Abstract
Alveolar type II (ATII) cell apoptosis and depressed fibrinolysis that promotes alveolar fibrin deposition are associated with acute lung injury (ALI) and the development of pulmonary fibrosis (PF). We therefore sought to determine whether p53-mediated inhibition of urokinase-type plasminogen activator (uPA) and induction of plasminogen activator inhibitor-1 (PAI-1) contribute to ATII cell apoptosis that precedes the development of PF. We also sought to determine whether caveolin-1 scaffolding domain peptide (CSP) reverses these changes to protect against ALI and PF. Tissues as well as isolated ATII cells from the lungs of wild-type (WT) mice with BLM injury show increased apoptosis, p53, and PAI-1, and reciprocal suppression of uPA and uPA receptor (uPAR) protein expression. Treatment of WT mice with CSP reverses these effects and protects ATII cells against bleomycin (BLM)-induced apoptosis whereas CSP fails to attenuate ATII cell apoptosis or decrease p53 or PAI-1 in uPA-deficient mice. These mice demonstrate more severe PF. Thus p53 is increased and inhibits expression of uPA and uPAR while increasing PAI-1, changes that promote ATII cell apoptosis in mice with BLM-induced ALI. We show that CSP, an intervention targeting this pathway, protects the lung epithelium from apoptosis and prevents PF in BLM-induced lung injury via uPA-mediated inhibition of p53 and PAI-1.
the lungs, and airway and alveolar epithelial cells in particular, are constantly exposed to a variety of insults. Extensive apoptosis of alveolar type II (ATII) cells, augmented p53 expression due to DNA damage, and chronic lung inflammation have collectively been implicated in the development of diffuse alveolar damage (DAD) and idiopathic pulmonary fibrosis (IPF) (61) and have each been found to promote accelerated PF in various animal models (36, 39, 44). Increased expression of p53 by apoptotic ATII cells surrounding the fibrotic lesions in IPF patients implicates p53 in the development of IPF. There is no effective pharmacological treatment to prevent or reverse IPF or other forms of pulmonary fibrosis (PF), so delineation of the key underlying events assumes paramount importance.
Fibrinolytic defect associated with acute lung injury (ALI) as well as lung remodeling in acute respiratory distress syndrome (7, 21–24) or interstitial lung diseases (2, 4, 7, 14, 21–23, 25, 43, 53, 58, 63) has been linked to loss of urokinase-type plasminogen activator (uPA) activity and inhibition of uPA by the disproportion increase in the expression of its inhibitor, plasminogen activator inhibitor-1 (PAI-1). uPA is mitogenic (26, 28, 30, 34, 35, 38, 46–51, 54) and enhances plasminogen activation in multiple cell types, including lung epithelial cells through self-induction as well as that of the uPA receptor, uPAR. These responses occur solely through posttranscriptional stabilization of the respective mRNAs (46, 48, 49) and involve extensive cross talk between the fibrinolytic system and p53, in which the p53 specifically binds to 35-, 37-, and 70-nucleotide 3′-untranslated region sequences of uPA, uPAR, and PAI-1 mRNAs, respectively. We also found that p53 destabilizes uPA (46) and uPAR (54) mRNAs and inhibits their expression, whereas it stabilizes PAI-1 mRNA and induces PAI-1 expression (55).
uPA-mediated maintenance of lung epithelial cell viability in vitro is due to inhibition of apoptosis (1, 51, 54) and/or induction of proliferation that depends on suppression of p53 in a dose-dependent manner (51–56). The viability of lung epithelial and carcinoma cells is regulated by coordinate expression of regulation of uPA, uPAR, and PAI-1 (46, 54–56). In a related vein, a recent report demonstrated that transplantation of exogenous ATII cells to the injured lung ameliorates bleomycin (BLM)-induced PF (45). This report strongly suggests that viable epithelial cells within the injured lung are salutary. Although this approach is not clinically feasible at this time, the findings support the alternate strategy of inhibiting ATII cell apoptosis to mitigate PF. The process requires uPA binding to uPAR at the cell surface and involves activation of β1-integrin. Furthermore, both caveolin-1 expression and src kinase activities are induced during injury (31, 32, 65) and caveolin-1 recruits active src kinase to β1-integrin-uPAR signaling complexes (31, 32, 66). Caveolin-1 scaffolding domain peptide (CSP) inhibits caveolin-1 interaction with active src kinase (15). We therefore inferred that targeting caveolin-1 and active src kinase using CSP could inhibit p53 and reverse p53-mediated changes in the fibrinolytic system to enhance ATII cell viability and prevent PF. To test this possibility, we used ATII cells and a murine model of ALI and PF induced by BLM (2, 4, 14, 20).
Here, we describe a new paradigm by which coordinate p53-mediated changes in uPA, uPAR, and PAI-1 expression contribute to ATII cell apoptosis and subsequent development of PF. We also found that mice deficient in expression of p53 and PAI-1 resist BLM-induced ATII cell apoptosis whereas mice lacking uPA expression are sensitive to ATII cell apoptosis. Most importantly, we now show that targeting of this pathway with CSP in wild-type (WT) mice protects the lung epithelium against BLM-induced ALI in an uPA-dependent manner and reverses BLM-induced PF in mice.
MATERIALS AND METHODS
Mice.
WT and p53-, uPA-, uPAR-, and PAI-1-deficient mice of C57BL-6 background were bred at The University of Texas Health Science Center at Tyler or purchased from Charles River (Boston, MA) and Jackson Laboratory (Bar Harbor, ME) were used. Anti-uPA, uPAR, PAI-1, p53, β-actin, and other antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), Ki-67 antibody was purchased from Abcam (San Francisco, CA).
DAD tissues.
Paraffin-embedded deidentified lung sections (5 μM) from patients without known lung disease and from patients with DAD were obtained from the tissue archives of the Department of Pathology, The University of Texas Health Science Center, Tyler. Lung tissues were subjected to terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining (Promega, Madison, WI) to assess changes in apoptosis. For immunohistochemical (IHC) analyses, UltraVision LPValue Detection System (Fremont, CA) was used as described earlier (5, 6, 55).
Determination of BLM-induced changes in primary ATII cell uPA, uPAR, p53, and PAI-1 expression and apoptosis in vitro.
All animal experiments were performed according to protocols approved by the Animal Care and Use Committee of The University of Texas Health Science Center, Tyler. ATII cells were isolated from 6-wk-old mice by following the procedure described by Corti and colleagues (10) with 90–95% purity. These cells were treated with or without anti-β1-integrin antibody (1 μg/ml), isotypic mouse IgG, uPA, or the aminoterminal fragment of uPA (20 nM), alone or in combination, CSP (NH2-DGIWKASFTTFTVTKYWFYR-COOH) (10 nM), or a corresponding control peptide (CP) of scrambled sequence, for 2 h. These cells were later exposed to BLM (40 μg/ml) for 28 h to induce injury. The conditioned media, cell lysates, and membrane proteins were analyzed for uPA, uPAR, PAI-1, p53, and β-actin by Western blotting. Alternatively, cells treated as above were detached by using trypsin-EDTA and analyzed for apoptosis by flow cytometry after staining with anti-annexin-V antibody-propidium iodide (PI) solution as described elsewhere (51).
