Abstract
Cardiac and vascular dysfunctions resulting from autonomic neuropathy (AN) are complications of diabetes, often undiagnosed. Our objectives were to: 1) determine sympathetic and parasympathetic components of compromised blood pressure (BP) regulation in patients with peripheral neuropathy and 2) rank noninvasive indexes for their sensitivity in diagnosing AN. We continuously measured electrocardiogram, arterial BP, and respiration during supine rest and 70° head-up tilt in 12 able-bodied subjects, 7 diabetics without, 7 diabetics with possible, and 8 diabetics with definite, sensory, and/or motor neuropathy (D2). During the first 3 min of tilt, systolic BP (SBP) of D2 decreased [−10.9 ± 4.5 (SE) mmHg] but increased in able-bodied (+4.8 ± 5.4 mmHg). Compared with able-bodied, D2 had smaller low-frequency (0.04–0.15 Hz) spectral power of diastolic BP, lower baroreflex effectiveness index (BEI), and more SBP ramps. Except for low-frequency power of SBP, D2 had greater SBP and smaller RR interval harmonic and nonharmonic components at rest across the 0.003- to 0.45-Hz region. In addition, our results support previous findings of smaller HF RR interval power, smaller numbers of baroreflex sequences, and lower baroreflex sensitivity in D2. We conclude that diabetic peripheral neuropathy is accompanied by diminished parasympathetic and sympathetic control of heart rate and peripheral vasomotion and diminished baroreflex regulation. A novel finding of this study lies in the sensitivity of BEI to detect AN, presumably because of its combination of parameters that measure reductions in both sympathetic control of vasomotion and parasympathetic control of heart rate.
Keywords: autonomic neuropathy, spectral power, baroreflex sensitivity, baroreflex effectiveness index, power spectral density
diabetic neuropathy is a common and serious complication of both type 1 and type 2 diabetes. In particular, autonomic neuropathy (AN), a common complication of diabetes, is associated with high mortality and morbidity, including sudden cardiac death, ventricular arrhythmias, and orthostatic hypotension (52). Therefore, early detection of AN leading to early medical intervention is of great clinical interest.
Current clinical methods used to diagnose diabetic AN rely on a battery of reflex tests called “Ewing tests” (22). Although useful in diagnosing AN, these tests require voluntary participation by patients, and test sensitivity is limited. Therefore, other techniques are being examined for their potential to provide in-depth, easy-to-administer, diagnostic tools. Comparisons of heart rate variability and low (LF)- and high (HF)-frequency components of heart rate (HR) oscillations have been correlated with abnormal results of Ewing tests (17, 26, 30, 59). Diabetic patients with abnormal Ewing results also demonstrated reduced LF spectral power of systolic blood pressure (SBP) compared with able-bodied controls or compared with diabetics with normal results during 1-h supine or a short period of standing (17, 59). In diabetics with no signs of AN, the numbers of spontaneous baroreflex sequences were reduced, and slopes of baroreflex sequences were smaller compared with controls (17, 25). The composite interpretation of these results points toward diabetic neuropathy-induced failure of both sympathetic and parasympathetic control of HR, baroreflex function, and vasomotion.
Studies of sinoaortic denervated (SAD) animals (cats and dogs), a form of “dysautonomia,” showed that not only were harmonic components (LF or HF peaks) of HR and blood pressure (BP) oscillations affected by SAD, but nonharmonic components were also altered over a wide frequency range (very low, low, and high frequencies) (14, 55). In addition to the alteration in HR and BP variability over a wide frequency range, the baroreflex effectiveness index (BEI), which represents the percentage of SBP ramps followed by reflex RR interval responses to all SBP ramps, was reduced by 89% in SAD cats (16). In humans with pure autonomic failure, HR and BP variability have been shown to be altered at all frequencies (44). Finally, we reported previously that spinal cord injury resulted in reduced BEI in tetraplegic patients compared with able-bodied controls (1). Results of these previous human and animal studies indicated similarities between diabetic AN, sinoaortic denervation, and primary autonomic failure that appeared to be proportional to the level of neuropathy and observable in noninvasive indexes of autonomic function.
In the present study, we compare sympathetic, parasympathetic, and baroreflex function of able-bodied individuals with that of individuals with diabetes with 1) absent, 2) possible, or 3) definite peripheral sensory and/or motor neuropathy. We hypothesized that the presence of definite peripheral sensory and/or motor neuropathy would be associated with the greatest deficits in both cardiac and peripheral autonomic regulation and that broadband power of HR variability, BP variability, and baroreflex function would be sensitive measures for early diagnosis of diabetic AN. Our tools included 1) spectral analysis techniques applied to HR and BP over a wide range of frequencies and 2) spontaneous baroreflex sequence technique to determine numbers of BP ramps, numbers of baroreflex sequences, baroreflex effectiveness, and baroreflex sensitivity (BRS).
METHODS
Subjects
Twelve healthy able-bodied volunteers were recruited from the local community using word of mouth, newspaper advertisements, and flyers mounted on boards across the university campus and hospital. Twenty-two diabetic subjects were recruited. The diabetic subjects were evaluated for distal symmetrical polyneuropathy using neuropathic symptoms, depressed ankle reflexes, distal sensory loss, distal muscle weakness or atrophy, and nerve conduction studies (8). Subjects were then distinguished by consensus criteria that determined the likelihood of neuropathy using an ordinal scale from highest four to lowest one (19). “Possible neuropathy” was defined by a likelihood ranking of two or three and “definite neuropathy” by the likelihood of four. Diabetic subjects without neuropathy had no signs or symptoms of neuropathy and normal nerve conduction studies. Based upon the results of these evaluations, the neuropathy of diabetic subjects was categorized as absent, possible, or definite. In addition to the tests for peripheral neuropathy, and for fasting blood glucose levels on the day of study, all subjects filled out a comprehensive questionnaire to assist with classification of any potential neuropathy and underwent a familiarization tilt and 12-lead electrocardiogram (ECG). Medications affecting autonomic function were discontinued for the day of study. Demographic characteristics of the participants are shown in Table 1.
Table 1.
Demographic characteristics of participants
| Diabetic |
||||
|---|---|---|---|---|
| Able Bodied | No peripheral neuropathy | Possible peripheral neuropathy | Definite peripheral neuropathy | |
| Age, yr | 48.2 ± 3.9 | 39.7 ± 5.6 | 53 ± 4.1 | 55.1 ± 2.7 |
| Weight, kg | 93.0 ± 9.0 | 84.2 ± 8.1 | 110.7 ± 15.9 | 97.9 ± 11.4 |
| Height, cm | 171.5 ± 3.0 | 164.6 ± 3.0 | 176.8 ± 4.6 | 174.2 ± 3.3 |
| n (male/female) | 12 (5/7) | 7 (1/6) | 7 (5/2) | 8 (5/3) |
| n of Type 1/Type 2 | 3/4 | 2/5 | 3/5 | |
Values are means ± SE; n, no. of subjects.
