Abstract
The prevalence of urinary symptoms increases with age and is a significant source of distress, morbidity, and expense in the elderly. Recent evidence suggests that symptoms in the aged may result from sensory dysfunction, rather than abnormalities of detrusor performance. Therefore, we employed a pressure/flow multichannel urethane-anesthetized mouse cystometry model to test the hypothesis that in vivo detrusor performance does not degrade with aging. Secondarily, we sought to evaluate sensory responsiveness to volume using pressure-volume data generated during bladder filling. Cystometric data from 2-, 12-, 22-, and 26-mo-old female C57BL6 mice were compared. All 2- and 12-mo-old mice, 66% of 22-mo-old mice, and 50% of 26-mo-old mice responded to continuous bladder filling with periodic reflex voiding. Abdominal wall contraction with voiding had a minimal contribution to expulsive pressure, whereas compliance pressure was a significant contributor. Maximum bladder pressure, estimated detrusor pressure, detrusor impulse (pressure-time integral), as well as indices of detrusor power and work, did not decrease with aging. Bladder precontraction pressures decreased, compliance increased, and nonvoiding contraction counts did not change with increasing age. Intervoid intervals, per-void volumes, and voiding flow rates increased with age. Calculations approximating wall stress during filling suggested loss of bladder volume sensitivity with increasing age. We conclude that aging is associated with an impaired ability to respond to the challenge of continuous bladder filling with cyclic voiding, yet among responsive animals, voiding detrusor contraction strength does not degrade with aging in this murine model. Furthermore, indirect measures suggest that bladder volume sensitivity is diminished. Thus, changes in homeostatic reserve and peripheral and/or central sensory mechanisms may be important contributors to aging-associated changes in bladder function.
Keywords: mouse cystometry, comparative aging study, sensory regulation
the prevalence of urinary symptoms increases with age, and is a significant source of distress, morbidity, and expense in the elderly (3, 13). Excluding outlet dysfunction, such as sphincteric failure and mechanical obstruction, symptoms of urgency or frequency, nocturia, incontinence, and retention are often regarded as the result of abnormal detrusor muscle activity during filling (detrusor overactivity, DO), and/or an underperforming detrusor during voiding (detrusor underactivity, DU) (2, 40).
Urodynamic studies suggest that aging may be associated with detrusor overactivity during filling and diminished detrusor function during voiding (34, 45), Incomplete bladder emptying, urinary retention, frequency, nocturia, and incontinence associated with DU have been attributed to impaired contractility (57). Morphological findings of diminished detrusor smooth muscle and nerve content, as well as increased collagen content within the detrusor (16, 21), seem consistent with studies demonstrating diminished detrusor strength (20, 41, 45, 47). Impaired substrate delivery, resulting in oxidative stress, may also contribute to age-associated diminished detrusor contractile force (20, 48). Conversely, increases in maximum in vivo voiding pressures in aged rats and increased isometric and isotonic in vitro responses to pharmacological stimulation in aged rats have been reported (32, 33, 46). No loss of contractile responses to cholinergic and field stimulation has been reported in aged human detrusor strips (61). Thus, urodynamic observations, suggesting detrusor underactivity, may reflect dysregulation of detrusor voiding control rather than intrinsic impairment of detrusor muscle strength (51). In fact, recent evidence has suggested the importance of sensory dysfunctions in the generation of symptoms in the aged (1).
Animal models offer the opportunities for detailed investigation of specific mechanistic aspects of the micturition cycle (17), with genetically modified mouse models offering the additional prospect of implicating specific genes. The influence of age on the micturition cycle has been previously studied using in vivo rodent cystometric models (8, 9, 62), with mixed results regarding urine storage and voiding characteristics. Several methodological considerations may explain these discrepancies. Comparisons of young to old animals risk confusing maturational effects with those of aging (10). Also, voiding expulsive pressure is not limited to detrusor contraction in mice and rats. The murine bladder is not highly compliant, causing bladder pressure to gradually rise, as the bladder is filled (36, 53, 56), thereby generating a relatively passive component of voiding expulsive pressure. Rodents also contract the abdominal wall with voiding (15, 54), potentially adding an abdominal pressure contribution, which must also be considered. Estimating detrusor contractility requires knowledge of volumes and flows in addition to expulsive pressures (22), but such approaches have not been incorporated in previous mouse studies.
Therefore, we used a multichannel pressure-flow mouse cystometric model at ages spanning a typical colony life span to test the hypothesis that changes in measures of voiding expulsive force and specifically detrusor contraction strength would not be diminished with aging. Filling phase was evaluated by standard pressure thresholds, compliance, and nonvoiding contractile activity. Furthermore, since bladder afferent activity is a function of both pressure and volume (30, 31), we also incorporated preliminary evaluation of bladder volume sensitivity and/or central receptivity to bladder afferent activity, derived from pressure and volume data at cystometrically important events.
METHODS
C57BL6 female mice procured from the National Institute on Aging's Aging Colony were used. Animal care protocols were approved by the University of Connecticut Health Center Animal Care Committee. Mice were acclimatized for 3–6 days prior to study. Four age groups were evaluated and compared. Young (2 mo), Mature (12 mo), Old (22 mo), and Oldest-old (26 mo) mice were studied. For comparisons of bladder filling and voiding dynamics, a multichannel pressure-flow cystometric method was developed, extending a previously reported pressure-flow mouse cystometric method (53).
Mice were anesthetized with urethane 1.2 g/kg ip, and a polyurethane (PE)-50 catheter was inserted into the dome of the bladder. This catheter was secured to the abdominal wall, keeping the catheter tip above the bladder base but not placing the bladder under any tension. A second PE-50 catheter was used to canulate an isolated and flushed segment of small bowel for the measurement of intra-abdominal pressure. Both catheters were tunneled subcutaneously to exit points near the forepaws, where they were secured to the skin. The bladder catheter was connected to an infusion pump with an inline pressure transducer (BLP1; World Precision Instruments, Sarasota FL). The abdominal catheter/bowel loop was filled to sufficient volume to register a positive response on an inline pressure transducer (BLP1). Small boluses of saline were required periodically throughout the study to accommodate volume loss via bowel absorption. Preliminary experiments had shown an isolated loop of small bowel to introduce no measurable bowel motion artifact, and it is highly compliant, making it a good pressure-sensing balloon for this nonsurvival methodology.
Instrumented mice were placed prone, with the urethral meatus overhanging a 5-ml cup filled with tissue paper in close proximity to the meatus. This cup was suspended on an FT03 load cell (Grass Technologies, West Warwick, RI). Output from all transducers was amplified ×1,000, a 60-Hz notch filter applied, and the signals were digitized at a sampling rate of 30 Hz (volume) or 20 Hz (pressure). Differential amplifiers were balanced, and transducers were calibrated prior to each cystometric study. Data were recorded using WinDAQ Pro+ data acquisition software (DataQ Instruments, Akron, OH).
Continuous infusion cystometry was conducted with bladder infusion of room-temperature normal saline at 1.5 ml/h. After the establishment of a reliable filling/voiding cycle, three sequential voiding cycles were recorded for analysis. Data recorded included bladder (Pves) and intra-abdominal pressure (Pabd), and voided volume. Postvoid residual volumes were not measured. Following completion of the cystometric study, the animal was euthanized with carbon dioxide, and the bladder was removed and weighed. Animals with evidence of compromised health status, significant weight loss, distress during cystometry, or obvious pathology on necropsy were excluded from analysis of cystometric data (38).
