Abstract
We have previously demonstrated that leptin-mediated activation of the central nervous system (CNS) melanocortin system reduces appetite and increases sympathetic activity and blood pressure (BP). In the present study we examined whether endogenous melanocortin system activation, independent of leptin's actions, contributes to the regulation of BP and metabolic functions in obese Zucker rats, which have mutated leptin receptors. The long-term cardiovascular and metabolic effects of central melanocortin-3/4 receptor (MC3/4R) antagonism with SHU-9119 were assessed in lean (n = 6) and obese (n = 8) Zucker rats. BP and heart rate (HR) were measured 24-h/day by telemetry and an intracerebroventricular cannula was placed in the brain lateral ventricle. After stable control measurements, SHU-9119 was infused intracerebroventricularlly (1 nmol/h) for 10 days followed by a 10-day recovery period. Chronic CNS MC3/4R antagonism significantly increased food intake and body weight in lean (20 ± 1 to 45 ± 2 g and 373 ± 11 to 432 ± 14 g) and obese (25 ± 2 to 35 ± 2 g and 547 ± 10 to 604 ± 11 g) rats. No significant changes were observed in plasma glucose levels in lean or obese rats, whereas plasma leptin and insulin levels markedly increased in lean Zucker rats during CNS MC3/4R antagonism. Chronic SHU-9119 infusion in obese Zucker rats reduced mean arterial pressure (MAP) and HR by 6 ± 1 mmHg and 24 ± 5 beats/min, whereas in lean rats SHU-9119 infusion reduced HR by 31 ± 9 beats/min while causing only a transient decrease in MAP. These results suggest that in obese Zucker rats the CNS melanocortin system contributes to elevated BP independent of leptin receptor activation.
Keywords: obesity, blood pressure, heart rate, central nervous system
the leptin-melanocortin system plays an important role in regulating energy balance and body weight by controlling appetite and energy expenditure (1, 3, 12, 13). This system also controls blood pressure (BP) and heart rate (HR) by increasing sympathetic nervous system (SNS) activity to various organs and tissues (1, 3, 8, 12, 14, 15, 18). Although the mechanisms implicated in the regulation of energy homeostasis and cardiovascular function by the leptin-melanocortin pathway are still not fully understood, strong evidence indicates that leptin-mediated activation of proopiomelanocortin (POMC) neurons leads to release of α-melanocyte stimulating hormone (α-MSH) and subsequent stimulation of melanocortin 3 and 4 receptors (MC3/4R) in several brain regions that regulate appetite and SNS activity (27–29).
We and others have demonstrated that the central nervous system (CNS) leptin-melanocortin system may also be an important link between obesity, increased SNS activity, and hypertension (8, 12, 18, 20, 25, 27, 28). For instance, leptin-deficient ob/ob mice are markedly obese but exhibit similar, or even lower, BP compared with lean control littermates and wild-type mice (21). Leptin deficiency in humans is also not associated with elevated BP despite morbid obesity and these individuals exhibit impaired SNS and renin-angiotensin system activation to various stimuli (14, 15). These observations support a potential role for leptin in the regulation of SNS activity and in obesity-induced hypertension.
The Zucker fatty rat is obese due to a mutation in the long form of the leptin receptor that causes hyperphagia and reduced energy expenditure. Despite the absence of a functional leptin receptor, the Zucker fatty rat appears to have normal or even increased SNS activity and develops hypertension during adulthood (2, 7, 24). One potential factor that could contribute to the development of hypertension in this model, despite lack of functional leptin receptors, is leptin-independent activation of the CNS melanocortin system. We have demonstrated that the CNS melanocortin system is downstream of the leptin-induced cascade of events leading to increases in SNS activity and BP. For example, pharmacological antagonism of the MC3/4R completely blocked leptin's ability to raise BP in lean Sprague-Dawley rats (8). In addition, MC4R knockout mice are markedly obese, hyperleptinemic, and hyperinsulinemic but do not develop hypertension, even at old age, and have no increase in BP during chronic leptin infusion (9, 27). The role of the melanocortin system in mediating the hypertensive actions of leptin is also evident in humans with MC4R mutation who, like humans with leptin deficiency, exhibit early-onset morbid obesity but lower prevalence of hypertension than obese control subjects (11).
Whether the CNS melanocortin system may contribute to BP regulation independent of leptin activation is still unclear. Therefore, the main goal of the present study was to determine whether leptin-independent activation of the CNS MC3/4R contributes to regulation of BP and metabolic functions in obese Zucker rats that have nonfunctional leptin receptors. Our results suggest a differential control of appetite and cardiovascular function by the CNS MC3/4R in lean compared with obese Zucker rats, and that the CNS melanocortin system may contribute to the elevated BP in obese Zucker rats.
METHODS
Animal surgeries.
The experimental procedures and protocols of this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Blood pressure telemetry probe implantation.
Male lean (n = 6) and obese (n = 8) Zucker rats (Harlan Sprague-Dawley, Indianapolis, IN) at 12 wk of age were anesthetized with isoflurane (1.5%), and atropine sulfate (0.37 mg/kg) was administered to prevent excessive airways secretion. A telemetric pressure transmitter device (model TA11PAC-40, Data Sciences International) was implanted into the abdominal aorta distal to the kidneys under sterile surgical conditions as previously described (8, 9). The catheter was fixed in the aorta with a small drop of cyanoacrylate adhesive, and the transmitter was secured to the abdominal wall by sutures. Mean daily 24-h BP data were derived from the average BP measured by bursts of 10 s every 10 min using the software (Dataquest 4.0) provided by the manufacturer.
