Abstract
The heritable form of pulmonary arterial hypertension (PAH) is typically caused by a mutation in bone morphogenic protein receptor type 2 (BMPR2), and mice expressing Bmpr2 mutations develop PAH with features similar to human disease. BMPR2 is known to interact with the cytoskeleton, and human array studies in PAH patients confirm alterations in cytoskeletal pathways. The goal of this study was to evaluate cytoskeletal defects in BMPR2-associated PAH. Expression arrays on our Bmpr2 mutant mouse lungs revealed cytoskeletal defects as a prominent molecular consequence of universal expression of a Bmpr2 mutation (Rosa26-Bmpr2R899X). Pulmonary microvascular endothelial cells cultured from these mice have histological and functional cytoskeletal defects. Stable transfection of different BMPR2 mutations into pulmonary microvascular endothelial cells revealed that cytoskeletal defects are common to multiple BMPR2 mutations and are associated with activation of the Rho GTPase, Rac1. Rac1 defects are corrected in cell culture and in vivo through administration of exogenous recombinant human angiotensin-converting enzyme 2 (rhACE2). rhACE2 reverses 77% of gene expression changes in Rosa26-Bmpr2R899X transgenic mice, in particular, correcting defects in cytoskeletal function. Administration of rhACE2 to Rosa26-Bmpr2R899X mice with established PAH normalizes pulmonary pressures. Together, these findings suggest that cytoskeletal function is central to the development of BMPR2-associated PAH and that intervention against cytoskeletal defects may reverse established disease.
Keywords: bone morphogenic protein receptor type 2, cytoskeleton, Rho-GTPase
pulmonary arterial hypertension (PAH) is a severe and progressive disease characterized by obstruction of small pulmonary arteries leading to increased pulmonary vascular resistance and right heart failure. Mutations in bone morphogenic protein receptor type 2 (BMPR2) are present in ∼80% of hereditable PAH (8, 23), and BMPR2 expression is decreased in PAH patients without BMPR2 mutations (33). However, the mechanism by which BMPR2 mutations cause PAH is unknown, as is the relevant cell type: BMPR2 mutations specific to both smooth muscle or endothelium are capable of causing PAH (43, 47).
Cytoskeletal dysfunction in pulmonary vascular cells may contribute to BMPR2-associated PAH. BMPR2 directly binds and modulates proteins related to cytoskeletal organization, including LIMK, TCTEX, and SRC (10, 27, 44), and has been shown to regulate cytoskeletal functions including adhesion (5) and migration (11).
Human PAH patients demonstrate significant alterations in cytoskeleton function on transcriptome-wide expression arrays, including two studies of whole lung (12, 33), and protein and expression array studies on both fresh and cultured patient lymphocytes (30, 40, 42). The Giessen group found dysregulation of Rho GTPases as the central feature in expression profiling of laser-dissected pulmonary arteries from PAH patients (25). Rho GTPases (Rac1, Cdc42, and RhoA) are part of the Ras superfamily of small signaling G proteins. They play a central role in cytoskeletal function controlling actin and microtubule dynamics, wound healing, cell polarization, cell migration, cell adhesion, and angiogenesis (2, 6, 21, 37).
Cytoskeletal defects are thus broadly seen in PAH patients and could be mechanistically linked to BMPR2 dysfunction. The goal of this study was to evaluate whether cytoskeletal dysfunction contributes to BMPR2-associated PAH. The sequence of experiments to test the hypothesis that BMPR2 mutations produced PAH through defects in cytoskeletal function was 1) create and validate a mouse model of a human Bmpr2 mutation (Rosa26-Bmpr2R899X); 2) analyze mRNA expression in whole lung of control and Rosa26-Bmpr2R899X mice for cytoskeletal changes; 3) test for abnormalities in cytoskeletal activity (Rac1 activity), architecture (actin, microtubules, adherens junctions), and function (wound healing) in Bmpr2 mutants; and 4) reverse both cytoskeletal abnormalities and pulmonary hypertension with angiotensin-converting enzyme 2 (ACE2).
ACE2 is an enzyme within the renin-angiotensin system and converts angiotensin II (ANG II) to ANG-(1–7). We selected ACE2 for our cytoskeletal intervention because it regulates Rac1 activation through ANG-(1–7) binding of the Mas1 receptor and has been shown to regulate angiogenesis and vascular permeability (18, 26, 50).
MATERIALS AND METHODS
Rosa26-Bmpr2R899X Phenotype
A transgenic mouse strain containing the modified reverse tetracycline transactivator, rtTA2-M2, driven by an 812 base pair segment of the ubiquitous promoter Rosa26 was created (20). These Rosa26-rtTA2 (Rosa26-control) mice were crossed to our previously described TRE-Bmpr2R899X transgenic mice to create an animal (Rosa26-rtTA2 X TetO7-Bmpr2R899X) in which universal expression of the Bmpr2R899X transgene could be induced by adding doxycycline to the diet (43). Male and female mice received doxycycline at 1 g/kg in chow for 1, 4, or 8 wk, and right ventricular systolic pressure (RVSP) was measured by closed-chest right heart catheterization at these time points. RVSP was directly measured via insertion of a 1.4F Mikro-tip catheter transducer (Millar Instruments Houston, TX) into a surgically exposed right internal jugular vein as previously described (43). The Institutional Animal Care and Use Committees at University of Colorado Health Sciences Center and Vanderbilt University approved all animal studies.
Affymetrix Arrays
Mouse Genome 430 2.0 microarrays (Affymetrix, Foster City, CA) were performed at 1 wk of gene activation in Rosa26-control Rosa26-Bmpr2R899X mice with normal RVSP as previously described (22). Each array consisted of a pool of two to three mice, and two arrays were used per condition. Gene arrays were also performed on RNA from 1) Rosa26-control with vehicle; 2) Rosa26-control with recombinant human angiotensin-converting enzyme 2 (rhACE2); 3) Rosa26-Bmpr2R899X mice with vehicle; and 4) Rosa26-Bmpr2R899X mice with rhACE2 after gene activation for 4 to 6 wk. Array results were submitted to the National Center for Biotechnology Information (NCBI) gene expression and hybridization array data repository (GEO, http://www.ncbi.nlm.nih.gov/geo/), accession no. GSE21583.
Generation of Murine Pulmonary Microvascular Endothelial Cells
Immortomouse X Rosa26-rtTA2 X TetO7-Bmpr2R899X triple transgenic mice were bred with transgenes verified by PCR genotyping of tail DNA. The immortomouse contains a transgenic insertion of the SV40 large T antigen, tsA58, under control of an interferon-inducible promoter (19). Addition of the immortomouse transgene was used to allow longer-term culture of pulmonary microvascular endothelial cells (PMVEC), which normally have very limited passage number. PMVEC were collected from adult mice as previously described (32) and verified by staining for endothelial markers von Willebrand factor (vWF), PECAM, and VE-cadherin.
