Abstract
Allergic airway disease is characterized by a T helper type 2 cell-mediated airway inflammation and airway hyperresponsiveness. Little is known about the role of hypoxia-mediated signaling in the progression of the disease. To address this knowledge gap, a mouse model was created in which doxycycline exposure induces the functional deletion of hypoxia inducible factor-1α from alveolar type II and Clara cells of the lung. When hypoxia inducible factor-1α deletion was induced during the early postnatal development period of the lung, the mice displayed an enhanced response to the ovalbumin model of allergic airway disease. These hypoxia inducible factor-1α-deficient mice exhibit increased cellular infiltrates, eosinophilia in the lavage fluid and parenchyma, and T helper type 2 cytokines, as compared with ovalbumin-treated control mice. Moreover, these hypoxia inducible factor-1α-deficient mice display increased airway resistance when compared with their control counterparts. Interestingly, if the loss of hypoxia inducible factor-1α was induced in early adulthood, the exacerbated phenotype was not observed. Taken together, these results suggest that epithelial hypoxia inducible factor-1α plays an important role in establishing the innate immunity of the lung and epithelial-specific deficiency in the transcription factor, during early postnatal development, increases the severity of inflammation and functional airway resistance, following ovalbumin challenge. Finally, these results might explain some of the chronic respiratory pathology observed in premature infants, especially those that receive supplemental oxygen. This early hyperoxic exposure, from normal ambient and supplemental oxygen, would presumably inhibit normal hypoxia inducible factor-1α signaling, mimicking the functional deletion described.
Keywords: hypoxia inducible factor-1α, ovalbumin, allergic airway, inflammation
asthma is a chronic inflammatory disease of the airways that affects ∼20 million Americans. Asthma symptoms include wheezing, coughing, and shortness of breath. Histopathological characteristics of asthma include mucous cell metaplasia, eosinophilic inflammation, expression of chitinase-like proteins, and T helper 2 (Th2)-mediated inflammation (3, 4). Preterm birth is one common risk factor for developing the disease, and the risk increases with the duration of supplemental oxygen (8, 15). The molecular mechanism for this increased risk is unknown.
Hypoxia, a decrease in oxygen reaching the cells and tissues of the body, plays a role in both metabolic reprogramming and inflammation. The cellular hypoxic sensing mechanism involves mitochondrial electron transport, prolyl hydroxylation, and a family of proteins, known as the hypoxia inducible factors (HIFs). HIFs are members of the Per-Arnt-Sim (PAS) superfamily of physiological sensors, and two of these factors, HIF-1α and HIF-2α, are critical for the proper development of the lungs (5, 30). The potential role these factors play in the development and progression of asthma remains largely unknown.
Recently it was shown that loss of HIF-1α from Clara and alveolar type II (ATII) cells early in development display profound eosinophilia, mucus cell metaplasia, and chitinase-like protein expression upon metal challenge. Control mice, however, resemble a Th1-mediated inflammatory response, including neutrophilia and TNF-α expression (29, 31). These results suggest that the loss of HIF-1α from only two epithelial cell types of the lung profoundly alters the inflammatory response of the tissue. Our present animal study was designed to test the hypothesis that neonatal loss of HIF-α causes an enhanced response to an allergen known to induce a Th2-mediated inflammation. Control and lung epithelial HIF-1α-deficient mice were subjected to ovalbumin (OVA) sensitization and challenge. When airway epithelium-specific HIF-1α deletion was induced early in postnatal development, the HIF-1α-deficient mice displayed increased eosinophils, Th2 cytokines, and total lung resistance when compared with their littermate controls. In contrast, when deletion was induced following the completion of postnatal development of the lung, the HIF-1α-deficient mice behaved in a similar manner as HIF-1α-sufficient control mice to OVA challenge. Taken together, these results suggest that epithelial-derived HIF-1α signaling plays a central role in determining the response of the lungs to allergen and HIF-1α-mediated transcriptional regulation is critical to the normal development of immunity in the lung. Finally, these results suggest a possible mechanism for the increased incidence of asthma in preterm infants. These infants' lungs are exposed to premature hyperoxia compared with the in utero environment, both from the ambient air and supplemental oxygen, if administered. The increased oxygen will decrease normal HIF-1α in the lungs, mimicking the loss of the transcription factor described in our present study (1, 2). Presumably, this disruption in normal HIF-1α-mediated lung development could lead to an increased asthma incidence in these children.
MATERIALS AND METHODS
Description of mice.
Matings between HIF-1αflox/flox (gift from Randall Johnson, University of California–San Diego) and SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg (gift from Jeffrey A. Whitsett, Cincinnati Children's Hospital Medical Center) transgenic mice generated the triple transgenic mice used in this study. These mice, SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αflox/flox, upon exposure to doxycycline (DOX), are able to undergo recombination in the floxed HIF-1α gene, specifically in the respiratory epithelium (28, 31). Previous studies have demonstrated that the Th2 bias is dependent upon all three transgenes and doxycycline exposure, thus eliminating the possibility that these observed inflammatory changes are due to doxycycline alone or Cre toxicity (31). All of the mice used in this study are maintained in a mixed C57BL/6 and FVB/N background. Genotyping of the mice was performed by PCR for the three loci as described previously (30).