Intracerebroventricular cannulation.
After telemetry probe implantation, a stainless steel cannula (26 gauge, 10 mm long) was implanted into the right lateral cerebral ventricle using coordinates as previously described (8, 9). The guide cannula was anchored into place with two stainless steel machine screws, a metal cap, and dental acrylic, and a stylet was inserted to seal the cannula to keep it from becoming clogged. During stereotaxic manipulation, anesthesia was maintained with 0.5–1.5% isofluorane. Several days after the rats recovered from surgery, accuracy of the cannula placement was tested by measuring the dipsogenic response (immediate drinking of at least 5 ml of water in 10 min) to an intracerebroventricular (ICV) injection of 100 ng of angiotensin II. After the experiment, the animals were killed and the brains removed, sectioned, and stained with cresyl violet to confirm the placement of the cannula in the right lateral ventricle.
After the surgical procedures, rats were housed individually in metabolic cages for determination of daily food consumption. Rats were provided a normal sodium diet (0.5 mmol sodium/g food, Harlan Teklad Diet no. 170955). The rats were allowed to recover for 10–12 days before control measurements were initiated, and then we began monitoring food intake, body weight, BP, and HR.
Experimental protocol.
Mean arterial pressure (MAP), HR, and food intake were recorded daily. After a 5-day control period, the MC3/4R antagonist SHU-9119 was infused ICV (1 nmol/h, 0.5 μl/h) for 10 consecutive days via osmotic minipump (model 2002, Durect). With the rat under isoflurane anesthesia, the minipump was implanted subcutaneously in the scapular region and connected to the ICV cannula using tygon tubing (Cole Parmer). The rate of SHU-9119 infusion was based on our previous studies showing that this dose effectively blocks MC3/4R and increases food intake and promotes weight gain (8, 9, 18). On the last day of ICV SHU-9119 infusion, the cannula connecting the minipump with the ICV cannula was severed, and the rats were followed for an additional 10-day posttreatment period. Fasting blood samples (250 μl) were obtained via a tail snip once during the control period, on day 10 of SHU-9119 infusion, and on day 5 of posttreatment period for determination of plasma glucose, insulin, and leptin levels. To examine the BP variability, frequency distribution of systolic BP, measured by telemetry 24 h/day, was performed for the 5-day control period and during the last 5 days of SHU-9119 infusion.
Spontaneous baroreflex sensitivity.
Spontaneous baroreflex sensitivity (BRS) was determined once during control, on the last day of SHU-9119 infusion, and on the last day of posttreatment period. BRS was calculated using continuous BP and HR recordings (120 min at 1,000 Hz, between 2:00 PM and 4:00 PM) by the sequence method based on quantification of sequences of at least three heartbeats in which systolic arterial pressure (SAP) consecutively increases (up sequence) or decreases (down sequence) accompanied by changes in the same direction of the RR intervals (RRIs) of the subsequent beats. The following criteria were used to estimate spontaneous BRS: 1) minimal RRI change, 1 ms; 2) minimal SAP changes, 1 mmHg; 3) minimal number of beats, 3 consecutive beats; 4) minimal correlation coefficient, 0.85; 5) delay of 5 beats for each pair of SAP and RRI series. Spontaneous BRS was calculated in the time (sequences up, down, and total) and frequency [low frequency (LF) and high frequency (HF)] domains.
Power spectral densities of SAP and RRI oscillations were computed by 512-point fast-Fourier transform and integrated over the specific frequency range (LF, 0.25–0.75 Hz; HF, 0.75–5.0 Hz) using Nevrokard software (Medistar, Ljubjana, Slovenia). When present, artifacts were removed and corrected by means of linear interpolation with the previous and following SAP and RRIs values. A Hanning window was applied, and the spectra of SAP and RRI series and their square-coherence modulus were computed if the coherence was >0.75. The square roots of the ratio of RRI and SAP powers were computed to calculate LF and HF components, which LF is a marker of sympathetic tone to the heart and BP, whereas HF component reflects parasympathetic tone to the heart and the influence of respiratory rhythm on BP. Power of RRI and SAP spectra in the LF and HF range were calculated in normalized units (nu) for each frequency.
Analytical methods.
Plasma leptin and insulin concentrations were measured using ELISA kits (R&D Systems and Crystal Chem, respectively). Plasma glucose concentrations were determined using the glucose oxidation method (Beckman glucose analyzer 2).
Statistical methods.
The results are expressed as means ± SE. The data were analyzed by paired t-test or one-way ANOVA with repeated measures followed by Dunnett's post hoc test for comparisons between control and experimental values within each group when appropriate. Comparisons between different groups were made by 2-way ANOVA followed by Bonferroni's post hoc test when appropriate. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Food and body weight responses to chronic MC3/4R antagonism.
