Abstract
Chronic hypoxia causes pulmonary vascular remodeling leading to pulmonary hypertension (PH) and right ventricle (RV) hypertrophy. Aberrant expression of microRNA (miRNA) is closely associated with a number of pathophysiologic processes. However, the role of miRNAs in chronic hypoxia-induced pulmonary vascular remodeling and PH has not been well characterized. In this study, we found increased expression of miR-21 in distal small arteries in the lungs of hypoxia-exposed mice. Putative miR-21 targets, including bone morphogenetic protein receptor (BMPR2), WWP1, SATB1, and YOD1, were downregulated in the lungs of hypoxia-exposed mice and in human pulmonary artery smooth muscle cells (PASMCs) overexpressing miR-21. We found that sequestration of miR-21, either before or after hypoxia exposure, diminished chronic hypoxia-induced PH and attenuated hypoxia-induced pulmonary vascular remodeling, likely through relieving the suppressed expression of miR-21 targets in the lungs of hypoxia-exposed mice. Overexpression of miR-21 enhanced, whereas downregulation of miR-21 diminished, the proliferation of human PASMCs in vitro and the expression of cell proliferation associated proteins, such as proliferating cell nuclear antigen, cyclin D1, and Bcl-xL. Our data suggest that miR-21 plays an important role in the pathogenesis of chronic hypoxia-induced pulmonary vascular remodeling and also suggest that miR-21 is a potential target for novel therapeutics to treat chronic hypoxia associated pulmonary diseases.
Keywords: pulmonary hypertension, micro-ribonucleic acid, smooth muscle cell
chronic hypoxia occur frequently in a number of pulmonary disorders, such as chronic obstructive pulmonary disease, obstructive sleep apnea, and diffuse interstitial fibrosis (42, 49, 54). Persistent hypoxia causes vascular structural remodeling, leading to pulmonary hypertension (PH) that results in right ventricle (RV) hypertrophy and ultimately RV failure (42, 49, 54). The response of PH due to vascular remodeling to current therapies is limited. Thus there is a pressing need to identify novel targets for developing more effective approaches to treat PH.
Chronic hypoxia-induced vascular remodeling occurs in all three layers of the pulmonary small arterial wall (49). In the adventitia, there is increased accumulation of pulmonary artery adventitial fibroblasts (PAAFbs) and excessive deposition of extracellular matrix (ECM) proteins (45, 46, 48, 50, 53). In the media, hypertrophy and hyperplasia of pulmonary arterial smooth muscle cells (PASMCs) represent one of the major pathological features (32, 49). Chronic hypoxia also induces endothelial cell proliferation that promotes intimal thickening (33).
Many contributing molecular mechanisms have been identified in chronic hypoxia-induced vascular remodeling. Chronic hypoxia has dramatic impacts on transcriptome in the resident cells of the pulmonary vascular wall (49). Upregulation of hypoxia inducible factor-1, a transcriptional factor that mediates responses to hypoxia in cells, is a characteristic finding in pulmonary vascular cells isolated during chronic hypoxia (15, 17). Furthermore, elevated expression in a number of mitogenic and vasoconstrictive small molecules, cytokines and growth factors, including TGF-β1, FGF-2, PDGF-BB, endothelin-1, and serotonin, has been found in the lungs of animals with chronic hypoxia-induced PH (1, 5, 6, 11, 19, 23–25, 29, 31, 38, 49, 52). These molecules act individually or synergistically to promote proliferation and resistance to apoptosis of PASMC, PAAFb, and PAEC, and trans-differentiation of PAAFb and PAEC, all of which are molecular signatures of the vascular remodeling process that accompanies chronic hypoxia-induced PH (1, 5, 6, 11, 19, 23–25, 29, 31, 38, 49, 52).
microRNAs (miRNAs) are 21–22 nucleotide (nt) noncoding small RNAs (2). The expression of miRNAs is regulated by transcriptional factors in response to extracellular stimuli (22). Aberrant expression of miRNAs is closely associated with pathophysiologic processes including diabetes, cancer, and cardiovascular disease (2, 8, 9). However, the role of miRNAs in chronic hypoxia-induced pulmonary vascular remodeling and PH remains less defined (3, 7).
In our previous studies (27), we found elevated levels of miR-21 in the lungs of mice with experimental pulmonary fibrosis and in the lungs of patients with idiopathic pulmonary fibrosis. We found that miR-21 expression is upregulated in TGF-β1-treated lung fibroblasts. We also demonstrated that sequestering miR-21 in lungs diminishes pulmonary remodeling and fibrosis in response to bleomycin-induced lung injury (27). Given that TGF-β1 is a central mediator in chronic hypoxia-induced pulmonary vascular remodeling (5), we hypothesized that miR-21 may also play a role in this pathological process. In the present study, we found that miR-21 regulates pulmonary vascular remodeling in mice undergoing hypoxia and that sequestering miR-21 diminishes the progression of the remodeling process as well as vascular remodeling associated RV hypertrophy.
MATERIALS AND METHODS
Chronic normobaric hypoxic exposure.
