Abstract
Chronic hypoxia causes pulmonary hypertension with vascular remodeling, increase in vascular tone, and altered reactivity to agonists. These changes involve alterations in multiple Ca2+ pathways in pulmonary arterial smooth muscle cells (PASMCs). We have previously shown that vanilloid (TRPV)- and melastatin-related transient receptor potential (TRPM) channels are expressed in pulmonary arteries (PAs). Here we found that TRPV4 was the only member of the TRPV and TRPM subfamilies upregulated in PAs of chronic hypoxic rats. The increase in TRPV4 expression occurred within 1 day of hypoxia exposure, indicative of an early hypoxic response. TRPV4 in PASMCs were found to be mechanosensitive. Osmo-mechanical stress imposed by hypotonic solution activated Ca2+ transients; they were inhibited by TRPV4 specific short interfering RNA, the TRPV blocker ruthenium red, and the cytochrome P450 epoxygenase inhibitor N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide. Consistent with TRPV4 upregulation, the Ca2+ response induced by the TRPV4 agonist 4α-phorbol 12,13-didecanoate and hypotonicity was potentiated in hypoxic PASMCs. Moreover, a significant myogenic tone, sensitive to ruthenium red, was observed in pressurized endothelium denuded small PAs of hypoxic but not normoxic rats. The elevated basal intracellular Ca2+ concentration in hypoxic PASMCs was also reduced by ruthenium red. In extension of these results, the development of pulmonary hypertension, right heart hypertrophy, and vascular remodeling was significantly delayed and suppressed in hypoxic trpv4−/− mice. These results suggest the novel concept that TRPV4 serves as a signal pathway crucial for the development of hypoxia-induced pulmonary hypertension. Its upregulation may provide a pathogenic feed-forward mechanism that promotes pulmonary hypertension via facilitated Ca2+ influx, subsequently enhanced myogenic tone and vascular remodeling.
Keywords: transient receptor potential vanilloid 4 channels, trpv4 knockout mice
acute reduction in alveolar O2 causes hypoxic pulmonary vasoconstriction, and prolonged exposure to hypoxia eventually leads to pulmonary vascular remodeling and pulmonary arterial hypertension. The pathogenesis of chronic hypoxic pulmonary hypertension is complex. Multiple mechanisms including O2-sensitive transcription factors (67), endothelium-derived factors (7, 55), circulating hormones/growth factors and their receptors (21, 23, 30), extracellular matrix (71), as well as reactive oxygen species (27) are all critically involved. Moreover, substantial evidence indicates that chronic hypoxia causes intrinsic changes in ionic balance and Ca2+ homeostasis in pulmonary arterial smooth muscle cells (PASMCs), leading to membrane depolarization, elevation of resting intracellular Ca2+ concentration ([Ca2+]i), and changes in electrophysiological and Ca2+ responses to vasoconstrictors and vasodilators (26, 41, 49–51, 53). These functional changes of PASMCs involve alterations in multiple Ca2+ pathways (45, 52), and they are crucial for the development of pulmonary hypertension.
The transient receptor potential (TRP) gene superfamily, which consists of three major subfamilies of canonical (TRPC), melastatin-related (TRPM), and vanilloid-related (TRPV) channels, and four lesser subfamilies, is known to encode a large repertoire of nonselective cation channels (33). To date, ≥10 TRP channels have been identified in vascular smooth muscle cells (VSMCs) and implicated in various vascular functions (2). In search for Ca2+ pathways participating in the development of chronic hypoxic pulmonary hypertension, we (26) have previously characterized TRPC channels in rat intralobar PASMCs and provided the first evidence that chronic hypoxia upregulates the store-operated TRPC1 and the receptor-operated TRPC6 expression in rat pulmonary arteries (PAs). Moreover, the enhanced store-operated Ca2+ entry was found to be responsible for the increase in resting [Ca2+]i and basal tone of PAs of hypoxic pulmonary hypertensive rats. A subsequent study by others (60) showed that the upregulation of TRPC1 and TRPC6 is the direct effect of hypoxia on PASMCs and requires the full expression of hypoxia inducible factor (HIF-1α). In addition, idiopathic pulmonary arterial hypertension has been shown to be associated with the overexpression of TRPC6 and TRPC3 in PASMCs, and inhibition of TRPC6 expression markedly attenuated proliferation of PASMCs from these patients (68). Interestingly, a functional single-nucleotide polymorphism in the TRPC6 gene promoter region was identified in patients with idiopathic pulmonary arterial hypertension, and was shown to enhance NF-κB-mediated promoter activity and stimulated TRPC6 expression in PASMCs (69). These studies revealed a critical involvement of TRPC channels in pulmonary hypertension.
We have further extended our efforts to identify TRPV and TRPM channels in rat PAs (65). Multiple channel transcripts including TRPV1, TRPV2, TRPV4, TRPM2, TRPM3, TRPM4, TRPM7, and TRPM8 were detected, with TRPV4 and TRPM8 being the most abundantly expressed TRPV and TRPM subtypes, respectively. Moreover, these channels constitute functional Ca2+ entry pathways in PASMC, inasmuch as the TRPV4 agonist 4α-phorbol 12,13-didecanoate (4α-PDD) and the TRPM8 agonist menthol elicited a significant increase in [Ca2+]i. Previous studies have indicated that several TRPM and TRPV channels play important physiological roles in the systemic circulation. It has been proposed that TRPM4 operates as a stretch-activated cation channel to mediate myogenic responses in isolated cerebral arteries and in cerebral arteries of intact animals (10, 34, 44). TRPM7 was identified as a functional regulator of Mg2+ homeostasis in mesenteric and aortic smooth muscle cells (17, 37, 57). Its expression and activity are modulated by angiotensin II and shear stress and are related to smooth muscle cell proliferation and vascular remodeling in hypertension and vascular injury. Furthermore, endothelial TRPV4 mediates Ca2+-dependent release of nitric oxide and endothelium-derived hyperpolarizing factors (EDHFs; Refs. 22, 32), which in turn can activate the TRPV4 channel of smooth muscle to cause membrane hyperpolarization and vasodilation through the activation of Ca2+ sparks and Ca2+-dependent-K+ channels (9).
Besides these studies in systemic VSMCs, the physiological functions and pathophysiological roles of TRPV and TRPM channels in PASMCs have not been investigated. In the present study, we compared the expression of TRPV and TRPM channels in PAs of normoxic and chronic hypoxic rats. We found that the TRPV4 is the only channel upregulated by chronic hypoxia. We further investigated their physiological functions in PASMCs and their roles in the pathogenesis of hypoxic pulmonary hypertension. Our results suggest that TRPV4 are osmo-mechanosensitive channels, which may serve as a signal transduction mechanism for the elevated pulmonary arterial pressure (Ppa) in hypoxia, and its upregulation may contribute to the enhanced vascular tone and vascular remodeling in hypoxic pulmonary hypertension.
MATERIALS AND METHODS
Rats and mouse models of chronic hypoxia-induced pulmonary hypertension.
Male Wistar rats (150 to 250 g), TRPV4 null (trpv4−/−) mice, and age-matched wild-type mice (C57BL6/J; 8–10 wk old) were used as the experimental animals. The generation of trpv4−/−mice has been previously described (25). They were placed in a hypoxic chamber and exposed to either normoxia or normobaric 10% O2 for 4 wk to induce hypoxic pulmonary hypertension as described previously (26). Animals were anesthetized with sodium pentobarbital (50 mg/kg ip). Pulmonary hypertension was evaluated in both models by measuring right ventricular (RV) systolic pressure (RVSP) and RV hypertrophy. RV pressure was measured with a Mikro-Tip pressure catheter (SPR-671; Millar Instruments) approached through cannulation of the right jugular vein. Mixed venous blood of mice was collected from the jungular vein or punctured vena cava with a heparizined micro-hematocrit capillary tube, and hematocrit was measured after centrifugation with a Damon IEC MB Centrifuge. Heart and lung were then removed after exsanguination. RV was separated from the left ventricle and septum (LV + S). The mass ratio of RV/(LV + S) was determined. All animal procedures were performed in accordance with the guidelines and approved by the Johns Hopkins Animal Care and Use Committee.
