Abstract
We recorded sleep electroencephalogram longitudinally across ages 9–18 yr in subjects sleeping at home. Recordings were made twice yearly on 4 consecutive nights: 2 nights with the subjects maintaining their ongoing school-night schedules, and 2 nights with time in bed extended to 12 h. As expected, school-night total sleep time declined with age. This decline was entirely produced by decreasing non-rapid eye movement (NREM) sleep. Rapid eye movement (REM) sleep durations increased slightly but significantly. NREM and REM sleep durations also exhibited different age trajectories when sleep was extended. Both durations exceeded those on school-night schedules. However, the elevated NREM duration did not change with age, whereas REM durations increased significantly. We interpret the adolescent decline in school-night NREM duration in relation to our hypothesis that NREM sleep reverses changes produced in plastic brain systems during waking. The “substrate” produced during waking declines across adolescence, because synaptic elimination decreases the intensity (metabolic rate) of waking brain activity. Declining substrate reduces both NREM intensity (i.e., delta power) and NREM duration. The absence of a decline in REM sleep duration on school-night sleep and its age-dependent increase in extended sleep pose new challenges to understanding its physiological role. Whatever their ultimate explanation, these robust findings demonstrate that the two physiological states of human sleep respond differently to the maturational brain changes of adolescence. Understanding these differences should shed new light on both brain development and the functions of sleep.
Keywords: brain maturation, adolescence, electroencephalogram
it is now accepted that adolescence is a period of extensive brain reorganization. Synaptic density, cerebral metabolic rate (CMR), and non-rapid eye movement (NREM) delta electroencephalogram (EEG) activity decline steeply and in parallel (21). These developmental brain changes presumably underlie the emergence of adult cognitive power (11). We recently delineated the longitudinal time course of the adolescent decline in NREM delta (1–4 Hz) EEG power (5). It falls by over 60% between ages 11 and 16.5 yr, when its rate of decline markedly slows. This period of rapid decline may define brain adolescence (5). NREM delta power continues to decline after age 16.5 yr, but at a slower rate. By late middle age, it has fallen a further 50% (22).
Another major change in sleep physiology during adolescence (at least in developed countries) is a substantial decline in school-night total sleep time (TST) (cf., Ref. 40). A recent cross-sectional survey of 4,032 Australian youth, 9–18 yr of age, reported that TST on school-nights decreased by an average of 12 min/yr (30). Questionnaire surveys cannot determine the relative contributions of NREM and rapid eye movement (REM) sleep to changes in TST. This distinction requires EEG recording. One major goal of the present study was to determine with longitudinal recordings how NREM and REM durations change as school-night TST declines across adolescence. We also investigated adolescent changes in NREM and REM durations during extended sleep by having subjects remain in bed for 12 h.
Our findings reveal robust differences in the adolescent trajectories of NREM and REM durations on both school-night and extended sleep schedules. NREM and REM sleep are qualitatively different states of brain organization, in which neuronal discharge rates, neuronendocrine patterns, and CMR differ grossly. Their markedly different trajectories across adolescence demonstrate differential effects of late brain maturation on NREM and REM sleep physiology.
METHODS
The methods of our longitudinal study have been previously described (5, 6) and are briefly summarized here.
Subjects
Data are from 67 subjects in two age cohorts. Cohort C9 (n = 30, 16 girls) entered the experiment at ∼9 yr of age (mean age at entry = 9.31 yr), and cohort C12 (n = 37, 19 girls) entered at ∼12 yr of age (mean age 12.29 yr). The data presented are from the first 6 yr of the study. The combined cohorts span ages 9–18 yr with 3 yr of overlap (ages 12–15 yr). All 67 subjects completed at least 3 yr of the study. Fifty-six subjects completed all 6 yr. Parents provided informed consent for all subjects, and subjects older than 12 yr provided assent. Subjects were paid for their participation. The University of California Davis Institutional Review Board approved all procedures.
Study Design
Twice a year, at ∼6-mo intervals, all-night EEG was recorded for 4 consecutive nights with subjects sleeping at home in their own beds. On the first 2 nights (usually Wednesday and Thursday), subjects went to bed at their current habitual weekday bedtime and arose at their weekday rise time. On the third and fourth nights (Friday and Saturday), subjects retired at their school-night bedtimes, but were instructed to sleep as long as possible (up to 12 h). While the school-night schedules are essentially naturalistic, the extended nights are not a model of the typical weekend sleep of adolescents. Adolescents typically go to bed later and awaken later on weekends. We required that bedtimes be kept to those of the ongoing school-night schedule, and that time in bed (TIB) be extended. This requirement was intended to minimize circadian changes on the patterns of extended sleep.
Below, we first present the results for NREM and REM sleep durations on school-nights (nights 1 and 2) and then report the findings for extended sleep (nights 3 and 4). For nights 1 and 2, of the 804 possible subject recordings (67 subjects × 12 recordings), 37 were lost due to subject attrition, and 30 recordings could not be used because of recorder or electrode failure on both nights 1 and 2. When both nights 1 and 2 were usable, we used the 2-night average as the data point for the semiannual recording. For night 3, the first extended night, 523 nights of data were usable. Of these 523 recordings, 414 also had usable night 4 data. Extended night data for nights 3 and 4 were treated separately rather than being averaged, because night 4 follows a shorter period of waking due to extended sleep on night 3.
To reduce night-to-night variability in sleep schedule, we required subjects to maintain their regular school-night schedules for the 5 nights before EEG recording and for the first 2 nights of study. Napping was prohibited throughout this period. Subjects wore actigraphy watches (Minimitter A16) to confirm compliance with the required schedules. Recordings were canceled and rescheduled if actigraphy revealed deviations from required schedules. Across the 6 yr of the study, adolescents progressively reduced their TIB on school-nights. We accepted these changes and performed our recordings on each individual's current school-night schedule. We scheduled recordings during the school year when possible, but it was necessary to perform some recordings (17%) during summer vacations to complete the study. For summer recordings, subjects maintained the weekday (school) sleep schedule of the preceding school year. Compliance was more difficult to achieve during summer vacations; therefore, we included “summer” as a covariate in the analyses.
