Abstract
In the pulmonary vasculature, cGMP levels are regulated by soluble guanylate cyclase (sGC) and phosphodiesterase 5 (PDE5). We previously reported that lambs with persistent pulmonary hypertension of the newborn (PPHN) demonstrate increased reactive oxygen species (ROS) and altered sGC and PDE5 activity, with resultant decreased cGMP. The objective of this study was to evaluate the effects of hydrocortisone on pulmonary vascular function, ROS, and cGMP in the ovine ductal ligation model of PPHN. PPHN lambs were ventilated with 100% O2 for 24 h. Six lambs received 5 mg/kg hydrocortisone every 8 h times three doses (PPHN-hiHC), five lambs received 3 mg/kg hydrocortisone followed by 1 mg·kg−1·dose−1 times two doses (PPHN-loHC), and six lambs were ventilated with O2 alone (PPHN). All groups were compared with healthy 1-day spontaneously breathing lambs (1DSB). O2 ventilation of PPHN lambs decreased sGC activity, increased PDE5 activity, and increased ROS vs. 1DSB lambs. Both hydrocortisone doses significantly improved arterial-to-alveolar ratios relative to PPHN lambs, decreased PDE5 activity, and increased cGMP relative to PPHN lambs. High-dose hydrocortisone also increased sGC activity, decreased PDE5 expression, decreased ROS, and increased total vascular SOD activity vs. PPHN lambs. These data suggest that hydrocortisone treatment in clinically relevant doses improves oxygenation and decreases hyperoxia-induced changes in sGC and PDE5 activity, increasing cGMP levels. Hydrocortisone reduces ROS levels in part by increasing SOD activity in PPHN lambs ventilated with 100% O2. We speculate that hydrocortisone increases cGMP by direct effects on sGC and PDE5 expression and by attenuating abnormalities induced by oxidant stress.
Keywords: phosphodiesterase 5, soluble guanylate cyclase, reactive oxygen species
at birth, pulmonary vascular resistance (PVR) dramatically decreases as the pulmonary vasculature adapts to the extrauterine environment. Persistent pulmonary hypertension of the newborn (PPHN) occurs when this transition fails, leading to persistently elevated PVR, right-to-left shunting, and hypoxemia. PPHN affects 1 in 500 term infants and is associated with multiple underlying conditions. In spite of recent treatment advances, PPHN continues to be associated with significant short-term and long-term morbidity, as well as a significant risk of death (24).
Inhaled nitric oxide (iNO) improves oxygenation and reduces the need for extracorporeal membrane oxygenation support but has not been shown to improve survival or long-term neurodevelopmental outcomes in PPHN (17). As many as half of infants with PPHN do not respond or sustain their response to iNO (9, 30a). High concentrations of inspired oxygen are also used to promote pulmonary vasodilation in PPHN, but their benefits and risks for this population are not well defined. Hyperoxia has been associated with both alveolar and vascular remodeling in bronchopulmonary dysplasia in preterm neonates (21), and a growing body of evidence suggests that even short-term exposure to 100% oxygen may cause oxidative injury in the pulmonary parenchyma and vasculature (25, 36). New therapies for PPHN are needed to augment current therapeutic strategies as well as to prevent long-term complications of standard management.
Glucocorticoids have been used in neonates for the treatment of pressor-resistant hypotension and adrenal insufficiency and for prophylaxis of bronchopulmonary dysplasia. They are potent anti-inflammatory agents that have been reported to decrease hospital stay and duration of oxygen dependence in meconium aspiration syndrome, a disease often associated with the development of PPHN (34, 39). Recent evidence from animal models of PPHN also suggests a potential role for glucocorticoids in restoring normal pulmonary vascular function. In a fetal lamb model of chronic intrauterine pulmonary hypertension, prenatally administered betamethasone attenuated oxidative stress and improved in vitro pulmonary artery (PA) response to vasodilators (8). In a porcine model of meconium aspiration, methylprednisolone improved oxygenation and attenuated the pulmonary hypertensive response (32). However, virtually nothing is known about the effects of postnatally administered glucocorticoids on pulmonary vascular reactivity, key pathways regulating cGMP levels, or modulation of oxidative stress in PPHN.
In the normal pulmonary vasculature, NO produced in the endothelium activates soluble guanylate cyclase (sGC) in the vascular smooth muscle to produce cGMP, a critical second messenger that initiates and maintains vasorelaxation. In the lung, cGMP is inactivated by phosphodiesterase 5 (PDE5). Recent evidence indicates that oxidant stress plays a significant role in PPHN pathogenesis (5, 23, 41) and promotes vascular dysfunction in part by oxidizing and reducing sGC activity (33) and by increasing PDE5 activity and expression in vascular smooth muscle cells. Together, these changes would be expected to decrease cGMP concentrations and increase vasoconstriction (12). We (13) recently demonstrated that administration of antioxidants such as SOD or catalase via the airway was sufficient to normalize PDE5 expression and activity, as well as increase cGMP concentrations in resistance PA. Taken together, these findings illustrate the importance of increased oxidative stress in pathogenesis of neonatal pulmonary hypertension (13). The goal of the present study was to investigate the effects of hydrocortisone on oxygenation, oxidative stress, and PDE5 expression and activity in PPHN. We used a well-established lamb model of PPHN to test the hypothesis that hydrocortisone restores normal postnatal patterns of sGC and PDE5 activity, leading to improvements in oxygenation.