Tilt Protocol
We utilized passive head-up tilt to challenge the autonomic regulation of arterial BP. Before entering the study, all subjects gave written informed consent, and the protocol was approved by the University of Kentucky Institutional Review Board. Before head-up tilt testing, subjects' height, age, weight, and diabetic status were recorded. Later, after the blood glucose level had been determined, an intravenous cannula was placed in an antecubital vein. Subjects then rested in the supine position for 30 min when instrumentation was applied followed by 10 min of supine control and 30 min of 70-degree head-up tilt. If presyncopal symptoms developed during tilt, subjects were brought back to supine immediately. All subjects were followed for a supine recovery period of 5 min.
Measured Variables
Mixed venous blood samples were drawn from the antecubital vein catheter at the end of control, at 3 and 7 min of tilt, and during the 2nd min of recovery. Results of blood assays are not reported here.
Three-lead ECG (Pilot Colin), noninvasive continuous arterial BP (Portapres model-2), respiratory activity (Respitrace), and tilt angle (Crossbow CXL 04LP3) were acquired continuously as described in detail elsewhere (1); recordings commenced during supine control and continued uninterrupted during the 70-degree head-up tilt and supine recovery. The BP sensor was maintained at heart level. Manual BP using an arm cuff was acquired at the end of supine control, the beginning of tilt, and at the end of recovery.
Data Acquisition and Analysis
BP, ECG, respiratory activity, and tilt angle recordings were digitized at 1,000 Hz by using a commercial data acquisition system (DATAQ).
Mean values.
HR and RR interval were computed by identifying R waves in the last 5 min of control; 0–3 min, 3–7 min, and 7–12 min of tilt; and the first 3 min of recovery. Data were analyzed using custom written programs in C++ and MATLAB (the MathWorks, Natick, MA). Artifacts in HR and BP signals, including premature beats and Portapres servo adjustments (∼5% of the beats/time series), were manually removed. The cleaned signals were then aligned in time. SBP and diastolic blood pressure (DBP) were determined by computing the maximum and minimum values of clean arterial BP for each heartbeat. Mean values of RR interval, SBP, and DBP were computed in each time segment. All subsequent data analyses were performed in MATLAB.
Spectral analysis.
RR interval, SBP, DBP, and respiratory activity in control, tilt, and recovery periods were resampled at 4 Hz using a cubic spline. Each segment was then linearly detrended. Power spectral densities of RR intervals, SBP, DBP, and respiratory activity were estimated using Welch's method of averaged periodograms (480-point Hamming windows with 440-point overlap). Spectral powers in the LF (0.04–0.15 Hz) and HF (0.15–0.4 Hz) regions were obtained using trapezoidal integration over the specified frequency range. Spectral power of DBP was used as an index of sympathetic control of vasomotion because DBP correlated better than did SBP with vascular resistance (4). Power spectral density of SBP, however, was used as an index of baroreflex-mediated buffering of BP. Power spectral densities were plotted in a log-power vs. log-frequency scale, and the slope of the linear regression of this plot within the LF range was calculated. This power law scaling relationship was quantified for RR interval, SBP, and DBP to determine their harmonic and nonharmonic characteristics (18, 28).
Baroreflex sequences.
We adopted the sequence method (3) to quantify the number of BP ramps and baroreflex sequences, as well as BRS. We identified sequences of three or more consecutive heartbeats, in which progressively increasing (or decreasing) SBP with at least 1-mmHg beat-to-beat change were followed within one heartbeat by progressively lengthening (or shortening) of RR interval with at least 4-ms beat-to-beat change. A sequence was accepted as a baroreflex sequence if the correlation coefficient of the regression line between SBP and RR interval within the sequence was 0.85 or greater (3). The slope of the regression line for each sequence was taken as spontaneous BRS. The ratio between the number of baroreflex sequences and the total number of increasing or decreasing SBP ramps determined the BEI (16). Because the numbers of SBP ramps and baroreflex sequences depend on the number of analyzed heartbeats, which varied among and within subjects, the numbers of SBP ramps and baroreflex sequences were normalized by the number of analyzed heartbeats of each subject in each time segment.
Arterial pulse transit time.
An index of arterial compliance, arterial pulse transit time (PTT), was measured by adapting the technique of Foo et al. (23). Arterial PTT was recorded as the time interval between the R peak in ECG and the SBP peak in the arterial pressure measured at the finger. Average values from 10 beats selected from clean data were taken in the last minute of supine control and the 2nd min of 70-degree head-up tilt.
Statistical analysis.
We used SAS (The SAS Institute, Cary, NC) software to test a linear mixed model for differences within and among four groups (able-bodied, diabetic without neuropathy, diabetic with possible neuropathy, diabetic with definite neuropathy) during supine control, at three time segments during tilt, and in recovery. The group factor was used to test differences among the four groups. The time factor was used to test the tilt effect. The group × time factor was used to test for differences in tilt effects across groups. The changes from control to the first 3 min of tilt were tested with one-way ANOVA and two-tailed t-tests. For a priori hypothesized differences between variables, for which we predicted a specific difference (greater or less) before we analyzed the data, changes from control were analyzed using one-tailed t-tests. Data were transformed by using logarithm or square root if the residual plots showed heteroscedasticity. Outliers were identified if residuals were larger than two standard deviations and thus were not included in the statistical testing. Differences were considered significant if P ≤ 0.05. Results are presented as means ± SE.
RESULTS
Upon testing, one able-bodied subject was diagnosed with nondiabetic sensory and motor neuropathy; his data were not included in any group. Five able-bodied subjects and three diabetics with possible neuropathy demonstrated symptoms of presyncope before finishing 30 min of head-up tilt and were returned to supine immediately. Typical RR interval, arterial BP, and tilt angle of an asymptomatic able-bodied subject (Fig. 1, left) and a diabetic patient with definite neuropathy (Fig. 1, right) during supine and during 30 min head-up tilt are shown in Fig. 1. Note the decrease of RR interval and the modest increase in BP during head-up tilt in the nonsyncopal, able-bodied subject and the decrease in BP and smaller change in RR interval in the diabetic patient with definite neuropathy.
Fig. 1.
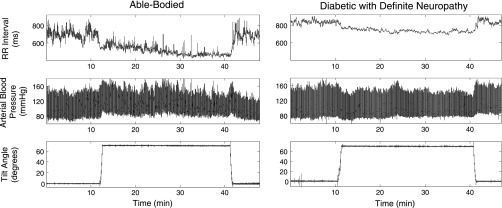
Illustrative data of RR interval, arterial blood pressure for one able-bodied (AB) subject and one diabetic patient with definite neuropathy (D2) undergoing shift from supine to 70° head-up tilt (HUT) for 30 min, followed by return to supine. RR interval decreased and arterial blood pressure was maintained during HUT in the AB subject, but blood pressure decreased and changes in RRI were smaller in the D2 subject.