EEG Recording
At the subjects' homes, technicians applied EEG electrodes at Fz, Cz, C3, C4, O1 and either O2 or Pz with A1 and A2 mastoid electrodes. Electrooculogram (EOG) electrodes were applied at the left and right outer canthi and referred to an electrode applied in the center of the forehead. Technicians did not stay to monitor the recording throughout the night.
EEG was recorded on Grass H2O ambulatory EEG recorders for the first nine semiannual recordings. We switched to Grass Aura recorders during the 10th recording period when Grass discontinued support for the H2O. (The H2O and Aura showed virtually identical frequency response curves across the 1- to 4-Hz frequency range used in the analyses of delta power.) Signals were digitized at 200 Hz for the H2O and 400 Hz for the Aura, saved on the recorder's disk, and downloaded to laboratory computers for analysis. The recorders have a push-button event marker that subjects were instructed to use to indicate “lights off” time. Of the 737 subject recordings used in the sleep duration analysis, only 562 had identifiable lights off time. On 175 recordings, the subjects either did not press the button, or the button press did not register. On these nights, we could not determine sleep onset latency or total TIB, although we could measure TST, NREM, and REM durations.
EEG Analysis
The digitized EEG was displayed on a computer monitor for visual sleep stage scoring using PASS PLUS (Delta Software, St. Louis, MO). Each 20-s epoch was scored as wake, stage 1, NREM, REM, or movement using Rechtschaffen and Kales (31) criteria modified by collapsing stages 2, 3, and 4 into one NREM stage. EEG was scored by one scorer and checked by a second scorer. Discrepancies were resolved by a senior lab scientist (I. G. Campbell). The data were collected over a 6-yr period and scored as they were collected. Scorers changed during the study with turnover of laboratory personnel. All scorers were trained by the same senior laboratory scientist and were required to achieve 95% agreement before they were allowed to score new recordings. Despite these efforts, statistical analysis revealed small but significant differences among scorers. We, therefore, included scorer as a covariate in the statistical analyses.
The main sleep duration variables were all-night TIB, sleep onset latency, wake after sleep onset, TST, and its two components, NREM and REM durations. Epochs with body movement, stage 1, and wake after sleep onset were not included in TST, but were included in TIB. Sleep onset latency was the time from the push button marker of lights out to the beginning of sustained sleep, defined as 5 consecutive min of NREM or REM sleep uninterrupted by an epoch scored as wake. TIB was the duration of recorded EEG from push button to final morning waking. Wake after sleep onset included both wake and movement time between sleep onset and the final morning awakening. We also measured the durations of consecutive NREM periods (NREMPs) and REM periods (REMPs) 1–4 on school-night sleep. We restricted these cycle analyses to subjects with four or more complete cycles. Cycles were defined according to Feinberg and Floyd criteria (16). In children, the first NREMP can be abnormally long if a skipped first REMP is not recognized (20, 24). We split these long NREMPs into two when the epoch-by-epoch plots of delta power showed two clear peaks separated by a valley of at least 10-min duration. In such cases, the REM duration for cycle 1 was 0 min.
We used the fast Fourier transform module of PASS PLUS to analyze EEG recorded from C3 or C4 vs. contralateral mastoid. Power in the 1- to 4-Hz (delta) frequency band was averaged over the first 5 h of NREM sleep. Fast Fourier transform analysis was performed on all artifact-free NREM epochs using 5.12-s Welch tapered windows with a 2.62-s overlap for eight windows per 20-s epoch. Since this power measure is expressed per unit time, it is independent of NREM sleep duration.
Statistical Analysis
Age-related changes in the sleep duration were evaluated with linear mixed-effect analysis (MEA) (SAS, Proc Mixed). The analysis provided estimates of the intercept and slope and their standard errors. This analysis also allowed us to determine the significance of the relation between a dependent variable and the independent variable, age. MEA is well-suited for longitudinal data, because it accounts for the inherent correlation of repeated measures from the same subject and effectively accommodates missing data (32, 37). We also used SAS Proc Mixed to examine relations with covariates controlled. As noted above, we evaluated the effects of “summer” and “scorer.” Also, we tested for sex differences in the age-change in sleep duration by including “sex” as a class variable.
We used bivariate mixed models to test whether the developmental trend in two variables was related. Thus we tested whether the age-related decline in NREM duration on school-nights was related to the age-related decline in delta power. We also tested if the age-related decline in TST on school-nights was related to the age-related increase in TST on extended nights. For both analyses, we used SAS Proc Mixed linear models for both variables with intercepts and slopes random and tests for significant covariance in their age-related rates of change (i.e., slopes). In addition to linear models, we used Winbugs (33) to fit Gompertz equations to the age-related changes in NREM duration and delta power and to test for covariance among the parameters of the Gompertz equations.
RESULTS
School-Night Schedules
TIB, sleep onset latency, and sleep efficiency.
