Abstract
The trace element molybdenum (Mo) is the catalytic component of important enzymes involved in global nitrogen, sulfur, and carbon metabolism in both prokaryotes and eukaryotes. With the exception of nitrogenase, Mo is complexed by a pterin compound thus forming the biologically active molybdenum cofactor (Moco) at the catalytic sites of molybdoenzymes. The physiological roles and biochemical functions of many molybdoenzymes have been characterized. However, our understanding of the occurrence and evolution of Mo utilization is limited. This article focuses on recent advances in comparative genomics of Mo utilization in the three domains of life. We begin with a brief introduction of Mo transport systems, the Moco biosynthesis pathway, the role of posttranslational modifications, and enzymes that utilize Mo. Then, we proceed to recent computational and comparative genomics studies of Mo utilization, including a discussion on novel Moco-binding proteins that contain the C-terminal domain of the Moco sulfurase and that are suggested to represent a new family of molybdoenzymes. As most molybdoenzymes need additional cofactors for their catalytic activity, we also discuss interactions between Mo metabolism and other trace elements and finish with an analysis of factors that may influence evolution of Mo utilization.
Keywords: Molybdenum, Molybdopterin, Molybdoenzyme, Comparative genomics, Evolution
1. Introduction
The trace element molybdenum (Mo) plays a critical role in several metabolic pathways and functions as a catalytic component of certain metalloenzymes that are essential for nearly all living organisms, including animals, plants, fungi and bacteria [1–3]. Enzymes containing Mo at their active sites catalyze oxo-transfer reactions in the metabolism of carbon, nitrogen and sulfur compounds [2–6]. With the exception of the multinuclear iron (Fe)-Mo cofactor in nitrogenase [7,8], all Mo-dependent enzymes (molybdoenzymes) use this metal in the form of the Mo cofactor (Moco), which consists of Mo coordinated to an organic tricyclic pyranopterin moiety, referred to as molybdopterin [3–5, 9–11]. In some microorganisms (mostly thermophilic archaea), tungsten (W) is also coordinated by pyranopterin (Wco) [12–14]. In addition, W can be selectively transported into prokaryotic cells by certain transporters [15,16] and is an essential element for enzymes within the aldehyde:ferredoxin oxidoreductase (AOR) family [17,18]. Due to the chemical and physical similarities between Mo and W, it is often impossible to distinguish the utilization of these two elements based on sequence analysis of proteins and analysis of metabolic pathways. In this review, the term Moco refers to the utilization of both metals.
Identification of Mo transport systems and the Moco biosynthesis pathway are essential for characterization of the Mo utilization trait. Three classes of high-affinity molybdate/tungstate ATP-binding-cassette (ABC) transport systems have been reported in prokaryotes, including ModABC, WtpABC and TupABC [15,16,19]. In contrast, eukaryotic Mo transport is poorly understood and only a high-affinity molybdate transport system, MOT1, was identified in land plants and green algae [20,21]. On the other hand, Moco biosynthesis is a conserved multi-step pathway that is similar in prokaryotes and eukaryotes. This process depends on a set of gene products that have been described in many research and review articles [1–6,9–11].
Molybdoenzymes (also including W-containing enzymes) catalyze important redox reactions in the global carbon, nitrogen, and sulfur cycles. More than 50 molybdoenzymes, mostly of bacterial origin, have been characterized so far [2–6]. These enzymes can be grouped into four major families: sulfite oxidase (SO), xanthine oxidase (XO), dimethylsulfoxide reductase (DMSOR) and AOR (W-containing). Each family could be further divided into a variety of subfamilies based on substrate preferences [22,23]. Members of all four families can be found in prokaryotes, whereas only a limited number of enzymes belonging to the SO and XO families occur in eukaryotes [9,24]. Very recently, novel Moco-binding proteins have been reported in both eukaryotes (mARC in pig mitochondria) [25] and bacteria (YiiM and YcbX in Escherichia coli) [26]. These newly identified Moco-binding proteins share significant homology with the C-terminal domain of eukaryotic Moco sulfurase (MOSC) and show catalytic activity strictly dependent on Moco, suggesting that they belong to a novel molybdoenzyme family.
Recent dramatic advances in high-throughput sequencing resulted in the generation of complete genomic sequences of a large number of organisms from the three domains of life. This information provides an opportunity to examine occurrence and evolutionary trends of biochemical pathways that an organism utilizes, including the pathway of Mo utilization. Comprehensive analyses of Mo transporters, the Moco biosynthesis pathway and molybdoproteins may lead to a better understanding of utilization and biological functions of Mo as well as their evolution. This review will focus on recent studies involving comparative genomics and phylogenetic analyses of Mo and discuss general evolutionary trends of Mo utilization.
2. Molybdenum transport and molybdenum cofactor biosynthesis
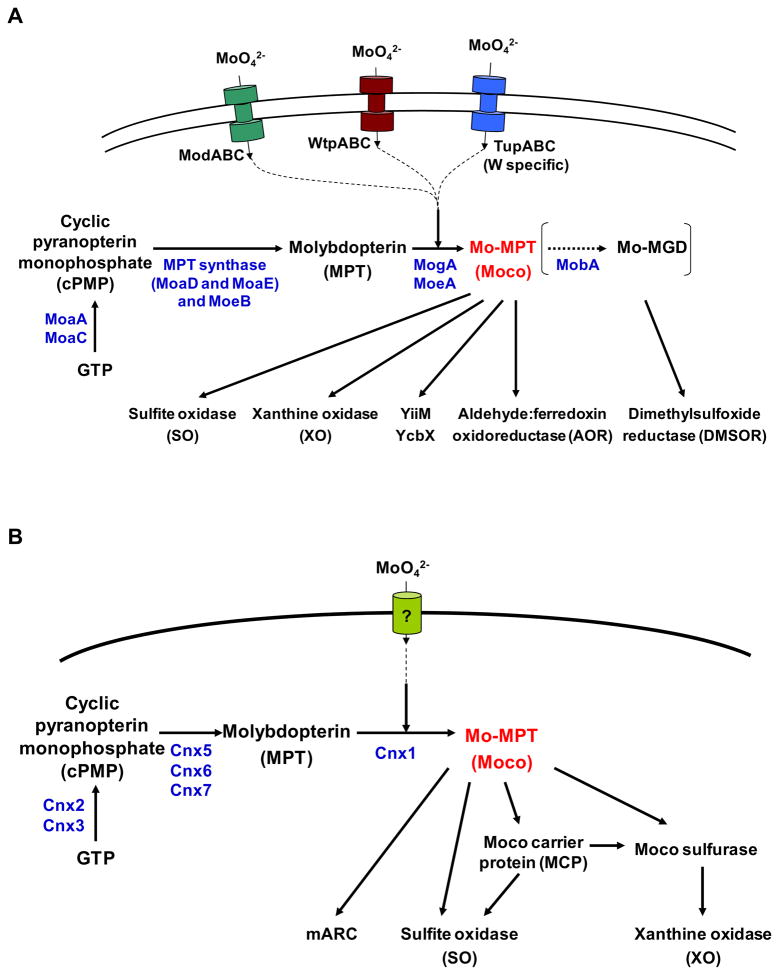
Biosynthesis of Moco and its insertion into molybdoenzymes depend on transport of molybdate anion, activation of molybdate, and finally incorporation of the activated Mo into molybdopterin [27]. A general scheme of Mo metabolism in prokaryotes and eukaryotes is shown in Fig. 1.
Fig. 1. Biosynthesis of molybdenum cofactor.
The pathway of Moco synthesis can be divided into three or four steps. (A) Biosynthesis of molybdenum cofactor in prokaryotes. (B) Biosynthesis of molybdenum cofactor in eukaryotes. The proteins from E. coli and A. thaliana catalyzing the respective steps are depicted and their names are given. MGD, molybdopterin guanine dinucleotide.
2.1. Molybdenum uptake systems
In bacteria, the first identified Mo transporter was the high-affinity ModABC transport system, which consists of ModA (molybdate-binding protein), ModB (membrane integral channel protein) and ModC (cytoplasmic ATPase) [19,28,29]. In E. coli, the modABC operon is regulated by ModE repressor, which may sense intracellular levels of Mo and bind the promoter region of modA [30,31]. E. coli ModE is composed of an N-terminal DNA-binding domain (ModE_N) and a C-terminal molybdate-binding domain. The latter contains a tandem repeat of the Mo-binding protein (Mop) domain, thus is also referred to as the Di-Mop domain. The ModABC-ModE system is widespread in prokaryotes; however, variations of ModE were also observed in Mo-utilizing organisms [32,33]. Besides, two additional classes of Mo/W ABC transport systems with different substrate affinity, WtpABC (dual specificity for Mo/W) and TupABC (W-specific), were identified [15,16]. Both transporter systems exhibit low similarity to ModABC. WtpA (periplasmic component of WtpABC) is homologous to ModA and contains the same domain (COG0725), whereas TupA (periplasmic component of TupABC) contains a different domain (COG4662), which has a low similarity to ModB. Very recently, it was reported that, in Campylobacter jejuni, a ModE-like protein which lacks the Mop domain can repress both ModABC (in the presence of both Mo and W) and TupABC (in the presence of W) transport systems [34]. However, the regulation of these two transporters is still unclear. Very recently, a member of a universal permease family, PerO, was found to import molybdate and other oxyanions in Rhodobacter capsulatus, which is the first reported bacterial molybdate transporter outside the ABC transporter family [35].
