Abstract
Placental hypoxia/ischemia has been implicated as a central factor in the development of preeclampsia. One particularly useful animal model to study the impact of placental ischemia is the reduced uterine perfusion pressure (RUPP) model. We have previously demonstrated that RUPP animals exhibit elevated placental oxidative stress, which plays an important role in the development of the associated maternal hypertension. Recently, we have demonstrated that cobalt protoporphyrin (CoPP)-mediated induction of heme oxygenase-1 (HO-1) attenuates RUPP-induced oxidative stress and consequent hypertension. However, signaling pathways that are involved in this process are virtually unknown. Here, we show that placentas from RUPP animals exhibit increased phosphorylation of JNK, STAT1, STAT3, and p52shc with a concomitant increase in caspase-3 activation and depletion of intracellular ATP. Treatment with CoPP decreased RUPP-induced phosphorylation of JNK and STAT1, while it increased phosphorylation of ERK and STAT3, leading to decreased caspase-3 activation and restoration of intracellular ATP content. Our data imply that RUPP induces oxidative stress and the consequent injurious state by increasing phosphorylation of mediators of injury (STAT1, JNK) and, to a lesser extent, survival (STAT3, p52shc) in placentas of pregnant rats. HO-1 induction shifts this balance to a prosurvival phenotype by augmenting phosphorylation of the prosurvival ERK and STAT3, while suppressing phosphorylation of JNK and STAT1. This attenuates the resulting injury, as indicated by caspase-3 activation and ATP depletion. These results demonstrate a novel therapeutic activity of HO-1 induction in placental cell survival during ischemia and support the HO-1 pathway as a promising therapeutic target for the management of preeclampsia.
Keywords: preeclampsia, Janus-activated kinase, extracellular signal-regulated kinase, heme oxygenase-1
preeclampsia is a serious and common complication in pregnancy, characterized by hypertension, proteinuria, and maternal endothelial dysfunction (37). Although the underlying mechanisms that initiate preeclampsia are not well understood, one common finding is an inadequate remodeling of the maternal vasculature. Remodeling of the maternal vascaulature is essential for adequate delivery of blood to the uteroplacental unit, as failure to remodel causes chronic placental ischemia (23, 26). In response to this ischemia, the oxidative stress in the placenta is significantly increased, as indicated by the increased production of lipid hydroperoxides and free isoprostane (44, 45). One experimental model that has proved useful for the study of preeclampsia is the reduced uterine perfusion pressure (RUPP) model, in which mechanical constriction of the blood vessels feeding the uteroplacental unit leads to chronic placental ischemia/hypoxia and mimics many of the pathological features of the human disorder (19, 43). Studies in the RUPP model have indicated an elevation in placental oxidative stress in pregnant rats (40). Increased oxidative stress is hypothesized to be an important mediator of placental inflammation and apoptosis during preeclampsia (7). Importantly, reduction of oxidative stress with the SOD mimetic tempol or the NADPH oxidase inhibitor apocynin led to a significant decrease in the maternal hypertension, demonstrating the importance of oxidative stress in the development of hypertension in response to placental ischemia (40). The end result is dysfunction of the maternal vascular endothelium (19), an underlying manifestation of preeclampsia.
Heme oxygenase-1 (HO-1) has been shown to have beneficial effects in a number of experimental forms of hypertension (5–6, 13, 39, 54). One way that HO-1 is hypothesized to attenuate hypertension is through its well-established ability to regulate oxidative stress (40, 47). The most prominent way that it does this is through the production of bilirubin, a potent antioxidant, during the degradation of heme (18, 46). Under ischemic conditions, HO-1 can exert cytoprotective and anti-inflammatory functions (49). We have recently demonstrated that induction of HO-1 by cobalt protoporphyrin (CoPP) significantly attenuated oxidative stress and hypertension in pregnant rats with RUPP (15). However, mediators of HO-1-dependent protection in the ischemic placenta are unknown. It is well established that oxidative stress or inflammation can modify phosphorylation of proteins that regulate survival or injury (33, 48). Those pathways that are affected by oxidative stress or inflammation include the MAPKs, such as ERK and JNK, STAT1 and STAT3, and members of the shc adaptor protein family, such as p52shc. Generally, activation (phosphorylation) of ERK (12), STAT3 (22), or p52shc (30) is considered to be prosurvival, while activation of JNK (12) or STAT1 (53) supports cell death under ischemic conditions. While the relationship between changes in activity/phosphorylation of these proteins and survival/injury in various ischemic organs are well established, their phosphorylation status during placental ischemia and HO-1 induction is virtually unknown. Accordingly, the aim of this study was to examine the effects of reduction in uterine perfusion pressure on phosphorylation status of survival- or injury-promoting signaling proteins in pregnant rats and determine whether HO-1 induction could ameliorate the activation of detrimental signaling cascades activated by placental ischemia.
MATERIALS AND METHODS
Animals.
Timed pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN) were received on day 10 or 11 of gestation. All protocols were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Rats were maintained on a 12:12-h light-dark cycle, at 23°C constant temperature and were provided food and water ad libitium.
