Abstract
The renin-angiotensin system (RAS) has mainly been categorized as a circulating and a local tissue RAS. A new component of the local system, known as the intracellular RAS, has recently been described. The intracellular RAS is defined as synthesis and action of ANG II intracellularly. This RAS appears to differ from the circulating and the local RAS, in terms of components and the mechanism of action. These differences may alter treatment strategies that target the RAS in several pathological conditions. Recent work from our laboratory has demonstrated significant upregulation of the cardiac, intracellular RAS in diabetes, which is associated with cardiac dysfunction. Here, we have reviewed evidence supporting an intracellular RAS in different cell types, ANG II's actions in cardiac cells, and its mechanism of action, focusing on the intracellular cardiac RAS in diabetes. We have discussed the significance of an intracellular RAS in cardiac pathophysiology and implications for potential therapies.
Keywords: renin-angiotensin system, cardiac, intracrine
the renin-angiotensin system (RAS) is an extensively studied hormonal system present in most vertebrates. Starting with the discovery of renin more than a century ago, the RAS has evolved into an extensive system of bioactive peptides that result from the action of several enzymes on a single precursor, angiotensinogen (AGT) (26). These peptides have specific receptors that produce unique cellular responses in multiple tissues. ANG II was the first and extensively investigated peptide of the RAS. Initially shown to regulate systemic blood pressure, via modulating salt and water homeostasis and vascular tone, ANG II also produces tissue-specific effects that include hypertrophy and fibrosis in the heart (2). The tissue effects of ANG II are generally independent of the systemic effects on blood pressure, which are separated, largely due to independent control of ANG II production in the circulation and tissues (61). Thus, ANG II is an endocrine, as well as an autocrine/paracrine hormone, based on the site of synthesis and action (47). ANG II binds primarily to two specific G protein-coupled receptors, ANG type I (AT1) and ANG type II (AT2), present on the plasma membrane of most cells. A new aspect of ANG II actions, as an intracrine hormone, has become evident recently. In the intracrine mode, ANG II actions begin from an intracellular location, instead of the interstitial space, similar to a typical peptide-receptor interaction (63). The intracrine aspect of ANG II adds a unique dimension to the RAS, which is significantly different from endocrine and autocrine/paracrine systems, in terms of the site and mode of ANG II synthesis and possible new intracellular receptors and mechanisms of action (78). These differences might potentially change our approach to inhibit the RAS in pathological conditions (46). Diabetes is a disease with predominant intracrine or intracellular RAS activation and in which classical RAS inhibitors have not been proven as effective as anticipated (86, 93). In this article, we will review studies that describe activation of the intracellular RAS by hyperglycemia, in different cell types, with particular emphasis on the actions of this system in the diabetic heart.
Classical RAS
Several decades ago, the RAS was characterized as a circulating system in which ANG II was generated in the circulation from AGT as a result of sequential cleavage by renin and angiotensin-converting enzyme (ACE) (44). The source of AGT in the circulation was mainly the liver, renin was secreted from kidneys, and ACE was primarily enriched on pulmonary endothelial cells. Circulating ANG II has an indisputable role in the pathophysiology of hypertension through multiple mechanisms, which include vasoconstriction, salt and water reabsorption, and aldosterone secretion. In the last two decades, attention has shifted from circulating to local generation of ANG II in tissues, particularly in the brain, heart, vasculature, adrenal glands, pancreas, and adipose tissue. Because of the access of circulating ANG II to tissues, it has been difficult to delineate the relative contribution of locally generated vs. circulating ANG II to overall tissue concentrations and biological effects. However, using tissue-specific genetic models of RAS components and other experimental designs, it is evident that the local RAS has a much greater role in tissue pathology than the circulating RAS (46). Most major organ systems have been reported to express all components of the RAS, which are regulated independently of the circulating system.
Cardiac RAS
All major components of the classical RAS, i.e., renin, AGT, ACE, AT1, and AT2, are expressed in the heart, although there is a lack of consensus on the source of renin (27, 43). Irrespective of the origin of these components, the majority of ANG II in the heart is produced in situ (87). Cardiac levels of renin and ANG II correlate well with plasma levels before and after nephrectomy in rats, promoting the hypothesis that circulating renin contributes significantly to ANG II production in the heart (9, 17). However, local ANG II levels are increased in pathological conditions, such as myocardial infarction (MI) and in the failing heart (10, 71). The difference in circulating and cardiac ANG II levels is particularly prominent in diabetes. Diabetes causes a decrease in circulating renin, while prorenin levels are increased several-fold, resulting in a reduced or normal ANG II concentration (38, 74). On the contrary, tissue RAS activity is significantly increased in diabetes, including in the heart (81). These observations support a local source of RAS components, including renin, in diabetes-induced ANG II production. We have reported increased renin protein and mRNA levels in isolated cardiac myocytes and fibroblasts in response to high glucose exposure (76, 80). In addition, alternative pathways of ANG II synthesis that involve cathepsins and chymase have been described and reviewed elsewhere (45).
As is evident from the foregoing discussion, renin and AGT are secretory proteins, and ACE and ANG receptors are localized on the plasma membrane. These characteristics of RAS components dictate that ANG II synthesis occurs outside of cells, and ligand-receptor interaction occurs at the level of the plasma membrane, as is typical of a hormonal system. The above is a well-documented and accepted characteristic of the RAS, which defines ANG II as an endocrine and autocrine/paracrine hormone. However, over the course of several years, investigators have made several observations that suggest an additional mechanism of ANG II functionality, termed an intracrine mechanism (60).
Intracellular RAS
The intracellular RAS and intracrine ANG II might appear to have the same meaning; however, there is a subtle difference between the two. Intracrine ANG II refers to actions of ANG II that originate from an intracellular location, irrespective of the source of ANG II. On the other hand, the intracellular RAS denotes the presence of a completely functional RAS inside cells, which includes intracellular ANG II synthesis and action (48).
Nuclear ANG II and ANG II receptors.