Generation of Stably Transfected PMVEC and Vascular Smooth Muscle Cells
The stably transfected A7r5 vascular smooth muscle cell lines were previously generated and described (22), and human PMVEC were made in a similar fashion. Briefly, cells were transfected with either empty vector (native) or vector containing wild-type BMPR2 (WT), or BMPR2 with C118W (extracellular domain, ED), R332X (kinase domain, KD), or 2579–2580delT (cytoplasmic domain, CD) mutations and stably selected using G418S.
PCR
cDNA was made using QuantiTect Reverse Transcription Kit (Qiagen) from 1 μg total RNA. Quantitative real-time PCR was performed using a total reaction volume of 25 μl containing 5 μl of diluted cDNA, 10.0 μl SYBR Green Supermix (Applied Biosystems, Foster City, CA), and 0.03 μl of each oligonucleotide primer (250 μM). PCR was carried out in a StepOnePlus Real Time PCR System (Applied Biosystems) using 40 cycles of 95°C for 15 s followed by 60°C for 1 min with a 10-min 95°C initial soak. Each measurement was made in triplicate and is expressed relative to the detection of the standard hypoxanthine-guanine phosphoribosyltransferase (HPRT). PCR was performed for the primer sets HPRT, rtTA2, and R899X (Integrated DNA Technologies IDT, Coralville, IA).
Histology
Hematoxylin and eosin staining was performed on paraffin-embedded lung sections from Rosa26-Bmpr2R899X mice. Elastin staining using Verhoeff's elastic stain kit was performed. Immunohistochemistry on paraffin-embedded lung tissue for Rosa26-Bmpr2R899X included the following antibodies: α-smooth muscle actin (ab5694, Abcam, Cambridge, MA), CD45 (sc25590, Santa Cruz, CA), and tenascin-C (sc20932, Santa Cruz).
Western Blot Analysis
Mouse lungs or PMVEC were homogenized in RIPA buffer (PBS, 1% Ipegal, 0.5% sodium deoxycholate, 0.1% SDS) with proteinase and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). Protein concentration was determined by Bradford assay. Primary antibodies used for Western blot included phospho-cofilin [3313, Cell Signaling Technologies (CST)], phospho-Src (2101, CST), phospho-Mypt1 (07-251, Upstate), VE-cadherin (ab8227, Abcam), and β-actin (ab8227, Abcam).
Rac1 Activity
Rac1 activity was determined using the EZ-Detect Rac1 Activation Kit (Pierce, Rockford, IL) in A7r5 vascular smooth muscle cells (Global Bioresource Center, Manassas, VA) and human PMVEC (gift from Rizwan Hamid, Vanderbilt University) transfected with wild-type BMPR2 or one of the BMPR2 mutations, cytoplasmic domain truncation (CD), kinase domain truncation (KD), or extracellular domain (ED). All three of these result in loss of some cytoplasmic tail domain signaling, but the CD mutation leaves SMAD signaling intact. The Rac1 Activation Kit uses glutathione S-transferase-fusion protein, containing the p21-binding domain of human p21-activated protein kinase 1 (Pak1), to pull-down active Rac1. Cells were grown to confluence, protein concentration was determined by Pierce BCA protein assay, and 500 μg of protein were used for each sample. In rhACE2-treated cells, 100 ng/ml ACE2 (R&D, Minneapolis, MN) was added 30 min before lysis. ImageJ software (National Institutes of Health, Bethesda, MD) was used for densitometry. Rac1 activity in whole mouse lung was measured using a homogenized lobe of the right lung of Rosa26-control or Rosa26-Bmpr2R899X mice treated with doxycycline for 4 wk, with or without two additional weeks of rhACE2 (Apeiron Biologics) via osmotic pump.
Immunohistochemistry
Immorto-Bmpr2R899X and immorto-control PMVEC were collected from adult mice as previously described (32) and verified by staining for endothelial markers PECAM (Sc-1506, Santa Cruz) and vWF (A0082, Dako, Denmark). Cells were stained with phalloidin TRITC (p1951, Sigma-Aldrich), tubulin (ab15246, Abcam), and VE-cadherin (Sc-28644, Santa Cruz).
Micro-X-ray Computed Tomography
Microfil (Flow Tech MV-122 Yellow) was mixed at 2.5 ml diluent, 2 ml compound, and 0.225 ml curing agent immediately before use. Immediately after euthanasia, the mouse chest wall was opened, the left atrium was removed, and PBS was perfused through the right ventricle and lungs until clear (∼5 ml) using a constant volume pump (5 ml/min). A stopcock was switched to the microfil line, and 2 ml microfil were infused at 0.25 ml/min. Pulmonary arteries and veins were clamped to prevent backflow, and the lungs were packed with ice for 30 min to allow the microfil to cure. After 30 min, the lungs were inflated with 0.8% agarose through the trachea and then fixed in 10% formalin at 4°C. The postcaval lobe of the right lung was detached for micro-X-ray computed tomography (microCT) imaging using a μCT40 (Scanco Medical, Bassersdorf, Switzerland). Images were acquired with a 8 μm voxel size at 45 kV, 114 μA, 1,000 projections per 180° rotation, 300 ms integration, and 2× frame averaging to reduce noise. An optimized density threshold and Gaussian noise filter were applied to the three-dimensional stack of images to segment the contrast-filled vessels from soft tissue and air and create a three-dimensional reconstruction of the entire vascular network. Analysis of vascular architecture was performed with the manufacturer's software using methodology similar to previous reports (38). Vessel thickness was calculated by direct thickness determination (16). Briefly, a sphere was fitted within each point of the cast reconstruction, the diameter of the sphere was recorded as vessel thickness at that point, and the distribution of all vessels in the vascular tree was compared between genotypes. Use of distance ridge calculation allows for inclusion of local neighboring voxels; while this method does not eliminate redundant points, it ensures that all points are included in the analysis. This calculation has no structural assumption.
Evans Blue
Mice were placed supine under anesthesia (Avertin) on a warm dissection table, and the internal jugular vein was exposed. One hundred microliters of 2% sterile Evans Blue in 1× PBS was injected into the jugular vein, and the Evans Blue dye circulated for 30 min while the mice were under anesthesia. The mice were then euthanized with a lethal dose of phenobarbital and the chest cavity was opened. Eight milliliters of 1× PBS was infused into the right ventricle. The lungs were then inflated with agarose, placed in 10% formalin, and photographed at 48 h.