Isolation and transient culture of PASMCs.
PASMCs were enzymatically isolated and transiently cultured as previously described (65). In brief, intralobar PAs (300–800 μm) were isolated, and the endothelium was removed by gently rubbing the luminal surface with a cotton swab. Deendothelialized PAs were allowed to recover for 30 min in cold (4°C) HBSS, followed by 20 min in reduced-Ca2+ (20 μM) HBSS at room temperature. The tissue was digested at 37°C for 20 min in 20 μM Ca2+ HBSS containing collagenase (type I; 1,750 U/ml), papain (9.5 U/ml), BSA (2 mg/ml), and dithiothreitol (1 mM) and then washed with Ca2+-free HBSS to stop digestion. PASMCs were dispersed gently by trituration with a small-bore pipette in Ca2+-free HBSS at room temperature. Cells were then placed on 25-mm glass coverslips. PASMCs from chronic hypoxic and normoxic animals were cultured transiently (16–24 h) in Ham's F-12 medium (with l-glutamine) supplemented with 0.5% FCS, 100 U/ml streptomycin, and 0.1 mg/ml penicillin inside a modular incubator chamber (Billups-Rothenberg) under 4% O2-5% CO2 and 21% O2-5% CO2, respectively, before use. The 4% O2 was used for providing a hypoxic environment for the transient culture of PASMCs of chronic hypoxic rats. It has been tested that transient culture of PASMCs for 24 h under this condition did not alter the expression of TRPV4 and TRPC1 channel proteins, as well as 4α-PDD and thapsigargin-induced store-operated Ca2+ entry.
RNA preparation and quantitative real-time RT-PCR.
Deendothelialized PAs frozen in liquid nitrogen were mechanically homogenized. Total RNA was extracted using RNeasy mini kit (Qiagen, Valencia, CA). Genomic DNA contamination was removed by TURBO DNA-free DNase (Ambion, Austin, TX). An aliquot of total RNA (0.5 μg) were used for first-strand cDNA synthesis using random hexamer primers and Superscript Ш RNase H− reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Quantitative real-time RT-PCR was used to quantify the changes in the expression of TRPV and TRPM subtypes. PCR reactions were set up with iQ SYBR Green PCR Supermix (Bio-Rad, Hercules, CA), using 1 μl of cDNA as the template in each 20-μl reaction mixture. Gene-specific real-time PCR primers for TRPV and TRPM subtypes were described previously (65). PCR protocol, consisting of an initial step at 95°C for 5 min, followed by 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 1 min, was performed using the iQ5 Multicolor real-time PCR detection system (Bio-Rad, Hercules, CA). Standard curves were generated from serial dilutions of purified PCR products of known copy number. Data were normalized with the amount of 18S rRNA in individual samples to correct for sample variability. Conventional RT-PCR for TRPV4 and TRPV2 was also performed in some experiments using primers and protocols described previously (65).
Western blotting.
PAs frozen in liquid nitrogen were crushed and homogenized using a mortar and pestle and resuspended in ice-cold lysis buffer containing 50 mM Tris·Cl (pH 7.4), 150 mM NaCl, 1% deoxycholic acid, 0.1% SDS, 0.5% NP-40, and protease inhibitor cocktail (Roche, Mannheim, Germany). The homogenate was centrifuged at 4°C with 1,000 g for 5 min, the supernatant was collected, and the protein concentration was estimated using the BCA assay. Protein samples of 20 µg were resolved in an 8% SDS-PAGE gel and electrotransferred onto a PVDF membrane (Millipore, Bedford, MA). The membrane was blocked with 5% (wt/vol) nonfat dry milk in PBS containing 0.02% Tween 20 (PBST) for 1 h at room temperature, and followed by incubation at 4°C overnight with a specific primary antibody. The primary antibodies were polyclonal rabbit anti-TRPV2 (1:1,000 dilution) and anti-TRPM8 (1:500 dilution) from Abcam (Cambridge, MA) and anti-TRPV4 (1:500 dilution) from Alomone (Jerusalem, Israel). β-Actin was used as loading control. After being washed, the membrane was incubated with peroxidase-conjugated goat-anti-rabbit secondary antibody (Bio-Rad, Hercules, CA) at 1:3,000 dilution at room temperature for an hour. After wash, protein bands were detected with enhanced chemiluminescence (Pierce, Rockford, IL) and imaged by a Gel Logic 200 image system (Kodak, New Haven, CT).
Measurement of intracellular [Ca2+]i.
[Ca2+]i was monitored using the membrane-permeable Ca2+-sensitive fluorescent dye fluo 3-AM. PASMCs were loaded with 5–10 μM fluo 3-AM (dissolved in DMSO with 20% pluronic acid) for 45 min at room temperature (∼23°C) in normal Tyrode solution containing the following (in mM): 137 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.4 (adjusted with NaOH). Cells were then washed and rested for 15–30 min to allow for complete deesterification of cytosolic dye. Fluo-3 was excited at 488 nm, and emission light at >515 nm was detected using a Nikon Diaphot microscope equipped with a microfluorometer. Protocols were executed and data collected online with a Digidata analog-to-digital interface and a pClamp software package (Axon Instruments, Foster City, CA). [Ca2+]i was calibrated using the equation [Ca2+]i = KD·(F − Fbg)/(Fmax − F), where Fbg was background fluorescence and Fmax was the maximum fluorescence determined in situ in cell superfused with 10 μM 4-B-Bromo A-23187. For the hypotonicity experiments, PASMCs were first equilibrated in modified Tyrode solutions with half of NaCl replaced with equi-osmol of mannitol. Hypotonicity-induced response was initiated by removal of mannitol from the external solution. All the experiments were conducted under normoxic condition at room temperature.
Mn2+ quenching of fura-2.
Rate of Ca2+ entry was quantified by quenching of fura-2 with Mn2+. PASMCs were loaded with fura-2 AM as described above. Fura-2 was excited at the isosbestic point (360 nm), and emission light was recorded at >510 nm. PASMCs were then bathed in a Ca2+-free (with 0.1 mM EGTA) nifedipine (1 μM) containing Tyrode solution. After a stable baseline fluorescence was attained, 4α-PDD was given to PASMCs for 15 min before 500 μM Mn2+ was applied through a multibarrel pipette positioned <50 μm from PASMCs. The rates of quenching of fura-2 fluorescence in PASMCs with/without treatments were determined.
Short interfering RNA knockdown of TRPV4.
PASMCs were isolated and seeded onto coverslips in 12-well cell culture plate and cultured for ∼24 h to 80% confluence. short interfering (si)RNA against TRPV4 (5′-CGUCCAAACCUGCGUAUGAUU-3′) or scrambled control oligonucleotide (5′-UUCUCCGAACGUGUCACGU-3′) synthesized by Dharmacon (Lafayette, CO) was transfected into PASMCs using Geneporter2 transfection reagent (Genlantis, San Diego, CA) according to the manufacturer's instructions. Then, 3.5 μg of siRNA were mixed with 25 μl of DNA dilution B solution and 5 μl of Geneporter2 transfection reagent was diluted into 20 μl of serum-free Ham's F-12 medium. The two solutions were then combined and incubated for 10 min at room temperature to allow complex formation. PASMCs were washed twice, and 50 μl of siRNA/Geneporter2 complexes were added to each well together with 650 μl of fresh Ham's F-12 medium without serum or antibiotics. The cells were incubated with the complexes for 4–6 h (37°C, 5% CO2), and 700 μl of Ham's F-12 containing 2% FBS were added for further incubation for 12 h. The medium was then changed, and the cells were cultured in growth medium for 36 h before use for experiments.
Analysis of vasomotor tone in pressurized microvessels.