Mean Values
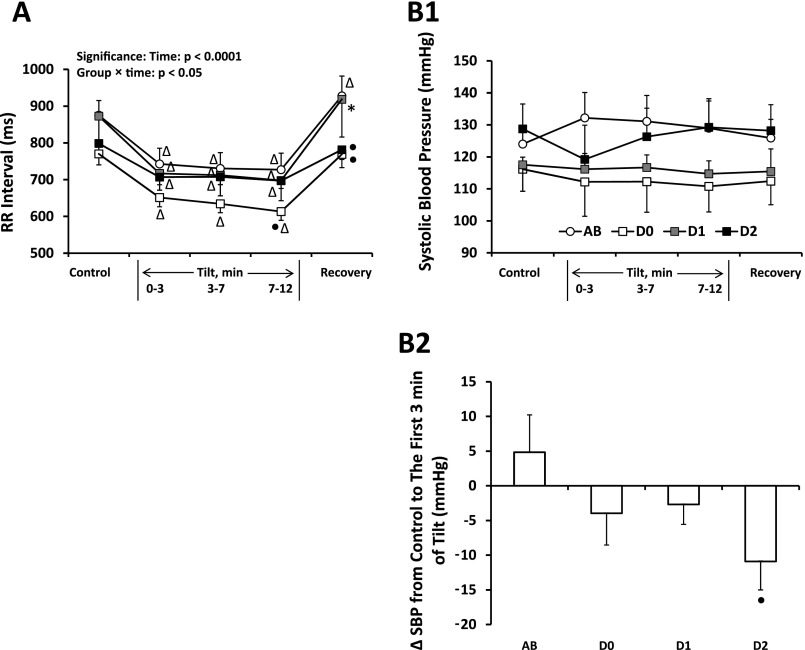
Figure 2 shows group-averaged (±SE) RR interval and SBP for able-bodied subjects, diabetics without neuropathy, diabetics with possible neuropathy, and diabetics with definite neuropathy. Data are given for supine control, three stages of tilt, and supine recovery. All groups decreased RR interval (Fig. 2A) in response to head-up tilt. The magnitudes of SBP responses to the first 3 min of head-up tilt (Fig. 2B2) were significantly different between able-bodied subjects and diabetics with definite neuropathy (one-tailed t-test); for able-bodied subjects, SBP increased from 124 ± 5.8 mmHg in supine control to 132 ± 7.9 mmHg; for diabetics with definite neuropathy, SBP decreased from 128.7 ± 7.8 mmHg in supine control to 119.2 ± 10.7 mmHg. Supine control values of RR interval and SBP were not different between the groups.
Fig. 2.
A: averaged ± SE RR interval for able-bodied subjects (AB; n = 11), diabetics without neuropathy (D0; n = 6), diabetics with possible neuropathy (D1; n = 5), and diabetics with definite neuropathy (D2; n = 8) in response to HUT. All groups decreased RR interval during HUT. △Significantly different from control for the same group. ●Significantly different from AB at the same time segment. *Significantly different from D0 at the same time segment. B1: averaged ± SE systolic blood pressure (SBP) for AB, D0, D1, and D2 in response to HUT. AB tended to increase SBP in response to HUT while D2 tended to decrease SBP in the first 3 min of tilt and recover during tilt 3–7 and 7–12 min. B2: changes of SBP from supine control to the first 3 min of tilt. The changes of SBP were significantly different (●) between AB and D2.
Spectral Power
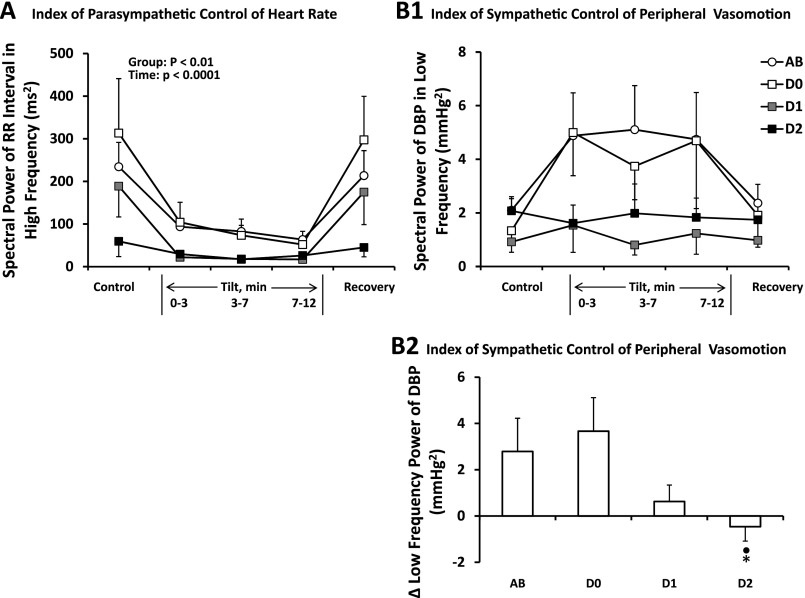
Figure 3 gives average high-frequency spectral power of RR interval (HFRRI; Fig. 3A, left) and low-frequency spectral power of diastolic blood pressure (LFDBP; Fig. 3B1, top right) for the same four groups. In Fig. 3A, HFRRI decreased during head-up tilt for all groups. In addition, able-bodied subjects and diabetics without neuropathy had higher HFRRI than did diabetics with definite neuropathy (group main effect). Figure 3B1 shows that diabetics with possible neuropathy and those with definite neuropathy tended to have lower LFDBP than did able-bodied subjects and diabetics without neuropathy during head-up tilt (group × time, P = 0.06). The change in LFDBP during the first 3 min of tilt (Fig. 3B2) differed significantly (P < 0.05) between able-bodied (2.1 ± 0.4 mmHg2 supine, to 4.9 ± 1.6 mmHg2) and diabetics with definite neuropathy (2.1 ± 0.5 mmHg2 supine, to 1.6 ± 0.7 mmHg2).
Fig. 3.
A: average ± SE spectral power of RR interval in the high-frequency region for AB (n = 11), D0 (n = 6), D1 (n = 5), and D2 (n = 8) subjects at rest and in response to HUT. For all groups, the high-frequency spectral power of RR interval decreased going from supine control to tilt with an immediate return during supine recovery. D2 had significantly smaller power than did AB and D0. B1: the spectral power of diastolic blood pressure (DBP) in the low-frequency region tended to increase in AB and D0 and not change in D1 and D2 in response to HUT. These group differences in response to the first 3 min of tilt are shown in B2. ●Significantly different from AB. *Significantly different from D0.