The first suggestion of intracrine functionality of ANG II stemmed from the studies of Robertson and Khairallah (67) in 1971, who demonstrated nuclear transport of radiolabeled ANG II injected in the left ventricle of adult rats (67). It is now well recognized that binding of extracellular ANG II to plasma membrane AT1 receptor results in internalization of the ANG II-AT1 complex (40, 85). Although a significant amount of internalized ANG II is degraded, a regulated portion is targeted to intracellular locations (such as the nucleus), where it could function as an intracrine mediator (31, 51, 72, 88). Specific binding of ANG II to nucleoprotein particles in the chromatin and increased RNA synthesis in isolated rat liver nuclei following exposure to ANG II were also reported (59, 61). We first characterized AT1-like ANG II binding sites on rat liver nuclei (6), which were subsequently shown to induce RNA transcription by others (30). Using electron microscopic and immunofluorescence immunocytochemistry techniques and an anti-AT1 antibody, ANG II receptors were detected in the sarcolemma, T-tubules, and nuclei of rat cardiomyocytes (37). More recently, both AT1 and AT2 receptors were detected on cardiomyocyte nuclei by Western blot analysis and confocal microscopy. ANG II application to cardiomyocyte nuclei enhanced NF-κB mRNA expression with approximately a twofold greater affinity, compared with when applied to intact cardiomyocytes (83). AT1-like nuclear ANG II receptors have also been reported in the renal cortex and medulla of rats (55). Isolated nuclei showed increased reactive oxygen species levels following exposure to ANG II, which were abolished by the AT1 antagonist losartan or the NADPH oxidase inhibitor DPI (56). The renal intracellular RAS has been reviewed in more detail elsewhere in this issue. These studies provided a framework for the concept of intracellular actions of ANG II.
Cellular effects of ANG II.
Some of the first evidence of intracellular effects of ANG II came from studies on cell communication in cardiomyocytes, isolated from adult rats. Intracellular dialysis of ANG I and ANG II decreased junctional conductance in cell pairs, which were suppressed by codialysis of an ACE inhibitor and an ANG receptor blocker (ARB), respectively (22). Similar observations were made when the cells were dialyzed with renin or renin plus AGT, suggesting intracellular generation of ANG II by cardiomyocytes (20). It was further demonstrated that endogenous ANG II was involved in the regulation of cell communication in cardiomyocytes, at an advanced stage of heart failure in hamsters, when the cardiac RAS was activated (19). Intracellular ANG II also decreased the cell volume of myocytes isolated from cardiomyopathic hamsters, an effect that was opposite to that of extracellular ANG II (21). In a similar study using vascular smooth muscle cells (VSMCs), microinjection of ANG II produced an increase in cytosolic and nuclear Ca2+ ions, due to an influx of extracellular Ca2+ ions. This effect of intracellular ANG II in VSMCs could be prevented by microinjection of an ARB, but not by extracellular application (39). In de-endothelized rat aorta rings, intracellular administration of ANG II, through liposomes, resulted in a dose-dependent contraction that was sensitive to an intracellular, but not extracellular ARB (8). Intracellular injection of ANG II increased cytosolic Ca2+ concentration in myometrial cells in a dose-dependent manner, likely through AT1-like receptors on lysosomes (25).
More recent studies utilized a recombinant approach to produce ANG II intracellularly. Overexpression of a nonsecretory truncated AGT, which resulted in ANG II generation in the cytoplasm, induced cell growth and PDGF expression in rat hepatoma cells (11, 15). We used a recombinant adenoviral expression vector to produce ANG II peptide in neonatal rat ventricular myocytes (NRVM). Cells expressing intracellular ANG II were significantly larger than control cells after 96 h of infection (3). The addition of extracellular ANG II to the culture medium led to an additional increase in cellular protein synthesis. Only extracellular effects of ANG II were blocked by the addition of the ARB losartan to the culture medium (3). These results were reproduced in adult mice, by tail vein injection of an intracellular ANG II expression plasmid, under the control of the α-myosin heavy chain promoter. The increase in intracellular ANG II expression in the heart caused a significant enlargement of ventricles in these mice, which was not prevented by losartan (3). Because ANG II expression was restricted to the heart, there was no change in blood pressure of these animals. Additionally, intracellular ANG II led to increased mRNA expression of several growth factors, including c-jun, IGF-1, and TGF-β in the heart. These results demonstrated for the first time, in vivo, that ANG II plays a critical role as an intracrine hormone in the heart. In cultured cardiac myocytes and fibroblasts, our studies suggested that intracellular ANG II, upregulated the expression of renin, AGT, and AT1, creating a positive feedback loop (77). The latter effect of intracellular ANG II in cardiac fibroblasts was in contrast to the effect of extracellular ANG II in these cells (28, 77). The positive feedback loop might have significant implications in the pathology of chronic diseases, such as cardiac remodeling, that involve persistent activation of the RAS. In cardiac fibroblasts, high glucose-induced intracellular ANG II augmented the production of TGF-β and collagen-1. The ARB candesartan partially attenuated, while renin and ACE inhibitors completely prevented, the latter effects of ANG II (76). The lack of inhibition of intracellular effects of ANG II, by ARBs, suggested that either these drugs did not internalize sufficiently or that intracellular ANG II acted independently of the AT1 receptor (4).
Mechanism of intracellular ANG II actions.
