Abstract
NADPH oxidase is a major source of superoxide anions in the pulmonary arteries (PA). We previously reported that intratracheal SOD improves oxygenation and restores endothelial nitric oxide (NO) synthase (eNOS) function in lambs with persistent pulmonary hypertension of the newborn (PPHN). In this study, we determined the effects of the NADPH oxidase inhibitor apocynin on oxygenation, reactive oxygen species (ROS) levels, and NO signaling in PPHN lambs. PPHN was induced in lambs by antenatal ligation of the ductus arteriosus 9 days prior to delivery. Lambs were treated with vehicle or apocynin (3 mg/kg intratracheally) at birth and then ventilated with 100% O2 for 24 h. A significant improvement in oxygenation was observed in apocynin-treated lambs after 24 h of ventilation. Contractility of isolated fifth-generation PA to norepinephrine was attenuated in apocynin-treated lambs. PA constrictions to NO synthase (NOS) inhibition with N-nitro-l-arginine were blunted in PPHN lambs; apocynin restored contractility to N-nitro-l-arginine, suggesting increased NOS activity. Intratracheal apocynin also enhanced PA relaxations to the eNOS activator A-23187 and to the NO donor S-nitrosyl-N-acetyl-penicillamine. Apocynin decreased the interaction between NADPH oxidase subunits p22phox and p47phox and decreased the expression of Nox2 and p22phox in ventilated PPHN lungs. These findings were associated with decreased superoxide and 3-nitrotyrosine levels in the PA of apocynin-treated PPHN lambs. eNOS protein expression, endothelial NO levels, and tetrahydrobiopterin-to-dihydrobiopterin ratios were significantly increased in PA from apocynin-treated lambs, although cGMP levels did not significantly increase and phosphodiesterase-5 activity did not significantly decrease. NADPH oxidase inhibition with apocynin may improve oxygenation, in part, by attenuating ROS-mediated vasoconstriction and by increasing NOS activity.
Keywords: reactive oxygen species, NADPH oxidase inhibition, nitric oxide signaling
when the pulmonary circulation fails to respond to various stimuli at birth, such as increased Po2 and ventilation, it does not undergo the shift from the high-resistance, low-flow state in utero to the low-resistance, high-flow system postnatally (16, 25). This failure results in persistent pulmonary hypertension of the newborn (PPHN) and is associated with impaired pulmonary gas exchange and oxygenation. Clinical strategies, such as mechanical ventilation with high concentrations of O2 to correct hypoxemia and inhaled nitric oxide (NO), are commonly used to promote pulmonary vasodilation, but sustained improvement is not observed in ∼40% of patients with PPHN (21). Hyperoxic ventilation also promotes formation of reactive oxygen species (ROS), such as superoxide anions, increases pulmonary artery (PA) contractility, and may contribute to the pathogenesis of PPHN (23, 24).
We recently showed that intratracheal recombinant human SOD (rhSOD) improved oxygenation and decreased oxidative stress and PA contractility in PPHN lambs ventilated with 100% O2 (24, 38). Intratracheal rhSOD also increased endothelial NO synthase (eNOS) protein expression and activity, in part by reducing the oxidation of the essential cofactor tetrahydrobiopterin (BH4) (15). ROS induce phosphodiesterase-5 (PDE5) enzyme activity and decrease intracellular concentrations of cGMP, an essential second messenger that mediates vasodilation (13). Intratracheal rhSOD decreased PDE5 activity and increased cGMP levels in the PA of ventilated PPHN lambs (14). These data suggest that therapies to decrease PA superoxide levels may be beneficial in the treatment of PPHN.
NADPH oxidase is a major source of superoxide anions in various models of pulmonary hypertension, including hypoxia-induced pulmonary hypertension in newborn piglets (9) and ductal ligation- and aortopulmonary shunt-induced pulmonary hypertension in neonatal lambs (6, 18). Pretreatment of PA isolated from PPHN lambs with the nonspecific NADPH oxidase inhibitor diphenyleneiodonium enhanced relaxation to S-nitrosyl-N-acetyl-penicillamine (SNAP), a NO donor (6). Apocynin, an ortho-methoxy-substituted catechol derived from the pharmacological plant Picrorhiza kurroa, directly interacts with the p47phox subunit and inhibits NADPH oxidase (39). We hypothesized that administration of intratracheal apocynin would reduce superoxide anion formation by NADPH oxidase and improve oxygenation, decrease oxidative stress, and increase PA eNOS activity in lambs with PPHN. We further hypothesized that intratracheal apocynin would decrease PA contractility and improve PA relaxation to NO donors.
METHODS
This protocol was approved by the Laboratory Animal Care Committees at the University at Buffalo and Northwestern University. Time-dated pregnant ewes were obtained from the Swartz family farm (Attica, NY). One-day spontaneously breathing (Control-1DSB) lambs were healthy newborn lambs that delivered spontaneously at comparable gestation to the experimental lambs, were fed normally, breathed room air, and then, at 24 h of life, were anesthetized with thiopental sodium (Pentothal) and killed by rapid exsanguination through a direct cardiac puncture. Fetal lambs underwent antenatal ligation of the ductus arteriosus at 126 days gestation (full-term ∼145 days) to induce pulmonary hypertension, as previously described (27, 48). Lambs were delivered 9 days later. Eight PPHN lambs were intubated, fetal lung liquid was drained, and a dose of calfactant (3 ml/kg; Infasurf, ONY Laboratories, Amherst, NY, a gift from Dr. Edmund A. Egan) was administered through the tracheal tube at birth (PPHN-Oxygen group). In five lambs, apocynin (3 mg/kg; Sigma Aldrich, St. Louis, MO) was mixed in calfactant prior to intratracheal administration of apocynin (PPHN-Apocynin group). Lambs were placed under a servo-controlled radiant warmer and ventilated with 100% O2 (Servo300 ventilator, Siemens, Mississauga, ON, Canada), as previously described (24). Umbilical arterial and venous lines were placed. Fentanyl (2–4 μg·kg−1·h−1) was given by continuous infusion. Additional boluses of fentanyl were administered for evidence of pain (spontaneous movement and increased heart rate and blood pressure). Intravenous fluids were administered at 100 ml·kg−1·day−1. Arterial blood gases were drawn soon after birth and every 2 h and as needed. At the end of 24 h of ventilation, lambs were killed with an overdose of thiopental sodium and exsanguination. Fifth-generation PA and lung tissue were collected for further analysis.
Isolated vessel protocol.