Model of BLM-induced lung injury in mice and therapeutic intervention.
Six-week-old mice were given 40 μg (from 15 units/15 mg stock) of BLM in 50 μl sterile saline by intranasal insufflations under anesthesia to induce lung injury (59). These mice were later exposed to CSP (18.75 mg/kg) or CP through the nose on days 2, 4, and 6 post-BLM injuries. Mice were euthanized on days 7 and 21 after BLM treatment, after which lung sections from half of the animals (n = 3) were subjected to TUNEL staining to evaluate apoptosis (55). Lung sections of mice euthanized on day 21 were also stained with Masson's trichrome to detect collagen deposition. The whole lung homogenates from the remaining mice were analyzed for the hydroxyproline content as described earlier (29). To determine whether systemic administration of CSP effectively treats established BLM-induced injury on 2, 7, or 14 days after BLM injury, mice were intraperitoneally (IP) implanted with a 0.1 ml microosmotic pump (Alzet, Cupertino, CA) containing 125 mg/kg of CSP or CP delivered over 14 days. As an alternate less invasive approach, mice were given IP injections at 2, 7, and 14 days post-BLM injury with CSP (125 mg/kg) or CP. These mice were euthanized 21 days post-BLM exposure and analyzed as described above.
Determination of BLM-induced changes in ATII cell uPA, uPAR, p53, and PAI-1 expression and apoptosis in mice.
ATII cells were isolated from the lungs of mice exposed to BLM for 1, 3, and 5 days and were analyzed for PAI-1, p53, cleaved poly(ADP-ribose) polymerase (Cl.PARP), and β-actin by Western blotting. In a separate experiment, mice exposed to saline or BLM were IP injected with or without CSP or CP 24 h following BLM injury. ATII cells isolated from the lungs on day 3 post-BLM injuries were tested for changes in uPA, uPAR, p53, PAI-1, β-actin, and Cl.PARP by Western blotting. Similarly flow cytometry analysis was also done after treating with anti-annexin-V antibody and PI, as described above. Total RNA isolated from ATII cells were analyzed for changes in uPA, uPAR, PAI-1, and β-actin mRNAs by RT-PCR. To determine whether p53-induced changes in PAI-1 and uPA contributes to ATII cell apoptosis, WT and uPA-, p53-, and PAI-1-deficient mice were exposed to BLM with or without CSP or CP as described above. ATII cells were analyzed for activation of cleaved caspase-3 (Cl.casp-3), p53, and PAI-1 expression by Western blotting. The lung sections of WT and uPA-, PAI-1-, and p53-deficient mice were also subjected to TUNEL staining and IHC analysis using anti-Cl.casp-3 or anti-Ki-67 antibodies as described earlier (5, 6, 55).
Measurement of lung compliance.
Lung compliance was measured as described earlier (68). Briefly, mice were anesthetized, intubated via the trachea, and mechanically ventilated by a computer-controlled piston ventilator with pulmonary function capability (FlexiVent, Scireq) to determine lung compliance.
Statistical analysis.
Student's t-test or one-way ANOVA was used to analyze the results from two or more different groups of treatment conditions and calculated values of P < 0.05 were taken as statistically significant.
RESULTS
uPA, p53, and PAI-1 expression in DAD lung tissues.
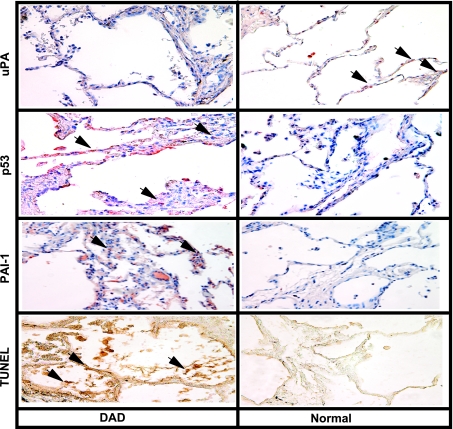
Microscopic examination of antibody-treated lung sections showed increased p53 and PAI-1 and reduced uPA staining of alveolar and airway epithelial cells in DAD tissues. However, no significant staining for p53 or PAI-1 antigen was observed in histologically normal lung tissues, whereas we found robust uPA staining in these cells. TUNEL staining also showed increased alveolar and airway epithelial cell apoptosis in DAD sections (Fig. 1).
Fig. 1.
p53, urokinase-type plasminogen activator (uPA), and plasminogen activator inhibitor-1 (PAI-1) expression in the human diffuse alveolar damage (DAD) tissues. Sections from DAD and normal lungs were subjected to immunohistochemistry (IHC) analyses using anti-p53, anti-uPA, or anti-PAI-1 monoclonal antibodies (1:50 dilution) or terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining to assess the changes in lung epithelial (LE) cell uPA, p53, and PAI-1 expression as well as LE cell apoptosis. Representative fields from 1 of 3 sections per subject are shown at ×200 magnification. Arrows indicate foci of strong staining in each of the representative fields.
CSP reverses BLM-induced expression of ATII cell uPA, uPAR, PAI-1, and p53.
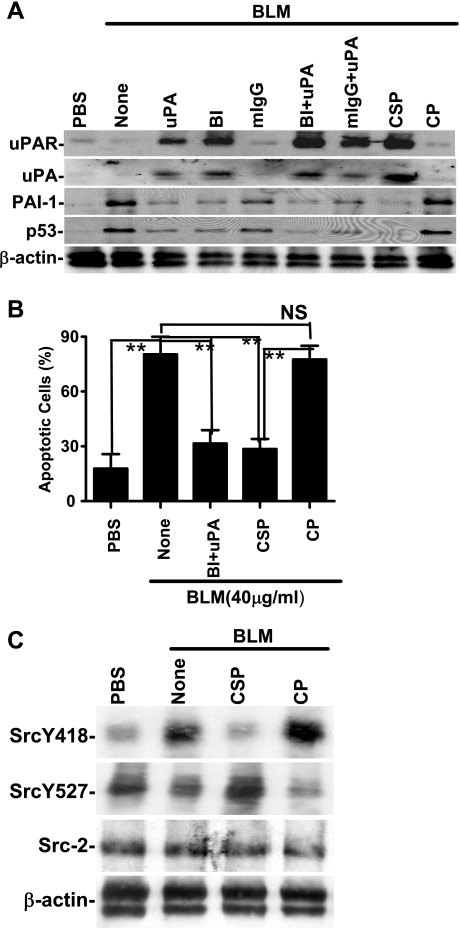
On the basis of the findings in DAD lung tissues, we speculated that interfering with p53-mediated changes in the expression of uPA, uPAR, and PAI-1 could inhibit ATII cell apoptosis in BLM-induced ALI. Activation of β1-integrin with anti-β1-integrin antibody ligation or treatment with uPA inhibits p53 expression by lung epithelial (LE) cells (50) suggesting that activation of β1-integrin enhances LE cell viability. Caveolin-1 also binds to Src kinase via the scaffolding domain (31, 65) and recruits active Src kinase to β1-integrin-uPAR complexes. Caveolin-1 expression and Src kinase activities are increased during injury, and CSP inhibits caveolin-1 interaction with Src kinase (32). Therefore, BLM-exposed ATII cells treated with anti-β1-integrin antibody, uPA, or CSP were tested for uPA, uPAR, p53, and PAI-1 expression. As shown in Fig. 2A, CSP alone or anti-β1-integrin antibody+uPA combined induced uPA and uPAR whereas both treatments reciprocally suppressed p53 and PAI-1. However, control cells treated with either isotypic antibody or CP failed to alter BLM-induced changes in p53 or components of the fibrinolytic system.