MATERIALS AND METHODS
Fetal surgery and ventilation protocols for neonatal lambs.
The study was approved by the Laboratory Animal Care Committees at the State University of New York at Buffalo and at Northwestern University. Pregnant ewes and newborn lambs were obtained from the Swartz family farm in Attica, NY. Pulmonary hypertension was established by surgical ligation of the ductus arteriosus in lambs at 126 days gestation as previously described (26). PPHN lambs were delivered by Cesarean section at 135 days gestation, representing 93% of the 143-day term gestation (31), placed under servo-controlled radiant warmers, intubated, and given 3 ml/kg calfactant (ONY, Amherst, NY; a gift from Dr. Edmund A. Egan). Three ventilated groups received 100% O2 alone (n = 6), 100% O2 plus 5 mg·kg−1·dose−1 hydrocortisone every 8 h for total of three doses (PPHN-hiHC; n = 6), or 100% O2 plus 3 mg/kg loading dose of hydrocortisone followed by 1 mg·kg−1·dose−1 times two doses every 8 h (PPHN-loHC; n = 5). Ventilator management included keeping positive end-expiratory pressure constant at 4 cmH2O and adjusting peak inflation pressure to a minimum of 18 cmH2O based on blood gas CO2 values. The subsequent care protocols have been described in detail previously (15). After 24 h of ventilation, lambs were anesthetized and killed by cardiac puncture and exsanguination. The heart and lungs were removed en bloc, and fifth-generation PA (inner diameters of 500 μm) were isolated. Tissue samples were snap frozen in liquid nitrogen and stored at −80°C until analysis. One-day spontaneously breathing lambs (1DSB) were healthy newborn lambs that delivered spontaneously at comparable gestation to the experimental lambs, fed normally, and breathed room air. 1DSB lambs were anesthetized with pentothal and killed by rapid exsanguination as described above. Fetal control lambs (n = 6) were unoperated twins of PPHN lambs, were killed before first breath, and served as reference controls for expression studies as previously reported (13, 15).
cGMP EIA.
cGMP content in snap-frozen PA tissue was measured by EIA in duplicate, using a commercially available kit (Cayman Chemical) as previously described (13). Results were measured using a Labsystems Multiskan EX automated plate reader at 420 nm. Results are shown as picomoles of cGMP per milligrams of frozen tissue.
Quantitative reverse transcription real-time PCR.
Frozen PA tissue was ground on liquid nitrogen. RNA was isolated using the Aurum total RNA mini kit (Bio-Rad, Hercules, CA) and quantified using the Quant-iT RiboGreen assay (Molecular Probes/Invitrogen, Carlsbad, CA). cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time PCR was performed using the iQ SYBR Green Supermix (Bio-Rad) with the iCycler iQ real-time PCR detection system (Bio-Rad) as previously described (12). Real-time PCR for PDE5 with a β-actin internal control was performed with 35 cycles of real-time data collection using 95°C for 10 s and 48.2°C for 45 s. PDE5 and β-actin primers were designed using Beacon Designer software (Premier BioSoft International, Palo Alto, CA) and have been previously described (12). For all primers, amplicons between 75 and 150 bp in length were produced, and there was a single product on melt-curve analysis with good correlation for efficiency and standard curves (r2 ≥ 0.98). All samples were analyzed in duplicate. Relative PDE5 amounts were normalized to β-actin using the ΔΔCT method (28). Data are shown as fold relative to fetal control lambs.
Western blot analysis.
Frozen PA tissue was homogenized, and total protein was collected using the PARIS kit (Ambion, Austin, TX) supplemented with protease (Sigma, St. Louis, MO) and phosphatase inhibitors (EMD Biosciences, San Diego, CA) as previously described (12, 15). Protein concentration was measured using the Bradford assay (4). PDE5 and sGC subunit protein expression was assessed in all animals via Western blot, which was performed as previously described (12, 13, 15). Membranes were blocked for 1 h at room temperature with 5% nonfat dry milk in TBS containing 0.1% Tween 20 (1× TBST) and incubated overnight at 4°C with primary antibody in 5% milk + 1× TBST at an appropriate dilution [1:500 for mouse anti-PDE5 (BD Transduction, San Jose, CA), 1:500 for rabbit anti-sGCα (BD Transduction Laboratories), and 1:2,000 for mouse β-actin (Sigma)]. The membranes were washed and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Pierce, Rockford, IL) diluted 1:1,000 in 5% milk + 1× TBST. Membranes were then washed and exposed via chemiluminescence (Pierce). Bands were analyzed using a Digital Science Image Station (Kodak, Rochester, NY). Expression was normalized to β-actin. Data are shown as fold relative to fetal control lambs.