Mice were exposed to hypoxia (10% O2) in a normobaric chamber for 2–3 wk as previously described (5). The ventricles were dissected free from the atria, and the RV was separated from the left ventricle and septum (LV + S). RV hypertrophy was expressed as the weight ratio of RV to LV + S and was used as an index of pulmonary hypertension. The animal protocol was approved by the University of Alabama Institutional Animal Care and Use Committee.
miRNA array analysis.
miRNA array analysis was performed as previously described (27). Briefly, total RNAs were isolated from mouse lungs of mice that were exposed to air or hypoxia (10% O2) for 14 days (3 mice per group) with miRNAeasy Mini kit (Qiagen). The miRNA array was performed by Exiqon using miRCURY LNA microRNA array (Exiqon).
Cell line.
Human primary PASMCs were purchased from Invitrogen and cultured according to the Invitrogen instructions.
Real-time PCR.
The assay was performed as previously described (27). Taqman probes for hsa-miR-21, hsa-miR-155, human RNU48, and mouse sno135 were purchased from Applied Biosystems. Primer sequences were as follows: mouse fibronectin (Fn): sense, TCTGGGAAATGGAAAAGGGGAATGG and antisense, CACTGAAGCAGGTTTCCTCGGTTGT; mouse smooth muscle actin-α (SMA-α): sense, GACGCTGAAGTATCCGATAGAACACG and antisense, CACCATCTCCAGAGTCCAGCACAAT; mouse caldesmon (Cald1): sense, GCCGTTCAAGTGCTTCACTCCTA and antisense TTTGTTCCCTCGATTGCATTGGT; mouse α-calponin (CNN1): sense, CCTACGGTACACGGCGTCACCT and antisense GCAGTGTTCCATGCCCAGACC; mouse transgellin (SM22-α): sense, AGACTGACATGTTCCAGACTGTTGACC and antisense GCTGTCTGTGAAGTCCCTCTTATGCT; mouse bone morphogenetic protein receptor (BMPR2): sense, GTTTTGATAGTCGCCTTATGTTTTGGA and antisense, GAAAATACTTTTACAGCAACTGGACGC; mouse SATB1: sense, TGGCGTTGCTGTCTCTAGGCTATTC and antisense, TACTGTGGTGTGCGACCATTGTTCA; mouse WWP1: sense, TGATGGATTAGTGATTGAGCAAGAGCC and antisense, CTACATGTGAACAGCAGGAGTTGGGAA; mouse YOD1: sense, GTCAGCGAATCCTCGTTGGCTACCC and antisense, TCTGCTGGGACTGCGGTTCTGGTAA; mouse endothelin-1 (ET-1): sense, CCGTGATCTTCTCTCTGCTGTTCGT and antisense, AACCTCCCAGTCCATACGGTACGAC; and mouse GAPDH: sense, CGACTTCAACAGCAACTCCCACTCTTCC and antisense, TGGGTGGTCCAGGGTTTCTTACTCCTT.
Western blotting.
Western blotting was performed as previously described (28). Rabbit anti GAPDH antibodies were from Santa Cruz. Mouse anti-SMA-α was from Sigma. Rabbit anti Bcl-xL, cyclin D1, and proliferating cell nuclear antigen (PCNA) antibodies were from Cell Signaling.
In situ hybridization.
In situ hybridization assays were performed as previously described (27). Light blue cytoplasmic staining is positive.
Immunohistochemistry.
Immunohistochemistry assays were performed as previously described (27).
Evaluation of pulmonary arterial muscularization.
Muscularization of pulmonary distal small arteries (30–150 μm in external diameter) was assessed using α-SMA-immunostained lung sections as previously described (5). Arterial muscularization was defined according to the degree of muscularization: muscularized arteries (α-SMA positive in >70% of wall area); partially muscularized arteries (α-SMA positive in between 30–70% of wall area); and nonmuscularized vessels (α-SMA positive in <30% of wall area) were distinguished and counted. Approximately 100 distal small arteries were evaluated for each slide. The ratio of distal small arteries with different degrees of muscularization to the total number of distal small arteries evaluated was calculated. Morphometric analysis was carried out by one examiner who was blinded with respect to the treatment assignment of the tissue samples examined.
Growth curve.
Human PASMCs were transfected with 10 nM control mimics (Ambion), 10 nM miR-21 mimics (Ambion), 20 nM control inhibitors (Ambion), or 20 nM miR-21 inhibitors (Ambion) using HIPerFect transfection reagents (Qiagen) for 1 day. The cells were then trypsinized, and the cell number was counted. The cells were then plated at the same number into sixwell plate. Cell number at days 4–7 was counted, and growth curve was prepared.
Statistical analysis.
One-way ANOVA followed by the Holm-Sidak or Tukey-Kramer test was performed for multiple group comparisons. The Student t-test was used for comparison between two groups. P < 0.05 was considered statistically significant.
RESULTS
miR-21 is upregulated in the lungs of mice in response to hypoxia.
To determine the role of miRNAs in the pathogenesis of chronic hypoxia-induced pulmonary vascular remodeling, we performed miRNA profiling on RNAs isolated from lungs of mice that were exposed to air or normobaric hypoxia (10% O2), a well-characterized model of PH (43, 51). A number of miRNAs, including miR-21, showed enhanced expression in the lungs of mice exposed to hypoxia, compared with those in the lungs of mice exposed to air (Table 1).