Fig. 2.
Caveolin-1 scaffolding domain peptide (CSP) reverses bleomycin (BLM)-induced changes in alveolar type II (ATII) cell uPA, uPA receptor (uPAR), PAI-1, and p53 in vitro. A: mouse ATII cells were treated for 28 h at 37°C with PBS or 40 μg/ml of BLM in the presence or absence of either full-length uPA or the aminoterminal fragment (ATF) of uPA (each 20 nM), 1 μg/ml each of anti-β1-integrin monoclonal antibody (BI) or mouse IgG (mIgG), BI+uPA, mIgG+uPA, CSP (10 nM), and control peptide (CP; 10 nM) at 37°C. The membrane proteins were immunoblotted with anti-uPAR antibody. In the case of uPA and PAI-1, the full-length uPA stimulus was substituted with ATF. The conditioned media were analyzed for uPA and PAI-1 while cell lysates were analyzed for p53 and β-actin by Western blotting. Experiments were repeated at least twice and representative results are illustrated. B: inhibition of BLM-induced apoptosis of ATII cells by anti-β1-integrin antibody and uPA or CSP. Mouse ATII cells treated as described above (A) were detached from the culture plates by using trypsin-EDTA solution. These cells were stained with anti-annexin-V antibody-propidium iodide (PI) solution and analyzed for apoptosis by flow cytometry. The percentage of apoptotic cells is presented as bars representing mean ± SD of 4 replications. Differences between treatments are statistically significant (**P < 0.001) or not statistically significant (NS) as confirmed by Student's t-test or 1-way ANOVA. C: CSP inhibits BLM-induced Src activation. Lysates of ATII cells treated with PBS or BLM with or without CSP or CP for 24 h were tested for tyrosine (Y418) phosphorylation and inhibitory tyrosine (Y527) phosphorylation by Western blotting using phosphospecific antibodies. The same membrane was then stripped and tested for total Src (Src-2) kinase and β-actin expression. Doublets found in bottom panels of A and C are due to the recognition of both β- and γ-actins by anti-β-actin antibody.
Flow cytometry analyses (Fig. 2B) showed that treatment of ATII cells with anti-β1-integrin antibody plus uPA or CSP alone significantly (P < 0.001) blocked BLM-induced apoptosis. The response to CSP alone was as potent as that to anti-β1-integrin antibody and uPA combined. We also found that BLM inhibits β1-integrin activation and that the effect was reversed by CSP (data not shown), suggesting an intricate link between p53-mediated changes in the fibrinolytic system, activation of β1-integrin, and viability of ATII cells. We next tested whether CSP alters Src kinase activity. Phosphorylation of a conserved Y418 in the activation of loop by Src kinase itself (3, 9) or other kinases (8, 19) enhances its activity. In contrast, phosphorylation of a conserved Y529 in the COOH-terminal tail by tyrosine kinase Csk or its family member Chk causes intramolecular binding of SH2 domain to the phosphorylated COOH terminus. This leads to binding of the SH3 domain to the linker between the SH2 and the catalytic domains, causing formation of an inactive conformation of Src (12, 40, 57, 67). We found that BLM induces Src kinase activation through phosphorylation of the Y418 residue, which was inhibited after CSP treatment through inhibitory (Y527) phosphorylation (Fig. 2C).
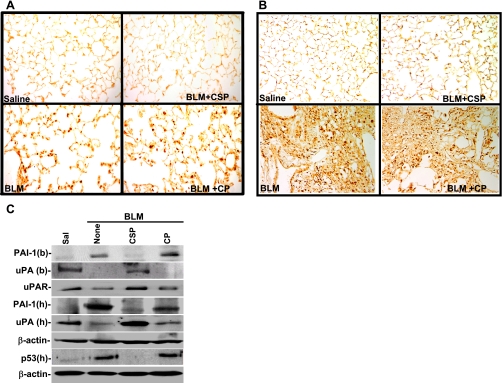
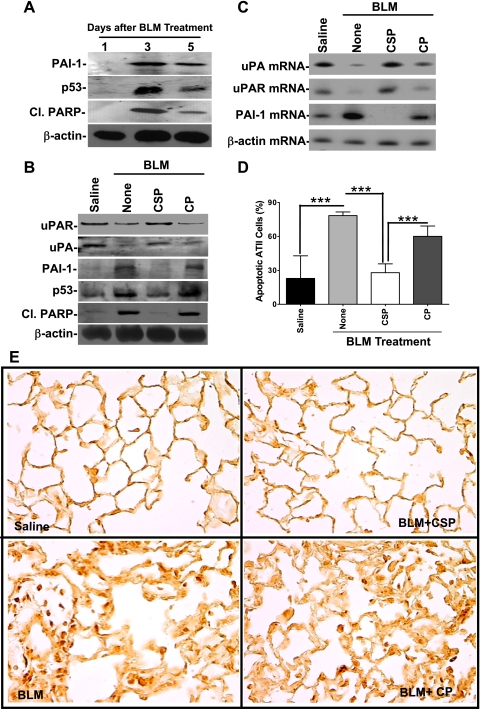
Since CSP is a 20-amino acid peptide with no enzymatic activity or globular conformation, we extended the in vitro observations and elucidated the effect of CSP on ALI/PF using mice with BLM-induced ALI/PF. Examination of TUNEL-stained lung sections at 7 (Fig. 3A) and 21 days (Fig. 3B) after BLM revealed ATII apoptosis. Treatment of CSP by intranasal or microosmotic pump administration after BLM injury blocked ATII cell apoptosis >95% whereas CP failed to protect ATII cells. Lung homogenates tested 7 days after BLM injury demonstrated increased p53 and PAI-1 expression and reciprocal suppression of uPA and uPAR (Fig. 3C). However, CSP reversed this process, whereas the control peptide had no effect.