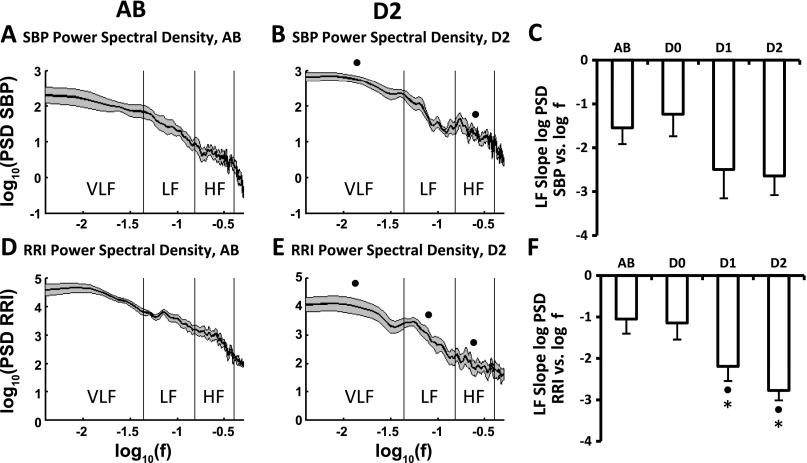
Figure 4 shows group-averaged log of power spectral density of SBP (PSD SBP; Fig. 4, A and B, top) and log of RR interval (PSD RRI; Fig. 4, D and E, bottom) vs. log of frequency for able-bodied subjects (Fig. 4, left) and diabetics with definite peripheral neuropathy (Fig. 4, middle). The analyses are based on recordings taken during 5 min of supine control. Data shown are means ± SE. Spectral power of SBP was significantly greater (4-fold, which is not immediately evident on logarithmic scales) in diabetics with definite neuropathy than in able-bodied subjects (Fig. 4, B vs. A) across the very-low-frequency (VLF, 0.003–0.04 Hz) and HF (0.15–0.4 Hz) regions. However, the SBP spectral power of diabetics with definite neuropathy dipped at frequencies around 0.1 Hz (Fig. 4B). In addition, the slope of the linear portion of this SBP curve in the LF region tended to be steeper in diabetics with definite neuropathy (−2.64 ± 0.44) than in able-bodied subjects (−1.54 ± 0.37) (Fig. 4C). SBP powers for diabetics without, and those with, possible neuropathy were similar to that of able-bodied subjects (data not shown). In contrast to SBP, the variability of RR interval for diabetics with definite neuropathy was significantly (4-fold) lower than that of able-bodied subjects over the range of frequencies (0.003–0.4 Hz) (Fig. 4, D vs. E). Log-log plots of RR interval for diabetics without neuropathy and diabetics with possible neuropathy were intermediate between able-bodied subjects and diabetics with definite neuropathy (data not shown). Slopes of the log-log RR interval/frequency curve were significantly steeper in the LF region (0.04–0.15 Hz) in diabetics with possible (−2.19 ± 0.35) and definite (−2.77 ± 0.24) neuropathy than slopes of able-bodied (−1.05 ± 0.35) and diabetics without neuropathy (−1.15 ± 0.4) (Fig. 4F). In contrast to systolic pressure, the log-log plot of the power spectral density of DBP was not different between able-bodied subjects and diabetics with definite neuropathy (data not shown).
Fig. 4.
Average ± SE logarithm of power spectral density of systolic blood pressure (PSD SBP) and RR interval (PSD RRI) taken from 5 min supine control (means shown in solid lines and SE shown in shaded area above and below the mean in A, B, D, and E). Plots A and B show log(PSD SBP) vs. log(f) for AB (n = 11) and D2 (n = 8), respectively. Values for D0 and D1 were similar to those of AB and are not shown. PSD SBP of D2 was significantly higher than AB across the very-low-frequency (VLF, 0.003–0.04 Hz) and high-frequency (HF, 0.15–0.4 Hz) regions. Plot C shows average values (±SE) of the slopes of the linear portion of log(PSD SBP) vs. log(f) in the low-frequency (LF; 0.04–0.15 Hz) region for all four groups. Plots D and E show log(PSD RRI) vs. log(f) for AB and D2, respectively. PSD RRI of D2 was significantly lower than AB across all frequencies. Plot F shows average values (±SE) of the slopes of log(PSD RRI) vs. log(f) in the low-frequency region for all four groups. ●Significantly different from AB. *Significantly different from D0.
Baroreflex Sequences
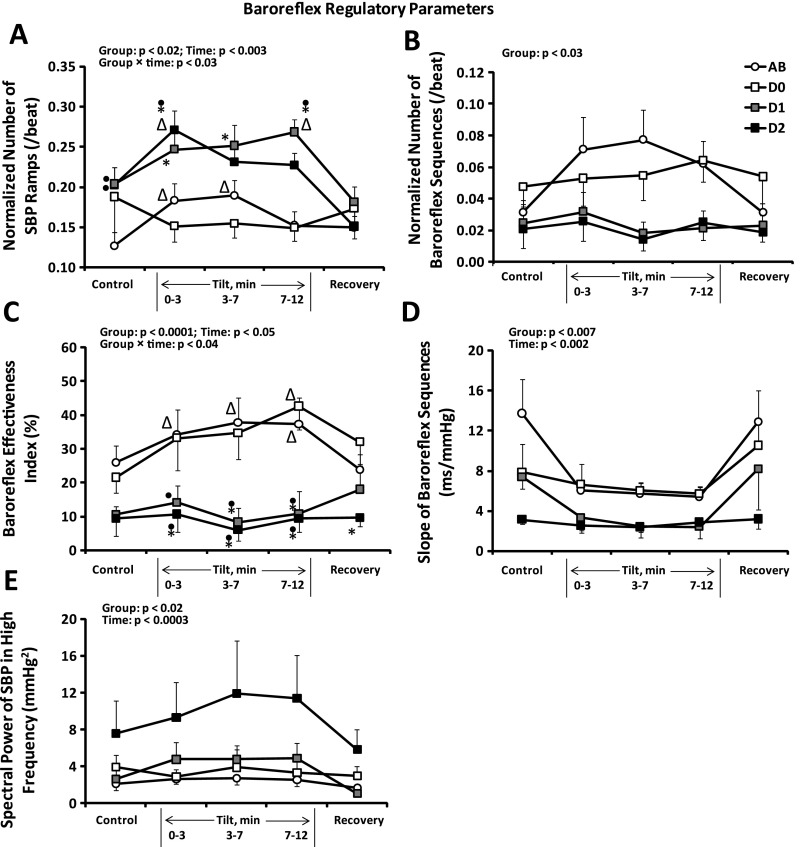
Figure 5 provides the average (±SE) normalized number of SBP ramps (Fig. 5A), normalized number of baroreflex sequences (Fig. 5B), BEI (Fig. 5C), baroreflex slope (Fig. 5D), and high-frequency spectral power of systolic blood pressure (HFSBP; Fig. 5E) for each of the four groups. In supine control, both diabetics with possible, and those with definite, neuropathy had greater numbers of SBP ramps than did able-bodied subjects (Fig. 5A). During head-up tilt, diabetics with definite neuropathy also had more SBP ramps than did diabetics without neuropathy during all three time segments, most clearly illustrated at 7–12 min of tilt (Fig. 5A). However, diabetics with definite neuropathy had a significantly smaller number of baroreflex sequences than did able-bodied subjects and diabetics without neuropathy (group main effect; Fig. 5B). As a result, BEI values for diabetics with possible, and for those with definite, neuropathy were significantly lower than those for able-bodied subjects and diabetics without neuropathy during head-up tilt (Fig. 5C). One able-bodied subject had BEI residuals at all time segments larger than two standard deviations. When this subject's data were excluded from the analysis, the group × time interaction became significant. Other factors remained significant with or without exclusion of this subject, with no effect on the direction of the results. In addition, baroreflex slope significantly decreased in all groups in response to head-up tilt (Fig. 5D). Diabetics with possible neuropathy had significantly lower baroreflex slopes than able-bodied subjects, and diabetics with definite neuropathy had significantly lower slopes than both able-bodied subjects and diabetics without neuropathy (group main effect). Finally, the diminution of baroreflex activity to buffer breathing frequency BP oscillations in diabetics with definite neuropathy is illustrated in Fig. 5E, indicating a fourfold greater magnitude of HFSBP oscillations in those subjects compared with the other groups (similar to the results illustrated in Fig. 4, A and B).
Fig. 5.