Immunohistochemistry.
After death, the right middle lobe of the lung was removed, and optimum cutting temperature compound was pushed gently into the deflated lobe and allowed to solidify as previously described (13, 15). Blocks and sections were prepared as previously described (13, 15). Sections were blocked with 5% BSA at room temperature for 1 h and then stained overnight at 4°C with primary antibody at a 1:50 dilution in 5% BSA [anti-PDE5 (BD Transduction) or anti-nitrotyrosine (Cayman Chemical)]. With the use of an Alexa Fluor 488 anti-mouse antibody (Molecular Probes/Invitrogen) for PDE5 or anti-rabbit antibody (Molecular Probes/Invitrogen) for 3-nitrotyrosine (3-NT) at 1:200 dilution in 5% BSA and a Nikon Eclipse TE-300 fluorescent microscope, the tissue localization and expression of PDE5 or 3-NT was visualized by fluorescence microscopy with excitation at 495 nm and emission at 519 nm (13).
PDE5 activity assay.
Protein was prepared from snap-frozen PA tissue as described above and was purified over a Centri-Spin 10 column to remove any phosphate contamination (Princeton Separations, Adelphia, NJ). Protein concentration was determined using the Bradford method. Total protein (5 μg) was assayed for cGMP hydrolytic activity using a commercially available colorimetric cyclic nucleotide phosphodiesterase assay kit with and without sildenafil (Biomol, Plymouth Meeting, PA) as described previously (12, 13). Results are shown as the PDE5-specific picomoles cGMP hydrolyzed per milligrams total protein per minute.
sGC activity assay.
Total lung protein was prepared as described above and assayed the same day as previously described (12). Protein concentration was determined as described above. Samples were prepared as previously preparation described (13). Each sample was dried, resuspended in cGMP EIA buffer, and acetylated according to the manufacturer's protocol. cGMP was measured by EIA in duplicate using a commercially available kit (Cayman Chemical). Results were measured using a Labsystems Multiskan EX automated plate reader at 420 nm. sGC activity results are shown as picomoles cGMP per milligram total protein per minute.
In situ analysis of superoxide generation.
Frozen lung sections were exposed to 5 μM dihydroethidium (DHE; Molecular Probes/Invitrogen) in PBS. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min. Ethidium-stained slices were observed by fluorescence microscopy with excitation at 518 nm and emission at 605 nm. Fluorescent images were captured using a CoolSnap digital camera with Metamorph imaging software (Molecular Devices, Sunnyvale, CA). Tissue sections were processed and imaged in parallel as previously described (15).
SOD activity assay.
Frozen lung tissue was ground on liquid nitrogen and sonicated in ice-cold 50 mM potassium phosphate buffer (pH 7.6) containing protease inhibitors. Protein concentration was determined by the Bradford method, and samples were analyzed immediately for enzyme activity using a commercially available SOD assay kit (Stressgen, Ann Arbor, MI). Activity was normalized to protein content and expressed relative to control samples (16).
Statistical analysis.
All data are expressed as the means ± SE with each n representing a single lamb studied. Results were analyzed by repeated-measures ANOVA with Bonferroni's multiple comparison test where appropriate using Prism software (GraphPad Software, San Diego, CA). Statistical significance was set at P < 0.05.
RESULTS
Hydrocortisone improves oxygenation in PPHN lambs ventilated with 100% O2.
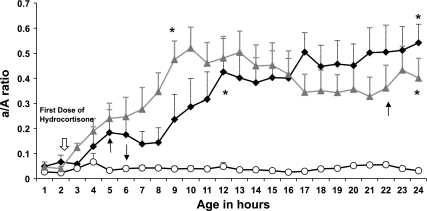
The arterial/alveolar (a/A) ratio was used to assess the effectiveness of oxygen exchange. PPHN lambs ventilated with 100% oxygen alone had persistently low a/A ratios throughout the 24-h study period. In comparison, both low-dose and high-dose hydrocortisone significantly improved a/A ratios in PPHN lambs (Fig. 1). Treatment with hydrocortisone did not significantly alter mean arterial blood pressure throughout the 24-h study period (Fig. 2) but did reduce the need for boluses and/or dopamine. All lambs in the oxygen only group received a fluid bolus and were transfused; one lamb required dopamine. One high-dose hydrocortisone lamb and two low-dose hydrocortisone lambs received fluid boluses and transfusions. While both groups of hydrocortisone-treated PPHN lambs had lower average ventilator rates compared with untreated PPHN lambs (Fig. 3B), there was no significant difference in mean airway pressure between the three groups (Fig. 3A). Treatment with hydrocortisone resulted in improved arterial pH and decreased Pco2 relative to untreated PPHN lambs (Fig. 3, C and D). Hydrocortisone treatment resulted in hyperglycemia requiring glucose infusion rate reduction to maintain normoglycemia (data not shown). One untreated PPHN lamb died at 6 h of age from severe hypoxemia. One low-dose hydrocortisone lamb died from pulmonary hemorrhage at 22 h of age, and one high-dose hydrocortisone lamb died at 5 h of age with severe hypoxemia.