Small branches of PAs (100–200 μm internal diameter) were dissected in ice-cold Krebs-Ringer bicarbonate solution containing the following (in mmol/l): 118.3 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 25.0 NaHCO3, and 11.1 glucose and transferred to a vessel chamber containing the same solution. The proximal end of the artery was cannulated with a tapered glass pipette, secured, and gently flushed to remove any blood from the lumen. The arterial lumen was rubbed with a strand of moose mane to disrupt the endothelium before the distal end of the artery was cannulated. The artery was stretched longitudinally to approximate its in situ length and pressurized to 15 mmHg with a servo-controlled peristaltic pump (Living Systems Instrumentation, Burlington, VT). Any arteries with apparent leaks were discarded. The chamber was superfused with Krebs-Ringer solution at 37°C, pH 7.4 (gassed with 16% O2-5% CO2), and placed on the stage of an inverted microscope (×20, Nikon TS-100) connected to a video camera (Sony, CCTV camera) and a video monitor. The internal diameter of the vessel was determined continuously by a video dimension analyzer (Living Systems) and recorded using a BIOPAC (Santa Barbara, CA) data-acquisition system. The effectiveness of endothelial disruption was verified by the lack of a vasodilatory response to acetylcholine (1 μM) in arteries constricted with U46619 (10–100 nM). Arteries were exposed to a series of 10-mmHg pressure steps (3 min each) beginning at 5 mmHg and ending at 55 mmHg to examine myogenic tone. Paired experiments were conducted in the absence or presence of ruthenium red (3 μM). To determine the passive diameter at each pressure step, a pressure-diameter curve was recorded after incubation (30 min) of the arteries with Ca2+-free control solution (containing 3 mM EGTA). Pressure-diameter curves were also obtained after incubation (15 min) of the arteries with Ca2+-free control solution containing 10 μM papaverine. Myogenic tone was calculated as the percent difference in internal diameter observed for Ca2+-containing vs. Ca2+-free Krebs plus papaverine at each pressure for all groups.
Pulmonary vascular morphometry.
Mice were exsanguinated after deep anesthesia with pentobarbital sodium (130 mg/kg ip). The trachea was cannulated and the lungs were inflated to 20 mmHg by injection of 0.5% UltraPure low melting point agarose PBS solution (gelling temperature of 24–28°C; Invitrogen). The inflated lungs were cooled on ice for 10 min, cut into smaller pieces, and fixed with 4% formaldehyde at 4°C. The fixed lungs were embedded in paraffin, sectioned into 5-μm slices at levels parallel to, but away from, the plane of the hilum to avoid the large vessels. The lung sections were stained with hematoxylin and eosin for gross morphology or with a rabbit polyclonal antibody against smooth muscle α-actin (AB15267; Abcam, Cambridge, MA) to identify muscularized pulmonary arteries. Lung sections were examined under an Olympus BX51 microscope, and images were taken with an Olympus Q-color 5 digital camera and the Qcapture software (Qimaging, Surrey, Canada) and analyzed with the SimplePCI software (Compix, Cranberry, PA). Vessels of internal diameter <100 μm were classified into nonmuscularized, partially muscularized (25–75%), and completely muscularized (>75%) vessels. Approximately 300 vessels were scored in multiple lung sections from three to four animals in each group. Percent wall thickness (%WT) was quantified for completely muscularized vessels based on area or diameter according to Yu et al. (67): %WT = [(AREAexternal − AREAinternal)/AREAexternal] × 100%, and %WT = [(diameterexternal − diameterinternal)/diameterexternal] × 100%. Dimensions were demarcated by the external and internal perimeters of the α-actin-stained smooth muscle layer. Frequency distributions of the diameter of muscularized microvessels were generated and compared.
Statistical analysis.
All data are expressed as means ± SE. The numbers of animals, tissues, preparations, and cells are specified in the text. Statistical significance (P < 0.05) of the changes was assessed by paired or unpaired Student's t-tests, the nonparametric Mann-Whitney U-tests, or by one- or two-way ANOVA with Tukey's range test for post hoc analysis, wherever applicable.
RESULTS
Chronic hypoxia upregulates TRPV4 expression in PASMCs.
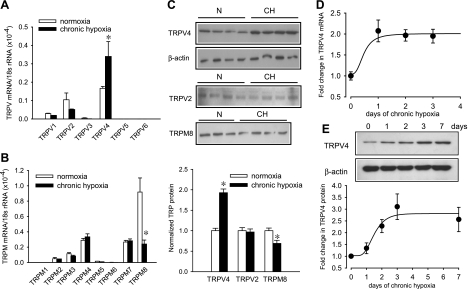
Pulmonary hypertension developed in rats after exposure to 10% O2 for 4 wk, as indicated by a significant increase in RVSP (normoxic rats: 23.6 ± 1.4 mmHg, n = 11; hypoxic rats: 64.4 ± 4.4 mmHg, n = 13; P < 0.001) and RV hypertrophy [RV/(LV + S): 0.284 ± 0.005 in normoxic (n = 31) and 0.595 ± 0.013 in hypoxic rats (n = 31); P < 0.001]. Expression of TRPV and TRPM channels in endothelium-denuded intralobar PAs of normoxic and chronic hypoxic rats was quantified by real-time RT-PCR. TRPV4 mRNA level was significantly increased by approximately double, whereas TRPM8 mRNA was reduced by 70%, and all other TRPV and TRPM channels were unchanged in hypoxic PAs (Fig. 1, A and B). Consistent with the mRNA data, Western blot analysis showed that TRPV4 protein in chronic hypoxic PAs was increased by 92 ± 9% (n = 8; P < 0.001), while TRPM8 was decreased by 31 ± 8% (n = 8; P < 0.01) and TRPV2 was unaltered (Fig. 1C). The increase of TRPV4 transcript was detected within 24 h, and the increase in protein was observed in the second day of hypoxia exposure (Fig. 1, D and E), suggesting that TRPV4 upregulation is an early response to hypoxia.
Fig. 1.
Effect of chronic hypoxia on the expression of vanilloid (TRPV)- and melastatin-related transient receptor potential (TRPM) channel mRNA and protein in rat deendothelialized intralobar pulmonary arteries (PAs). A and B: real-time RT-PCR analysis of the relative expression of TRPV and TRPM subtypes mRNA in intralobar PAs of normoxic and 4-wk hypoxic rat (n = 5 animals for each group). Data are expressed as percent normalized to 18S rRNA. C: Western blot analysis of TRPV2, TRPV4, and TRPM8 proteins in PAs of normoxic (N) and hypoxic rats (CH; n = 8). D and E: time-course of change in TRPV4 mRNA and protein expression induced by hypoxia, respectively (n = 5 animals for each time point). *P < 0.05, significant difference from control.
TRPV4 as a mechanosensitive Ca2+ entry pathway in PASMCs.
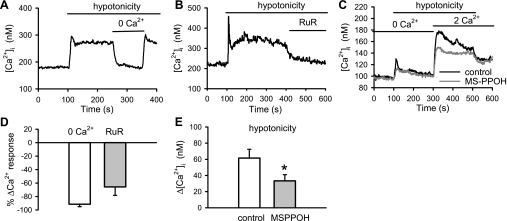
Previous studies suggest that TRPV4 operates as an osmo-mechanosensitive cation channel in many cell types. As the first step to elucidate the physiological functions of TRPV4 in pulmonary vasculature, we examined the effects of hypotonic challenge, a surrogate for mechanical stretch, on the Ca2+ response of PASMCs. In the presence of nifedipine (1 μM), lowering the osmolarity of extracellular solution from 320 to 186 mosmol activated an initial increase followed by a sustained rise in [Ca2+]i (Fig. 2A). The Ca2+ response was completely reversed by the removal of extracellular Ca2+ (baseline: 178 ± 19 nM; 2 mM Ca2+ hypotonic solution: 279 ± 19 nM, P < 0.001; Ca2+-free-hypotonic solution: 188 ± 19 nM, n = 15, P < 0.001) and was inhibited significantly by the TRPV channel antagonist ruthenium red (ruthenium red, 3 μM, n = 11, P < 0.001; Fig. 2, A, B, and D). Hypotonicity-induced Ca2+ entry was further examined using the Ca2+ add-back protocol. Application of Ca2+-free hypotonic solution elicited a small Ca2+ release in Ca2+-free solution. Reintroduction of 2 mM Ca2+ in the presence of nifedipine (1 μM) elicited a Ca2+ transient, which was significantly reduced by the cytochrome P450 epoxygenase inhibitor N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide (MS-PPOH; 30 μM, P < 0.01; Fig. 2, C and E). These results were consistent with previous reports (58, 59, 61) that osmo-mechanical stimuli activate TRPV4 through the generation of arachidonic acid metabolite epoxyeicosatrienoic acids by cytochrome P450 epoxygenase.