RUPP procedure and CoPP treatment.
On gestational day 14, all animals were anesthetized, and pregnancy was confirmed by manipulation of the abdomen. RUPP-treated animals were subjected to aortic and bilateral ovarian artery constriction, as previously described (2). Briefly, animals were anesthetized by controlled 3% isoflurane (Webster, Devens, MA), and a midline abdominal incision was made. After externalization of both uterine horns, one single 0.203-mm silver surgical clip was placed on the lower abdominal aorta above the iliac bifurcation. One 0.100-mm silver surgical clip was placed on both the left and right ovarian arteries that supply the uterus to prevent compensatory flow. Rats absorbing all pups as a result of the procedure were excluded from the study. For HO-1 induction, CoPP (Frontier Scientific, Logan, UT) was injected intraperitoneally at a dose of 5 mg/kg on day 14 of gestation, at doses comparable to those described previously (24, 38). Sham procedures were not performed in the current study. Although some investigators have reported effects of sham manipulation of the abdominal aorta (20, 32), the noted effects seem to be minor and limited to metabolic measurements of the offspring. However, sham operations in studies originating from our group have demonstrated no significant effects on blood pressure or vascular reactivity in pregnant rats (1, 9), and virgin rats undergoing the RUPP procedure show no phenotypic effects on blood pressure (2).
Placenta lysates, Western blot analysis.
On gestational day 19, the uterus was externalized and incised, and placentas were removed mechanically and snap frozen in liquid nitrogen. A random placenta from each animal was homogenized in a RIPA buffer, as described elsewhere (3). Protein content was determined by using a Bio-Rad Protein Determination assay (Bio-Rad, Hercules, CA). One-hundred micrograms of tissue lysate were separated by SDS-PAGE electrophoresis and transferred to a PVDF membrane (Invitrogen, Grand Isle, NY). The filters were hybridized with the appropriate primary antibodies followed by an HRP-conjugated secondary antibody. First Anti-pERK (1:1,000), -ERK (1:1,000), -JNK (1:1,000), -pTyrSTAT3 (1:1,000), -STAT3 (1:2,000), -pTyrSTAT1 (1:1,000), and -STAT1 (1:1,000) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Anti-pJNK (1:500) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), anti-shc (1:1,000) from BD Transduction Laboratories (San Jose, CA), anti-Tyr-shc (1:1,000) from Enzo Life Sciences (Farmingdale, NY). Secondary antibodies were purchased from Cell Signaling Technology and used in 1:5,000 dilutions. First, the appropriate anti-phospho antibody was used and after stripping, the unphosphorylated antibody was applied in each separate case; i.e., separate blots were obtained for pER/ER, pJnk/JNK, pSTAT3/STAT3, pSTAT1/STAT1, and pTyr-shc/shc. The bands were visualized by an ECL method (Amersham) and quantified by densitometry (UnScan-It; Silk Scientific, Orem, UT). Blots were stripped in Restore Plus Western blot stripping buffer (Thermo Scientific, Rockford, IL), as suggested by the manufacturer. Briefly, after washing, the blots in Tris-buffered saline supplemented with 0.05% Tween-20 (TBS-T) for 2 × 15 min at room temperature, they were transferred into Restore Plus buffer and incubated for 30 min at 37°C followed by 2 × 15 min washing in TBS-T at room temperature. After this, blots were hybridized, as described previously.
Intracellular ATP content.
ATP content was determined from tricholoroacetic acid-extracted tissue lysates using the ENLITEN luminescent ATP assay kit (Promega, Madison, WI), as suggested by the manufacturer. ATP content was calculated as relative luminescent unit (RLU)/mg protein. Values were compared as a percentage of the normal pregnant values.
Caspase-3 activation.
Caspase-3 activity was determined from tissue lysates using the Caspase Glo 3/7 assay system (Promega, Madison, WI), as described elsewhere (8). Caspase-3 activity was calculated as RLU per milligram protein. Values were compared as a percentage of the normal pregnant values.
Statistics.
Continuous variables were expressed as mean + SD. One-way ANOVA was used to evaluate the differences between groups. Means of treatment groups were pairwise compared with controls by using ANOVA with a Holm-Sidak post hoc test. A P value of ≤ 0.05 was considered statistically significant. All analyses were performed using a SigmaStat 3.5 software package. Differences between means were considered significant if P < 0.05.
RESULTS
Effect of RUPP and CoPP treatment on placental phosphorylation of various ERK1, ERK2, and JNK.