In contrast to the well-studied Mo uptake and homeostasis in bacteria, information on Mo transport in eukaryotes is limited. MOT1 is the only known Mo transporter which was first characterized in Arabidopsis thaliana [20] and Chlamydomonas reinhardtii [21]. MOT1 belongs to the sulfate transporter superfamily. It is strongly expressed in the roots of plants [36]. Nothing is known about Mo uptake mechanism in animals which lack MOT1, implying that either there is a currently unknown Mo transport system, or molybdate could be nonspecifically taken up through the sulfate or phosphate uptake systems [24].
2.2. Molybdenum cofactor (Moco) biosynthesis and storage/transfer
Moco is synthesized by an evolutionarily conserved multi-step pathway in all three domains of life [1–6]. The overall process includes (i) conversion of GTP into cyclic pyranopterin monophosphate, (cPMP, also known as precursor Z); (ii) transformation of cPMP into molybdopterin; (iii) metal incorporation; and (iv) maturation to an active cofactor in some organisms, e.g., formation of a dinucleotide form (molybdopterin guanine dinucleotide, MGD) or substitution of a terminal oxygen ligand of Moco with a sulfur ligand. In E. coli, the proteins required for biosynthesis and regulation of Moco are encoded in the moa-mog operons (Fig. 1A) [37,38]. In eukaryotes, at least six proteins (named Cnx1–3 and Cnx5–7 in plants) are involved in Moco biosynthesis (Fig. 1B), which are homologous to their counterparts in bacteria [3–5,39–41]. As different nomenclature has been used in humans and plants [39,42], here we use the plant nomenclature to refer to the eukaryotic Moco synthetic genes. Moreover, a Moco sulfurase, catalyzing the generation of the sulfurylated form of Moco that is needed for activation of all XO family proteins such as xanthine dehydrogenase (XDH) and aldehyde oxidase (AO), has been identified in both plants and humans [43,44]. A recent study also showed that, in A. thaliana, the first step of Moco biosynthesis is localized in the mitochondrial matrix, and a mitochondrial ABC transporter ATM3 (previously implicated in the maturation of extramitochondrial Fe-S proteins) has a crucial role in Moco biosynthesis by transporting cPMP [45].
As Moco is highly unstable and oxygen-sensitive [1,4], after synthesis, it should be either transferred immediately to the molybdoenzymes or bound to a storage/carrier protein until further insertion. In bacteria, many molybdoenzymes have chaperones, such as NarJ for nitrate reductase and DmsD for DMSOR, which can bind Moco and assist in cofactor incorporation [46–48]. Little is known about Moco buffering in eukaryotes. Recently, a Moco carrier protein (MCP) has been identified in C. reinhardtii [49]. MCP belongs to the lysine decarboxylase family and can bind Moco with high affinity, whereas Mo-free molybdopterin is not bound, suggesting a specific metal-mediated interaction with MCP. Crystal structure of MCP showed a symmetric homotetramer with the monomers arranged in a Rossmann-like fold, and a proposed Moco-binding site that was confirmed by structure-guided mutagenesis [49]. In addition, several homologous Moco-binding proteins (MoBP), which also belong to lysine decarboxylase family, were discovered in land plants that might be involved in the cellular distribution of Moco [9,50]. However, the mechanism of Moco protection, storage and transfer in mammals is still unclear.
3. Molybdoenzymes
Molybdoenzymes illustrate the metabolic pathways in which Mo is involved in. As mentioned above, on the basis of cofactor composition and catalytic function, there are two groups: (i) Mo-dependent nitrogenase that contains an Fe-Mo cofactor in the active site, and (ii) all other molybdoenzymes that bind Moco. Table 1 lists the majority of known molybdoenzymes.
Table 1.
Classification of molybdoenzymes
| Group | Family | Protein |
|---|---|---|
| Moco-binding proteins | sulfite oxidase | sulfite oxidase nitrate reductase (assimilatory) |
| xanthine oxidase | xanthine oxidase xanthine dehydrogenase aldehyde oxidase aldehyde oxidoreductase 4-hydroxybenzoyl-CoA reductase CO dehydrogenase quinoline 2-oxidoreductase isoquinoline 1-oxidoreductase quinoline 4-carboxylate-2-oxidoreductase quinaldine 4-oxidoreductase quinaldic acid 4-oxidoreductase nicotinic acid hydroxylase 6-hydroxynicotinate hydroxylase nicotine dehydrogenase picolinate hydroxylase pyridoxal oxidase nicotinate hydroxylase |
|
| dimethylsulfoxide reductase | dimethylsulfoxide reductase biotin sulfoxide reductase trimethylamine-N-oxide reductase nitrate reductase (dissimilatory) formate dehydrogenase formylmethanofuran dehydrogenase polysulfide/thiosulfate/arsenate reductase arsenite oxidase pyrogallol-phloroglucinol transhydroxylase |
|
| aldehyde:ferredoxin oxidoreductase (W-containing) | aldehyde:ferredoxin oxidoreductase formaldehyde ferredoxin oxidoreductase glyceraldehyde-3-phosphate ferredoxin oxidoreductase carboxylic acid reductase hydroxycarboxylate viologen oxidoreductase aldehyde dehydrogenase |
|
| MOSC-containing | mARC/YcbX YiiM |
|
| Fe-Mo-binding protein | nitrogenase | nitrogenase |
Nitrogenase is required for biological nitrogen fixation, which is an essential step in the nitrogen cycle in the biosphere. It reduces atmospheric dinitrogen to ammonia under room temperature and atmospheric pressure with high-energy input in the form of ATP [7,8,51]. There are four known types of nitrogenases, each of which has different combination of metals in the active site [51–53]. The most abundant and widely studied is the Fe-Mo-dependent nitrogenase, which contains MoFe3S3 and Fe4S3 cuboidal subunits triply joined by three bridging sulfurs [51,52].
The second group of proteins which utilize Moco as cofactor contains sulfite oxidase, xanthine oxidase, dimethylsulfoxide reductase, and aldehyde:ferredoxin oxidoreductase (mostly Wco-containing) families and the novel Moco-binding proteins. Each family includes a variety of subfamilies based on sequence similarity, spectroscopic properties and substrate preferences (Table 1). Compared to prokaryotes which contain diverse members belonging to the four major families, eukaryotes only have four typical molybdoenzymes, including nitrate reductase and SO (members of the SO family), as well as XDH and AO (members of the XO family) [4,9,24]. Two additional Moco-binding enzymes of the XO family were also reported: pyridoxal oxidase and nicotinate hydroxylase, which were found exclusively in Drosophila melanogaster and Aspergillus nidulans, respectively [4].
SO family members generally catalyze net oxygen atom transfer to or from a heteroatom lone electron pair rather than hydroxylation of a carbon center [54]. Typical enzymes belonging to this family include sulfite oxidase (the name-giving enzyme of the SO family) and assimilatory nitrate reductase. Moco in this protein family is covalently bound as Mo-MPT by a sulfur of a conserved cysteine residue that links Mo with the apo-protein [9]. Sulfite oxidase is mainly found in eukaryotes and is located in the mitochondrial intermembrane space where it catalyzes the oxidation of sulfite to sulfate, the terminal reaction in the oxidative degradation of sulfur-containing amino acids cysteine and methionine [4,23,55]. Assimilatory nitrate reductase catalyzes the reduction of nitrate to nitrite and is responsible for the first step in the uptake and utilization of nitrate [24,56]. So far this enzyme is only found in autotrophic organisms such as plants and fungi.
The XO family contains the largest and most diverse Moco-containing enzymes, with members from all three domains of life (Table 1). Members of this protein family are characterized by a unique third terminal sulfur ligand in their Mo-MPT like cofactor [9]. They catalyze oxidative hydroxylation of a wide range of aldehydes and aromatic heterocycles [2,4]. The major enzymes of the XO family include AO (catalyzes the oxidation of a variety of aromatic and nonaromatic heterocycles and aldehydes, thereby converting them to the respective carboxylic acid) [57], XDH (an essential enzyme of purine degradation that oxidizes hypoxanthine to xanthine and xanthine to uric acid) [58] and a variety of bacterial enzymes such as aldehyde oxidoreductase [59], 4-hydroxybenzoyl-CoA reductase [60] and quinoline 2-oxidoreductase [61].