Fifth-generation (<500 μm ID) PA were carefully dissected, cut into rings, and studied in isolated vessel baths bubbled with 20% O2 and 6% CO2, as described elsewhere (23, 24). They were pretreated with propranolol (10−6 M) and constricted with increasing concentrations (10−8–10−6 M) of norepinephrine (NE). Some PA were pretreated with N-nitro-l-arginine (l-NA, 10−3 M) to inhibit endogenous NO synthase (NOS) activity and then constricted with NE. The enhancement of contraction induced by pretreatment with l-NA was taken as a measure of endogenous PA NOS activity (15). Finally, the tissues were washed and contracted with 118 mM potassium chloride solution. At the end of the experiment, the tissues were blotted dry and weighed. Force of contraction was expressed as grams of force per gram of tissue. Some PA rings were constricted to half-maximal contraction and relaxed with 10−8–10−6 M A-23187 (a calcium ionophore and stimulant of eNOS) or SNAP (a NO donor). Relaxations are expressed as percentage of NE constriction.
In situ analysis of superoxide generation.
Lung sections were prepared and stained as described previously (13, 15). Briefly, the right middle lobe of the lung was removed, and optimal cutting temperature compound (OCT, VWR Scientific, West Chester, PA) was pushed into the deflated lobe and allowed to solidify on ice for 15–20 min. Blocks were prepared and cut into 8- to 10-μm sections, which were mounted onto charged slides and stored at −80°C. The sections were allowed to reach room temperature and exposed to 5 μM dihydroethidium (DHE; Molecular Probes) in PBS. Sequential sections were additionally treated with 100 U/ml polyethylene glycol-SOD (PEG-SOD; Sigma, St. Louis, MO). Slides were incubated in a light-protected humidified chamber at 37°C for 15 min. Slides were washed three times in PBS and observed using a fluorescence microscope (Eclipse TE-300, Nikon) with excitation at 518 nm and emission at 605 nm. Fluorescent images were captured using a CoolSnap digital camera, and the average fluorescence intensities (to correct for differences in pixel number) of PA were quantified using Metamorph imaging software (Fryer). Tissue sections were processed and imaged in parallel. Fluorescence intensity from the PEG-SOD-treated sections was subtracted from that of the corresponding untreated section to give the SOD-inhibited DHE fluorescence for each PA.
Western blot analysis.
Isolated frozen lung and PA tissue was homogenized, and total protein was collected using the PARIS kit (Ambion, Austin, TX), as previously described (13). Protein concentration was measured using the Bradford method (5). Total protein (40 μg) was separated on a 4–20% SDS-polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to a nitrocellulose membrane (Amersham, Arlington Heights, IL). Western blotting was performed as previously described (13, 15). Briefly, membranes were blocked at room temperature with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (1× TBST) and then incubated overnight at 4°C with the primary antibody in 5% milk + 1× TBST at the appropriate dilution: 1:1,000 for mouse anti-eNOS (BD Transduction, San Jose, CA), 1:500 for rabbit anti-Nox2, anti-p22phox, and anti-p47phox (Santa Cruz Biotechnology, Santa Cruz, CA), 1:333 for mouse anti-PDE5 (BD Transduction), and 1:2,000 for mouse β-actin (Sigma). The membranes were washed and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Pierce, Rockford, IL) diluted 1:1,000 in 5% milk + 1× TBST. Membranes were washed and exposed via chemiluminescence (Pierce). Bands were analyzed using a Digital Science Image Station (Kodak, Rochester, NY). Expression within each Western blot was normalized to β-actin.
Immunoprecipitation.
Lung protein was prepared as described above, and 500 μg were incubated with 4 μg of anti-p47 antibody (Santa Cruz Biotechnology) at 4°C overnight with gentle rocking using the Catch-and-Release Immunoprecipitation System according to the manufacturer's instructions (Millipore, Billerica, MA). Eluted protein was analyzed by Western blotting using an anti-p22 antibody (Santa Cruz Biotechnology), as described above.
In situ analysis of NO generation.
Lung sections were prepared as described above for superoxide detection. The sections were allowed to reach room temperature and exposed to 10 μM 4,5-diaminofluorescein diacetate (DAF2-DA; Molecular Probes) in PBS. Slides were incubated in a light-protected humidified chamber at 37°C for 15 min, washed three times in PBS, and observed using a fluorescence microscope (Eclipse TE-300, Nikon) with excitation at 495 nm and emission at 519 nm. Fluorescent images were captured, and intensities within the endothelium of PA were quantified using MetaMorph imaging software, as described above.
Immunohistochemistry.
Frozen lung sections prepared as described above were allowed to reach room temperature and then fixed with acetone (Sigma) for 10 min at 4°C, allowed to air-dry, and then washed with 1× PBS (Mediatech, Herndon, VA). Sections were blocked with 5% BSA (Sigma) + 1× TBST at room temperature for 1 h and then stained overnight at 4°C with an anti-3-nitrotyrosine (3-NT) antibody (1:50 dilution; Cayman Chemical, Ann Arbor, MI). Sections were washed and probed with the appropriate secondary antibody [an Alexa Fluor 488 anti-mouse antibody (Molecular Probes/Invitrogen) or a rhodamine red goat anti-rabbit secondary antibody (Molecular Probes/Invitrogen)] at a 1:200 dilution in 5% BSA. Tissue localization and expression were visualized with a fluorescence microscope (Eclipse TE-300, Nikon) with excitation at 495 nm and emission at 519 nm for Alexa Fluor 488 or with excitation at 518 nm and emission at 605 nm for rhodamine red. Fluorescent images were captured using a CoolSnap digital camera with Metamorph imaging software (Molecular Devices, Sunnyvale, CA).
Determination of total biopterin levels by HPLC.
PA biopterin content was measured by HPLC analysis and a differential oxidation method, as previously described (24, 26). Briefly, frozen PA tissue was ground to a powder on liquid nitrogen. The powder was dissolved into biopterin extraction buffer (50 mM Tris, 1 mM DTT, and 1 mM EDTA). BH4 levels were determined from the difference between the acid extraction [total biopterins: biopterin + BH4 + dihydrobiopterin (BH2)] and the alkaline extraction (BH2 + biopterin). A 5-μm C-18 reverse chromatography column (Waters, Milford, MA) was used for HPLC with a solvent system of 5% methanol (Fisher Scientific, Pittsburgh, PA)-95% water at a flow rate of 1 ml/min. Biopterins were detected by fluorescence at 350 nm for excitation and 450 nm for emission. Peak areas were compared with a biopterin standard curve (Sigma) and then normalized for micrograms of total protein in each sample, as measured by the Bradford assay (3).
cGMP enzyme immunoassay.
cGMP levels were measured in sheep PA tissue snap-frozen in liquid nitrogen, weighed, and homogenized in 10 volumes of 5% trichloroacetic acid (Sigma). Precipitate was removed by centrifugation at 1,500 g for 10 min. The trichloroacetic acid was extracted using water-saturated ether according to the manufacturer's protocol (Cayman Chemical). cGMP content of the PA samples was measured by acetylated enzyme immunoassay in duplicate using a commercially available kit (Cayman Chemical). Results were measured using a Labsystems Multiskan EX automated plate reader (Thermo Electron) at 420-nm wavelength. Results are shown as picomoles of cGMP per milligram of frozen tissue.
