Abstract
Over the course of the past decade, increasing evidence has implicated alveolar epithelial cell injury and dysfunction in the pathogenesis of idiopathic pulmonary fibrosis (IPF). Genetic factors, cigarette smoking, and other environmental exposures have been identified as potential factors leading to a population of vulnerable alveolar epithelial cells. In addition, molecular techniques have demonstrated herpesviruses are commonly detectable in the lungs of patients with IPF, raising suspicion that, in the setting of a vulnerable alveolar epithelium, lytic (or latent) herpesvirus infection may act as a “second hit” leading to the development of pulmonary fibrosis. Intriguingly, in vivo modeling has shown that herpesvirus infection induces or worsens lung fibrosis when combined with immunodeficiency or other injurious stimuli. Here, we discuss potential mechanisms through which herpesvirus infection may contribute to the pathogenesis of IPF. Ultimately, antiviral therapy may hold promise for halting the progression of this deadly disease.
Keywords: idiopathic pulmonary fibrosis, lung, endoplasmic reticulum stress, epithelial mesenchymal transition, alveolar epithelial cell
in recent years, it has become increasingly clear that idiopathic pulmonary fibrosis (IPF) is a disease characterized by alveolar epithelial cell (AEC) injury and abnormal repair mechanisms leading to exuberant fibroblast activation, collagen and matrix deposition, and progressive remodeling of lung parenchyma (12). A viral etiology of IPF was first suspected more than half a century ago (8), but for decades there was little convincing evidence to support this suspicion. In general, there has been controversy regarding whether the presence of herpesviruses in the lungs of IPF patients represents reactivation of latent herpesvirus infection secondary to immune suppressive treatment and extensive lung remodeling or whether herpesviruses could have a primary role in disease pathogenesis. Despite this uncertainty, a series of intriguing observations has established a compelling association between human herpesvirus infection and IPF. In addition, herpesvirus infection has been linked with chronic allograft rejection and/or fibrosis following solid organ transplants of lung (3), liver (9), and kidney (27). Given the ubiquity of herpesvirus exposure and the relative rarity of IPF, it is unlikely herpesviruses are singular etiologic agents causing this disease. Rather, current evidence suggests that, in a susceptible individual, when combined with specific genetic factors or environmental exposures, herpesvirus infection may act as a “second hit” resulting in additive or synergistic injury to vulnerable AECs, culminating in clinically evident lung fibrosis.
Human Studies
The first link between herpesvirus infection and IPF was reported in 1984 in a small series that found Epstein-Barr Virus (EBV) seropositivity in 12/13 patients with IPF but 0/12 of subjects with other interstitial lung diseases (35). Subsequently, there have been multiple reports of herpesvirus detection by serology or PCR with increased frequency in subjects with IPF (4, 11, 36). Using PCR, Tang et al. (32) found that 97% of patients with IPF had herpesvirus DNA present in lung tissue [including EBV, cytomegalovirus (CMV), human herpesvirus 7, and/or human herpesvirus 8] compared with 36% of normal lungs. More compelling evidence for a possible pathogenic role in IPF has been the recognition that herpesviruses infect not just the proximal airway epithelium but also the distal alveolar epithelium. With the use of immunohistochemistry, herpesvirus antigens have been detected in type II AECs in biopsy specimens from patients with IPF (7, 15, 32), a pattern not seen in normal lung tissue. Thus, while herpesviruses can be detected at the molecular level in both affected and unaffected lungs, it may be that their presence specifically within the alveolar epithelium is critical in facilitating the development of fibrosis.
Demonstrating herpesviruses are present within AECs before the development of IPF is important for establishing potential pathogenicity. Because most patients with IPF have advanced disease at the time of presentation, characterizing the role of herpesviruses in the early pathogenesis of IPF has not been possible until recently. An ongoing study in our laboratory has enrolled asymptomatic first-degree relatives of patients with familial interstitial pneumonia (FIP), the inherited form of IPF. Because FIP is typically inherited in an autosomal dominant pattern (16), one-half of the enrolled subjects is expected to carry genetic risk for IPF. Intriguingly, we have identified herpesvirus antigens in transbronchial biopsy specimens of 24/45 subjects at risk for FIP (unpublished data). This finding suggests that, in a group of individuals at high risk for IPF, viral proteins are detectable in AECs before the development of clinically evident lung fibrosis. Long-term follow-up of these individuals will be crucial for establishing a clear pathogenic relationship.