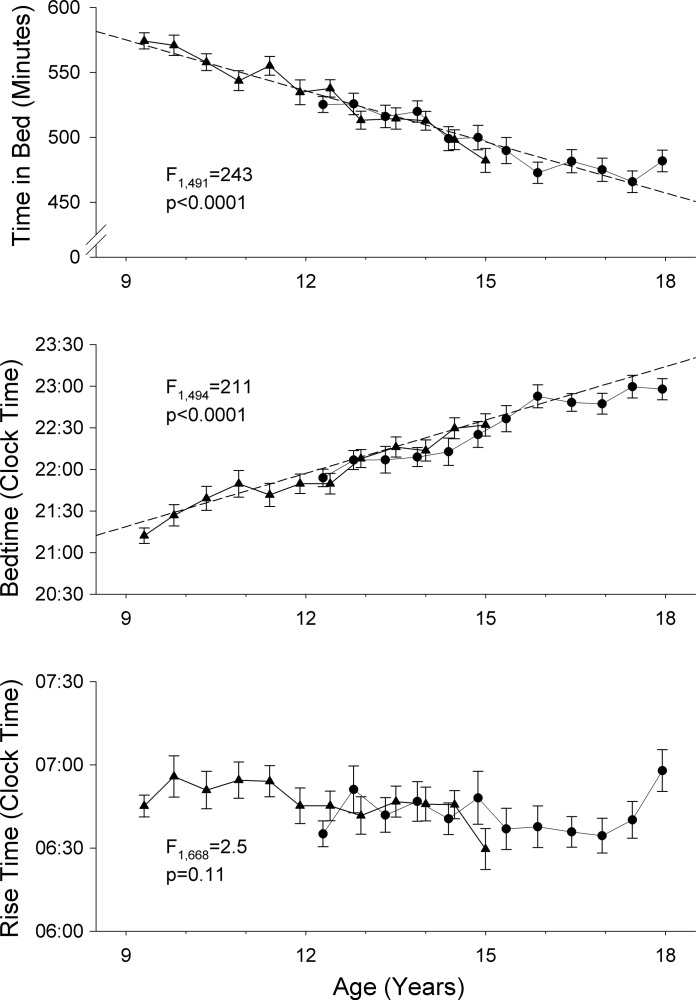
Across ages 9–18 yr, adolescents significantly (F1,491 = 243, P < 0.0001) reduced their TIB (Fig. 1A). Linear MEA estimated the decline at 13.1 ± 0.8 min/yr (means ± SE) from the intercept of 575 ± 4 min at age 9 yr. The change in TIB resulted from progressively later bedtimes (Fig. 1B; F1,494 = 211, P < 0.0001), which increased by 12.8 ± 0.9 min/yr from the estimated bedtime of 21:15 ± 0:05 at age 9 yr. Rise time (Fig. 1C) did not change significantly (F1,668 = 2.5, P = 0.11) from an average of 06:50 ± 0:04 at age 9 yr.
Fig. 1.
Average ± SE time in bed (A), bedtime (B), and rise time (C) at each semiannual recording for the C9 cohort (subjects who entered the experiment at ∼9 yr of age; ▴) and C12 cohort (subjects who entered the experiment at ∼12 yr of age; ●). Results of mixed-effect analysis of age effects (F and P values) are shown on each plot. Linear trend lines (dashed lines) computed by mixed-effect analysis are shown for variables that changed significantly with age. Time in bed decreased across adolescence because bed times became later and rise times did not change.
Sleep onset latency varied greatly between subjects and from one recording period to the next. However, sleep onset latency did not change significantly across ages 9–18 yr (F1,490 = 0.14, P = 0.70). MEA estimated the intercept for sleep onset latency at age 9 yr as 15.9 ± 1.1 min. Sleep efficiency increased significantly (F1,491 = 33.7, P < 0.0001) by 0.38 ± 0.07%/yr from 89.4 ± 0.42% at age 9 yr. Wake after sleep onset was ∼35.1 ± 1.6 min at age 9 yr and decreased significantly (F1,668 = 113, P < 0.0001) by 2.53 ± 0.24 min/yr.
TST, NREM, and REM sleep durations.
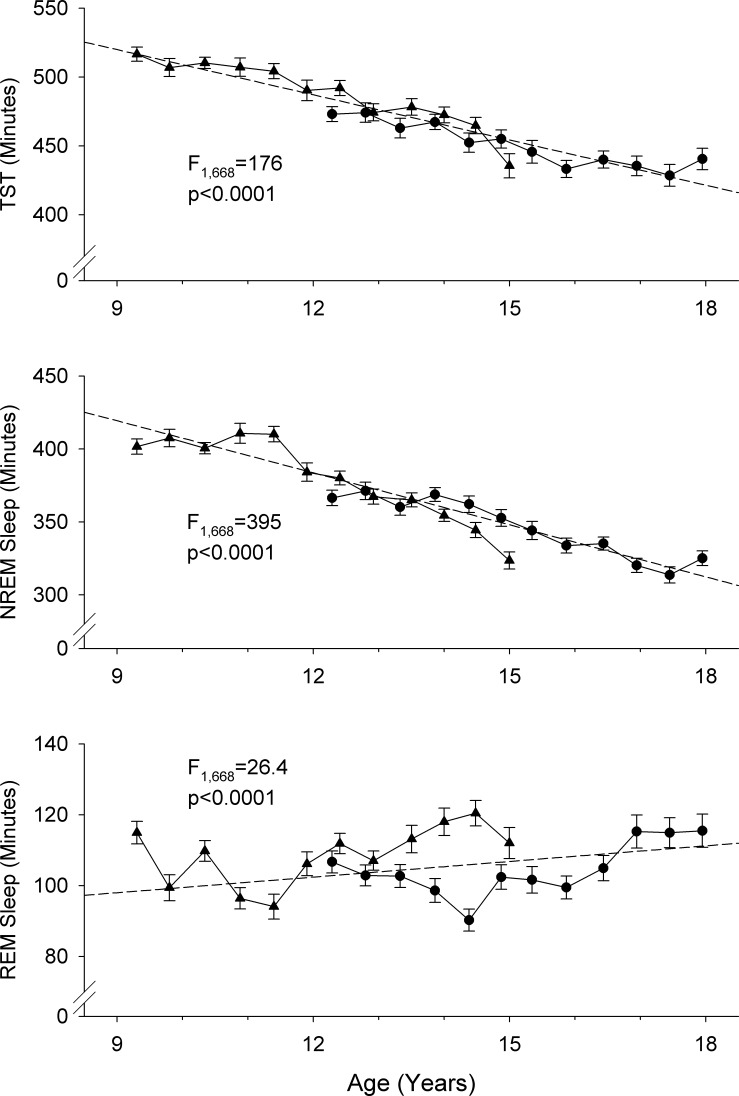
TST declined significantly (F1,668 = 176, P < 0.0001) by 10.3 ± 0.8 min/yr from an intercept of 515.9 ± 4.2 min at age 9 yr (Fig. 2A). TST declined across adolescence entirely because of a significant (F1,668 = 395, P < 0.0001) reduction in NREM sleep duration (Fig. 2B), which decreased by 12.0 ± 0.6 min/yr from 417.9 ± 3.9 min at age 9 yr. In contrast, REM sleep duration (Fig. 2C) increased slightly but significantly (F1,668 = 26.4, P < 0.0001) at a rate of 1.9 ± 0.37 min/yr. The intercept for REM duration at age 9 yr was 97.5 ± 2.0 min. As a result of the declining NREM duration and increasing REM duration, the REM-to-NREM ratio increased significantly (F1,668 = 262, P < 0.0001) by 0.016 per year from 0.22 at age 9 yr.