For each analyzed void, the following data were tabulated: baseline pressure (Pbase, lowest bladder pressure between voids), voiding contraction threshold pressure (Pthresh, bladder pressure at which voiding contraction begins), and maximum bladder pressure (Pmax, maximum pressure achieved during the voiding contraction). The pressure-time (equivalent to pressure-volume due to a constant filling rate) slope (ΔP/Δt) was calculated between Pbase and Pthresh, and a hypothetical pressure on this slope at flow onset was calculated, Pcomp. Compliance was calculated as the inverse of the pressure-time slope, multiplied by the infusion rate, to give the standard ΔV/ΔP. Since the maximum voiding bladder pressure coincides with the onset of flow (53, 56), detrusor pressure at Pmax (Pdetmax) was calculated by subtracting Pcomp from Pmax. Per-void volume (postvoid minus prevoid volume) and flow rates (Qave, slope of volume voided tracing over entire change in volume during void) were determined for each void.
The pressure-time interval (PTI) is the expulsive impulse and is linearly related to external work in cardiac contractility models (50). Therefore, we used this quantity to estimate the expulsive work components by the detrusor contraction (i.e., excluding the heat loss associated with muscle contraction), the pressure associated with compliance, and the abdominal pressurization associated with voiding. The PTI was calculated by determining the area under the curve of the relevant pressure curve during the event of interest. Division of the PTI by the event duration yields a mean pressure, which could be used in calculations of mean simple work and power. Bladder PTIs for the complete voiding contraction and the preflow, intraflow, and postflow segments of the voiding contraction were determined. Since Pabd was not absolute, the voiding-associated abdominal PTI was calculated as the area of Pabd during flow minus the area of the baseline pressure over the preceding same interval.
The compliance contribution to the measured preflow pressurization of the voiding contraction was estimated by calculating the compliance slope PTI between Pthresh and Pcomp. Because compliance-associated pressure dropped from Pcomp to zero during flow, an estimate of compliance-derived expulsive PTI during flow was made by calculating the area of a triangle bounded by Pcomp, and time at flow start and flow end on the time axis. The abdominal PTI and compliance PTI during flow were subtracted from the bladder pressure PTI during flow, to estimate the detrusor PTI during flow. Similarly, the compliance PTI during preflow (Pthresh to Pcomp) and flow regions and the abdominal PTI were subtracted from the total bladder pressure PTI between onset of voiding contraction until resolution to estimate detrusor impulse associated with the complete voiding contraction. These calculations are illustrated in Fig. 1. The contributions of detrusor, compliance, and abdominal PTI during flow and voiding contraction were calculated as percentages of total PTI. Detrusor PTIs were divided by event duration to obtain mean detrusor pressure values, which were multiplied by voided volume and flow rates to obtain mean work and power indices.
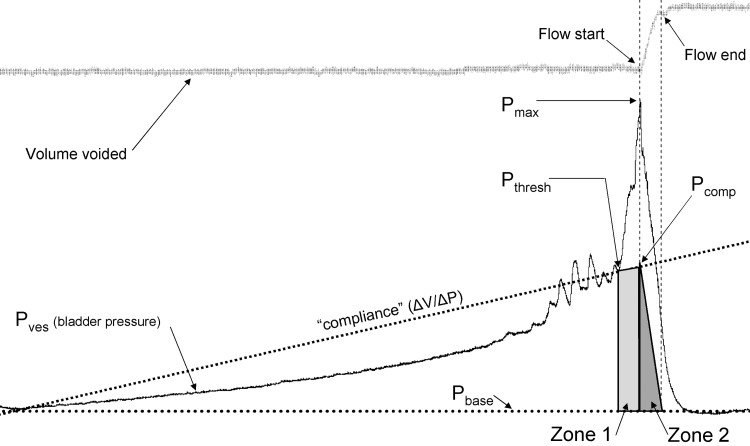
Fig. 1.
Method of analysis. Tracing of voided volume (top) and measured bladder pressure during one filling-voiding cycle in a 12-mo-old mouse. Voiding flow was demonstrated by increase in voided volume, onset coincident with maximum bladder pressure (Pmax), ending prior to resolution of voiding contraction. Indicated pressure points are those relevant to the analysis. Compliance line demonstrates the method of estimating the contribution of compliance to expulsive pressure. Compliance contribution during flow only is indicated by Zone 2. Compliance contribution to entire voiding contraction expulsive pressure is indicated by Zone 1 and 2.
Nonvoiding contraction (NVC) activity has been observed in awake and anesthetized murine cystometric models (5, 42, 53). It has been proposed that this activity contributes to the volume sensory process via activation of mechanoreceptors during filling (55). Defining an NVC as any phasic pressure rise over the interpolated pressure curve due to compliance, NVC activity was evaluated. Data recorded for comparison between groups were the pressure and volume at NVC onset, number of NVCs for each filling and voiding cycle, calculation of a mean NVC frequency by division of number of NVCs by duration of NVC activity.
The relationship of bladder volume to centrally mediated responses to volume depends upon the level of afferent activity per unit volume, and/or upon central sensitivity to afferent outflow. The best correlation of bladder afferent outflow to filling parameters is a linear relationship to wall stress (treating the bladder as a thin-walled sphere). The simple product of bladder volume and pressure has the same correlation (r = 0.81) when bladder tissue volume is small compared with overall volume (30). In all groups in this study, mean bladder volumes (estimated from bladder weight, assuming 1 g = 1 ml) were less than 20% of minimum bladder volume (assuming negligible postvoid residual volumes) at voiding contraction threshold. Therefore, as an index of system sensitivity to bladder volume, the product of pressure and volume (“stress function”) at the contraction threshold pressure was calculated, equating filling time with bladder volume at Pthresh and assuming equivalent postvoid residual volumes across groups. In a similar fashion, the stress function at the onset of NVC activity was calculated by multiplying pressure and volume at the onset of NVC activity.
Analysis.
Cystometric data files were coded, and data analysis was conducted en bloc, remote from the conduct of the cystometric studies, allowing analysis blinded to animal age. After all cystometry tracings had been analyzed, the analysis data were assigned to the appropriate age group for subsequent comparisons. Data were analyzed using WinDAQ software and MS Excel, and comparative statistics were generated with Prism 5 (GraphPad Software, Palo Alto CA), P < 0.05 regarded as statistically significant. Data distribution normality was tested with Kolmogorov-Smirnov test. Mean values were calculated for each measured and calculated parameter for each group, and age-associated trends evaluated were made with ANOVA with post hoc test for linear trend when all data were normally distributed. ANOVA with Bonferroni multiple-comparisons test or Kruskal-Wallace test with Dunn's multiple comparisons test was applied as indicated to evaluate individual group differences in all cases. All data are shown as means ± SE. Associated overall P values for the overall comparisons, and where appropriate, for linear trend, are shown in the table and figures for each comparison. Differences between groups achieving statistical significance P < 0.05 are denoted within each parameter.