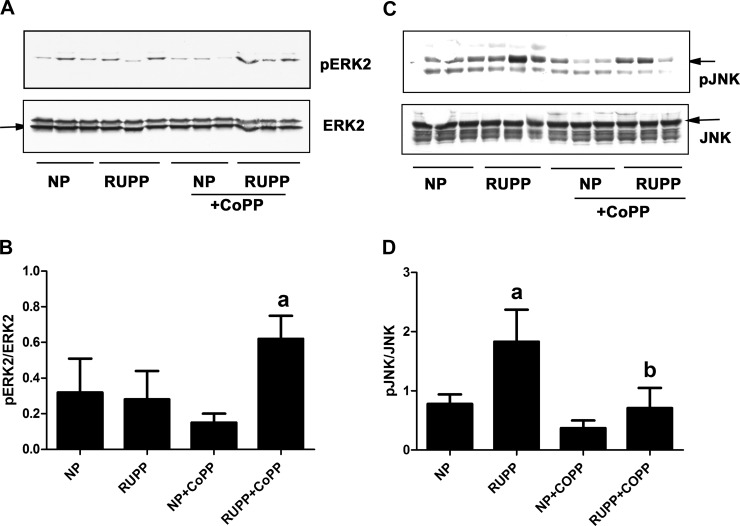
Phosphorylation of ERK and JNK was determined in placenta lysates by Western blot analysis. As Fig. 1A demonstrates that ERK2, but not ERK1 phosphorylation, was detectable in placentas. Although there were no significant differences between normal pregnant (NP) and RUPP placentas, CoPP treatment significantly increased ERK2 phosphorylation in the placentas of rats with RUPP (Fig. 1, A and B). On the other hand, phosphorylation of the stress kinase JNK was significantly higher in the RUPP placentas compared with NP placentas (Fig. 1, C and D), which was significantly decreased upon treatment with CoPP.
Fig. 1.
Reduced uterine perfusion pressure (RUPP) and cobalt protoporphyrin (CoPP) treatment affect placental phosphorylation of MAPKs in rats. Pregnant rats were subjected to RUPP then treated with CoPP or vehicle, as described in materials and methods. A group of normal pregnant (NP) rats were also treated with either CoPP or vehicle. On gestational day 19, placentas were excised and processed to obtain protein lysates. All groups included three placentas from different animals. A: phosphorylation of ERK was determined by Western blot analysis and compared with the unphosphorylated ERK. NP, normal pregnant; RUPP, reduced uterine perfusion pressure; CoPP, cobalt protoporphyrin. B: densitometry of data in A. Ratio of pERK2/ERK2 was calculated. aP < 0.05 compared with RUPP. C: phosphorylation of JNK was determined by Western blot analysis and compared with the unphosphorylated JNK. NP, normal pregnant; RUPP, reduced uterine perfusion pressure; CoPP, cobalt protoporhyrin. D: densitometry of data in C. Ratio of pJNK/JNK was calculated. aP < 0.05 compared with NP. bP < 0.05 compared with RUPP.
Effect of RUPP and CoPP treatment on placental tyrosine phosphorylation of STAT3 and STAT1.
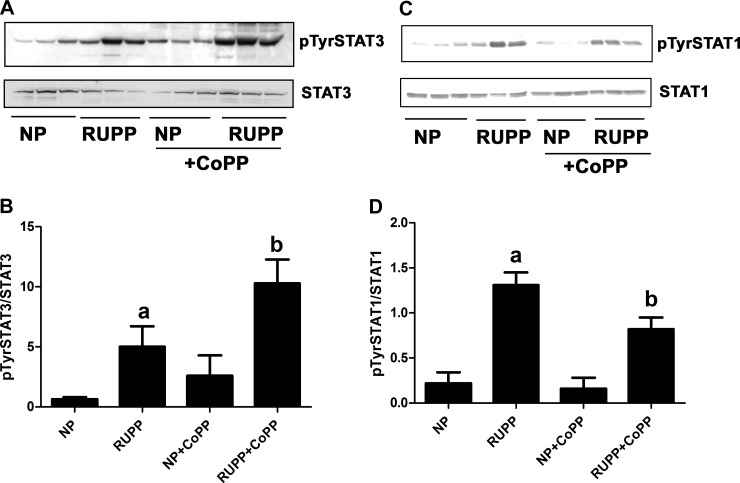
Next, we determined tyrosine phosphorylation of STAT3 and STAT1 in various placenta samples. As seen in Fig. 2, A and B, RUPP significantly increased tyrosine phosphorylation of STAT3, which was further enhanced after treatment with CoPP. Although CoPP increased STAT3 tyrosine phosphorylation in NP placentas, changes are not statistically significant. Similar to STAT3, tyrosine phosphorylation of STAT1 was significantly enhanced in RUPP placentas (Fig. 2, C and D). However, as opposed to STAT3, CoPP significantly attenuated tyrosine phosphorylation of STAT1 (Fig. 2, C and D).
Fig. 2.
RUPP and CoPP treatment changes placental phosphorylation of STATs in rats. Pregnant rats were subjected to RUPP and then treated with CoPP or vehicle as described in materials and methods. A group of NP rats were also treated with either CoPP or vehicle. On gestational day 19, placentas were excised and processed to obtain protein lysates. All groups included three placentas from different animals. A: tyrosine phosphorylation of STAT3 was determined by Western blot analysis and compared with the unphosphorylated STAT3. B: densitometry of data in A. Ratio of pTyrSTAT3/STAT3 was calculated. aP < 0.05 compared with NP, bP < 0.05 compared with RUPP. C: tyrosine phosphorylation of STAT1 was determined by Western blot analysis and compared with the unphosphorylated STAT1. D: densitometry of data in C. Ratio of pTyrSTAT1/STAT1 was calculated. aP < 0.05 compared with NP; bP < 0.05 compared with RUPP.