Members of the DMSOR family are exclusively found in bacteria and archaea and bind a Mo-MGD cofactor consisting of one Mo atom complexed by two MGD molecules [2–4,13]. They are very diverse in reaction, function and structure [62]. Most of these enzymes function as terminal reductases under anaerobic conditions where their respective cofactors serve as terminal electron acceptors in respiratory metabolism. DMSOR (the name-giving enzyme for the family) is found in a wide range of bacteria and catalyzes reductive deoxygenation of dimethyl sulfoxide to dimethyl sulfide. It is a periplasmic single-subunit protein in some bacteria (such as Rhodobacter sphaeroides [63]), whereas a membrane-bound protein composed of three subunits (Moco-containing, four [4Fe–4S] cluster-containing and transmembrane subunits) in some other bacteria (such as E. coli [64]). Formate dehydrogenase, another widespread member of DMSOR family, catalyzes the oxidation of formate to bicarbonate and is also a selenocysteine (Sec)-containing enzyme in many organisms [65]. Other members include dissimilatory nitrate reductase, pyrogallol-phloroglucinol transhydroxylase and several additional enzymes exhibiting substantial sequence homology [2–4,13,62].
W-containing AOR catalyzes the interconversion of aldehydes and carboxylates and was the first member of the AOR family to be structurally characterized as a protein containing a Wco cofactor that shows Mo-MPT like structure with the tungsten center coordinated by two pyranopterin dithiolenes [66,67]. Other members include formaldehyde ferredoxin oxidoreductase, glyceraldehyde-3-phosphate ferredoxin oxidoreductase, carboxylic acid reductase and hydroxycarboxylate viologen oxidoreductase (a Moco-containing protein identified in Proteus vulgaris [68]).
Additionally to the four major molybdoenzyme families, novel Moco-binding proteins were recently identified in both pig liver and E. coli [25,26]. In the pig, a Moco-dependent protein was found in the outer mitochondrial membrane and named mitochondrial amidoxime reducing component (mARC). In contrast to XO and SO family proteins, mARC binds a Mo-MPT like Moco that carries neither a terminal sulfur ligand like XO nor a covalently bound cysteine residue like SO, suggesting that these proteins represent a new family of molybdoenzymes [69]. Recent studies have shown that human mARC proteins catalyze the N-reduction of a variety of N-hydroxylated substrates such as N-hydroxy-cytosine and Nω-hydroxy-L-arginine albeit with different specificities [69,70]. So, it might play a major role in drug metabolism and nitric oxide regulation system [25,70,71].
Very recently, Cvetkovic et al. developed an elegant approach to characterize metals an organism assimilates and identify its metalloproteins on a genome-wide scale [72]. They identified new metalloproteins (including novel Mo-containing proteins) in several microorganisms, suggesting that metalloproteomes are more extensive and diverse than previously recognized. However, considering that the metal dependency and affinity of these new Mo-binding proteins have not been analyzed yet, in this review, we only focus on known molybdoenzymes.
4. Comparative genomics of molybdenum utilization
While the majority of studies in the field focused on the identification and characterization of Mo uptake systems, Moco biosynthesis pathways, and Mo-dependent enzymes in individual organisms, comprehensive analyses of the occurrence and evolutionary trends in Mo utilization, which could greatly benefit our understanding of Mo utilization and its evolutionary changes, have been limited. In recent years, following the availability of a large number of newly sequenced organisms, several computational and comparative studies have been carried out to investigate the phylogeny of Mo utilization in prokaryotes and eukaryotes. In the following sections, we highlight the significant contributions from these studies.
4.1. General trends in molybdenum utilization
We carried out comparative genomics analyses to examine the occurrence and dynamics of Mo utilization in sequenced bacteria, archaea, and eukaryotes at the level of Mo transport, the Moco biosynthesis, and molybdoenzymes [73–75]. Details about the strategy and procedures of comparative genomics approaches have been recently reviewed [75]. Overall, these studies provided a first glance at Mo utilization in all three domains of life and showed its widespread occurrence, yet limited use of this metal in individual organisms.
A wide distribution of genes encoding Mo transporters, the Moco biosynthesis pathway and Mo-containing proteins was found in sequenced genomes, and almost all Mo-utilizing organisms contained both Moco biosynthesis proteins and at least one known molybdoenzyme [73,74]. 74% bacteria, 98% archaea and 66% eukaryotes utilize Moco. In bacteria and archaea, Mo was utilized by almost all phyla (except Mollicutes and Chlamydiae), suggesting that Mo utilization is an ancient and essential trait that is common to essentially all organisms in these two domains. In eukaryotes, Mo is used by all animals, land plants, algae, certain fungi (all pezizomycotina and some basidiomycota) and stramenopiles; however, parasites, yeasts (saccharomycotina and schizosaccharomycetes) and free-living ciliates lack the Mo utilization trait [73]. It is possible that many protozoa, especially parasites, lost the ability to utilize Mo.
4.2. Comparative analyses of molybdenum uptake and Moco biosynthesis
An early computational study of the occurrence of the ModABC-ModE system in deltaproteobacteria revealed that all analyzed deltaproteobacteria have ModABC transporters, whereas the full-length E. coli-type ModE was only observed in a few of them [33]. However, a single ModE_N protein was found in some other deltaproteobacteria, suggesting that a somewhat different regulatory mechanism may be present in these organisms.
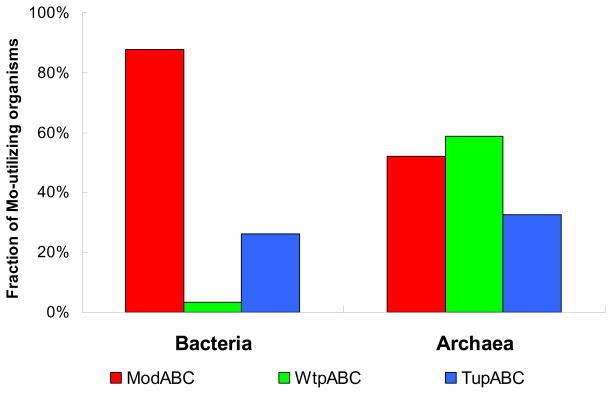
Comparative analyses of Mo/W transport systems in all sequenced prokaryotes revealed that Mo/W transporters are often present in single copies [73,74]. Among them, ModABC is the most common Mo transporter, which is present in approximately 90% of Mo-utilizing bacteria (Fig. 2). The occurrence of the other two transporters, WtpABC and TupABC, is much more restricted, especially WtpABC, which is only detected in 3% of Mo-utilizing bacteria. In contrast, WtpABC is the most frequently used transporter in archaea, whereas ModABC showed a restricted distribution in these organisms (Fig. 2). It appeared that WtpABC is mainly an archaeal Mo/W transporter, whereas ModABC functions predominantly in bacteria. The TupABC transport system has similar occurrence in Mo-utilizing bacteria (26.2%) and archaea (32.6%), implying a comparable role of this W-specific transport system in both domains.
Fig. 2. Distribution of Mo/W transporters in Mo-utilizing organisms in bacteria and archaea.
Three classes of high-affinity Mo/W ABC transport systems are known: ModABC, WtpABC and TupABC.
Although ModA and WtpA proteins contain the same conserved domain, previous experiments showed that they have different anion affinities. WtpA has a higher affinity for tungstate than ModA and TupA, and the affinity for molybdate is similar to that of ModA [15,17]. Phylogenetic analysis of ModA and WtpA proteins showed that they may have evolved from a common ancestral protein and functionally diverged during evolution [73]. Crystal structures showed that the residues involved in molybdate binding in E. coli ModA (1AMF) and tungstate binding in Archaeoglobus fulgidus WtpA (2ONS) were different [76,77]. Interestingly, sequence alignment of ModA and WtpA proteins from different organisms revealed that the residues involved in substrate binding are not highly conserved, not only between WtpA and ModA, but also within the same family [73,75].
E. coli-type ModE regulation of ModABC transporters only occurred in less than 30% of Mo-utilizing organisms, suggesting the presence of novel or unspecific regulatory pathways for molybdate uptake in many other organisms such as Gram-positive bacteria and Cyanobacteria [32,73]. On the other hand, separate ModE_N and Mop/Di-Mop proteins, orphan ModE_N proteins (lacking Mop protein in the same organism), and novel domain fusions for either ModE_N or Mop were observed in a variety of organisms that lack full-length ModE, indicating complexity of ModE-related regulation. So far, five types of novel fusion forms were observed for Mop (three types) and ModE_N (two types), mostly in bacteria (Table 2). Genomic context analyses of these ModE-related variations suggested potential correlations with ModABC transporters as most of these genes are close to or even in the same operon with modABC [73]. The functions of these ModE-related variants are unclear. It was previously thought that a separate ModE_N and Mop/Di-Mop proteins together may have a function similar to that of full-length ModE, although ModE_N may already have a weak role in DNA binding for ModABC regulation [78]. Among these fusions, the MerR-Mop form that contains MerR-like transcription factor domain could be a novel candidate regulator for ModABC or other Mo-related genes in organisms such as Actinobacteria. The ModE_N-COG1910 (periplasmic molybdate-binding protein/domain) form may have the similar function as E. coli ModE, in which the Di-Mop domain is replaced with another Mo-binding domain). In contrast, almost half of ModABC-containing organisms lack both full-length ModE and its variants, indicating the presence of new regulators of ModABC in these organisms. Although the regulatory mechanisms of the other two Mo transporters are not clear, it was assumed that, in some organisms, TupABC and WtpABC transporters may also be regulated by ModE-like mechanisms based on the genomic context analysis [73]. This hypothesis was recently verified by a study showing that a ModE-like protein (Unknown3-ModE_N in Table 2) could repress both ModABC and TupABC transport systems in C. jejuni [34].