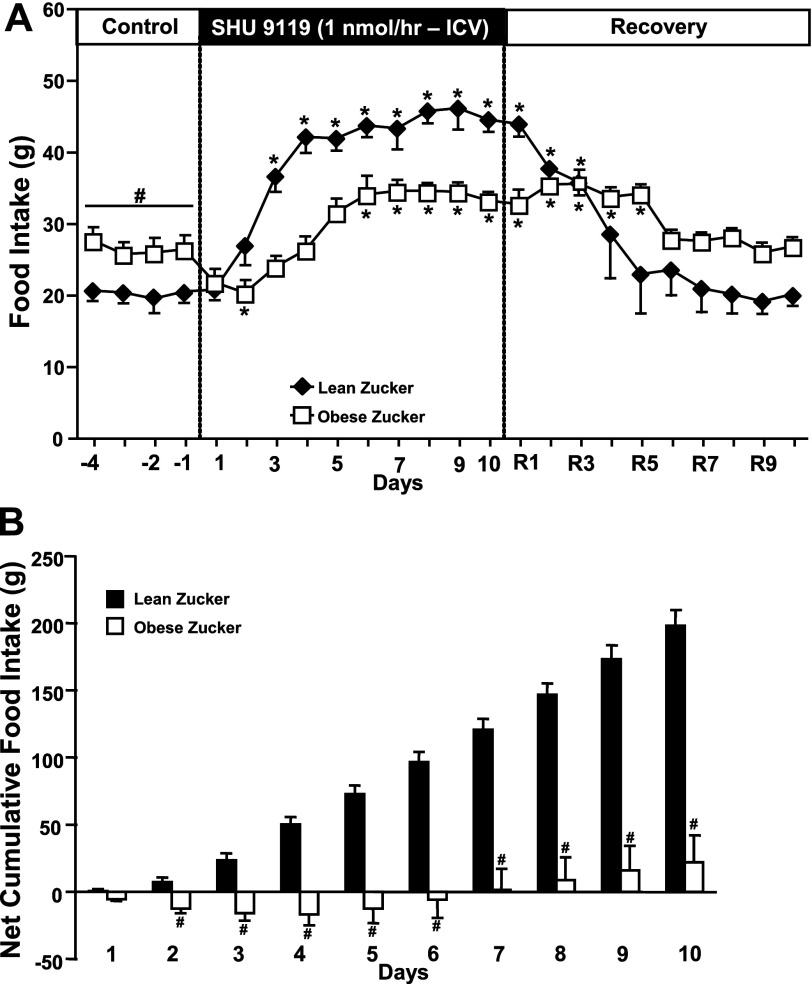
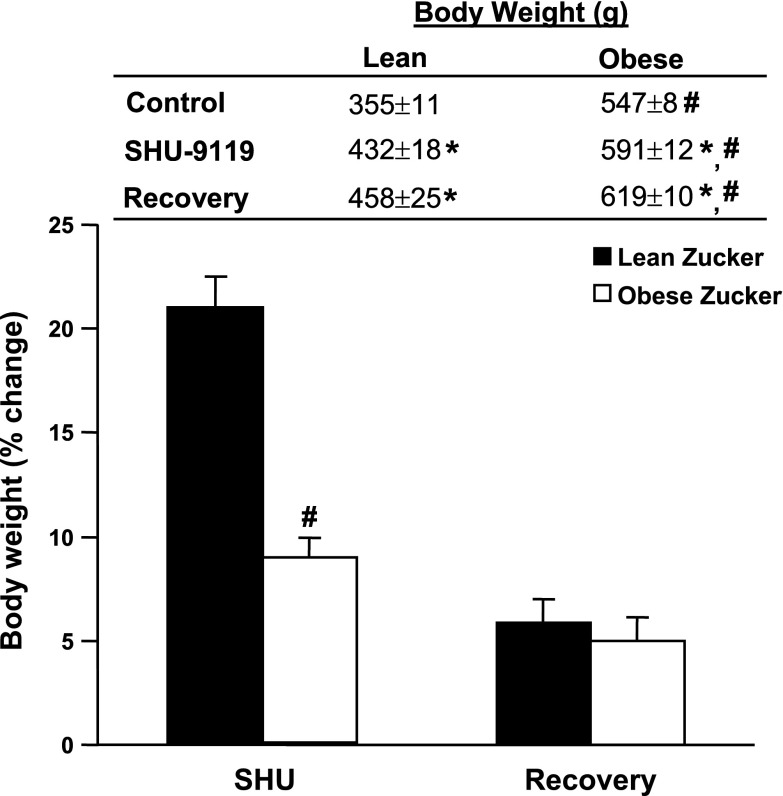
In lean Zucker rats, chronic antagonism of the MC3/4R for 10 consecutive days markedly increased appetite, with food intake more than doubling by the end of the 10-day MC3/4R blockade period (Fig. 1A). This increase in food intake was associated with a 22% increase in body weight (Fig. 2). In obese Zucker rats, however, MC3/4R antagonism transiently reduced food intake, and this was followed by a 35% increase in food consumption (Fig. 1A) and modest, but significant, weight gain (8% increase in body weight, Fig. 2). The greater effect of MC3/4R antagonism on food intake in lean compared with obese Zucker rats is even more evident when analyzing the net cumulative increase in food intake during the 10 days of MC3/4R antagonist infusion (Fig. 1B). After the infusion of the MC3/4R antagonist SHU-9119 was stopped, food intake gradually decreased and returned all the way back to baseline values in lean Zucker rats by day 4 of recovery period, whereas it took 2 additional days for food intake to return to baseline values in obese rats (Fig. 1A).
Fig. 1.
A: food intake responses to chronic intracerebraventricular (ICV) infusion of the MC3/4R antagonist SHU-9119 (1 nmol/h). B: net cumulative food intake in response to MC3/4R antagonist in lean (n = 6) and obese (n = 8) Zucker rats. Data are presented as means ± SE. *P < 0.05 vs. control period (1-way ANOVA repeated measures). #P < 0.05 compared with lean Zucker rats. Comparisons between groups were made using 2-way ANOVA with repeated measures.