Electron Microscopy
Lungs from Rosa26-rtTA2 (control) mice or Rosa26-rtTA2 X TetO7CMV-Bmpr2R899X mice were inflated and fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, then washed and postfixed in 1% aqueous osmium tetroxide. Following fixation, the samples were dehydrated through a graded series of ethanols to 100%. The samples were then embedded in Spurr resin. Thin sections of the cured resin were viewed using an FEI CM12 transmission electron microscope operated at 80 KeV. Lung sections containing small vessels were selected (away from broncus and large arteries) for electron microscopy. Pulmonary vessels were identified by presence of blood, which was not flushed for this experiment.
rhACE2 Treatment
Seventy-nine five-month-old female Rosa26-control and Rosa26-Bmpr2R899X mice were fed oral doxycycline for gene activation for 4 and 6 wk. During gene activation with doxycycline, mice received either rhACE2 (1.2 mg·kg−1·day−1, Apeiron Biologics) or vehicle (pH 7.5, 100 mM glycine, 150 mM NaCl, 50 μM ZnCl2, Apeiron Biologics) through a micro-osmotic pump for two additional weeks and then underwent two-dimensional echocardiography and right heart catheterization. ACE2 activity was measured in mouse plasma using the fluorescent peptide substrate Mca-Ala-Pro-Lys (Dnp)-OH. Cleavage was measured in diluted samples using excitation and emission wavelengths of 320 and 430 nm, respectively, in the presence of 100 μM substrate in 50 mM MES, 300 μM NaCl, 10 μM ZnCl2, and 0.01% Brij-30 at pH 6.5. Evaluation was performed by comparing the maximal slope of the fluorescence/time curves to respective maximal slopes of a serial rhACE2 dilution in normal mouse serum. The ACE2 specific inhibitor DX600 was used to confirm ACE2 specificity of the assay. ELISA was a sandwich ELISA using human ACE2-specific antibodies, and immune response was detected and quantified via ELISA. rhACE2 was coated on plates, and specific humoral immune response was quantified by using mouse IgG- or IgM-specific detection antibodies.
Statistics
Overrepresentation of KEGG groups and gene ontology groups was determined using the hypergeometric test within the WebGestalt program (48). Other statistical tests were either one-way or two-way ANOVA with post hoc Fisher's least significant difference test performed using the JMP program (SAS, Cary, NC).
RESULTS
Rosa26-Bmpr2R899X Mice Phenotype
To study the molecular effects of Bmpr2 mutation in whole lung without dilution effects, we generated a transgenic mouse strain containing the modified reverse tetracycline transactivator, rtTA2-M2, driven by the ubiquitous promoter Rosa26 (20). These Rosa26-rtTA2 mice were crossed to our previously described TRE-Bmpr2R899X transgenic mice, producing an animal in which universal expression of the Bmpr2R899X transgene (Rosa26-Bmpr2R899X) could be induced by addition of doxycycline to the diet (43). To verify the mouse model, quantitative RT-PCR for both rtTA2-M2 and Bmpr2R899X transgenes was tested in the double transgenic mice with 1 wk of induction and compared with uninduced animals. Statistically even expression of both transgenes across tissues was observed, with a median of 17× induction of Bmpr2R899X transgene expression with doxycycline (49) (data not shown).
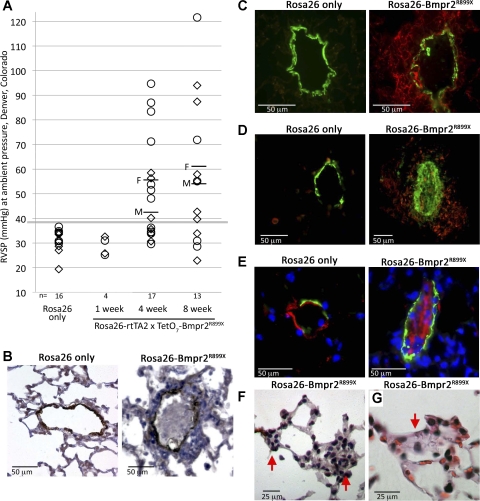
To characterize pulmonary hypertension in Rosa26-Bmpr2R899X mice, a time course of induction was performed. Male and female mice received doxycycline at 1 g/kg in chow for 1, 4, or 8 wk, and RVSP was measured by closed-chest right heart catheterization at these time points. With 1 wk of induction, there was no change in RVSP. By 4 wk, roughly half had increased RVSP, and by 8 wk, roughly three-fourths had elevated RVSP (Fig. 1A). There was no significant difference in systemic blood pressure by tail cuff or cardiac output by echocardiography between Rosa26-control and Rosa26-Bmpr2R899X mice in this experiment. The variable penetrance may relate to stochastic processes involved in the precise level of occlusion of vessels, diet, or inflammatory burden.
Fig. 1.
A: Rosa26-Bmpr2R899X mice have elevated right ventricular systolic pressure (RVSP) by 4 wk of transgene activation (P < 0.01 by ANOVA, Kruskal-Wallis). Each symbol indicates a measurement from an individual mouse; males (M) are marked with diamonds and females (F) are marked with circles. Average male and female RVSP are marked with horizontal lines for 4- and 8-wk time points. Measurements were made at Denver, CO, altitude; thus all animals have a slightly elevated RVSP compared with Nashville, TN, measurements in Fig. 6. B: example of a complex lesion in Rosa26-BMPR2R899X mice, including occlusion of the intima by nucleated cells and a greatly thickened adventitia; stain is for actin, with counterstaining by hematoxylin and eosin. C: adventitial lesions are surrounded by tenascin-C (red, right), normally not visible in control animals (left). Actin is in green. D: cells filling the lumen can be actin positive (green); adventitia usually contain a large number of CD45+ cells, presumably of circulating origin (red). E: cells filling the lumen can be von Willebrand factor (vWF) positive (red), presumably endothelial cells. F and G: far more common than the complex lesions above is the occlusion (partial or complete) of very small vessels with cells of unknown origin; two examples are indicated by red arrows. These simple alterations of very small vessels are found in every Rosa26-BMPR2R899X animal, usually with many instances per 10× field.
Some Rosa26-Bmpr2R899X mice developed complex vascular lesions in small- to medium-sized pulmonary vessels comparable to human PAH patients (Fig. 1B). These lesions showed increased tenascin-C similar to human lesions (Fig. 1C) and a large number of inflammatory cells (Fig. 1D) (17). The cells occluding the lumen included smooth muscle cells (Fig. 1D) and endothelial cells (Fig. 1E). The cells occluding these vessels did not generally form into the well-organized structures seen in either human PAH patients or the SM22-only version of the Bmpr2R899X model (43). This failure to form well-organized structures may relate to expression of the mutation in circulating cells. However, these complex lesions were present in a small proportion of animals with elevated pressure and probably were a consequence of disease, not the cause. Much more common, and present in many vessels in every Rosa26-Bmpr2R899X mouse, was occlusion or obstruction of very small vessels with an unidentifiable cell type (Fig. 1F).