Table 2.
Distribution of novel fusion forms of Mop and ModE_N in prokaryotes
| Fusion form | Occurrence |
|---|---|
| MerR-Mop | Actinobacteria Acidobacteria Alphaproteobacteria |
| Unknown1-Mop | Bacteroidetes Chlorobi Epsilonproteobacteria |
| Unknown2-Mop | Cyanobacteria |
| Unknown3-ModE_N | Epsilonproteobacteria |
| ModE_N-COG1910 | Alphaproteobacteria Betaproteobacteria Gammaproteobacteria Archaea |
As discussed above, in eukaryotes, MOT1 is the only characterized Mo transporter and was detected in less than 40% Mo-utilizing organisms (land plants, green algae, pezizomycotina and stramenopiles). Most Mo-utilizing species (including all animals) lack this transporter family, suggesting the presence of currently unknown Mo transport systems encoded in their genomes.
The majority of known proteins involved in Moco biosynthesis pathways (Fig. 1) could be detected in essentially all Mo-utilizing organisms. However, a very small number of prokaryotes which contain homologs of molybdoenzymes lack genes for either Moco biosynthesis trait components or Mo/W transporters [74]. It is possible that Moco is dispensable for the molybdoprotein homologs in these organisms. An alternative possibility, although unlikely, is that there is an unknown Mo utilization pathway in these organisms. Nevertheless, these comparative studies suggested a very good correspondence between occurrence of the Moco biosynthesis trait and Moco-dependent enzymes in all three domains of life.
4.3. Comparative analyses of molybdoenzymes
Previously, several studies have been conducted to investigate the evolution of certain molybdoenzymes in a limited number of organisms, such as nitrate reductase and other DMSOR family members [79,80], which showed complexity in their evolutionary trajectories. Based on recent comparative analyses of Moco-utilizing enzymes and nitrogenase, we characterized the distribution of each molybdoenzyme family in sequenced genomes in the three domains of life (Fig. 3).
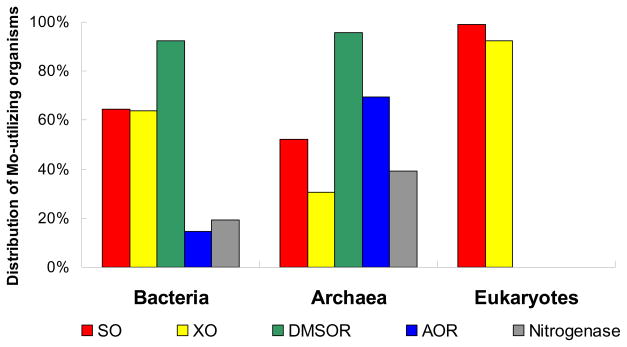
Fig. 3. Distribution of molybdoenzymes in Mo-utilizing organisms in the three domains of life.
SO, sulfite oxidase; XO, xanthine oxidase; DMSOR, dimethylsulfoxide reductase, AOR, aldehyde:ferredoxin oxidoreductase. 100% represents all Mo-utilizing organisms in each domain of life.
In bacteria, DMSOR, SO and XO families were widespread, especially DMSOR whose members (mostly DMSOR, dissimilatory nitrate reductase and formate dehydrogenase) were detected in more than 90% Mo-utilizing organisms [73,74]. In contrast, the W-containing AOR family was only detected in ~15% Mo/W-utilizing organisms. In archaea, DMSOR was also the most abundant molybdoenzyme family, which was found in more than 95% Mo-utilizing organisms. Interestingly, members of AOR family had a much higher occurrence in archaea (~70%). The FeMo-utilizing molybdoenzyme, nitrogenase, was detected in ~20% Mo-utilizing bacteria (almost all also used Moco) and methanogenic archaea.
Further investigation of molybdoenzyme sets (molybdoproteome) of each organism revealed both many molybdoprotein homologs and variable occurrence of these proteins. Proteobacteria appeared to have larger molybdoproteomes than other organisms [74]. To date, the largest molybdoproteome in prokaryotes was observed in a dehalorespiring bacterium, Desulfitobacterium hafniense [81]. It contains at least 63 molybdoproteins, 95% of which are members of the DMSOR family, suggesting particularly important roles of DMSOR members in this organism [74].
As mentioned above, two major molybdoenzyme families, SO and XO, have been reported in eukaryotes. Comparative genomics studies of known molybdoenzyme families in this kingdom confirmed this fact [73–75]. Essentially all Mo-utilizing organisms had both families. Land plants possessed the largest molybdoproteomes in eukaryotes (10–11 molybdoproteins). On the other hand, all sequenced saccharomycotina (e.g., Saccharomyces cerevisiae) and schizosaccharomycetes (e.g., Schizosaccharomyces pombe) had neither known molybdoenzymes nor Moco biosynthesis proteins. Although a small number of unsequenced yeast species, such as Candida nitratophila, Pichia anomala and P. angusta, utilize Mo-containing assimilatory nitrate reductase [82–84], the fact that homologs of this protein and the Moco biosynthesis pathway are absent in all sequenced yeast genomes suggests the loss of Mo utilization in these organisms.
4.4. in silico analyses of novel Moco-binding proteins
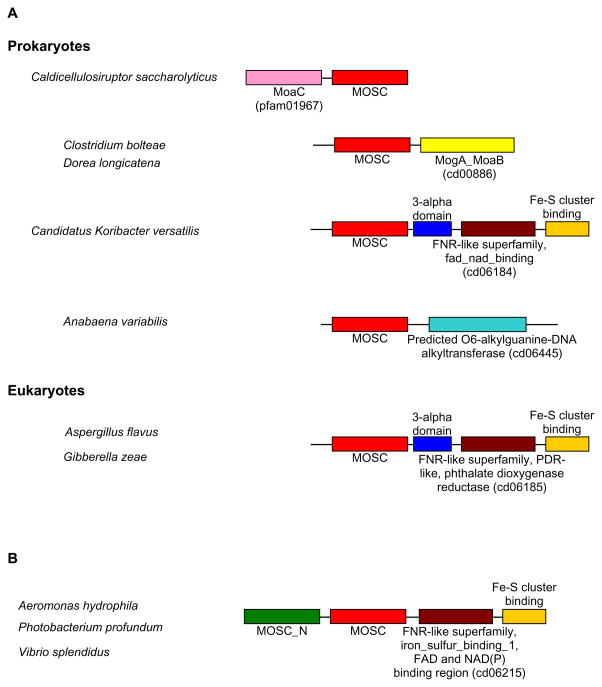
Novel Moco-binding proteins were recently identified in addition to the four major molybdoenzyme families [25,26,69–71]. The mammalian mARC protein consists of two conserved domains, N-terminal MOSC_N (pfam03476) and C-terminal MOSC (pfam03473) domains, which are also present in Moco sulfurases (Fig. 4). The MOSC domain is a superfamily of beta-strand-rich domains identified in the Moco sulfurase and several other proteins from both prokaryotes and eukaryotes [85]. The MOSC domain of eukaryotic Moco sulfurase is involved in Moco binding with high affinity and its Moco carries a terminal sulfur ligand due to the catalytic activity of pyridoxal-5′-phosphate-dependent NifS-like domain [86]. On the other hand, Moco bound to the MOSC domain of mARC showed no terminal sulfur ligand [69]. The function of the MOSC_N domain is unknown; however, it is predicted to adopt a beta barrel fold.
Fig. 4. Domain organizations of Moco sulfurase and novel Moco-containing proteins.
Different domains are shown by different colors. MOSC, C-terminal domain of the eukaryotic Moco sulfurase.
Two additional Moco-dependent proteins were characterized in E. coli, YcbX and YiiM, which may represent novel enzymatic activities involved in the detoxification pathway of N-hydroxylated base analogs [26]. Both proteins contain the MOSC domain (Fig. 4). The E. coli YcbX also contains the MOSC_N and an additional Fe-S cluster binding domain (cd00207), whereas YiiM has a C-terminal 3-alpha domain (pfam03475). Based on a similar domain organization (MOSC_N + MOSC) and significant sequence similarity (48%) between E. coli YcbX and mammalian mARC, they could be considered as orthologs of the same family (designated mARC/YcbX family hereafter) in different kingdoms. On the other hand, no significant sequence similarity could be detected between YiiM and mARC/YcbX, suggesting that they belong to different families of the MOSC superfamily. In general, mARC/YcbX and YiiM can be easily separated based on the presence of MOSC_N and sequence similarity.