Fig. 2.
Body weight and percent change in body weight in response to chronic ICV infusion of MC3/4R antagonist SHU-9119 (1 nmol/h) in lean (n = 6) and obese (n = 8) Zucker rats. Data are presented as means ± SE. *P < 0.05 vs. control period (1-way ANOVA repeated measures). #P < 0.05 compared with lean Zucker rats.
MAP and HR responses to chronic MC3/4R antagonism.
Baseline MAP was higher in obese (111 ± 1 mmHg) compared with lean (100 ± 1 mmHg) Zucker rats (Fig. 3). MC3/4R antagonism reduced MAP in lean and obese rats, but the decrease in BP was transient in lean rats (days 2 and 3 of SHU-9119 infusion) and was not significantly different from control after 4 days of SHU-9119 infusion. In contrast, SHU-9119 caused a slow, gradual decrease in BP in obese Zucker rats that reached significance on day 6 and was sustained until infusion of SHU-9119 was stopped (Fig. 3, A and B). The more pronounced chronic effect of MC4R antagonism to reduce BP in obese compared with lean Zucker rats is more evident when analyzing the total area under the curve (AUC) of BP during SHU-9119 infusion (Fig. 3C), which was ∼78% greater in obese compared with lean Zucker rats.
Fig. 3.
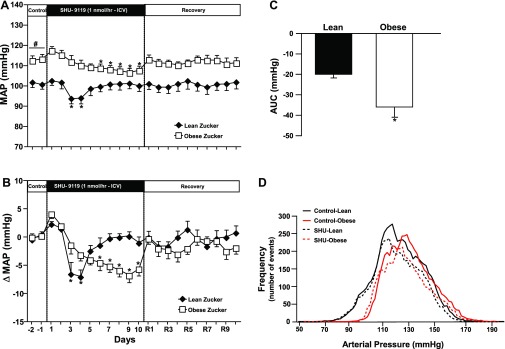
A: mean arterial pressure (MAP) responses to chronic ICV infusion of MC3/4R antagonist SHU-9119 (1 nmol/h). B: changes in mean arterial pressure (MAP) during MC3/4R antagonism in lean (n = 6) and obese (n = 8) Zucker rats. C: area under the curve (AUC) of blood pressure (BP) during SHU-9119 infusion. D: frequency distribution of systolic BP during the 5-day control and the last 5-days of SHU-9119 infusion. Data are presented as means ± SE. *P < 0.05 vs. control period (1-way ANOVA repeated measures). #P < 0.05 compared with lean Zucker rats. Comparisons between groups were made using 2-way ANOVA with repeated measures.
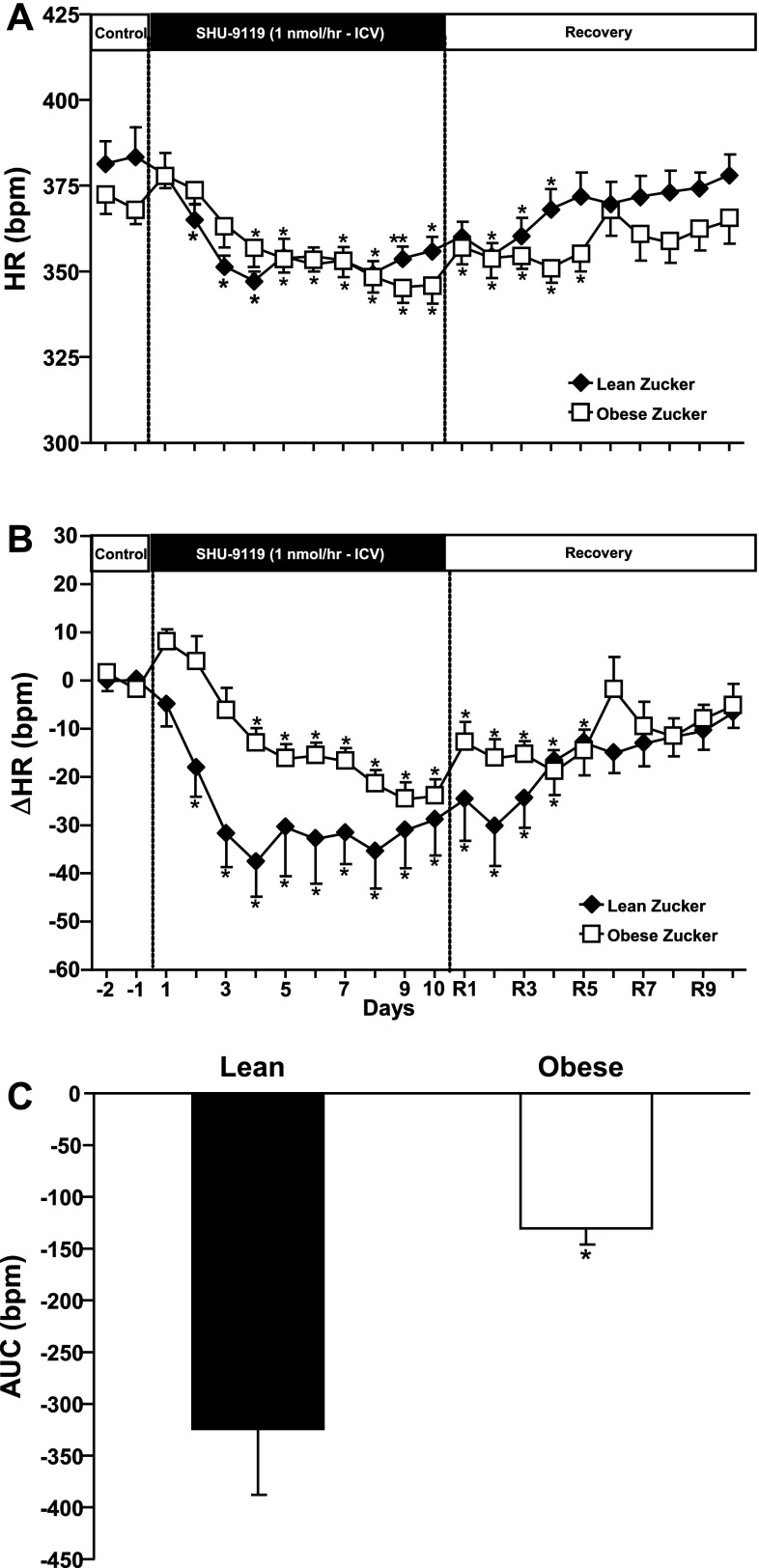
Baseline HR was higher in lean than in obese Zucker rats, and the reduction in HR during SHU-9119 infusion was more pronounced in lean rats (Fig. 4, A and B). This is also more evident when looking at the AUC of HR during SHU-9119 infusion, which was ∼248% larger in lean compared with obese Zucker rats (Fig. 4C). After the infusion of the MC3/4R antagonist was stopped, HR gradually increased and returned to baseline values in both groups, but it returned faster in lean rats than in obese Zucker rats, which required almost 10 days for HR to fully return to baseline values (Fig. 4, A and B).
Fig. 4.