Fig. 2.
Average ± SE school-night total sleep time (TST; A), non-rapid eye movement (NREM) sleep duration (B), and rapid eye movement (REM) sleep duration (C) plotted against age. Format is the same as in Fig 1. The adolescent decline in TST was entirely due to a significant decrease in NREM sleep duration. REM sleep duration increased slightly but significantly. ▴, C9 cohort; ●, C12 cohort.
Covariate evaluation.
Controlling for the covariate “scorer” did not alter the significance of the age-related reductions in TST and NREM sleep duration, which remained robust (P < 0.0001). However, the significance of the increase in REM duration became somewhat weaker (P = 0.0072). Statistically controlling for scorer had little effect on the parameter estimates for the intercept or slope of the age-related declines in TST or NREM sleep duration (Table 1). Although subjects were instructed to keep their habitual school year schedule during the summer, their TST, NREM, and REM durations were all significantly longer in summer recordings (Table 2). However, these longer durations did not affect the age-related changes described above. When both summer recordings and scorer were statistically controlled, TST and NREM duration still declined significantly with age (P < 0.0001), and the REM duration still increased significantly with age (P = 0.0034). There were no significant sex differences in the age-related declines in TST and NREM duration (P = 0.067 and P = 0.096) or in the age-related increase in REM sleep duration (P = 0.77).
Table 1.
Age-related changes in school-night sleep durations with scorer statistically controlled
| Intercept, min |
Slope, min/yr |
Age |
Scorer |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | F1,661 | P | F7,253 | P | |
| TST | 519.9 | 5.7 | −10.94 | 1.1 | 107 | <0.0001 | 1.01 | 0.42 |
| NREM | 419.3 | 5.3 | −11.89 | 0.87 | 187 | <0.0001 | 3.96 | 0.0004 |
| REM | 98.0 | 3.0 | +1.47 | 0.55 | 7.27 | 0.0072 | 4.39 | 0.0001 |
Results are of linear mixed-effect analysis of changes in sleep duration between age 9 and 18 yr with scorer statistically controlled. Included are estimates of intercept and slope (with SE of estimates) and statistical tests of the significance of age-related changes and scorer differences in sleep duration. Intercept is the sleep duration at age 9 yr. Slope is the change in sleep duration per year. TST, total sleep time; REM, rapid eye movement; NREM, non-REM.
Table 2.
Age-related changes in school-night sleep durations with summer statistically controlled
| Intercept, min |
Slope, min/yr |
Age |
Summer |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | F1,660 | P | Mean, min | SE, min | F1,660 | P | |
| TST | 514.5 | 5.7 | −10.61 | 1.0 | 103 | <0.0001 | 19.1 | 3.1 | 36.8 | <0.0001 |
| NREM | 416.2 | 5.2 | −11.71 | 0.85 | 188 | <0.0001 | 11.5 | 2.5 | 20.6 | <0.0001 |
| REM | 95.8 | 3.0 | +1.62 | 0.55 | 8.63 | 0.0034 | 7.5 | 1.6 | 21.3 | <0.0001 |
Analysis of age effects on sleep duration with effects of summer recordings and scorer (not shown) statistically controlled. Format is the same as in Table 1.
The age decline for school-night NREM duration parallels that for NREM delta power.
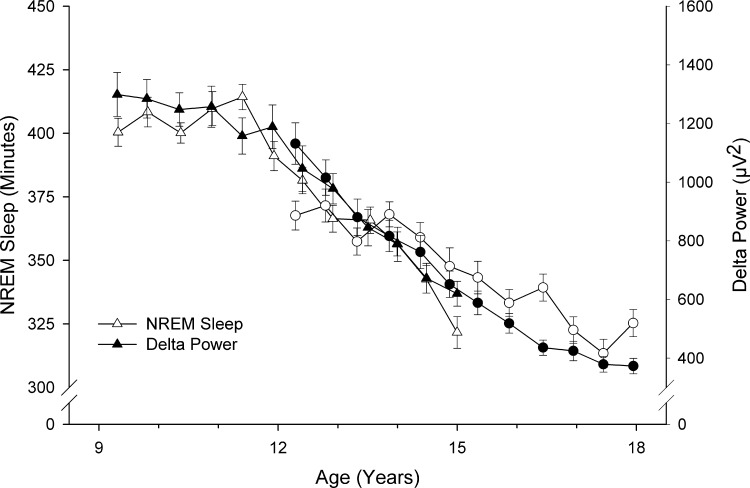
We previously reported that the decline in delta EEG power across late childhood and adolescence can be described with a Gompertz function that drops steeply from an upper to lower asymptote (5). Figure 3 shows that the age curve for NREM duration is similar to the curve that we recently published for NREM delta power (5). Both curves show little change between ages 9 and 11.5 yr, then decline steeply to about age 16.5 yr, and then begin to flatten. We, therefore, fit a Gompertz equation to the NREM decline and obtained the following parameters: upper asymptote = 415 min, lower asymptote = 277 min, age of most rapid decline = 13.5 yr, relative rate of decline = 0.336 yr−1. Despite the similar shape of the two curves, bivariate growth function analysis did not reveal a significant covariance between their Gompertz parameters. Correlations calculated from the covariance of these parameters ranged from −0.1 to 0.57, and the 95% confidence intervals for the covariances included 0. Bivariate linear mixed analysis also failed to demonstrate significant covariance between the rate of decline in NREM duration and the rate of decline in NREM delta power (P = 0.29).
Fig. 3.
Average ± SE school-night NREM sleep duration (open symbols) plotted with average delta power (solid symbols). The developmental curves for these two variables are similar. Both curves show a period of rapid decline between ages 11.5 and 16.5 yr of age. However, statistical analysis did not show a significant covariance in the parameters of the equations describing the declines. Note the different scaling for the two measures. While the shapes of the curves are similar, the decline in delta power is steeper than that of NREM duration. Triangles, C9 cohort; circles, C12 cohort.