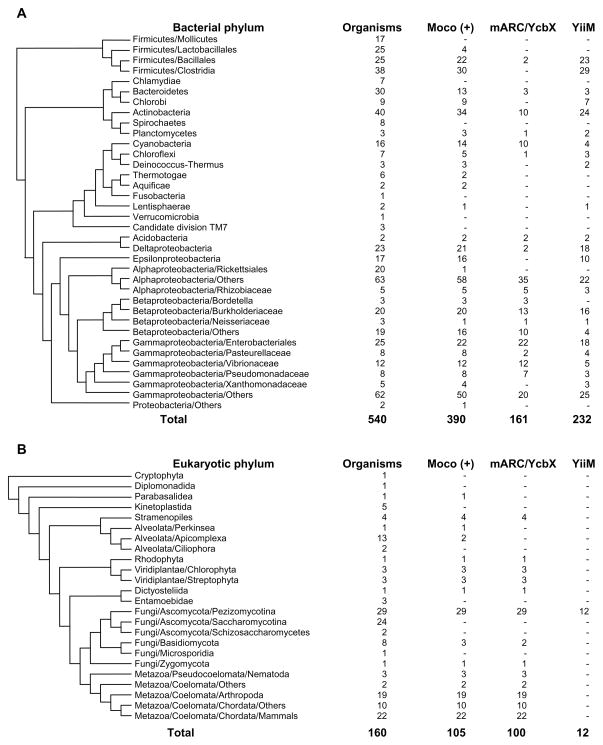
To further investigate the distribution of these novel MOSC-containing molybdoproteins, we carried out a computational analysis using a similar strategy previously applied for known molybdoenzyme families [73,74]. All sequenced genomes in the three domains of life were retrieved for BLAST-based homology search. Orthologs of mARC/YcbX and YiiM were verified based on domain composition, genomic context and phylogenetic analyses. In bacteria, both mARC/YcbX and YiiM were widespread but only detected in Moco-utilizing organisms (Fig. 5A). Compared to E. coli sequences, many proteins only contain the prominent domains (MOSC_N + MOSC for mARC/YcbX, MOSC for YiiM). In contrast, the occurrence of these two families in archaea is limited, where only organisms belonging to Euryarchaeota/Halobacteriales had mARC/YcbX proteins (data not shown). It is possible that archaea acquired this gene via horizontal gene transfer from bacteria. In eukaryotes (Fig. 5B), mARC proteins were detected in more than 95% Mo-utilizing organisms, suggesting a wide distribution of this novel molybdoenzyme family. Interestingly, several YiiM-like proteins were detected in a small number of pezizomycotina, all of which contain MOSC and additional domains (see below).
Fig. 5. Occurrence of mARC/YcbX and YiiM proteins in different phyla in bacteria and eukaryotes.
(A) Bacteria; (B) Eukaryotes. Moco (+), organisms containing the Moco biosynthesis pathway.
Genomic context analyses of genes for mARC/YcbX and YiiM revealed that, in some organisms, a single-MOSC-containing YiiM gene is clustered with either modABC or Moco biosynthesis genes such as moaA, moaB, moaE and mobB. In addition, novel domain fusion forms were also observed, especially for YiiM family. In prokaryotes, four types of new fusions were observed for YiiM family (YiiM-like MOSC domain plus new domains, Fig. 6A). Among them, two are Moco biosynthesis components (MoaC and MogA_MoaB domains), suggesting that they may be involved in Moco biosynthesis pathway in these organisms. The third form is the extension of the E. coli-type YiiM, which contains an additional FNR-like superfamily (cd06184, FAD_NAD(P)H binding domain of flavohemoglobin) and Fe-S cluster binding domains at the C terminus. Interestingly, all YiiM-like proteins detected in eukaryotes (pezizomycotina) have very similar domain organization that also includes a FNR-like superfamily (cd06185, phthalate dioxygenase reductase FMN binding domain) and an Fe-S cluster binding domain, implying they may have evolved from the bacterial fusion form (Fig. 6A). Only one novel fusion was identified for the mARC/YcbX family which was present in several gammaproteobacteria (Fig. 6B). Again, a FNR-like domain (cd06215, FNR_iron_sulfur_binding_1) was inserted between the MOSC and Fe-S cluster binding domains. Although the roles of these fusion proteins are unclear, it appeared that both FAD/FMN and Fe-S cluster may be essential for their function.
Fig. 6. Novel fusion forms of MOSC-containing proteins.
(A) New fusions of YiiM. (B) New fusions of mARC/YcbX. Different domains are shown by different colors.
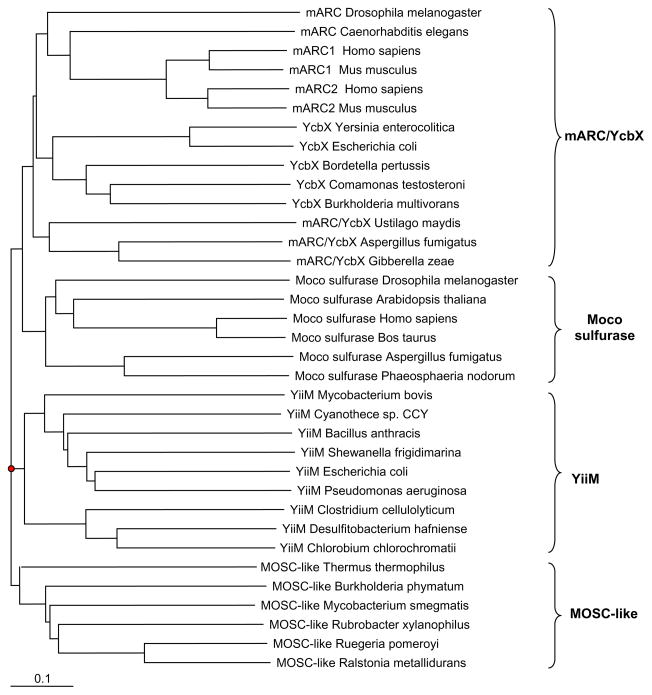
Interestingly, a novel group of MOSC-containing proteins (designated MOSC-like) was identified in a variety of prokaryotes (unpublished data). These proteins were relatively small and had low sequence similarity to mARC/YcbX and YiiM proteins. Phylogenetic analyses showed that the MOSC-like proteins formed a separate branch of the MOSC proteins (Fig. 7). The MOSC domain is the only domain detected in almost all sequences of this group, but its function is still unclear. Considering the facts that all organisms containing MOSC-like proteins are Moco-utilizing organisms and that, in some organisms, genes for MOSC-like proteins are located close to Moco biosynthesis components or molybdoenzymes such as MoaC and formate dehydrogenase, it is possible that these MOSC-like proteins may serve as Moco chaperone involved in Moco transfer or storage.
Fig. 7. Phylogenetic tree of MOSC-containing proteins.
Measurement of a distance for the branch length is indicated. Four MOSC-containing protein families are included: Moco sulfurase, mARC/YcbX, YiiM and MOSC-like protein.
All above studies suggested complexity and diverse roles of the MOSC superfamily, whose proteins may be (i) involved in Moco modification pathway (Moco sulfurase); (ii) new molybdoenzymes (mammalian mARC, E. coli YcbX and YiiM); (iii) potential Moco chaperones (MOSC-like); and (iv) involved in other functions (unrelated to Moco utilization). Further experiments are needed to better understand the functions of MOSC-containing proteins.
4.5. Crosstalk between Mo and other trace elements
The interactions between Mo utilization and that of other trace elements have been reported in several studies. These crosslinks not only highlight complexity of Mo metabolism, but also reveal complexity of trace element utilization in general.
It was previously reported that Mo metabolism is linked to Fe metabolism at different levels. The majority of Mo-enzymes need Fe (as either Fe-S cluster or heme). For example, the maturation of Fe-Mo-dependent nitrogenase heavily relies on the synthesis of complex Fe-S clusters [7,8]. In addition, enzymes involved in the first step of Moco biosynthesis contain two [4Fe–4S] clusters [87,88]. Additionally, Moco-containing hydroxylases (XO family) and several members of DMSOR family also bind Fe-S clusters for intramolecular electron transfer [58,62], whereas some members of SO family contain heme [55]. A recent study on the mitochondrial ABC transporter ATM3 from A. thaliana revealed a dual function: exporting both Fe-S cluster precursors and cPMP from mitochondria to the cytosol [45].
The molecular link between Mo and copper metabolism was recently identified in A. thaliana. The C-terminal domain of Cnx1 (named Cnx1G) that catalyzes the insertion of Mo into molybdopterin binds copper, although the function of copper during Moco biosynthesis is unknown [89]. Copper may act as a protecting group for MPT and/or play a role in molybdenum insertion [90]. Investigation of crystal structures revealed that either two water molecules (original Cnx1G) or one water molecule and His618 (a Ser583Ala variant) are the copper ligands. However, further examination of the in vivo and/or in vitro activities of two molybdoenzymes, DMSOR and NR, in E. coli and R. sphaeroides, showed that their activities were not affected when copper was depleted from the media [91]. Moreover, comparative analysis of Cnx1G orthologs in various organisms showed that His618 was not conserved [74]. Although copper-binding function could not be excluded for many Cnx1G proteins that lack His618, it is possible that while copper may be utilized during Moco biosynthesis in some organisms such as plants, it does not appear to be strictly required for Moco biosynthesis in many other organisms. Furthermore, another link between Mo and copper has been reported in a XO family protein of Oligotropha carboxidovorans, as its carbon monoxide dehydrogenase is characterized by a Mo-MPT cofactor forming a special dinuclear Cu–S–Mo centre [92].