Because observations in diseased human tissues can confer only an association with disease and not causality, these striking clinical findings regarding the presence of herpesviruses in IPF lungs have led several groups to utilize in vivo mouse models in hopes of defining mechanistic relationships.
Murine Models of Herpesvirus Infection
Herpesviridae characteristically have high species specificity; thus, in vivo studies of herpesvirus infection have typically employed murine herpesvirus 68 (MHV-68), a naturally observed murine γ-herpesvirus that readily infects the respiratory tract. MHV-68 has high homology with human γ-herpesviruses and has been shown to be an informative model of human herpesvirus infection in a variety of disease states (1).
Multiple groups have demonstrated that infecting the respiratory tract of healthy mice with MHV-68 does not lead to significant lung fibrosis (18, 24). However, in the setting of impaired cellular immunity or other injurious/fibrotic stimuli, MHV-68 infection substantially worsens lung fibrosis. BALB/C mice express bleomycin hydrolase, a hepatic enzyme that rapidly metabolizes bleomycin, and are thus typically resistant to bleomycin-induced lung fibrosis (18). However, BALB/C mice infected with MHV-68 concurrent with intraperitoneal bleomycin develop significant histological fibrosis and increased lung collagen content compared with mice exposed to bleomycin or MHV-68 infection alone (18).
In the fluorescein isothiocyanate (FITC)-induced lung fibrosis model, MHV-68 infection two weeks after FITC exposure led to increased histological fibrosis and lung collagen content (20). In addition, mice with latent MHV-68 infection then exposed to FITC had greater inflammation, higher lung collagen content, and increased fibrosis scores compared with uninfected controls (34). Interestingly, in this model, infection with a mutant form of MHV-68 lacking v-cyclin, a key mediator of lytic reactivation, produced similar fibrosis to infection with MHV-68 with intact v-cyclin expression, suggesting lytic reactivation was not necessary to augment fibrosis.
Another group has extensively explored herpesvirus infection in a Th2-biased interferon receptor-γ (IFNγR) knockout mouse (24). This model results in marked impairment in the control of acute viral infection. IFNγR-deficient mice show persistent lytic viral infection, persistent inflammation, and the development of lung fibrosis. Antiviral therapy with cidofovir beginning 45–60 days after initial infection attenuated lung fibrosis (23). In contrast to the FITC model, infection of IFNγR-deficient mice with mutant MHV-68 deficient in v-cyclin did not lead to significant fibrosis.
Potential Mechanisms
Human and in vivo mouse studies suggest that, in combination with a variety of risk factors and exposures, herpesvirus infection can act as a second hit that precipitates or worsens lung fibrosis. The principal mechanisms by which this occurs remain unclear, although several potential explanations are discussed below.
Epithelial cell injury.
AEC injury and death likely play a significant role in the pathogenesis of IPF (37). In a mouse model, targeted injury of type II AECs using diphtheria-toxin was shown to be sufficient for induction of lung fibrosis (30). When combined with certain genetic factors or environmental exposures, herpesvirus infection may lead to particularly severe or persistent injury. Alternatively, recurrent cycles of lytic replication and subsequent AEC death could lead to chronic activation of injury-repair mechanisms resulting in fibrosis. To date there are conflicting data as to whether herpesvirus reactivation is a necessary step in the development of lung fibrosis, likely related to differences between models and timing of assessment (23, 34). Latent EBV infection does not appear to significantly alter human AEC proliferation or viability in vitro (19), although this has not been studied in the setting of additional injurious stimuli.
Inflammation and cytokine production.