RESULTS
Forty-six mice were prepared for cystometry. We define “maturation” as referring to the transition from Young (2 mo) to Mature (10 mo). “Aging” refers to the transition from Mature to Old (22 mo) or Oldest-old (26 mo), based upon reported age-associated changes in weight and survival in the C57BL6 female mouse (38). Bladder and body weights, as well as the ratio of bladder weight to body weight, tended to increase with maturation and aging, as shown in Fig. 2. Periodic voiding in response to continuous filling, yielding an interpretable cystometrogram, was obtained in 11/11 Young, 10/10 Mature, 10/15 Old, and 5/10 Oldest-old mice (χ2-test, P < 0.05). Representative tracings, including pressure and voided volume waveforms from each group, are demonstrated in Fig. 3. The remaining animals failed to respond to this fluid challenge, did not develop a voiding pattern, and filled until continuous leakage resulted. Since these animals did not provide an analyzable voiding pattern, they are not included in the cystometric data analysis. Overall, measures of contractility showed a maturational increase but not an aging effect. Bladder filling parameters generally demonstrated significant trends with maturation and aging.
Fig. 2.
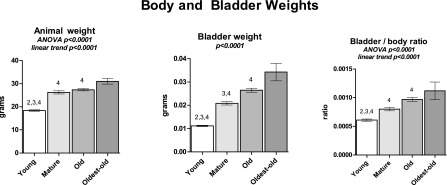
Body and bladder weights for the four age groups. Body weights are preanesthesia, while bladder weights are empty wet. Weights and ratio trend upward with increasing age. Bars show means ± SE; P values for overall comparison. ANOVA was used where noted; otherwise, a Kruskal-Wallace test was performed. 2P < 0.05 vs. Mature. 3P < 0.05 vs. Old. 4P < 0.05 vs. Oldest-old. P for linear trend is reported where applicable.
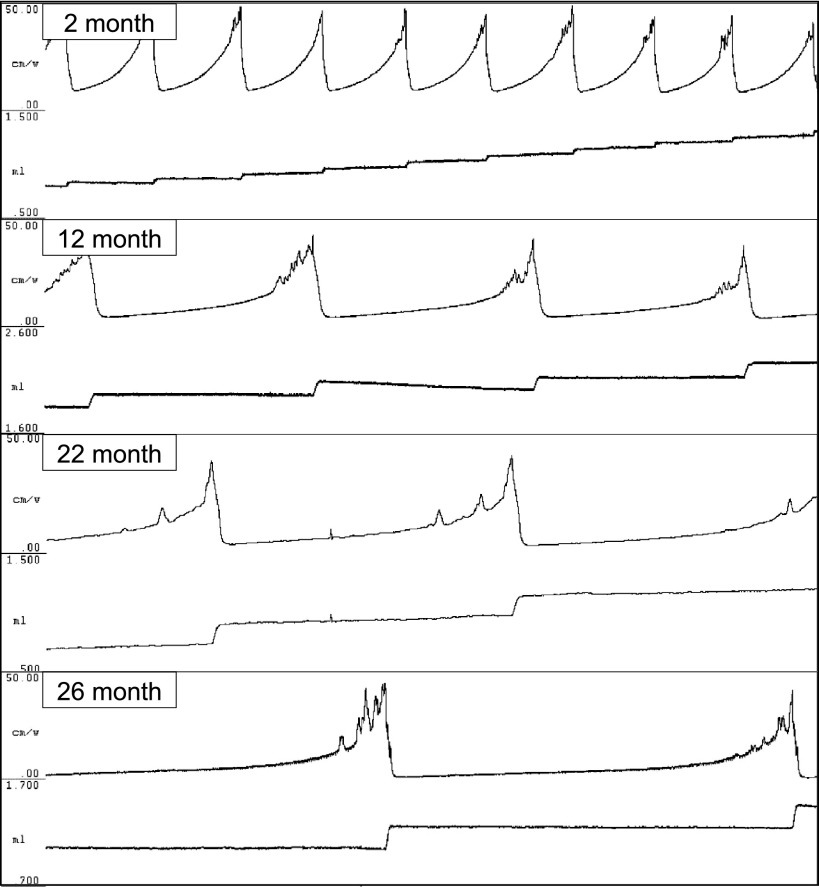
Fig. 3.
Representative bladder pressure tracings from each age group, obtained during continuous-fill cystometry under urethane anesthesia. Boxes from top to bottom: Young, 2 mo; Mature, 12 mo; Old, 22 mo; Oldest-old, 26 mo. Top tracing in each box is bladder pressure (0–50 cm/W), bottom tracing in each box is voided volume (scale: 1 ml). Tracings obtained in response to constant filling at 1.5 ml/h via catheter placed through the dome of the bladder. Voided urine collection is as described in methods. Time (horizontal) scale is the same for all tracings.
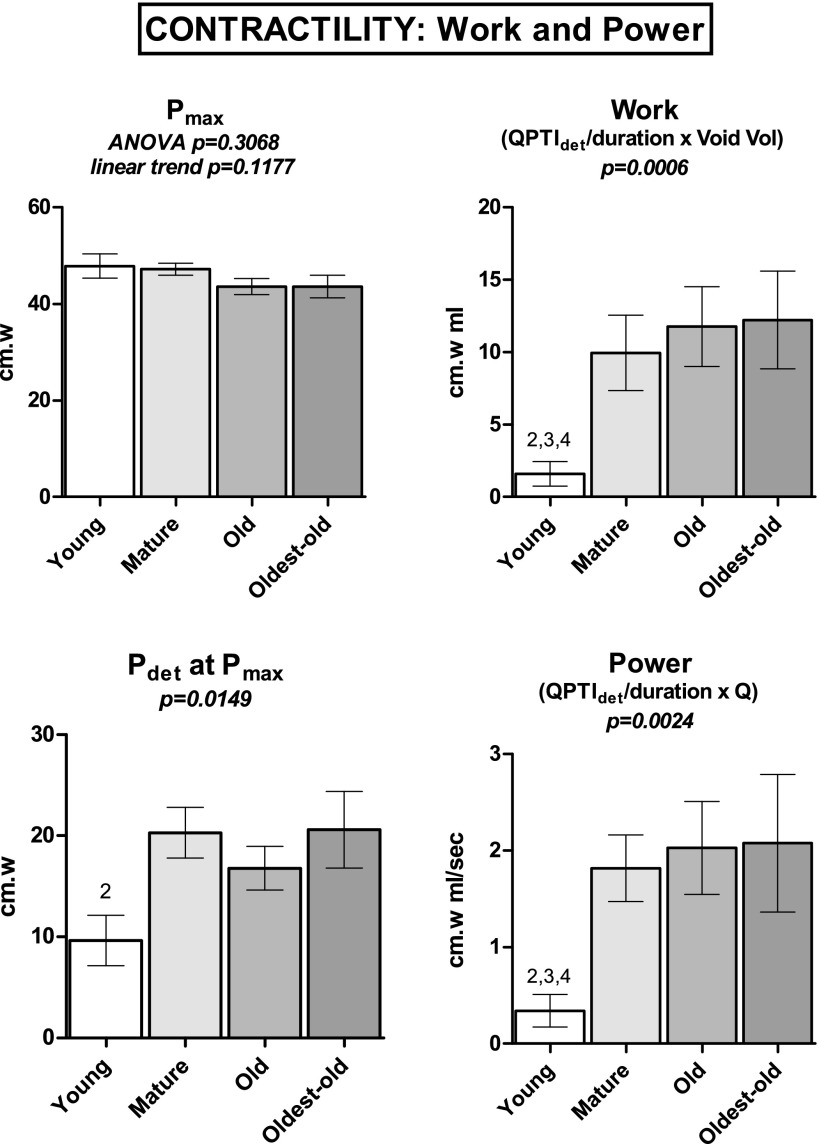
Contractility indices are presented in Table 1 and Figs. 4 and 5. Maximum voiding contraction pressure (Pmax), accompanied by the onset of flow, did not statistically differ among groups, nor did it trend with age (P = 0.1177). Pdetmax data were not normally distributed; however, Pdetmax increased with maturation, reaching statistical significance only for comparison of Young vs. Mature. The components of the PTI associated with the entire voiding contraction were separated into abdominal, compliance, and detrusor components. Overall contraction PTI data were not normally distributed; thus, no trends were formally evaluated. The total PTI of Young was significantly less than Mature and Oldest-old, thus increasing with maturation. A similar pattern was observed with the detrusor PTI over the voiding contraction. PTI due to estimated compliance demonstrated an upward trend with maturation and aging, achieving statistical significance for Young vs. Old and Oldest-old.