Baroreflex regulation: average ± SE normalized no. of SBP ramps (A), normalized no. of baroreflex sequences (B), baroreflex effectiveness index (C), baroreflex slope (D), and high-frequency spectral power of SBP (E) for AB (n = 11), D0 (n = 6), D1 (n = 5), and D2 (n = 8) subjects at rest and in response to HUT. Note diminished baroreflex activity in diabetics with neuropathy. ▵Significantly different from control (same group). ●Significantly different from AB (same time segment). *Significantly different from D0 (same time segment).
Gender
Because of known gender differences in autonomic regulation of HR (parasympathetic dominance in HF power of HR in women compared with men) and peripheral vasculature (sympathetic dominance in control of vascular regulation in men) (2, 21, 29, 39), we examined HR, BP, and baroreflex variables for gender differences. The results of these analyses indicated that, for these groups, gender effects were obscured by effects of diabetes (data not shown).
Arterial PTT
Arterial PTT was not significantly different among the four groups in supine or during the 2nd min of tilt, nor were the tilt-induced increases of PTT significantly different among the four groups.
DISCUSSION
This study tested noninvasive indexes of sympathetic, parasympathetic, and baroreflex control of circulatory function for their ability to discriminate between able-bodied subjects, diabetics without peripheral neuropathy, diabetics with possible and diabetics with definite, length-dependent peripheral neuropathy. Peripheral neuropathy was determined by standard clinical neurological tests. We now report five major new findings. First, VLF SBP power spectral density in the supine state was four times greater in diabetic patients with length-dependent peripheral neuropathy compared with able bodied. Second, we also report an increase in HFSBP spectral power in supine patients suffering from diabetic neuropathy; as will be discussed below, this contrasts with other published findings. Third, the BEI was lower in diabetic patients with definite neuropathy, but without chronic renal failure, compared with the control group and with diabetic patients without length-dependent sensory or motor neuropathy. In fact, our data indicate that BEI is a more sensitive index of diabetic dysautonomia than is LF spectral power of SBP. Fourth, we found that the baroreflex played an important role in regulating BP over a wide frequency range between 0.003 and 0.45 Hz, and that, as a consequence, RR interval spectral power in diabetic patients with definite neuropathy was one-fourth that of able-bodied controls across the frequency range. Finally, the slope (i.e., “beta”) of the log-log relationship between the power spectral density of RR interval in the low frequencies for recordings made during supine rest was significantly more negative in both the patients with possible diabetic neuropathy and patients with definite diabetic neuropathy compared with able bodied, and there was a dip in LF SBP spectral power in the log-log plot centered around ∼0.1 Hz in the group with definite diabetic neuropathy. The latter finding supports the conclusion that baroreflex control of sympathetically mediated vasomotion is impaired in diabetic patients with neuropathy. In summary, the BP regulatory capability of diabetics with neuropathy was significantly diminished because of diminution of sympathetic and parasympathetic control of HR and peripheral vasculature and an almost total loss of baroreflex regulation of HR. As we explain below, the unique contributions of the present study lie in documenting mechanisms associated with failure of autonomic control and in identifying easily obtained, noninvasive measures to quantify those failures. In addition, results of our study support the hypothesis that patients with diabetic neuropathy resemble patients with primary autonomic failure (44) and SAD animals (14, 55). These findings potentially provide quantitative assessments of a serious complication in arterial pressure regulation in diabetic patients secondary to diabetic dysautonomia that go beyond standard clinical neurological tests, and could ultimately be of clinical use.
Mean Values
The inability of diabetics to increase, or even maintain, BP during tilt (Fig. 2) indicates that sympathetic pathways to cardiovascular effectors were impaired in diabetics with peripheral neuropathy compared with able-bodied subjects. Moreover, the importance of the baroreflex in regulating BP over longer time periods is illustrated in the VLF region of Fig. 4. These results also support the comment made by many of the patients that they had learned to move slowly into the upright position, and if they move slowly they are okay. The slow speed (∼38 s to move from supine to 70° head up) of our tilt was probably responsible for the fact that presyncopal symptoms were relatively rare in our diabetic subjects.
RR Interval Spectral Power Indexes of Sympathetic and Parasympathetic Control of HR
The magnitude of HFRRI is a marker of vagal control of the sinoarotic node (46). The rapid decline of HFRRI in response to head-up tilt and rapid recovery of HFRRI when able-bodied subjects were returned to supine (within 1 min of the change in posture, Fig. 3A) supports neural, reflex-mediated modulation of HR at respiratory frequencies in these people. During supine rest and head-up tilt, diabetics with definite neuropathy had lower HFRRI than did able-bodied subjects or diabetics without neuropathy, indicating impaired parasympathetic control of HR in diabetics with signs of peripheral neuropathy (51). The reduced reserve of parasympathetic control of HR in response to head-up tilt in diabetics with possible or definite neuropathy also confirmed a diminished orthostatic response and progressive loss of parasympathetic control of HR with increasing neuropathy (44).
A previous study of SAD cats determined that the wide band spectra of pulse interval was significantly lower in SAD cats than that in controls (14). In our study, the decreases of wide-band RR interval spectra in diabetics with definite neuropathy may be due to diminished baroreflex function, or perhaps more specifically, to cardiac parasympathetic neuropathy. Moreover, the LF RR interval component was different in diabetics with definite neuropathy: a steeper slope of the 1/f relationship was evident at the LF range of diabetics with definite neuropathy. This 1/f relationship of power spectrum exists in dynamic systems that have multiple control mechanisms with different time constants, and the steeper slope of the 1/f relationship suggests a less complex control system (6, 7, 28, 55) in diabetics with definite neuropathy. If so, the complexity of the sympathetic regulation of HR in diabetics with definite neuropathy was diminished, possibly reflecting a diminution of sympathetic regulation of the sinoaortic node, perhaps secondary to diabetic sympathetic neuropathy. In patients with Chagas disease, which damages autonomic neurons, the slope of the linear part of the log-log plot of PSD RRI was significantly steeper than that of controls, indicating dysautonomia in these patients (12, 49). Therefore, the slope of the log-log plot of HR power spectral density of resting diabetic subjects calculated from a 5-min measurement appears to be a sensitive discriminator of AN in the LF region.
BP Spectral Power Index of Sympathetic Control of Peripheral Vasculature
Significant coherence between LFDBP and muscle sympathetic nerve activity (SNA) has been reported in able-bodied humans (11). In particular, muscle SNA increased with head-up tilt and remained elevated during tilt in able-bodied subjects (27). In our study, LFDBP increased in response to tilt and remained high in able-bodied and in diabetic subjects without neuropathy (Fig. 3B). Conversely, in diabetics with possible, and those with definite, neuropathy, LFDBP did not change, indicating that reflex-mediated sympathetic pathways to peripheral vasculature were impaired in diabetics, including those with milder symptoms of neuropathy.
The log-log plot (Fig. 4, A and B) of the power spectral density of systolic blood pressure (PSD SBP) taken during 5 min of supine rest showed a significant elevation of harmonic and nonharmonic components of BP variability in the VLF and the HF regions but not in the LF range (presumably because of a loss of the harmonic component) in diabetics with definite neuropathy compared with able-bodied subjects. These findings confirm the previously reported reduction of sympathetically mediated harmonic vasomotion in diabetic AN (17, 59).