Doxycycline treatment and animal husbandry.
Exposing triple transgenic mice to DOX in utero is lethal upon birth (30). Postnatal exposure leads to almost complete loss of HIF-1α from Clara and ATII cells. In the developmental model, lactating dams were exposed to DOX-containing feed (625 mg doxycycline/kg; Harlan Teklad, Madison, WI) and drinking water (0.8 mg/ml; MP Biochemicals, Solon, OH) beginning on postnatal day 4 (PN4) until weaning. This dose of doxycycline is slightly lower than the concentration that has been used to induce recombination without any observable toxicity or impact on alveolarization (34). The triple transgenic mice were maintained on the same DOX-containing food and water until they were ∼4 weeks of age (PN28). DOX treatment was terminated ∼2 weeks before the first intraperitoneal injection of OVA (Fig. 1). These mice will be referred to as PN4 mice throughout the paper. In the young adult model, mice were treated with DOX for 10 days, from PN32 to PN42. DOX was again terminated ∼2 weeks before the first intraperitoneal injection of OVA (Fig. 1). These mice will be referred to as PN32 mice throughout the paper. The third DOX treatment regimen began at PN4, but only went until PN14. This was to match the shortened length of DOX exposure in the PN32 mice. These mice will be referred to as PN4–14. The OVA sensitizations for this group began at the same age as those in the PN4 group described previously. All DOX treated mice will be referred to as HIF-1α-deficient throughout this paper. Animals used as controls in this study were also triple transgenic [SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αflox/flox] mice, which were given normal food and water ad libitum. All the procedures regarding the handling, maintenance, and necropsy protocols of the mice used in this study were approved by the university laboratory animal resource (ULAR) regulatory unit at Michigan State University.
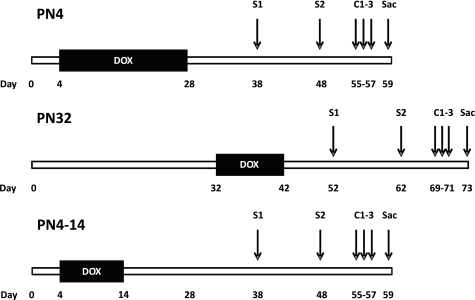
Fig. 1.
Experimental design. Hypoxia inducible factor-1α (HIF-1α)-deficient mice were generated through 3 different postnatal doxycycline (DOX) treatment schemes: postnatal day (PN)4, PN32, or PN4–14. For PN4, DOX was delivered from PN4 to PN28. For PN32, DOX was delivered from PN32 to PN42. For PN4–14, DOX was delivered from PN4 to PN14. Following at least 10 days on normal food and water, HIF-1α-sufficient and HIF-1α-deficient female mice were sensitized (S1) intraperitoneally with saline or ovalbumin (OVA) plus alum (250 μl) and 10 days later sensitized again (S2) with saline or OVA (no alum, 250 μl). Seven days later, the mice were challenged (C1–3) with saline or OVA (1% solution) via inhalation 30 min per day for 3 days. Animals were euthanized 48 h after their final challenge.
Ovalbumin exposure, tissue harvesting, and processing.
To determine the effect of the lung-specific, epithelial loss of HIF-1α in the OVA model of allergic airway disease, control and HIF-1α-deficient female mice were randomly assigned to saline or OVA treatment groups. Approximately 2 weeks after terminating DOX exposure, OVA group mice were injected with OVA/alum (20 μg/1 mg ip in 250 μl saline). Saline group animals were injected with 250 μl saline alone. Ten days later, OVA group animals were given an OVA boost consisting of an injection of OVA alone (20 μg ip in 250 μl saline) and 250 μl saline for the saline group. One week after the OVA boost, mice were challenged with either OVA (OVA group) or saline (saline group) via inhalation (Fig. 1). Briefly, mice were exposed to OVA (1% in saline) or saline alone in a mass dosing chamber (Buxco, Wilmington, NC) with a nebulizer (Aerogen, Galway, Ireland) for 30 min at 100% duty and a flow rate of 2.5 l/min, once daily, for 3 consecutive days. Mice were anesthetized with pentobarbital sodium (50 mg/kg) 48 h after final OVA/saline challenge, and a midline laparotomy was performed. Tissue isolation and bronchoalveolar lavage fluid (BALF) were collected as described previously (29). Total and differential cell counts were performed on BALF samples using a hemacytometer and Diff-Quik reagent (Baxter, Deerfield, IL), respectively. The right lung lobes were removed and snap frozen in liquid nitrogen. The left lung lobe was perfused with 10% neutral buffered formalin for 1 h at 30 cm pressure and then stored for at least 24 h in a large volume of fixative before processing for histopathological analysis. After fixation, the left lobe was microdissected along the main axial airway, and two transverse tissue blocks were excised at the level of the fifth (proximal) and eleventh (distal) airway generation (G5 and G11, respectively) for further processing, as has been reported previously (11).
Histopathology and immunohistochemistry.