Table 1.
Contractility indices
| Young |
Mature |
Old |
Oldest-Old |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Note | Percent | Mean | SE | Percent | Mean | SE | Percent | Mean | SE | Percent | |
| Pmax, cm/W (P = 0.1431) | 48 | 2.5 | 100% | 47 | 1.2 | 100% | 44 | 1.7 | 100% | 44 | 2.3 | 100% | |
| Pdet at Pmax, cm/W (P = 0.0149) | 10 | 2.5 | 2 | 21% | 20 | 2.5 | 43% | 17 | 2.2 | 39% | 21 | 3.8 | 47% |
| PTI, voiding contraction, cm·W−1·s−1 | |||||||||||||
| Total (P = 0.0020) | 376 | 64.8 | 2, 4 | 100% | 612 | 48.5 | 100% | 587 | 37.5 | 100% | 892 | 171.0 | 100% |
| Abdominal (P = 0.1231) | 1.8 | 0.52 | 0.5% | 2.4 | 0.70 | 0.4% | 0.4 | 0.27 | 0.1% | 2.0 | 2.00 | 0.2% | |
| Compliance (P = 0.00480) | 124 | 34.2 | 2, 3 | 33% | 149 | 14.6 | 24% | 189 | 19.8 | 32% | 333 | 105.4 | 37% |
| Detrusor (P = 0.0019) | 251 | 35.4 | 2, 4 | 67% | 462 | 44.9 | 75% | 399 | 26.2 | 68% | 558 | 79.5 | 63% |
| PTI, during flow, cm·W−1·s−1 | |||||||||||||
| Total (P = 0.0094) | 112 | 12.4 | 2, 3 | 100% | 193 | 26.5 | 100% | 193 | 13.2 | 100% | 183 | 30.0 | 100% |
| Abdominal (P = 0.1231) | 1.8 | 0.52 | 1.6% | 2.4 | 0.70 | 1.2% | 0.4 | 0.27 | 0.2% | 2.0 | 2.00 | 1.1% | |
| Compliance (P = 0.0600) | 43 | 5.8 | 38% | 47 | 9.4 | 24% | 67 | 10.2 | 35% | 81 | 25.2 | 44% | |
| Detrusor (P = 0.0333) | 67 | 14.2 | 2 | 60% | 145 | 25.9 | 75% | 126 | 11.1 | 65% | 100 | 32.2 | 55% |
| Detrusor voiding PTI work and power | |||||||||||||
| Work, cm·W−1· ml−1 (P = 0.0006) | 1.60 | 0.86 | 2, 3, 4 | 10.0 | 2.60 | 11.8 | 2.75 | 12.2 | 3.37 | ||||
| Power, cm·W−1· ml−1·s−1 (P = 0.0024) | 0.340 | 0.168 | 2, 3, 4 | 1.82 | 0.45 | 2.03 | 0.48 | 2.08 | 0.71 | ||||
Bladder and detrusor pressures, pressure-time integrals (PTI), and PTIdetrusor-derived work (W) and power. Calculations described in the text. Means ± SE are reported. Component contribution to total percentages reported for detrusor pressure at maximum voiding bladder pressure (Pdet at Pmax) and PTI during voiding contraction and during flow. Comparisons between groups by ANOVA with Bonferroni or Kruskal-Wallace with Dunn's post hoc tests, as appropriate. Only Young vs. other groups demonstrated significant differences, as indicated in notes column: 2vs. Mature; 3vs. Old; 4vs. Oldest-Old.
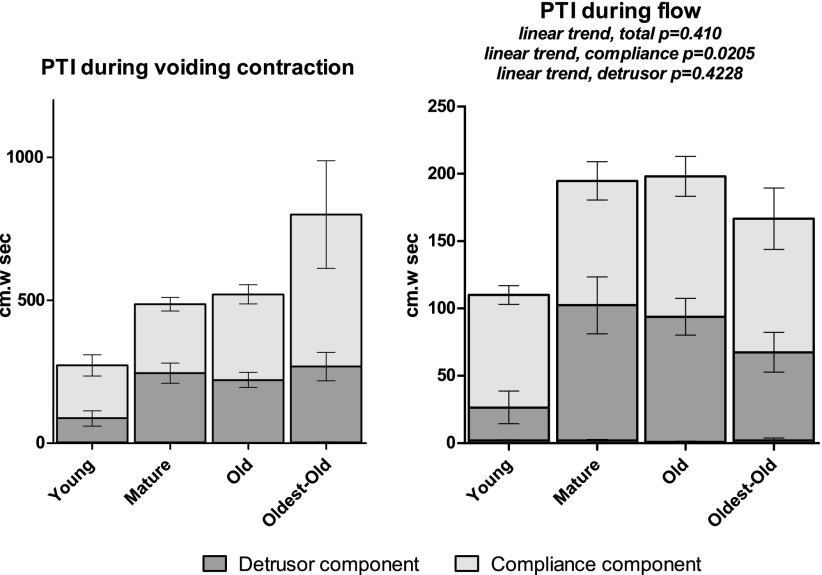
Fig. 4.
Pressure-time interval (PTI) contractility results. Total height of each group bar is the summed PTI for total voiding contraction (left) and during flow (right). Each bar is divided into detrusor contraction, compliance, and abdominal PTI, although the latter is very small compared with detrusor and compliance PTI and, therefore, does not show. Error bars show SE for individual components. Linear trend not reported for PTI during voiding contraction as data were not normally distributed.
Fig. 5.
Maximum pressure and calculated power and work indices were obtained during continuous-fill cystometry under urethane anesthesia. Pressure component of power and work calculations obtained by dividing detrusor PTI during flow (QPTI) by duration of flow. Bars show means ± SE. ANOVA was used where noted; otherwise, a Kruskal-Wallace test was performed. 2P < 0.05 vs. Mature. 3P < 0.05 vs. Old. 4P < 0.05 vs. Oldest-old. P for linear trend is reported where applicable.
The flow-associated PTI data for total, compliance, and detrusor components were normally distributed. Total PTI during flow trended upward (P = 0.0410), primarily attributable to the estimated compliance component (P = 0.0205) rather than the estimated detrusor component (P = 0.4228). The trend in total flow-associated PTI was primarily maturational, as Young vs. Mature and Young vs. Old proved significantly different. Although no significant trend was observed for estimated detrusor PTI, Young vs. Mature significantly differed, suggesting a maturational effect on flow-associated detrusor impulse. Abdominal PTI data were not normally distributed; however, they did not differ among groups and constituted less than 2% of the PTI during flow. Indices of detrusor work and power were not normally distributed; only maturational increase (Young vs. Mature/Old/Oldest-old) in work and power indices achieved statistical significance.