In the above described studies using dialysis or microinjection of ANG II into cardiomyocytes or VSMCs, ANG II actions could be blocked by intracellular, not extracellular, application of an ARB, suggesting involvement of an intracellular AT1-like receptor. In these cells, AT1-like binding sites were associated with PLC and PKC activation (33). However, in A7r5 VSMCs and CHO cells, which do not express functionally relevant amounts of AT1 receptor, intracellular ANG II produced growth effects, suggesting the presence of novel intracellular ANG II binding sites (4, 34, 35). Coexpression of ANG II and AT1 receptor in COS-7 and CHO-K1 cells caused greater nuclear localization of the AT1 receptor and stimulated cAMP response element-binding protein activity (13). The latter involved activation of p38 MAPK, but not ERK1/2, in A10 VSMCs (12). Recently, it was reported that the effects of intracellular dialysis of ANG II on the L-type inward calcium current in the failing heart of cardiomyopathic hamsters were inhibited by eplerenone, a mineralocorticoid receptor antagonist. Aldosterone reversed the inhibition by eplerenone, suggesting that the aldosterone system also interacts with the intracellular RAS (24). In a yeast two-hybrid screen, intracellular ANG II was reported to bind to mitochondrial proteins of the NADH dehydrogenase complex (14). This latter finding was confirmed in renal proximal tubule cells. The interaction of ANG II with mitochondrial proteins modified oxidative phosphorylation and reactive oxygen species production (14), which might have contributed to the development of hypertension in transgenic mice expressing intracellular ANG II (65). A recently described non-AT1, non-AT2 ANG II binding site was identified to be a membrane-bound variant of neurolysin (41, 91). Neurolysin is an endopeptidase with wide tissue distribution and cytosolic, mitochondrial, and plasma membrane localization on cells (91). Interestingly, neurolysin is similar to the previously described soluble angiotensin-binding protein in the liver (82). The pathophysiological function of these novel ANG II binding proteins remains to be elucidated. Nonetheless, these studies point to plurality of the mechanisms of intracellular ANG II actions, which might depend on the source of ANG II, i.e., internalized or synthesized intracellularly, which, in turn, might affect the intracellular location and function. Of additional interest are yet unidentified intracellular ANG II binding sites, such as those speculated in A7r5 VSMCs and CHO cells and those described on chromatin (4, 35, 64), identification of which is required for complete understanding of this system and therapeutic modulation in pathological states.
Intracellular cardiac ANG II synthesis.
Although ANG II that is internalized via AT1 receptor could possibly act as an intracrine factor in a pathophysiological setting, in situ generation of ANG II would provide a more convincing argument in favor of intracellular effects. As alluded to earlier, the secretory nature of AGT and renin suggests only extracellular synthesis of ANG II. However, observations, such as uptake and activation of prorenin by cardiomyocytes, with a resultant increase in intracellular ANG II levels, indicate that intracellular ANG II synthesis might occur following sequestration of prorenin and AGT by these cells (53, 57, 89). Additionally, an intracellular exon 1A isoform of renin is expressed in rat heart, levels of which are increased four-fold in the infarcted heart, which could contribute to intracellular ANG II generation (10). The intracellular isoform of renin appears to be active as overexpression of this isoform increases mitochondrial apoptosis in H9c2 cells and increases aldosterone production in transgenic rats (58, 92). Further, studies on renin and AGT dialysis into cardiomyocytes, described above, and those on isolated perfused rat hearts suggested intracellular generation of ANG II (18, 20). In the latter study, tissue ANG II levels were increased following perfusion of the heart either with renin plus AGT, ANG I, or ANG II. The addition of losartan to the renin plus AGT perfusion had no significant effect, whereas losartan in the perfusions with ANG I or ANG II decreased tissue ANG II to undetectable levels. These observations suggested renin and AGT uptake and intracellular ANG II production in the heart (18). More recently, a newly identified metabolite of AGT, ANG-(1–12), has been reported to be internalized by rat cardiomyocytes and metabolized to bioactive ANG peptides, including ANG II (1). The conversion of ANG-(1–12) into ANG II, which was independent of renin but likely involved chymase, was significantly enhanced in spontaneously hypertensive rats compared to Wistar-Kyoto rats. All of the above studies supported intracellular ANG II production by cardiomyocytes; however, a direct demonstration in a pathophysiological setting was lacking. We performed detailed studies to determine the mechanism and site of ANG II generation in cardiomyocytes and fibroblasts, as described below.
Mechanism of intracellular ANG II synthesis in cultured cardiomyocytes.
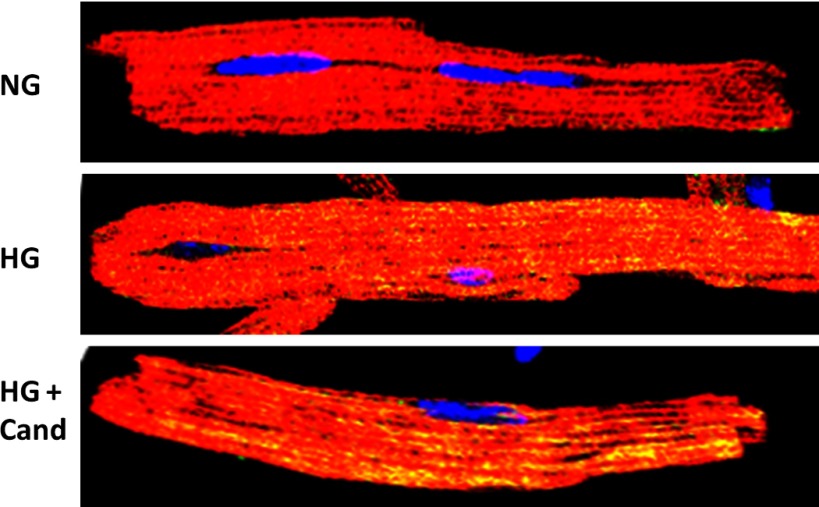
ANG II synthesis in NRVM was stimulated by isoproterenol and a high glucose concentration in serum-free culture medium (80). Changes in ANG II levels in the cell lysate and culture medium were measured separately in the presence or absence of the ARB candesartan. Under these conditions, ANG II would be synthesized from endogenously produced, not internalized, RAS components. Further, an increase in ANG II levels in the cell lysate, in the presence of candesartan, would indicate intracellular synthesis, not uptake, from the culture medium. Exposure to isoproterenol increased ANG II levels, both in the cell lysate and culture medium, suggesting intracellular synthesis, as well as secretion and/or extracellular synthesis of ANG II. In the case of high glucose, ANG II levels were increased only in the cell lysate. Further experiments showed that AGT and renin were retained intracellularly in the presence of high glucose, the reason for which is unknown. Confocal microscopy revealed that isoproterenol-stimulated ANG II was localized mainly along actin filaments, likely in secretory vesicles; whereas ANG II staining was observed in the perinuclear region and inside of nuclei of cardiomyocytes exposed to high glucose. These results indicated that intracellular ANG II was synthesized at different sites in response to isoproterenol and high glucose, likely because of redistribution of RAS components under these conditions. Using specific enzyme inhibitors, we observed that isoproterenol-induced ANG II synthesis was catalyzed by renin and ACE; whereas the high-glucose-mediated ANG II generation involved renin and chymase. Similar to NRVM, adult mouse cardiomyocytes showed enhanced intracellular ANG II production when exposed to high-glucose medium (Fig. 1). These studies demonstrated that cardiomyocytes synthesized ANG II solely intracellularly in high glucose conditions (80).