All samples from each treatment group were analyzed for histopathological changes as described previously (29). Briefly, fixed left lung lobe tissues were embedded in paraffin, and sections (5 μm) were mounted on glass slides and stained with hematoxylin and eosin, Alcian Blue (pH 2.5)/PAS (AB/PAS) to detect total mucosubstances (acidic and neutral) in airway epithelium, or immunostained for major basic protein (MBP) within eosinophils using a polyclonal rabbit antibody directed against murine MBP (1:500; Mayo Clinic, Scottsdale, AZ), or HIF-1α antibody (Novus Biologicals, Littleton, CO).
Morphometry of intraepithelial mucosubstances.
To determine the amount of stored mucosubstances in the epithelium lining, the proximal axial airway (generation 5) in the left lung lobe volume densities of AB/PAS stained samples were analyzed using computerized image analysis. The area of positive AB/PAS staining was calculated using the Scion Image program (Scion Corporation, Frederick, MD). The length of the basal lamina underlying the airway surface epithelium was calculated from the contour length of the digitized image of the basal lamina. The volume of stored mucosubstances (volume density [Vs]) per unit of surface area was estimated using the method described previously in detail by Harkema et al. (17).
Pulmonary function analysis.
Mice were anesthetized with pentobarbital sodium (120 mg/kg ip), intubated, and ventilated with a small animal ventilator (SAV, flexiVent; SCIREQ, Montreal, Quebec, Canada) at an initial frequency of 120–150 breaths/minute and volume of 1.5 ml/kg. Increasing doses of aerosolized methacholine were given via an Aeroneb nebulizer (Aerogen), and total lung resistance was calculated during ventilator-controlled oscillations in breathing (sinusoidal forced oscillations) as determined by protocols specific to the flexiVent pulmonary function testing system (SnapShot-150; SCIREQ). Data collection took ∼10 min, after which animals were euthanized and tissues were collected as described above.
Cytokine bead array.
BALF was analyzed for cytokine expression (IL-2, IL-4, IL-5, INF-γ, TNF-α, IL-13, KC, and IL-1β) using Cytometric Bead Array kits and reagents (BD Bioscience, Franklin Lakes, NJ) according to manufacturer's protocols. In brief, cytokine-specific antibody coated beads are mixed with the BALF samples and then run through a flow cytometer for analysis based on the specific fluorescent signature of each bead. Cytokine concentrations were calculated using standard curves generated from samples supplied by the manufacturer.
Quantitative analysis.
Treatment and HIF-1α-dependent differences in BALF cells and cytokines, intraepithelial mucosubstances, and airway resistance were analyzed by one-way ANOVA followed by Fishers test (OriginPro; OriginLab, Northampton, MA). All error bars represent SE. Statistical differences of P value <0.05 were considered significant.
RESULTS
Cell counts from lavage fluid of HIF-1α-sufficient and PN4 HIF-1α-deficient animals.
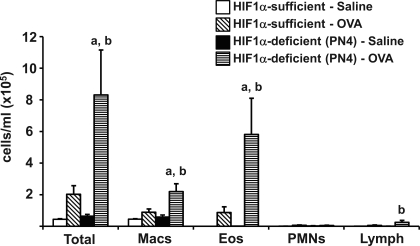
Total cells recovered from BALF increased slightly with OVA treatment in HIF-1α-sufficient control mice, and this rise in total cells was exacerbated in the PN4 HIF-1α-deficient mice treated with OVA. Differential staining of the cells recovered in the BALF showed that MBP-containing eosinophils were the predominant cell type causing the increase in total BALF inflammatory cells in both the OVA-treated control and PN4 HIF-1α-deficient groups. In both HIF-1α-sufficient and HIF-1α-deficient saline-treated groups, eosinophils accounted for ∼0–4% of the total cells. In comparison, with OVA treatment of HIF-1α-sufficient animals, this number rose to 33%, but with OVA treatment in PN4 HIF-1α-deficient animals it almost doubled, reaching 59%. In addition, macrophages were also significantly increased in the OVA-treated PN4 HIF-1α-deficient mice compared with both the saline-treated deficient mice and the OVA-treated HIF-1α-sufficient mice (Fig. 2). Despite this increase, the overall percentage of macrophages was actually reduced from 92–97% of total cells in saline-treated mice to 62% and 38% of total cells in HIF-1α-sufficient and HIF-1α-deficient OVA-treated mice, respectively. The number of lymphocytes was also significantly increased in the OVA-treated PN4 HIF-1α-deficient mice compared with saline-treated, deficient mice; however, the percentage of lymphocytes in the total number of cells remained relatively steady in these two groups at 2.6% and 1.4%, respectively.
Fig. 2.
The effect of OVA on bronchoalveolar lavage fluid (BALF) cellularity (PN4 mice). HIF-1α-sufficient (white and diagonally hatched bars) and PN4 HIF-1α-deficient (black and horizontally hatched) mice were sensitized/challenged with saline (white and black bars) or OVA (diagonally and horizontally hatched bars). Total cells, as well as the numbers of macrophages (Macs), eosinophils (Eos), neutrophils (PMNs), and lymphocytes (Lymph) were determined from BALF. Values are means ± SE (n ≥ 9). a, Significantly different from HIF-1α-sufficient within treatment P < 0.05; b, significantly different from saline treated within genotype P < 0.05.