Table 1.
miRNAs with enhanced expression in the lungs of hypoxia-exposed mice
| microRNAs | Hypoxia/Normoxia (fold change) |
|---|---|
| mmu-miR-210 | 2.84 |
| mmu-miR-144 | 2.29 |
| mmu-miR-451 | 2.10 |
| mmu-miR-376a | 1.99 |
| mmu-miR-144* | 1.94 |
| mmu-miR-486 | 1.68 |
| mmu-miR-136 | 1.66 |
| mmu-miR-434-3p | 1.60 |
| mmu-miR-379 | 1.59 |
| mmu-miR-377 | 1.58 |
| mmu-miR-127 | 1.51 |
| mmu-miR-411 | 1.49 |
| mmu-miR-322 | 1.43 |
| mmu-miR-193 | 1.34 |
| mmu-miR-503 | 1.34 |
| mmu-miR-541 | 1.34 |
| mmu-miR-21 | 1.31 |
| mmu-miR-335-5p | 1.29 |
| mmu-miR-15b | 1.28 |
| mmu-miR-208b | 1.28 |
| mmu-miR-10b | 1.24 |
| mmu-miR-3084 | 1.24 |
| mmu-miR-3084* | 1.22 |
| mmu-miR-218 | 1.21 |
| mmu-miR-150 | 1.20 |
| mmu-miR-140* | 1.20 |
| mmu-miR-130a | 1.20 |
| mmu-miR-351 | 1.20 |
mi, Micro.
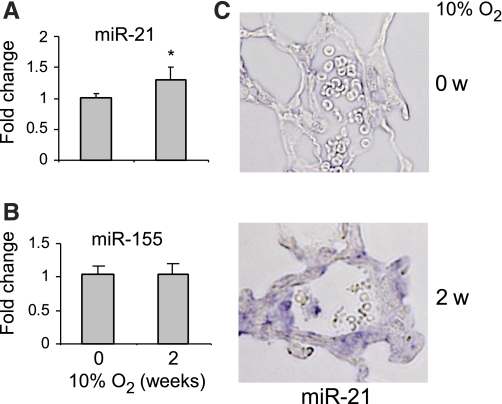
We (27) previously found that miR-21 regulates pulmonary interstitial remodeling in response to bleomycin-induced lung injury. Therefore, we initially chose to characterize the role of miR-21 in chronic hypoxia-induced pulmonary vascular remodeling. To validate the data from the miRNA array assay, which showed upregulation of miR-21 in the lungs of hypoxia-exposed mice, we performed real-time PCR on the same set of samples that were used for the profiling assay. We found a modest, but consistent and significant, increase in miR-21 expression in the lungs of mice exposed to hypoxia (Fig. 1A). However, the levels of miR-155, a miRNA that was previously shown to be induced in fibrotic lungs (39), remained unchanged (Fig. 1B). These data suggest that pulmonary interstitial remodeling and chronic hypoxia-induced pulmonary vascular remodeling are regulated via both common and distinct mechanisms. Although the increase in miR-21 expression, as revealed by real-time PCR using RNA isolated from whole lungs, was not striking, we reasoned that greater increases in specific compartments of the lungs, such as pulmonary distal arteries, might be concealed by the unaltered basal levels of miR-21 in other compartments of the lungs of hypoxia-exposed mice. To examine this hypothesis, we performed in situ hybridization assay for miR-21 and found that miR-21 expression was increased in the pulmonary distal arteries (30–150 μm in external diameter) of mice exposed to hypoxia (Fig. 1C). The enhanced expression of miR-21 appeared to be primarily in the media and adventitia of the pulmonary distal arteries. Taken together, these data suggest that alterations in miR-21 expression may participate in the pathogenesis of chronic hypoxia-induced vascular remodeling.
Fig. 1.
micro(mi)R-21 is upregulated in the lungs of mice in response to hypoxia. Mice were exposed to air or hypoxia (10% O2) for 2 wk (w). RNA from whole lungs was isolated and the levels of miR-21 (A) and miR-155 (B) in the lungs were determined by real-time PCR (n = 5; means ± SD). *P < 0.05. C: lungs from mice exposed to air or hypoxia were inflated and fixed with 10% formalin. Lung sections were prepared and were then blocked with hybridization solution for 4 h at room temperature, followed by incubation with digoxigenin (Dig)-conjugated miR-21 probes (Exiqon) or Dig-conjugated control probes with scrambled sequence (Exiqon) overnight. Sections were washed with 0.2× SSC followed by incubation with horseradish-peroxidase-conjugated anti-Dig antibody (Roche) overnight at 4°C. After being washed 3 times with buffer B1, the sections were developed with NBT/BCIP (Roche) for 24 h, with light blue cytoplasmic staining being positive.
Sequestration of miR-21, either before or after hypoxia exposure, diminishes chronic hypoxia-induced PH.
Given the increase in miR-21 expression in pulmonary small arteries of mice exposed to hypoxia, we next asked if blocking miR-21 in vivo is able to attenuate chronic hypoxia-induced PH. To answer this question, we designed and produced locked nucleic acid modified anti-miR-21 knockdown probes. Locked nucleic acid anti-miR probes bind to and form heteroduplexes with target miRNA, thereby sequestering and preventing the miRNA from binding to the 3′ UTR of its target mRNAs (14).