Fig. 1.
Hydrocortisone improves the arterial to alveolar (a/A) ratio in persistent pulmonary hypertension of the newborn (PPHN) lambs. a/A ratios were measured in three groups of lambs: PPHN lambs (n = 6; white circles), lambs that received 3 mg/kg hydrocortisone followed by 1 mg·kg−1·dose−1 times two doses (PPHN-loHC lambs; n = 5; grey triangles), and lambs that received 5 mg/kg hydrocortisone every 8 h times three doses (PPHN-hiHC; n = 6; black diamonds). There was 1 death in each of the 3 groups (shown by arrows). Data are shown as means ± SE. *P < 0.05 vs. PPHN lambs.
Fig. 2.
Mean systemic blood pressure in PPHN lambs ventilated with 100% oxygen with and without hydrocortisone. Mean systemic blood pressure in PPHN lambs (n = 6; white circles), PPHN-loHC lambs (n = 5; grey triangles), and PPHN-hiHC lambs (n = 6; black diamonds). There was no significant difference in mean systemic blood pressure between PPHN lambs ventilated with 100% oxygen alone and PPHN lambs ventilated with 100% oxygen receiving hydrocortisone.
Fig. 3.
Ventilator settings and blood gas variables in PPHN lambs ventilated with 100% oxygen with and without hydrocortisone. Changes in mean airway pressure (MAP; A), ventilator rate (B), pH (C), and Pco2 (D) in PPHN lambs (white circles), PPHN-loHC lambs (grey triangles), and PPHN-hiHC lambs (black diamonds) during the study period. Data are shown as means ± SE. *P < 0.05 vs. PPHN lambs by ANOVA over time.
Hydrocortisone increases steady-state cGMP in PA from PPHN lambs.
Previous studies (35) have reported decreased cGMP levels in PA from fetal lambs with PPHN. Here, we demonstrate that while cGMP levels were not different in PA from PPHN lambs ventilated with 100% O2 compared with 1DSB lambs (0.02 ± 0.006 vs. 0.03 ± 0.008 pmol/mg tissue; Fig. 4), hydrocortisone increased pulmonary vascular cGMP levels vs. untreated PPHN lambs (3.6 ± 0.9- and 10 ± 2-fold, respectively; P < 0.05; Fig. 4).
Fig. 4.
Hydrocortisone increases cGMP in pulmonary arteries from PPHN lambs. cGMP concentrations were measured in resistance pulmonary arteries from 1-day spontaneously breathing lambs (1DSB) lambs (n = 4), PPHN lambs (n = 6), PPHN-loHC lambs (n = 5), and PPHN-hiHC lambs (n = 6). cGMP concentration was assayed by enzyme-linked immunoassay and normalized for milligrams of total protein. Data are shown as means ± SE. *P < 0.05 vs. PPHN lambs.
High-dose hydrocortisone increases sGC activity in resistance PA from PPHN lambs.
We (13, 35) previously reported decreased sGCα expression in fetal PPHN lambs vs. healthy fetal controls. Here, we demonstrate that 1-day-old ventilated PPHN lambs have sGCα protein expression similar to that observed in healthy 1DSB lambs (Fig. 5A). Treatment with hydrocortisone had no effect on sGCα expression (Fig. 5A). A representative blot is shown in Fig. 5B. sGC activity was decreased by 72 ± 3% in PPHN lambs relative to 1DSB lambs, while treatment with high-dose hydrocortisone significantly increased sGC activity by 81 ± 25% vs. PPHN lambs (Fig. 5C).
Fig. 5.
High-dose hydrocortisone increases sGC activity but does not affect soluble guanylate cyclase-α (sGCα) protein expression in lambs with PPHN. sGCα protein expression (A) was measured in resistance pulmonary arteries from 1DSB lambs (n = 4), PPHN lambs (n = 6), PPHN-loHC lambs (n = 5), and PPHN-hiHC lambs (n = 6) via Western blot and normalized to β-actin. Representative blot is shown for sGCα and β-actin (B). sGC activity (C) was measured in lung tissue by EIA and normalized for milligrams of total protein. Data are shown as means ± SE relative to fetal controls for sGCα expression and as means ± SE for sGC activity. #P < 0.05 vs. 1DSB; *P < 0.05 vs. PPHN.
High-dose hydrocortisone decreases PDE5 protein expression and activity in PA of PPHN lambs ventilated with 100% oxygen.