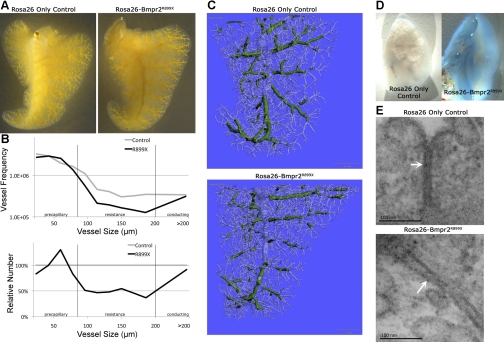
To quantify this occlusion or partial occlusion of vessels, we perfused the lung vasculature with a CT contrast agent, Microfil (Fig. 2A). The postcaval lobe of the right lung was used for microCT imaging, because as the smallest lobe it would fit entirely within the imaging tube and remove artifacts associated with differences in the section of lung used. We were able to precisely quantify the perfusable vessels of each size, finding that there was a roughly 50% loss of resistance level arteries (Fig. 2B). The resolution of the microCT was not sufficient to determine loss of vessels <25 μm. Examination of the pattern of loss of perfusable area in resistance level arteries shows that, in general, there was not occlusion of entire vessels: rather, the vessels were partially obstructed at many points along their length, particularly at junctions (Fig. 2C).
Fig. 2.
Rosa26-Bmpr2R899X mice have a decrease in perfusable pulmonary vessel area compared with Rosa26-only controls. A: postcaval lobe of the right lung from Rosa26-only and Rosa26-Bmpr2R899X mice perfused with microfil, an X-ray computed tomography contrast agent. B: quantification of perfusable vessel size shows a loss of 50% of resistance level artery area. C: this loss of area is not primarily due to complete occlusion of vessels, but partial occlusion or narrowing at multiple points (vessels between 80 and 200 μm marked in green). Note that in Bmpr2R899X mice the green is discontinuous. D: Evans blue test of vascular leak shows that Bmpr2R899X mice have much higher levels of leak than do control mice. E: electron microscopy shows that Bmpr2R899X mice have larger gaps, indicated by white arrows, between adjacent pulmonary vascular endothelial cells than do control mice.
Finally, because evidence that BMPR2 mutation is associated with vascular leak has been accumulating for 40 years (5, 29), we tested vascular leak using Evans Blue. We found that vascular leak was dramatically worse in Rosa26-Bmpr2R899X mice than in Rosa26-only controls (Fig. 2D), associated with increased distance between pulmonary vascular endothelial cells in Rosa26-Bmpr2R899X mice as determined by electron microscopy (Fig. 2E).
Cytoskeletal Pathway Gene Expression Is Altered in Rosa26-Bmpr2R899X Mice
To determine the molecular consequences of the Bmpr2R899X mutation, we used Affymetrix gene arrays to measure gene expression in lungs of adult Rosa26-Bmpr2R899X and Rosa26-control mice with transgenes activated for 1 wk. This 1 wk time point was selected to allow the effects of activation of the mutation to be well established without confounding effects of increased pulmonary pressure.
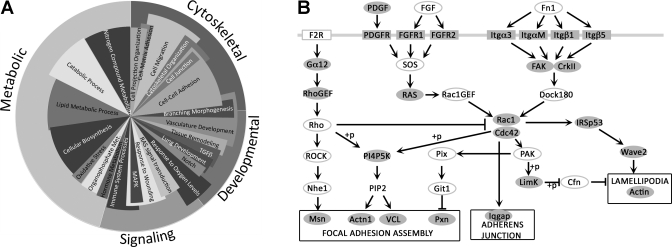
Changes in gene expression in whole lungs from Rosa26-Bmpr2R899X mice compared with Rosa26-controls were dramatic, with 832 genes changed more than 3× (false discovery rate of 0.1%) (Supplemental Table S1; Supplemental Material for this article is available on the Journal website). A total of 635 of 832 (76%) genes were sorted into statistically overrepresented gene ontology consortium biological process groups (1), and 588 of the 635 genes (93%) fit into one of four broad categories: cytoskeletal, signaling, developmental, and metabolic (Fig. 3A). Further examination of Rosa26-Bmpr2R899X gene expression data revealed a central disrupted pathway, the Ras/Rho GTPase/cytoskeletal pathway (Fig. 3B).
Fig. 3.
Genes dysregulated in Rosa26-Bmpr2R899X whole lung at 1 wk. A: pie chart showing relative numbers of genes in statistically significant dysregulated gene ontology groups. Outer circle (cytoskeletal, developmental, signaling, and metabolic) totals 588 genes with minimum 3× change that fall into statistically overrepresented top level gene ontology groups. Inner circle contains representative lower level ontology groups. Angular width of categories in the inner circle indicates number of genes altered. Radius is a relative indicator of significance, where the longest categories were significantly dysregulated at ∼P < 1 × 10−8, and the shortest categories were dysregulated at ∼P < 1 × 10−3 by hypergeometric test. Categories in the inner circle sum to >100%, because many genes are in more than one ontology group. B: there is extensive Bmpr2R899X-associated regulation of cytoskeleton (based on KEGG; 31, 48). Shaded genes show altered expression in Rosa26-Bmpr2R899X whole lung after 1 wk of activation (all decreased compared with controls, except actin expression increased). Genes marked with open ovals/rectangles do not have significant changes in mRNA expression. Phosphorylation designated as (+p).
Each of the other three broad categories, signaling, developmental, and metabolic, is also associated with cytoskeletal function. The signaling category includes wound healing and Ras and receptor-tyrosine kinase pathways upstream of Rho GTPases. Furthermore, some of the developmental genes are required for cytoskeletal development and planar-polarity (7). Lastly, metabolic problems may result from defects in the cytoskeleton's role in mitochondrial fission and fusion (22). In summary, the goal of this array study, to identify important pathways regulated by Bmpr2 mutation, implicates cytoskeletal pathways, in particular Rho GTPases, in the etiology of PAH.
These results, using unbiased analysis of expression arrays, support the hypothesis that BMPR2 regulates cytoskeletal dynamics in vivo.
Bmpr2 Mutation Produces Defects in Cytoskeletal Structure and Function in Cell Culture
Bmpr2 mutation produces defects in cytoskeletal architecture.
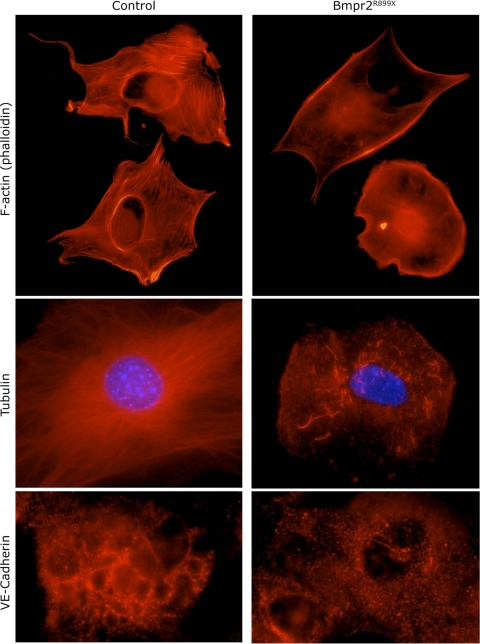
To determine functional consequences of the alterations in cytoskeletal genes seen in Rosa26-Bmpr2R899X whole lung, we moved to cell culture, using PMVEC isolated from control and Bmpr2R899X mice. We were interested in assays related to defects in angiogenesis or endothelial migration, because PAH in the Bmpr2R899X model appears to be driven by dropout of small vessels (43). We characterized the PMVEC isolated from Bmpr2R899X and control mice by staining for vWF, VE-cadherin, tubulin, and F-actin in sparsely plated cells grown on collagen. Both Bmpr2R899X and control cells had strong staining for vWF (data not shown). However, in all three categories of cytoskeletal architecture, Bmpr2R899X cells showed defects compared with controls (Fig. 4). By phalloidin staining for F-actin, Bmpr2 mutant cells lacked well-defined stress fibers compared with controls, although they retained surface-associated F-actin comparable to controls. Control cells had extensive networks of microtubules, extending from the nucleus to the cell surface in all directions, while Bmpr2 mutants had limited and truncated microtubule networks. VE-cadherin staining in controls was organized into a network, whereas staining was punctate in Bmpr2 mutant cells. Thus, immunohistochemistry indicated broad defects in cytoskeletal architecture.