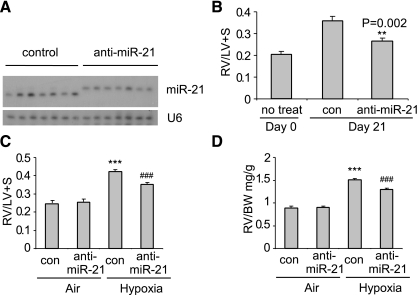
As shown in Fig. 2A and consistent with our previous study (27), intratracheal delivary of anti-miR-21 probes completely sequestered endogenous miR-21, as demonstrated by retarded migration of the miR-21 band due to the greater molecular weight of the miR-21:anti-miR-21 duplexes. Next, we examined the effects on chronic hypoxia-induced PH of blocking miR-21 before hypoxic exposure. As shown in Fig. 2B, sequestration of miR-21 reduced the ratio of the RV weight to the combined weights of LV and septum (RV/LV + S), a widely accepted and reliable index on the severity of RV hypertrophy and PH (26).
Fig. 2.
Sequestration of miR-21, either before or after hypoxia exposure, diminishes chronic hypoxia-induced pulmonary hypertension (PH). A: mice were given intratracheally control probes or anti-miR-21 probes at 10 mg/kg 2 times separated by 1 day. At 1 day after the second administration of the probes, the mice were exposed to hypoxia for 3 wk. RNA from lungs was isolated and miR-21 levels determined by Northern blotting assay. B: mice were treated as in A or left untreated. Three weeks after hypoxia exposure, hearts were harvested and right ventricle (RV)/left ventricle and septum (LV + S) determined (n = 3 in day 0 group; n = 7 in each of the 2 day 21 groups). C and D: mice were exposed to air or hypoxia for a week and then given intratracheally control (con) probes or anti-miR-21 probes at 10 mg/kg two times separated by 1 day. Three weeks after the hypoxia exposure, RV/LV + S (C) and RV weight to body weight (RV/BW; D) were determined (n = 4 in air groups; n = 7 in hypoxia groups). Data are means ± SE. ***P < 0.001, compared with air-con groups. ###P < 0.001, compared with the hypoxia-con group.
To determine if sequestration of miR-21 has the therapeutic potential to treat chronic hypoxia-induced PH, we administered anti-miR-21 probes intratracheally 7 days after the initiation of hypoxic exposure and then determined the severity of PH at day 21 of hypoxia. We found that the anti-miR-21 probes significantly attenuated chronic hypoxia-induced PH. Specifically, RV/LV + S in hypoxia-exposed mice that were given anti-miR-21 probes was decreased compared with that in hypoxia-exposed mice that were given control probes (Fig. 2C). Another index of RV hypertrophy, the ratio of the RV weight to body weight, was reduced in hypoxia-exposed mice that were given anti-miR-21 probes, compared with that found in hypoxia-exposed mice given control probes (Fig. 2D).
Sequestration of miR-21 attenuates chronic hypoxia-induced pulmonary vascular remodeling.
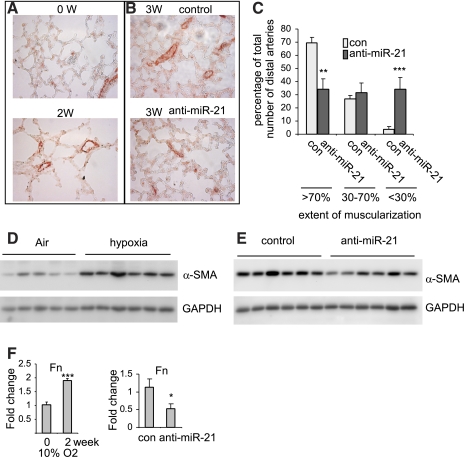
One of the pathological hallmarks for chronic hypoxia-induced pulmonary vascular remodeling is muscularization of pulmonary small distal arteries, caused by hyperplasia of PASMCs and/or trans-differentiation of PAAfbs and PAECs into smooth muscle cell-like cells (41, 49). We performed immunohistochemistry for α-SMA, a marker of SMCs, to evaluate the muscularization of pulmonary small distal arteries. As shown in Fig. 3A, mice exposed to hypoxia for 2 wk demonstrated marked increase in muscularization of pulmonary small distal arteries (i.e., those arteries with external diameter of 30–150 μm). Furthermore, we found that muscularization of pulmonary small distal arteries was attenuated in hypoxia-exposed mice that were given anti-miR-21 probes compared with hypoxia-exposed mice given control probes (Fig. 3B). Specifically, the percentage of small arteries with >70% muscularization were significantly decreased, and the percentage of small arteries with <30% muscularization increased in hypoxia-exposed mice that were given anti-miR-21 probes, compared with hypoxia-exposed mice treated with control probes (Fig. 3C). Next, we compared the levels of α-SMA in the lungs of mice that were exposed to air and hypoxia. As shown in Fig. 3D, α-SMA levels were increased in the lungs of hypoxia-exposed mice. However, the elevated levels of α-SMA were diminished in the lungs of hypoxia-exposed mice that were given anti-miR-21 probes, compared with mice that were given control probes (Fig. 3E). Additionally, we determined the expression of an ECM protein, Fn, in the lungs of mice exposed to hypoxia and treated with anti-miR-21 or control probes. As shown in Fig. 3F, Fn expression was increased in the lungs of mice exposed to hypoxia. However, the enhanced expression of Fn was attenuated by miR-21 sequestration. Taken together, these data suggest that miR-21 participates in chronic hypoxia-induced pulmonary vascular remodeling and participates in enhancing the expression of ECM proteins and increasing the muscularization of pulmonary small distal arteries.
Fig. 3.