Fig. 3.
CSP inhibits BLM-induced ATII cell apoptosis and alters uPA, uPAR, p53, and PAI-1 in mouse lungs. Mice exposed to BLM were then treated with CSP (18.75 mg/kg body wt) or CP by intranasal instillation on days 2, 4, and 6 after BLM treatment. Lung sections of mice euthanized 7 (A) and 21 (B) days after BLM injury were analyzed for apoptosis by TUNEL staining. TUNEL-stained sections (magnification ×200) are representative of 9 fields/mouse from each of 3 mice. C: bronchoalveolar lavage (BAL) fluids (b) and lung homogenates (h) of mice at 1 wk after BLM injury as described in A were analyzed for uPA, p53, PAI-1, and β-actin proteins by Western blotting. Membrane proteins were immunoblotted for uPAR expression by using mouse-specific antibodies. Results are representative of at least 3 independent repetitions.
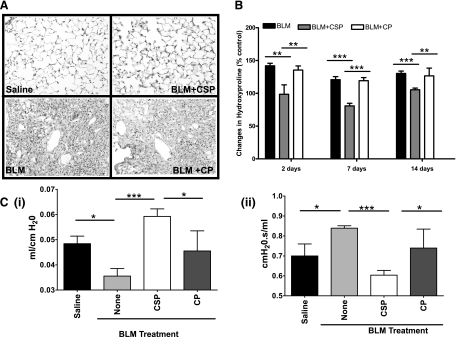
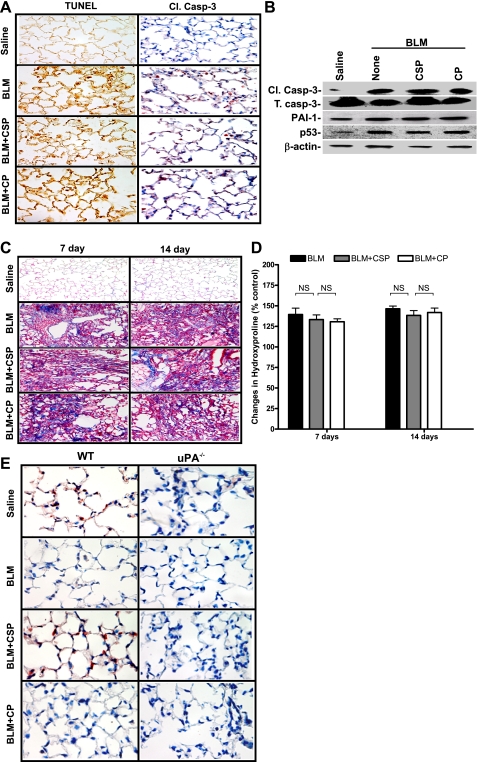
TUNEL-stained lung sections showed massive consolidation and interstitial thickening (Fig. 3B) 21 days after BLM injury consistent with the development of fibrosis besides ATII cell apoptosis. Massive collagen deposits were seen as illustrated by the blue stain with Masson's trichrome staining of BLM-injured lungs (Fig. 4A). Treatment of mice with CSP suppressed collagen deposition. Although the histological responses were remarkably consistent, we analyzed lung homogenates for hydroxyproline as a quantitative, independent measure of changes in PF. Consistent with the findings of trichrome staining, BLM significantly increased the amount of hydroxyproline in the lungs. Treatment of mice with CSP but not CP significantly reduced BLM-induced hydroxyproline levels (Fig. 4B).
Fig. 4.
CSP prevents BLM-induced collagen deposition in mouse lungs. A: lung sections of mice euthanized on day 21 after exposure to BLM as described in Fig. 3 were subjected to trichrome staining. Stain indicates the collagen deposition. Figure (magnification ×200) is representative of 9 fields/mouse; n = 3 mice. B: inhibition of lung fibrosis by treatment with CSP in mice. Mice exposed to BLM were implanted with a 0.1-ml microosmotic pump filled with 125 mg/kg body wt of CSP (BLM+CSP) or CP (BLM+CP) on 2 or 7 or 14 days after initiation of injury. The lung homogenates from mice euthanized 21 days after BLM injury were analyzed for hydroxyproline content. Bars represent mean ± SD of at least 3 repetitions (n = 3 mice/group). Differences between treatments are statistically significant (**P < 0.05) or (***P < 0.01) as confirmed by Student's t-test or 1-way ANOVA. C: CSP restores lung functions after BLM injury. BLM-treated mice were intraperitoneally (IP) injected with or without 125 mg/kg body wt of CSP or CP on days 2, 4, and 6 after initial injury. These mice were tested for changes in lung compliance (i) and resistance (ii) to assess respiratory function 21 days after BLM injury as described in materials and methods. Bars represent means ± SD of at least 2 repetitions (n = 3 mice/group). Significant differences (***P < 0.005 and *P < 0.05) in lung compliance or resistance between different experimental groups were confirmed by Student's t-test or 1-way ANOVA.
To test the ability of CSP to reverse established ALI and PF, we treated mice with BLM by intranasal instillation to induce fibrosis. Seven and 14 days later, we inserted microosmotic pump containing CSP or CP. The animals were euthanized on day 21 and lung homogenates were analyzed for hydroxyproline. As shown in Fig. 4B, treatment with CSP on day 7 or 14 after exposure to BLM significantly suppressed collagen deposition compared with mice exposed to BLM or BLM+CP. Morphometry also confirmed remarkable protection against BLM-induced ALI and PF in CSP-treated mice compared with those of BLM alone or BLM+CP controls (data not shown). We found that intranasal instillation of BLM caused inflammation of the nares. Intranasal administration CSP to these mice 24 h after BLM injury proved cumbersome and resulted in 20–30% mortality. To avoid higher mortality associated with intranasal CSP administration after BLM, we switched to microosmotic pump to release CSP continuously to the systemic circulation and found better protection. Having achieved protection using microosmotic pump, we finally refined our mode of CSP administration by using IP injections since the latter approach is less invasive. Similar protection was achieved when the CSP was given thrice by IP injection. Lung function testing confirmed a significant compromise in lung compliance 21 days after injury in WT mice exposed to BLM or BLM+CP, whereas mice exposed to saline (BLM vehicle) alone or CSP after BLM had no change in lung compliance (Fig. 4Ci). By contrast, lung resistance was significantly increased in WT mice after BLM injury compared with uninjured mice exposed to intranasal saline. However, lungs of mice treated with CSP after BLM injury showed minimal change in resistance compared those exposed to BLM alone or BLM+CP (Fig. 4Cii) compatible with protection against airway and lung injury.