A: heart rate (HR) responses to chronic ICV infusion of MC3/4R antagonist SHU-9119 (1 nmol/h) on HR. B: changes in HR during chronic MC3/4R infusion in lean (n = 6) and obese (n = 8) Zucker rats. C: area under the curve of HR during SHU-9119 infusion. Data are presented as means ± SE. *P < 0.05 vs. control period (1-way ANOVA repeated measures). Comparisons between groups were made using 2-way ANOVA with repeated measures.
We also observed that the systolic BP frequency distribution was slightly shifted to the right in obese compared with lean Zucker rats, highlighting their elevated baseline BP. However, the overall frequency distribution of BP was not different between groups (Fig. 3D).
Chronic effect of MC3/4R antagonism on spontaneous BRS and power spectral analysis of systolic arterial pressure and RR interval oscillations.
To assess the impact of MC3/4R antagonism on reflex control of cardiovascular function in lean and obese Zucker rats, we measured spontaneous BRS using the sequence method. Spontaneous BRS was analyzed by time domain during control, day 10 of the MC3/4R antagonist infusion, and during the recovery posttreatment period (Table 1). Chronic SHU-9119 infusion for 10 consecutive days did not significantly alter baroreflex function in lean or obese Zucker rats.
Table 1.
Spontaneous BRS measured by the sequence method in lean and obese Zucker rats
| Groups | Seq Up, ms/mmHg | Seq Down, ms/mmHg | Seq All, ms/mmHg |
|---|---|---|---|
| Lean Zucker (n = 6) | |||
| Control | 1.24 ± 0.13 | 1.19 ± 0.14 | 1.27 ± 0.13 |
| SHU-9119 | 1.12 ± 0.07 | 1.17 ± 0.22 | 1.17 ± 0.15 |
| Recovery | 1.14 ± 0.19 | 1.29 ± 0.22 | 1.22 ± 0.20 |
| Obese Zucker (n = 8) | |||
| Control | 1.34 ± 0.20 | 1.32 ± 0.30 | 1.33 ± 0.20 |
| SHU-9119 | 1.96 ± 0.51 | 1.63 ± 0.41 | 1.77 ± 0.40 |
| Recovery | 1.42 ± 0.22 | 1.46 ± 0.33 | 1.45 ± 0.24 |
Values are means ± SE. BRS; n, number of rats. spontaneous baroreflex sensitivity. Sequences up (Seq Up), down (Seq Down) and all (Seq All) values were obtained on day 5 of control, day 10 of chronic SHU-9119 infusion (1 nmol/h), and on the last day of the recovery posttreatment period.
Spectral analyses data of HR and BP oscillations between 0.2 and 0.7 Hz (LF region) were used to assess sympathetic modulation, whereas oscillations of HR in the HF region between 0.7 and 2.0 Hz were used to assess parasympathetic tone. At baseline, the HF range of HR, reflecting the parasympathetic tone to the heart, was slightly higher in obese compared with lean Zucker rats, although the difference did not reach statistical significance (Table 2). Chronic MC3/4R blockade significantly increased the HF component in both lean and obese Zucker rats (Table 2). Chronic MC3/4R antagonism reduced the LF component of systolic blood pressure by 37% in obese Zucker rats, whereas no significant changes were observe in lean Zucker rats (Table 2).
Table 2.