Fig. 1.
Intracellular ANG II in adult mouse cardiac myocytes. Cardiac myocytes were isolated from young adult mice using the Langendorff method. Myocytes were grown in medium containing normal glucose (NG; 5.5 mM), high glucose (HG; 25 mM), or HG + candesartan (Cand; 1 μM). After 24 h, cells were coimmunostained for ANG II (green) and α-sarcomeric actin (red) and visualized with a confocal microscope. A high degree of intracellular ANG II staining (yellow) was observed in HG medium compared with NG. Candesartan did not have any effect on levels of intracellular ANG II, suggesting intracellular synthesis.
Mechanism of intracellular ANG II synthesis in cultured cardiac fibroblasts.
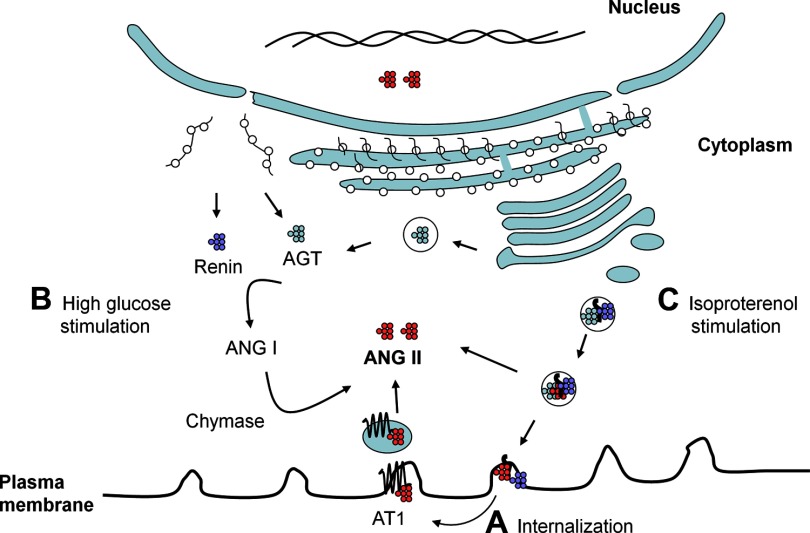
Fibroblasts constitute the second major cell type in the heart, which have a significant role in cardiac remodeling in various pathological conditions. Fibroblasts have been reported to contain a complete RAS and respond to stimulation with ANG II by extracellular matrix production (69). The close proximity to and interaction with cardiac myocytes make cardiac fibroblasts of critical importance when considering paracrine and intracrine production of ANG II in the heart. We performed experiments in these cells similar to those described in cardiomyocytes (76). It was observed that treatment of cardiac fibroblasts with either high glucose or isoproterenol induced both intracellular and extracellular production of ANG II. ANG II production, intracellular and extracellular, was dependent on renin and ACE, not chymase, both with high glucose and isoproterenol treatment. Immunocytochemical analysis showed punctate cytoplasmic and perinuclear staining for ANG II. Taken together, our studies in cardiac myocytes and fibroblasts suggested that the site of ANG II synthesis, i.e., intracellular (secretory vesicles or elsewhere) or extracellular, and the enzymes involved, largely depend on the cell type and the stimulus (Fig. 2).
Fig. 2.
Sources of intracellular ANG II in cardiomyocytes. A, internalization: ANG II-AT1 receptor complex undergoes internalization in the process of cellular signaling. The complex dissociates intracellularly, resulting in the degradation or release of ANG II in the cytoplasm. B, high-glucose stimulation: intracellular synthesis following stimulation with high glucose. The precise intracellular site of ANG II synthesis is not known; however, it appears to occur outside of the secretory pathway. In cardiomyocytes, renin and chymase are involved in intracellular ANG II synthesis. C, isoproterenol stimulation: intracellular synthesis following stimulation with isoproterenol: In this case, ANG II synthesis likely occurs inside the secretory vesicles, due to cosorting of AGT, renin, and ACE. Part of the secretory vesicle ANG II content is released into the cytoplasm, while what remains is secreted outside of the cell. Cytoplasmic ANG II can freely diffuse into nucleus due to the small size. ANG II likely has multiple interaction sites in the cytoplasmic organelles and nucleus.
Stimulation of the intracellular RAS by glucose in other cell types.
The most significant observation regarding the intracellular cardiac RAS is the high glucose-induced intracellular synthesis and complete retention of ANG II in cardiomyocytes. These observations are important not only for the understanding of the RAS in diabetes, but also for clinical management in diabetic cardiomyopathy. Interestingly, the conversion of a secretory system to an intracellular system by glucose is not unique to cardiomyocytes. It was reported in rat VSMCs that ANG II production was mainly extracellular and ACE-mediated in normal glucose conditions, becoming largely intracellular and chymase-catalyzed in high glucose medium (50). Podocytes have an important role in the pathogenesis of proteinuria in diabetic nephropathy. Exposure of podocytes to high glucose doubled intracellular ANG II levels, which could be blocked by chymostatin, but not by captopril (29). Other investigators also reported increased ANG II levels in the cell lysate of podocytes exposed to high glucose conditions (94, 95). Similarly, increased intracellular ANG II levels in response to high glucose have been described in renal mesangial and proximal tubular cells (16, 84, 90). These studies suggest that several major cell types produce ANG II intracellularly and that the intracellular RAS is significantly upregulated by high glucose conditions.