Sequestration of miR-21 attenuate chronic hypoxia-induced pulmonary vascular remodeling. A: mice were exposed to hypoxia for 0 and 2 wk. Lung sections were prepared and α-smooth muscle actin (α-SMA) expression was assessed by immunohistochemistry (IHC) assays. B: Mice were exposed to air or hypoxia for a week and then given intratracheally control locked nucleic acid (LNA) probes or anti-miR-21 probes at 10 mg/kg, once every other day for a total of 2 times. Three weeks after hypoxia exposure, lung sections were prepared and α-SMA expression was assessed by IHC assays. C: muscularization of small distal arteries in B was determined by an observer blinded to the experimental conditions. Percentages of small distal arteries with >70%, 30–70%, <30% muscularization were calculated. Data are means ± SE. **P < 0.01, ***P < 0.001, compared with con group. D: mice were exposed to hypoxia for 0 and 3 wk. Lung tissue extracts were prepared and α-SMA expression assessed by Western blotting. GAPDH was used as controls. E: experiments were performed as in B. Lung tissue extracts were prepared and α-SMA expression assessed by Western blotting. F: experiments were performed as in A (left) or in B (right). RNA from lungs was isolated and fibronectin (Fn) expression was determined by real-time PCR. Data are means ± SD. **P < 0.01, compared with con group. *P < 0.05, compared with 0 wk.
miR-21 sequestration attenuates suppression of miR-21 targets in the lungs of hypoxia-exposed mice.
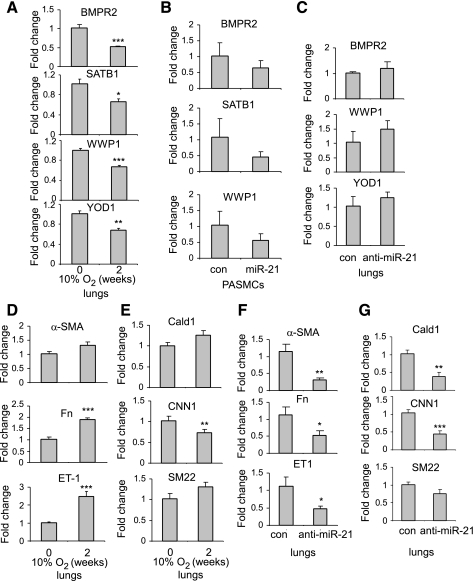
As shown in our initial experiments, miR-21 levels are increased in the lungs of hypoxia-exposed mice, suggesting that miR-21 may suppress expression of specific targets in the lungs of these mice. As shown in Fig. 4A, levels of putative miR-21 targets (30), including BMPR2, WWP1, SATB1, and YOD1, were decreased in the lungs of hypoxia-exposed mice, compared with air-exposed control mice. Transfection of miR-21 mimics into PASMCs also leads to a trend of reduced expression of BMPR2, SATB1, and YOD1 (Fig. 4B). As it has been shown previously that reduced expression of BMPR2 contributes to chronic hypoxia-induced PH in mice (35, 36), our data indicate that miR-21 targets may play a negative role in chronic hypoxia-induced pulmonary vascular remodeling.
Fig. 4.
miR-21 sequestration attenuates suppression of miR-21 targets in the lungs of hypoxia-exposed mice. A, D, and E: mice were exposed to hypoxia for 0 and 2 wk. RNA from lungs was isolated and the expression of bone morphogenetic protein receptor 2 (BMPR2), SATB1, WWP1, YOD1, endothelin-1 (ET-1), Col1A1, Fn, α-SMA, Cald1, CNN1, and SM22-α was determined by real-time PCR (n = 5 in each group). Data are means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, compared with air group. B: human pulmonary artery smooth muscle cells (PASMCs) were transfected with 10 μM control mimics or miR-21 mimics. Three days after transfection, RNA from cells was isolated and levels of BMPR2, SATB1, and WWP1 determined by real-time PCR. C, F, and G: mice were exposed to air or hypoxia for a week and then given intratracheally control LNA probes or anti-miR-21 probes at 10 mg/kg, once every other day for a total of 2 times. Three weeks after hypoxia exposure, RNA from lungs was isolated and the expression of BMPR2, SATB1, WWP1, YOD1, ET-1, Fn, α-SMA, Cald1, CNN1, and SM22-α was determined by real-time PCR (n = 7 in each group). Data are means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, compared with con group.
The suppression in the expression of miR-21 targets showed a trend of attenuation in the lungs of hypoxia-exposed mice that were given anti-miR-21 probes (Fig. 4C). Enhancing the expression of miR-21 targets may contribute to the beneficial effects of anti-miR-21 probes in attenuating chronic hypoxia-induced pulmonary vascular remodeling. Of note, levels of ET-1, Fn, α-SMA, and collagen I were increased in hypoxia-exposed lungs, consistent with previous studies (Fig. 4D). Similar to α-SMA, Cald1, and SM22-α showed elevated levels in lungs of hypoxia-exposed mice (Fig. 4E). Although α-SMA, Cald1, and SM22-α have been shown to have reduced expression in proliferative smooth muscle cells (12), the increased levels of α-SMA, Cald1 and SM22-α in the whole lungs of hypoxia-exposed mice simply represent an increase in the absolute number of smooth muscle cells in the lungs. The enhanced expression of ET-1, Fn, α-SMA, Cald1, and SM22-α was diminished in the lungs of hypoxia-exposed mice that were given anti-miR-21 probes (Fig. 4, F and G), consistent with attenuated pulmonary vascular remodeling in this group.
miR-21 regulates the proliferation of PASMCs in vitro.