PDE5 activity assay.
Protein was prepared fresh from snap-frozen PA tissue as described above, with the lysis buffer supplemented with protease and phosphatase inhibitors. The total PA protein was immediately placed on ice and assayed on the same day, as previously described (13). The protein was purified over a Centri-Spin 10 column (Princeton Separations, Adelphia, NJ) to remove any phosphate contamination. Protein concentration was determined. Total protein (5 μg) was assayed for cGMP hydrolytic activity using a commercially available colorimetric cyclic nucleotide phosphodiesterase assay kit (Biomol, Plymouth Meeting, PA). Each sample was read in four wells, two without sildenafil and two with sildenafil (100 nM), to determine PDE5-specific cGMP hydrolytic activity. The samples were incubated at 30°C for 30 min and then incubated with the Biomol Green reagent with shaking at room temperature for 20 min. Results were measured using a Labsystems Multiskan EX automated plate reader at 620-nm wavelength. The difference between the amount of cGMP hydrolyzed per milligram of total protein per minute without and with sildenafil represents the PDE5-specific cGMP hydrolytic activity. Results are shown as the PDE5-specific amount of cGMP hydrolyzed per milligram of total protein per minute for each sample.
Statistical analysis.
Values are means ± SE. Oxygenation (arterial-to-alveolar Po2 ratio) and isolated PA constriction and relaxation responses were compared by repeated-measures ANOVA with Bonferroni's post hoc analysis to compare multiple groups (Statview 4.0, Abacus Concepts, Berkeley, CA). In vitro results were analyzed by factorial ANOVA with Bonferroni's post hoc analysis using Prism software (GraphPad, San Diego, CA). Statistical significance was set at P < 0.05.
RESULTS
Intratracheal apocynin improves oxygenation in lambs with PPHN.
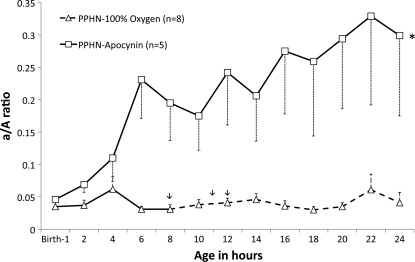
Birth weight and gender distribution were similar in the PPHN-Oxygen and PPHN-Apocynin groups (data not shown). Oxygenation was significantly impaired in both groups at birth (arterial-to-alveolar Po2 ratio = 0.04 ± 0.007 in the PPHN-Oxygen group and 0.045 ± 0.005 in the PPHN-Apocynin group; Fig. 1). Three lambs in the PPHN-Oxygen group died (1 at 8 h, 1 at 11 h, and 1 at 12 h), and oxygenation remained low at 24 h among the survivors (arterial-to-alveolar Po2 ratio at 24 h = 0.04 ± 0.016; Fig. 1). All five lambs in the PPHN-Apocynin group survived the 24-h period, and oxygenation improved by 6 h of age and remained significantly higher than in the PPHN-Oxygen group (arterial-to-alveolar Po2 ratio at 24 h = 0.30 ± 0.12; Fig. 1). In the Control-1DSB group, the arterial-to-alveolar Po2 ratio was 0.55 ± 0.03 at 24 h.
Fig. 1.
Apocynin improves oxygenation in ventilated lambs with persistent pulmonary hypertension of the newborn (PPHN). Arterial-to-alveolar Po2 ratio (a/A ratio) was measured in 1 group of lambs with PPHN ventilated with 100% O2 (PPHN-100 O2) and 1 group of lambs with PPHN ventilated with 100% O2 and treated with apocynin (3 mg/kg) administered intratracheally at birth (PPHN-Apocynin). PPHN lambs ventilated with 100% O2 were critically ill, and 3 died: 1 at ∼8 h, 1 at 11 h, and 1 at 12 h (arrows). Dashed line beyond this point represents mean ± SE for surviving lambs. *P < 0.05 vs. PPHN-100% O2.
Intratracheal apocynin reduces PA contractility and enhances relaxation via NO signaling.
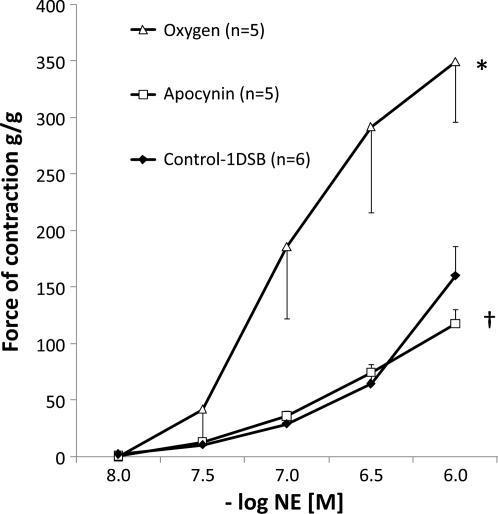
Fifth-generation PA rings were obtained from five lambs in the PPHN-Oxygen group and five lambs in the PPHN-Apocynin group that were killed at 24 h and compared with PA rings from six Control-1DSB lambs. Pharmacomechanical constriction to receptor-mediated agonist (NE) was studied. Isolated PA rings from the Control-1DSB lambs constricted in a dose-dependent manner to increasing concentrations of NE (Fig. 2). As previously described (23, 24), PA rings from the PPHN-Oxygen group showed a marked increase in constriction to NE, while constriction to NE was significantly decreased in PA rings isolated from the PPHN-Apocynin group (Fig. 2).
Fig. 2.
Intratracheal apocynin reduces pulmonary artery (PA) contractility and enhances relaxation in ventilated PPHN lambs. Cumulative concentration-response curves to increasing doses of norepinephrine (NE) in 5th-generation PA isolated from 1-day spontaneously breathing (Control-1DSB) lambs, PPHN lambs ventilated for 24 h with 100% O2 (Oxygen), and PPHN lambs ventilated for 24 h with 100% O2 and treated with intratracheal apocynin. *P < 0.05 vs. Control-1DSB. †P < 0.05 vs. Oxygen.