NREMP-REMP durations.
NREMP and REMP durations change systematically across sleep (10, 16). The decline in total school-night NREM duration and the increase in total REM duration imply corresponding changes in individual NREMPs and REMPs. Table 3 illustrates these changes. There were significant age-related decreases in NREMP durations and age-related increases in REMP durations on school-nights.
Table 3.
Age-related changes in school-night NREM period and REM period durations
| Intercept, min |
Slope, min/yr |
Age |
||||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | F1,636 | P | |
| NREMP1 | 87.7 | 2.4 | −2.31 | 0.38 | 36.5 | <0.0001 |
| NREMP2 | 86.4 | 1.8 | −1.43 | 0.35 | 16.2 | <0.0001 |
| NREMP3 | 79.7 | 1.6 | −0.97 | 0.28 | 12.4 | 0.0005 |
| NREMP4 | 68.0 | 1.5 | −0.55 | 0.25 | 4.8 | 0.029 |
| REMP1 | 1.41 | 0.61 | 1.05 | 0.15 | 51.6 | <0.0001 |
| REMP2 | 18.4 | 1.0 | 0.72 | 0.17 | 18.4 | <0.0001 |
| REMP3 | 20.5 | 1.0 | 1.05 | 0.19 | 31.1 | <0.0001 |
| REMP4 | 25.4 | 1.1 | 0.91 | 0.22 | 17.3 | <0.0001 |
Analyses only included school-nights with 4 complete sleep cycles. Format is as in Table 1. NREMP, NREM period; REMP, REM period.
Extended Sleep
First extended night.
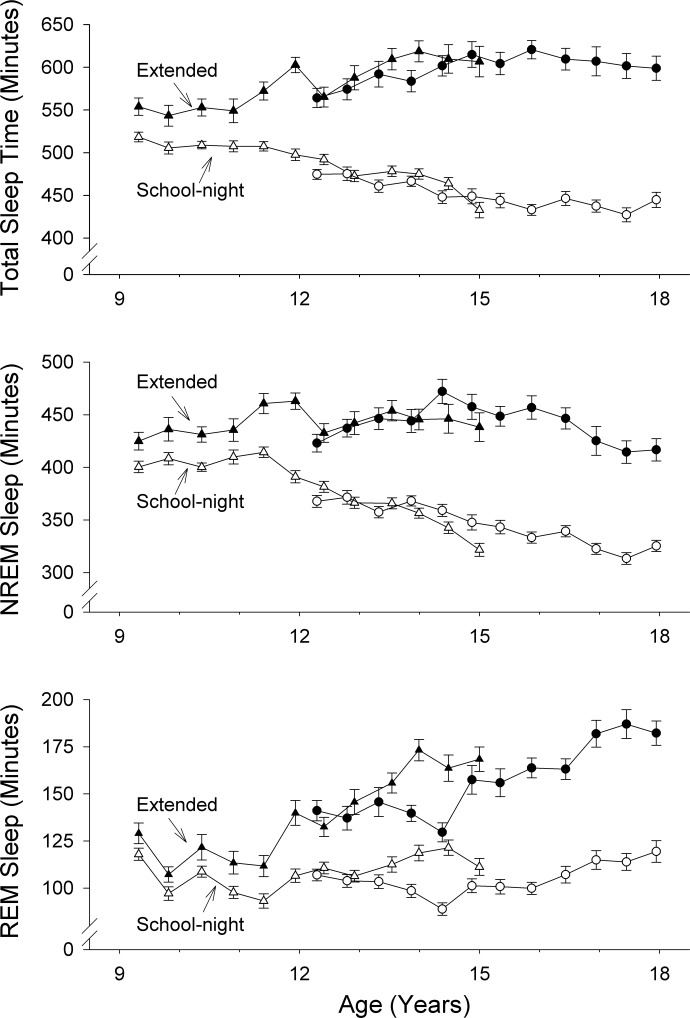
Figure 4 compares the age-related changes in sleep durations on school-nights and on the first extended night. Statistical analyses show that, with scorer variability controlled, TST on the first extended night increased across ages 9–18 yr by an average of 10.6 ± 1.8 min/yr (P < 0.0001) from its intercept of 540 min at age 9 yr (Fig. 4A). Although our subjects showed longer NREM sleep durations on extended nights, these NREM durations did not change with age (P = 0.81), hovering around an average of 442 ± 8.3 min (Fig. 4B). In contrast, REM durations in extended sleep increased significantly (P < 0.0001) with age by 9.9 min/yr from 100 min at age 9 yr (Fig. 4C). To clarify, NREM sleep duration on the first extended night was greater than on school-nights, but did not increase significantly with age. REM duration was also greater on the first extended night than on school-nights and did increase significantly with age. As on school-nights, the disproportionate age-related changes in NREM and REM durations on extended nights increased the REM-to-NREM ratio by 0.023 per year from 0.23 at age 9 yr (P < 0.0001).
Fig. 4.
Average ± SE TST (A), NREM sleep duration (B), and REM sleep duration (C) on the first extended night (solid symbols) and school-nights (open symbols). Format is the same as in Fig 1. TST on extended nights increased with age, as did the difference between TST on extended nights and school-nights. NREM duration on the extended night did not change with age. Differences between NREM duration on extended nights vs. school-nights increased across adolescence, because school-night NREM durations declined. The age-related increase in TST on extended nights was produced by significant age-related increases in REM sleep duration. Triangles, C9 cohort; circles, C12 cohort.
Second extended night.
Compared with the first extended night, TST on the second extended night increased across adolescence at a slower rate, by 4.1 min/yr (P = 0.016) from 560 min at age 9 yr. On the second extended night, NREM durations again did not change significantly (P = 0.12) with age from 445 min at age 9 yr. The age-trend suggested by the P = 0.12 value was actually a decrease of 2.1 min/yr. REM sleep on the second extended night again increased significantly with age, rising by 6.1 min/yr (P < 0.0001) from 113 min at age 9 yr. Thus, on both the first and second extended nights, TST increased with age because REM durations increased with age.