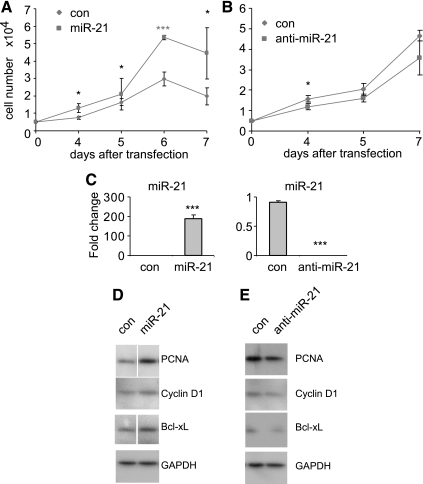
Muscularization of pulmonary distal arterials is partially caused by hyperplasia of PASMCs. Because blocking miR-21 attenuates muscularization of pulmonary distal arteries in hypoxia-exposed mice, we examined if miR-21 regulates PASMC proliferation in vitro. As shown in Fig. 5A, transfection of miR-21 mimics significantly accelerated PASMC growth, compared with that present in PASMC transfected with control mimics. Furthermore, blocking endogenous miR-21 by transfecting anti-miR-21 probes decreased PASMC growth (Fig. 5B). As expected, transfection of miR-21 mimics significantly increased, whereas transfection of anti-miR-21 decreased the levels of miR-21 in PASMCs (Fig. 5C).
Fig. 5.
miR-21 regulates the proliferation of PASMCs in vitro. A: human PASMCs were transfected with 10 nM control mimics or miR-21 mimics. One day after the transfection, the cells were trypsinized and plated onto 6-well plate at the same number. Cell number at day 4–7 after plating was counted. Growth curve was prepared. B: human PASMCs were transfected with 20 nM control inhibitors or anti-miR-21 inhibitors. One day after the transfection, the cells were trypsinized and plated onto 6-well plate at the same number. Cell number at days 4–7 after plating was counted. Growth curve was prepared. C: Human PASMCs were transfected with 10 nM control mimics or miR-21 mimics or transfected with 20 μM control inhibitors or anti-miR-21 inhibitors. Three days after transfection, RNA from cells was isolated and miR-21 levels determined by real-time PCR. D and E: human PASMCs were transfected with 10 nM control mimics or miR-21 mimics (D) or transfected with 20 μM control inhibitors or anti-miR-21 inhibitors (E). Three days after transfection, cell extracts were prepared and the levels of proliferating cell nuclear antigen (PCNA), cyclin D1, Bcl-xL, and control GAPDH determined by Western blotting. Lanes separated by a white line were from same gels after taking out unrelated experiments. *P < 0.05, **P < 0.01, ***P < 0.001, compared with con group.
miR-21 regulates the expression of proteins involved in cell cycle, cell proliferation and apoptosis in PASMCs in vitro.
As shown in Fig. 5D, transfection of miR-21 mimics enhanced the expression of PCNA, a nuclear protein that participates in active cell proliferation (37), and cyclin D1, which promotes G1-S phase transition (21), in PASMCs. Transfection of miR-21 mimics also increased the expression of Bcl-xL (47), an anti-apoptotic protein, in PASMCs. These data suggest that miR-21 can enhance proliferation and resistance to apoptosis in PASMCs, effects that may contribute to hyperplasia of PASMCs in vivo. As expected and in contrast to the effects of miR-21 mimics, anti-miR-21 decreased the expression of PCNA, cyclin D1, and Bcl-xL in PASMCs (Fig. 5E).
DISCUSSION
Although miRNAs have been shown to regulate numerous physiological and pathological processes, the role of miRNAs in the pathogenesis of chronic hypoxia-induced pulmonary vascular remodeling and pulmonary hypertension has not been well characterized. Previously, Courboulin et al. (7) found that miR-204 is downregulated in PASMCs from patients with pulmonary arterial hypertension (PAH) and PASMCs from rats with monocrotaline (MCT)-induced PAH. They showed that reconstitution of miR-204 attenuated MCT-induced PAH in rats (7). Caruso et al. (3) performed miRNA profiling and found that a number of miRNAs demonstrated significantly altered expression in the lungs of rats that were exposed to hypoxia or were given MCT. These data suggested that miRNAs participate in the pathogenesis of PAH in both patients and rodent models.
In our previous study, we found that the expression of miR-21 is enhanced in the lungs of mice with bleomycin-induced pulmonary fibrosis and in the lungs of patients with idiopathic pulmonary fibrosis (27). Blocking miR-21 attenuated interstitial remodeling in the lungs, thereby diminishing experimental pulmonary fibrosis (27). In the current study, we demonstrated that blocking miR-21 reduced chronic hypoxia-induced pulmonary vascular remodeling. Taken together, these data suggest that the remodeling processes in the lungs in response to bleomycin-induced injury and to chronic hypoxia may be regulated by common molecular mechanisms. Indeed, TGF-β1, one of the most important mediators in tissue regeneration and remodeling, is known to play important roles in both chronic hypoxia-induced pulmonary vascular remodeling and bleomycin-induced pulmonary interstitial remodeling (10, 20). Furthermore, it has been shown that TGF-β1 induces miR-21 expression in both lung fibroblasts and PASMCs in vitro (13, 27). Although miR-21 is upregulated in the lungs of hypoxia-exposed mice, we found no enhanced expression of miR-21 in human PASMCs undergoing hypoxia in vitro (data not shown). These data suggest that the upregulated expression of miR-21 in hypoxia-exposed lungs is not caused by activation of hypoxia associated transcriptional factors, such as hypoxia inducible factor-1, but rather by mediators that are involved in chronic hypoxia-induced vascular remodeling, such as TGF-β1.