Bladder filling and voiding results are presented in Figs. 6 and 7. Baseline bladder pressure (Pbase) trended downward with age, with Young vs. Old and Oldest-old proving statistically significant. Likewise, Pthresh trended downward, with Young vs. Mature and Oldest-old comparisons achieving statistical significance. Bladder compliance increased with age; Young vs. Oldest-old was statistically significant. The voiding contraction stress function increased with aging, but specific comparisons were statistically significant only for Young vs. Oldest-old. Intervoid interval and per-void volume increased with maturation and aging. Oldest-old demonstrated a significantly greater average flow rate than Young and Mature groups, and voiding flow rate trended upward with increasing age.
Fig. 6.
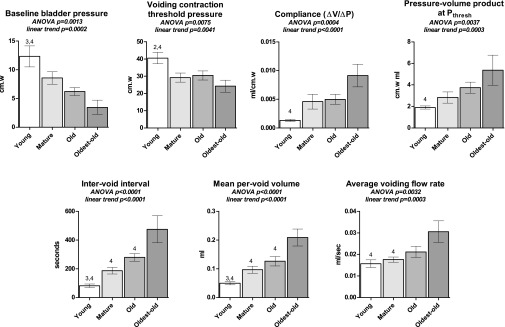
Cystometric filling and voiding data for the four age groups, obtained during continuous-fill cystometry under urethane anesthesia. Pressures trend downward with aging, compliance trends upward with maturation and aging. Intervoid interval, voided volume, and average flow rate increase with age. Bars show means ± SE. ANOVA was used where noted; otherwise, a Kruskal-Wallace test was performed. 2P < 0.05 vs. Mature. 3P < 0.05 vs. Old. 4P < 0.05 vs. Oldest-old. P for linear trend is reported where applicable.
Fig. 7.
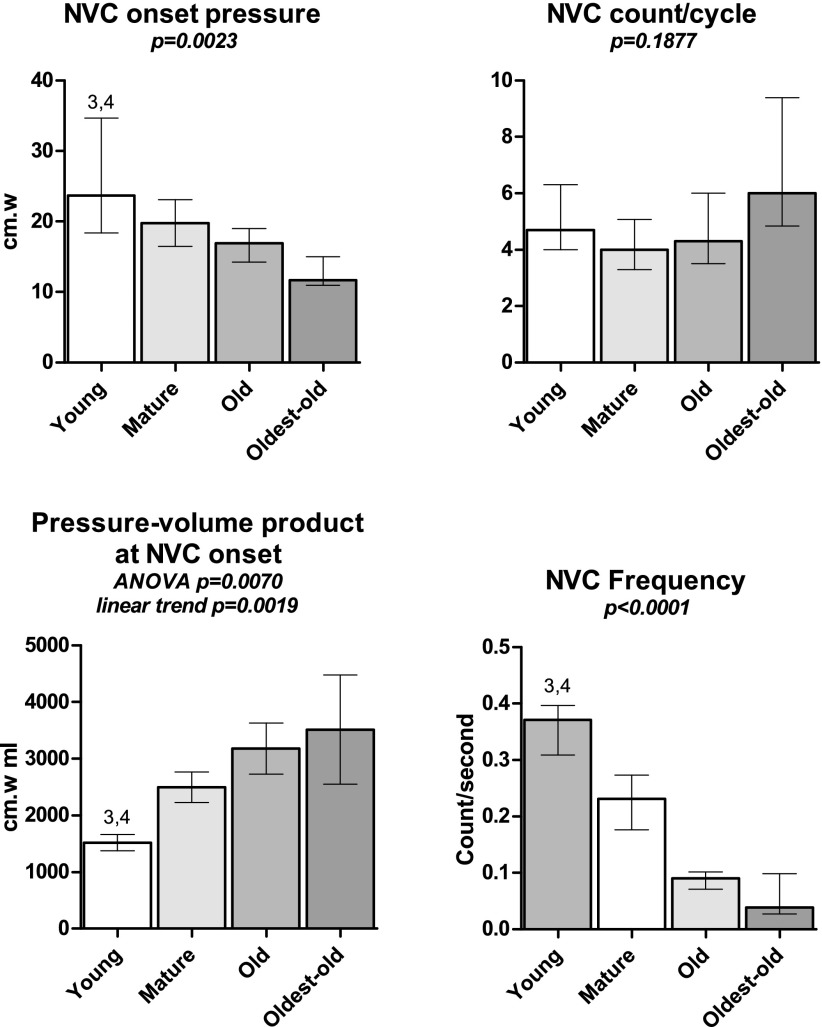
Nonvoiding contraction (NVC) results from cystometry. NVC onset threshold bladder pressure decreased, and bladder wall stress function (pressure-volume product) at NVC onset increases with age. NVC frequency also decreased with age. Total NVC count during filling did not differ among groups. Bars show means ± SE. ANOVA was used where noted; otherwise, a Kruskal-Wallace test was performed. 3P < 0.05 vs. Old. 4P < 0.05 vs. Oldest-old. P for linear trend is reported where applicable.
Nonvoiding contraction count during filling phase did not differ between groups. NVC onset threshold and frequency were not normally distributed; however, comparisons between groups demonstrated Young vs. Old and Oldest-old being statistically significant. The NVC onset wall stress function (pressure × volume) demonstrated the opposite response, increasing with aging (P = 0.0019), with significant differences between Young vs. Old and Oldest-old. Frequency of NVC contractions declined from almost 0.4 Hz to <0.1 Hz, with Young vs. Old and Oldest-old proving statistically significant.
DISCUSSION
These experiments yielded three principal findings. First, aging was associated with a diminishing likelihood of voiding in response to continuous bladder filling, suggesting impaired homeostatic mechanisms following a fluid challenge to the aged bladder. Second, addressing our primary hypothesis, we demonstrated positive changes in detrusor expulsive effort that are maturational rather than an effect of aging. No decline with maturation or aging in total expulsive force or isolated detrusor effort during the voiding contraction or flow was demonstrated by our multiple measures of voiding function. Finally, our evidence suggests that not only does the bladder become less sensitive to volume (mediated via increased compliance), central determinants of voiding initiation may become less sensitive to afferent input with aging. Multiple filling indices demonstrated changes that may be characterized as both maturational and aging.
An unexpected, but not entirely surprising, finding is the impaired responsiveness to the cystometric methodology among the Old and Oldest-old animals. Aging is associated with impaired homeostasis (diminished or less appropriate responsiveness to stressors) rather than measurable declines in baseline system function (29). This loss of homeostatic ability associated with aging has been termed homeostenosis. In these experiments, the mode of failure in aged mice was failure to respond to the continuous infusion at cystometry, rather than degraded detrusor function during successive filling-voiding cycles. Thus, a primary finding of this study is the confirmation of a homeostenotic effect of aging in the mouse cystometric model.