We (13) recently reported increased PA PDE5 protein expression and activity in PPHN lambs ventilated with 100% oxygen for 24 h relative to fetal PPHN lambs. In the current study, we found that hydrocortisone significantly increased PDE5 mRNA (Fig. 6A) but decreased PA PDE5 protein expression by 54 ± 7% (Fig. 6, B and C). As expected, PDE5 protein expression localized to PA smooth muscle (Fig. 6D). PDE5 activity was dramatically increased in PPHN lambs ventilated with 100% O2 relative to 1DSB lambs (5 ± 0.5-fold; Fig. 7). Treatment with both low and high-dose hydrocortisone significantly decreased PDE5 enzyme activity compared with untreated PPHN lambs (49 ± 20 and 93 ± 2%, respectively; Fig. 7), with high-dose hydrocortisone decreasing PDE5 activity to levels similar to those observed in healthy 1DSB lambs (Fig. 7).
Fig. 6.
High-dose hydrocortisone decreases phosphodiesterase 5 (PDE5) protein expression in resistance pulmonary arteries from PPHN lambs. PDE5 mRNA and protein expression were measured in resistance pulmonary arteries from 1DSB lambs (n = 4), PPHN lambs (n = 6), PPHN-loHC lambs (n = 5), and PPHN-hiHC lambs (n = 6). A: PDE5 mRNA expression was measured by real-time PCR and normalized to β-actin. B: PDE5 protein expression was analyzed via Western blot, normalized to β-actin. Data are shown as means ± SE. C: representative Western blot is shown for PDE5 and β-actin. D: phase-contrast images (20×) of frozen lamb lung sections (top) with the corresponding sections stained for PDE5 shown in red (bottom). #P < 0.05 vs. 1DSB; *P < 0.05 vs. PPHN.
Fig. 7.
Hydrocortisone decreases PDE5 activity in pulmonary arteries from PPHN lambs. PDE5 activity was measured in resistance pulmonary arteries from 1DSB lambs (n = 4), PPHN lambs (n = 6), PPHN-loHC lambs (n = 5), and PPHN-hiHC lambs (n = 6). PDE5-specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total milligrams of protein. Data are shown as mean ± SE. #P < 0.05 vs. 1DSB; *P < 0.05 vs. PPHN.
Hydrocortisone reduces oxidative stress in PPHN lambs ventilated with 100% oxygen.
We (5, 15) have previously demonstrated that ventilation with high levels of O2 increases reactive oxygen species (ROS) in PPHN lambs and that one mechanism is likely reduced activity of SOD. We first sought to determine effects of hydrocortisone treatment on oxidative stress by measuring DHE fluorescence, a marker of superoxide production. As expected, oxidative stress was increased by 3.07 ± 0.2-fold in PPHN lambs ventilated with 100% oxygen relative to 1DSB lambs (P < 0.05, Fig. 8A). DHE fluorescence was decreased by 70 ± 8% in lambs treated with high-dose hydrocortisone but was not significantly affected by low-dose hydrocortisone (Fig. 8A). Total SOD activity was decreased by 53 ± 6% in ventilated PPHN lambs vs. 1DSB lambs (P < 0.05; Fig. 8B), and high-dose hydrocortisone increased SOD activity by 90 ± 30%, and to levels equivalent to the 1DSB lambs (Fig. 8B). We also investigated the effects of hydrocortisone on 3-NT, a marker of peroxynitrite-dependent oxidative protein damage. 3-NT levels were increased by 2.6 ± 0.4-fold in PPHN lambs relative to 1DSB animals (Fig. 9), and treatment with high-dose hydrocortisone significantly decreased 3-NT levels by 47 ± 9% (Fig. 9).
Fig. 8.
Hydrocortisone decreases oxidative stress in lambs with PPHN. Dihydroethidium (DHE) fluorescence (A) and total SOD activity (B) were measured in lung tissue from 1DSB lambs (n = 4), PPHN lambs (n = 6), PPHN-loHC lambs (n = 5), and PPHN-hiHC lambs (n = 6). A: frozen lung sections were incubated with DHE and imaged using fluorescence microscopy. Conversion of DHE by superoxide to ethidium results in red nuclear fluorescence. Values are means ± SE (5–7 vessels quantified per animal imaged) relative to 1DSB lambs. Representative images of DHE staining are shown at bottom. B: total SOD activity was measured using a commercially available colorimetric assay. Activity was normalized to protein content and expressed relative to control samples. #P < 0.05 vs. 1DSB. *P < 0.05 vs. PPHN.
Fig. 9.
High-dose hydrocortisone decreases 3-NT levels. 3-NT levels were measured in resistance pulmonary arteries from 1DSB lambs (n = 4), PPHN lambs (n = 4), PPHN-loHC lambs (n = 3), and PPHN-hiHC lambs (n = 4). 3-Nitrotyrosine (3-NT) expression was quantified by immunohistochemistry. Data are shown as means ± SE relative to fetal controls. Representative fluorescent images of pulmonary arteries with corresponding light microscopy images from various groups of lambs are shown at bottom. #P < 0.05 vs. 1DSB. *P < 0.05 vs. PPHN.