Baroreflex
The greater numbers of BP ramps at rest and during tilt in diabetics with definite and suspected AN (Fig. 5A) were an unexpected finding of the present study. The combination of increased numbers of BP ramps with reduced numbers of baroreflex sequences (25, 59) suggests that diabetics with neuropathy experience diminished effectiveness of the baroreflex in driving the sinus node. As is clearly illustrated in Fig. 5C, BEI was significantly increased compared with supine control in able-bodied subjects during all phases of head-up tilt and in diabetics without neuropathy during 7–12 min of tilt, but remained unchanged in diabetics with possible, or definite, neuropathy. Furthermore, Fig. 5 clearly illustrates the ability of this measure to differentiate diabetics with neuropathy (definite or possible) from diabetics without neuropathy. Therefore, in our study, BEI was the most sensitive discriminator of autonomic neuropathy. Other studies have proposed LF power of SBP (17) as a sensitive discriminator of AN, but, for our study, BEI was significantly better at discriminating differences, perhaps because this measure combines loss of sympathetic modulation of vasomotion with loss of parasympathetic control of HR into one parameter. Previous studies showed a severe impairment of baroreflex effectiveness in diabetic chronic renal failure patients compared with nondiabetic patients at supine rest (32). However, as far as we can determine, the present study is the first to demonstrate a reduction in baroreflex effectiveness in diabetics with neuropathy, without the simultaneous complication of chronic renal failure.
BRS has been proposed to reflect the strength of the baroreflex when it is effective (16). In our study, all groups demonstrated decreased baroreflex slope in response to head-up tilt (Fig. 5D) as expected (33, 42, 43). However, during both supine control and head-up tilt, diabetics with definite neuropathy had significantly smaller BRS than did able-bodied subjects and diabetics without neuropathy. During supine rest, diabetics without neuropathy tended to have smaller BRS than able-bodied subjects and larger BRS than diabetics with definite neuropathy. These findings support the widely held notion that diabetes reduces BRS and that reduced BRS is an early sign of AN (25, 48, 59). When we compared our significant reduction of BRS and BEI with the trend toward greater arterial PTT in diabetics with definite neuropathy, we conclude that, for our study, baroreflex impairment was a more sensitive indicator of neuropathy than alterations in arterial stiffness (50).
Increased BP variability has been shown to predict nephropathy and retinopathy (35) and to correlate with endothelial and cardiovascular damage (13, 57) and higher mortality (34). BP variability, measured by standard deviation over 24 h, has been reported to be significantly higher in diabetic patients (24, 31, 40, 56) and was highest in those with cardiovascular AN (10). In our study, we further explored the specific frequency ranges of increased BP variability and determined that diabetics with definite neuropathy had significantly higher VLF and HF power spectral density of SBP compared with able-bodied subjects. In addition, in all frequency ranges, our able-bodied subjects demonstrated an ability to engage HR to buffer SBP fluctuations while diabetics with neuropathy demonstrated a reduced ability.
Arterial baroreflex buffering at respiratory frequencies may be more dominant in older persons. The fact that HF power of SBP was greater in our diabetics with neuropathy than in able-bodied subjects, but has been shown to be smaller in diabetic children and adolescents than in healthy controls (36), may be a matter of age but may, more accurately, reflect the duration of diabetes in our subjects. In addition, in middle-aged to older diabetics, HF power of SBP was reported to be smaller than in able-bodied during conditions of controlled breathing (17). The act of controlling breathing itself, however, may be responsible for this effect since we previously determined that controlled breathing reduced cardiovascular parasympathetic influence (47).
An important indicator of impaired baroreflex buffering of BP lies in the fourfold increase in the harmonic and nonharmonic components of VLF power spectral density of SBP in diabetics with definite neuropathy compared with able-bodied subjects (Fig. 4, A and B). Although enhanced VLF SBP has been reported to result from SAD in animals (9, 15), VLF SBP power in humans is believed to reflect renin-angiotensin system activity, endothelial factors, thermoregulation, etc. (46). However, in patients with primary autonomic failure, enhanced VLF oscillations of SBP have been documented (44). Our study's enhanced VLF SBP power and decreased VLF RR interval power in diabetics with definite neuropathy indicated that intact baroreflex function is important for normal BP regulation in the VLF range for both harmonic and nonharmonic components in humans. Finally, in our study, VLF SBP was similar between able-bodied and diabetics without neuropathy. This phenomenon has been reported previously in children and adolescents with type 1 diabetes mellitus who have not developed other signs of neuropathy (36). These results suggest that, in addition to BEI, increased VLF SBP spectral power may be a strong, early, indicator of diabetic neuropathy.
Our study did not show significantly increased standard deviation of SBP in diabetics with neuropathy. Similar to the finding of Frattola et al. (25), this may be due to the relatively short period of time over which the standard deviation was calculated (3–5 min in our study and 15 min in the study by Frattola et al.) compared with the greater standard deviation measured from 24-h recordings (10, 24, 31, 40, 56).
At frequencies centered around 0.1 Hz (Fig. 4B), the log-log power spectral density curve of SBP of diabetics with definite neuropathy demonstrated a dip, indicating that the loss of the harmonic component at LF (Fig. 3B) is apparent in the log-log plot of SBP. This loss of power in the LF region is similar to that previously reported following SAD in animals (14, 54) and total autonomic failure in humans (44). SNA with a frequency around 0.1 Hz in humans is similar to the 0.4-Hz rhythm in rats and, at this frequency, there is a tight coherence in unanesthetized rats between changes in SNA and changes in arterial BP (5). We conclude that the dip in oscillations of SBP in the LF region of diabetics with definite neuropathy is consistent with effects of neuropathy on sympathetically mediated vasomotion. This change is exposed by the corresponding loss of parasympathetically (baroreflex) driven changes in HR in subjects with definite neuropathy. In addition, altered BP and RR interval power spectral densities over the range of frequencies between 0.003 and 0.45 Hz in diabetics with neuropathy indicate that an intact baroreflex is an important component of healthy human BP regulation at all frequencies. Each of these measures is relatively easy to determine from a short (5 min) recording of continuous BP and HR from resting subjects.
Limitations
The time of onset, the prevalence, and the development of diabetic neuropathy and treatment effects have been reported to be different between type 1 and type 2 diabetes (53, 58). Because of our limited number of subjects, diabetic patients were not separated based on the type of diabetes. Therefore, the relative ability of our indexes of autonomic dysfunction to identify AN in type 1 vs. type 2 diabetes is unknown. Direct measures of sympathetic autonomic function (like MIBG SPECT imaging) were not performed; thus, comparisons between direct measures and our indexes are not available. Again, because of the limited numbers of subjects in each group, we were not able to establish age effects on the autonomic indexes we report. The group with possible diabetic neuropathy was younger than the group with definite diabetic neuropathy; however, major differences in this study lay between able-bodied and definite diabetic neuropathy groups where there was no meaningful difference in age. Greater numbers of subjects would be required to establish significant interactions of study variables with gender and age.