Contractility is a difficult and poorly defined term, but it is intended to express the strength of the detrusor muscle. A formal expression of contractility requires knowledge of the muscle work. This is represented as length-dependent curves (including y and x intercepts) of force vs. velocity of shortening (analogous to pressure vs. flow rate) and muscle thermodynamics (26). This is not feasible in an in vivo system, and so approximations using cystometrically obtainable data have been proposed in humans (22). As the dynamics of rodent voiding are considerably different from those in humans, these approximations are inappropriate for a rodent cystometric model. To our knowledge, there are no reports of direct investigation of the thermodynamics of bladder contractility and aging, potentially a serious limitation to any reported methodology [although, aged bladders are more sensitive to oxidative stress (20), suggesting either impaired basal substrate delivery and/or diminished thermodynamic efficiency with aging]. We based our primary indices of contractility on the PTI, including calculations of simple work and power, thereby integrating expulsive force with volume and flow (49).
Abdominal wall contraction during voiding has been reported in rats (15, 54), and we observed abdominal pressurization during voiding in mice. We found no clear difference in PTI due to abdominal contraction between age groups; however, variances within groups were high, suggesting that animal-to-animal variability greatly exceeds any variability attributable to maturation or aging. Additionally, the abdominal component comprised only a very small contribution (<2%) to the overall PTI and can, therefore, probably be safely ignored in future studies.
We observed increased compliance with increasing age, contrary to some previous reports. Kohan et al. (28) reported greater compliance in young vs. mature, aged, and senescent Wistar rats. While capacity volume in that study did not change, the voiding contraction pressure threshold increased in aged animals, consistent with loss of system sensitivity to bladder filling. Lluel et al. (33) reported no effect of age on compliance in Wistar rats. Consistent with biological membranes, the bladder pressure as a function of volume during filling exhibits an exponential-type curve (23). The shape of this curve is determined by both passive (extracellular matrix/collagen) and active (detrusor muscular) elements, thus previously described age-associated changes in bladder wall composition logically affect the shape and location of this curve (11, 16). Despite the complex relationship of pressure to the volume of the filling bladder, compliance is typically expressed as a linear calculation of volume per unit pressure. The determinants of baseline and voiding thresholds may not be shared across species; thus, different segments of an isolated pressure-volume curve may be the basis for “compliance” calculations, precluding comparison of simple numeric representations of compliance across species. The bladder is more compliant in the Wistar rat (28) than our mouse observations (0.15 ml·cm−1·W−1 vs. 0.0033 ml·cm−1·W−1 for 12 mo mice), suggesting that a different segment of the pressure-volume curve is being sampled, accounting for the divergent results.
The age-associated increase in intervoid interval and voided volume demonstrates that aging is associated with diminished bladder volume sensitivity. To the contrary, Pthresh decreased with aging. These apparently contradictory findings are resolved by considering the central role of wall stress in the transduction of volume information to bladder afferent activity. Using the pressure-volume product, the estimated wall stress at Pthresh significantly increased with aging, suggesting that either the slope of the afferent activity vs. pressure-volume product decreases with aging, signifying an altered mechanotransduction mechanism, and/or there is diminished central sensitivity to afferent activity. Age-associated diminished activity in relevant brain areas in response to bladder stimulation has been observed by functional MRI study (24), consistent with either diminished afferent inflow or central receptivity. In either case, the finding of diminished volume sensitivity in combination with preserved detrusor contractility supports the emerging view that sensory dysfunction, rather than primary detrusor motor dysfunction, may underlie nonobstructive voiding disorders in the aged (1, 51).
The NVC activity results may also reflect age-associated changes in the regulation of volume mechanotransduction. NVCs in animal models have previously been interpreted as analogous to DO in humans, such as in spinal cord injury (7) and obstruction (59) models. However, as producers of localized wall stresses, NVCs have been postulated to be part of the continual mechanosensory process as the bladder fills (19, 55), and as outward evidence of detrusor autonomous (micromotional) activity, related to the compliance-determining mechanics (12). Early in filling (and presumably throughout filling in the normal human bladder), tension induced by local autonomous contractile activity of the detrusor is diffused by bladder compliance; however, as that compliance is lost as the bladder fills, local contractile activity induces measurable pressure rises, evident as NVC activity. Therefore, the threshold of onset of measurable NVC activity reflects interplay between bladder wall mechanics and regulators of autonomous contractile behavior. The wall stress function for NVC onset increased with aging, parallel to Pthresh and consistent with a loss or downward modulation of system sensitivity. The downward trend of the NVC pressure threshold is offset by a higher volume of onset. The trend toward a decrease in frequency of NVC, significantly lower for Oldest-old vs. Young bladders, suggests that NVC activity in older bladders may not be the result of the same regulatory processes as in younger bladders. Cystometric events are generally regarded as static pressure/time markers; however, the unchanged NVC count across age groups, despite compliance differences, suggests that the rate of change of pressure, volume, or wall stress may have a role in the sensory transduction process. Given the probable importance of autonomous (and thus NVC) activity to volume mechanotransduction, this topic deserves further exploration.
Our choice of age groups was intended to include groups representing immature animals (2 mo), young but mature animals (12 mo), Old but intact mature animals (22 mo), and Oldest-old animals toward the limits of routine survival (26 mo). Food consumption, weight, and survival curves steadily rise from birth to about 10–12 mo. The weight vs. age curve for ad libitum-fed female C57BL6 mice maximizes at about 22 mo and achieves 80% of this value at about 12 mo. Survival in the mouse colony drops off after 26 mo (58). As the maximum rate of increase in weight and food consumption occurs between 2 mo and 12 mo, we assigned “maturation” to this interval. Between 12 mo and 22 and 26 mo, growth slows/stops, food consumption does not increase, and survival begins to diminish; therefore, we assigned these intervals to “aging.” These categories are intended as representative of measures of overall life span processes in this organ system-specific study, parallel to aging categorization within the geriatric clinical literature (4, 14, 18, 39).
This study addresses several significant limitations of previous aging cystometric studies. An animal model incorporating animals at the extremes of the expected life span, as well as mature animals, with an isolated focus on urinary function, allows separation of the effects of maturation from those of aging (10). Our cystometric methodology avoided the impact of urethral stimulation by transurethral catheter, allowed estimation of all potential expulsive pressures, incorporated volume and flow data into indices of contractility, and allowed post hoc calculations estimating system responsiveness to bladder volume.
The incorporation of voiding volumes and flows into contractility estimates is to our knowledge unique in rodent cystometric studies of detrusor contractility, despite its accepted role in estimating contractility in humans. This is facilitated by the use of anesthesia, as the animal remains still, allowing voiding flow rate determination (53). Unlike most other anesthesia agents, the micturition reflex and bladder filling and voiding dynamics are preserved under urethane (6, 35, 37). In sample sizes typical of rodent research, pressure and timing responses to continuous filling cystometry do not differ between awake and urethane-anesthetized animals (53). Urethane modestly potentiates inhibitory GABAA, glycine, and nicotinic receptor activity and inhibits stimulatory ionotropic glutamate receptor (NMDA, AMPA) activity (25), and thus, it may have some suppressive effect on bladder volume sensitivity. We cannot exclude the possibility that older animals are more sensitive to this central suppression, thus amplifying the magnitude of differences for bladder filling parameters. Normal voiding is dependent upon bladder and urethral afferent activity (27, 31, 43, 44), and thus, the lack of degradation in voiding performance in the older animals suggests that any urethane-induced artifact during filling is small.