DISCUSSION
Corticosteroids have been investigated as a potential treatment for critical illnesses such as sepsis and acute lung injury, and limited data indicate they may benefit infants with meconium aspiration (2, 11, 34), the leading cause of neonatal pulmonary hypertension. The effect of hydrocortisone on PPHN without parenchymal lung disease is not known. In the present study, we sought to determine whether hydrocortisone improved oxygenation in PPHN lambs and whether its mechanism of action included effects on pulmonary vascular regulation of cGMP concentrations and/or oxidative stress. We tested two different dosing strategies to study the dose-response effect of hydrocortisone. We found that hydrocortisone treatment of hyperoxia-exposed PPHN lambs resulted in improved oxygenation, increased steady-state cGMP concentrations, increased sGC activity, decreased PDE5 protein expression and activity, and decreased ROS production in resistance PA. We propose that the changes in PDE5 expression and activity are attributable, in part, to decreased oxidative stress in hydrocortisone-treated lambs.
We found that hydrocortisone improved oxygenation in PPHN lambs, as evidenced by increased a/A oxygen ratios. Three other therapeutic agents, iNO, recombinant human SOD (rhSOD), and PEG-catalase have also been reported to improve systemic oxygenation in this animal model (26, 41). In the current study, we found that while the improvement with hydrocortisone occurred at a later time point relative to rhSOD and iNO (Fig. 1), the overall degree of oxygenation improvement was comparable with no adverse effects on systemic blood pressure (Fig. 2). Although treatment with hydrocortisone resulted in lower ventilator rates and improved gas exchange (Fig. 3), we believe that the changes in oxygenation were likely secondary to normalization of cGMP concentrations and PDE5 activity. Our previous findings that ventilation of healthy near-term neonatal sheep with 100% O2 increases PDE5 expression and activity (12) and, similarly, that ventilation of PPHN lambs with 100% O2 increased PDE5 expression and activity leading to impaired cGMP-medicated vasodilation (15) underscore the importance of oxidative stress as a key player in the ductal-ligation model of PPHN.
We did not observe a difference in vascular cGMP concentrations in PPHN lambs ventilated with 100% O2 vs. 1DSB lambs in this study (Fig. 4) (13). There was, however, a significant increase in steady-state cGMP concentrations (Fig. 4) in hydrocortisone-treated lambs compared with untreated PPHN animals. The observed increases in cGMP in both hydrocortisone-treated groups were comparable to the levels we previously observed with the use of iNO, rhSOD, and PEG-catalase in the lamb model of PPHN (13, 16) and might, at least in part, explain the improvements in oxygenation seen in lambs receiving hydrocortisone.
To further investigate the mechanisms responsible for increasing cGMP levels, we sought to determine the effects of hydrocortisone on sGC and PDE5, two key regulators of cGMP. Reductions in sGC expression and activity under conditions of oxidative stress have been previously described in the literature (33, 40, 42). For instance, sGCα subunit protein expression and sGC activity were decreased in PA from fetal PPHN lambs relative to healthy control lambs (3, 35). In the current study, we found no significant differences in sGCα expression between 1DSB and ventilated PPHN lambs and no effect of hydrocortisone treatment on sGCα expression (Fig. 5A). However, sGC activity was significantly decreased in ventilated PPHN lambs relative to 1DSB lambs (Fig. 5B), and hydrocortisone treatment of PPHN lambs significantly increased sGC activity (Fig. 5B). These findings are consistent with other reports of glucocorticoid-mediated stimulation of sGC activity in other types of tissue, such as rat glomeruli and small intestine mucosa (27, 30).
We found that hydrocortisone reduced PDE5 protein expression and activity (Figs. 6 and 7), which would also be expected to increase cGMP levels. Steroids have been reported to affect a small subgroup of PDE enzymes, predominantly PDE3 and PDE4 (1, 10, 20). To our knowledge, this study is the first report that glucocorticoids also affect PDE5, an important modulator of pulmonary vascular tone. Hydrocortisone's effects on PDE5 are likely complex and act through multiple mechanisms as evidenced by increased PDE5 mRNA (Fig. 6A) but decreased protein expression (Fig. 6B) and activity (Fig. 7). The observed differences between expression and activity might be due to posttranslational modifications of PDE5, which are independent of expression and have also been previously described by other investigators (19).
We and others (5, 13, 15, 23) have reported increased oxidative stress in the pulmonary vasculature of lambs with pulmonary hypertension. Because ROS are known to alter sGC and PDE5 activity (12, 13, 40), we explored whether hydrocortisone also reduced oxidant stress. Interestingly, while both low and high-dose hydrocortisone significantly decreased PDE5 activity and increased cGMP levels, lambs treated with high-dose hydrocortisone also had significantly lower ROS levels compared with control PPHN lambs (Figs. 8 and 9). Lambs treated with high-dose hydrocortisone additionally had higher sGC activity, lower PDE5 activity, and higher cGMP levels compared with lambs treated with lower doses.