Microscopic examination of lung sections of mice IP injected with biotin-labeled CSP revealed that the peptide was predominantly distributed in the alveolar and airway epithelium of the BLM-injured lungs compared with uninjured epithelium of the saline-treated lungs (data not shown). This indicates that the lung injury facilitates CSP adsorption, which was independently confirmed by increased (34% vs. 2.6% of total isotope originally found in the lungs) uptake of 125I-labeled CSP by the isolated ATII cells from BLM-injured mice compared with uninjured mice.
We next isolated ATII cells from mice exposed to BLM for 0–5 days and analyzed for changes in p53 and PAI-1 expression, and PARP degradation. PARP is a major enzyme involved in DNA repair and preservation of genomic integrity (41). PARP is cleaved by activated caspase-3, leading to nuclease-mediated genomic DNA fragmentation and cellular apoptosis. As shown in Fig. 5A, BLM induced maximum ATII cell apoptosis as indicated by the presence of Cl.PARP on day 3, parallel with maximum p53 and PAI-1 induction, indicating that p53-induced changes in the fibrinolytic system contribute to ATII cell damage. We next tested ATII cells from mice treated with saline, BLM, BLM+CSP, or BLM+CP for 72 h for changes in uPA, uPAR, p53, PAI-1, and PARP degradation. As shown in Fig. 5B, BLM induced p53 and PAI-1 with a concurrent reduction in ATII cell uPA and uPAR expression. These changes were also associated with increased PARP degradation, demonstrating ATII cell damage. Treatment with CSP, however, reversed p53 and PAI-1 expression and increased both uPA and uPAR associated with reduced PARP degradation, indicating that CSP contributes to ATII cell survival. CSP also suppressed induction of PAI-1 mRNA caused by BLM injury and increased expression of uPA and uPAR mRNAs (Fig. 5C). Protection against ATII cell apoptosis was independently confirmed by flow cytometry. Consistent with the PARP degradation, treatment with CSP significantly (P < 0.001) prevented BLM-induced ATII cell apoptosis (Fig. 5D). TUNEL staining (Fig. 5E) and IHC for Cl.casp-3 (data not shown) also confirmed the anti-apoptotic effect of CSP. Analysis of ATII cells from BLM-injured mice treated IP with 0–125 mg/kg body wt of CSP for Cl.casp-3 demonstrated maximum protection against apoptosis with 1.5 mg/kg peptide (data not shown).
Fig. 5.
CSP reverses BLM-induced changes in ATII cell uPA, uPAR, p53, and PAI-1 expression and apoptosis in vivo. A: ATII cells were isolated from mice on day 1, 3, or 5 after BLM injury and were immunoblotted for PAI-1 and p53 expression and cleaved poly(ADP-ribose) polymerase (Cl.PARP). The same membrane was later stripped and analyzed for β-actin. B: mice exposed to saline or BLM for 24 h were given IP injection of CSP or CP. ATII cells extracted from the lungs of these mice 3 days after BLM injury were analyzed for uPA, uPAR, p53, PAI-1, Cl.PARP, and β-actin by Western blotting. C: total RNA extracted ATII cells from mice treated as described in B were tested for changes or uPA, uPAR, PAI-1, and β-actin mRNA by RT-PCR. D: ATII cells isolated from mice as described in B were treated with anti-annexin-V antibody-PI solution and subjected to flow cytometry. The percentage of apoptotic cells is presented as a bar graph representing mean ± SD of 4 replications. Significant differences (***P < 0.001) between the groups were confirmed by Student's t-test or 1-way ANOVA. E: mice exposed to BLM were treated with or without CSP or CP 24 h after BLM injury. Lung sections from these mice euthanized 3 days after initial BLM injury and control mice were analyzed for apoptosis by TUNEL staining.
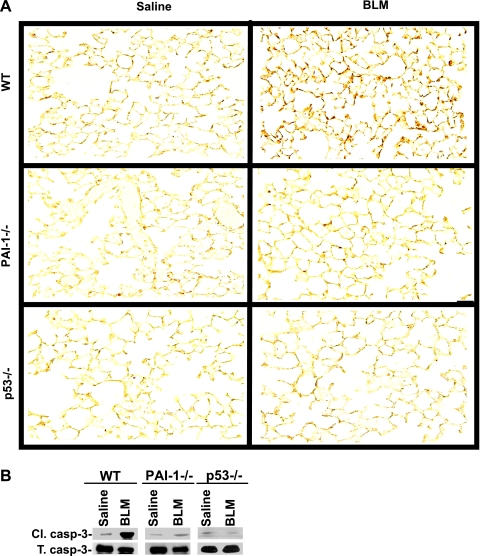
Analysis of lung sections (Fig. 6A) and ATII cells (Fig. 6B) isolated from the BLM-treated WT and p53- or PAI-1-deficient mice showed that both p53- and PAI-1-deficient mice resist BLM-induced ATII cell apoptosis, whereas WT mice showed increased apoptosis. Resistance to BLM-induced LE cell apoptosis in p53 or PAI-1 small interfering RNA (siRNA)-treated cells in vitro (55) and lack of ATII cell apoptosis by p53- and PAI-1-deficient mice after BLM injury (Fig. 6) suggest that induction of p53 and PAI-1 is directly linked. These observations are supported by the inhibition of both BLM-induced p53 and PAI-1 expression with parallel protection against ATII cell apoptosis after CSP in vivo or in vitro. In addition, p53 as a specific mRNA-binding protein inhibits uPA and uPAR and induces PAI-1 in LE cells (46, 54, 55). TUNEL staining and IHC for Cl.casp-3 in lung sections of uPA-deficient mice treated with CSP after BLM demonstrated lack of protection against ATII cell apoptosis (Fig. 7A). Furthermore, immunoblotting for Cl.casp-3, PAI-1, and p53 using ATII cell lysates of uPA-deficient mice exposed to saline, BLM, BLM+CSP, or BLM+CP confirmed that BLM induced p53 and PAI-1 and apoptosis in those cells (Fig. 7B). These observations demonstrate that lung protection by CSP requires inhibition of p53 and PAI-1 and increased uPA expression.
Fig. 6.
p53- and PAI-1-deficient mice resist ATII cell apoptosis. A: lung sections of wild-type (WT), p53-, and PAI-1-deficient mice euthanized 3 days after the exposure to saline or BLM were subjected to TUNEL staining. The stained sections (magnification ×200) are representative of 9 fields/mouse (n = 3 mice). B: ATII cells isolated from WT and p53- and PAI-1-deficient mice 3 days after the exposure to saline or BLM were analyzed for active (Cl.) and total (T) caspase-3 (casp-3) by Western blotting to assess apoptosis.