Intracellular RAS in Diabetic Cardiomyopathy
Diabetic cardiomyopathy is defined as heart failure manifested independently of other risk factors and is closely associated with diabetes mellitus (42). The etiology of this disease remains unclear, but it may be related to several factors, including abnormal metabolism of glucose in myocardial tissue. Involvement of the RAS in diabetic cardiomyopathy has been demonstrated by experimental and clinical studies. As discussed above, high glucose upregulated the intracellular RAS in cardiomyocytes (79), cardiac fibroblasts (76), VSMCs (50), and renal mesangial cells (75). Thus, diabetes mellitus provided an important pathological condition to study the role of the intracellular RAS in vivo. Our in vitro studies suggested that in the diabetic heart, cardiomyocytes will generate primarily intracellular ANG II, via renin and chymase, whereas fibroblasts will generate both intracellular and extracellular ANG II through renin and ACE. Thus, ANG II will have both intracrine and autocrine/paracrine effects on cardiac cells in the diabetic heart. ACE inhibitors will prevent ANG II synthesis only in cardiac fibroblasts. Significantly, in humans, intracellular ANG II levels in cardiac myocytes were 3.4-fold higher in diabetic patients, compared with nondiabetics and an additional twofold higher in diabetic hypertensive patients, compared with diabetic nonhypertensive patients (36). Interestingly, these patients were on ACE inhibitor therapy. We have reported previously that ARBs do not abolish intracellular ANG II-mediated effects in the heart (3). Therefore, current therapeutic modalities utilizing ACE inhibitors and ARBs may only be partially effective in diabetic cardiomyopathy. The benefits of ACE inhibitors and ARBs during and after MI have been found to be greater in diabetics than nondiabetics and activation of the RAS has been implicated in diabetes (52). However, following MI, the incidence of heart failure and mortality rates is increased twofold in patients with diabetes, compared with nondiabetics (5, 54). There is a growing consensus that inhibition of the RAS using ARBs and ACE inhibitors has not provided as much cardiovascular benefit as anticipated (86, 93).
We utilized a streptozotocin-induced type-1 model of diabetes in rats to study activation of the cardiac intracellular RAS and the role in cardiac remodeling (81). One week of diabetes significantly increased intracellular ANG II levels in cardiac myocytes isolated from diabetic hearts compared with controls. Intracellular levels of ANG II were not normalized by candesartan, suggesting that ANG II was synthesized intracellularly, not internalized through AT1 receptor. Similar to the in vitro studies discussed previously, an increase in intracellular ANG II levels in cardiac myocytes was prevented by treatment of diabetic rats by aliskiren (renin inhibitor), but not by benazeprilat. Diabetes-induced superoxide production and cardiac fibrosis were partially inhibited by candesartan and benazepril treatment, whereas aliskiren produced complete inhibition (81). These observations suggested that renin inhibition has a more pronounced effect than ARBs and ACE inhibitors, on diabetic complications and may be clinically more efficacious.
We further extended these observations by studying comparative efficacy of RAS inhibition at the level of renin, ACE, and AT1 receptor, on cardiac function in a mouse model of streptozotocin-induced diabetes (70). Diastolic cardiac dysfunction evident at 10 wk of diabetes, measured by mitral annulus flow (E/A ratio), was completely prevented by treatment with all three agents. However, aliskiren resulted in a better E/A ratio at 10 wk compared with time 0. The increase in isovolumetric relaxation time was completely prevented by aliskiren and benazeprilat, while valsartan was only partially effective. A significant reduction in ejection fraction (EF), fractional shortening (FS), and cardiac output in diabetic animals were prevented by all three drugs. However, aliskiren and benazeprilat treatment showed statistically significant improvement in EF and FS, at 10 wk compared with time 0. There was no difference in the mean arterial pressure between control, diabetic, or diabetic groups treated with RAS inhibitors, suggesting that any effect of these drugs on cardiac function was independent of blood pressure. These data showed that renin inhibition provided similar/better cardiac protection compared with ACE inhibitors and ARBs in diabetic cardiomyopathy (70). Protective effects of ACE inhibitors and ARBs may have been partially mediated through non-RAS-related mechanisms, such as an effect on the kallikrein-kinin system and PPARγ activation (32, 68). A genetic model, such as AT1 receptor-deficient mice, will likely provide better insight on the role of intracellular vs. extracellular ANG II in diabetic conditions.
Direct Effect of ANG II in the Heart: Extracellular vs. Intracellular ANG II
Multiple in vitro studies have shown that ANG II has a direct effect on cell signaling, resulting in hypertrophy in cardiac myocytes and proliferation of cardiac fibroblasts (7, 28, 73). ANG II causes changes in gene expression, resulting in the secretion of growth factors and extracellular matrix proteins. However, direct effects of ANG II on cardiac remodeling are difficult to study in vivo, as it is not possible to prevent systemic effects of ANG II, such as hypertension, from affecting cardiac function. Several experimental and genetic models have been utilized to study a direct effect of the RAS in the heart. Results of these studies have not been consistent, which has prompted some investigators to suggest that ANG II alone, independent of blood pressure, does not produce cardiac hypertrophy and fibrosis (66). A requirement of other factors, such as oxidative stress, inflammation, and aldosterone, has been proposed for ANG II to produce pathological effects in the heart (49). However, these factors may very well be the product of ANG II actions. These studies using genetic models were designed to activate the extracellular cardiac RAS. A recent report described a transgenic mouse expressing intracellular ANG II in fusion with a fluorescent protein (65). The fusion protein was detected in several tissues, including kidneys and the heart. These mice were hypertensive and developed thrombotic microangiopathy. However, no significant histological changes were observed in the heart, which is in contrast to our observation of significant cardiac hypertrophy in mice injected with an intracellular ANG II-expressing vector (3). One of the differences in these two studies was the use of ANG II as a fusion protein vs. native peptide. No cardiac histological change in the fusion protein study was particularly noteworthy in view of the elevated blood pressure in these animals. Effects on cardiac function or other parameters, such as oxidative stress, were not reported. As discussed by De Mello and Frohlich (23), caution should be used when interpreting results of studies utilizing different experimental conditions, and direct but subtle effects of ANG II, including those on cellular communication and metabolism, should not be ignored (23). It will be interesting to study cardiac pathology in intracellular ANG II transgenic mice in the diabetic state, which is associated with upregulation of the endogenous intracellular RAS.