Histopathology of allergic airways.
Only mice sensitized and challenged with OVA had conspicuous histopathological changes in the lung that were consistent with allergic airway disease. OVA-induced changes consisted of both periairway inflammation and airway epithelial remodeling (i.e., mucous cell metaplasia) and were more prominent in the hilar region of the lung lobe (e.g., G5 lung section) than in the more distal aspects of the lung lobe (i.e., G11 lung section). Both inflammatory and epithelial changes were also more apparent in the large-diameter, proximal bronchioles (e.g., axial airway) than in the more distal, small-diameter bronchioles (i.e., preterminal and terminal bronchioles).
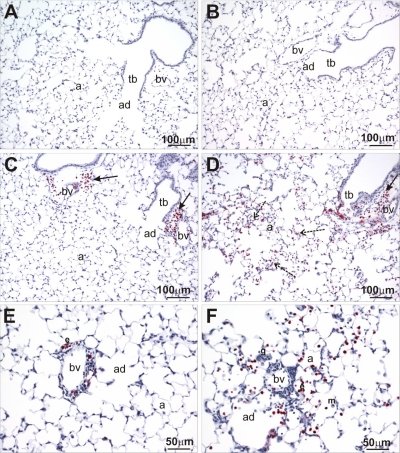
OVA-induced airway inflammation was characterized by a mixed inflammatory cell infiltrate composed of numerous eosinophils, lymphocytes, and lesser numbers of neutrophils, along with edema in the interstitium surrounding the airway (peribronchiolar inflammation). This eosinophilic and mononuclear cell infiltrate was localized in the surrounding interstitium, predominantly on the pulmonary artery/arteriole side of the airway, and also circumscribed this airway-associated blood vessel (perivascular inflammation; Fig. 3). A similar perivascular inflammatory cell infiltrate was also present in the interstitium surrounding some of the pulmonary veins embedded in the alveolar parenchyma located remotely from conducting airways.
Fig. 3.
Major basic protein (MBP) immunohistochemistry. Lung sections from HIF-1α-sufficient (A, C, and E) and PN4 HIF-1α-deficient (B, D, and F) mice following sensitization and challenge with saline (A and B) or OVA (C, D, E, and F) were stained for MBP, an eosinophil-specific marker, via immunohistochemistry. Solid arrows indicate eosinophils located around terminal bronchioles and blood vessels, whereas dashed arrows indicate eosinophils located out in the parenchyma. a, Alveolus; bv, blood vessel; tb, terminal bronchiole; ad, alveolar duct.
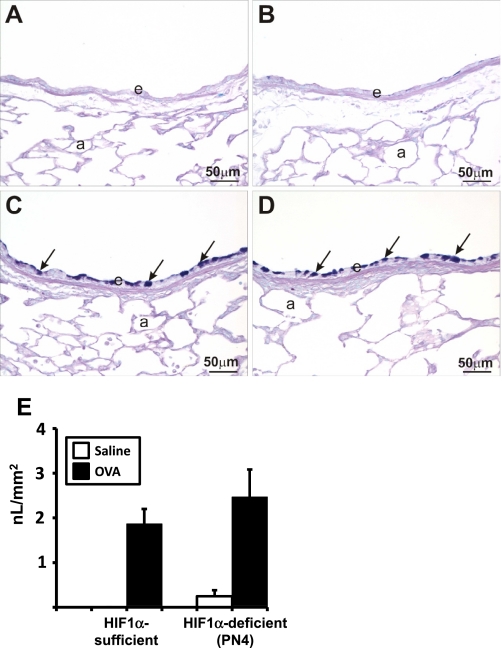
OVA-induced changes to the airway epithelium included epithelial thickening (hypertrophy) and mucous cell metaplasia (MCM; Fig. 4). MCM is defined as the presence of AB/PAS-stained mucous goblet cells in airway epithelium that is normally devoid or contains only occasional secretory cells of this phenotype. Epithelial MCM and hypertrophy were not present in the airways of saline-treated HIF-1α-sufficient control mice. MCM was a consistent airway epithelial change in the large-diameter proximal axial airways (e.g., G5) in all of the OVA-treated mice. This alteration was less evident in the epithelium lining of the more distal small-diameter bronchioles. The magnitude of this OVA-induced remodeling of the airway epithelium was similar among all the OVA-treated groups, regardless of HIF-1α phenotype (Fig. 4E).
Fig. 4.
Alcian Blue-Periodic Acid Schiff (AB-PAS) staining and mucous quantification. Lung sections from saline (A and B) and ovalbumin (C and D) sensitized and challenged HIF-1α-sufficient (A and C) and PN4 HIF-1α-deficient (B and D) mice were stained with AB-PAS. Solid arrows indicate areas of positive AB-PAS staining. a, Alveolus; e, epithelium. Quantification of volume density of mucous (in nl/mm2) from AB-PAS immunohistochemistry from saline (white bars) and OVA (black bars) control and PN4 HIF-1α-deficient mice was performed as described in materials and methods (E).