Herpesviruses may contribute to lung fibrosis by affecting lung inflammation. Latent herpesvirus infection has been associated with elevated levels of proinflammatory cytokines in humans and in animal models (2, 17). MHV-68-infected IFNγR-deficient mice, which progress to a fibrotic phenotype, have more inflammatory cells, higher levels of proinflammatory cytokines, and delayed resolution of inflammation compared with MHV-68-infected wild-type controls (23, 24), a pattern also seen with MHV-68-infected/FITC-treated wild-type mice (20, 34). Following MHV-68 infection, IFNγR-deficient mice infected with MHV-68 also develop fibrosis in the liver and spleen (5). In this model, herpesvirus infection increased the number of alternatively activated macrophages, which are thought to participate in tissue remodeling (22).
Profibrotic mediators.
Also of interest is a potential role for herpesviruses to induce production of profibrotic mediators. Human A549 cells infected with EBV show increased production of transforming growth factor-β (TGF-β) during latency, with a further increase during lytic infection (19). Human mononuclear cells have also been found to increase TGF-β production following herpesvirus infection (21). Cultured AECs (19) and mesenchymal cells (31) from mice latently infected with MHV-68 have increased TGF-β production compared with AECs from uninfected mice (34). In renal tubular epithelial cells, infection with CMV can lead to activation of extracellular TGF-β1 activation via matrix metalloproteinase-2 production (28). The mechanisms by which locally produced (and/or activated) TFG-β interacts with viral proteins and the cellular and innate immune response elements to impact epithelial injury and lung remodeling require further evaluation.
Endoplasmic reticulum stress.
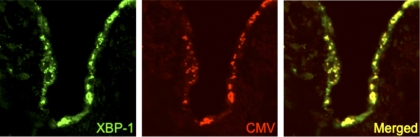
A promising new area of investigation involves the role of endoplasmic reticulum (ER) stress in the development of fibrosis. ER stress markers are increased in the alveolar epithelium in lung biopsies from individuals with IPF (13, 15). Furthermore, recent mouse models of ER stress, induced either by expression of mutant surfactant protein C or by tunicamycin exposure, led to enhanced AEC death and lung fibrosis in the intratracheal bleomycin model (14). Thus, underlying ER stress may confer vulnerability of the AEC population to a second hit like herpesvirus infection, overwhelming homeostatic responses and resulting in enhanced injury and fibrosis. In addition, herpesvirus infection can promote ER stress and activation of the unfolded protein response (UPR) (10). Interestingly, herpesvirus antigens colocalize with ER stress markers in AECs from IPF lung tissue (15) (Fig. 1), raising questions as to whether herpesviruses may contribute to disease pathogenesis through induction or modulation of ER stress responses. In vivo mouse modeling holds promise to determine potential mechanistic relationships linking herpesvirus infection to ER stress and subsequent lung fibrosis.
Fig. 1.
Colocalization of X-box-binding protein 1 (XBP-1) and cytomegalovirus (CMV) late antigen in alveolar epithelial cells from an individual with usual interstitial pneumonia. Confocal laser-scanning microscopy with Z-stack was performed on lung tissue sections. Immunofluorescence for XBP-1 (green), a marker of endoplasmic reticulum (ER) stress, and CMV late antigen (red) identify expression in epithelial cells lining areas of adjacent fibrosis. Coexpression of these proteins (yellow) is detected within the alveolar epithelium. Magnification ×200. Reproduced from Lawson et al. (15).
Epithelial-mesenchymal transition.
Another area of current research interest is the role of epithelial-mesenchymal transition (EMT) in the pathogenesis of IPF (33). In IFNγR-deficient mice, MHV-68 infection leads to dramatic upregulation of Twist, a transcription factor that promotes EMT (26). In vitro, transient knockdown of Twist with small-interfering RNA led to a restoration of an epithelial phenotype. EBV latent membrane protein 1 (LMP1) mimics a constitutively active tumor necrosis factor-α superfamily receptor, and a recent in vitro study revealed that LMP1 can augment the effects of TGF-β in the induction of EMT in lung epithelial cells (29). There is need for further investigation in this area, including in vivo modeling to determine mechanisms by which herpesvirus infection may impact EMT pathways within the lung.