Effect of RUPP and CoPP treatment on placental tyrosine phosphorylation of shca isoforms.
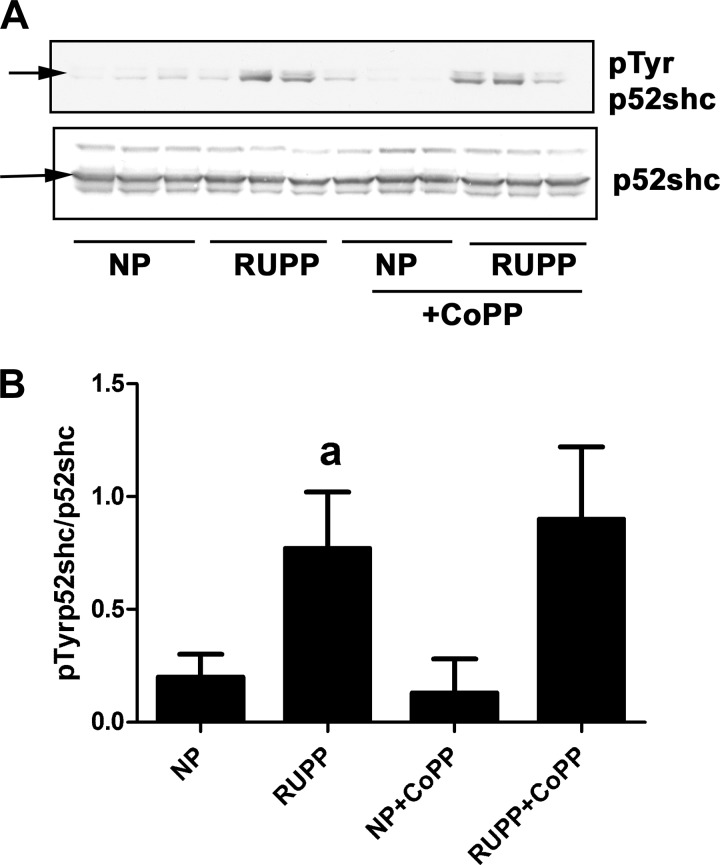
Out of the three shcA isoform, p52shc tyrosine phosphorylation was significantly elevated in RUPP placentas (Fig. 3, A and B). Neither p47shc nor p66shc was significantly affected (data not shown). Interestingly, CoPP administration had no significant effect on any of the shcA isoforms.
Fig. 3.
RUPP and CoPP treatment changes placental phosphorylation of shc proteins in rats. Pregnant rats were subjected to RUPP and then treated with CoPP or vehicle, as described in materials and methods. A group of NP rats were also treated with either CoPP or vehicle. On gestational day 19, placentas were excised and processed to obtain protein lysates. All groups included three placentas from different animals. A: tyrosine phosphorylation of shc proteins was determined by Western blot analysis and compared with the unphosphorylated shc. B: densitometry of data in A. Ratio of pTyrp52shc/p52shc was calculated. aP < 0.05 compared with NP.
Effect of RUPP and CoPP treatment on placental ATP content and caspase-3 activation.
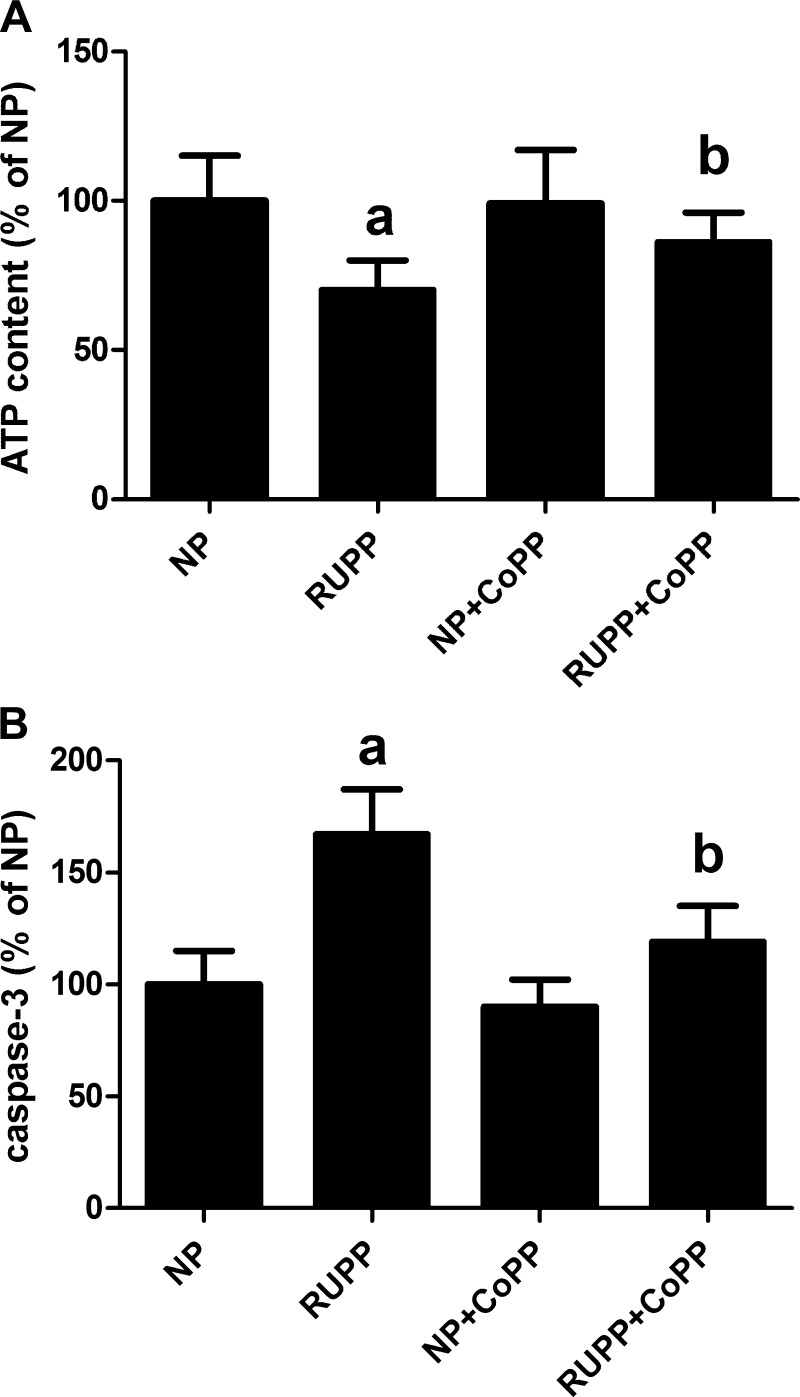
To evaluate whether RUPP caused any injury in placentas, ATP content as well as caspase-3 activation were determined in placenta lysates. As seen in Fig. 4, RUPP significantly decreased ATP content and also activated caspase-3. Importantly, while CoPP treatment had no effect in NP animals, in RUPP-treated animals, it restored intracellular ATP to normal levels and attenuated caspase-3 activation (Fig. 4).
Fig. 4.
RUPP and CoPP treatment alters placental viability in rats. Pregnant rats were subjected to RUPP and then treated with CoPP or vehicle, as described in materials and methods. A group of NP rats were also treated with either CoPP or vehicle. On gestational day 19, placentas were excised and processed to obtain protein lysates. All groups included three placentas from different animals. A: cellular ATP content was determined as described in materials and methods and equalized for the same amount of protein. Data are expressed as a percentage of values determined in NP placentas. aP < 0.05 compared with NP. bP < 0.05 compared with RUPP. B: activation of caspase-3 was determined in placental lysates, as described in materials and methods and equalized for the same amount of protein. Data are expressed as a percentage of values determined in NP placentas. aP < 0.05 compared with NP. bP < 0.05 compared with RUPP.
DISCUSSION