Fig. 7.
uPA is involved in CSP-mediated protection against BLM-induced apoptosis of ATII cells and prevention of PF. A: lung sections of uPA-deficient mice exposed to BLM with or without CSP or CP euthanized 3 days after BLM injury were tested for apoptosis by TUNEL staining and IHC using an antibody that detects Cl.caspase-3 (1:50 dilution). Mice exposed to saline were used as controls. Representative sections from 3 mice are shown at ×400 magnification. B: ATII cells isolated 72 h after BLM injury from uPA-deficient mice treated with saline, BLM, BLM+CSP or BLM+CP as described in A were analyzed for Cl./T. caspase-3, PAI-1, p53, and β-actin proteins by Western blotting. C: 7 or 14 days after BLM injury, uPA-deficient mice were IP injected with or without CSP or CP. Saline-treated mice were used as controls. Lung sections from these mice euthanized 21 days after BLM injury were subjected to trichrome staining. Sections representative of 9 fields/mouse (n = 3 mice) are shown at ×200 magnification. D: lung homogenates from mice treated as described in C were analyzed for the changes in total hydroxyproline contents. Bars represent means ± SD of at least 3 repetitions (n = 3 mice/group). Significant differences between treatment and control groups were confirmed by Student's t-test or 1-way ANOVA. E: representative lung sections of WT and uPA-deficient mice exposed to BLM with or without CSP or CP euthanized 3 days after BLM injury (n = 3 mice/group) were stained for the proliferation marker Ki-67 by using an anti-Ki-67 antibody (1:50 dilution). Mice exposed to saline were used as controls. Representative sections from 3 mice are shown at ×400 magnification.
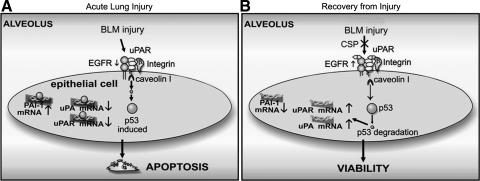
To determine whether uPA expression is crucial to suppress evolving PF, we next treated uPA-deficient mice with CSP or CP 7 and 14 days after BLM injury and analyzed the lung sections (Fig. 7C) as well as homogenates (Fig. 7D) for fibrosis and hydroxyproline content, respectively, on day 21 post-BLM. We found that CSP failed to attenuate BLM-induced fibrosis in uPA-deficient mice. IHC analysis of lung sections showed increased nuclear staining (red) for Ki-67 antigen in WT mice treated with CSP compared with CSP-treated uPA-deficient mice (Fig. 7E), indicating that uPA-mediated proliferation contributes to maintenance of the LE cell viability and alveolar epithelium apart from suppression of ATII cell apoptosis. As shown in Fig. 8, p53 expression is increased during lung injury, including that induced by BLM; p53 in turn binds uPA, uPAR, and PAI-1 mRNA 3′-untranslated region and inhibits uPA and uPAR while inducing PAI-1. Reversal of these changes by CSP treatment enhances viability of ATII cells and resistance to pulmonary fibrosis.
Fig. 8.
Regulation of LE cell viability through cross talk between p53 and components of fibrinolytic system. A: p53 is induced during acute lung injury then binds transcripts for uPA, uPAR, and PAI-1 and inhibits uPA and uPAR mRNA through destabilization of the transcripts (46, 54). PAI-1 is induced through p53-mediated stabilization of PAI-1 mRNA (55); the end result is reciprocal suppression of uPA and uPAR and increased PAI-1 expression by LE cells. These conditions result in enhanced LE cell apoptosis. B: expression of p53 can be inhibited by activation of the β1-integrin-uPAR-EGFR complex through CSP, resulting in competitive inhibition of caveolin-1. The paucity of p53 in LE cells prevents p53 binding to uPA, uPAR, and PAI-1 mRNA, so uPA and uPAR transcripts remain stable whereas PAI-1 mRNA is destabilized. uPA and uPAR levels in LE cells are preserved and serve as survival signals whereas PAI-1 expression is inhibited.
DISCUSSION
In normal as well as mildly injured lungs, ATII cells proliferate and differentiate into ATI cells as part of epithelial repair. Apoptosis is essential to clear the cells that are damaged beyond repair as they otherwise prolong inflammation and delay epithelial regeneration (13, 18). In this study, we found that CSP inhibits ATII cell p53 and PAI-1 and reciprocally reverses suppression of uPA and uPAR caused by BLM. These changes in turn prevent ATII cell apoptosis, indicating that reversal of p53-mediated coordinate changes in the components of fibrinolytic system contribute to preservation of ATII cells in this model of ALI.
We found that, unlike WT mice, p53-deficient mice exposed to BLM by intranasal instillation resisted ATII cell apoptosis. This is in agreement with the earlier observation reported by Okudela and colleagues (42). We suspect that the increased LE cell apoptosis of p53-deficient mice reported by Davis et al. (13) can be explained by the very high amount of BLM (50 U/kg body wt) used in that study compared with 2–20 U used in the present study or that reported by Kuwano et al. (27) or others (37). In addition, the responses of p53 to DNA damage could also vary depending on tissue and cell specificity (62). The evidence that p53-induced PAI-1 contributes to ATII cell apoptosis in vivo was further supported by resistance against BLM-induced ATII cell apoptosis in both p53- and PAI-1-deficient mice. Earlier reports showed that increased susceptibility to BLM-induced PF occurs in mice that overexpress PAI-1 whereas targeted deletion of PAI-1 gene is protective (20). Our findings further demonstrate that reduced p53-PAI-1-mediated ATII cell apoptosis contributed to prevention of PF or actually reverses evolving PF after BLM injury. Interestingly, p53-null mice showed focal inflammation followed by PF whereas both PAI-1- and p53-deficient mice resist apoptosis. This inconsistency could be explained by increased inflammation in p53−/− mice from accumulation of apoptotic cells due to less thrombospondin-induced phagocytosis (11).
In this study, we used a peptide-based therapeutic approach to inhibit ATII cell apoptosis and reverse PF in mice with BLM-induced injury. Remarkable protection by CSP against ATII cell apoptosis was noted throughout the 3- to 21-day course of the lung injury. CSP administered in either a preventive or a treatment mode through nasal insufflations, microosmotic pump or IP injection all inhibited ATII cell apoptosis and PF. This approach is therefore promising as a potential avenue for clinical intervention.