Relation of sleep durations on extended and school-nights.
If increasing TST on the first extended night was a response to decreasing TST on school-nights, one might expect significant covariance between the age changes in these measures. We tested this possibility using bivariate mixed analysis. The results were nonsignificant. Using linear models, the age-dependent increase of TST on the first extended night did not co-vary significantly (P = 0.92) with the age-dependent decline in school-night TST. To maximize the possibility of detecting a relationship, we repeated the analysis for ages 11–16.5 yr, the period in which both measures changed most steeply. Again, the changing TSTs did not covary significantly (P = 0.12).
DISCUSSION
These longitudinal data demonstrate that NREM and REM sleep durations exhibit distinctly different trajectories across adolescence on both school-night and extended sleep schedules. School-night NREM durations decline across adolescence, whereas REM durations increase slightly. The decline in NREM durations accounts for the reduction in TST across adolescence. These differential changes significantly increase the REM-to-NREM ratio.
On extended nights, subjects retired at their current school-night bedtimes, but remained in bed for 12 h, attempting to sleep as much as possible. This longer TIB increases both NREM and REM durations above the school-night levels. However, extended-night NREM durations do not change with age, whereas REM sleep durations increase significantly. Consequently, extended-night TSTs and their REM-to-NREM ratios increase across adolescence. The following discussion briefly reviews relevant prior studies of adolescent sleep and then interprets our results in relation to maturational changes in brain biology and our restorative model of NREM sleep.
Prior Studies of Adolescent Sleep EEG
Different NREM-REM trajectories on school-night sleep have not been discussed in previous reports of adolescent sleep. The most comprehensive review of sleep EEG changes across adolescence is by Ohayon et al. (29), whose meta-analysis of age changes includes the adolescent period. Ohayon et al. summarize results as percentages of TST, making them difficult to compare with our experimental results. Therefore, we reviewed each paper on adolescent sleep cited by Ohayon et al., as well as subsequent studies. We will not discuss here the age changes in adolescent sleep EEG observed with experimenter-imposed bed schedules (e.g., Refs. 7, 22). Such studies provide useful information, but they do not assess the changes in NREM and REM durations as school-night TIB decreases across adolescence, which is a major focus of our study.
In what was perhaps the first longitudinal study of adolescent sleep EEG, Karacan et al. (25) recorded school-night sleep EEG in seven boys 12.5–15.8 yr and 10 girls 14–15.8 yr of age. TST and percent slow-wave sleep decreased across the 4 yr, along with a slight, nonsignificant increase in percent REM sleep. Two later large cross-sectional EEG studies also reported decreased school-night TST across adolescence. These studies reported that slow-wave sleep stage percentages (34) or duration (8) decreased, but REM percentage (34) or duration (8) did not change. These findings appear consistent with our results here. However, neither these nor previous investigators of adolescent sleep have reported that NREM and REM durations exhibit different trajectories across adolescence.
Adolescent Changes in NREM and REM Durations on School-nights
TST.
The decline in school-night TST we find with longitudinal EEG recording parallels the decline recently reported by Olds et al. (30) in a cross-sectional survey of school-night sleep in 4,032 Australian youth over the same 9- to 18-yr age range. Similarly to our findings, the Olds et al. subjects went to bed progressively later while maintaining the same rise times. The resulting decrease in TIB produced a 12 min/yr decline in inferred TST. This decline is similar in magnitude to the 10 min/yr decline we measured in sleep EEG across the same age range in Davis, California.
NREM sleep duration.
We show here that the decline in school-night TST across 9–18 yr of age is entirely produced by declining NREM sleep durations. Before attempting to relate these sleep EEG changes to adolescent brain maturation, we emphasize that a selective loss of NREM sleep could not be produced by sleep restriction. We make this point because many investigators believe that the dominant factor in adolescent sleep is sleep restriction that produces chronic sleep debt. We agree that it is likely that many, if not most, adolescents suffer some degree of sleep deprivation due to insufficient TIB. One of our observations, decreasing time awake after sleep onset, is consistent with this possibility, but another, the absence of an age-related decrease in sleep latency, is not. Nevertheless, sleep restriction could not have produced the differential age changes in NREM and REM durations we observed. Experiments imposing acute or chronic limitations of TIB disproportionately reduce REM rather than NREM sleep (1, 17, 36, 38). The proportion of NREM sleep decreases in late sleep cycles at all ages so that truncating sleep disproportionately reduces REM rather than NREM sleep. However, our longitudinal data show that REM duration increases across adolescence, and NREM durations decline. Moreover, our cycle analyses show that these changes result from significant age-dependent shortening of NREMPs 1–4 and lengthening of REMPs 1–4. Such pervasive changes in the cyclic organization of sleep would not be expected from sleep restriction, nor have they ever been reported.
Parallel maturational curves of NREM duration and delta power.
It is now widely accepted in sleep research that the steep decline in NREM delta power during adolescence reflects brain maturation. The similar adolescent trajectories of NREM duration and delta power support the view that the brain maturation contributes to the adolescent decline in NREM duration. However, the absence of significant between-subject covariance in the two measures suggests that their age trends might reflect somewhat different maturational processes. Alternatively, external factors, such as changing sleep schedules, may have a greater impact on NREM duration than on delta power. Whatever the factors involved, we think it unlikely that the parallel age curves of delta power and NREM duration result from chance.
Physiological significance of the decline in NREM duration across adolescence.
Our laboratory's 1974 model (10) proposed that NREM sleep performs a restorative function for changes induced during waking in plastic neuronal systems. The intensity of restorative processes is proportional to the level of high-amplitude delta waves. The amount of restoration needed (i.e., the “substrate” for NREM sleep) depends both on prior waking duration and the intensity of waking neuronal activity (cf., Ref. 15). The intensity of waking brain activity is directly proportional to waking CMR. The notion that it is NREM sleep that reverses the effects of waking neuronal activity and that the intensity of the reversal process is proportional to high-amplitude delta waves was later included in the “two-process” model, which proposed a second, circadian process and a mathematical formulation (3, 9).