Placental ischemia is a central factor in the development of preeclampsia, activating numerous pathways leading to maternal hypertension (17). One of the best characterized is increased placental oxidative stress. Placentas from preeclamptic women demonstrate markedly increased production of oxidative stress indicators (44–45). It has also been shown that oxidative stress is an important mediator of placental ischemia-induced hypertension in pregnant rats (40), and oxidative stress may be responsible for inflammation, injury, and endothelial dysfunction in the maternal placenta. Despite these observations, the signaling pathways that mediate these effects in the placenta are poorly characterized. Recently, we have reported that administration of a pharmacological inducer of HO-1, CoPP, was capable of attenuating the hypertension associated with placental ischemia in the rat. Furthermore, the HO-1 induction attenuated the increased placental oxidative stress associated with the RUPP model, possibly through increased expression of HO-1 in the ischemic placenta (14). Intriguingly, we have recently demonstrated that in response to chronic hypoxic exposure, the placental vasculature exhibits a decrease in HO-1 expression in vitro, which correlates with an increase in oxidative stress in this tissue (16). Here, we have attempted to elucidate the biochemical pathways associated with oxidative stress in the ischemic placenta and to determine whether decreased oxidative stress, brought about by HO-1 induction, would reverse the changes seen in these pathways.
MAPKs transduce signals from the cell surface to the nucleus in response to cytokines, growth factors, stress, and other factors (34). ERK and the stress kinase JNK exert opposite effects on cells in response to oxidative stress, while ERK mostly supports survival, JNK is mostly associated with cell injury (52). Unopposed activation of JNK leads to cell death, while concomitant activation of ERK and JNK supports cell survival during renal ischemia (11) or oxidative stress in cultured renal proximal tubule cells (4). Our present study demonstrates that JNK, but not ERK, was activated (phosphorylated) by RUPP treatment (Fig. 1), suggesting a phenotype that is associated with injury. This supports observations by Lin et al. (28), who reported that CoPP inhibited LPS-induced activation of JNK in RAW264.7 cells. Interestingly, CoPP treatment increased ERK phosphorylation and also attenuated phosphorylation of JNK in RUPP samples (Fig. 1). Thus, this scenario is consistent with an increased CoPP-mediated cell survival signaling. It should be noted that limited examination of these proteins in human samples have not been equivocal, most likely because of differences in the selection of tissue from a limited area of the placenta (42).