Our calculations are based on several assumptions that may affect the magnitude, but unlikely the direction, of the results. First, the pressure-time (volume) graph (and hence compliance) is not linear; rather, it has an exponential shape. Therefore, we may have underestimated Pcomp with our assumption of linearity. Similarly, we may have overestimated the contribution of compliance during flow. However, lengthening of the voiding interval with age means that any underestimation (and, therefore, overestimation of detrusor contribution to expulsive effort) is more pronounced in younger animals than Old animals. The relative rapidity of the flow phase suggests that the impact of an assumption of pressure-time linearity on the results is minimal.
Second, our chosen indices of contractility and central sensitivity to afferent outflow are not validated instruments. Maximum bladder pressure (Pmax) is typically reported in rodent cystometry as a measure of “contractility.” However, it does not isolate detrusor expulsive pressure. Furthermore, since the attainment of Pmax marks flow onset and, therefore, the relationship of pressure head to urethral resistance, it does not necessarily express the maximum isovolumetric pressure. The use of PTI as the basis for assessing contractility is based on the linear relationship of external work to PTI, independent of preload (analogous to bladder volume), but varying inversely with afterload (analogous to urethral resistance to flow) (50). Therefore, direct comparison of PTI between groups requires the assumption that urethral flow dynamics are consistent between groups. The observed greater flow rates with similar pressures in older animals suggest that if this assumption is not valid, then the urethral resistance function is likely lower in older animals. Therefore, the work vs. PTI slope would be greater, and thus, the similar value of the measured PTI in older animals would reflect more external work.
Our estimation of system sensitivity to volume based on the stress function at Pthresh may underestimate the effect. For the stress function calculations, we assumed that PVR was minimal in all groups. If this is not true, it is quite likely that PVR is greater in the Old groups, as suggested by the standard perception of aging and supported by human studies (34). If PVR is greater in Old/Oldest-old mice, volume at Pthresh would be even greater, further increasing the stress function, and indicating an even greater loss of either afferent outflow as a function of wall stress and/or central sensitivity to afferent outflow. Thus, while our methodology does not directly assess afferent outflow and central sensitivity, wall stress approximations (via the pressure-volume product) strongly suggest an age-associated diminished central sensitivity during filling, assuming a constant slope of afferent outflow vs. wall stress. However, the effect of aging on the transduction of wall stress to afferent outflow is not known, and therefore, the observed effects may be peripherally and/or centrally mediated. We acknowledge that our results are suggestive, not conclusive, but serve as the basis for stimulating future investigations of the effect of aging on bladder afferent outflow and central receptivity.
The consistency of all measures supports our interpretation that in vivo contractility increases as a function of maturation and that this parameter does not decline with aging. Conversely, our data indirectly suggest that aging is associated with diminished volume sensitivity. The finding of decreased resilience in aged animals is consistent with the human concept of homeostenosis, in which the aging process is associated with diminishing abilities to cope with homeostatic stressors rather than organ failure per se (29).
Perspectives and Significance
The principle finding of this study is that aging is associated with impaired resilience, i.e., homeostenosis; however, if responsiveness to bladder filling is preserved, there is no in vivo degradation of detrusor voiding function. Functional detrusor contractility is not degraded with age. Secondarily, system responsiveness to bladder volume diminishes, as assessed by traditional measures of intervoid interval and compliance, and by surrogates for direct measures of the mechanotransduction process. The latter suggests that either the local mechanotransduction process is impaired (via diminished afferent activity per unit bladder wall stress) and/or decreased central responsiveness to transmitted afferent activity.
The reported effect of aging on in vitro detrusor muscle strip tension-generating capabilities has been variable, suggesting contractility is diminished or does not change (41, 46–48, 60). Furthermore, compliance is actively enhanced during bladder filling (52). Resolving our findings with previous work suggests two possibilities that are not mutually exclusive. First, in vivo voiding contractility may not represent the maximum contractile capabilities of the detrusor, i.e., a functional buffer exists that accommodates detrusor aging. Second, compliance may be actively enhanced to delay the voiding threshold. Assuming detrusor smooth muscle demonstrates a Frank-Starling-type relationship of active tension and length, allowing greater bladder volumes at voiding initiation provides accommodation of an age-displaced active tension vs. length curve of isolated muscle strips. This would be consistent with the observed age-related increase in postvoid residual volumes in previous animal and human studies.
Follow-up of the current study requires at least three lines of investigation. First, in vitro tension-length studies across age groups will resolve the impact of aging on the maximum contractile potential and its dependence upon strip length (and by implication bladder volume). Second, direct measurement of bladder afferent activity across age groups is needed to begin the evaluation of the impact of aging on the mechanotransduction process. Finally, study of the impact of aging on the active regulation of bladder filling would provide insight into the role of central regulation of the bladder function. By focusing on the determinants of system responsiveness rather than end-organ performance, we anticipate that completion of these studies will contribute to an integrated model of bladder filling and voiding functions.
GRANTS
Research was funded by Dennis A. Jahnigen Scholars Career Development Award, American Geriatrics Society (to P. P. Smith, principal investigator), and National Institutes of Health R01AG028657 (to G. A. Kuchel, principal investigator).
DISCLOSURES
P. P. Smith is a consultant for Astellas Pharmaceuticals. A. DeAngelis and G. A. Kuchel have no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Author contributions: P.P.S. and G.A.K. conception and design of research; P.P.S. and A.D. performed experiments; P.P.S. analyzed data; P.P.S., A.D., and G.A.K. interpreted results of experiments; P.P.S. prepared figures; P.P.S. drafted manuscript; P.P.S. and A.D. edited and revised manuscript; P.P.S., A.D., and G.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Richard Feinn (biostatistician, University of Connecticut Health Center) for his review of our data and analysis.