OVA-induced inflammatory changes were microscopically more prominent in the lungs of PN4 HIF-1α-deficient mice compared with similarly treated HIF-1α-sufficient mice. Interestingly, the targeted depletion of HIF-1α in ATII cells and Clara cells resulted in an extension of the OVA-induced eosinophilic and mononuclear inflammatory response into the proximal alveolar ducts and adjacent alveolar parenchyma deep in the lung (Fig. 3) in these mice. HIF-1α deficiency also enhanced the inflammatory response around small diameter airways (i.e., preterminal and terminal bronchioles). The allergen-induced eosinophilic alveolitis was also reflected in the statistically significant increase in eosinophils and other inflammatory cells in the BALF from the PN4 HIF-1α-deficient mice.
Lung resistance in allergic airways.
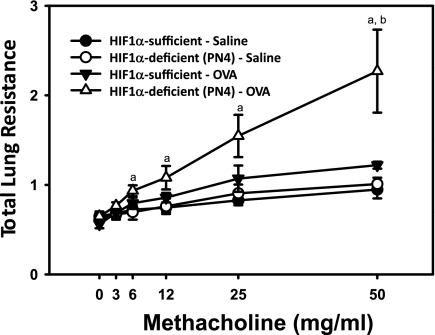
In addition to the histopathology data, treatment and HIF-1α-related differences in total lung resistance were determined. In the control mice, methacholine-induced resistance was not statistically different between saline and OVA challenge, although a modest trend for OVA-induced enhancement was apparent (Fig. 5). Following developmental recombination of HIF-1α, the PN4 HIF-1α-deficient, OVA-treated mice had the highest airway resistance of the treatment groups. At the highest dose of methacholine, the PN4 HIF-1α-deficient, OVA-treated mice were significantly different than control mice treated with OVA. At the 6, 12, 25, and 50 mg/ml methacholine doses, the PN4 HIF-1α-deficient, OVA-treated group had a significantly higher airway resistance than their genotype controls.
Fig. 5.
Total lung resistance. Allergic airway resistance (cmH2O·s·ml−1) was measured using a Flexivent protocol in HIF-1α-sufficient (black) and PN4 HIF-1α-deficient (white) mice treated with saline (circles) or OVA (triangles). a, Significantly different from HIF-1α-deficient (PN4)/saline group; b, significantly different than HIF-1α-sufficient/OVA group; ANOVA (P < 0.05).
Characterization of PN32 HIF-1α-deficient mice.
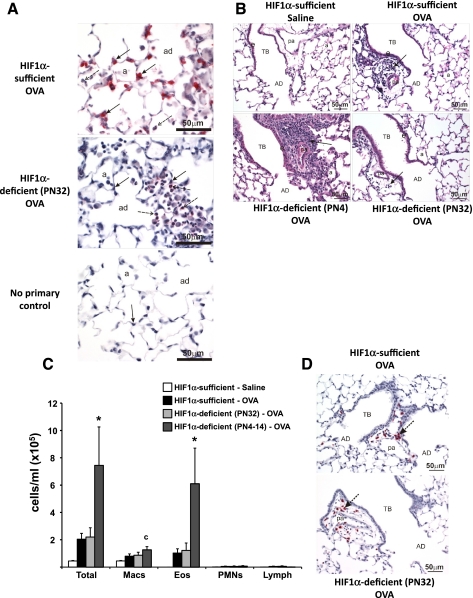
Administration of DOX for 10 days (PN32-PN42) was adequate to induce recombination and significantly reduce HIF-1α levels in Clara and type II cells (Fig. 6A). This level of recombination was similar to that observed in PN4 mice (27). In contrast with the PN4 HIF-1α-deficient mice, the enhanced allergic inflammatory response in the lung, as compared with OVA-treated HIF-1α-sufficient mice, was not evident in the PN32 HIF-1α-deficient mice similarly treated with OVA. For example, a minimal mixed inflammatory cell infiltrate is present around the terminal bronchiole and associated pulmonary artery in the OVA-treated HIF-1α-sufficient and -deficient PN32 mice. In contrast, there is a marked peribronchiolar and -vascular inflammatory cell infiltrate in the OVA-treated HIF-1α-deficient PN4 mouse. No centriacinar inflammation is present in the HIF-1α-sufficient mouse. (Fig. 6B). The total and differential cell counts in BALF and MBP staining confirmed the similar pattern of inflammation between HIF-1α-sufficient and PN32 HIF-1α-deficient OVA-treated mice (Fig. 6, C and D). The makeup of BALF in these PN32 HIF-1α-deficient, OVA-treated mice was ∼50% eosinophils and 50% macrophages, which is not significantly different from OVA-treated, HIF-1α-sufficient mice. Loss of HIF-1α after completion of the alveolarization stage in the OVA model had no noticeable effect upon the tissues when analyzed via AB-PAS (data not shown). The PN32 mice were exposed to DOX for 10 days. In contrast, the PN4 mice were exposed for 28 days, and this difference might explain the lack of phenotype in the PN32 mice. To test this possibility, mice were exposed to DOX from PN4 to PN14. These PN4–14 mice displayed an exacerbated response to OVA treatment, similar to PN4 mice (Fig. 6C). These results suggest that loss of HIF-1α during the first two weeks of postnatal alveolarization impacts the innate immunity of the lung.