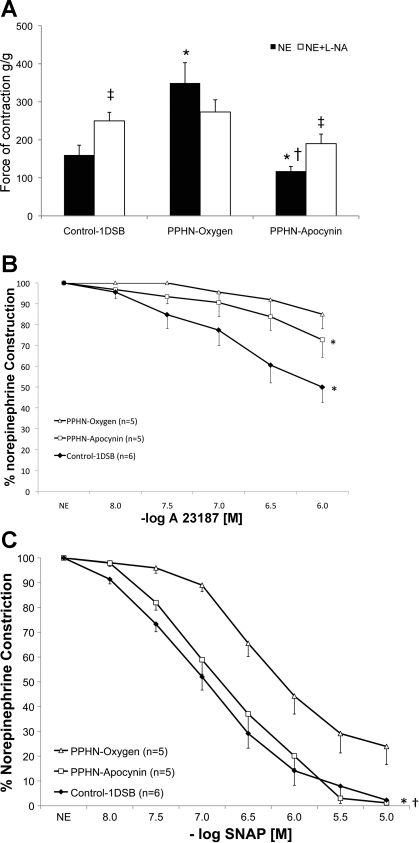
Pretreatment with the NOS inhibitor l-NA did not significantly alter the constriction responses in PA isolated from lambs in the PPHN-Oxygen group but significantly enhanced the constriction to NE in PA isolated from lambs in the PPHN-Apocynin and Control-1DSB groups (Fig. 3A). Relaxations to the endothelium-dependent eNOS agonist A-23187 were similarly enhanced in PA isolated from lambs in the PPHN-Apocynin and Control-1DSB groups (Fig. 3B) compared the PPHN-Oxygen group. Relaxations to the endothelium-independent NO donor SNAP were significantly better in PA isolated from lambs in the PPHN-Apocynin and Control-1DSB groups than the PPHN-Oxygen group (Fig. 3C).
Fig. 3.
A: apocynin restores endogenous nitric oxide synthase (NOS) activity in ventilated PPHN lamb PA. Contraction response of PA to 10−6 M NE was measured with or without pretreatment with the NOS inhibitor N-nitro-l-arginine (l-NA, 1 mM) in 1DSB-Control lambs (n = 6), PPHN lambs ventilated for 24 h with 100% O2 (PPHN-Oxygen, n = 5), and PPHN lambs ventilated for 24 h with 100% O2 and treated with intratracheal apocynin (PPHN-Apocynin, n = 5). *P < 0.05 vs. Control-1DSB. †P < 0.05 vs. PPHN-Oxygen. ‡P < 0.05 vs. corresponding PA without l-NA. B: apocynin reduces PA contractility and enhances relaxation to the NOS stimulant A-23187. Response was measured in 5th-generation PA that were constricted to half-maximal capacity with NE and relaxed with A-23187. *P < 0.05 vs. PPHN-Oxygen at 10−6 M only. C: apocynin reduces PA contractility and enhances relaxation to the NO donor S-nitrosyl-N-acetyl-penicillamine (SNAP). Response was measured in 5th-generation PA constricted to half-maximal capacity with NE and relaxed with SNAP. *P < 0.05, PPHN-Apocynin vs. PPHN-Oxygen. †P < 0.05, Control-1DSB vs. PPHN-Oxygen.
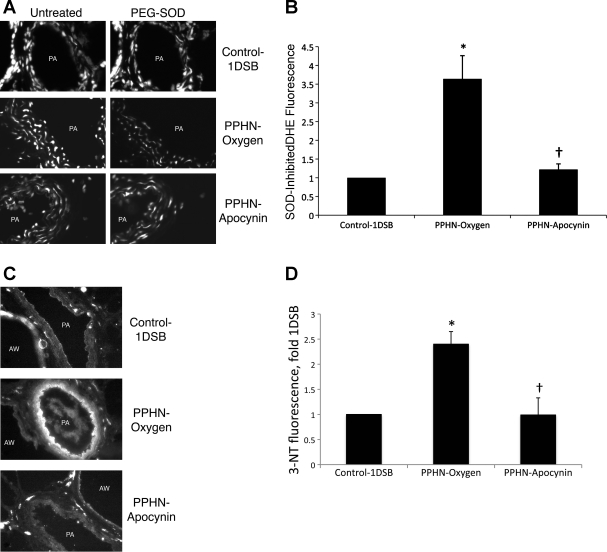
Intratracheal apocynin decreases superoxide anions and 3-NT in the PA.
We previously found (15) that ventilation with 100% O2 markedly increased superoxide anions in the PA of PPHN lambs relative to 1DSB control lambs, as detected by DHE fluorescence. Intratracheal apocynin significantly decreased PA DHE fluorescence in ventilated PPHN PA (Fig. 4, A and B). Since other molecules can oxidize DHE (28), we incubated sequential sections with PEG-SOD and determined the SOD-inhibited DHE fluorescence for each PA (Fig. 4, A and B). PA superoxide levels correlated with PA 3-NT levels, which were also significantly higher in lambs from the PPHN-Oxygen group than Control-1DSB group and were significantly decreased in lambs from the PPHN-Apocynin group (Fig. 4, C and D).
Fig. 4.
Apocynin decreases reactive oxygen species levels in ventilated PPHN PA. A: frozen lung sections were probed with dihydroethidium (DHE), which is oxidized by superoxide to a fluorescent red product. Sequential sections were additionally treated with polyethylene glycol (PEG)-SOD to determine SOD-inhibited DHE fluorescence. Images show PA and airways (AW). B: average SOD-inhibited fluorescence intensities from PA were quantified by MetaMorph and are expressed relative to Control-1DSB. C: lung sections were fixed and probed with an antibody against 3-nitrotyrosine (3-NT). Fluorescence from an appropriate secondary antibody was evident within PA and airways. D: 3-NT fluorescence intensities within the PA were quantified by MetaMorph and are expressed relative to Control-1DSB. Values are means ± SE; n = 6 Control-1DSB, n = 6 PPHN-Oxygen, n = 5 PPHN-Apocynin. *P < 0.05 vs. Control-1DSB. †P < 0.05 vs. PPHN-Oxygen.
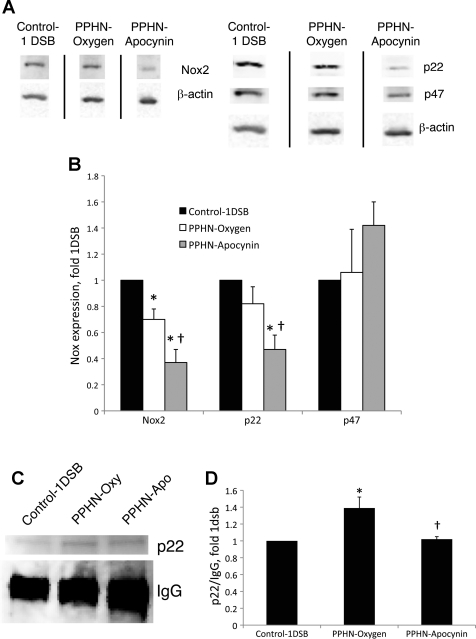
Intratracheal apocynin decreases Nox2 and p22phox expression and p22phox/p47phox subunit binding.