Fig. 2.
Ca2+ response induced by hypotonic solution on intracellular Ca2+ concentration ([Ca2+]i) in rat pulmonary arterial smooth muscle cells (PASMCs). Effects of Ca2+-free solution (A) and the TRPV channel antagonist ruthenium red (RuR; 3 μM; B) on the Ca2+ transients induced by hypotonic solution on [Ca2+]i in rat PASMCs. C: effect of N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide (MS-PPOH; 30 μM) on Ca2+ influx induced by hypotonic solution. D: mean percent change in hypotonic solution-induced Ca2+ response after removal of Ca2+ (n = 15 cells) and application of RuR (n = 11 cells). E: average change in [Ca2+]i (Δ[Ca2+]i) induced by hypotonic solution in the presence (n = 17) or absence (n = 16) of MS-PPOH. Experiments were conducted in the presence of 1 μM nifedipine. *P < 0.05, significant difference from control.
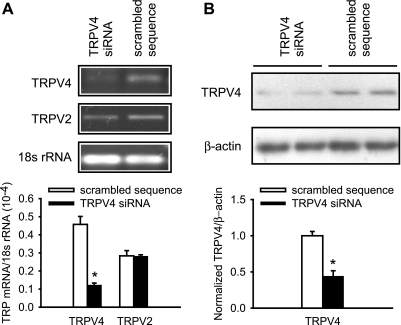
To examine whether TRPV4 indeed mediates the hypotonicity-induced Ca2+ entry in PASMCs, TRPV4 expression was inhibited specifically using siRNA (Fig. 3, A and B). Transfection of normoxic PASMCs with siRNA against TRPV4 resulted in a 75% reduction in TRPV4 mRNA and 56% reduction in protein expression, compared with PASMC transfected with a nonsilencing random sequence. Expression of the closely related TRPV2 mRNA was unaltered, confirming that the siRNA-mediated gene knockdown was specific. Moreover, there was no change in mRNA levels of TRPM4 (scrambled sequence: 0.50 ± 0.04, n = 8; siRNA: 0.47 ± 0.03, n = 8), TRPC1 (scrambled sequence: 0.12 ± 0.01, n = 8; siRNA: 0.13 ± 0.01, n = 8), and TRPC6 (scrambled sequence: 0.07 ± 0.02, n = 8; siRNA: 0.07 ± 0.01, n = 8), which have been implicated as mechanosensitive channels in other vascular smooth muscle cells.
Fig. 3.
Short interfering (si)RNA knockdown of TRPV4 mRNA and protein in PASMCs. A: conventional (top) and real-time (bottom) RT-PCR analysis of TRPV4 and TRPV2 mRNA expression in PASMCs transfected with siRNA against TRPV4 or control scrambled sequence (n = 8). B: Western blot of TRPV4 protein in PASMCs transfected siRNA against TRPV4 or a control sequence (n = 12). *P < 0.05, significant difference from control.
Knockdown of TRPV4 function was verified by examining cation entry elicited by the TRPV4 agonist 4α-PDD (0.3 μM). The maximum rate of Mn2+-quenching of fura-2 fluorescence, an index of nonselective cation entry, was significantly attenuated in PASMCs transfected with TRPV4 siRNA (0.77 ± 0.17% s−1, n = 13), compared with the control sequence (1.33 ± 0.19% s−1, n = 13; P < 0.05; Fig. 4, A and B). 4α-PDD-mediated Ca2+ entry was significantly reduced in PASMCs transfected with TRPV4 siRNA (control: 266.58 ± 64.25 nM, n = 13; TRPV4 siRNA: 122.53 ± 36.71 nM, n = 16; P < 0.05; Fig. 4, C and D). Furthermore, Ca2+ entry elicited by hypotonic solution was greatly reduced in TRPV4 siRNA transfected PASMCs (TRPV4 siRNA: 18.65 ± 6.81 nM, n = 14; control: 80.16 ± 25.48 nM, n = 14; P < 0.05; Fig. 4, E and F). These results for the first time show that TRPV4 channels operate as osmo-mechanosensitive Ca2+ channels in rat PASMCs.
Fig. 4.
Effect of TRPV4 knockdown on 4α-phorbol 12,13-didecanoate (4α-PDD) and hypotonic solution induced cation entry. A: time course of 4α-PDD-induced Mn2+ quenching in TRPV4 and scrambled siRNA transfected PASMCs. B: average maximum rate of 4α-PDD-induced Mn2+ quenching in TRPV4 siRNA transfected (n = 13) and control sequence (n = 13) transfected PASMCs. C and D: time-course and average maximum change in the amplitude of 4α-PDD induced Ca2+ entry recorded in TRPV4 siRNA transfected (n = 16) and control sequence (n = 13) transfected PASMCs. E and F: time course and the average maximum change in the amplitude of hypotonic solution-induced Ca2+ entry in the TRPV4-siRNA (n = 14) and control sequence (n = 14) transfected PASMCs. *P < 0.05, significant difference from control.
Chronic hypoxia enhances TRPV4-mediated Ca2+ entry in PASMCs.
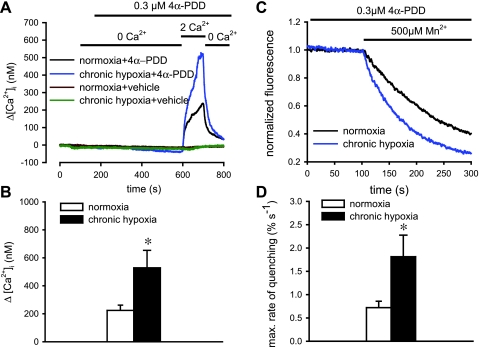
The effect of chronic hypoxia on the activity of TRPV4 in PASMCs was evaluated by measuring Ca2+ entry induced by 4α-PDD (0.3 μM; Fig. 5, A and B). Ca2+ entry was augmented significantly in PASMCs isolated from 4-wk chronic hypoxic rats [normoxic PASMCs: 224.14 ± 37.88 nM (n = 30); hypoxic PASMCs: 528.69 ± 124.70 nM, n = 25; P < 0.01]. There was no significant Ca2+ response when vehicle solution (DMSO 1:1,000) was applied instead of 4α-PDD (normoxic cells: −3.9 ± 3.6 nM, n = 8; hypoxic cells: 0.2 ± 0.9 nM, n = 12). The maximal rate of quenching induced by 4α-PDD was doubled in the chronic hypoxic PASMCs (normoxic PASMCs: 0.72 ± 0.14% s−1, n = 8; hypoxic PASMCs: 1.81 ± 0.46% s−1, n = 8; P < 0.01; Fig. 5, C and D). Similarly, Ca2+ transients activated by hypotonic solution were significantly larger in chronic hypoxic PASMCs, with a large transient Ca2+ release followed by a sustained elevation of [Ca2+]i due to Ca2+ influx (Fig. 6, A and B). The initial Ca2+ release recorded in Ca2+-free solution was 22.8 ± 4.51 (n = 25) nM normoxic and 90.76 ± 17.91 nM (n = 22; P < 0.01) in hypoxic PASMCs. The Ca2+ entry signal recorded after readmission of extracellular Ca2+ was significantly greater in the hypoxic cells (hypoxic: 57.68 ± 8.07 nM, n = 22; normoxic: 29.73 ± 2.73 nM, n = 25; P < 0.01; Fig. 6, C and D). Furthermore, the sustained Ca2+ increase elicited by hypotonic solution in normoxic and hypoxic PASMCs were both blocked by 3 μM ruthenium red (normoxia: −65.78 ± 12.62%, n = 11 and hypoxia: −67.96 ± 7.27%, n = 13; Fig. 6, E and F). These results clearly indicate that the functional activity of TRPV4 channels in PASMCs was augmented after chronic exposure to hypoxia.