Fig. 4.
Pulmonary microvascular endothelial cells (PMVEC) cultured from Bmpr2R899X mice have defective cytoskeletal architecture, including lack of stress fibers (top), few and truncated microtubules (middle), and disorganized VE-cadherin (bottom). VE-cadherin and F-actin rows used similar exposure times between control and Bmpr2 mutant PMVEC; exposure time was roughly 10× increased for Bmpr2 mutant tubulin to show that there were some truncated tubulin structures. For both F-actin and tubulin, gamma was set to 1.5 in postprocessing to allow both strong and faint structures to be seen simultaneously. In each case, cells chosen were representative. There was little variation across control cells, while some Bmpr2 mutant cells completely lacked structures, and others had slightly more extensive cytoskeletal structures.
BMPR2 mutation produces confluence-dependent defects in cytoskeletal protein phosphorylation.
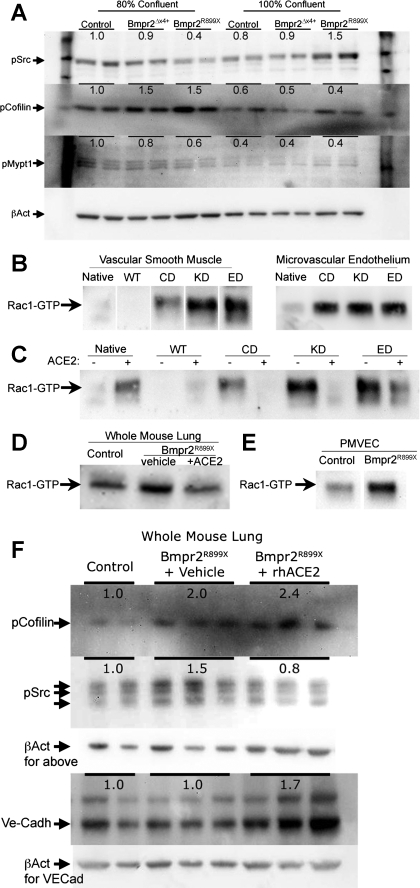
BMPR2 has been published to signal through direct interactions with Src, Limk, and Tctex1. Limk1 regulates cofilin phosphorylation; Tctex1 regulates a Rho guanine exchange factor and thus Rho-GTPase function (28). To assess the impact of BMPR2 mutation on these signaling pathways, PMVEC isolated from control, Bmpr2delx4+, or Bmpr2R899X mice were grown to either an 80% or an 100% confluent state and used for Western blotting. Different confluence was used because of the suggestion from Fig. 2, D and E, that Bmpr2 mutation may alter cell-cell contact sensing. We found strong confluence-dependent dysregulation of Src and Mypt1 phosphorylation in Bmpr2R899X but not Bmpr2delx4+ cells (Fig. 5A). Cofilin phosphorylation was increased in both Bmpr2R899X and Bmpr2delx4+ murine PMVEC, but only when subconfluent.
Fig. 5.
A: PMVEC derived from Bmpr2delx4+ or Bmpr2R899X mice have confluence-dependent changes in cytoskeletal proteins including Src, cofilin, and myosin phosphatase targeting protein 1 (Mypt1). Numbers are densitometric averages of two lanes derived from separate plates. B: both vascular smooth muscle cells and human PMVEC stably transfected with three classes of BMPR2 mutation have greatly increased Rac1 activation at confluence compared with control cells. WT, wild-type; ED, extracellular domain; KD, kinase domain; CD, cytoplasmic domain. C: treatment of vascular smooth muscle cells with recombinant human angiotensin-converting enzyme 2 (rhACE2) reverses Rac1 activation in BMPR2 mutant cells. D: homogenized lung from Rosa26-Bmpr2R899X mice has increased Rac1 activation compared with control, reversible with 2 wk of rhACE2 treatment. E: PMVEC isolated from Rosa26-Bmpr2R899X mice have increased Rac1 activation at confluence than those from control mice. F: homogenized lung from Rosa26-Bmpr2R899X mice have increased cofilin and Src phosphorylation. ACE2 treatment resolves the increased Src but not cofilin phosphorylation and causes a new increase in VE-cadherin (Ve-Cadh) protein levels, possibly compensatory for poor localization seen in Fig. 4. Figure parts with white space are identically processed nonadjacent lanes from the same blot.
BMPR2 mutation produces defects in Rac1 activation.
Analysis of the gene expression defects in Rosa26-Bmpr2R899X mice placed Rho GTPases (Rac1, Cdc42, and RhoA) at the center of cytoskeletal defects (Fig. 3B). We previously found that intervention against RhoA was ineffective in preventing PAH in Rosa26-Bmpr2R899X mice (46), and therefore we chose to concentrate on Rac1 (as proof of concept: CDC42 may be similarly dysregulated). To test the hypothesis that defects in Rac1 signaling are secondary to BMPR2 mutation, we measured levels of active, GTP-bound, Rac1 in both A7r5 vascular smooth muscle cells and human PMVEC stably transfected with three classes of BMPR2 mutation. In confluent culture, there was a dramatic increase in Rac1 activation in BMPR2 mutants compared with controls in both vascular cell types (Fig. 5, B and E). Note that Rac1 activation is functionally increased, while Rac1 expression is decreased, possibly due to a counterregulatory mechanism. This discordance between expression and function is a common finding in array expression studies: alterations in a pathway by expression points to a problem in the pathway, but functional studies are needed to identify the impact.
rhACE2 Reverses Some Cytoskeletal Signaling Defects in Rosa26-Bmpr2R899X Mice
BMPR2-induced Rac1 activation is reversed with rhACE2 administration.