Future Directions
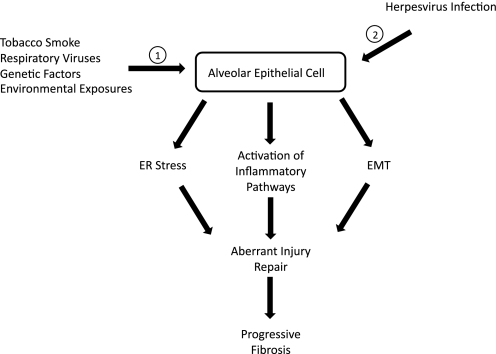
Given the compelling evidence associating herpesvirus infection with IPF and promising in vivo and in vitro studies indicating these viruses interact with multiple pathways relevant to the development of fibrosis (Fig. 2), further investigation exploring these mechanisms is needed. The manner by which herpesviruses interact with the aging process (25) as well as other genetic and environmental factors to impact IPF pathogenesis remains to be elucidated. In addition, studies are needed to determine the roles of latent and lytic virus in lung fibrosis, as well as to define mechanisms linking different stages of viral infection to profibrotic pathways and cellular phenotypes.
Fig. 2.
Hypothesized role of herpesvirus infection in a “two-hit” model of idiopathic pulmonary fibrosis. 1) Over time, through exposures to environmental stimuli, including tobacco smoke or respiratory viruses, or through genetic factors that lead to epithelial cell dysfunction, a “vulnerable” alveolar epithelial cell phenotype develops. 2) Exposure to common human herpesviruses leads to acute lytic and latent infection of vulnerable epithelial cells. This results in activation of inflammatory signaling, increased ER stress, and induction of epithelial-mesenchymal transition (EMT). Together these insults result in an aberrant injury response cascade with impaired reepithelialization of injured alveoli and local activation of fibroblasts. Activated fibroblasts produce collagen and deposit extracellular matrix, leading to marked distortion of normal pulmonary parenchyma. Without interruption, this aberrant injury response culminates in clinically evident pulmonary fibrosis.
Finally, we suggest the time has come for a pilot study of antiviral treatment in humans with IPF. Anecdotal reports (32) and one very small series (6) have reported promising results of disease stabilization using antivirals directed against herpesvirus in patients with IPF. Ultimately, this strategy may warrant evaluation in the setting of an adequately powered, well-designed, randomized, controlled trial, particularly if lytic replication of specific herpesviruses can be identified in the lungs of study participants. With increased understanding of the complex interplay of herpesvirus infection in the pathogenesis of lung fibrosis, we anticipate that this knowledge will be useful for designing new and effective therapies for IPF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-085317, HL-092870, and HL-105479 and by the Department of Veterans Affairs.
DISCLOSURES
All authors confirm that they have no competing interests regarding the content, investigations, and results outlined in this manuscript.
REFERENCES
- 1. Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol 29: 351– 397, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med 34: 842– 849, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costa C, Astegiano S, Terlizzi ME, Sidoti F, Curtoni A, Solidoro P, Baldi S, Bergallo M, Cavallo R. Evaluation and significance of cytomegalovirus-specific cellular immune response in lung transplant recipients. Transplant Proc 43: 1159– 1161, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Dworniczak S, Ziora D, Kapral M, Mazurek U, Niepsuj G, Rauer R, Wilczok T, Kozielski J. Human cytomegalovirus DNA level in patients with idiopathic pulmonary fibrosis. J Physiol Pharmacol 55, Suppl 3: 67– 75, 2004. [PubMed] [Google Scholar]
- 5. Ebrahimi B, Dutia BM, Brownstein DG, Nash AA. Murine gammaherpesvirus-68 infection causes multi-organ fibrosis and alters leukocyte trafficking in interferon-gamma receptor knockout mice. Am J Pathol 158: 2117– 2125, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Egan JJ, Adamali HI, Lok SS, Stewart JP, Woodcock AA. Ganciclovir antiviral therapy in advanced idiopathic pulmonary fibrosis: an open pilot study (Abstract). Pulm Med 2011: 240805, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax 50: 1234– 1239, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golden A, Bronk TT. Diffuse interstitial fibrosis of lungs; a form of diffuse interstitial angiosis and reticulosis of the lungs. AMA Arch Intern Med 92: 106– 114, 1953. [PubMed] [Google Scholar]
- 9. Herzog D, Soglio DB, Fournet JC, Martin S, Marleau D, Alvarez F. Interface hepatitis is associated with a high incidence of late graft fibrosis in a group of tightly monitored pediatric orthotopic liver transplantation patients. Liver Transpl 14: 946– 955, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol 79: 6890– 6899, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 166: 510– 513, 2002. [DOI] [PubMed] [Google Scholar]