Enzyme activity of the Nox2 isoform of NADPH oxidase requires the assembly of additional subunits, including p22phox, p47phox, and p67phox (8, 32). We previously reported that increased superoxide levels were associated with increased expression of p67phox in PA of fetal PPHN lambs (6). We also found that lung protein levels of Nox2 and p22phox, but not p47phox, were increased in fetal PPHN lambs relative to fetal controls (data not shown). Here we report that expression of Nox2 and p22phox, but not p47phox, was decreased in lambs from the PPHN-Apocynin group relative to the PPHN-Oxygen group (Fig. 5, A and B). Furthermore, Nox2 levels were significantly higher in the Control-1DSB group than the PPHN-Oxygen group (Fig. 5, A and B). Immunohistochemistry revealed that Nox2, p22phox, and p47phox were strongly expressed in the PA (data not shown). Apocynin inhibits Nox2 activity by preventing the assembly of the p47phox subunit within the enzyme complex (39). Since p47phox levels were not significantly different between the three groups, we immunoprecipitated total lung proteins using an antibody to p47phox. There was a decrease in p22phox and p47phox subunit association in lungs from lambs in the PPHN-Apocynin group, as detected by immunoprecipitation (Fig. 5, C and D). Furthermore, association was lower in the Control-1DSB group than the PPHN-Oxygen group. We were unable to normalize association to p47phox levels, since p47phox comigrates with the 50-kDa IgG subunit in the immunoprecipitation reaction (Fig. 5C). When normalized to the IgG band to correct for loading, immunoprecipitated p22phox levels were significantly higher in the PPHN-Oxygen group than the Control-1DSB group, while apocynin restored p22phox to Control-1DSB levels (Fig. 5D).
Fig. 5.
Apocynin decreases Nox2 and p22phox expression and p22phox/p47phox association in ventilated PPHN lungs. A: representative Western blots for Nox2, p22phox, and p47phox. Bands separated by a black line were from the same Western blot membrane and exposure, and images were processed identically. B: band intensities were normalized to β-actin and are expressed relative to Control-1DSB. Values are means ± SE; n = 6 Control-1DSB, n = 6 PPHN-Oxygen, n = 5 PPHN-Apocynin. *P < 0.05 vs. Control-1DSB. †P < 0.05 vs. PPHN-Oxygen. C: lung protein was immunoprecipitated using an antibody against p47phox, and Western blot was probed with an anti-p22phox antibody. Also depicted is precipitating IgG protein from the immunoprecipitation reaction. D: p22 band intensities were normalized to the IgG band and are expressed relative to Control-1DSB. Values are means ± SE; n = 5 Control-1DSB, n = 5 PPHN-Oxygen, n = 5 PPHN-Apocynin. *P < 0.05 vs. Control-1DSB. †P < 0.05 vs. PPHN-Oxygen.
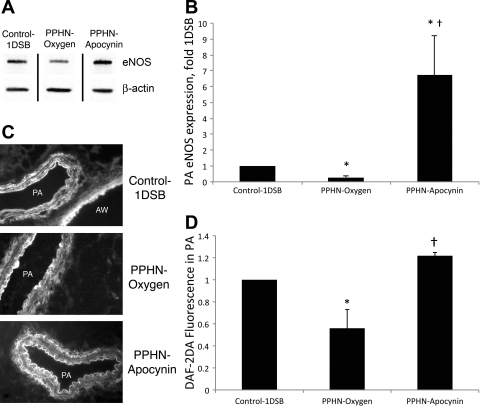
Intratracheal apocynin increases PA eNOS expression, NO levels, and BH4-to-BH2 ratio.
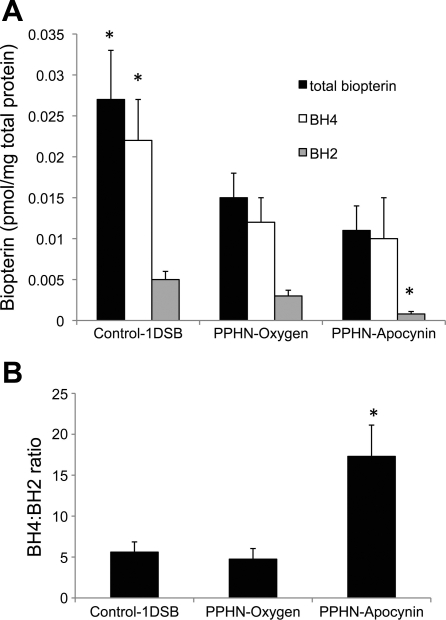
We previously showed that eNOS expression was decreased in PPHN lambs ventilated with 100% O2 relative to 1DSB control lambs (15). To explain the increased NOS activity in apocynin-treated lambs in isolated PA studies, we evaluated expression of eNOS protein in PA. A 6.7-fold increase in eNOS protein levels in the PPHN-Apocynin group relative to the Control-1DSB group was detected by Western blotting (Fig. 6, A and B). Apocynin treatment significantly increased NO levels in the endothelium relative to the PPHN-Oxygen group, as detected by the NO-sensitive fluorescent probe DAF2-DA (Fig. 6, C and D). Immunohistochemistry also demonstrated an increase in endothelial expression of eNOS within the PA of apocynin-treated PPHN lambs (data not shown). BH4 is an essential cofactor for eNOS, and its oxidation to BH2 can result in uncoupling of eNOS, with reduced NO and increased superoxide anion production (18, 26). Intratracheal apocynin significantly decreased BH2 levels (Fig. 7A) and increased the BH4-to-BH2 ratio (Fig. 7B) in ventilated PPHN PA. However, there was no difference in total biopterin and BH4 levels (Fig. 7A).
Fig. 6.
Apocynin increases endothelial NOS (eNOS) expression and nitric oxide levels in ventilated PPHN lungs. A: representative Western blot. Bands separated by a black line were from the same Western blot membrane and exposure, and images were processed identically. B: band intensities were normalized to β-actin and are expressed relative to Control-1DSB. C: frozen lung sections were probed with 4,5-diaminofluorescein diacetate (DAF2-DA), which fluoresces when it interacts with nitric oxide. Sections show PA and airways. D: average DAF2-DA fluorescence intensities from endothelium of PA were quantified by MetaMorph and are expressed relative to Control-1DSB. Values are means ± SE; n = 6 Control-1DSB, n = 6 PPHN-Oxygen, n = 5 PPHN-Apocynin. *P < 0.05 vs. Control-1DSB. †P < 0.05 vs. PPHN-Oxygen.
Fig. 7.
Apocynin increases the ratio of tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2) in ventilated PPHN PA. A: total biopterin, BH4, and BH2 levels were determined by HPLC. B: BH4-to-BH2 ratios were calculated for each group. Values are means ± SE; n = 6 Control-1DSB, n = 4 PPHN-Oxygen, n = 4 PPHN-Apocynin. *P < 0.05 vs. PPHN-Oxygen.