The rate of presyncope (5 of 11) in the able-bodied subjects of the present study was similar to able-bodied subjects we have reported in the past (38). In that study, 8 of 16 controls had symptoms within 30 min of head-up tilt. In another recent study of subjects who underwent 70° head-up tilt, 56% (9 of 16) had presyncopal symptoms during the 30-min tilt (20). Results from a third study of head-up tilt-mediated presyncope in healthy humans suggested that unexplained syncope was a result of altered cardiorespiratory interaction involving cerebral hemodynamics but with a normal neural control system (37). Similar to our results, other investigators have determined that the initial response to head-up tilt was similar between patients with vasovagal syncope and nonsyncopal subjects, and SNA withdrawal did not occur until symptoms began (41). We do not believe that the incidence of presyncopal events in the able-bodied subjects is remarkable, nor does it call into question any aspect of the interpretation of our findings.
Finally, abnormal findings in the battery of Ewing tests have been reported in 13% of the normal population (45). In our study, two able-bodied subjects with tingling of hands and/or feet were found to be free of peripheral neuropathy by standard clinical tests. Both subjects, however, decreased their BEI to near 0% in response to tilt while other able-bodied subjects increased or maintained their BEI at >10%. In the group of diabetics with definite neuropathy, six of eight subjects had BEI smaller than 10%. The similar behavior of the two able-bodied subjects to diabetics with definite peripheral neuropathy suggests that BEI may be an index of AN that is more sensitive, and/or more selective, for autonomic involvement than are standard sensory/motor neuropathy findings. Also for other variables, when we removed these two subjects from the able-bodied group, the statistical significance of the group differences reported here was dramatically increased, but, without a physiological reason to remove them, the results reported here include these subjects with the able-bodied group.
Perspectives and Significance
The much-reduced ability to regulate BP, characterized by decreased RR interval buffering of an increased number of SBP ramps, the fourfold increases in VLF and HF SBP power spectral densities, and the fourfold decrease in VLF, LF, and HF RR interval power spectral densities indicate serious deficits in maintenance of cardiovascular homeostasis in diabetics with neuropathy. The major contributor to this loss of BP regulation appeared to be the combination of a loss of sympathetically mediated control of vasomotion with a reduced contribution from parasympathetically mediated responses of HR to buffer these changes in BP. Our results indicate that preservation of baroreflex function needs to become a focus of diabetic neuropathy treatment. The present study also indicates that, in healthy subjects, baroreflex function is an important component of BP regulation in all (very low, low, and high) frequency regions and pertains to both self-similar and harmonic components. Finally, the results of our study indicate that noninvasive indexes of autonomic regulation were able to discriminate diabetics with AN from subjects with reduced, or no, neuropathic damage.
GRANTS
This study was supported by nih (NIH) Grant RO1 NS39774, Kentucky NASA EPSCOR Grant WKU 52611, UK GCRC USPHS Grant M01RR-02602, and the University of Kentucky NIH CTSA Grant UL1RR-033173.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
Our first acknowledgement is to our subjects who willingly gave their time and information to this study. We acknowledge the contributions of A. Hartman in recruiting subjects, D. Silcox and R. Schneider for helping to acquire data, and L. Krompak, L. Mohney, and C. Ferguson for help with data analysis. Adam Lindstrom, of the UK SSTARS Center guided our statistical analysis. Dr. David Brown, Biomedical Engineering, University of Kentucky, wrote the program for R wave detection. Natalia Arzeno, NASA Johnson Space Center Cardiovascular Laboratory, developed the software used for beat-to-beat analysis. We thank Janet Kaenzig and other staff of the UK GCRC for their expert assistance in the conduct of these studies.
REFERENCES
- 1. Aslan SC, Randall DC, Donohue KD, Knapp CF, Patwardhan AR, Mc Dowell SM, Taylor RF, Evans JM. Blood pressure regulation in neurally intact human vs. acutely injured paraplegic and tetraplegic patients during passive tilt. Am J Physiol Regul Integr Comp Physiol 292: R1146–R1157, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL, Nelson JC, Lipsitz LA. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension 33: 1195–1200, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985 [PubMed] [Google Scholar]
- 4. Bourgeois MJ, Gilbert BK, Donald DE, Wood EH. Characteristics of aortic diastolic pressure decay with application to the continuous monitoring of changes in peripheral vascular resistance. Circ Res 35: 56–66, 1974 [DOI] [PubMed] [Google Scholar]
- 5. Brown DR, Brown LV, Patwardhan A, Randall DC. Sympathetic activity and blood pressure are tightly coupled at 0.4 Hz in conscious rats. Am J Physiol Regul Integr Comp Physiol 267: R1378–R1384, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Brown DR, Cassis LA, Silcox DL, Brown LV, Randall DC. Empirical and theoretical analysis of the extremely low frequency arterial blood pressure power spectrum in unanesthetized rat. Am J Physiol Heart Circ Physiol 291: H2816–H2824, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Butler GC, Yamamoto Y, Xing HC, Northey DR, Hughson RL. Heart rate variability and fractal dimension during orthostatic challenges. J Appl Physiol 75: 2602–2612, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, Parma G, Lissoni A, Fei F, Cundari S, Zanna C. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology 61: 1297–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: assessment by spectral analysis. Am J Physiol Heart Circ Physiol 266: H1993–H2000, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Combe H, Bauduceau B, Chanudet X, Chau NP, Rabasa R, Meyer L, Mayaudon H, Larroque P, Gautier D. Cardiovascular autonomic neuropathy and blood pressure variability in insulin-dependent diabetes. Arch Mal Coeur Vaiss 86: 1149–1152, 1993 [PubMed] [Google Scholar]
- 11. Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol 587: 4987–4999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Sousa MR, Huikuri HV, Lombardi F, Perez AA, Gomes ME, Barros MV, Barros VC, Rocha MO, Ribeiro AL. Abnormalities in fractal heart rate dynamics in Chagas disease. Ann Noninvasive Electrocardiol 11: 145–153, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Flaviani A, Picconi F, Di Stefano P, Giordani I, Malandrucco I, Maggio P, Palazzo P, Sgreccia F, Peraldo C, Farina F, Frajese G, Frontoni S. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care 34: 1605–1609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Rienzo M, Castiglioni P, Parati G, Mancia G, Pedotti A. Effects of sino-aortic denervation on spectral characteristics of blood pressure and pulse interval variability: a wide-band approach. Med Biol Eng Comput 34: 133–141, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Di Rienzo M, Parati G, Castiglioni P, Omboni S, Ferrari AU, Ramirez AJ, Pedotti A, Mancia G. Role of sinoaortic afferents in modulating BP and pulse-interval spectral characteristics in unanesthetized cats. Am J Physiol Heart Circ Physiol 261: H1811–H1818, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G, Pedotti A. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol 280: R744–R751, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Ducher M, Cerutti C, Gustin MP, Abou-Amara S, Thivolet C, Laville M, Paultre CZ, Fauvel JP. Noninvasive exploration of cardiac autonomic neuropathy. Four reliable methods for diabetes? Diabetes Care 22: 388–393, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Eke A, Herman P, Kocsis L, Kozak LR. Fractal characterization of complexity in temporal physiological signals. Physiol Meas 23: R1–R38, 2002 [DOI] [PubMed] [Google Scholar]