One question that arises is whether intracellular and extracellular ANG II work independently of or in concert with each other. Re (62) has proposed an intracrine hypothesis, a key feature of which is that intracrine factors participate in the formation of cellular memory through positive feedback loops. The latter might be important for long-term potentiation of autocrine/paracrine effects of intracrine peptides. As alluded to earlier, extracellular ANG II binding to AT1 causes internalization of the receptor, causing downregulation of AT1-mediated signaling. The latter appears to be a protective mechanism to prevent continuous activation of the system, which otherwise might produce deleterious effects. Regulation of blood pressure and fluid and electrolyte homeostasis likely falls in this category of acute effects of extracellular ANG II. To produce chronic effects, such as cardiac hypertrophy, the system needs to be continuously activated. Our studies have suggested that intracellular ANG II is a positive regulator of the RAS, i.e., increases expression of AGT, renin, and AT1 receptor, both in cardiomyocytes and fibroblasts (77). Thus, in addition to having independent cellular effects, intracellular ANG II likely keeps AT1 receptor levels sufficiently high for long-term activity of extracellular ANG II. Thus, the intracellular and extracellular RAS work in concert rather than as separate systems. However, the above hypothesis needs to be verified in a suitable in vivo model.
Perspectives and Significance
Several studies have demonstrated effects of intracellular ANG II in multiple cell types. The occurrence of ANG II intracellularly does not solely depend on AT1-mediated internalization; instead, all major cell types synthesize ANG II intracellularly under certain pathophysiological conditions (Fig. 2). Hyperglycemia appears to be a common condition that significantly enhances intracellular ANG II generation in several cells. The intracellular site of ANG II synthesis and the participating enzymes may differ between cells. The latter may influence modulation of the RAS in a pathological state, particularly diabetes. From our current understanding of the cardiac intracellular RAS, it appears that therapeutic modalities, such as a renin inhibitor, will provide more complete blockade of the cardiac RAS in diabetes than an ARB or ACE inhibitor. The latter may apply to diabetic nephropathy and retinopathy as well; however, that remains to be determined. More studies are required to delineate specific roles of intracellular ANG II, independent of and in conjunction with extracellular ANG II. Additionally, the mechanism of intracellular ANG II actions, mediated by AT1-like or other intracellular binding sites, needs to be determined. Knowledge of the intracellular RAS will also enhance our understanding of other peptide hormonal systems, which have demonstrated similar intracrine modes of action.
GRANTS
This work was supported by National Institutes of Health Grant 5R01HL090817.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.K. conception and design of research; R.K., Q.C.Y., and C.M.T. analyzed data; R.K., Q.C.Y., and C.M.T. interpreted results of experiments; R.K. prepared figures; R.K., Q.C.Y., and C.M.T. drafted manuscript; R.K. and K.M.B. edited and revised manuscript; Q.C.Y. and C.M.T. performed experiments; K.M.B. approved final version of manuscript.
REFERENCES
- 1. Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS One 6: e15759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bader M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu Rev Pharmacol Toxicol 50: 439–465, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept 120: 5–13, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol 291: C995–C1001, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Boccara F, Cohen A. Interplay of diabetes and coronary heart disease on cardiovascular mortality. Heart 90: 1371–1373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Booz GW, Conrad KM, Hess AL, Singer HA, Baker KM. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology 130: 3641–3649, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Booz GW, Dostal DE, Baker KM. Paracrine actions of cardiac fibroblasts on cardiomyocytes: implications for the cardiac renin-angiotensin system. Am J Cardiol 83: 44H–47H, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Brailoiu E, Filipeanu CM, Tica A, Toma CP, de Zeeuw D, Nelemans SA. Contractile effects by intracellular angiotensin II via receptors with a distinct pharmacological profile in rat aorta. Br J Pharmacol 126: 1133–1138, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell DJ, Kladis A, Duncan AM. Nephrectomy, converting enzyme inhibition, and angiotensin peptides. Hypertension 22: 513–522, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Clausmeyer S, Reinecke A, Farrenkopf R, Unger T, Peters J. Tissue-specific expression of a rat renin transcript lacking the coding sequence for the prefragment and its stimulation by myocardial infarction. Endocrinology 141: 2963–2970, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Cook JL, Giardina JF, Zhang Z, Re RN. Intracellular angiotensin II increases the long isoform of PDGF mRNA in rat hepatoma cells. J Mol Cell Cardiol 34: 1525–1537, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol 40: 696–707, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol 36: 75–90, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Cook JL, Singh A, Aguiluz RN, Re RN, Alam J. Intracellular angiotensin II binds to mitochondrial proteins of the NADH dehydrogenase complex and modifies oxidative phosphorylation. Hypertension 56: E135–E135, 2010 [Google Scholar]
- 15. Cook JL, Zhang Z, Re RN. In vitro evidence for an intracellular site of angiotensin action. Circ Res 89: 1138–1146, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Cristovam PC, Arnoni CP, de Andrade MC, Casarini DE, Pereira LG, Schor N, Boim MA. ACE- and chymase-dependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med (Maywood) 233: 1035–1043, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Danser AH, Saris JJ, Schuijt MP, van Kats JP. Is there a local renin-angiotensin system in the heart? Cardiovasc Res 44: 252–265, 1999 [DOI] [PubMed] [Google Scholar]
- 18. de Lannoy LM, Danser AH, Bouhuizen AM, Saxena PR, Schalekamp MA. Localization and production of angiotensin II in the isolated perfused rat heart. Hypertension 31: 1111–1117, 1998 [DOI] [PubMed] [Google Scholar]
- 19. De Mello WC. Further studies on the effect of intracellular angiotensins on heart cell communication: on the role of endogenous angiotensin II. Regul Pept 115: 31–36, 2003 [DOI] [PubMed] [Google Scholar]
- 20. De Mello WC. Influence of intracellular renin on heart cell communication. Hypertension 25: 1172–1177, 1995 [DOI] [PubMed] [Google Scholar]