Spectral analysis data of RR interval and systolic arterial pressure during control, day 10 of SHU-9119 infusion, and recovery period in lean and obese Zucker rats
| RRI |
SAP |
|||||||
|---|---|---|---|---|---|---|---|---|
| Groups | LF, Hz | HF, Hz | LF, nu | HF, nu | LF, Hz | HF, Hz | LF, nu | HF, nu |
| Lean Zucker (n = 6) | ||||||||
| Control | 0.27 ± 0.01 | 1.38 ± 0.15 | 10.1 ± 2.7 | 17.3 ± 4.1 | 0.38 ± 0.10 | 1.35 ± 0.12 | 21.8 ± 3.4 | 21.1 ± 4.2 |
| SHU-9119 | 0.32 ± 0.03 | 1.59 ± 0.17* | 7.9 ± 1.8 | 19.9 ± 3.7 | 0.39 ± 0.01 | 1.68 ± 0.13 | 18.2 ± 2.6 | 17.5 ± 2.1 |
| Recovery | 0.32 ± 0.02 | 1.46 ± 0.19 | 10.3 ± 1.8 | 19.1 ± 3.5 | 0.36 ± 0.03 | 1.16 ± 0.08 | 25.4 ± 2.3 | 19.7 ± 0.1 |
| Obese Zucker (n = 8) | ||||||||
| Control | 0.37 ± 0.04 | 1.58 ± 0.19 | 17.3 ± 3.2 | 33.6 ± 5.5 | 0.31 ± 0.02 | 1.29 ± 0.19 | 24.4 ± 3.7 | 16.1 ± 4.5 |
| SHU-9119 | 0.30 ± 0.02 | 1.77 ± 0.18* | 7.9 ± 0.7* | 24.9 ± 2.2 | 0.30 ± 0.02 | 1.52 ± 0.21 | 15.39 ± 3.9* | 17.3 ± 3.9 |
| Recovery | 0.31 ± 0.01 | 1.98 ± 0.06 | 12.2 ± 2.1 | 33.4 ± 5.1 | 0.35 ± 0.01 | 1.92 ± 0.08 | 27.85 ± 3.5 | 19.0 ± 4 |
Values are mean ± SE; n, number of rats. RRI, RR interval; SAP, systolic arterial pressure; LF, low frequency; HF, high frequency; nu, normalized units (represent the relative value of each power component in proportion to the total power minus very low frequency (VLF) component. Values were obtained on day 5 of control, day 10 of chronic ICV SHU-9119 infusion (1 nmol/h), and on the last day of the recovery posttreatment period.
P < 0.05 compared with control period.
Plasma glucose, insulin, and leptin responses to chronic MC3/4R antagonism.
Chronic MC3/4R antagonism did not significantly alter plasma glucose levels in lean (97 ± 4 to 103 ± 6 mg/dl) or obese (111 ± 3 to 101 ± 6 mg/dl) Zucker rats. At baseline, obese Zucker rats exhibited significantly higher plasma levels of leptin (41 ± 3 vs. 6 ± 1 ng/ml) and insulin (139 ± 14 vs. 27 ± 4 μU/ml) compared with lean rats. Chronic MC3/4R antagonism increased plasma leptin and insulin levels in lean (from 6 ± 1 to 24 ± 2 ng/ml and 27 ± 4 to 69 ± 9 μU/ml, P < 0.05) and in obese Zucker rats (from 41 ± 3 to 51 ± 2 ng/ml and 139 ± 14 to 177 ± 27 μU/ml, P < 0.05). These results suggest that inhibition of MC3/4R was associated with development of insulin resistance in lean Zucker rats and further impaired insulin sensitivity in obese Zucker rats. The fourfold increase in plasma leptin levels observed in lean rats was likely caused by the marked weight gain in this group during MC3/4R blockade, whereas the modest, but significant, increases in leptin levels in obese Zucker rats were also proportional to the less pronounced weight gain in this group.
DISCUSSION
In the present study we demonstrated that chronic blockade of the CNS MC3/4R markedly increased appetite and promoted weight gain in lean and obese Zucker rats, while at the same time causing sustained reductions in HR. Although the hyperphagia, weight gain, and the bradycardia associated with MC3/4R antagonism were more pronounced in lean compared with obese Zucker rats, chronic CNS MC3/4R blockade caused sustained reduction in BP only in obese Zucker rats, suggesting that the CNS melanocortin system may contribute to the elevated BP in obese Zucker rats.
Our results also indicate that the CNS melanocortin pathway is active in obese Zucker rats via mechanisms distinct from leptin receptor activation. Although these mechanisms are still poorly understood they may involve activation of POMC neurons by other factors such as insulin and cholecystokinin (4) or by increased sensitivity or enhanced postreceptor signaling downstream of the MC4R. Additional studies are needed to address these possibilities.
We also observed that chronic MC3/4R antagonism caused a fourfold increase in fasting plasma leptin and a 2.5-fold increase in insulin levels in lean Zucker rats. These results, together with the observations that MC3/4R blockade caused a greater bradycardia in lean rats while causing a sustained decrease in BP only in obese Zucker rats, suggest a differential control of appetite, BP, and HR by the CNS MC3/4R in lean compared with obese Zucker rats.
Metabolic and hormonal effects of chronic MC3/4R antagonism.
