubMed] [Google Scholar]
- 29.Tan J JY, Tjandra J J. Laparoscopic surgery for Crohn's disease: a meta-analysis. Dis Colon Rectum. 2007;50(5):576–585. doi: 10.1007/s10350-006-0855-0. [DOI] [PubMed] [Google Scholar]
- 30.Marcello P W, Milsom J W, Wong S K, et al. Laparoscopic restorative proctocolectomy: case-matched comparative study with open restorative proctocolectomy. Dis Colon Rectum. 2000;43(5):604–608. doi: 10.1007/BF02235570. [DOI] [PubMed] [Google Scholar]
- 31.Tan J JY, Tjandra J J. Laparoscopic surgery for ulcerative colitis - a meta-analysis. Colorectal Dis. 2006;8(8):626–636. doi: 10.1111/j.1463-1318.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- 32.Wexner S D, Reissman P, Pfeifer J, Bernstein M, Geron N. Laparoscopic colorectal surgery: analysis of 140 cases. Surg Endosc. 1996;10(2):133–136. doi: 10.1007/BF00188358. [DOI] [PubMed] [Google Scholar]
- 33.Smith L E, Gordon P H. Laparoscopic colon and rectum surgery. In: Gordon P H, Nivatvongs S, editors. Principles and Practice of Surgery for the Colon, Rectum and Anus. 3rd ed. New York: Informa Health Care USA; 2007. pp. 1204–1261. [Google Scholar]
- 34.Smadja C, Sbai Idrissi M, Tahrat M, et al. Elective laparoscopic sigmoid colectomy for diverticulitis. Results of a prospective study. Surg Endosc. 1999;13(7):645–648. doi: 10.1007/s004649901065. [DOI] [PubMed] [Google Scholar]
- 35.Huscher C, Silecchia G, Croce E, et al. Laparoscopic colorectal resection. A multicenter Italian study. Surg Endosc. 1996;10(9):875–879. doi: 10.1007/BF00188473. [DOI] [PubMed] [Google Scholar]
- 36.Pandya S, Murray J J, Coller J A, Rusin L C. Laparoscopic colectomy: indications for conversion to laparotomy. Arch Surg. 1999;134(5):471–475. doi: 10.1001/archsurg.134.5.471. [DOI] [PubMed] [Google Scholar]
- 37.Schwandner O, Schiedeck T H, Bruch H. The role of conversion in laparoscopic colorectal surgery: do predictive factors exist? Surg Endosc. 1999;13(2):151–156. doi: 10.1007/s004649900927. [DOI] [PubMed] [Google Scholar]
- 38.Delaney C P, Pokala N, Senagore A J, et al. Is laparoscopic colectomy applicable to patients with body mass index >30? A case-matched comparative study with open colectomy. Dis Colon Rectum. 2005;48(5):975–981. doi: 10.1007/s10350-004-0941-0. [DOI] [PubMed] [Google Scholar]
- 39.Leroy J, Ananian P, Rubino F, Claudon B, Mutter D, Marescaux J. The impact of obesity on technical feasibility and postoperative outcomes of laparoscopic left colectomy. Ann Surg. 2005;241(1):69–76. doi: 10.1097/01.sla.0000150168.59592.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwandner O, Farke S, Schiedeck T H, Bruch H P. Laparoscopic colorectal surgery in obese and nonobese patients: do differences in body mass indices lead to different outcomes? Surg Endosc. 2004;18(10):1452–1456. doi: 10.1007/s00464-003-9259-6. [DOI] [PubMed] [Google Scholar]
- 41.Scheidbach H, Benedix F, Hügel O, Kose D, Köckerling F, Lippert H. Laparoscopic approach to colorectal procedures in the obese patient: risk factor or benefit? Obes Surg. 2008;18(1):66–70. doi: 10.1007/s11695-007-9266-0. [DOI] [PubMed] [Google Scholar]
- 42.Tuech J J, Regenet N, Hennekinne S, Pessaux P, Bergamaschi R, Arnaud J P. Laparoscopic colectomy for sigmoid diverticulitis in obese and nonobese patients: a prospective comparative study. Surg Endosc. 2001;15(12):1427–1430. doi: 10.1007/s00464-001-9023-8. [DOI] [PubMed] [Google Scholar]
- 43.Blee T H, Belzer G E, Lambert P J. Obesity: is there an increase in periopoerative complications in those undergoing elective colon and rectal resection for carcinoma? Am Surg. 2002;68(2):163–166. [PubMed] [Google Scholar]
- 44.Canedo J, Pinto R A, Regadas S, Regadas F S, Rosen L, Wexner S D. Laparoscopic surgery for inflammatory bowel disease: does weight matter? Surg Endosc. 2010;24(6):1274–1279. doi: 10.1007/s00464-009-0759-x. [DOI] [PubMed] [Google Scholar]
- 45.Wexner S D, Boutros M. Springer. In press Laparoscopic segmental colectomy. In: Scott-Connor C, Nguyen N, Soper N, editors. SAGES Fundamentals Vol II. 3rd ed. New York: [Google Scholar]