In cell culture, rhACE2 at 100 ng/ml was capable of reversing BMPR2-induced Rac1 activation in confluent vascular smooth muscle cells (Fig. 5C). In homogenized lung from Rosa26-Bmpr2R899X mice, Rac1 activation increased roughly 50%, and treatment with rhACE2 via osmotic pumps for 2 wk reversed Bmpr2-induced Rac1 activation (Fig. 5D) and Src phosphorylation (Fig. 5F). Treatment with rhACE2 did not, however, reduce the increase in cofilin phosphorylation, and it caused a new in crease in expression of VE-cadherin (Fig. 5F). If increased VE-cadherin is improving cell-cell junction defects (Fig. 2, D and E, Fig. 4), this increase may be therapeutic.
rhACE2 reverses cytoskeletal signaling defects on gene arrays.
To determine ACE2 effect in live mice, adult female Rosa26-control or Rosa26-Bmpr2R899X mice had transgenes activated with doxycycline for 1, 4, or 6 wk. Some mice at each time point were euthanized for baseline measurements while others received either rhACE2 or vehicle via osmotic pump for an additional 2 wk (at the 4 and 6 wk time points) (for numbers, see Fig. 6). rhACE2 treatment was confirmed by ELISA for antibodies to the human form of ACE2 in serum from mice.
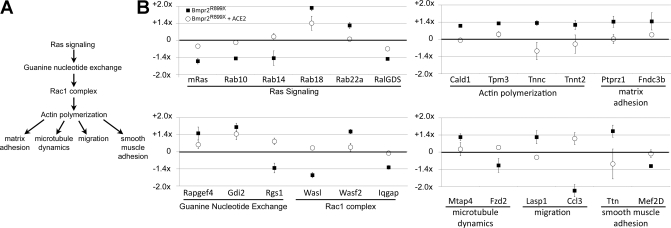
Fig. 6.
rhACE2 resolves most of the Ras/Rac1/cytoskeletal pathway genes altered in Rosa26-Bmpr2R899X mice. A: Ras signaling through guanine nucleotide exchange factors regulates Rac1 complex activation. The Rac1 complex is a central regulator of actin polymerization, with roles in matrix adhesion, microtubule dynamics, migration, and smooth muscle adhesion. B: gene expression arrays on whole lung from Bmpr2R899X mice show strong alteration in many genes in this pathway (■) as compared with control (Rosa26-only) mice (the “zero” line, marked with a heavy horizontal line). Treatment with rhACE2 (○) for 2 wk brings the dysregulated genes closer to control values. Genes depicted are examples selected from a much larger set (Supplemental Table S2). All mice used for array analysis had normal RVSP, to avoid massive expression changes associated with elevated pressures, and had transgenes activated for 6 wk starting as adults. Error bars are SE.
Affymetrix expression gene arrays were obtained for four different groups: 1) Rosa26-control with vehicle; 2) Rosa26-control with rhACE2; 3) Rosa26-Bmpr2R899X mice with vehicle; and 4) Rosa26-Bmpr2R899X mice with rhACE2. To avoid the overwhelming signal associated with elevated RVSP, only animals with normal RVSP were included in gene arrays (39). Two arrays per group were submitted with RNA pooled from two to three animals per array. This treatment protocol gave animals 4 wk to develop a well-established phenotype and 2 wk for rhACE2 to resolve that phenotype.
Compared with Rosa26-controls, Rosa26-Bmpr2R899X mice had 347 probe sets altered at greater than ×1.3. Of the 347 genes with altered expression in Rosa26-Bmpr2R899X mice at 6 wk, a remarkable 268 (77%) were brought back to control values by treatment with rhACE2. rhACE2 did not produce alterations in control mice; thus, rhACE2 effect was specific to mice expressing mutation. The full list of genes altered by Bmpr2R899X expression, rhACE2, or both is given in Supplemental Table S2. The central finding from these arrays is that gene expression changes in the Ras/Rho GTPase/cytoskeleton signaling cascade are corrected with rhACE2 treatment; a depiction of the pathway is shown in Fig. 6A, with examples of genes in this pathway dysregulated by Bmpr2R899X expression but corrected by rhACE2 treatment shown in Fig. 6B.
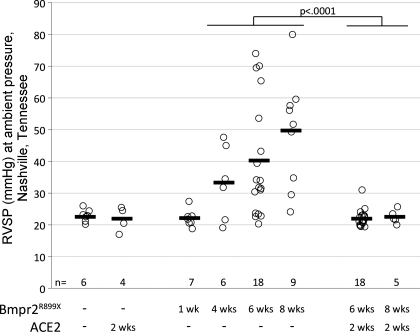
rhACE2 Treatment Reverses Established Pulmonary Hypertension in Rosa26-Bmpr2R899X Mice
Hemodynamic phenotyping was performed on Rosa26-control and Rosa26-Bmpr2R899X mice receiving 4 and 6 wk of doxycycline with an additional 2 wk of rhACE2 or vehicle. A total of 17/18 mice at the 6-wk time point and 5/5 mice at the 8-wk time point had normalization of RVSP (Fig. 7). Importantly, Rosa26-Bmpr2R899X mice treated with rhACE2 had significantly lower RVSP than untreated Rosa26-Bmpr2R899X mice euthanized at 4 and 6 wk (P < 0.0001) (Fig. 7). Therefore, rhACE2 reversed established PAH. rhACE2 had no effect on RVSP in Rosa26-control animals also fed doxycycline. An acute bolus of rhACE2 administered during catheterization did not decrease RVSP over 10 min; its action was thus not as a direct vasodilator. rhACE2 treatment in Rosa26-Bmpr2R899X mice did not have a significant effect on heart rate, systemic pressure, aortic diameter, left ventricular end systolic and diastolic diameters, cardiac output, or ejection fraction. In the 6-wk group, cardiac outputs for Bmpr2R899X mice averaged 9.3 ml/min, not statistically different from 8.8 ml/min in Rosa26-only mice; ACE2-treated mice averaged 8.6 ml/min, not statistically different from 9.5 ml/min in untreated animals.
Fig. 7.
Rosa26-Bmpr2R899X mice develop progressively increased RVSP as transgene is activated for 1, 4, 6, or 8 wk (middle). The disease appears to be driven by vascular dropout rather than increased tone, and so there is large variability in RVSP from animal to animal. After 2 wk of treatment with rhACE2 (right), the RVSP decreased back to wild-type levels (left). Each circle represents measurements from an individual mouse; heavy bars are means. Comparisons are by three-way ANOVA (genotype; treatment time; ACE2) with Fisher's least significant difference test. ACE2 response is also significant by Kruskal-Wallis signed-rank test at P < 0.0001.