Fig. 6.
HIF-1α immunohistochemistry, hematoxylin and eosin (H&E), and BALF cellularity for PN32 and PN4–14 mice. HIF-1α immunohistochemistry of HIF-1α-sufficient (top) and PN32 HIF-1α-deficient (middle) mice after OVA sensitization and challenge is shown. No primary antibody control for HIF-1α from a HIF-1α-sufficient, saline-treated mouse is also pictured (bottom). Solid arrows indicate alveolar type II cells and dashed arrows indicate macrophages (A). Light photomicrographs of a centriacinar region from the lungs of HIF-1α-sufficient (top left and right) and HIF-1α-deficient PN4 (bottom left) and P32 (bottom right) mice treated with saline (top left) or OVA (top right and bottom left and right). Tissues were stained with H&E. Arrows indicate areas of cellular infiltrate (B). HIF-1α-sufficient (white and black bars), PN32 HIF-1α-deficient (light gray bars), and PN4–14 HIF-1α-deficient (dark gray bars) mice were sensitized/challenged with saline (white bars) or OVA (black, light gray, and dark gray bars). Total cells, as well as the numbers of macrophages (Macs), eosinophils (Eos), neutrophils (PMNs), and lymphocytes (lymph) were determined from BALF (C). Values are the mean ± SE (n ≥ 7). *Significantly different from all other groups P < 0.05; c, significantly different from saline treated. MBP immunohistochemistry performed on PN32 HIF-1α-deficient (bottom) verifying the lack of a significant increase in eosinophils in these OVA-treated lungs as compared with HIF-1α-sufficient (top) mice is shown. Arrows indicate similar areas of eosinophilic infiltration surrounding the pulmonary artery (D). AD, alveolar duct; a, alveolus; TB, terminal bronchiole; pa, associated pulmonary artery.
Determination of cytokines in BALF.
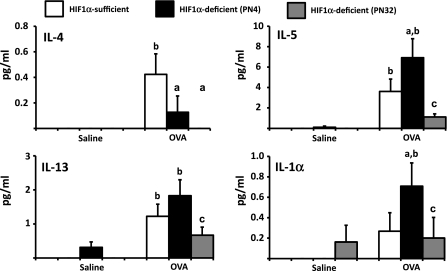
The cellular and histopathological results suggest that loss of HIF-1α during postnatal development of the lung exacerbated OVA-induced inflammation, whereas loss after this developmental stage resembled control animals in response to OVA. To determine whether these enhanced responses were correlated with increased production of Th2 type cytokines, the concentrations of IL-2, IL-4, IL-5, IFN-γ, TNF-α, IL-3, keratinocyte chemoattractant (KC), and IL-1β were analyzed from BALF. From this panel of cytokines, IL-4, IL-5, IL-13, and IL-1β showed significant differences between treatments or DOX exposure paradigms (Fig. 7). IL-5 and IL-1β showed significant increases in BALF from OVA-treated PN4 HIF-1α-deficient mice when compared with the OVA-treated HIF-1α-sufficient controls. A similar pattern was not observed in the PN32 HIF-1α-deficient mice. IL-4 showed a significant decrease in BALF from OVA-treated PN4 and PN32 HIF-1α-deficient mice compared with the OVA-treated HIF-1α-sufficient mice. In contrast, IL-13 showed significant increases in BALF of both HIF-1α-sufficient and PN4 HIF-1α-deficient OVA treated mice compared with their respective saline controls. This pattern was replicated in the PN32 HIF-1α-deficient mice; however, the levels did not reach significance. Taken together, these results suggest that the enhanced inflammation observed following deletion of HIF-1α is dependent upon the developmental timing of the deletion, and this enhanced response is evident at the level of cellular infiltrate, functional responses, and cytokine production.
Fig. 7.
The effect of OVA on cytokine levels. Cytokine levels in BALF were determined using a Th1/Th2 bead array panel (BD Bioscience). Samples were collected from HIF-1α-sufficient (white bars), PN4 HIF-1α-deficient (black bars), and PN32 HIF-1α-deficient (gray bars) mice sensitized/challenged with saline or with OVA. Data is represented as means ± SE (n ≥ 9). a, Significantly different from HIF-1α-sufficient within treatment, P < 0.05; b, significantly different from saline treatment within genotype, P < 0.05; c, significantly different from PN4 HIF-1α-deficient within treatment, P < 0.05.