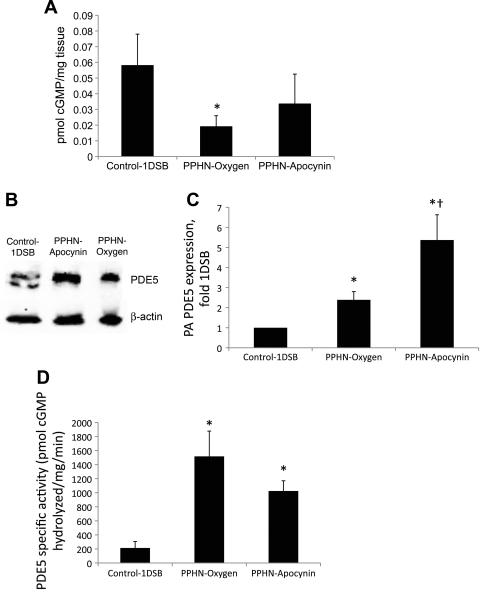
Apocynin does not significantly increase cGMP levels or decrease PDE5 activity in PA.
cGMP levels did not increase in the PPHN-Apocynin group (Fig. 8A), despite a significant increase in eNOS protein and NO levels (Fig. 6). We evaluated expression, localization, and activity of the cGMP-hydrolyzing enzyme PDE5 in PA to explain this discrepancy. We previously reported an increase in PDE5 protein expression and activity in PPHN lambs ventilated with 100% O2 relative to 1DSB control lambs (14) and found that intratracheal apocynin further increased PDE5 protein levels (Fig. 8, B and C). Conversely, there was a trend toward decreased PDE5 activity in the PPHN-Apocynin group, although the decrease did not reach statistical significance compared with the PPHN-Oxygen group (Fig. 8D). Immunohistochemistry also demonstrated an increase in endothelial expression of PDE5 in the PPHN-Apocynin group (data not shown).
Fig. 8.
A: apocynin increases cGMP levels in ventilated PPHN PA. cGMP levels were determined by enzyme immunoassay and normalized to protein content. Values are means ± SE; n = 6 Control-1DSB, n = 5 PPHN-Oxygen, n = 4 PPHN-Apocynin. *P < 0.05 vs. Control-1DSB. B–D: apocynin increases phosphodiesterase 5 (PDE5) protein expression but decreases activity in ventilated PPHN PA. B: representative Western blot. C: band intensities normalized to β-actin and expressed relative to Control-1DSB. Values are means ± SE; n = 5 Control-1DSB, n = 8 PPHN-Oxygen, n = 5 PPHN-Apocynin. *P < 0.05 vs. Control-1DSB. †P < 0.05 vs. PPHN-Oxygen. D: specific activity of PDE5 in resistance PA. Values are means ± SE; n = 4 Control-1DSB, n = 6 PPHN-Oxygen, n = 5 PPHN-Apocynin. *P < 0.05 vs. Control-1DSB.
DISCUSSION
ROS such as superoxide anions play an important role in the development of multiple vascular diseases, including PPHN (41). ROS can lead to vascular dysfunction and remodeling through decreased bioavailability of NO (11), decreased eNOS and increased PDE5 activity (13, 15), and impaired pulmonary angiogenesis (41). Increased NADPH oxidase expression has been reported in models of pulmonary hypertension (9, 18), and we previously reported increased expression of p67phox in the pulmonary vasculature in the ductal ligation model of PPHN utilized in this study (6). We now report that intratracheal administration of apocynin, an inhibitor of NADPH oxidase assembly, reduces ROS and restores eNOS expression and vascular reactivity in neonatal lambs with PPHN. Oral apocynin reduced systemic blood pressure in spontaneously hypertensive rats (3) and aldosterone-infused rats (29). While previously reported in vitro studies have shown that apocynin reduced production of ROS and enhanced pulmonary vascular responses to NO (9, 18), to our knowledge, this study is the first attempt to administer in vivo apocynin for PPHN.
Apocynin inhibits the generation of superoxide anions from NADPH oxidase by preventing the membrane translocation of p47phox, an event critical to subunit assembly of the active enzyme complex (39). The dose of apocynin was calculated with the assumption of an immediate volume of distribution of fetal lung liquid volume (∼20–30 ml/kg) (33) and a target concentration of 0.5–1 mM in the lung liquid. This concentration has been shown to be effective in various cell studies (17, 19). We chose the intratracheal route because of our prior success with intratracheal rhSOD (24) and because we wished to induce selective pulmonary vasodilation without systemic hypotension. Inhaled apocynin has been tested in human volunteers and shown to be well tolerated and effective in reducing ozone- and methacholine-induced airway hyperresponsiveness (31). In the present study, intratracheal apocynin improved oxygenation in lambs with PPHN, and we observed fewer deaths in the PPHN-Apocynin group than the PPHN-Oxygen group. We did not perform formal measurements of pulmonary hemodynamics, as a thoracotomy significantly reduces 24-h survival in PPHN lambs in our laboratory. As we previously reported (23, 24), hyperoxic ventilation of PPHN lambs markedly increased PA contractility to NE relative to 1DSB lambs, while intratracheal apocynin markedly reduced contractility in a NOS-dependent fashion. We previously demonstrated that intratracheal administration of rhSOD similarly decreased the contraction response to NE by restoring endogenous eNOS function (15). In the present study, we found that using intratracheal apocynin to block superoxide production similarly enhanced PPHN PA relaxations to the eNOS agonist A-23187 and the NO donor SNAP. NO reacts rapidly with superoxide to form peroxynitrite (ONOO), and reducing superoxide production may have increased bioavailable NO in these experiments. Furthermore, ONOO has been reported to be a potent vasoconstrictor within the neonatal pulmonary circulation (4). From these studies, we speculate that intratracheal apocynin reduces pulmonary vascular resistance and enhances pulmonary vasodilation in ventilated PPHN lambs by augmenting NO-mediated PA vasorelaxation and by attenuating superoxide- and ONOO-mediated PA vasoconstriction.
We next determined the effects of intratracheal apocynin on vascular ROS levels. We previously found that superoxide was elevated in the PA of fetal PPHN lambs relative to fetal control lambs (6) and in ventilated PPHN lambs relative to fetal PPHN lambs and 1DSB lambs (47). These data suggest that ventilation with high levels of inspired O2 stimulates further superoxide generation in PPHN. We recently demonstrated that smooth muscle cells isolated from PPHN PA had elevated ROS levels relative to control cells and that exposure to hyperoxia generated a further increase in ROS (47). In addition, endothelial cells isolated from PPHN PA were demonstrated to have increased superoxide levels relative to controls (41), suggesting that both cell types may contribute to increased ROS in ventilated PPHN lambs. In the present study, intratracheal apocynin decreased PA superoxide and ONOO levels relative to ventilated PPHN lambs, which may be due to attenuation of PPHN- and O2-induced superoxide production by NADPH oxidase. These data support our hypothesis that administration of intratracheal apocynin decreases superoxide levels within the PA, resulting in reduced ONOO formation and increased bioavailable NO.