- 12. King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949– 1961, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838– 846, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108: 10562– 10567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294: L1119– L1126, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Lawson WE, Loyd JE, Degryse AL. Genetics in pulmonary fibrosis–familial cases provide clues to the pathogenesis of idiopathic pulmonary fibrosis. Am J Med Sci 341: 439– 443, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Limaye AP, Boeckh M. CMV in critically ill patients: pathogen or bystander? Rev Med Virol 20: 372– 379, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lok SS, Haider Y, Howell D, Stewart JP, Hasleton PS, Egan JJ. Murine gammaherpes virus as a cofactor in the development of pulmonary fibrosis in bleomycin resistant mice. Eur Respir J 20: 1228– 1232, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Malizia AP, Keating DT, Smith SM, Walls D, Doran PP, Egan JJ. Alveolar epithelial cell injury with Epstein-Barr virus upregulates TGFbeta1 expression. Am J Physiol Lung Cell Mol Physiol 295: L451– L460, 2008. [DOI] [PubMed] [Google Scholar]
- 20. McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, van Dyk LF, Toews GB. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med 177: 771– 780, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mendez-Samperio P, Hernandez M, Ayala HE. Induction of transforming growth factor-beta 1 production in human cells by herpes simplex virus. J Interferon Cytokine Res 20: 273– 280, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 35: 466– 473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mora AL, Torres-Gonzalez E, Rojas M, Xu J, Ritzenthaler J, Speck SH, Roman J, Brigham K, Stecenko A. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice. Am J Respir Crit Care Med 175: 1139– 1150, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham KL, Stecenko AA. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol 289: L711– L721, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med 4: 759– 771, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pozharskaya V, Torres-Gonzalez E, Rojas M, Gal A, Amin M, Dollard S, Roman J, Stecenko AA, Mora AL. Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS One 4: e7559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebekova K, Feber J, Carpenter B, Shaw L, Karnauchow T, Diaz-Mitoma F, Filler G. Tissue viral DNA is associated with chronic allograft nephropathy. Pediatr Transplant 9: 598– 603, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Shimamura M, Murphy-Ullrich JE, Britt WJ. Human cytomegalovirus induces TGF-beta1 activation in renal tubular epithelial cells after epithelial-to-mesenchymal transition. PLoS Pathog 6: e1001170, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sides MD, Klingsberg RC, Shan B, Gordon KA, Nguyen HT, Lin Z, Takahashi T, Flemington EK, Lasky JA. The Epstein-Barr virus latent membrane protein 1 and transforming growth factor–beta1 synergistically induce epithelial–mesenchymal transition in lung epithelial cells. Am J Respir Cell Mol Biol 44: 852– 862, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254– 263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stoolman JS, Vannella KM, Coomes SM, Wilke CA, Sisson TH, Toews GB, Moore BB. Latent infection by gammaherpesvirus stimulates profibrotic mediator release from multiple cell types. Am J Physiol Lung Cell Mol Physiol 300: L274– L285, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA, Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 41: 2633– 2640, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, Lawson WE. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 180: 657– 665, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med 181: 465– 477, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vergnon JM, Vincent M, de The G, Mornex JF, Weynants P, Brune J. Cryptogenic fibrosing alveolitis and Epstein-Barr virus: an association? Lancet 2: 768– 771, 1984. [DOI] [PubMed] [Google Scholar]
- 36. Yonemaru M, Kasuga I, Kusumoto H, Kunisawa A, Kiyokawa H, Kuwabara S, Ichinose Y, Toyama K. Elevation of antibodies to cytomegalovirus and other herpes viruses in pulmonary fibrosis. Eur Respir J 10: 2040– 2045, 1997. [DOI] [PubMed] [Google Scholar]
- 37. Zoz DF, Lawson WE, Blackwell TS. Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci 341: 435– 438, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]