STATs are transcription factors that are tyrosine phosphorylated in response to cytokines, hormones, or growth factors, and thus, they activate transcription of various genes that are involved in inflammation, immune responses, and cell growth (31). Activated STAT3 protects against ischemia in the heart (22), while the activated STAT1 supports injury (50). The role of STATs in placental ischemia or preeclampsia is unknown. Fig. 2, A and B shows that RUPP treatment increased tyrosine phosphorylation of STAT3, which was further enhanced after treatment with CoPP. STAT1 phosphorylation was also enhanced by RUPP (Fig. 2, C and D), but CoPP treatment, in contrast to STAT3, decreased it. Considering that the activated STAT3 is prosurvival, while activated STAT1 supports injury, CoPP treatment may establish a condition that is favorable for survival. Similar results were observed in liver ischemia by others: CoPP-mediated activation of HO-1 showed cytoprotective and anti-inflammatory function through, among others, inhibiting STAT1 phosphorylation (49). It is plausible that the increased tyrosine phosphorylation in STAT3 may also activate an anti-inflammatory cascade (27) in the placenta.
The shcA gene encodes three different proteins through differential usage of transcription/translation initiation sites and splicing (29). It has been demonstrated that the tyrosine phosphorylated p52 shcA isoform couples the activated EGFR to ras/ERK activation during growth factor stimulation (36) or oxidative stress (35). This signaling pathway is associated with survival during oxidative stress. Tyrosine phosphorylation of the p52shc isoform is highly increased in placentas from rats with RUPP compared with placentas from NP rats (Fig. 3, A and B). CoPP treatment did not change tyrosine phosphorylation of p52shc, either in NP or RUPP rats, remaining elevated in both RUPP and RUPP rats treated with CoPP. This may suggest that events that regulate p52shc phosphorylation are upstream from CoPP-dependent pathways. Further studies are needed to explain this phenomenon.
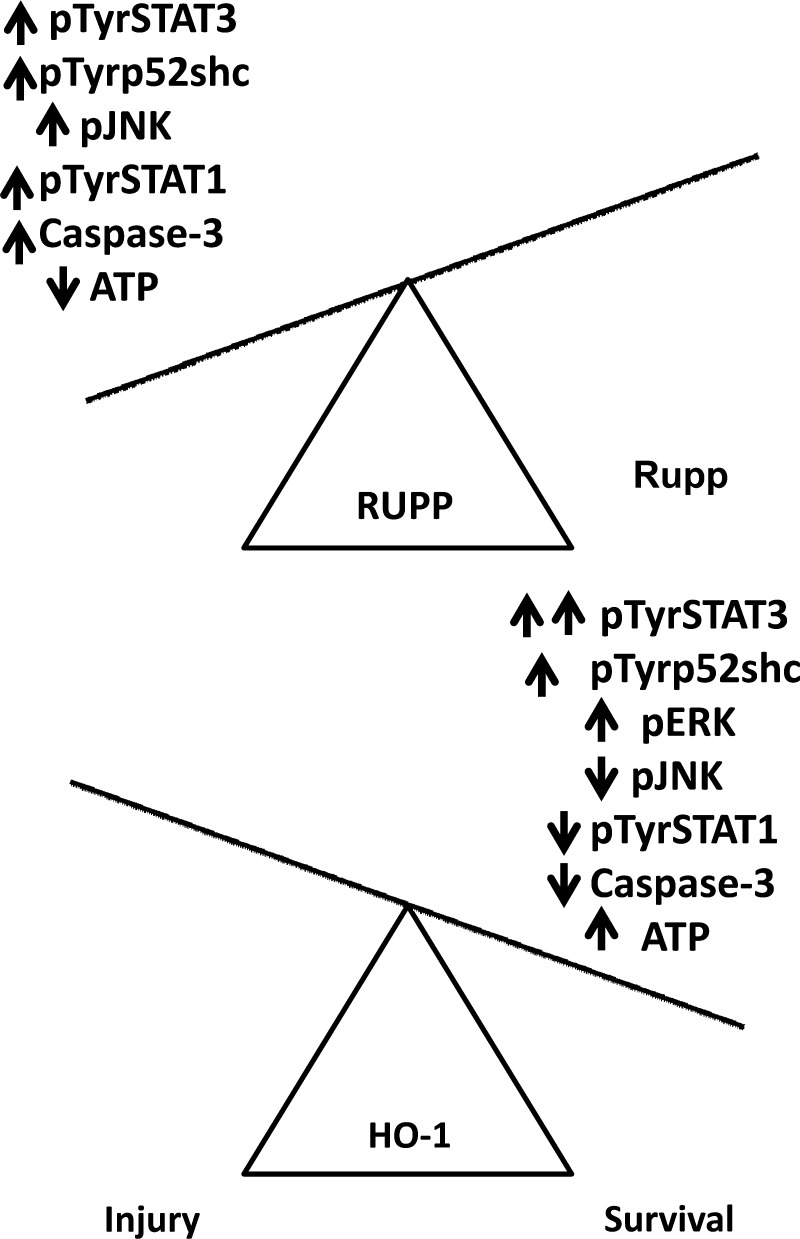
Oxidative stress is involved in RUPP-mediated hypertension and maternal endothelial dysfunction (19). Activation of HO-1 via CoPP decreases RUPP-induced placental oxidative stress and hypertension in experimental rats (14). Thus, it is possible that the observed changes in phosphorylation of the investigated proteins may be associated with HO-1-dependent decrease in oxidative stress. Various cell types respond to oxidative stress in a dose-dependent manner: a low level of oxidative stress activates protective signaling, while a high level of oxidative stress induces injury (10, 25). Earlier, we showed that a low level of oxidative stress activates both ERK and JNK in renal tubule cells and cells survive, while a high level of oxidative stress inhibits ERK but not JNK activation, and consequently, cells die (4). Hence, we speculate that excess ROS production—due to RUPP—activates proinjury pathways with a concomitant activation of prosurvival signaling in placentas with RUPP, resulting in an environment that is suitable for injury (Fig. 5). In contrast, attenuated