- 19. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 64: 199–207, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Evans JM, Leonelli FM, Ziegler MG, McIntosh CM, Patwardhan AR, Ertl AC, Kim CS, Knapp CF. Epinephrine, vasodilation and hemoconcentration in syncopal, healthy men and women. Auton Neurosci 93: 79–90, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol 91: 2611–2618, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 285: 916–918, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foo JY, Lim CS. Dual-channel photoplethysmography to monitor local changes in vascular stiffness. J Clin Monit Comput 20: 221–227, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Frattola A, Parati G, Castiglioni P, Paleari F, Ulian L, Rovaris G, Mauri G, Di Rienzo M, Mancia G. Lacidipine and blood pressure variability in diabetic hypertensive patients. Hypertension 36: 622–628, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Frattola A, Parati G, Gamba P, Paleari F, Mauri G, Di Rienzo M, Castiglioni P, Mancia G. Time and frequency domain estimates of spontaneous baroreflex sensitivity provide early detection of autonomic dysfunction in diabetes mellitus. Diabetologia 40: 1470–1475, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Freeman R, Saul JP, Roberts MS, Berger RD, Broadbridge C, Cohen RJ. Spectral analysis of heart rate in diabetic autonomic neuropathy. A comparison with standard tests of autonomic function. Arch Neurol 48: 185–190, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD, Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol 577: 679–687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldberger AL, West BJ. Fractals in physiology and medicine. Yale J Biol Med 60: 421–435, 1987 [PMC free article] [PubMed] [Google Scholar]
- 29. Gregoire J, Tuck S, Yamamoto Y, Hughson RL. Heart rate variability at rest and exercise: influence of age, gender, and physical training. Can J Appl Physiol 21: 455–470, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Howorka K, Pumprla J, Schabmann A. Optimal parameters of short-term heart rate spectrogram for routine evaluation of diabetic cardiovascular autonomic neuropathy. J Auton Nerv Syst 69: 164–172, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Ishaque MR, Ahmed SA, Abid I. Comparison of the ambulatory blood pressure variability in diabetic hypertensive and non diabetic hypertensive patients. Ann Pak Inst Med Sci 5: 174–177, 2009 [Google Scholar]
- 32. Johansson M, Gao SA, Friberg P, Annerstedt M, Bergstrom G, Carlstrom J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, Nielsen FD, Strombom U. Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens 18: 995–1016, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Kardos A, Rudas L, Simon J, Gingl Z, Csanady M. Effect of postural changes on arterial baroreflex sensitivity assessed by the spontaneous sequence method and Valsalva manoeuvre in healthy subjects. Clin Auton Res 7: 143–148, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: The Ohasama Study. Hypertension 36: 901–906, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Kilpatrick ES, Rigby AS, Atkin SL. The role of blood pressure variability in the development of nephropathy in type 1 diabetes. Diabetes Care 33: 2442–2447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krause M, Rudiger H, Bald M, Nake A, Paditz E. Autonomic blood pressure control in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes 10: 255–263, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Krishnamurthy S, Wang X, Bhakta D, Bruce E, Evans J, Justice T, Patwardhan A. Dynamic cardiorespiratory interaction during head-up tilt-mediated presyncope. Am J Physiol Heart Circ Physiol 287: H2510–H2517, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Leonelli FM, Wang K, Evans JM, Patwardhan AR, Ziegler MG, Natale A, Kim CS, Rajikovich K, Knapp CF. False positive head-up tilt: hemodynamic and neurohumoral profile. J Am Coll Cardiol 35: 188–193, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability–the ARIC study. Atherosclerosis risk in communities. Am J Cardiol 76: 906–912, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Mokhtar RH, Ayob A, Mohd Noor N. Blood pressure variability in patients with diabetes mellitus. Asian Cardiovasc Thorac Ann 18: 344–348, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KUO, Kuusela TA, Diedrich AM. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation 96: 2509–2513, 1997 [DOI] [PubMed] [Google Scholar]
- 42. O'Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol 88: 769–774, 2003 [DOI] [PubMed] [Google Scholar]
- 43. O'Leary DD, Lin DC, Hughson RL. Determination of baroreflex gain using auto-regressive moving-average analysis during spontaneous breathing. Clin Physiol 19: 369–377, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Omboni S, Parati G, Di Rienzo M, Wieling W, Mancia G. Blood pressure and heart rate variability in autonomic disorders: a critical review. Clin Auton Res 6: 171–182, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Pagani M, Malfatto G, Pierini S, Casati R, Masu AM, Poli M, Guzzetti S, Lombardi F, Cerutti S, Malliani A. Spectral analysis of heart rate variability in the assessment of autonomic diabetic neuropathy. J Auton Nerv Syst 23: 143–153, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 25: 1276–1286, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Patwardhan AR, Vallurupalli S, Evans JM, Bruce EN, Knapp CF. Override of spontaneous respiratory pattern generator reduces cardiovascular parasympathetic influence. J Appl Physiol 79: 1048–1054, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Philip J, Weston MAJ, Ronnie Panerai B, Paul McNally G, Potter JF, Thurston H. Evidence of defective cardiovascular regulation in insulin-dependent diabetic patients without clinical autonomic dysfunction. Diabetes Res Clin Prac 42: 141–148, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Ribeiro AL, Lombardi F, Sousa MR, Lins Barros MV, Porta A, Costa Val Barros V, Gomes ME, Santana Machado F, Otavio Costa Rocha M. Power-law behavior of heart rate variability in Chagas' disease. Am J Cardiol 89: 414–418, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Ruiz J, Monbaron D, Parati G, Perret S, Haesler E, Danzeisen C, Hayoz D. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension 46: 162–167, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Sundkvist G. Autonomic nervous function in asymptomatic diabetic patients with signs of peripheral neuropathy. Diabetes Care 4: 529–534, 1981 [DOI] [PubMed] [Google Scholar]
- 52. Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med 68: 928–944, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 26: 1553–1579, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Wagner CD, Mrowka R, Nafz B, Persson PB. Complexity and “chaos” in blood pressure after baroreceptor denervation of conscious dogs. Am J Physiol Heart Circ Physiol 269: H1760–H1766, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Wagner CD, Persson PB. Two ranges in blood pressure power spectrum with different 1/f characteristics. Am J Physiol Heart Circ Physiol 267: H449–H454, 1994 [DOI] [PubMed] [Google Scholar]
- 56. White WB. Diurnal blood pressure and blood pressure variability in diabetic normotensive and hypertensive subjects (Abstract). J Hypertens 10: S43, 1992 [DOI] [PubMed] [Google Scholar]
- 57. Zhang Q, Zhang X, Chang B, Qiu B, Zhang Y, Li J, Zeng Z. Blood pressure variability correlates with target-organ damage in elderly patients with hypertension (Abstract). J Sichuan Univ Med Sci Ed 42: 252, 2011 [PubMed] [Google Scholar]
- 58. Ziegler D. Diabetic cardiovascular autonomic neuropathy. Diabetic Neuropathy 200: 140–169, 2001 [Google Scholar]
- 59. Ziegler D, Laude D, Akila F, Elghozi JL. Time- and frequency-domain estimation of early diabetic cardiovascular autonomic neuropathy. Clin Auton Res 11: 369–376, 2001 [DOI] [PubMed] [Google Scholar]