Superoxide generation by the Nox2 isoform of NADPH oxidase requires the assembly of the p67phox, p47phox, and p22phox subunits into the enzyme complex. We showed previously that increased superoxide levels in the PA of fetal PPHN lambs were associated with increased PA expression of p67phox (6). In the present study, we found that apocynin decreased lung p22phox and Nox2 protein expression in ventilated PPHN lambs. Our results are in agreement with the reported decrease in p22phox mRNA in the aortas of apocynin-treated hypertensive rats (29) and consistent with the finding that NADPH oxidase-derived H2O2 amplifies its own production via a feedforward mechanism in vascular disease (7). Nox2 expression is increased by the transcription factor NF-κB (2), which may stimulate a positive-feedback loop via ROS-induced NF-κB activation in PPHN. However, Nox2 expression was also significantly higher in 1DSB lungs than in ventilated PPHN lambs, suggesting that other mechanisms may regulate Nox2 expression in the transitional pulmonary vasculature. We analyzed p47phox and p22phox association by immunoprecipitation as an indicator of Nox2 activity. We chose to immunoprecipitate lung extracts because of the large amount of input protein required, but our immunohistochemical analysis of lung sections localized p47phox and p22phox expression primarily within the PA (data not shown). Our immunoprecipitation studies, demonstrating a decrease in association between the p47phox and p22phox subunits, suggest a reduction in Nox2 activity in apocynin-treated PPHN lambs. Furthermore, p47phox and p22phox association was greater in ventilated PPHN lambs than 1DSB lambs, suggesting a corresponding increase in Nox2 activity, despite the differences in expression. Nox2 activity may be regulated by additional pathways independent of subunit expression, including protein phosphorylation (30). However, the mechanisms of apocynin-mediated decreases in Nox2 activity in ventilated PPHN lambs are unknown. It has been argued that apocynin functions as an antioxidant, rather than an NADPH oxidase inhibitor, in vascular cells in vitro, in part because these cells lack the myeloperoxidase activity required to convert apocynin to its active inhibitory form (17, 19). However, others have shown that vascular endothelial cells take up myeloperoxidase released by white blood cells (42, 45), and other peroxidases expressed in vascular cells also have the potential to influence apocynin activity (42). Our data suggest that apocynin may reduce ROS levels in PPHN PA by several different mechanisms, since it can function as an inhibitor of NADPH oxidase subunit assembly and as a negative regulator of Nox2 and p22phox expression, in addition to its antioxidant properties.
We next investigated the effects of intratracheal apocynin on downstream targets of ROS signaling in PPHN. eNOS expression is decreased in PPHN lamb lungs (35), and ROS were shown to decrease eNOS expression in vitro (46). Here we report that intratracheal apocynin markedly increased eNOS protein in PPHN PA, with an associated increase in enzyme function, as evidenced by the isolated vessel studies. Apocynin also significantly increased NO levels within the endothelium of PPHN PA, although these increases were not as dramatic as the increases in eNOS expression. Increases in eNOS protein and activity have also been observed in endothelial cells (40) and cardiac cells (20) treated with apocynin. These findings are similar to our previous observation of an increase in PA eNOS expression and function in PPHN lambs treated with intratracheal rhSOD (15). One potential mechanism of superoxide-mediated eNOS enzyme inhibition occurs via the oxidation of the essential cofactor BH4 to BH2, which promotes eNOS uncoupling (43). Although total biopterin and BH4 levels were not increased by apocynin, there was a significant reduction in BH2 levels resulting in an increase in the BH4-to-BH2 ratio. A decrease in the BH4-to-BH2 ratio has been shown to increase superoxide production by eNOS (1, 44), and uncoupled eNOS is a source of superoxide in persistent pulmonary hypertension (18, 26). Our data suggest that the vascular effects of apocynin may include promotion of the recoupling of eNOS. However, the BH4-to-BH2 ratios were similar between the Control-1DSB and PPHN-Oxygen groups, suggesting that other factors also regulate eNOS activity in PPHN. In contrast, our previously reported increase in eNOS activity in PPHN lambs treated with intratracheal rhSOD was associated with an increase in total biopterin and BH4 levels (15). This may be explained by the different antioxidant properties of apocynin and rhSOD, as well as by their sites of action within the pulmonary vasculature. BH4 levels are upregulated by H2O2-mediated induction of GTP cyclohydrolase (36, 37), and we speculate that dismutation of superoxide to H2O2 by rhSOD may account for the increase in BH4 (15). Conversely, apocynin inhibits superoxide generation by NADPH oxidase and has been reported to scavenge H2O2 in vitro (17, 19). Ventilation with NO also reduced superoxide levels in PPHN PA but had less pronounced effects on eNOS expression and did not affect BH4 or BH2 levels (15). NO reacts with superoxide to form ONOO, which may account for the reduced superoxide levels but persistent depression of BH4 levels.
NO signaling increases cGMP levels within the PA smooth muscle layer, resulting in vasodilation, and we previously showed that cGMP levels were lower in ventilated PPHN lambs than in 1DSB control lambs (14). In the present study, even though apocynin increased eNOS expression and activity, we did not find a corresponding increase in cGMP levels. PDE5 is the enzyme primarily responsible for cGMP degradation in the pulmonary vasculature. We were surprised to find that apocynin treatment significantly increased PDE5 expression relative to ventilated PPHN lambs, although we did not observe a similar increase in PDE5 activity. These findings are very different from our observations following intratracheal rhSOD, which significantly decreased PDE5 expression and activity and significantly increased cGMP levels in ventilated PPHN lambs (14). We previously reported ROS-induced increases in PDE5 activity in PA smooth muscle cells from control and PPHN lambs (12, 13) and noted that the increased PDE5 activity in smooth muscle cells isolated from PPHN lambs was attenuated by overexpression of catalase within the mitochondrial matrix (12). We speculate that apocynin was not sufficient to alleviate this H2O2-mediated stimulation of PDE5 in PPHN. Together, these data highlight the importance of PDE5 activity in maintaining cGMP levels, as well as the complex regulation of PDE5 by ROS. We also note that while apocynin increased the arterial-to-alveolar Po2 ratio in ventilated PPHN lambs from 0.05 to 0.3 after 24 h, previous studies showed a greater improvement in the arterial-to-alveolar Po2 ratios of ventilated PPHN lambs treated with intratracheal rhSOD (22) and catalase (47). The greater improvement in oxygenation in lambs treated with these antioxidants may reflect their ability to decrease PDE5 expression and activity. We speculate that the specific antioxidant properties and cellular sites of action of apocynin, rhSOD, and catalase may account for their different effects on cGMP and PDE5 in PA from PPHN lambs.
Improved oxygenation and decreased PA constriction in apocynin-treated ventilated PPHN lambs suggest that apocynin may also influence cGMP-independent vasodilation. In spontaneously hypertensive rats, Rho kinase inhibition and apocynin treatment attenuated acetylcholine-stimulated contraction of common carotid arteries (10), while apocynin was implicated in Rho kinase inhibition in renal intralobar arteries (34). Additional studies are needed to identify a potential role for Rho kinase-mediated PA contraction in ventilated PPHN lambs and to determine the impact of intratracheal apocynin on Rho kinase activity in our model.