Fig. 5.
4α-PDD-induced cation entry in PASMCs of normoxic and chronic hypoxic rats. A: Ca2+ transients elicited by readmission of Ca2+ to PASMCs pretreated with 0.3 μM 4α-PDD or vehicle solution in Ca2+-free solution. B: average change in peak Ca2+ transients elicited by 4α-PDD in normoxic (n = 30) and chronic hypoxic cells (n = 25). C: time course of Mn2+ quenching of fura-2 fluorescence stimulated by 4α-PDD in normoxic and chronic hypoxic cells. D: average maximum rate of 4α-PDD-induced Mn2+-quenching in normoxic (n = 8) and chronic hypoxic PASMCs (n = 8). *P < 0.01, significant difference from normoxia.
Fig. 6.
Hypotonic solution induced Ca2+ transients in PASMCs of normoxic and chronic hypoxic rats. A and B: representative tracings and the average change of initial peak and the sustained plateau of Ca2+ transients elicited by hypotonic solution in normoxic (n = 22) and hypoxic cells (n = 25). C and D: Ca2+ transients and the average change in [Ca2+]i elicited by hypotonic solution in Ca2+-free solution and after readmission of 2 mM Ca2+ in normoxic (n = 22) and hypoxic PASMCs (n = 25). E and F: inhibitory effects of 3 μM RuR on the sustained plateau of Ca2+ transients elicited by hypotonic solution in normoxic (n = 11) and chronic hypoxic PASMCs (n = 13). *P < 0.05, significant difference from normoxia.
Chronic hypoxia enhances myogenic tone in pulmonary microvessels.
The enhanced Ca2+ entry via osmo-mechanosensitive TRPV4 channels in hypoxic PASMCs might facilitate vasoconstriction in response to elevated Ppa. This possibility was investigated in isolated endothelium-denuded pressurized small PAs (internal diameter <200 μm) of normoxic and chronic hypoxic rats. The effectiveness of endothelial disruption was verified by the lack of a vasodilatory response to acetylcholine (1 μM) in arteries constricted with U46619 (10–100 nM). Elevation of transmural pressure from 5 to 55 mmHg (in 10-mmHg steps) caused a monotonic increase in the steady-state diameter of microvessels of normoxic rats. The pressure-diameter relation was unaffected by the removal of extracellular Ca2+ (in the presence of 3 mM EGTA) or by the addition of papaverine (10 μM) in Ca2+-free solution to induce maximal vasodilatation (Fig. 7A), indicating that there was no active myogenic tone in normoxic PAs. In contrast, significant myogenic tone developed in small PAs of chronic hypoxic rats. These arteries dilated significantly in Ca2+ free solution with or without papaverine (repeated-measures ANOVA, P < 0.001), and the differences in the pressure-diameter relation were readily noticeable at intraluminal pressure between 25–55 mmHg (Fig. 7B). Myogenic tone was further analyzed by calculating the percentage difference in internal diameter recorded in Ca2+-containing vs. papaverine containing Ca2+-free solution at each pressure (Fig. 7D). The active tone of chronic hypoxic arteries increased from 5.9 ± 1.5% at 5 mmHg to a maximum of 13.8 ± 3.5% at 35 mmHg (n = 9; P < 0.01). Moreover, the myogenic tone of size-matched PAs from the same animal was abolished by ruthenium red (3 μM; Fig. 7, C and D). The effect of ruthenium red was not related to nonspecific effects on vasoconstriction because it did not affect the U46619-induced response (Fig. 7E) nor was the effect related to the inhibition of store-operated Ca2+ entry, because 3 μM ruthenium red had no significant influence on the thapsigargin-induced Ca2+ influx in PASMCs (control: 294.2 ± 34.9 nM, n = 18; ruthenium red: 260.7 ± 26.1 nM, n = 18; Fig. 8, A and B). Moreover, ruthenium red (3 μM), which had no significant effect on the basal [Ca2+]i of normoxic PASMCs (before ruthenium red: 104.3 ± 9.5 nM; after RuR: 96.7 ± 6.5 nM, n = 9), caused a significant reduction in the elevated basal [Ca2+]i of the hypoxic PASMCs (before ruthenium red: 134.0 ± 6.7 nM; after ruthenium red: 110.4 ± 7.6 nM, n = 10; P < 0.001; Fig. 8, C and D). La3+ (10 μM), which had been shown to normalize the elevated [Ca2+]i in hypoxic PASMCs (26), also blocked the 4α-PDD induced Ca2+ influx (control: 476.2 ± 64.7 nM, n = 11; La3+: 52.0 ± 5.7, n = 11; P < 0.001; Fig. 8, E and F). Since TRPV4 is the only TRPV channel upregulated by chronic hypoxia, our results suggest that it is likely to be responsible for the enhanced myogenic tone and contributes to the elevated basal [Ca2+]i in chronic hypoxic PAs.
Fig. 7.
Effects of chronic hypoxia on the myogenic tone of isolated pressurized deendothelialized PAs. A and B: pressure-diameter relations generated from small PAs isolated from normoxic (n = 5) and hypoxic rats (n = 9) in Ca2+-containing (red), Ca2+-free (blue), or Ca2+-free + papaverine solution (black). *P < 0.05, significant difference from Ca2+-free solution by Tukey test. C: pressure-diameter curves generated from chronic hypoxic PAs (n = 8) in the presence of 3 μM RuR. D: analysis of myogenic tone of normoxic (black) and chronic hypoxic PAs in the presence (blue) or absence of RuR (red). Myogenic tone was expressed as % difference between vessel diameters obtained in Ca2+-containing and Ca2+-free + papaverine solution. *P < 0.05, significant difference between with or without RuR treatment by Tukey test. E: concentration-response curve of U-46619 generated in the presence or absence of RuR (n = 4).
Fig. 8.
Effect of RuR on store-operated Ca2+ entry and basal [Ca2+]i in PASMCs. A: store-operated Ca2+ entry was elicited by readmission of Ca2+ to PASMCs pretreated with 10 μM thapsigargin in Ca2+-free solution with or without 3 μM RuR. B: average peak Ca2+ response elicited by thapsigargin in the presence (n = 18) or absence (n = 18) of RuR. C: Ca2+ transients showing the effects 3 μM RuR on the basal [Ca2+]i of normoxic and hypoxic PASMCs. D: average basal [Ca2+]i of normoxic (n = 17) and hypoxic PASMCs (n = 18) before and 20 mins after application of RuR. #P < 0.05, significant difference from normoxic cells. *P < 0.05, significant difference from before application of RuR. E: Ca2+ transients elicited by readmission of Ca2+ to PASMCs activated by 4α-PDD in Ca2+-free solution with or without 10 μM La3+. F: average peak Ca2+ response elicited by 4α-PDD in the presence (n = 11) or absence (n = 11) of La3+.
TRPV4 gene deletion reduces chronic hypoxia-induced pulmonary hypertension.
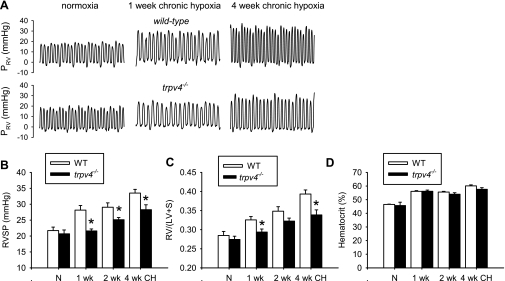
As a test of the concept that TRPV4 contributes to the development of chronic hypoxic pulmonary hypertension, TRPV4 null (trpv4−/−) mice and age-matched wild-type mice were exposed to 10% O2 for 0, 1, 2, and 4 wk. Increase in RVSP and RV/(LV + S) mass ratio were clearly evident in the wild-type mice after 1 wk of hypoxia exposure, and progressed to higher levels in the second and the fourth week (Fig. 9, B and C). These changes were virtually undetectable in trpv4−/− mice after 1 wk of hypoxia, and the increases in RVSP and RV/(LV + S) in the subsequent weeks were significantly attenuated compared with the wild-type controls. Polycythemia was developed to the similar extent in the trpv4−/− and the wild-type mice at all the time-points examined (Fig. 9D). These results show for the first time that the trpv4 gene contributes the development of hypoxic pulmonary hypertension.