- 21. De Mello WC. Intracellular and extracellular renin have opposite effects on the regulation of heart cell volume. Implications for myocardial ischaemia. J Renin Angiotensin Aldosterone Syst 9: 112–118, 2008 [DOI] [PubMed] [Google Scholar]
- 22. De Mello WC. Is an intracellular renin-angiotensin system involved in control of cell communication in heart? J Cardiovasc Pharmacol 23: 640–646, 1994 [DOI] [PubMed] [Google Scholar]
- 23. De Mello WC, Frohlich ED. On the local cardiac renin angiotensin system. Basic and clinical implications. Peptides 32: 1774–1779, 2011 [DOI] [PubMed] [Google Scholar]
- 24. De Mello WC, Gerena Y. Eplerenone inhibits the intracrine and extracellular actions of angiotensin II on the inward calcium current in the failing heart. On the presence of an intracrine renin angiotensin aldosterone system. Regul Pept 151: 54–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deliu E, Tica AA, Motoc D, Brailoiu GC, Brailoiu E. Intracellular angiotensin II activates rat myometrium. Am J Physiol Cell Physiol 301: C559–C565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dell'italia LJ. Translational success stories: Angiotensin receptor 1 antagonists in heart failure. Circ Res 109: 437–452, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res 85: 643–650, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Dostal DE, Booz GW, Baker KM. Regulation of angiotensinogen gene expression and protein in neonatal rat cardiac fibroblasts by glucocorticoid and beta-adrenergic stimulation. Basic Res Cardiol 95: 485–490, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol 294: F830–F839, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Eggena P, Zhu JH, Clegg K, Barrett JD. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension 22: 496–501, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Erdmann B, Fuxe K, Ganten D. Subcellular localization of angiotensin II immunoreactivity in the rat cerebellar cortex. Hypertension 28: 818–824, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Erdos EG, Tan F, Skidgel RA. Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function. Hypertension 55: 214–220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eto K, Ohya Y, Nakamura Y, Abe I, Iida M. Intracellular angiotensin II stimulates voltage-operated Ca2+ channels in arterial myocytes. Hypertension 39: 474–478, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Filipeanu CM, Henning RH, de Zeeuw D, Nelemans A. Intracellular angiotensin II and cell growth of vascular smooth muscle cells. Br J Pharmacol 132: 1590–1596, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filipeanu CM, Henning RH, Nelemans SA, de Zeeuw D. Intracellular angiotensin II: from myth to reality? J Renin Angiotensin Aldosterone Syst 2: 219–226, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Fu ML, Schulze W, Wallukat G, Elies R, Eftekhari P, Hjalmarson A, Hoebeke J. Immunohistochemical localization of angiotensin II receptors (AT1) in the heart with anti-peptide antibodies showing a positive chronotropic effect. Receptors Channels 6: 99–111, 1998 [PubMed] [Google Scholar]
- 38. Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab 16: 120–126, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res 79: 765–772, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal Physiol 283: F1003–F1010, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Karamyan VT, Speth RC. Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Res 1143: 83–91, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Khavandi K, Khavandi A, Asghar O, Greenstein A, Withers S, Heagerty AM, Malik RA. Diabetic cardiomyopathy—a distinct disease? Best Pract Res Clin Endocrinol Metab 23: 347–360, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Krop M, Danser AH. Circulating versus tissue renin-angiotensin system: on the origin of (pro)renin. Curr Hypertens Rep 10: 112–118, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Kumar R, Baker KM, Pan J. Activation of the renin-angiotensin system in heart failure. In: Heart Failure: A Companion to Braunwald's Heart Disease, edited by Mann DL. Philadelphia, PA: Saunders, 2011, p. 134–151 [Google Scholar]
- 45. Kumar R, Boim MA. Diversity of pathways for intracellular angiotensin II synthesis. Curr Opin Nephrol Hypertens 18: 33–39, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system—implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens 17: 168–173, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system in the heart. Curr Hypertens Rep 11: 104–110, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab 18: 208–214, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Kurdi M, Booz GW. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension 57: 1034–1038, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res 101: 455–464, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O'Dowd BF. Agonist-independent nuclear localization of the apelin, angiotensin AT1 and bradykinin B2 receptors. J Biol Chem 279: 7901–7908, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Lim HS, MacFadyen RJ, Lip GY. Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch Intern Med 164: 1737–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Mercure C, Ramla D, Garcia R, Thibault G, Deschepper CF, Reudelhuber TL. Evidence for intracellular generation of angiotensin II in rat juxtaglomerular cells. FEBS Lett 422: 395–399, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Okin PM, Devereux RB, Gerdts E, Snapinn SM, Harris KE, Jern S, Kjeldsen SE, Julius S, Edelman JM, Lindholm LH, Dahlof B. Impact of diabetes mellitus on regression of electrocardiographic left ventricular hypertrophy and the prediction of outcome during antihypertensive therapy: the Losartan Intervention For Endpoint (LIFE) reduction in hypertension study. Circulation 113: 1588–1596, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun 384: 149–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MGF, Mullins JJ. Functional significance of prorenin internalization in the rat heart. Circ Res 90: 1135–1141, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Peters J, Wanka H, Peters B, Hoffmann S. A renin transcript lacking exon 1 encodes for a non-secretory intracellular renin that increases aldosterone production in transgenic rats. J Cell Mol Med 12: 1229–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Re R, Parab M. Effect of angiotensin II on RNA synthesis by isolated nuclei. Life Sci 34: 647–651, 1984 [DOI] [PubMed] [Google Scholar]