In the present experiments, we found modest increases in miR-21 levels in whole lungs of hypoxia-exposed mice. Caruso et. al. (3) previously found a slightly decreased miR-21 expression in the lungs of rat with MCT-induced PH and in the lungs of patients with PAH, the group I of the six groups of PH according to the World Health Organization classifications. It needs to point out that although both the chronic hypoxia-induced PH model in mice and MCT-induced PH model in rats have been used to study pulmonary hypertensive process of human PAH, neither is perfect to recapitulate the molecular pathogenesis of PAH (51). In addition, the mouse model of chronic hypoxia-induced PH and the rat model of MCT-induced PH also differ significantly in molecular mechanisms leading to PH (51). Chronic hypoxia-induced PH in mice may represent a better model for the group III of human PH (pulmonary hypertension owing to lung diseases and/or hypoxia). Consistent with our findings, Pullamsetti et. al. (40) recently found that miR-21 is upregulated in the lungs of hypoxia-exposed mice. Despite modestly increased expression of miR-21 in the whole lungs of hypoxia-exposed mice, we demonstrated upregulation of miR-21 within the walls of pulmonary distal arteries. Furthermore, the enhanced expression of miR-21 appeared to be located in PASMCs and PAAFbs. Pulmonary distal arteries appear to be the primary sites of chronic hypoxia-induced pulmonary vascular remodeling (4). Thus our data suggest that elevated expression of miR-21, particularly in distal pulmonary arteries, contributes to the remodeling process that accompanies hypoxic PH, likely through regulating pathogenic activity of PASMCs and PAAFbs.
We found that sequestration of miR-21, either before or after hypoxia exposure, diminished chronic hypoxia-induced pulmonary vascular remodeling and PH-associated RV hypertrophy. Reduced pulmonary vascular remodeling and PH-associated RV hypertrophy in hypoxia-exposed mice with presequestered miR-21 indicate that miR-21 contributes to such a pathological progression. In addition, the findings that administering anti-miR-21 probes after hypoxia exposure attenuates the developing pulmonary vascular remodeling and RV hypertrophy suggest that blocking miR-21 may have therapeutic benefit in treating chronic hypoxia-induced pulmonary vascular remodeling and remodeling associated PH.
The present studies demonstrated that selected miR-21 targets, including BMPR2, WWP1, YOD1, and SATB1, were downregulated in the lungs of hypoxia-exposed mice. Among these targets, BMPR2 is known to have diminished expression in lungs exposed to hypoxia (35, 36). Reduced expression of BMPR2 has been shown to promote pulmonary vascular remodeling by enhancing proliferation of PASMCs (35, 36). WWP1, an E3 ubiquitin ligase, is not known to participate in tissue regeneration and remodeling. However, WWP1 was previously shown to be a negative regulator of TGF-β1 signaling, presumably through targeting Smad4 for degradation (34). The relationship among miR-21, WWP1, and TGF-β suggests a potential mechanism by which miR-21 promotes pulmonary vascular remodeling, a setting where TGF-β1 has a crucial role. YOD1 is a deubiquitinating enzyme and SATB1 is a global chromatin organizer and transcription factor that participates in integrating higher order chromatin architecture (16, 18). The exact role of YOD1 and SATB1 in chronic hypoxia-induced pulmonary vascular remodeling remains to be defined, although they may be involved in the regulation of the expression and/or degradation of important mediators in the remodeling process.
We found that miR-21 sequestration partially reverses the suppressed expression of miR-21 targets in hypoxia-exposed lungs. However, we must acknowledge that the de-repression of miR-21 targets is only modest. These data suggest that the beneficial effects of miR-21 sequestration found in hypoxia-exposed mice may result from the de-repression of multiple miR-21 targets that regulate vascular remodeling in the lungs. Our data are consistent with the notion that miRNAs function through not one but multiple targets in cells (2).
In these experiments, overexpression of miR-21 enhanced, whereas knockdown of miR-21 diminished, the proliferation of PASMCs in vitro. These data are consistent with previous studies (44) investigating the role of miR-21 in chronic hypoxia-induced PASMC proliferation and also suggest that the muscularization of pulmonary distal small arteries observed in hypoxia-exposed mice may result from enhanced proliferation of PASMCs as a result of elevated miR-21 expression. The beneficial effects of anti-miR-21 probes in hypoxia-exposed animals may result from the diminished proliferation of PASMCs in pulmonary distal small arteries. It should be pointed out that there are no predicated binding sites for miR-21 within the 3′-UTR of PCNA, cyclin D1, or Bcl-xL, indicating that these genes are not the direct targets of miR-21 and that the change in their expression may be a result of altered proliferation following manipulation of miR-21 expression in the cells.