Fig. 9.
Comparison of hypoxia-induced pulmonary hypertension in wild-type (WT) and trpv4−/−mice. A: representative tracings of right ventricular pressure (PRV) recorded in wild-type and trpv4−/− mice exposed to normoxia or 10% O2 for 1 and 4 wk. Mean group data of right ventricular (RV) systolic pressure (RVSP; B), RV to left ventricle and septum mass ratio [RV/(LV + S)] (C), and hematocrit (D) measured from wild-type and trpv4−/− mice after 1, 2, and 4 wk are shown. There were 5–9 mice in each group. *Significant difference between trpv4−/− and wild-type mice.
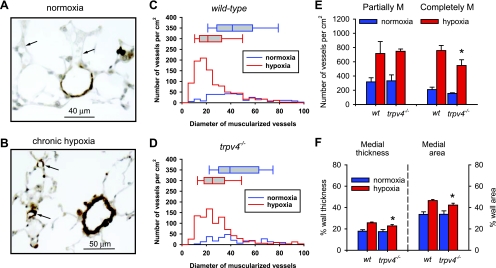
Morphological analysis was performed to compare vascular remodeling in wild-type and trpv4−/− mice exposed to 4 wk of hypoxia. Smooth muscle was clearly observed in small PAs and precapillary alveolar arterioles in wild-type hypoxic lungs, as a hallmark of hypoxic pulmonary hypertension, in contrast to the thin medial layer in small arteries and the absence of smooth muscle in arterioles of normoxic lungs (Fig. 10, A and B). Frequency distributions of resistance PAs (inner diameter <100 μm) show a dramatic increase in the muscularized small PA (<30 μm) in hypoxic lungs (Fig. 10, C and D). Median diameter of muscularized vessels was shifted from a median of 41.3 μm in normoxic lungs to 21.2 μm in hypoxic lungs (P < 0.001). Muscularization was observed, but to a less extent, in small PAs of chronic hypoxic trpv4−/− mice. Median diameter of muscularized PAs in hypoxic trpv4−/− lungs was 24.9 μm, which is significantly different from the hypoxic wild-type lung (Mann-Whitney rank sum test, P = 0.001). Moreover, the density of the completely muscularized vessels was lower, and the thickness of medial layers (expressed in %diameter or %area) was significantly decreased in the hypoxic trpv4−/− lungs (Fig. 10, E and F). Even though there is a possibility that residual vascular tone in the lungs of trpv4−/− mice before fixation may affect the percent wall thickness and area, our results on the density of muscularized vessels clearly suggest that the trpv4 gene contributes to the hypertrophy and/or hyperplasia of smooth muscle in small PAs of hypoxic animals. Nevertheless, the significant increase in muscularization of PA in hypoxic trpv4−/− lungs indicates that other mechanisms in additional to TRPV4 are also involved.
Fig. 10.
Morphological analysis of pulmonary vascular remodeling in wild-type and trpv4−/− mice exposed to 4-wk hypoxia. A and B: lung sections of normoxic and hypoxic wild-type mice immunostained with smooth muscle α-actin antibody (brown). Arrows indicate small peri-alveolar vessels. C and D: Size distributions of muscularized vessels in lungs of normoxic and hypoxic wild-type and trpv4−/− mice. Box plots show the median and range of the diameter of muscularized vessels. E: density of partially (partially M; 25–75% actin positive) and completely muscularized (completely M; >75% actin positive) arteries in normoxic and hypoxic lungs. F: %medial thickness and %cross-sectional area of completely muscularized vessels. Both the density and the thickness of the completely muscularized vessels were significantly less in hypoxic trpv4−/− mice. More than 300 vessels were analyzed from ≥4 mice in each group.
DISCUSSION
The purpose of the present study is to identify the vanilloid- and melastatin-related TRP channels that are regulated by chronic hypoxia, as an endeavor to discover alternative Ca2+ pathways contributing to hypoxic pulmonary hypertension. Our survey on the 14 known TRPV and TRPM isoforms found that TRPV4 was the only channel upregulated in PA of rats after four wk of hypoxia exposure. This specific upregulation of TRPV4 mRNA expression was an early response to hypoxia occurring within the first day of challenge and persisted throughout the development of pulmonary hypertension. Functional experiments performed in PASMCs showed that TRPV4 could be activated by osmo-mechanical stress, and its activity was significantly enhanced by chronic hypoxia. The upregulation of TRPV4 in PASMCs was associated with the appearance of a pressure-induced myogenic constrictor response, which was abolished by ruthenium red, in small PAs of chronic hypoxic rats. The elevated basal [Ca2+]i in hypoxic PASMCs was also reduced by ruthenium red. Moreover, deletion of the trpv4 gene delayed and suppressed the development of pulmonary hypertension and right heart hypertrophy, as well as reduced the muscularization of resistance PAs in hypoxic trpv4−/− mice. These results for the first time provide direct evidence at the molecular, cellular, organ, and animal levels, indicating that the expression and function of TRPV4 channels in PAs are regulated by chronic hypoxia, and they are required for the full development of hypoxic pulmonary hypertension.
Our previous study (26) showed that chronic hypoxia enhances the expression of store-operated TRPC1 and receptor-operated TRPC6 channels in rat PAs. The enhanced store-operated Ca2+ entry is thought to be responsible for the increase of basal [Ca2+]i in PASMCs and resting tone in PA of chronic hypoxic rats (26, 28, 60). The present study has placed TRPV4 as the third member of the TRP superfamily regulated by chronic hypoxia. The early transcriptional regulation of TRPV4 by hypoxia suggests that it could be a target gene of hypoxia-dependent transcription pathways, such as HIF-1. This notion is concordant with previous studies showing hypoxia-induced structural and physiological changes in pulmonary vasculatures, including membrane depolarization, increase in resting [Ca2+]i, reduction in KV currents, and upregulation of Na+/H+ exchange which all require the full expression of HIF-1α (48, 49, 67), and HIF-1α regulates the increase in expression of TRPC1 and TRPC6 in hypoxic PASMCs (60). Alternatively, TRPV4 expression could be regulated by other oxygen-sensitive or Ca2+-dependent transcription factors such as early growth-response gene, NF-κB, and nuclear factor of activated T cells (4, 5, 64), which are known to be activated by hypoxia.
The novel observation of TRPV4 upregulation in hypoxic PAs prompted us to investigate its physiological functions in pulmonary vasculature and its pathophysiological roles in pulmonary hypertension. TRPV4 has a widespread expression in many cell types and is best recognized as an osmo-mechanosensitive channel (24). It can be activated multimodally by hypotonicity, mechanical, and thermal (warmth) stimuli, as well as by naturally occurring and synthetic chemical compounds such as the arachidonic acid metabolites epoxyeicosatrienoic acids (EETs) and the phorbol ester derivative 4α-PDD (9, 59, 61). It has been established in certain cell types that osmo-mechanical stimuli can activate phospholipase A2 to generate arachidonic acid and its downstream cytochrome P450 epoxygenase metabolites 5,6-EET and 8–9-EET to activate TRPV4 channels (58, 59, 61).
Our present study clearly suggests that TRPV4 can operate as an osmo-mechanosensitive cation channel in PASMCs. It is based on a series of evidence that cell swelling induced by hypotonic solution, a common surrogate for mechanical stretch, activated a significant Ca2+ transient in PASMCs. The Ca2+ response was dependent on extracellular Ca2+ and blocked by the TRPV antagonist ruthenium red and the cytochrome P450 epoxygenase inhibitor MS-PPOH. Specific knockdown of TRPV4 with siRNA attenuated both the Ca2+ response induced by 4α-PDD and hypotonic solution. Mechanosensitive cation channels have been recorded in adult rabbit PASMCs (39, 40). The density of these channels is significantly higher, and the threshold for activation is lower in PAs than in systemic arteries, suggesting adaptive tuning for the low pressure in pulmonary circulation (40). The preferential expression of mechanosensitive channels in rabbit PA is consistent with our previous (65) finding that TRPV4 is abundantly expressed in rat PASM with a transcript level significantly higher than aorta.