We also found an interesting link between Mo and selenium. A major member of the DMSOR family, formate dehydrogenase alpha subunit, is also a Sec-containing protein that may be responsible for maintaining the Sec utilization trait in sequenced prokaryotes [93]. Comparison of the distribution of Mo- and Sec-utilizing organisms in the three domains of life revealed that Sec-utilizing organisms were essentially a subset of Mo- utilizing organisms in prokaryotes [73,74]. Thus, the Sec utilization trait depends on the Mo utilization trait in prokaryotes, most likely because of formate dehydrogenase, which is not only a widespread molybodoenzyme but is also the major user of Se in prokaryotes.
4.6. Analysis of factors that may affect evolution of Mo utilization
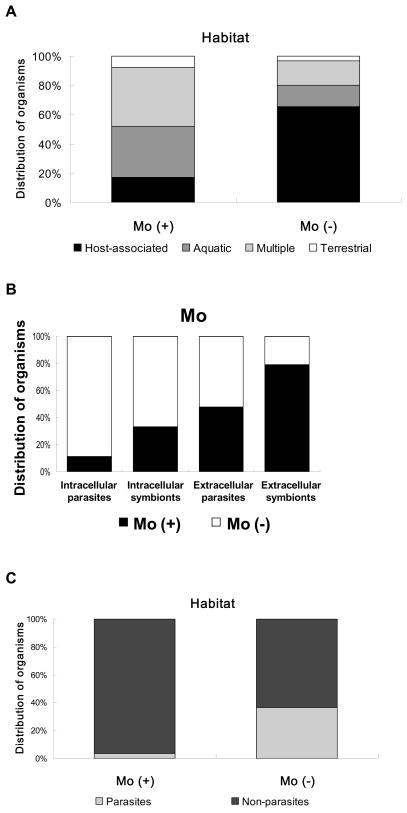
Since the Moco biosynthesis trait and molybdoenzymes were present in many but not all organisms, it is possible that certain common factors may have affected acquisition/loss of Mo utilization during evolution. We previously analyzed the roles of several factors, including environmental conditions (e.g., habitat, oxygen requirement, optimal temperature and optimal pH) and other factors (genome size, gram strain and G+C content), on Mo utilization [73,74]. First, the majority of bacteria that do not utilize Moco were host-associated organisms (Fig. 8A), suggesting that host-associated lifestyle may leads to the loss of Mo utilization. Considering differences in host-associated conditions (intra- or extracellular) and the relationship between these organisms and their hosts (symbiotic or parasitic), we further divided host-associated organisms into four groups: obligate intracellular symbionts, extracellular symbionts, obligate intracellular parasites and extracellular parasites. The majority of intracellular parasites and symbionts lost the ability to utilize Mo, whereas more than 80% of extracellular symbionts utilized the metal (Fig. 8B). Because most obligate intracellular parasites and symbionts had more condensed genomes than the organisms in the extracellular milieu, it is possible that Mo utilization is not essential for intracellular parasitic life and hence has been lost due to limited bioavailability of Mo and the evolutionary pressure on genome size (although these organisms may still use Mo-dependent proteins of the host). In contrast, Mo utilization was mostly preserved in extracellular symbionts. Most parasitic eukaryotes also did not use Mo (Fig. 8C). Similar effects were observed for some other trace elements, such as nickel and cobalt [74,94], suggesting that host-associated habitats (except extracellular symbionts) affect the utilization of multiple trace elements. Other factors, such as oxygen requirement, optimal temperature and pH, did not appear to have a significant influence on the evolution of Mo utilization.
Fig. 8. Relationship between environmental factors and the Mo utilization trait.
Organisms were split into two groups: Mo (+), i.e. containing the Mo utilization trait; Mo (−), i.e. lacking Mo utilization. (A) Habitat. (B) Different host-associated life styles. (C) Relationship between parasitic lifestyles and Mo utilization trait in eukaryotes.
Similar host-associated trends were also found for molybdoenzyme families. Moreover, additional features were observed for individual molybdoenzymes: organisms possessing W-containing AOR proteins appear to favor an anaerobic environment, whereas organisms containing SO or XO proteins favor aerobic conditions [73]. On the other hand, organisms possessing nitrogenase favor both anaerobic and relatively warm conditions. These data suggest that although being dependent on the same processes, such as Mo availability and Moco synthesis, different Mo enzymes are subject to independent and dynamic evolutionary processes. Previous studies on several trace elements such as selenium and copper showed that the size of cuproproteomes and selenoproteomes correlates with oxygen utilization and/or aquatic environment [93,95,96]. However, no significant correlation was observed between various factors examined and the size of Mo-dependent metalloproteomes [74]. It would be very important to examine additional factors that may influence composition of Mo-dependent metalloproteomes.
4.7. Mo pathways provide evolutionary origins for other functions in eukaryotes
Mo utilization is an ancient and essential trait and during evolution some proteins in the Moco biosynthesis pathway became precursors for other functions in eukaryotes. The Moco biosynthesis protein MoaD has an ubiquitin-like fold and is adenylated at its C-terminal glycine residue by MoeB in a similar way as it is known from the ubiquitin-activating enzyme Uba1. This observation explains the evolutionary origin of ubiquitin-like protein conjugation [97,98]. Furthermore, the mammalian homolog of Cnx1 (Gephyrin) was shown to be a neuroreceptor anchor protein involved in the formation of glycinergic synapses and the postsynaptic aggregation of glycine receptors [99–101]. Additionally, proteins utilizing Moco gained further functions (beyond their specific catalytic activity), e.g., mammalian xanthine dehydrogenase is involved in the formation of milk fat droplets within the lactating mammary epithelium acting as a membrane-associated structural protein [102,103]. Future investigations may find additional functions of Mo enzymes.
5. Concluding remarks
In recent decades, information on utilization and biological function of Mo has been rapidly developing. Most of the relevant proteins, including Moco biosynthesis components and Mo-dependent enzymes, have been characterized and their basic functions are now known. However, understanding of evolution and general features of Mo utilization is limited and lags behind these functional studies.
Our review is written to provide an overview of the general features, phylogeny and evolution of Mo utilization, and to broaden the scope of Mo research by considering insights offered by recent studies on comparative genomics of Mo utilization. We discuss how bioinformatics and comparative genomics can be used to examine evolution and function of Mo utilization. Currently known Mo-binding proteins are mostly well characterized and their dependence on Mo is evolutionarily conserved. Although there is no tool available for prediction of the whole set of Mo-dependent proteins in organisms, comprehensive analyses of homologs of known molybdoenzymes, Mo transporters and Moco biosynthesis genes may lead to significant advances in our understanding of Mo utilization and its evolutionary trends at different levels, and in turn may offer new insights and ideas for further experimental studies. In recent years, several reports have been published that used comparative genomics approaches to analyze the occurrence and evolution of Mo uptake systems, Moco biosynthesis pathways and molybdoenzymes. These studies may not only help decipher the general principles of utilization of Mo across the three domains of life but also help explain how its utilization changed during evolution and which environmental conditions and factors may play a role in shaping up the current Mo requirement for life. The potential interactions between Mo and other trace elements (such as selenium) may provide clues regarding common features and differences in their use. It may be expected that future use of comparative genomics tools will bring new insights into the biology of Mo.
Research highlights.
Comparative genomics can be used to examine evolution and function of Mo utilization;
Molybodoenzymes are widespread and their dependence on Mo is evolutionarily conserved;
Many organisms lost the ability of use Mo;
Links between Mo utilization and that of other trace elements are discussed.
Acknowledgments
Supported by NIH Grant GM061603 and by a postdoctoral fellowship from the German Academic Exchange Service (DAAD).