DISCUSSION
Asthma is a complex disease that involves interactions between the various cell types of the lung, the cytokines they produce, as well as the genetics and other predisposition factors of the individual. Allergic asthma is characterized by various pulmonary pathologic features including eosinophilic inflammation in tracheobronchial airways, mucus cell metaplasia of airway epithelium, airway hyperreactivity, and the presence of certain cytokines related to a Th2-mediated response. Although recent literature has demonstrated a role for hypoxia and HIF-mediated signaling in inflammation, little is known about their role in the etiology of asthma (6, 20, 32). The results presented here suggest that HIF-1α, specifically in the epithelial cells of the lung, plays a central role in establishing the immunity of the lung. Loss of HIF-1α led to an enhanced response to OVA sensitization and challenge. This was evidenced by the significant increase in eosinophils and airway hyperreactivity, as well as the extension of the eosinopilic inflammatory response into the proximal alveolar ducts and adjacent alveolar parenchyma deep in the lung. This exacerbation of the inflammatory and functional responses did not include a significant increase in mucus cell metaplasia, suggesting the signal responsible for this phenotype is HIF-1α-independent. Finally, the phenotypic changes were dependent upon the developmental timing of HIF-1α deletion, but not dependent on the length of DOX treatment.
The interesting difference in responses to OVA seen between the PN4 and PN32 mice suggests that HIF-1α-derived from the alveolar epithelium, Clara, and ATII cells specifically, is required during early postnatal development of the lung to establish the proper immune responses of tissues to allergens. Our findings suggest that early events in postnatal development induce HIF-1α to direct proper immune maturation. For example, early exposure to inhaled bacteria and allergens will induce inflammation that can lead to HIF-1α signaling via changes in local oxygen levels and the production of cytokines (21, 22, 35). As such, HIF-1α signaling might contribute to airway immune development during early-life exposure to pathogens. In a model of influenza, it has been shown that neonatal hyperoxia exacerbates the response of the mouse to influenza as an adult (27). This was apparent by increased leukocyte recruitment to the lungs and increased proinflammatory cytokines, similar to what was observed in the P4 HIF-1α-deficient mice.
Recently, the role of the airway epithelium in allergen-induced airway disease has started gaining attention (10, 18). This interest is driven, at least in part, by the variability or lack of efficacy of treatments that target the inflammatory cells (13, 16, 24, 26). These conflicting findings suggest that targeting inflammatory cells, such as eosinophils, is not productive for a broad range of asthmatics and other cell types must be considered. If it is viewed in this way, it might be possible to target HIF-1α signaling early in development for at-risk patients, such as preterm infants, and lessen the severity of allergic airway diseases.
In other studies, mice deficient in hypoxia signaling are partially protected from OVA-induced inflammation (14, 19). In these models, the deficiency in hypoxia-mediated signaling occurs in all of the cells of the mouse, including macrophages. As described above, macrophages require HIF-1α for proper function and this function plays a role in allergen-induced airway disease (6, 25). In contrast, the HIF-1α-deficient mice described here have targeted deficiencies in Clara and ATII cells only. These cells act as a signaling mediator between macrophages and dendritic cells and are critical to the progression of airway disease and remodeling (23). Taken together, these results suggest that global loss of HIF-1α protects against allergen-induced lung damage, most likely through inhibiting HIF-1α signaling in inflammatory cells, whereas specific loss from alveolar epithelium during postnatal development results in an increased allergic airway hyperreactivity phenotype.
The opposite signaling cascade would be true for oxygen-dependent preterm infants. There is extensive literature linking premature birth and an increased asthma susceptibility (8, 9, 12). This link between prematurity and asthma is complicated and involves many different facets, including abnormal lung development and cellular differentiation, bronchopulmonary dysplasia, and abrupt and severe changes in the oxygen exposure to the lungs of the neonate. Although complicated, it is interesting to hypothesize that the premature birth will expose the lungs to ambient levels of oxygen prior to the maturation of the tissue. This premature hyperoxia might inhibit normal HIF-1α-mediated signaling that is essential for normal fetal lung development (1, 30). Moreover, this hyperoxic environment, especially in those infants placed on ventilators with oxygen levels higher than ambient air, might act to functionally remove HIF-1α signaling from the processing of environmental signals necessary to develop a normal immunity. Functionally, this would mirror the results demonstrated in the PN4 HIF-1α-deficient mice. In fact, small molecule-induced stimulation of the HIF-1 signaling cascade during hyperoxia can alleviate some of the structural deficits induced by neonatal hyperoxia exposure (2). Clinicians, therefore, might explore the relationship between oxygenation, HIF-1α-mediated changes in inflammation, and susceptibility to chronic lung disease in preterm infants (15, 33). It should be noted that the link between preterm birth and changes in allergen-induced inflammation might only be beneficial in certain conditions. A recent report has demonstrated a decreased risk of allergic rhinitis is correlated with a low gestational age at birth (7).
In summary, the results from our current study indicate that HIF-1α plays a role in programming the ability of the immune system to respond to insults, particularly aerosolized allergens. Only when HIF-1α is deleted from the epithelial cells of the lung in a narrow window postnatally (PN4–14) is an exacerbation of the Th2-mediated response observed. Other studies have implicated neonatal hyperoxia in affecting the programming of the immune system, which implies that because HIF-1α is responsive to oxygen, low levels of HIF-1α may have this same effect, as would the mice in our study that lack HIF-1α in their lung epithelial cells. Further investigation into the way in which the immune system is altered and the signaling involved in this change is needed to determine a mechanism for this alteration.