The prominent expression of the mechanosensitive TRPV4 in PASMCs and their immediate upregulation after hypoxia exposure suggest that mechanical stimulus, an underappreciated factor in pulmonary hypertension, may play a significant role in the pathogenesis of chronic hypoxic pulmonary hypertension. In systemic circulation, an increase in intravascular pressure is known to activate mechanosensitive channels to generate myogenic tone (10, 54, 63), and mechanical stretch of VSMCs can stimulate cell proliferation and vascular remodeling (6, 18, 43, 47). In pulmonary circulation, Ppa and vascular resistance are inherently low, and vascular tone is minimal or nonexistent. However, hypoxia exposure initiates hypoxic pulmonary vasoconstriction and subsequent development of significant vascular tone (3, 26, 38, 50). The elevated Ppa may exert sufficient mechanical stretch to activate the upregulated TRPV4 channels to generate myogenic tone. This is evident in the present study of the pressurized deendothelized small PAs of chronic hypoxic rats. The myogenic tone, which was ruthenium red sensitive, increased with transmural pressure to a maximum of ∼10% reduction in diameter at 35 mmHg. Since the activation of myogenic tone (15–35 mmHg) occurs well within the dynamic range of Ppa of hypoxic animals, and 10% reduction in vessel diameter can account for significant elevation in vascular resistance (Poiseuille's law: resistance ∞ 1/r4), the hypoxia-induced myogenic tone could contribute in part to hypoxic pulmonary hypertension. Several other TRP channels including TRPC1, TRPC6, TRPM4, and TRPV2 have been implicated as mechanosensitive cation channels in VSMCs (10, 31, 35, 54, 63). However, TRPV4 is the only mechanosensitive TRP channel that is upregulated by hypoxia and blocked by ruthenium red. Hence, it is most likely the Ca2+ influx pathway responsible for the ruthenium red sensitive myogenic tone observed in the chronic hypoxic PAs.
It is noteworthy that ruthenium red reduces the elevated basal [Ca2+]i in PASMCs of chronic hypoxic rats. Our previous study (26) showed that La3+, at a low concentration (10 μM) that blocks store-operated Ca2+ entry, normalized the elevated basal [Ca2+]i in PASMCs of chronic hypoxic rats. Since ruthenium red does not inhibit store-operated Ca2+ entry (Fig. 8, A and B) and La3+ can inhibit 4α-PDD induced Ca2+ entry (Fig. 8, E and F), it is likely that the facilitation of Ca2+ influx via TRPV4 contributes at least in part to the elevated basal [Ca2+]i of these cells. However, it has been shown that siRNA knockdown of TRPC1 and TRPC6 reduced store-operated Ca2+ entry and partially reduced the elevated [Ca2+]i in the hypoxic PASMCs (28). Hence, the relative contributions and interactions of TRPV4 channels, TRPC channels, and store-operated Ca2+ entry in the regulation of basal [Ca2+]i of hypoxic PASMCs require further investigations.
In addition to myogenic tone and basal [Ca2+]i, the increased expression of TRPV4 in PAs may contribute to hypoxic pulmonary hypertension through other potential mechanisms. EETs, the endogenous activators of TRPV4 channels, are derived from cytochrome P450 epoxygenases. Cytochrome P450 epoxygenases are highly expressed in pulmonary endothelial cells, PASMCs, as well as airway epithelial and SMCs (19). A previous study (42) showed that exposure of mice to hypoxia upregulates P450 epoxygenases and increase the production of EETs (8,9-EET, 11,12-EET, and 13,14-EET) in the lungs. Since all EET regioisomers, including the putative systemic EDHF 11,12-EET, have been reported to cause vasoconstriction in pressurized rabbit PAs (72), the enhanced EET production in hypoxic lung cells may facilitate pulmonary vasoconstriction through paracrine activation of TRPV4 in PAs. Moreover, serotonin is capable of activating a TRPV4 like nonselective cation channels in rat PASMCs (8) presumably through receptor-mediated phospholipase A2 activation and EET production (8, 14). Since the serotonin signaling pathway is intricately linked to hypoxic pulmonary hypertension (11, 21, 29), the upregulation of TRPV4 in hypoxic PAs may contribute to the serotonin-dependent mechanisms. It is also recognized that chronic hypoxia enhances basal vascular tone and reactivity to agonists in part through Rho/Rho kinase signaling pathway (3, 12, 36, 62). The enhanced myofilament Ca2+ sensitivity mediated by Rho kinase, and the increased Ca2+ influx via TRPV4 channels may function synergistically to promote pulmonary vascular reactivity in hypoxic PAs.
The notion that TRPV4 channels are critically involved in the pathogenesis of hypoxic pulmonary hypertension is strongly supported by our results from trpv4−/− mice. The complete absence of pulmonary hypertension after 1 wk of hypoxia; the significant suppression of subsequent increase in Ppa and right heart hypertrophy; and the reduction in the density and medial thickening of muscularized small PAs in hypoxic trpv4−/− mice indicate that TRPV4 participates in both the early development and the maintenance of hypoxic pulmonary hypertension. These effects are unrelated to a generalized impairment of the HIF-1 pathway because the levels of polycythemia in the wild-type and trpv4−/− mice were similar throughout the period of hypoxia exposure. Since vascular remodeling is mild in the early stage of hypoxic pulmonary hypertension in mice, the dramatic differences between the wild-type and trpv4−/− mice in the first week of hypoxia are likely related to the TRPV4-dependent vascular tone. Moreover, the reduction in muscularization of perialveolar arterioles and medial thickening of distal PAs in the 4 wk hypoxic trpv4−/− mice suggests that TRPV4 may participate in PASMC hyperplasia and/or hypertrophy during vascular remodeling. It has been established that TRPC1 and TRPC6 upregulation are associated with mitogen/growth factor-induced PASMC proliferation, and knockdown of these channels inhibits PASMCs proliferation (13, 56, 70). The TRPV4-dependent muscularization of resistance PAs may provide an additional mechanism to further strengthen the vasoconstriction mediated by Ca2+ influx through TRPV4 channels and contribute to the progression of pulmonary hypertension.
It is recognized that systemic deletion of TRPV4 in trpv4−/− mice may affect other pulmonary cells that are involved in hypoxic pulmonary hypertension. For example, endothelial TRPV4 are critical for shear-stress-induced vasodilatation, nitric oxide, and EDHF production (16, 22, 46), as well as the increase in alveolar barrier permeability in high vascular pressure- and ventilator-induced lung injury (1, 15, 20, 66). These processes are not functioning normally in trpv4−/− mice. Since the development of hypoxic pulmonary hypertension depends on the complex interplays between PASMCs and other pulmonary cells, the specific contributions of TRPV4 of PASMCs and other lung cells require future investigations perhaps in tissue-targeted knockout animals.
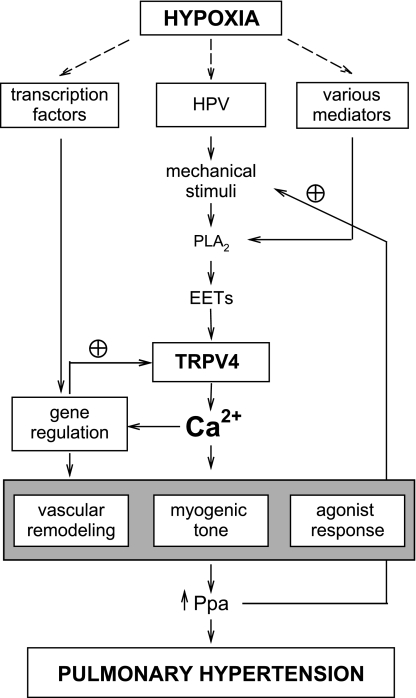
In summary, we have identified the osmo-mechanosensitive TRPV4 channel in PASMCs as a novel Ca2+ entry pathway upregulated by chronic hypoxia. It contributes to the enhanced myogenic response and vascular response in hypoxic PAs and is required for the full development of hypoxic pulmonary hypertension. These results lay out an attractive concept that TRPV4 acts as a signal transducer for the elevated Ppa and other stimulants. Its upregulation during hypoxia exposure provides a feed-forward mechanism to facilitate Ca2+ influx to enhance myogenic tone and vascular remodeling (see schematics in Fig. 11). Together with the upregulated store-operated TRPC1 and receptor-operated TRPC6 that contribute to the increased resting [Ca2+]i and agonist induced responses, respectively (26, 28), TRPV4 served as another multifaceted Ca2+ entry pathway participating in the exacerbation of hypoxic pulmonary hypertension. In view of its significant contribution to pulmonary hypertension, TRPV4 channel can be considered as a potential therapeutic target for the treatment of this dreadful disease.