Abbreviations
- Moco
molybdenum cofactor
- AOR
aldehyde:ferredoxin oxidoreductase
- ABC
ATP-binding-cassette
- SO
sulfite oxidase
- XO
xanthine oxidase
- DMSOR
dimethylsulfoxide reductase
- MOSC
Moco sulfurase
- Mop
Mo-binding protein
- cPMP
cyclic pyranopterin monophosphate
- MGD
molybdopterin guanine dinucleotide
- XDH
xanthine dehydrogenase
- AO
aldehyde oxidase
- MCP
Moco carrier protein
- MoBP
Moco-binding protein
- mARC
mitochondrial amidoxime reducing component
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajagopalan KV, Johnson JL. J Biol Chem. 1992;267:10199–10202. [PubMed] [Google Scholar]
- 2.Hille R. Chem Rev. 1996;96:2757–2816. doi: 10.1021/cr950061t. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz G, Mendel RR. Annu Rev Plant Biol. 2006;57:623–647. doi: 10.1146/annurev.arplant.57.032905.105437. [DOI] [PubMed] [Google Scholar]
- 4.Mendel RR, Bittner F. Biochim Biophys Acta. 2006;1763:621–635. doi: 10.1016/j.bbamcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Mendel RR. Biology J Exp Bot. 2007;58:2289–2296. doi: 10.1093/jxb/erm024. [DOI] [PubMed] [Google Scholar]
- 6.Hille R. Trends Biochem Sci. 2002;27:360–367. doi: 10.1016/s0968-0004(02)02107-2. [DOI] [PubMed] [Google Scholar]
- 7.Lawson DM, Smith BE. Met Ions Biol Syst. 2002;39:75–119. [PubMed] [Google Scholar]
- 8.Rubio LM, Ludden PW. Annu Rev Microbiol. 2008;62:93–111. doi: 10.1146/annurev.micro.62.081307.162737. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz G, Mendel RR, Ribbe MW. Nature. 2009;460:839–847. doi: 10.1038/nature08302. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz G. Cell Mol Life Sci. 2005;62:2792–2810. doi: 10.1007/s00018-005-5269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendel RR. Dalton Trans. 2005;21:3404–3409. doi: 10.1039/b505527j. [DOI] [PubMed] [Google Scholar]
- 12.Chan MK, Mukund S, Kletzin A, Adams MW, Rees DC. Science. 1995;267:1463–1469. doi: 10.1126/science.7878465. [DOI] [PubMed] [Google Scholar]
- 13.Romão MJ. Dalton Trans. 2009;21:4053–4068. doi: 10.1039/b821108f. [DOI] [PubMed] [Google Scholar]
- 14.Hille R. Trends Biochem Sci. 2002;27:360–367. doi: 10.1016/s0968-0004(02)02107-2. [DOI] [PubMed] [Google Scholar]
- 15.Bevers LE, Hagedoorn PL, Krijger GC, Hagen WR. J Bacteriol. 2006;188:6498–6505. doi: 10.1128/JB.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makdessi K, Andreesen JR, Pich A. J Biol Chem. 2001;276:24557–24564. doi: 10.1074/jbc.M101293200. [DOI] [PubMed] [Google Scholar]
- 17.Andreesen JR, Makdessi K. Ann N Y Acad Sci. 2008;1125:215–229. doi: 10.1196/annals.1419.003. [DOI] [PubMed] [Google Scholar]
- 18.Roy R, Adams MW. Met Ions Biol Syst. 2002;39:673–697. [PubMed] [Google Scholar]
- 19.Grunden AM, Shanmugam KT. Arch Microbiol. 1997;168:345–354. doi: 10.1007/s002030050508. [DOI] [PubMed] [Google Scholar]
- 20.Tomatsu H, Takano J, Takahashi H, Watanabe-Takahashi A, Shibagaki N, Fujiwara T. Proc Natl Acad Sci USA. 2007;104:18807–18812. doi: 10.1073/pnas.0706373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tejada-Jiménez M, Llamas A, Sanz-Luque E, Galván A, Fernández E. Proc Natl Acad Sci USA. 2007;104:20126–20130. doi: 10.1073/pnas.0704646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisker C, Schindelin H, Rees DC. Annu Rev Biochem. 1997;66:233–267. doi: 10.1146/annurev.biochem.66.1.233. [DOI] [PubMed] [Google Scholar]
- 23.Kisker C, Schindelin H, Baas D, Retey J, Meckenstock RU, Kroneck PM. FEMS Microbiol Rev. 1998;22:503–521. doi: 10.1111/j.1574-6976.1998.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 24.Mendel RR. Biofactors. 2009;35:429–434. doi: 10.1002/biof.55. [DOI] [PubMed] [Google Scholar]
- 25.Havemeyer A, Bittner F, Wollers S, Mendel R, Kunze T, Clement B. J Biol Chem. 2006;281:34796–34802. doi: 10.1074/jbc.M607697200. [DOI] [PubMed] [Google Scholar]
- 26.Kozmin SG, Leroy P, Pavlov YI, Schaaper RM. Mol Microbiol. 2008;68:51–65. doi: 10.1111/j.1365-2958.2008.06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinton SM, Dean D. Crit Rev Microbiol. 1990;17:169–188. doi: 10.3109/10408419009105724. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Angermüller S, Klipp W. J Bacteriol. 1993;175:3031–3042. doi: 10.1128/jb.175.10.3031-3042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pau RN, Lawson DM. Met Ions Biol Syst. 2002;39:31–74. [PubMed] [Google Scholar]
- 30.Grunden AM, Ray RM, Rosentel JK, Healy FG, Shanmugam KT. J Bacteriol. 1996;178:735–744. doi: 10.1128/jb.178.3.735-744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson LA, Palmer T, Price NC, Bornemann S, Boxer DH, Pau RN. Eur J Biochem. 1997;246:119–126. doi: 10.1111/j.1432-1033.1997.00119.x. [DOI] [PubMed] [Google Scholar]
- 32.Studholme DJ, Pau RN. BMC Microbiol. 2003;3:24. doi: 10.1186/1471-2180-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodionov DA, Dubchak I, Arkin A, Alm E, Gelfand MS. Genome Biol. 2004;5:R90. doi: 10.1186/gb-2004-5-11-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taveirne ME, Sikes ML, Olson JW. Mol Microbiol. 2009;74:758–771. doi: 10.1111/j.1365-2958.2009.06901.x. [DOI] [PubMed] [Google Scholar]
- 35.Gisin J, Müller A, Pfänder Y, Leimkühler S, Narberhaus F, Masepohl B. J Bacteriol. 2010;192:5943–5952. doi: 10.1128/JB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxter I, Muthukumar B, Park HC, Buchner P, Lahner B, Danku J, Zhao K, Lee J, Hawkesford MJ, Guerinot ML, Salt DE. PLoS Genet. 2008;4:e1000004. doi: 10.1371/journal.pgen.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanmugam KT, Stewart V, Gunsalus RP, Boxer DH, Cole JA, Chippaux M, DeMoss JA, Giordano G, Lin EC, Rajagopalan KV. Mol Microbiol. 1992;6:3452–3454. doi: 10.1111/j.1365-2958.1992.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 38.Mendel RR, Schwarz G. Met Ions Biol Syst. 2002;39:317–368. [PubMed] [Google Scholar]
- 39.Mendel RR, Hänsch R. J Exp Bot. 2002;53:1689–1698. doi: 10.1093/jxb/erf038. [DOI] [PubMed] [Google Scholar]
- 40.Millar LJ, Heck IS, Sloan J, Kana’n GJ, Kinghorn JR, Unkles SE. Mol Genet Genomics. 2001;266:445–453. doi: 10.1007/s004380100543. [DOI] [PubMed] [Google Scholar]
- 41.Reiss J, Cohen N, Dorche C, Mandel H, Mendel RR, Stallmeyer B, Zabot MT, Dierks T. Nat Genet. 1998;20:51–53. doi: 10.1038/1706. [DOI] [PubMed] [Google Scholar]
- 42.Reiss J, Johnson JL. Hum Mutat. 2003;21:569–576. doi: 10.1002/humu.10223. [DOI] [PubMed] [Google Scholar]
- 43.Bittner F, Oreb M, Mendel RR. J Biol Chem. 2001;276:40381–40384. doi: 10.1074/jbc.C100472200. [DOI] [PubMed] [Google Scholar]
- 44.Ichida K, Matsumura T, Sakuma R, Hosoya T, Nishino T. Biochem Biophys Res Commun. 2001;282:1194–1200. doi: 10.1006/bbrc.2001.4719. [DOI] [PubMed] [Google Scholar]
- 45.Teschner J, Lachmann N, Schulze J, Geisler M, Selbach K, Santamaria-Araujo J, Balk J, Mendel RR. F Bittner Plant Cell. 2010;22:468–480. doi: 10.1105/tpc.109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sargent F. Microbiology. 2007;153:633–651. doi: 10.1099/mic.0.2006/004762-0. [DOI] [PubMed] [Google Scholar]
- 47.Vergnes A, Pommier J, Toci R, Blasco F, Giordano G, Magalon A. J Biol Chem. 2006;281:2170–2176. doi: 10.1074/jbc.M505902200. [DOI] [PubMed] [Google Scholar]
- 48.Ilbert M, Méjean V, Iobbi-Nivol C. Microbiology. 2004;150:935–943. doi: 10.1099/mic.0.26909-0. [DOI] [PubMed] [Google Scholar]
- 49.Fischer K, Llamas A, Tejada-Jimenez M, Schrader N, Kuper J, Ataya FS, Galvan A, Mendel RR, Fernandez E, Schwarz G. J Biol Chem. 2006;281:30186–30194. doi: 10.1074/jbc.M603919200. [DOI] [PubMed] [Google Scholar]
- 50.Kruse T, Gehl C, Geisler M, Lehrke M, Ringel P, Hallier S, Hänsch R, Mendel RR. J Biol Chem. 2010;285:6623–6635. doi: 10.1074/jbc.M109.060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seefeldt LC, Hoffman BM, Dean DR. Annu Rev Biochem. 2009;78:701–722. doi: 10.1146/annurev.biochem.78.070907.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983–3011. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 53.Eady RR. Chem Rev. 1996;96:3013–3030. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]