DISCUSSION
In this project, expression arrays from Rosa26-Bmpr2R899X transgenic mice demonstrate that a central in vivo molecular consequence of Bmpr2 mutation is altered cytoskeletal pathways (Fig. 3). PMVEC cultured from Bmpr2R899X mice have defects in cytoskeletal architecture (Fig. 4) and activation of multiple cytoskeletal pathway components (Fig. 5A). We demonstrate that cytoskeletal defects and Rho GTPase defects (Rac1 activation) are common to all classes of BMPR2 mutation (Fig. 5B) and can be corrected in cell culture and in vivo (Fig. 5, C–F) through administration of exogenous rhACE2. rhACE2 reverses defects in the Ras/Rho GTPase/cytoskeletal signaling cascade in Rosa26-Bmpr2R899X mice on gene arrays (Fig. 6), reverses Rac1 activation in cell culture, and also reverses established Bmpr2-associated PAH in mice (Fig. 7). Together, these results suggest that cytoskeletal defects are central to the development of BMPR2-associated PAH and that intervention against cytoskeletal dysfunction may treat established PAH. Note that we have used Rac1 activation because it is a direct target of BMPR2 signaling through TCTEX-1; however, the expression array data suggest that there may be broader Rho GTPase defects in, for instance, CDC42 or RhoA activation.
While this study outlines a pathway of potentially central relevance to BMPR2-related PAH, several important questions remain. First, the mechanism by which BMPR2 regulates cytoskeletal dynamics is unclear. Second, we do not know how defects in the cytoskeleton lead to PAH. Finally, we do not know exactly how rhACE2 corrects these cytoskeletal defects and reverses PAH. However, for each of these questions, there is sufficient information in the literature to propose a hypothesis.
BMPR2 interacts directly with several proteins relevant to regulation of the actin cytoskeleton, including LIMK, TCTEX, and SRC (10, 27, 44). LIMK is directly responsible for phosphorylation of cofilin (the dephosphorylated form of cofilin is the active form) and is central to F-actin severing; lungs from Bmpr2R899X mice have increased cofilin phosphorylation (Fig. 5). TCTEX-1 is a dynein motor light chain that which binds and regulates the Rac guanine nucleotide exchange factor (ARHGEF2) (28); TCTEX-1 could be directly responsible for the alteration in Rac1 activation seen in Fig. 5. Finally, while SRC signals through many pathways, it can induce Rac1 activation through Ras and guanine nucleotide exchange factors (35). Therefore, while we do not know directly how BMPR2 mutation results in the cytoskeletal defects seen in this study, BMPR2-interacting proteins are involved in every level of the Ras/Rho GTPase/cytoskeletal cascade.
Although several independent array studies have identified cytoskeletal pathway defects in human PAH samples, none address downstream mechanisms. We propose that an imbalance in Rho GTPase activity leads to pulmonary vascular dysfunction. Rho GTPases (RhoA, Rac1, and Cdc42) control actin and microtubule dynamics, cell migration, cell polarization, cell-cell adhesions, and angiogenesis (2, 6, 21, 37). RhoA disrupts interendothelial junctions by inducing actin stress fiber formation and cytoskeleton retraction and is counteracted by Rac1 and Cdc42 (41). The lack of stress fibers in Bmpr2 mutant cells (Fig. 4) is thus consistent with increased Rac1 activation and decreased RhoA. Rac1 and Cdc42 stabilize endothelial barriers through cadherins and catenins; VE-cadherin structure is abnormal in our Bmpr2 mutant cells compared with controls as demonstrated by punctate VE-cadherin staining (Fig. 4). Rac1 and Cdc42 also reestablish cell-cell contact by stimulating actin assembly into cytoplasmic protrusions, lamellipodia and filopodia (41). Therefore, imbalances in Rho GTPases may prevent pulmonary vascular cells from interacting properly with each other and their environment. Furthermore, regulation of F-actin dynamics impacts intracellular transport(4), mitochondrial fission and fusion (3, 22), NADPH function (15), and caveoli transport. All of these cytoskeletal functions are plausible candidates for the mechanism of PAH.
Finally, we address the question of how rhACE2 could correct cytoskeletal defects. ACE2 was chosen as our cytoskeletal pharmaceutical intervention because it both stimulates endothelial cell migration (26) and decreases vascular permeability (18). ACE2 is a natural inhibitor of the renin-angiotensin pathway. Tissues are capable of synthesizing ACE2 locally, and it is found in over 72 tissues including the lung, heart, kidney, spleen, liver, bone, and brain (14). In the lung, ACE2 is found in arterial and venous endothelial cells, pulmonary epithelial cells, and arterial smooth muscle cells (13). ACE2 degrades ANG II to generate the heptapeptide ANG-(1–7), which binds to the Mas1 receptor and counterbalances ANG II. Targeting the ACE2 pathway has improved pulmonary hypertension in several animal models. Using the monocrotaline rat model, researchers prevented pulmonary hypertension with XNT, a synthetic activator of ACE2 (9). In addition, pulmonary hypertension was prevented and reversed via gene transfer with ACE2 and ANG-(1–7) lentiviral vectors in monocrotaline models (36, 45). Success with both the monocrotaline model and our genetic model supports ACE2 as an effective treatment for PAH.
ACE2 treatment of BMPR2-related cytoskeletal defects in Rosa26-Bmpr2R899X mice is likely through Mas1 interaction with Rho GTPases. ANG-(1–7) binding at Mas1 alters cytoskeletal dynamics. In pig aortic endothelial cells, Mas1 receptor activation induced lamellipodium formation and membrane ruffling through a Rac1-related protein (50). Silencing ACE2 in isolated human endothelial cells resulted in decreased endothelial cell migration and tube formation, while overexpression of ACE2 improved endothelial cell migration and angiogenesis (26). Therefore, Rho GTPase activation through ANG-(1–7) binding to Mas1 may be the mechanism behind ACE2 treatment of PAH.
In conclusion, we show that defects in cytoskeleton, at the level of expression and function, are important molecular consequences of Bmpr2 mutation, and that small molecule reversal of those defects with rhACE2 reverses established PAH. Pharmacologic agents targeting cytoskeletal function could provide a novel drug class for PAH treatment.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-82694 and Vanderbilt Allergy, Pulmonary, and Critical Care Division internal funds. All microarray experiments were performed in the Vanderbilt Microarray Shared Resource. The Vanderbilt Microarray Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Digestive Disease Center (P30 DK58404), and the Vanderbilt Vision Center (P30 EY08126).
DISCLOSURES
rhACE2 for animal experiments was supplied by Apeiron Biologics, and two Apeiron scientists assisted with those elements of study design (but not data collection or interpretation). The authors otherwise have no conflicts of interest. The manuscript has been read and approved by all authors. The requirements for authorship have been met by all authors, and each author believes the manuscript represents honest original work.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.J., M.S., H.L., Y.T., J.W.H., K.B.L., K.A.F., and J.W. conception and design of the research; J.A.J., A.R.H., D.S.P., M.S., L.J.R., S.G., S.B., T.R.B., Y.T., J.W.H., M.T., and J.W. performed the experiments; J.A.J., A.R.H., D.S.P., M.S., H.L., Y.T., J.W.H., M.T., K.B.L., K.A.F., and J.W. analyzed the data; J.A.J., D.S.P., L.J.R., S.G., S.B., Y.T., J.W.H., M.T., K.B.L., K.A.F., and J.W. interpreted results of experiments; J.A.J. and J.W. prepared the figures; J.A.J., K.B.L., and J.W. drafted the manuscript; J.A.J., A.R.H., D.S.P., M.S., L.J.R., S.G., H.L., S.B., T.R.B., Y.T., J.W.H., M.T., K.B.L., K.A.F., and J.W. approved the final version of the manuscript; D.S.P., K.B.L., K.A.F., and J.W. edited and revised the manuscript.