- 60. Re RN. The intracellular renin angiotensin system: the tip of the intracrine physiology iceberg. Am J Physiol Heart Circ Physiol 293: H905–H906, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Re RN. Mechanisms of disease: local renin-angiotensin-aldosterone systems and the pathogenesis and treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med 1: 42–47, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Re RN. Toward a theory of intracrine hormone action. Regul Pept 106: 1–6, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Re RN, Cook JL. The intracrine hypothesis: An update. Regul Pept 133: 1–9, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Re RN, Vizard DL, Brown J, Bryan SE. Angiotensin II receptors in chromatin fragments generated by micrococcal nuclease. Biochem Biophys Res Commun 119: 220–227, 1984 [DOI] [PubMed] [Google Scholar]
- 65. Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, Sigmund CD, Tang WW, Cook JL. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol 298: H1807–H1818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reudelhuber TL, Bernstein KE, Delafontaine P. Is angiotensin II a direct mediator of left ventricular hypertrophy? Time for another look. Hypertension 49: 1196–1201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Robertson AL, Jr, Khairallah PA. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science 172: 1138–1139, 1971 [DOI] [PubMed] [Google Scholar]
- 68. Rong X, Li Y, Ebihara K, Zhao M, Naowaboot J, Kusakabe T, Kuwahara K, Murray M, Nakao K. Angiotensin II type 1 receptor-independent beneficial effects of telmisartan on dietary-induced obesity, insulin resistance and fatty liver in mice. Diabetologia 53: 1727–1731, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Sanghi S, Kumar R, Smith M, Baker KM, Dostal DE. Activation of protein kinase A by atrial natriuretic peptide in neonatal rat cardiac fibroblasts: role in regulation of the local renin-angiotensin system. Regul Pept 132: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Seqqat R, Thomas C, Yong QC, Feldman DL, Baker KM, Kumar R. Aliskiren prevents diabetic cardiomyopathy, independent of blood pressure, in a mouse model: Comparison with an ACE inhibitor and angiotensin receptor blocker. Hypertension 58: e128, 2011 [Google Scholar]
- 71. Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, Bandinelli B, Bertolozzi I, Polidori G, Toscano T, Maccherini M, Modesti PA. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res 88: 961–968, 2001 [DOI] [PubMed] [Google Scholar]
- 72. Sherrod M, Liu X, Zhang X, Sigmund CD. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol 288: R539–R546, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Shivakumar K, Dostal DE, Boheler K, Baker KM, Lakatta EG. Differential response of cardiac fibroblasts from young adult and senescent rats to ANG II. Am J Physiol Heart Circ Physiol 284: H1454–H1459, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Sica DA, Ichihara A. The renin report. J RAAS 7: 247–251, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Singh R, Leehey DJ. Effect of ACE inhibitors on angiotensin II in rat mesangial cells cultured in high glucose. Biochem Biophys Res Commun 357: 1040–1045, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol 294: H1675–H1684, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Singh VP, Baker KM, Kumar R. Intracellular angiotensin II is a positive regulator of the cardiac renin-angiotensin system. Hypertension 52: E91–E92, 2008 [Google Scholar]
- 78. Singh VP, Baker KM, Kumar R. Novel aspects of the cardiac renin-angiotensin system. In: The Local Cardiac Renin Angiotensin-Aldosterone System, edited by Frohlich ED, Re RN. New York: Springer Science+Business Media, 2009, p. 75–89 [Google Scholar]
- 79. Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol 293: H939–H948, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High glucose-induced regulation of intracellular angiotensin II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol 293: H939–H948, 2007 [DOI] [PubMed] [Google Scholar]
- 81. Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57: 3297–3306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sugiura N, Hagiwara H, Hirose S. Molecular cloning of porcine soluble angiotensin-binding protein. J Biol Chem 267: 18067–18072, 1992 [PubMed] [Google Scholar]
- 83. Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem 285: 22338–22349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Takao T, Horino T, Kagawa T, Matsumoto R, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Taguchi T, Morita T, Terada Y. Possible involvement of intracellular angiotensin II receptor in high-glucose-induced damage in renal proximal tubular cells. J Nephrol 24: 218–224, 2011 [DOI] [PubMed] [Google Scholar]
- 85. Thomas WG, Thekkumkara TJ, Baker KM. Cardiac effects of AII. AT1A receptor signaling, desensitization, and internalization. Adv Exp Med Biol 396: 59–69, 1996 [PubMed] [Google Scholar]
- 86. Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, Woodward M, MacMahon S. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 165: 1410–1419, 2005 [DOI] [PubMed] [Google Scholar]
- 87. van Kats JP, Danser AHJ, van Maegen J, Sassen LMA, Verdouw PD, Schalekamp MADH. Angiotensin production in the heart: a quantitative study with use of radiolabelled angiotensin infusions. Circulation 98: 73–81, 1998 [DOI] [PubMed] [Google Scholar]
- 88. van Kats JP, de Lannoy LM, Jan Danser AH, van Meegen JR, Verdouw PD, Schalekamp MA. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension 30: 42–49, 1997 [DOI] [PubMed] [Google Scholar]
- 89. van Kesteren CA, Danser AH, Derkx FH, Dekkers DH, Lamers JM, Saxena PR, Schalekamp MA. Mannose 6-phosphate receptor-mediated internalization and activation of prorenin by cardiac cells. Hypertension 30: 1389–1396, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286: F1039–F1045, 2004 [DOI] [PubMed] [Google Scholar]
- 91. Wangler NJ, Santos KL, Schadock I, Hagen FK, Escher E, Bader M, Speth RC, Karamyan VT. Identification of membrane-bound variant of metalloendopeptidase neurolysin (EC 3.4.24.16) as the non-AT1, non-AT2 angiotensin binding site. J Biol Chem In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wanka H, Kessler N, Ellmer J, Endlich N, Peters BS, Clausmeyer S, Peters J. Cytosolic renin is targeted to mitochondria and induces apoptosis in H9c2 rat cardiomyoblasts. J Cell Mol Med 13: 2926–2937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weber MA, Giles TD. Inhibiting the renin-angiotensin system to prevent cardiovascular diseases: do we need a more comprehensive strategy? Rev Cardiovasc Med 7: 45–54, 2006 [PubMed] [Google Scholar]
- 94. Xu ZG, Yoo TH, Ryu DR, Cheon Park H, Ha SK, Han DS, Adler SG, Natarajan R, Kang SW. Angiotensin II receptor blocker inhibits p27Kip1 expression in glucose-stimulated podocytes and in diabetic glomeruli. Kidney Int 67: 944–952, 2005 [DOI] [PubMed] [Google Scholar]
- 95. Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, Choi HY, Kim JS, Kim HJ, Han SH, Lee JE, Han DS, Kang SW. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int 71: 1019–1027, 2007 [DOI] [PubMed] [Google Scholar]