Fig. 11.
Schematic diagram illustrating the proposed roles of TRPV4 channels in hypoxic pulmonary hypertension. HPV, hypoxic pulmonary vasoconstriction; PLA2, phospholipase A2; EETs, epoxyeicosatrienoic acids; Ppa, pulmonary arterial pressure; plus circle symbol, positive or feed-forward regulation.
GRANTS
This work is in part supported by National Institutes of Health Grants HL-075134 and HL-071835 and an American Heart Association (Mid-Atlantic) Grant-in-Aid (to J. S. K. Sham).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.-R.Y., A.H.L., J.M.H., N.A.F., and Y.-N.C. performed experiments; X.-R.Y., A.H.L., J.M.H., and J.S.K.S. analyzed data; X.-R.Y., J.M.H., N.A.F., W.L., and J.S.K.S. interpreted results of experiments; X.-R.Y., A.H.L., and J.S.K.S. prepared figures; X.-R.Y., W.L., and J.S.K.S. edited and revised manuscript; J.S.K.S. conception and design of research; J.S.K.S. drafted manuscript; J.S.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Omkar Paudel for technical support.
REFERENCES
- 1. Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beech DJ. Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin Exp Pharmacol Physiol 32: 597–603, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Cummins EP, Comerford KM, Scholz C, Bruning U, Taylor CT. Hypoxic regulation of NF-kappaB signaling. Methods Enzymol 435: 479–492, 2007 [DOI] [PubMed] [Google Scholar]
- 5. de Frutos S, Spangler R, Alo D, Bosc LV. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with alpha-actin upregulation. J Biol Chem 282: 15081–15089, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dethlefsen SM, Shepro D, D'Amore PA. Comparison of the effects of mechanical stimulation on venous and arterial smooth muscle cells in vitro. J Vasc Res 33: 405–413, 1996 [DOI] [PubMed] [Google Scholar]
- 7. DiCarlo VS, Chen SJ, Meng QC, Durand J, Yano M, Chen YF, Oparil S. ETA-receptor antagonist prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol Lung Cell Mol Physiol 269: L690–L697, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Ducret T, Guibert C, Marthan R, Savineau JP. Serotonin-induced activation of TRPV4-like current in rat intrapulmonary arterial smooth muscle cells. Cell Calcium 43: 315–323, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Esteve JM, Launay JM, Kellermann O, Maroteaux L. Functions of serotonin in hypoxic pulmonary vascular remodeling. Cell Biochem Biophys 47: 33–44, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Guibert C, Marthan R, Savineau JP. 5-HT induces an arachidonic acid-sensitive calcium influx in rat small intrapulmonary artery. Am J Physiol Lung Cell Mol Physiol 286: L1228–L1236, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L923–L932, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLos One 2: e827, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res 96: 207–215, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can J Physiol Pharmacol 83: 869–875, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Jacobs ER, Zeldin DC. The lung HETEs (and EETs) up. Am J Physiol Heart Circ Physiol 280: H1–H10, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 38: 386–392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT(1B) receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT(1B)-receptor knockout mice and the 5-HT(1B/1D)-receptor antagonist GR127935. Circ Res 89: 1231–1239, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Launay JM, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 8: 1129–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol 567: 53–58, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA 100: 13698–13703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JSK. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Liu JQ, Zelko IN, Erbynn EM, Sham JSK, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Lu W, Ran P, Zhang D, Peng G, Li B, Zhong N, Wang J. Sildenafil inhibits chronically hypoxic upregulation of canonical transient receptor potential expression in rat pulmonary arterial smooth muscle. Am J Physiol Cell Physiol 298: C114–C123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacLean MR, Herve P, Eddahibi S, Adnot S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol 131: 161–168, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MacLean MR, Morecroft I. Increased contractile response to 5-hydroxytryptamine1-receptor stimulation in pulmonary arteries from chronic hypoxic rats: role of pharmacological synergy. Br J Pharmacol 134: 614–620, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Marrelli SP, O'Neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1390–H1397, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Montell C. The TRP superfamily of cation channels. Sci STKE 2005: re3, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, Brayden JE. Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharmacol Sci 103: 417–426, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res 98: 245–253, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Oka M, Morris KG, McMurtry IF. NIP-121 is more effective than nifedipine in acutely reversing chronic pulmonary hypertension. J Appl Physiol 75: 1075–1080, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Park KS, Kim Y, Lee YH, Earm YE, Ho WK. Mechanosensitive cation channels in arterial smooth muscle cells are activated by diacylglycerol and inhibited by phospholipase C inhibitor. Circ Res 93: 557–564, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Park KS, Lee HA, Earm KH, Ko JH, Earm YE, Kim SJ. Differential distribution of mechanosensitive nonselective cation channels in systemic and pulmonary arterial myocytes of rabbits. J Vasc Res 43: 347–354, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol 280: L801–L812, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, Vermeersch P, Gillijns H, Pellens M, Grimminger F, van Zonneveld AJ, Collen D, Busse R, Janssens S. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension 47: 762–770, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Predel HG, Yang Z, von Segesser L, Turina M, Buhler FR, Luscher TF. Implications of pulsatile stretch on growth of saphenous vein and mammary artery smooth muscle. Lancet 340: 878–879, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Reading SA, Brayden JE. Central role of TRPM4 channels in cerebral blood flow regulation. Stroke 38: 2322–2328, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Remillard CV, Yuan JX. High altitude pulmonary hypertension: role of K+ and Ca2+ channels. High Alt Med Biol 6: 133–146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Shaw A, Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: an update. Curr Vasc Pharmacol 1: 41–58, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 291: L941–L949, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Shimoda LA, Manalo DJ, Sham JSK, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 281: L202–L208, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Shimoda LA, Sham JSK, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Shimoda LA, Sylvester JT, Sham JSK. Chronic hypoxia alters effects of endothelin and angiotensin on K+ currents in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 277: L431–L439, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 13: 657–670, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol Heart Circ Physiol 266: H365–H370, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101: 2468–2477, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Touyz RM. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol 294: H1103–H1118, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97: 908–915, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101: 396–401, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Yan SF, Zou YS, Gao Y, Zhai C, Mackman N, Lee SL, Milbrandt J, Pinsky D, Kisiel W, Stern D. Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA 95: 8298–8303, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang XR, Lin MJ, McIntosh LS, Sham JSK. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Yin J, Hoffmann J, Kaestle SM, Neye N, Wang L, Baeurle J, Liedtke W, Wu S, Kuppe H, Pries AR, Kuebler WM. Negative-feedback loop attenuates hydrostatic lung edema via a cGMP-dependent regulation of transient receptor potential vanilloid 4. Circ Res 102: 966–974, 2008 [DOI] [PubMed] [Google Scholar]
- 67. Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JSK, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103: 691–696, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY, Thistlethwaite PA, Channick RN, Robbins IM, Loyd JE, Ghofrani HA, Grimminger F, Schermuly RT, Cahalan MD, Rubin LJ, Yuan JX. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 119: 2313–2322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 71. Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 105: 516–521, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Zhu D, Bousamra M, II, Zeldin DC, Falck JR, Townsley M, Harder DR, Roman RJ, Jacobs ER. Epoxyeicosatrienoic acids constrict isolated pressurized rabbit pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 278: L335–L343, 2000 [DOI] [PubMed] [Google Scholar]