Previous studies (18, 38) have shown that prenatal glucocorticoids accelerate the late gestation increase in lung antioxidant enzyme activity and decrease ROS. At least one isoform of SOD, copper-zinc SOD, has been found to contain glucocorticoid responsive elements in the 5′-flanking region of the gene promoter (22). In addition, a recent report (8) indicates that antenatal betamethasone reduces vascular oxidant stress, increases MnSOD expression, and improves the in vitro pulmonary vascular response to vasodilators in a lamb model of PPHN. Our findings suggest that postnatal administration of glucocorticoids may also help restore normal vascular function, in part through reducing ROS-mediated alterations in sGC and PDE5 function. As pulmonary hypertension is seldom identified before birth, our study provides important evidence that early postnatal administration of hydrocortisone may enhance total vascular SOD activity and reduce levels of ROS such as superoxide and peroxynitrite.
Similar to other causes of acute lung injury, inflammation is believed to play an important role in the pathophysiology of PPHN due to meconium aspiration, pneumonia, and other causes; several studies (7, 37) illustrate the importance of both inflammation and oxidative stress in the pathophysiology of pulmonary hypertension due to meconium aspiration syndrome. Activation of inflammatory cells results in increased production of ROS, creating an important link between inflammation and oxidative stress. On the other hand, glucocorticoids may decrease pulmonary oxidative stress in the presence of inflammation (29). Hydrocortisone treatment could therefore decrease ROS directly through altering expression or activity of prooxidant and antioxidant enzymes or indirectly by modulation of the inflammatory response. While both the dosing regimens used in this study would be expected to be anti-inflammatory, it is also possible that the higher hydrocortisone dose had a greater antioxidant effect. Future studies are planned to delineate whether the effects we observed are due to anti-inflammatory vs. antioxidant effects of hydrocortisone.
We previously demonstrated that hyperoxia induces PDE5 expression and activity and diminishes cGMP response to exogenous NO in fetal PA smooth muscle cells and that these effects can be reversed by antioxidants (12). We and others (5, 15, 23) have also shown that increased oxidative stress is at least partly responsible for the impaired pulmonary vasodilatation in pulmonary hypertension. High concentrations of oxygen are commonly used to attempt to reverse the hypoxemia associated with PPHN. However, PPHN lambs remain hypoxemic even when breathing 100% O2 (Fig. 1), and this level of hyperoxia exaggerates levels of oxidative stress and increases PDE5 activity to levels that are much greater than in control lambs (5, 13, 15). In contrast, treatment with antioxidants such as rhSOD or catalase normalized PDE5 expression and activity as well as cGMP levels in ventilated PPHN lambs (13). The current study demonstrates that hydrocortisone treatment similarly normalizes PDE5 protein expression and activity, leading to increases in steady-state cGMP levels. Our proposed model is that hydrocortisone reduces oxidative stress that, in turn, results in increased sGC activity and decreased PDE5 expression and activity.
We acknowledge several limitations to the current study. While we observed a benefit of hydrocortisone in the ovine ductal ligation model of PPHN with associated vascular remodeling, whether these results are generalizable to human infants with pulmonary hypertension and parenchymal lung disease remains unknown. In addition, while we conclude that the benefits of hydrocortisone on oxygenation are explained in part by decreased oxidative stress and PDE5 activity, hydrocortisone might have also improved lung compliance or reduced lung injury, leading to improvements in Pco2 and pH as observed (Fig. 3). We did not perform direct measurements of pulmonary or systemic hemodynamics because placement of the invasive catheters and transducers required for such measurements significantly increases mortality in these critically ill animals (26). Further, while our findings could be explained by an effect of hydrocortisone on the overall level of pulmonary vascular oxidant stress, it is difficult to elucidate a specific molecular mechanism. We suggest that the use of glucocorticoids in PPHN deserves further study in both animal and clinical settings.
In conclusion, we found that hydrocortisone treatment decreased oxidative stress and normalized PDE5 expression and activity and sGC activity in a neonatal lamb model of PPHN. We speculate that hydrocortisone may normalize PDE5 and sGC activity by attenuation of oxidant stress, thus improving pulmonary vascular reactivity. These effects were associated with an improvement in oxygenation in the ventilated PPHN lambs that was similar in magnitude to our previously published studies with inhaled NO, as well as the antioxidants rhSOD and PEG-catalase. rhSOD and catalase are not clinically available, and up to 50% of human infants do not respond to iNO (17). Thus our findings support a potential future role for hydrocortisone, which is clinically available, in treatment of human neonates with PPHN.
GRANTS
These studies have been funded by a Hearst Foundation Fellowship and an Ikaria Advancing Newborn Medicine Fellowship as well as by National Heart, Lung, and Blood Institute Grants HL-086715 (to K. N. Farrow) and HL-54705 (to R. H. Steinhorn).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P., S.L., J.A.R., K.N.F., and R.H.S. conception and design of research; M.P., S.L., S.W., L.C., S.F.G., and K.N.F. performed experiments; M.P., S.L., S.F.G., and K.N.F. analyzed data; M.P., S.L., K.N.F., and R.H.S. interpreted results of experiments; M.P. and S.W. prepared figures; M.P. drafted manuscript; M.P., S.L., S.W., K.N.F., and R.H.S. edited and revised manuscript; M.P., S.L., S.W., L.C., S.F.G., J.A.R., K.N.F., and R.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sharron Francis and Jackie Corbin, Vanderbilt University, for providing sildenafil.