REFERENCES
- 1. Andersson KE. Detrusor underactivity/underactive bladder: new research initiatives needed. J Urol 184: 1829–1830, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology 62: 3–10, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Asplund R. Nocturia, nocturnal polyuria, and sleep quality in the elderly. J Psychosom Res 56: 517–525, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Baltes PB, Smith J. New frontiers in the future of aging: from successful aging of the young old to the dilemmas of the fourth age. Gerontology 49: 123–135, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci 69: 1193–1202, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187: 445–454, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Chun AL, Wallace LJ, Gerald MC, Levin RM, Wein AJ. Effect of age on in vivo urinary bladder function in the rat. J Urol 139: 625–627, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Chun AL, Wallace LJ, Gerald MC, Wein AJ, Levin RM. Effects of age on urinary bladder function in the male rat. J Urol 141: 170–173, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Coleman P, Finch C, Joseph J. The need for multiple time points in aging studies. Neurobiol Aging 25: 3–4, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Coolsaet BL. Bladder compliance and detrusor activity during the collection phase. Neurourol Urodyn 4: 263–273, 1985 [Google Scholar]
- 12. Coolsaet BL, van Mastrigt R, van Duyl WA, van Rees Vellinga F. Some influences of the contractile element on the visco-elastic properties of bladder wall strips. Eur Urol 4: 450–456, 1978 [DOI] [PubMed] [Google Scholar]
- 13. Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 101: 1388–1395, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Crews DE, Zavotka S. Aging, disability, and frailty: Implications for universal design. J Physiol Anthropol 25: 113–118, 2006 [PubMed] [Google Scholar]
- 15. Cruz Y, Downie JW. Abdominal muscle activity during voiding in female rats with normal or irritated bladder. Am J Physiol Regul Integr Comp Physiol 290: R1436–R1445, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Elbadawi A, Diokno AC, Millard RJ. The aging bladder: morphology and urodynamics. World J Urol 16 Suppl 1: S10–S34, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Fry CH, Daneshgari F, Thor K, Drake M, Eccles R, Kanai AJ, Birder LA. Animal models and their use in understanding lower urinary tract dysfunction. Neurourol Urodyn 29: 603–608, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Garfein AJ, Herzog AR. Robust aging among the young-old, old-old, and oldest-old. J Gerontol B Psychol Sci Soc Sci 50: S77–S87, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Gillespie J, van Koeveringe G, de Wachter S, de Vente J. On the origins of the sensory output from the bladder: the concept of afferent noise. BJU Int 103: 1324–1333, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Aging impairs neurogenic contraction in guinea pig urinary bladder: role of oxidative stress and melatonin. Am J Physiol Regul Integr Comp Physiol 293: R793–R803, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Gosling JA. Modification of bladder structure in response to outflow obstruction and ageing. Eur Urol 32 Suppl 1: 9–14, 1997 [PubMed] [Google Scholar]
- 22. Griffiths DJ. Assessment of detrusor contraction strength or contractility. Neurourol Urodyn 10: 1–18, 1991 [Google Scholar]
- 23. Griffiths DJ. Urodynamics: The Mechanics and Hydrodynamics of the Lower Urinary Tract.Bristol, UK: Adam Hilger, 1980 [Google Scholar]
- 24. Griffiths DJ, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the lower urinary tract: how age-related changes might predispose to urge incontinence. NeuroImage 47: 981–986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94: 313–318, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc London, Ser B, Biol Sci 126: 136–195, 1938 [Google Scholar]
- 27. Jung SY, Fraser MO, Ozawa H, Yokoyama O, Yoshiyama M, De Groat WC, Chancellor MB. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol 162: 204–212, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Kohan AD, Danziger M, Vaughan ED, Jr, Felsen D. Effect of aging on bladder function and the response to outlet obstruction in female rats. Urol Res 28: 33–37, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Kuchel GA. Aging and Homeostatic Regulation. In: Hazzard's Principles of Geriatric Medicine and Gerontology, (6th ed.), edited by Halter JB, Hazzard W, Ouslander JG, Tinetti M, Woolard N, Studenski S, High K, Asthana S. New York, NY: McGraw-Hill, 2009 [Google Scholar]
- 30. Le Feber J, Van Asselt E, Van Mastrigt R. Afferent bladder nerve activity in the rat: a mechanism for starting and stopping voiding contractions. Urol Res 32: 395–405, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Le Feber J, Van Asselt E, Van Mastrigt R. Neurophysiological modeling of voiding in rats: bladder pressure and postganglionic bladder nerve activity. Am J Physiol Regul Integr Comp Physiol 272: R413–R421, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Lieu PK, Sa'adu A, Orugun EO, Malone-Lee JG. The influence of age on isometric and isotonic rat detrusor contractions. J Gerontol 52: M94–M96, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, Corman B, Martin DJ. Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol 278: R964–R972, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Madersbacher S, Pycha A, Schatzl G, Mian C, Klingler CH, Marberger M. The aging lower urinary tract: a comparative urodynamic study of men and women. Urology 51: 206–212, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia 42: 531–537, 1986 [DOI] [PubMed] [Google Scholar]
- 36. Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods 15: 157–167, 1986 [DOI] [PubMed] [Google Scholar]
- 37. Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn 19: 87–99, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Miller RA, Nadon NL. Principles of Animal Use for Gerontological Research. New York, NY: http://www.afar.org/animal.html 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neugarten BL. Age groups in American society and the rise of the young-old. Ann Am Acad Polit Soc Sci 9: 287–298, 1974 [Google Scholar]
- 40. Nordling J. The aging bladder—a significant but underestimated role in the development of lower urinary tract symptoms. Exp Gerontol 37: 991–999, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Pagala MK, Tetsoti L, Nagpal D, Wise GJ. Aging effects on contractility of longitudinal and circular detrusor and trigone of rat bladder. J Urol 166: 721–727, 2001 [PubMed] [Google Scholar]
- 42. Pandita RK, Nylen A, Andersson KE. Oxytocin-induced stimulation and inhibition of bladder activity in normal, conscious rats—influence of nitric oxide synthase inhibition. Neuroscience 85: 1113–1119, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn 25: 388–396, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Peng CW, Chen JJ, Cheng CL, Grill WM. Improved bladder emptying in urinary retention by electrical stimulation of pudendal afferents. J Neural Eng 5: 144–154, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM. The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc 54: 405–412, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Saito M, Gotoh M, Kato K, Kondo A. Influence of aging on the rat urinary bladder function. Urol Int 47 Suppl 1: 39–42, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Saito M, Kondo A, Gotoh M, Kato K. Age-related changes in the rat detrusor muscle: the contractile response to inorganic ions. J Urol 146: 891–894, 1991 [DOI] [PubMed] [Google Scholar]
- 48. Saito M, Ohmura M, Kondo A. Effect of ageing on blood flow to the bladder and bladder function. Urol Int 62: 93–98, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Schäfer W. Analysis of active detrusor function during voiding with the bladder working function. Neurourol Urodyn 10: 19–35, 1991 [Google Scholar]
- 50. Sela G, Landesberg A. The external work-pressure time integral relationships and the afterload dependence of Frank-Starling mechanism. J Mol Cell Cardiol 47: 544–551, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Smith PP. Aging and the underactive detrusor: A failure of activity or activation? Neurourol Urodyn 29: 408–412, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Smith PP, DeAngelis A, Kuchel GA. Evidence of central modulation of bladder compliance during filling phase. Neurourol Urodyn In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn 29: 1344–1349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith PP, Smith CP, Boone TB, Somogyi GT. Is abdominal wall contraction important for normal voiding in the female rat? BMC Urol 7: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Streng T, Hedlund P, Talo A, Andersson KE, Gillespie JI. Phasic non-micturition contractions in the bladder of the anaesthetized and awake rat. BJU Int 97: 1094–1101, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Streng T, Santti R, Talo A. Similarities and differences in female and male rat voiding. Neurourol Urodyn 21: 136–141, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Taylor JA, 3rd, Kuchel GA. Detrusor underactivity: Clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc 54: 1920–1932, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol Ser A: Biol Sci Med Sci 54: B492–B501, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Velasco C, Guarneri L, Leonardi A, Testa R. Effects of intravenous and infravesical administration of suramin, terazosin and BMY 7378 on bladder instability in conscious rats with bladder outlet obstruction. BJU Int 92: 131–136, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Wuest M, Morgenstern K, Graf EM, Braeter M, Hakenberg OW, Wirth MP, Ravens U. Cholinergic and purinergic responses in isolated human detrusor in relation to age. J Urol 173: 2182–2189, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Wuest M, Morgenstern K, Graf EM, Braeter M, Hakenberg OW, Wirth MP, Ravens U. Cholinergic and purinergic responses in isolated human detrusor in relation to age. J Urol 173: 2182–2189, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Zhao W, Aboushwareb T, Turner C, Mathis C, Bennett C, Sonntag WE, Andersson KE, Christ G. Impaired bladder function in aging male rats. J Urol 184: 378–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]