As reported in previous studies in lean rats (8, 18), chronic central MC3/4R antagonism with SHU-9119 more than doubled food intake during the 10-day period of treatment in lean Zucker rats, resulting in rapid weight gain. In obese rats, chronic MC3/4R antagonism also increased food intake, but to a much lesser extent and this was preceded during the first few days of treatment by a transient reduction in food intake. Another difference in the appetite response to SHU-9119 between lean and obese Zucker rats is that food intake continued to be elevated compared with baseline values in obese Zucker rats for at least 6 days after cessation of SHU-9119 infusion, whereas food intake returned to baseline values in lean rats by day 4 of the posttreatment period. The likely explanation for the delayed return of food intake to control values is that obese Zucker rats exhibit delayed sensitivity to MC3/4R antagonism than lean Zucker rats and more time is needed to clear the effect of SHU-9119. This prolonged response to MC3/4R blockade and delayed recovery of food intake toward baseline values in obese Zucker rats may be caused by impaired changes in compensatory factors aimed at restraining the hyperphagia caused by MC3/4R antagonism, such as suppression of neuropeptide Y (6, 17). Whether obese Zucker rats exhibit impaired modulation of orexigenic/anorexigenic factors in response to chronic MC3/4R blockade is, however, outside the scope of the present study and requires further investigation.
Chronic MC3/4R antagonism also caused significant increases in plasma insulin and leptin levels that paralleled the degree of hyperphagia and weight gain caused by MC3/4R antagonism in lean and obese Zucker rats. Previous acute studies suggest that the CNS melanocortin system may modulate insulin sensitivity and glucose uptake by peripheral tissues independent of its effects on appetite and body weight homeostasis (10, 18, 19, 22). However, we demonstrated in a previous study that preventing the hyperphagia and weight gain caused by chronic MC3/4R antagonism completely prevented the hyperinsulinemia and insulin resistance associated with MC3/4R inhibition (18). These observations suggest that the majority of the chronic metabolic effects observed during MC3/4R blockade in lean and obese Zucker rats are due to hyperphagia and weight gain.
Cardiovascular responses to chronic MC3/4R antagonism.
Our finding that blockade of the CNS MC3/4R caused a sustained fall in BP in obese Zucker rats, as evidenced by a larger area under the curve of the fall in BP during SHU-9119 infusion, but failed to cause a sustained lowering of BP in lean Zucker rats suggests that the central melanocortin system plays a role in the maintenance of high BP in obese Zucker rats and that this effect is independent of leptin receptor activation. However, blockade of MC4R did cause a transient decrease in BP in lean Zucker rats. One potential explanation for the failure of MC4R antagonism to cause a sustained BP reduction in lean rats is that compensatory mechanisms that oppose the BP lowering effects of MC3/4R antagonism may be more potent in lean than in obese rats. In addition, the hyperphagia and weight gain in lean Zucker rats were more pronounced and may also contribute to buffering the BP effects of SHU-9119.
Since chronic MC3/4R antagonism reduced the average HR to a greater extent in lean than obese Zucker rats over the 10-day infusion period, as shown in Fig. 4, the CNS mechanisms involved in mediating the larger BP response in obese rats are likely different from those involved in HR regulation. We previously showed that only a modest portion of the bradycardia observed during MC3/4R antagonism in spontaneous hypertensive rats was due to suppression of SNS activity to the heart (7). Furthermore, MC4R expression is abundant in the dorsal motor nucleus of the vagus (26, 29) and other brainstem areas that not only regulate SNS activity but also parasympathetic activity (20, 26, 28), suggesting that at least part of the bradycardia caused by chronic MC3/4R inhibition may be also due to increased parasympathetic activity to the heart; this possibility is supported by our spectral analyses data showing higher HF component of HR oscillations, reflecting increased parasympathetic tone to the heart, during chronic MC3/4R antagonism. It is important to note, however, that although overall reduction in HR was greater in lean rats, MC3/4R antagonism caused a gradual fall in HR in obese rats that reached the same magnitude observed in lean rats by day 9 of treatment. Thus our results suggest that the melanocortin pathway is active in obese Zucker rats via mechanisms independent of leptin. However, further experiments are needed to examine other CNS pathway involved in the activation of the melanocortin system in obesity.
Previous studies have shown that obese Zucker rats have impaired baroreflexes at 13 wk of age and normal SNS activity at 7–8 wk of age (24). In addition, impaired baroreflex sensitivity was found when examined by changes in BP and HR using vasoactive drugs (i.e., Oxford method) in Zucker rats that also could be responsible for the increased blood pressure in obese Zucker rats (5). In the present study we found that chronic MC3/4R antagonism did not alter baroreflex sensitivity in lean or obese rats. In our study, however, baroreflex sensitivity was assessed by spontaneous increases and decreases in BP with parallel changes in HR under physiological condition, without changing BP using vasoactive drugs. One limitation of the sequence method is that the limited range of fluctuations in systolic BP and HR in resting condition (daytime for rodents) and moment-to-moment variability could underestimate the measure of baroreflex sensitivity. Therefore, it is possible that the method used was not sensitive enough to find a difference in baroreceptor sensitivity in our study. However, the sequence method has been shown to correlate with the baroreflex sensitivity evaluated by use of phenylephrine in humans (23), and the fact that we did not observe differences in the frequency distribution of BP in lean and obese rats also supports our finding that BRS is not impaired in lean and obese Zucker rats during SHU-9119 infusion.
The reduction in HR in lean and obese Zucker rats and the sustained fall in MAP in obese rats during chronic MC3/4R blockade occurred despite the increase in food intake and body weight in both groups, which normally would be expected to elevate HR and MAP. This suggests that blockade of the CNS MC3/4R blunted the usual effect of weight gain to cause elevations in BP and HR.