Supplementary Material
REFERENCES
- 1. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25– 29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci 115: 1123– 1136, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bereiter-Hahn J, Voth M, Mai S, Jendrach M. Structural implications of mitochondrial dynamics. Biotechnol J 3: 765– 780, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bu W, Lim KB, Yu YH, Chou AM, Sudhaharan T, Ahmed S. Cdc42 interaction with N-WASP and Toca-1 regulates membrane tubulation, vesicle formation and vesicle motility: implications for endocytosis. PLoS One 5: e12153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burton VJ, Ciuclan LI, Holmes AM, Rodman DM, Walker C, Budd DC. Bone morphogenetic protein receptor-II regulates pulmonary artery endothelial cell barrier function. Blood 117: 333– 341, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Connolly JO, Simpson N, Hewlett L, Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol Biol Cell 13: 2474– 2485, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Jesus Perez VA, Alastalo TP, Wu JC, Axelrod JD, Cooke JP, Amieva M, Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol 184: 83– 99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67: 737– 744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 179: 1048– 1054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 162: 1089– 1098, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gamell C, Osses N, Bartrons R, Ruckle T, Camps M, Rosa JL, Ventura F. BMP2 induction of actin cytoskeleton reorganization and cell migration requires PI3-kinase and Cdc42 activity. J Cell Sci 121: 3960– 3970, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 88: 555– 562, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631– 637, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532: 107– 110, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Hassanain HH, Gregg D, Marcelo ML, Zweier JL, Souza HP, Selvakumar B, Ma Q, Moustafa-Bayoumi M, Binkley PF, Flavahan NA, Morris M, Dong C, Goldschmidt-Clermont PJ. Hypertension caused by transgenic overexpression of Rac1. Antioxid Redox Signal 9: 91– 100, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14: 1167– 1174, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Ihida-Stansbury K, McKean DM, Lane KB, Loyd JE, Wheeler LA, Morrell NW, Jones PL. Tenascin-C is induced by mutated BMP type II receptors in familial forms of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 291: L694– L702, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112– 116, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096– 5100, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol 214: 128– 138, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci 121: 989– 1001, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lane KL, Talati M, Austin E, Hemnes AR, Johnson JA, Fessel JP, Blackwell T, Mernaugh RL, Robinson L, Fike C, Roberts LJ, 2nd, West J. Oxidative injury is a common consequence of BMPR2 mutations. Pulm Circ 1: 72– 83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. International PPH Consortium. Nat Genet 26: 81– 84, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Laumanns IP, Fink L, Wilhelm J, Wolff JC, Mitnacht-Kraus R, Graef-Hoechst S, Stein MM, Bohle RM, Klepetko W, Hoda MA, Schermuly RT, Grimminger F, Seeger W, Voswinckel R. The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 40: 683– 691, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, Slutsky AS, Peterson MD, Backx PH, Penninger JM, Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 295: H1377– H1384, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Machado RD, Rudarakanchana N, Atkinson C, Flanagan JA, Harrison R, Morrell NW, Trembath RC. Functional interaction between BMPR-II and Tctex-1, a light chain of Dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum Mol Genet 12: 3277– 3286, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Meiri D, Greeve MA, Brunet A, Finan D, Wells CD, LaRose J, Rottapel R. Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation. Mol Cell Biol 29: 5963– 5973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meyrick B, Clarke SW, Symons C, Woodgate DJ, Reid L. Primary pulmonary hypertension: a case report including electronmicroscopic study. Br J Dis Chest 68: 11– 20, 1974 [DOI] [PubMed] [Google Scholar]
- 30. Meyrick BO, Friedman DB, Billheimer DD, Cogan JD, Prince MA, Phillips JA, 3rd, Loyd JE. Proteomics of transformed lymphocytes from a family with familial pulmonary arterial hypertension. Am J Respir Crit Care Med 177: 99– 107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okuda S, Yamada T, Hamajima M, Itoh M, Katayama T, Bork P, Goto S, Kanehisa M. KEGG atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res 36: W423– W426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA 97: 2202– 2207, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol 298: H1235– H1248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem 278: 34339– 34346, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Diez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 182: 1065– 1072, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soga N, Connolly JO, Chellaiah M, Kawamura J, Hruska KA. Rac regulates vascular endothelial growth factor stimulated motility. Cell Commun Adhes 8: 1– 13, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Sparks EE, Perrien DS, Huppert KA, Peterson TE, Huppert SS. Defects in hepatic Notch signaling result in disruption of the communicating intrahepatic bile duct network in mice. Dis Model Mech 4: 359– 367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tada Y, Majka S, Carr M, Harral J, Crona D, Kuriyama T, West J. Molecular effects of loss of BMPR2 signaling in smooth muscle in a transgenic mouse model of PAH. Am J Physiol Lung Cell Mol Physiol 292: L1556– L1563, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Ulrich S, Taraseviciene-Stewart L, Huber LC, Speich R, Voelkel N. Peripheral blood B lymphocytes derived from patients with idiopathic pulmonary arterial hypertension express a different RNA pattern compared with healthy controls: a cross sectional study. Respir Res 9: 20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134– 145, 2008 [DOI] [PubMed] [Google Scholar]
- 42. West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, Lane K, Meyrick B, Loyd J. Gene expression in BMPR2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC Med Genomics 1: 45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol 295: L744– L755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong WK, Knowles JA, Morse JH. Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 33: 438– 446, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamazato Y, Ferreira AJ, Hong KH, Sriramula S, Francis J, Yamazato M, Yuan L, Bradford CN, Shenoy V, Oh SP, Katovich MJ, Raizada MK. Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension 54: 365– 371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yasuda T, Tada Y, Tanabe N, Tatsumi K, West J. Rho-kinase inhibition alleviates pulmonary hypertension in transgenic mice expressing a dominant-negative type II bone morphogenetic protein receptor gene. Am J Physiol Lung Cell Mol Physiol 301: L667– L674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yelle D, Deng YP, West J, Stewart DJ. Endothelial-targeted BMPR2 loss-of-function mutations cause increased apoptosis and pulmonary arterial hypertension. Circulation 120: S1021– S1021, 2009 [Google Scholar]
- 48. Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33: W741– 748, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu Z, Ma B, Homer RJ, Zheng T, Elias JA. Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J Biol Chem 276: 25222– 25229, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Zohn IE, Symons M, Chrzanowska-Wodnicka M, Westwick JK, Der CJ. Mas oncogene signaling and transformation require the small GTP-binding protein Rac. Mol Cell Biol 18: 1225– 1235, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.