GRANTS
Funding was obtained from National Institutes of Health Grant Number R01ES-12186.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.K.G., S.P.P., Y.S., D.N.J.-H., J.G.W., J.R.H., and J.J.L. conception and design of research; K.K.G., S.P.P., Y.S., L.A.B., D.N.J.-H., J.G.W., J.R.H., and J.J.L. performed experiments; K.K.G., S.P.P., L.A.B., D.N.J.-H., J.G.W., J.R.H., and J.J.L. analyzed data; K.K.G., S.P.P., D.N.J.-H., J.G.W., J.R.H., and J.J.L. interpreted results of experiments; K.K.G., J.G.W., J.R.H., and J.J.L. prepared figures; K.K.G., S.P.P., J.R.H., and J.J.L. drafted manuscript; K.K.G., S.P.P., Y.S., J.G.W., J.R.H., and J.J.L. edited and revised manuscript; K.K.G., S.P.P., Y.S., L.A.B., D.N.J.-H., J.G.W., J.R.H., and J.J.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Randall Johnson (University of California San Diego) for the conditional HIF-1α mice, Dr. Jeffrey A. Whitsett (Cincinnati Children's Hospital Medical Center) for the SP-C-rtTA/(tetO)7-CMV-Cre transgenic, and the Michigan State University Investigative Histopathology Lab for expertise and technical assistance with immunohistochemistry.
REFERENCES
- 1. Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia-inducible factors and vascular endothelial growth factor in primate lungs. Pediatr Pulmonol 40: 538– 546, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am J Physiol Lung Cell Mol Physiol 291: L588– L595, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc 6: 301– 305, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med 357: 2016– 2027, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2[alpha] and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702– 710, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1± is essential for myeloid cell-mediated inflammation. Cell 112: 645– 657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and risk of allergic rhinitis in young adulthood. J Allergy Clin Immunol 127: 1173– 1179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics 127: e913– e920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doyle LW. Respiratory function at age 8–9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol 41: 570– 576, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fahy JV, Locksley RM. The airway epithelium as a regulator of Th2 responses in asthma. Am J Respir Crit Care Med 184: 390– 392, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Farraj AK, Harkema JR, Jan TR, Kaminski NE. Immune responses in the lung and local lymph node of A/J mice to intranasal sensitization and challenge with adjuvant-free ovalbumin. Toxicol Pathol 31: 432– 447, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 182: 237– 245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, Robinson D, Wenzel S, Busse W, Hansel TT, Barnes NC. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 176: 1062– 1071, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Guo J, Lu W, Shimoda LA, Semenza GL, Georas SN. Enhanced interferon-gamma gene expression in T cells and reduced ovalbumin-dependent lung eosinophilia in hypoxia-inducible factor-1-alpha-deficient mice. Int Arch Allergy Immunol 149: 98– 102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagan R, Minutillo C, French N, Reese A, Landau L, LeSouef P. Neonatal chronic lung disease, oxygen dependency, and a family history of asthma. Pediatr Pulmonol 20: 277– 283, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 360: 973– 984, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harkema JR, Plopper CG, Hyde DM, St George JA, Dungworth DL. Effects of an ambient level of ozone on primate nasal epithelial mucosubstances. Quantitative histochemistry. Am J Pathol 127: 90– 96, 1987 [PMC free article] [PubMed] [Google Scholar]
- 18. Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol 120: 1233– 1244, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Huerta-Yepez S, Baay-Guzman GJ, Bebenek IG, Hernandez-Pando R, Vega MI, Chi L, Riedl M, Diaz-Sanchez D, Kleerup E, Tashkin DP, Gonzalez FJ, Bonavida B, Zeidler M, Hankinson O. Hypoxia inducible factor promotes murine allergic airway inflammation and is increased in asthma and rhinitis. Allergy 66: 909– 918, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120: 2699– 2714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 17: 2115– 2117, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. J Biochem 370: 1011– 1017, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 376: 835– 843, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356: 2144– 2148, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol 42: 595– 603, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 360: 985– 993, 2009 [DOI] [PubMed] [Google Scholar]
- 27. O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med 177: 1103– 1110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 99: 10482– 10487, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saini Y, Greenwood KK, Merrill C, Kim KY, Patial S, Parameswaran N, Harkema JR, LaPres JJ. Acute cobalt-induced lung injury and the role of hypoxia-inducible factor 1alpha in modulating inflammation. Toxicol Sci 116: 673– 681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saini Y, Harkema JR, LaPres JJ. HIF1α is essential for normal intrauterine differentiation of alveolar epithelium and surfactant production in the newborn lung of mice. J Biol Chem 283: 33650– 33657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saini Y, Kim KY, Lewandowski R, Bramble L, Harkema JR, LaPres JJ. The role of hypoxia inducible factor 1 (α) in modulating cobalt-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 298: L139– L147, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol 5: 712– 721, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol 3: 1088– 1100, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whitsett JA, Perl AK. Conditional control of gene expression in the respiratory epithelium: a cautionary note. Am J Respir Cell Mol Biol 34: 519– 520, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Zhou J, Schmid T, Brüne B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol Biol Cell 14: 2216– 2225, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]