oxidative stress (RUPP+CoPP treatment) supports activation of prosurvival signaling with a concomitant inhibition of proinjury signaling (Fig. 5). Indeed, CoPP restored RUPP-mediated depletion of intracellular ATP and also decreased caspase-3 activation in placentas (Fig. 4). This has a potentially important role for the management of preeclampsia, as syncytiotrophoblast microvillus membrane microparticles arising from syncytiotrophoblast cell death are found in the maternal circulation during preeclampsia and are thought to be responsible, in part, for the activation of the maternal innate immune response (41). Mounting evidence supports this activation of the maternal immune response as a key player in the symptomatic phase of preeclampsia (21, 51). The connection between those signaling events and ATP depletion/caspase-3 activation, however, needs further investigation.
Fig. 5.
Summary of the effects of RUPP treatment and HO-1 induction on the cell survival signaling pathways in the placenta. In response to RUPP treatment, signaling mediators of cell injury (i.e., STAT1 and JNK) are activated in the placenta, indicating a proinjury state. Induction of HO-1, however, attenuates the activation of these pathways, and increases the activation of prosurvival signaling, such as ERK and STAT3. In consequence, the induction of caspase-3 and depletion of ATP seen in the RUPP placenta are significantly reduced, shifting the balance of cell signaling from a proinjury state to a prosurvival state.
Perspectives and Significance
Here, we have elucidated the biochemical mechanisms that lead from ischemia to cellular dysfunction and death in a model of chronic placental ischemia. We further demonstrate that administration of an HO-1 inducer in rats undergoing placental ischemia significant attenuates the activation of cell death pathways and promotes cell survival in the ischemic placenta. These data support past studies showing beneficial effects of HO-1 induction on chronic placental ischemia in the rat and clarifies the mechanisms underpinning HO-1's positive effects in this model. Together, these results further support the HO-1 pathway as a possible therapeutic target for the management of preeclampsia.
GRANTS
These studies were supported by an American Heart Association Midwest Affiliate Grant-in-Aid (10GRNT3790019; to I. Arany), an Intramural Research Support Program Award from the University of Mississippi Medical Center (to I. Arany), a National Heart, Lung, and Blood Institute Grant 1T32HL-105324-01 (to E. George), a postdoctoral fellowship from the American Heart Association (11POST7840039; to E. George), and HL-51971.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.M.G. and I.A. were responsible for the conception and design of research; E.M.G. and I.A. performed the experiments; E.M.G. and I.A. interpreted the results of the experiments; E.M.G. and I.A. prepared the figures; E.M.G. and I.A. drafted the manuscript; E.M.G. and I.A. edited and revised the manuscript; E.M.G. and I.A. approved the final version of manuscript; I.A. analyzed the data.
ACKNOWLEDGMENTS
The authors thank Kathy Cockrell and Marietta Arany for their technical expertise.