Our findings also broaden our understanding of the role of uPA in the pathogenesis of ALI. We found that uPA-deficient mice showed maximum apoptosis after BLM injury. uPA interacts with uPAR to promote LE cell proliferation and migration (49). In addition, inhibition of both uPA and uPAR expression by siRNA in neuroblastoma cells (16–17, 60) or by uPA mRNA-specific antisense oligonucleotide in mammary carcinoma cells (33) promotes activation of caspase-3. uPA plays a critical role in preventing the development of PF through protection against ATII cell apoptosis in addition to maintaining alveolar proteolysis. This conclusion is buttressed by the inability of CSP to mitigate BLM-induced ATII cell apoptosis in uPA-deficient mice. Administration of CSP at 7 or 14 days post-BLM injury in uPA-deficient mice failed to inhibit PF, underscoring the critical role played by uPA. Inability of CSP to block ATII cell p53 or PAI-1 expression in uPA-deficient mice exposed to BLM indicates that inhibition of p53 and downstream PAI-1 expression by uPA is likewise crucial to the salutary effects of CSP.
The peptide-based approach is supported by the earlier observations of Tourkina et al. (64) that a chimeric CSP peptide containing a Penetratin sequence suppressed LE cell apoptosis when administered prior to BLM injury. The present study independently extends this finding and demonstrates that CSP, which lacks a penetration sequence, confers effective protection against BLM-induced ALI and PF. In addition, we demonstrate the mechanistic link between p53 and the fibrinolytic system during ALI and PF (Fig. 8). Our findings confirm that cross talk between p53 and the fibrinolytic system represents an appropriate target to protect against apoptosis of ATII cells. Reversal of the proapoptotic changes by CSP implicates the interaction of p53 with uPA, uPAR, and PAI-1 mRNA in the response.
ATII cell apoptosis continues during the progression of ALI and fibrotic repair. We found that CSP effectively blocks PF irrespective of whether it is administered early or late after BLM-induced injury and that reversal of PF involves protection against ATII cell apoptosis. CSP failed to inhibit BLM induced p53 expression and ATII cell apoptosis or induce Ki-67 (proliferation marker) in uPA-deficient mice, whereas WT mice showed increased staining for Ki-67 after CSP treatment. CSP also induces uPA expression in vitro and in vivo, activates β1-integrins and inhibits of LE cell apoptosis (Fig. 2). These observations collectively suggest that CSP-induced uPA contributes to ATII cell viability and lead to the contention that uPA-mediated proliferation is, at least in part, responsible for its protection. Alternatively, CSP could block both ATII cell apoptosis and inhibit epithelial myofibroblast transformation, which are possibilities to be investigated in future studies. Although the full scope of the response remains to be elucidated, the present study clearly shows that CSP represents an effective treatment that reverses the fibrotic outcome of BLM-induced ALI and that cross talk between p53 and components of the fibrinolytic system contributes to the response.
GRANTS
This work was supported in part by grants from Flight Attendant Medical Research Institute Clinical Innovator Award (FAMRI-ID-082380) and National Heart, Lung, and Blood Institute Grant R21-HL093547.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Harold Chapman Jr. for helpful discussion concerning the manuscript.
REFERENCES
- 1. Alfano D, Franco P, Vocca I, Gambi N, Pisa V, Mancini A, Caputi M, Carriero MV, Iaccarino I, Stoppelli MP. The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thromb Haemost 93: 205– 211, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Barazzone C, Belin D, Piguet PF, Vassalli JD, Sappino AP. Plasminogen activator inhibitor-1 in acute hyperoxic mouse lung injury. J Clin Invest 98: 2666– 2673, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barker SC, Kassel DB, Weigl D, Huang X, Luther MA, Knight WB. Characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochemistry 34: 14843– 14851, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, Chapman HA., Jr Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med 322: 890– 897, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Bhandary YP, Shetty SK, Shetty R, Idell S, Starcher B, Shetty S. Regulation of lung epithelial apoptosis by coordinate expression of components of the fibrinolytic system (Abstract). Am J Respir Crit Care Med 181: A4185, 2010 [Google Scholar]
- 6. Bhandary YP, Velusamy T, Shetty P, Shetty RS, Idell S, Cines DB, Jain D, Bdeir K, Abraham E, Tsuruta Y, Shetty S. Post-transcriptional regulation of urokinase-type plasminogen activator receptor expression in lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med 179: 2882– 98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chapman HA. Disorders of lung matrix remodeling. J Clin Invest 113: 148– 157, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiang GG, Sefton BM. Phosphorylation of a Src kinase at the autophosphorylation site in the absence of Src kinase activity. J Biol Chem 275: 6055– 6058, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Cooper JA, MacAuley A. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc Natl Acad Sci USA 85: 4232– 4236, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 14: 309– 315, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 265: 1582– 1584, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Davidson D, Chow LM, Veillette A. Chk, a Csk family tyrosine protein kinase, exhibits csk-like activity in fibroblasts, but not in an antigen-specific T-cell line. J Biol Chem 272: 1355– 1362, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Davis DW, Weidner DA, Holian A, McConkey DJ. Nitric oxide-dependent activation of p53 suppresses bleomycin-induced apoptosis in the lung. J Exp Med 192: 857– 869, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 97: 232– 237, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. Reciprocal regulation of neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for human breast cancer. J Biol Chem 273: 20448– 20455, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol 31: 19– 27, 2007 [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta R, Rao Gogineni V, Nalla AK, Chetty C, Klopfenstein JD, Tsung AJ, Mohanam S, Rao JS. Oncogenic role of p53 is suppressed by si-RNA bicistronic construct of uPA, uPAR and cathepsin-B in meningiomas both in vitro and in vivo. Int J Oncol 38: 973– 983, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 17: 272– 278, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Hardwick JS, Sefton BM. Activation of the Lck tyrosine protein kinase by hydrogen peroxide require the phosphorylation of Tyr-394. Proc Natl Acad Sci USA 92: 4527– 4531, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 106: 1341– 1350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol 38: 78– 87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Idell S. Endothelium and disordered fibrin turnover in the injured lung: newly recognized pathways. Crit Care Med 30: S274– S280, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Idell S. Extravascular coagulation and fibrin deposition in acute lung injury. New Horiz 2: 566– 574, 1994 [PubMed] [Google Scholar]
- 24. Idell S. Coagulation, fibrinolysis and fibrin deposition in lung injury and repair. In: Pulmonary Fibrosis, edited by Phan SH, Thrall RS. New York: Dekker, 1995, p. 743– 776 [Google Scholar]