Our present study indicates that intratracheal apocynin improves oxygenation and diminishes vascular dysfunction in PPHN via several mechanisms. Apocynin inhibits NADPH oxidase subunit expression and association, resulting in decreased PA superoxide and ONOO levels, augmented NO-mediated vasodilation, and attenuated ROS-mediated vasoconstriction. Intratracheal apocynin treatment may have advantages over protein antioxidants because of a direct effect on production of ROS, as well as its potentially greater permeability and half-life within the pulmonary vasculature. In addition, an increased understanding of its mode of action may identify other antioxidants that can be used in conjunction with apocynin to provide better treatment strategies for PPHN. For instance, antioxidants such as rhSOD and catalase will decrease PDE5 activity and increase cGMP levels (12). These approaches, in combination with apocynin-mediated decreases in production of superoxide and ONOO, may prove to be more effective in preventing ROS-induced vasoconstriction than either treatment alone.
GRANTS
This study was supported by National Institutes of Health Grants HL-54705 (R. H. Steinhorn), K08 HL-086715 (K. N. Farrow), and HD-060138 (S. Lakshminrusimha).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.W., S.L., K.N.F., J.A.R., and R.H.S. are responsible for conception and design of the research; S.W., S.L., K.N.F., L.C., S.F.G., F.S., and R.H.S. performed the experiments; S.W., S.L., K.N.F., L.C., S.F.G., F.S., J.A.R., and R.H.S. analyzed the data; S.W., S.L., K.N.F., L.C., S.F.G., F.S., J.A.R., and R.H.S. interpreted the results of the experiments; S.W., S.L., K.N.F., and R.H.S. prepared the figures; S.W., S.L., K.N.F., and R.H.S. drafted the manuscript; S.W., S.L., K.N.F., and R.H.S. edited and revised the manuscript; S.W., S.L., K.N.F., L.C., S.F.G., F.S., J.A.R., and R.H.S. approved the final version of the manuscript.
REFERENCES
- 1. Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Anrather J, Racchumi G, Iadecola C. NF-κB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281: 5657–5667, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Baumer AT, Kruger CA, Falkenberg J, Freyhaus HT, Rosen R, Fink K, Rosenkranz S. The NAD(P)H oxidase inhibitor apocynin improves endothelial NO/superoxide balance and lowers effectively blood pressure in spontaneously hypertensive rats: comparison to calcium channel blockade. Clin Exp Hypertens 29: 287–299, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Belik J, Pan J, Jankov R, Tanswell A. Peroxynitrite inhibits relaxation and induces pulmonary artery muscle contraction in the newborn rat. Free Radic Biol Med 37: 1384–1392, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 6. Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 96: 818–822, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chamseddine AH, Miller FJ., Jr gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. Am J Physiol Heart Circ Physiol 285: H2284–H2289, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denniss SG, Jeffery AJ, Rush JW. RhoA-Rho kinase signaling mediates endothelium- and endoperoxide-dependent contractile activities characteristic of hypertensive vascular dysfunction. Am J Physiol Heart Circ Physiol 298: H1391–H1405, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24: 1367–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Farrow K, Wedgwood S, Lee K, Czech L, Gugino S, Lakshminrusimha S, Schumacker P, Steinhorn R. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 174: 272–281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102: 226–233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino S, Davis JM, Russell JA, Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 299: L109–L116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Firth AL, Yuan JX. Bringing down the ROS: a new therapeutic approach for PPHN. Am J Physiol Lung Cell Mol Physiol 295: L976–L978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukatsu A, Hayashi T, Miyazaki-Akita A, Matsui-Hirai H, Furutate Y, Ishitsuka A, Hattori Y, Iguchi A. Possible usefulness of apocynin, an NADPH oxidase inhibitor, for nitrate tolerance: prevention of NO donor-induced endothelial cell abnormalities. Am J Physiol Heart Circ Physiol 293: H790–H797, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 290: L1069–L1077, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi N, Honda T, Yoshida K, Nakano S, Ohno T, Tsubokou Y, Matsuoka H. Critical role of bradykinin-eNOS and oxidative stress-LOX-1 pathway in cardiovascular remodeling under chronic angiotensin-converting enzyme inhibition. Atherosclerosis 187: 92–100, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am 56: 579–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lakshminrusimha S, Russell J, Wedgwood S, Gugino S, Kazzaz J, Davis J, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 174: 1370–1377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 59: 137–141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 174: 1370–1377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol 26: 601–619, 1999 [PubMed] [Google Scholar]
- 26. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morin FC., 3rd Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245–250, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Papapostolou I, Patsoukis N, Georgiou CD. The fluorescence detection of superoxide radical using hydroethidine could be complicated by the presence of heme proteins. Anal Biochem 332: 290–298, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Park Y, Park M, Suh Y, Park J. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun 313: 812–817, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters EA, Hiltermann JT, Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 31: 1442–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Pfister RE, Ramsden CA, Neil HL, Kyriakides MA, Berger PJ. Volume and secretion rate of lung liquid in the final days of gestation and labour in the fetal sheep. J Physiol 535: 889–899, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schluter T, Steinbach AC, Steffen A, Rettig R, Grisk O. Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 80: 271–279, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC., 3rd Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 272: L1005–L1012, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Shimizu S, Hiroi T, Ishii M, Hagiwara T, Wajima T, Miyazaki A, Kiuchi Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through the induction of GTP-cyclohydrolase I and increases nitric oxide synthase activity in vascular endothelial cells. Int J Biochem Cell Biol 40: 755–765, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Shimizu S, Shiota K, Yamamoto S, Miyasaka Y, Ishii M, Watabe T, Nishida M, Mori Y, Yamamoto T, Kiuchi Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through the induction of GTP-cyclohydrolase I and increases nitric oxide synthase activity in vascular endothelial cells. Free Radic Biol Med 34: 1343–1352, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med 164: 834–839, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Stolk J, Hiltermann T, Dijkman J, Verhoeven A. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Taye A, Saad AH, Kumar AH, Morawietz H. Effect of apocynin on NADPH oxidase-mediated oxidative stress-LOX-1-eNOS pathway in human endothelial cells exposed to high glucose. Eur J Pharmacol 627: 42–48, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297: L184–L195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Touyz R. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension 51: 172–174, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters B, Karoui H, Tordo P, Pritchard KJ. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733–739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vita J, Brennan M, Gokce N, Mann S, Goormastic M, Shishehbor M, Penn M, Keaney JJ, Hazen S. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 110: 1134–1139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 288: L480–L487, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Wedgwood S, Lakshminrusimha S, Fukai T, Russell J, Schumacker P, Steinhorn R. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 15: 1497–1506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zayek M, Cleveland D, Morin FC., 3rd Treatment of persistent pulmonary hypertension in the newborn lamb by inhaled nitric oxide. J Pediatr 122: 743–750, 1993 [DOI] [PubMed] [Google Scholar]