REFERENCES
- 1. Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension 38: 742–745, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Arany I, Faisal A, Nagamine Y, Safirstein RL. p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. J Biol Chem 283: 6110–6117, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65: 1231–1239, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Botros FT, Schwartzman ML, Stier CT, Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int 68: 2745–2755, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cao J, Inoue K, Li X, Drummond G, Abraham NG. Physiological significance of heme oxygenase in hypertension. Int J Biochem Cell Biol 41: 1025–1033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia: from placental oxidative stress to maternal endothelial dysfunction. Placenta 30, Suppl. A: S55–S65, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Clark JS, Faisal A, Baliga R, Nagamine Y, Arany I. Cisplatin induces apoptosis through the ERK-p66shc pathway in renal proximal tubule cells. Cancer Lett 297: 165–170, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Davies KJ. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life 48: 41–47, 1999 [DOI] [PubMed] [Google Scholar]
- 11. di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol Renal Physiol 277: F195–F203, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007 [DOI] [PubMed] [Google Scholar]
- 13. George EM, Arany M, Cockrell K, Storm MV, Stec DE, Granger JP. Induction of heme oxygenase-1 attenuates sFlt-1-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol 301: R1495–R1500, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. George EM, Colson D, Dixon J, Palei AC, Granger JP. Heme oxygenase-1 attenuates hypoxia-induced sFlt-1 and oxidative stress in placental villi through its metabolic products CO and bilirubin. Int J Hypertens 2012: 486053, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George EM, Granger JP. Recent insights into the pathophysiology of preeclampsia. Expert Rev Obstet Gynecol 5: 557–566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122: 383–392, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Heltemes A, Gingery A, Soldner EL, Bozadjieva N, Jahr KN, Johnson BK, Gilbert JS. Chronic placental ischemia alters amniotic fluid milieu and results in impaired glucose tolerance, insulin resistance and hyperleptinemia in young rats. Exp Biol Med (Maywood) 235: 892–899, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Herse F, Staff AC, Hering L, Muller DN, Luft FC, Dechend R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med 86: 697–703, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther 107: 131–137, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol 2: 543–549, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Jiang K, Cheng L, Wang J, Li J, Nie J. Heme oxygenase-1 expression in rats with acute lung rejection and implication. J Huazhong Univ Sci Technolog Med Sci 29: 84–87, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Khan AU, Wilson T. Reactive oxygen species as cellular messengers. Chem Biol 2: 437–445, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Khong Y, Brosens I. Defective deep placentation. Best Pract Res Clin Obstet Gynaecol 25: 301–311, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Kurdi M, Booz GW. Deciphering STAT3 signaling in the heart: plasticity and vascular inflammation. Congest Heart Fail 16: 234–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin HY, Shen SC, Lin CW, Wu MS, Chen YC. Cobalt protoporphyrin inhibition of lipopolysaccharide or lipoteichoic acid-induced nitric oxide production via blocking c-Jun N-terminal kinase activation and nitric oxide enzyme activity. Chem Biol Interact 180: 202–210, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Luzi L, Confalonieri S, Di Fiore PP, Pelicci PG. Evolution of Shc functions from nematode to human. Curr Opin Genet Dev 10: 668–674, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Migliaccio E, Mele S, Salcini Anna E, Pelicci G, Lai KMan V, Superti-Furga G, Pawson T, Paolo Di Fiore P, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J 16: 706–716, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol 178: 2623–2629, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Nusken KD, Dotsch J, Rauh M, Rascher W, Schneider H. Uteroplacental insufficiency after bilateral uterine artery ligation in the rat: impact on postnatal glucose and lipid metabolism and evidence for metabolic programming of the offspring by sham operation. Endocrinology 149: 1056–1063, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol 64: 765–770, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 26: 3100–3112, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Rao GN. Hydrogen peroxide induces complex formation of SHC-Grb2-SOS with receptor tyrosine kinase and activates Ras and extracellular signal-regulated protein kinases group of mitogen-activated protein kinases. Oncogene 13: 713–719, 1996 [PubMed] [Google Scholar]
- 36. Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene 20: 6322–6330, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 41: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Rosa AO, Egea J, Lorrio S, Rojo AI, Cuadrado A, Lopez MG. Nrf2-mediated haeme oxygenase-1 up-regulation induced by cobalt protoporphyrin has antinociceptive effects against inflammatory pain in the formalin test in mice. Pain 137: 332–339, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension 38: 210–215, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol 64: 159–169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shin JK, Jeong YT, Jo HC, Kang MY, Chang IS, Baek JC, Park JK, Lee SA, Lee JH, Choi WS, Paik WY. Increased interaction between heat shock protein 27 and mitogen-activated protein kinase (p38 and extracellular signal-regulated kinase) in pre-eclamptic placentas. J Obstet Gynaecol Res 35: 888–894, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Staff AC, Halvorsen B, Ranheim T, Henriksen T. Elevated level of free 8-iso-prostaglandin F2alpha in the decidua basalis of women with preeclampsia. Am J Obstet Gynecol 181: 1211–1215, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Staff AC, Ranheim T, Khoury J, Henriksen T. Increased contents of phospholipids, cholesterol, and lipid peroxides in decidua basalis in women with preeclampsia. Am J Obstet Gynecol 180: 587–592, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 47. Takahashi T, Shimizu H, Morimatsu H, Inoue K, Akagi R, Morita K, Sassa S. Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini Rev Med Chem 7: 745–753, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Tsuchihashi S, Zhai Y, Bo Q, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation 83: 1628–1634, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Wagner M, Siddiqui MA. Signaling networks regulating cardiac myocyte survival and death. Curr Opin Investig Drugs 10: 928–937, 2009 [PubMed] [Google Scholar]
- 51. Xia Y, Kellems RE. Is preeclampsia an autoimmune disease? Clin Immunol 133: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci 98: 9050–9055, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang L, Quan S, Nasjletti A, Laniado-Schwartzman M, Abraham NG. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension 43: 1221–1226, 2004 [DOI] [PubMed] [Google Scholar]