- 25. Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 164: S28– S38, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Jo M, Thomas KS, Marozkina N, Amin TJ, Silva CM, Parsons SJ, Gonias SL. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem 280: 17449– 17457, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kuwano K, Hagimoto N, Tanaka T, Kawasaki M, Kunitake R, Miyazaki H, Kaneko Y, Matsuba T, Maeyama T, Hara N. Expression of apoptosis-regulatory genes in epithelial cells in pulmonary fibrosis in mice. J Pathol 190: 221– 229, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Lau HK, Ho J. Regulation of plasminogen activator inhibitor-1 secretion by urokinase and tissue plasminogen activator in rat epithelioid-type smooth muscle cells. Br J Haematol 117: 151– 158, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Lazar MH, Christensen PJ, Du M, Yu B, Subbotina NM, Hanson KE, Hansen JM, White ES, Simon RH, Sisson TH. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am J Respir Cell Mol Biol 31: 672– 678, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Li C, Zhang J, Jiang Y, Gurewich V, Chen Y, Liu JN. Urokinase-type plasminogen activator up-regulates its own expression by endothelial cells and monocytes via the u-PAR pathway. Thromb Res 103: 221– 232, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Lisanti MP, Tang Z, Scherer PE, Kubler E, Koleske AJ, Sargiacomo M. Caveolae, transmembrane signalling and cellular transformation. Mol Membr Biol 12: 121– 124, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Liu J, Lee P, Galbiati F, Kitsis RN, Lisanti MP. Caveolin-1 expression sensitizes fibroblastic and epithelial cells to apoptotic stimulation. Am J Physiol Cell Physiol 280: C823– C835, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci 114: 3387– 3396, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Mazar AP, Henkin J, Goldfarb RH. The urokinase plasminogen activator system in cancer: implications for tumor angiogenesis and metastasis. Angiogenesis 3: 15– 32, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Mazzieri R, Blasi F. The urokinase receptor and the regulation of cell proliferation. Thromb Haemost 93: 641– 646, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Migliaccio E, Giorgio M, Pelicci PG. Apoptosis and aging: role of p66Shc redox protein. Antioxid Redox Signal 8: 600– 608, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Mishra A, Doyle NA, Martin WJ. Bleomycin-mediated pulmonary toxicity: evidence for a p53-mediated response. Am J Respir Cell Mol Biol 22: 543– 549, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Montuori N, Mattiello A, Mancini A, Taglialatela P, Caputi M, Rossi G, Ragno P. Urokinase-mediated posttranscriptional regulation of urokinase-receptor expression in non small cell lung carcinoma. Int J Cancer 105: 353– 360, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Murphy M, Mabruk MJ, Lenane P, Liew A, McCann P, Buckley A, Flatharta O, Hevey D, Billet P, Robertson W, Javed S, Leader M, Kay E, Murphy GM. Comparison of the expression of p53, p21, Bax and the induction of apoptosis between patients with basal cell carcinoma and normal controls in response to ultraviolet irradiation. J Clin Pathol 55: 829– 833, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 351: 69– 72, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37– 43, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Okudela K, Ito T, Mitsui H, Hayashi H, Udaka N, Kanisawa M, Kitamura H. The role of p53 in bleomycin-induced DNA damage in the lung. A comparative study with the small intestine. Am J Pathol 155: 1341– 1351, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olman MA, Mackman N, Gladson CL, Moser KM, Loskutoff DJ. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin Invest 96: 1621– 1630, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 21: 7435– 7451, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 176: 1261– 1268, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Shetty P, Velusamy T, Bhandary YP, Shetty RS, Liu MC, Shetty S. Urokinase expression by tumor suppressor protein p53: a novel role in mRNA turnover. Am J Respir Cell Mol Biol 39: 364– 372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shetty S, Idell S. Urokinase induces expression of its own receptor in Beas2B lung epithelial cells. J Biol Chem 276: 24549– 24556, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Shetty S, Idell S. Urokinase/urokinase receptor-mediated signaling in cancer. In: Apoptosis, Cell Signaling and Human Diseases: Molecular Mechanisms, edited by Srivastava R. Totowa, NJ: Humana, 2006, vol. 2, p. 167– 177. [Google Scholar]
- 49. Shetty S, Pendurthi UR, Halady PK, Azghani AO, Idell S. Urokinase induces its own expression in Beas2B lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 283: L319– L328, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Shetty S, Bdeir K, Cines DB, Idell S. Induction of plasminogen activator inhibitor-1 by urokinase in lung epithelial cells. J Biol Chem 278: 18124– 18131, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Shetty S, Gyetko MR, Mazar AP. Induction of p53 by urokinase in lung epithelial cells. J Biol Chem 280: 28133– 28141, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Shetty S, Rao GN, Cines DB, Bdeir K. Urokinase induces activation of STAT3 in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L772– L780, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Shetty S, John J, Idell S. Fibrosis including fibrinolytic pathways. In: Textbook of Pleural Diseases (2nd ed.), edited by Light RW, Lee YCG. London: Hodder Arnold, 2007, p. 101– 112. [Google Scholar]
- 54. Shetty S, Velusamy T, Idell S, Shetty P, Mazar AP, Bhandary YP, Shetty RS. Regulation of urokinase receptor expression by p53: novel role in stabilization of uPAR mRNA. Mol Cell Biol 27: 5607– 5618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. J Biol Chem 283: 19570– 19580, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shetty S, Padijnayayveetil J, Tucker T, Stankowska D, Idell S. The fibrinolytic system and the regulation of lung epithelial cell proteolysis, signaling, and cellular viability. Am J Physiol Lung Cell Mol Physiol 295: L967– L975, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature 385: 602– 609, 1997 [DOI] [PubMed] [Google Scholar]
- 58. Sisson TH, Hattori N, Xu Y, Simon RH. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum Gene Ther 10: 2315– 2323, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Starcher B, Kuhn C. Combining histology and biochemical measurements of connective tissue components in small samples of lung: application to bleomycin-induced fibrosis in the mouse. Exp Lung Res 29: 179– 194, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Subramanian R, Gondi CS, Lakka SS, Jutla A, Rao JS. siRNA-mediated simultaneous downregulation of uPA and its receptor inhibits angiogenesis and invasiveness triggering apoptosis in breast cancer cells. Int J Oncol 28: 831– 839, 2006 [PMC free article] [PubMed] [Google Scholar]
- 61. Sutherland LM, Edwards YS, Murray AW. Alveolar type II cell apoptosis. Comp Biochem Physiol A Mol Integr Physiol 129: 267– 285, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Tamura T, Ishihara M, Lamphier MS, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak TW, Taki S, Taniguchi T. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature 376: 596– 599, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Tiddens H, Silverman M, Bush A. The role of inflammation in airway disease: remodeling. Am J Respir Crit Care Med 162: S7– S10, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Tourkina E, Richard M, Gooz P, Bonner M, Pannu J, Harley R, Bernatchez PN, Sessa WC, Silver RM, Hoffman S. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 294: L843– L861, 2008 [DOI] [PubMed] [Google Scholar]
- 65. Wanaski SP, Ng BK, Glaser M. Caveolin scaffolding region and the membrane binding region of SRC form lateral membrane domains. Biochemistry 42: 42– 56, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. A role of caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol 144: 1285– 1294, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385: 595– 602, 1997 [DOI] [PubMed] [Google Scholar]
- 68. You D, Ripple M, Balakrishna S, Troxclair D, Sandquist D, Ding L, Ahlert TA, Cormier SA. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol 181: 3486– 3494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]