REFERENCES
- 1. Ahlstrom M, Pekkinen M, Huttunen M, Lamberg-Allardt C. Dexamethasone down-regulates cAMP-phosphodiesterase in human osteosarcoma cells. Biochem Pharmacol 69: 267–275, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Basu S, Kumar A, Bhatia BD, Satya K, Singh TB. Role of steroids on the clinical course and outcome of meconium aspiration syndrome-a randomized controlled trial. J Trop Pediatr 53: 331–337, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5. Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cayabyab RG, Kwong K, Jones C, Minoo P, Durand M. Lung inflammation and pulmonary function in infants with meconium aspiration syndrome. Pediatr Pulmonol 42: 898–905, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chandrasekar I, Eis A, Konduri GG. Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension. Pediatr Res 63: 67–72, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 342: 469–474, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Crocker IC, Church MK, Ohia SE, Townley RG. Beclomethasone decreases elevations in phosphodiesterase activity in human T lymphocytes. Int Arch Allergy Immunol 121: 151–160, 2000 [DOI] [PubMed] [Google Scholar]
- 11. da Costa DE, Nair AK, Pai MG, Al Khusaiby SM. Steroids in full term infants with respiratory failure and pulmonary hypertension due to meconium aspiration syndrome. Eur J Pediatr 160: 150–153, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102: 226–233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, Russell JA, Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 299: L109–L116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farrow KN, Wedgwood S, Lee KJ, Czech L, Gugino SF, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 174: 272–281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev CD000399, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Frank L, Lewis PL, Sosenko IR. Dexamethasone stimulation of fetal rat lung antioxidant enzyme activity in parallel with surfactant stimulation. Pediatrics 75: 569–574, 1985 [PubMed] [Google Scholar]
- 19. Hanson KA, Ziegler JW, Rybalkin SD, Miller JW, Abman SH, Clarke WR. Chronic pulmonary hypertension increases fetal lung cGMP phosphodiesterase activity. Am J Physiol Lung Cell Mol Physiol 275: L931–L941, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Hermsdorf T, Richter W, Dettmer D. Effects of dexamethosone and glucagon after long-term exposure on cyclic AMP phosphodiesterase 4 in cultured rat hepatocytes. Cell Signal 11: 685–690, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Kim HT, Kim YH, Nam JW, Lee HJ, Rho HM, Jung G. Study of 5′-flanking region of human Cu/Zn superoxide dismutase. Biochem Biophys Res Commun 201: 1526–1533, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Konduri GG, Bakhutashvili I, Eis A, Pritchard K., Jr Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am 56: 579–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, Swartz DD, III, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 59: 137–141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 174: 1370–1377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewko B, Wendt U, Stepinski J, Angielski S. Dexamethasone sensitizes soluble guanylate cyclase in the rat renal glomeruli. Biochem Biophys Res Commun 197: 826–832, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Majori M, Vachier I, Godard P, Farce M, Bousquet J, Chanez P. Superoxide anion production by monocytes of corticosteroid-treated asthmatic patients. Eur Respir J 11: 133–138, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Marnane WG, Tai YH, Decker RA, Boedeker EC, Charney AN, Donowitz M. Methylprednisolone stimulation of guanylate cyclase activity in rat small intestinal mucosa: possible role in electrolyte transport. Gastroenterology 81: 90–100, 1981 [PubMed] [Google Scholar]
- 30a. The Neonatal Inhaled Nitric Oxide Study Group Inhaled nitric oxide in full-term, and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336: 597–604, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Smith ID. Breed differences in the duration of gestation in sheep. Aust Vet J 43: 63–64, 1967 [DOI] [PubMed] [Google Scholar]
- 32. Soukka H, Halkola L, Aho H, Rautanen M, Kero P, Kaapa P. Methylprednisolone attenuates the pulmonary hypertensive response in porcine meconium aspiration. Pediatr Res 42: 145–150, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, HSA, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tripathi S, Saili A. The effect of steroids on the clinical course and outcome of neonates with meconium aspiration syndrome. J Trop Pediatr 53: 8–12, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Tzao C, Nickerson PA, Russell JA, Gugino SF, Steinhorn RH. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr Pulmonol 31: 97–105, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 107: 642–647, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Vidyasagar D, Zagariya A. Studies of meconium-induced lung injury: inflammatory cytokine expression and apoptosis. J Perinatol 28, Suppl 3: S102–107, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Walther FJ, David-Cu R, Mehta EI, Polk DH, Jobe AH, Ikegami M. Higher lung antioxidant enzyme activity persists after single dose of corticosteroids in preterm lambs. Am J Physiol Lung Cell Mol Physiol 271: L187–L191, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Ward M, Sinn J. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev CD003485, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weber M, Lauer N, Mulsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic Biol Med 31: 1360–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 15: 1497–1506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]