Taken together, these findings indicate that blockade of the CNS melanocortin pathway have a different impact on appetite, HR, and BP regulation in lean compared with obese Zucker rats. Although our data support previous studies showing greater endogenous melanocortin tone in lean compared with obese Zucker rats, our results also suggest that the CNS melanocortin system contributes to the elevated BP in obese Zucker rats, even in the absence of leptin receptor activation, and may be an important mechanism contributing to elevations in arterial pressure in obesity.
Perspectives and Significance
Chronic inhibition of the CNS MC3/4R results in bradycardia and lowers BP in obese Zucker rat despite hyperphagia and body weight gain. The reduction in BP in obese rats during MC3/4R antagonism suggests that endogenous activation of CNS melanocortin system may play a role in the development of hypertension in this model. The mechanisms responsible for this selective enhanced BP response to MC3/4R antagonism in the face of reduced appetite and HR responses, however, are still unclear. Potential mechanisms may involve differential control of SNS and parasympathetic activation to the kidneys versus the heart, for example, or interaction with the renin-angiotensin system, which has been shown to also play a role in the development and maintenance of the hypertension in this model. Unraveling the mechanisms by which the CNS melanocortin pathway may be capable of exerting differential control of appetite, HR and BP may provide important insights to our understanding of the development of hypertension in obesity.
GRANTS
This research was supported by the National Heart, Lung and Blood Institute Grant PO1HL-51971 (to J. E. Hall) and by an American Heart Association Scientist Development Grant (to A. A. da Silva and J. M. do Carmo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.d.C. and A.A.d.S. conception and design of research; J.M.d.C. and J.S.R. performed experiments; J.M.d.C. analyzed data; J.M.d.C., A.A.d.S., and J.E.H. interpreted results of experiments; J.M.d.C. prepared figures; J.M.d.C. drafted manuscript; J.M.d.C., A.A.d.S., and J.E.H. edited and revised manuscript; J.M.d.C., A.A.d.S., J.S.R., and J.E.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Haiyan Zhang for the insulin and leptin assays, and Calvin Torrey for technical support.
REFERENCES
- 1. Adage T, Scheurink de Boer SF, de Vries K, Konsman JP, Kuipers F, Adan RAH, Baskin DG, Schwartz MW, Dijk GV. Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats. J Neurosc 21: 3639–3645, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension 28: 1047–1054, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell B. Divergence of melanocortin pathway in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet 3: 589–600, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bunag RD, Barringer DL. Obese Zucker rats, though still normotensive, already have impaired chronotropic baroreflexes. Clin Exp Hypertens 10: 257–262, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24: 155–163, 1999 [DOI] [PubMed] [Google Scholar]
- 7. da Silva AA, do Carmo JM, Kanycska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneous hypertensive rats. Hypertension 51: 1–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal and metabolic actions of leptin. Hypertension 43: 1312–1317, 2004 [DOI] [PubMed] [Google Scholar]
- 9. do Carmo JM, Tallam LS, Roberts JV, Brandon EL, Bigliane J, da Silva AA, Hall JE. Impact of obesity on renal structure and function in presence or absence of hypertension: evidence of melanocortin-4-deficient mice. Am J Physiol Regul Integr Comp Physiol 297: R803–R812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology 141: 3072–3079, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl Med 360: 44–52, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin and melanocortin. J Biol Chem 285: 17271–17276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrold JA, Williams G, Widdowson PS. Changes in hypothalamic agouti-related protein (AGRP), but not alpha-MSH or pro-opiomelanocortin concentrations in dietary-obese and food-restricted rats. Biochem Biophys Res Commun 258: 574–577, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haynes Sivitz WIWG, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension 30: 619–623, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Huber D, Schreihofer A. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515–1525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol 281: R444–R451, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Kuo JJ, da Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 41: 768–774, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Li G, Mobbs CV, Scarpace PJ. Central pro-opiomelanocortin gene delivery results in hypophagia, reduced visceral adiposity, and improved insulin sensitivity in genetically obese Zucker rats. Diabetes 52: 1951–1957, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Li SJ, Archer P, Hruby VJ, Sharma SD, Kesterson RA. Melanocortin antagonists define two distinct pathways of cardiovascular control by α- and δ-melanocyte-stimulating hormones. J Neurosc 16: 5182–5188, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens 17: 1949–1953, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest 108: 1079–1085, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parlow J, Viale JP, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension 25: 1058–1068, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Schreihofer A, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor in obese Zucker rats. Am J Physiol Heart Circ Physiol 293: H2543–H2549, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Rahmouni K, Haynes WG, Morgan DA, Mark AL. role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23: 5998–6004, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tai MH, Weng WT, Lo WC, Chan JY, Lin CJ, Lam Tseng CJHC. Role of nitric oxide in alpha-melanocyte-stimulating-hormone-induced hypotension in in the nucleus tractus solitarii of the spontaneous hypertensive rats. J Pharmacol Exp Ther 321: 455–461, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 48: 58–64, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Versteeg DHG, Bergen PV, Adan RAH, De Wildt DJ. Melanocortin and cardiovascular regulation. Eur J Pharmacol 360: 1–14, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Ye ZY, Li DP. Activation of the melanocortin-4 receptor causes enhanced excitation in presympathetic paraventricular neurons in obese Zucker rats. Regul Pept 166: 112–120, 2010 [DOI] [PubMed] [Google Scholar]