Our laboratory's 1974 model (10) also holds that more intense waking brain activity, indicated by higher CMR, produces larger amounts of the “substrate” for NREM reversal. These high substrate levels produce more intense NREM sleep, which is manifested by the high levels of NREM delta power at the onset of adolescence. Synaptic elimination during adolescence (23) decreases the intensity (CMR) of waking brain activity; this decreases the amount of substrate for NREM reversal. Lower levels of substrate decrease NREM intensity (delta power). They also reduce the total requirement for NREM reversal, reducing NREM duration. An analogous pattern occurs within the night: after the more intense (high delta power) NREM EEG wanes, NREMPs become shorter (10).
The relation between waking CMR and NREM sleep that we proposed gains interest, because it is now known that CMR falls below waking levels by an average of 20–30% in NREM sleep (4, 26) and by as much as 40% in its intense, high-amplitude, slow-wave component (27). The finding that NREM sleep is a hypometabolic state compared with both waking and REM sleep suggests that NREM achieves its restorative function through neuronal inactivity or “rest”. Alternatively, this recuperative function might involve processes of active synaptic restitution (cf., Refs. 18, 28, 35) that require neurons to be “offline” in a hypometabolic state.
REM sleep durations.
The finding that school-night REM sleep durations do not decline in the face of markedly decreasing NREM sleep durations adds to the challenge of understanding the enigma of REM physiology. One might be tempted to interpret this finding as indicating that school-night REM duration is biologically critical and must be maintained through adolescent brain development. However, abundant data from studies of naps, total and partial sleep deprivation, and extended sleep indicate that the duration of REM sleep is not closely regulated (see summary in Ref. 10). Thus, neither the “additional” (above baseline levels) REM duration provided by naps, nor the huge increase in REM duration (to nearly twice baseline levels) in extended sleep delays the onset of REM in recovery sleep, or reduces its total amount (13). Similarly, the amount of REM lost in total and partial sleep deprivation does not increase REM durations in recovery sleep. Taken together, these findings indicate that the total quantity of REM sleep is not biologically critical. Nevertheless, it is also true that REM deprivation by repeated awakenings causes tenacious attempts to reenter REM sleep. (Even in these experiments, subjects do not make up the lost REM durations when allowed recovery sleep.) Elsewhere, we proposed that this pattern of REM behavior indicates that periodic episodes of REM sleep are required to allow NREM episodes to resume until the need for NREM recuperation has been met, and we suggested several ways by which REM sleep might serve this function (19). We acknowledge the speculative nature of this proposal and the need for more basic investigation into this active sleep state.
The plots of REM sleep duration for the two cohorts diverge between ages 13 and 15 yr. This divergence prompted us to conduct several post hoc statistical analyses on the age-related change in REM sleep duration. Analyzing the cohorts separately showed that, despite the divergence, REM sleep increased with age in both the C9 (2.3 min/yr, P < 0.0001) and C12 cohorts (2.1 min/yr, P < 0.0001). Furthermore, including cohort as a grouping factor did not show a significant (P = 0.71) cohort difference in the age-related REM sleep increase.
Adolescent Changes in NREM and REM Durations on Extended Nights
Although EEG and eye movement patterns during extended sleep provide insights into sleep dynamics and are important in sleep theory (cf., Refs. 2, 14, 39), these issues are not directly related to the maturational changes in NREM and REM durations that are our focus here. Our study reveals two new findings on the adolescent trajectories of NREM and REM durations in extended sleep. First, although NREM durations in extended sleep are longer than in school-night sleep, they do not change with age. Second, REM durations in extended sleep increase significantly across adolescence, producing an age-related increase in TST on extended nights. Thus, in extended as well as in school-night sleep schedules, the trajectories of NREM and REM durations differ across adolescence.
The age-related increase in TST on the second extended night was smaller than that on the first. This finding raises the possibility that part of the age-related increase on the first extended night was a compensatory response to increasing sleep loss across adolescence, i.e., to the progressive decrease in TST, on school-nights. However, two observations argue against this interpretation. First, the age-related increase in TST on the first extended night was not significantly related to age-related reduction in TST on the preceding school-night. Second, it is declining NREM durations that reduce school-night TST across adolescence, but it is increasing REM durations that produce the age-related increase in TST on extended nights.
Since the age-related increase in REM durations in extended sleep is not attributable to sleep deprivation, it seems likely that it is another effect of adolescent brain maturation on sleep physiology. One possible mechanism is that brain development alters circadian systems to permit sleep to continue into the morning hours. Circadian activating signals from the suprachiasmatic nucleus increase arousal level and contribute to waking in the morning (9). These signals might become weaker with brain maturation, allowing sleep extension. However, this explanation still leaves unanswered the question of why extended-night NREM duration remains unchanged and only REM duration increases across adolescence.
One possibility is that the essentially constant NREM duration in extended sleep indicates the amount of NREM biologically needed. In that case, NREM sleep durations become increasingly insufficient across adolescence so that, by age 18 yr, our subjects are suffering from an average of 100 min of NREM deprivation. Again, the finding that NREM durations in extended sleep do not increase across adolescence argues against this possibility. In fact, the nonsignificant trend for NREM duration on the second extended night (night 4) was negative; i.e., NREM duration was decreasing rather than increasing with age. The question of how much sleep adolescents need must be resolved with different experimental designs. The need for such investigations was emphasized by Olds et al. (30), who emphasized the dearth of empirical data relating TIB to biological sleep need across adolescence.
Perspectives and Significance
The findings here demonstrate anew the value of the sleep EEG as a noninvasive, relatively inexpensive tool for the study of brain maturation in human subjects (cf., Ref. 12). These results also illustrate the power of longitudinal research designs to detect statistically robust maturational changes not reported in previous cross-sectional sleep research. Apart from the first years of life, human sleep electrophysiology changes most rapidly during adolescence; these changes provided one of the initial clues that the human brain undergoes a pervasive reorganization during adolescence (11). Understanding their biological mechanisms and significance should ultimately shed light on late brain maturation and on the physiological function(s) of the two mammalian sleep states.