- 54.Workun GJ, Moquin K, Rothery RA, Weiner JH. Microbiol Mol Biol Rev. 2008;72:228–248. doi: 10.1128/MMBR.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng C, Tollin G, Enemark JH. Biochim Biophys Acta. 2007;1774:527–539. doi: 10.1016/j.bbapap.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell WH. Cell Mol Life Sci. 2001;58:194–204. doi: 10.1007/PL00000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garattini E, Fratelli M, Terao M. Cell Mol Life Sci. 2008;65:1019–1048. doi: 10.1007/s00018-007-7398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hille R. Arch Biochem Biophys. 2005;433:107–116. doi: 10.1016/j.abb.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Romão MJ, Archer M, Moura I, Moura JJ, LeGall J, Engh R, Schneider M, Hof P, Huber R. Science. 1995;270:1170–1176. doi: 10.1126/science.270.5239.1170. [DOI] [PubMed] [Google Scholar]
- 60.Boll M, Fuchs G, Meier C, Trautwein A, El Kasmi A, Ragsdale SW, Buchanan G, Lowe DJ. J Biol Chem. 2001;276:47853–47862. doi: 10.1074/jbc.M106766200. [DOI] [PubMed] [Google Scholar]
- 61.Bonin I, Martins BM, Purvanov V, Fetzner S, Huber R, Dobbek H. Structure. 2004;12:1425–1435. doi: 10.1016/j.str.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Rothery RA, Workun GJ, Weiner JH. Biochim Biophys Acta. 2008;1778:1897–1929. doi: 10.1016/j.bbamem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Satoh T, Kurihara FN. J Biochem. 1987;102:191–197. doi: 10.1093/oxfordjournals.jbchem.a122032. [DOI] [PubMed] [Google Scholar]
- 64.Weiner JH, Rothery RA, Sambasivarao D, Trieber CA. Biochim Biophys Acta. 1992;1102:1–18. doi: 10.1016/0005-2728(92)90059-b. [DOI] [PubMed] [Google Scholar]
- 65.Jormakka M, Byrne B, Iwata S. Curr Opin Struct Biol. 2003;13:418–423. doi: 10.1016/s0959-440x(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 66.Chan MK, Mukund S, Kletzin A, Adams MWW, Rees DC. Science. 1995;267:1463–1469. doi: 10.1126/science.7878465. [DOI] [PubMed] [Google Scholar]
- 67.Hu Y, Faham S, Roy R, Adams MW, Rees DC. J Mol Biol. 1999;286:899–914. doi: 10.1006/jmbi.1998.2488. [DOI] [PubMed] [Google Scholar]
- 68.Trautwein T, Krauss F, Lottspeich F, Simon H. Eur J Biochem. 1994;222:1025–1032. doi: 10.1111/j.1432-1033.1994.tb18954.x. [DOI] [PubMed] [Google Scholar]
- 69.Wahl B, Reichmann D, Niks D, Krompholz N, Havemeyer A, Clement B, Messerschmidt T, Rothkegel M, Biester H, Hille R, Mendel RR. F Bittner J Biol Chem. 2010;285:37847–37859. doi: 10.1074/jbc.M110.169532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotthaus J, Wahl B, Havemeyer A, Kotthaus J, Schade D, Garbe-Schönberg D, Mendel R, Bittner F, Clement B. Biochem J. 2010 doi: 10.1042/BJ20100960. in press. [DOI] [PubMed] [Google Scholar]
- 71.Gruenewald S, Wahl B, Bittner F, Hungeling H, Kanzow S, Kotthaus J, Schwering U, Mendel RR, Clement B. J Med Chem. 2008;51:8173–8177. doi: 10.1021/jm8010417. [DOI] [PubMed] [Google Scholar]
- 72.Cvetkovic A, Menon AL, Thorgersen MP, Scott JW, Poole FL, 2nd, Jenney FE, Jr, Lancaster WA, Praissman JL, Shanmukh S, Vaccaro BJ, Trauger SA, Kalisiak E, Apon JV, Siuzdak G, Yannone SM, Tainer JA, Adams MW. Nature. 2010;466:779–782. doi: 10.1038/nature09265. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Gladyshev VN. J Mol Biol. 2008;379:881–899. doi: 10.1016/j.jmb.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Gladyshev VN. J Biol Chem. 2010;285:3393–3405. doi: 10.1074/jbc.M109.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Gladyshev VN. Chem Rev. 2009;109:4828–4861. doi: 10.1021/cr800557s. [DOI] [PubMed] [Google Scholar]
- 76.Hu Y, Rech S, Gunsalus RP, Rees DC. Nature Struct Biol. 1997;4:703–707. doi: 10.1038/nsb0997-703. [DOI] [PubMed] [Google Scholar]
- 77.Hollenstein K, Frei DC, Locher KP. Nature. 2007;446:213–216. doi: 10.1038/nature05626. [DOI] [PubMed] [Google Scholar]
- 78.McNicholas PM, Mazzotta MM, Rech SA, Gunsalus RP. J Bacteriol. 1998;180:4638–4643. doi: 10.1128/jb.180.17.4638-4643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stolz JF, Basu P. Chembiochem. 2002;3:198–206. doi: 10.1002/1439-7633(20020301)3:2/3<198::AID-CBIC198>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 80.Tomiki T, Saitou N. J Mol Evol. 2004;59:158–176. doi: 10.1007/s00239-004-2610-2. [DOI] [PubMed] [Google Scholar]
- 81.Nonaka H, Keresztes G, Shinoda Y, Ikenaga Y, Abe M, Naito K, Inatomi K, Furukawa K, Inui M, Yukawa H. J Bacteriol. 2006;188:2262–2274. doi: 10.1128/JB.188.6.2262-2274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hipkin CR, Kau DA, Cannons AC. J Gen Microbiol. 1993;139:473–478. doi: 10.1099/00221287-139-3-473. [DOI] [PubMed] [Google Scholar]
- 83.Barbier GG, Joshi RC, Campbell ER, Campbell WH. Protein Expr Purif. 2004;37:61–71. doi: 10.1016/j.pep.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 84.Fischer K, Barbier GG, Hecht HJ, Mendel RR, Campbell WH, Schwarz G. Plant Cell. 2005;17:1167–1179. doi: 10.1105/tpc.104.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anantharaman V, Aravind L. FEMS Microbiol Lett. 2002;207:55–61. doi: 10.1111/j.1574-6968.2002.tb11028.x. [DOI] [PubMed] [Google Scholar]
- 86.Wollers S, Heidenreich T, Zarepour M, Zachmann D, Kraft C, Zhao Y, Mendel RR, Bittner F. J Biol Chem. 2008;283:9642–9650. doi: 10.1074/jbc.M708549200. [DOI] [PubMed] [Google Scholar]
- 87.Hänzelmann P, Hernández HL, Menzel C, García-Serres R, Huynh BH, Johnson MK, Mendel RR, Schindelin H. J Biol Chem. 2004;279:34721–34732. doi: 10.1074/jbc.M313398200. [DOI] [PubMed] [Google Scholar]
- 88.Hänzelmann P, Schindelin H. Proc Natl Acad Sci USA. 2006;103:6829–6834. doi: 10.1073/pnas.0510711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuper J, Llamas A, Hecht HJ, Mendel RR, Schwarz G. Nature. 2004;430:803–806. doi: 10.1038/nature02681. [DOI] [PubMed] [Google Scholar]
- 90.Llamas A, Otte T, Multhaup G, Mendel RR, Schwarz G. J Biol Chem. 2006;281:18343–18350. doi: 10.1074/jbc.M601415200. [DOI] [PubMed] [Google Scholar]
- 91.Morrison MS, Cobine PA, Hegg EL. J Biol Inorg Chem. 2007;12:1129–1139. doi: 10.1007/s00775-007-0279-x. [DOI] [PubMed] [Google Scholar]
- 92.Dobbek H, Gremer L, Kiefersauer R, Huber R, Meyer O. Proc Natl Acad Sci USA. 2002;99:15971–15976. doi: 10.1073/pnas.212640899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Romero H, Salinas G, Gladyshev VN. Genome Biol. 2006;7:R94. doi: 10.1186/gb-2006-7-10-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Rodionov DA, Gelfand MS, Gladyshev VN. BMC Genomics. 2009;10:78. doi: 10.1186/1471-2164-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lobanov AV, Fomenko DE, Zhang Y, Sengupta A, Hatfield DL, Gladyshev VN. Genome Biol. 2007;8:R198. doi: 10.1186/gb-2007-8-9-r198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ridge PG, Zhang Y, Gladyshev VN. PLoS One. 2008;3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lake MW, Wuebbens MM, Rajagopalan KV, Schindelin H. Nature. 2001;414:325–329. doi: 10.1038/35104586. [DOI] [PubMed] [Google Scholar]
- 98.Lee I. H Schindelin Cell. 2008;134:268–278. doi: 10.1016/j.cell.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 99.Schwarz G, Schrader N, Mendel RR, Hecht HJ, Schindelin H. J Mol Biol. 2001;312:405–418. doi: 10.1006/jmbi.2001.4952. [DOI] [PubMed] [Google Scholar]
- 100.Kirsch J, Betz H. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- 101.Kirsch J, Wolters I, Triller A, Betz H. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- 102.Vorbach C, Scriven A, Capecchi MR. Genes Dev. 2002;16:3223–3235. doi: 10.1101/gad.1032702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McManaman JL, Palmer CA, Wright RM, Neville MC. J Physiol. 2002;545:567–579. doi: 10.1113/jphysiol.2002.027185. [DOI] [PMC free article] [PubMed] [Google Scholar]