Our data suggest that miR-21 plays a role in the pathogenesis of chronic hypoxia-induced pulmonary vascular remodeling. Our results also suggest that miR-21 is a potential target for novel therapeutics to treat chronic hypoxia associated pulmonary diseases, including PH.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-105473, HL-097218, and HL-076206 and American Heart Association Award 10SDG4210009.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.Y., S.B., A.d.F., and G.L. performed experiments; S.Y., S.B., A.d.F., E.A., and G.L. analyzed data; S.Y., S.B., A.d.F., E.A., and G.L. interpreted results of experiments; S.Y., S.B., A.d.F., H.C., N.X., E.A., and G.L. approved final version of manuscript; A.d.F., H.C., N.X., E.A., and G.L. edited and revised manuscript; E.A. and G.L. drafted manuscript; G.L. conception and design of research; G.L. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Yiu-Fai Chen at the University of Alabama at Birmingham for providing hypoxia chamber and intellectual inputs.
REFERENCES
- 1. Aaronson PI, Robertson TP, Ward JP. Endothelium-derived mediators and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 132: 107–120, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 23: 175–205, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 30: 716–723, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Chen YF, Feng JA, Li P, Xing D, Ambalavanan N, Oparil S. Atrial natriuretic peptide-dependent modulation of hypoxia-induced pulmonary vascular remodeling. Life Sci 79: 1357–1365, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Oparil S. Dominant negative mutation of the TGF-β receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol 100: 564–571, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chen YF, Oparil S. Endothelin and pulmonary hypertension. J Cardiovasc Pharmacol 35: S49–S53, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 10: 704–714, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 122: 6–7, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Cutroneo KR, White SL, Phan SH, Ehrlich HP. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol 211: 585–589, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol 168: 1793–1807, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis-Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol 31: 2370–2377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature 452: 896–899, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 94: 4273–4278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ernst R, Mueller B, Ploegh HL, Schlieker C. The otubain YOD1 is a deubiquitinating enzyme that associates with p97 to facilitate protein dislocation from the ER. Mol Cell 36: 28–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 277: 38205–38211, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Galande S, Purbey PK, Notani D, Kumar PP. The third dimension of gene regulation: organization of dynamic chromatin loopscape by SATB1. Curr Opin Genet Dev 17: 408–414, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50: 697–702, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene 25: 1620–1628, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kulshreshtha R, Ferracin M, Negrini M, Calin GA, Davuluri RV, Ivan M. Regulation of microRNA expression: the hypoxic component. Cell Cycle 6: 1426–1431, 2007 [PubMed] [Google Scholar]
- 23. Kwapiszewska G, Wilhelm J, Wolff S, Laumanns I, Koenig IR, Ziegler A, Seeger W, Bohle RM, Weissmann N, Fink L. Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res 6: 109, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Launay JM, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 8: 1129–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Li P, Oparil S, Novak L, Cao X, Shi W, Lucas J, Chen YF. ANP signaling inhibits TGF-β-induced Smad2 and Smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J Appl Physiol 102: 390–398, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med 15: 1289–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207: 1589–1597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK) -1-mediated NF-kappaB activation requires cytosolic and nuclear activity. FASEB J 22: 2285–2296, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation 119: 566–576, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc Natl Acad Sci USA 108: 10144–10149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, Eddahibi S. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med 168: 487–493, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Meyrick B, Gamble W, Reid L. Development of Crotalaria pulmonary hypertension: hemodynamic and structural study. Am J Physiol Heart Circ Physiol 239: H692–H702, 1980 [DOI] [PubMed] [Google Scholar]
- 33. Meyrick B, Reid L. Endothelial and subintimal changes in rat hilar pulmonary artery during recovery from hypoxia. A quantitative ultrastructural study. Lab Invest 42: 603–615, 1980 [PubMed] [Google Scholar]
- 34. Moren A, Imamura T, Miyazono K, Heldin CH, Moustakas A. Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J Biol Chem 280: 22115–22123, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 104: 790–795, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Nakaoka T, Gonda K, Ogita T, Otawara-Hamamoto Y, Okabe F, Kira Y, Harii K, Miyazono K, Takuwa Y, Fujita T. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. J Clin Invest 100: 2824–2832, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naryzhny SN. Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci 65: 3789–3808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 30: 364–372, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Pottier N, Maurin T, Chevalier B, Puissegur MP, Lebrigand K, Robbe-Sermesant K, Bertero T, Lino Cardenas CL, Courcot E, Rios G, Fourre S, Lo-Guidice JM, Marcet B, Cardinaud B, Barbry P, Mari B. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLos One 4: e6718, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 2011. December 8 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118: 2372–2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 51: 363–370, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 43: 629–634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 299: L861–L871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res 89: 1111–1121, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol 286: C416–C425, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Soussi T. p53 alterations in human cancer: more questions than answers. Oncogene 26: 2145–2156, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 21: 134–145, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Stenmark KR, Gerasimovskaya E, Nemenoff RA, Das M. Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest 122: 326S–334S, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Ten Freyhaus H, Dagnell M, Leuchs M, Vantler M, Berghausen E, Caglayan E, Weissmann N, Dahal BK, Schermuly RT, Ostman A, Kappert K, Rosenkranz S. Hypoxia enhances PDGF signaling in the pulmonary vasculature by downregulation of protein tyrosine phosphatases. Am J Respir Crit Care Med 183: 1092–1102, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Tozzi CA, Christiansen DL, Poiani GJ, Riley DJ. Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am J Respir Crit Care Med 149: 1317–1326, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Weitzenblum E, Chaouat A, Canuet M, Kessler R. Pulmonary hypertension in chronic obstructive pulmonary disease and interstitial lung diseases. Semin Respir Crit Care Med 30: 458–470, 2009 [DOI] [PubMed] [Google Scholar]