GRANTS
This research was supported by United States Public Health Service Grant R01 MH62521.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.F. and I.G.C. conception and design of research; I.F. and I.G.C. interpreted results of experiments; I.F. drafted manuscript; I.F., N.M.D., E.d.B., K.J.G., and I.G.C. edited and revised manuscript; I.F., N.M.D., E.d.B., K.J.G., and I.G.C. approved final version of manuscript; N.M.D., E.d.B., and I.G.C. performed experiments; N.M.D., E.d.B., K.J.G., and I.G.C. analyzed data; N.M.D. prepared figures.
ACKNOWLEDGMENTS
We thank the subjects and their families for generous participation in this study.
REFERENCES
- 1. Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep 32: 217–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aserinsky E. The maximal capacity for sleep: rapid eye movement density as an index of sleep satiety. Biol Psychiatry 1: 147–159, 1969 [PubMed] [Google Scholar]
- 3. Borbély AA. A two process model of sleep regulation. Hum Neurobiol 1: 195–204, 1982 [PubMed] [Google Scholar]
- 4. Buchsbaum MS, Gillin JC, Wu J, Hazlett E, Sicoote N, Dupont RM, Bunny WE., Jr Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci 45: 1349–1356, 1989 [DOI] [PubMed] [Google Scholar]
- 5. Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A 106: 5177–5180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell IG, Higgins LM, Trinidad JM, Richardson P, Feinberg I. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep 30: 1677–1687, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep 2: 453–460, 1980 [DOI] [PubMed] [Google Scholar]
- 8. Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. I. Findings using standard measurement methods. Sleep 7: 289–303, 1984 [DOI] [PubMed] [Google Scholar]
- 9. Daan S, Beersma DGM, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol Regul Integr Comp Physiol 246: R161–R178, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res 10: 283–306, 1974 [DOI] [PubMed] [Google Scholar]
- 11. Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 17: 319–334, 1982/1983 [DOI] [PubMed] [Google Scholar]
- 12. Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn 72: 56–65, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Feinberg I, Fein G, Floyd TC. Computer-detected patterns of electroencephalographic delta activity during and after extended sleep. Science 215: 1131–1133, 1982 [DOI] [PubMed] [Google Scholar]
- 14. Feinberg I, Fein G, Floyd TC. EEG patterns during and following extended sleep in young adults. Electroencephalogr Clin Neurophysiol 50: 467–476, 1980 [DOI] [PubMed] [Google Scholar]
- 15. Feinberg I, Floyd TC. The regulation of human sleep. Clues from its phenomenology. Hum Neurobiol 1: 185–194, 1982 [PubMed] [Google Scholar]
- 16. Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology 16: 283–291, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Feinberg I, Floyd TC, March JD. Acute deprivation of the terminal 3.5 hours of sleep does not increase delta (0–3-Hz) electroencephalograms in recovery sleep. Sleep 14: 316–319, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Feinberg I, Koresko RL, Heller N. EEG sleep patterns as a function of normal and pathological aging in man. J Psychiatr Res 5: 107–144, 1967 [DOI] [PubMed] [Google Scholar]
- 19. Feinberg I, March JD. Observations on delta homeostasis, the one-stimulus model of NREM-REM alternation and the neurobiologic implications of experimental dream studies. Behav Brain Res 69: 97–108, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Feinberg I, March JD, Flach K, Maloney T, Chern WJ, Travis F. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (delta) electroencephalogram of human sleep. Brain Dysfunct 3: 183–192, 1990 [Google Scholar]
- 21. Feinberg I, Thode HC, Jr, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol 142: 149–161, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res 10: 165–172, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Huttenlocher PR. Synaptic density in human frontal cortex–developmental changes and effects of aging. Brain Res 163: 195–205, 1979 [DOI] [PubMed] [Google Scholar]
- 24. Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep 27: 774–783, 2004 [PubMed] [Google Scholar]
- 25. Karacan I, Anch M, Thornby JI, Okawa M, Williams RL. Longitudinal sleep patterns during pubertal growth: four-year follow up. Pediatr Res 9: 842–846, 1975 [DOI] [PubMed] [Google Scholar]
- 26. Kennedy C, Gillin JC, Mendelson W, Suda S, Miyaoka M, Ito M, Nakamura RK, Storch FI, Pettigrew K, Mishkin M, Sokoloff L. Local cerebral glucose utilization in non-rapid eye movement sleep. Nature 297: 325–327, 1982 [DOI] [PubMed] [Google Scholar]
- 27. Maquet P, Dive D, Salmon E, Sadzot B, Franco G, Poirrier R, von Frenckell R, Franck G. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-d-glucose method. Brain Res 513: 136–143, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Moruzzi G. The functional significance of sleep with particular regard to brain mechanisms underlying consciousness. In: Brain and Conscious Experience, edited by Eccles JC. New York: Springer-Verlag, 1966, p. 345–388 [Google Scholar]
- 29. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27: 1255–1273, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Olds T, Maher C, Blunden S, Matricciani L. Normative data on the sleep habits of Australian children and adolescents. Sleep 33: 1381–1388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington, DC: Public Health Services, US Government Printing Office, 1968 [Google Scholar]
- 32. Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 23: 323–355, 1998 [Google Scholar]
- 33. Spiegelhalter DJ, Thomas A, Best NG, Gilks WR. BUGS: Bayesian Inference Using Gibbs Sampling, Version 0.30. Cambridge, UK: MRC Biostatistics Unit, 1994 [Google Scholar]
- 34. Stores G, Crawford C, Selman J, Wiggs L. Home polysomnography norms for children. Technol Health Care 6: 231–236, 1998 [PubMed] [Google Scholar]
- 35. Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull 62: 143–150, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Travis F, Maloney T, Means M, March JD, Feinberg I. Acute deprivation of the terminal four hours of sleep does not increase delta (0-3-Hz) electroencephalograms: a replication. Sleep 14: 320–324, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology. Cambridge, UK: Cambridge University Press, 2003 [Google Scholar]
- 38. Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26: 117–126, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Verdone P. Sleep satiation: extended sleep in normal subjects. Electroencephalogr Clin Neurophysiol 24: 417–423, 1968 [DOI] [PubMed] [Google Scholar]
- 40. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev 69: 875–887, 1998 [PubMed] [Google Scholar]