Abstract
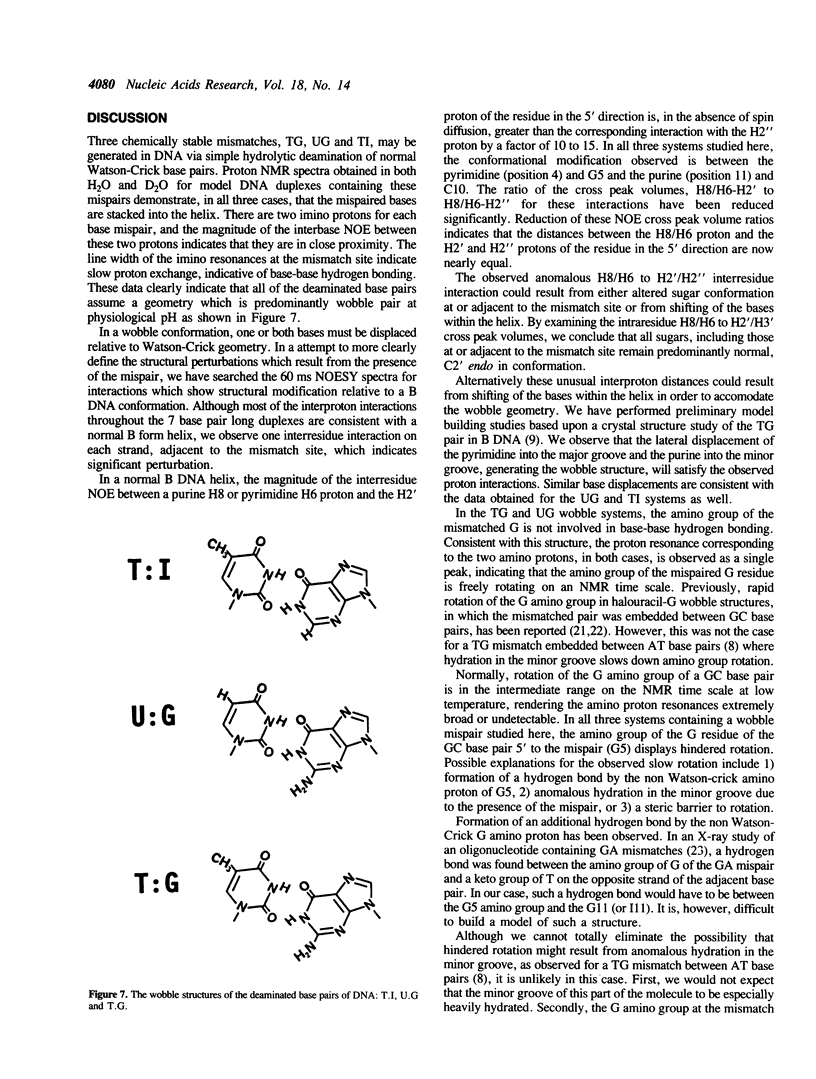
The structurally aberrant base pairs TG, UG and TI may occur in DNA as a consequence of deamination of 5-methylcytosine, cytosine and adenine respectively. Results of NMR spectroscopic studies are reported here for these deaminated base pairs in a model seven base pair long oligonucleotide duplex. We find that in all three cases, the DNA helix is a normal B form and both mispaired bases are intrahelical and hydrogen bonded with one another in a wobble geometry. Similarly, in all three cases, all sugars are found to be normal C2' endo in conformation. Symmetric structural perturbations are observed in the helix twist on the 3' side of the mispaired pyrimidine and on the 5' side of the mispaired purine. In all three cases, the amino group of the G residue on the 3' side of the mispaired pyrimidine shows hindered rotation. Although less thermodynamically stable than helices containing only Watson-Crick base pairs, these helices melt normally from the ends and not from the mispair outwards.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caradonna S. J., Cheng Y. C. Uracil DNA-glycosylase. Purification and properties of this enzyme isolated from blast cells of acute myelocytic leukemia patients. J Biol Chem. 1980 Mar 25;255(6):2293–2300. [PubMed] [Google Scholar]
- Cuniasse P., Sowers L. C., Eritja R., Kaplan B., Goodman M. F., Cognet J. A., Le Bret M., Guschlbauer W., Fazakerley G. V. Abasic frameshift in DNA. Solution conformation determined by proton NMR and molecular mechanics calculations. Biochemistry. 1989 Mar 7;28(5):2018–2026. doi: 10.1021/bi00431a009. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V., Téoule R., Guy A., Fritzsche H., Guschlbauer W. NMR studies on oligodeoxyribonucleotides containing the dam methylation site GATC. Comparison between d(GGATCC) and d(GGm6ATCC). Biochemistry. 1985 Aug 13;24(17):4540–4548. doi: 10.1021/bi00338a009. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V., van der Marel G. A., van Boom J. H., Guschlbauer W. Helix opening in deoxyribonucleic acid from a proton nuclear magnetic resonance study of imino and amino protons in d(CG)3. Nucleic Acids Res. 1984 Nov 12;12(21):8269–8279. doi: 10.1093/nar/12.21.8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigon J., Leupin W., Denny W. A., Kearns D. R. Two-dimensional proton nuclear magnetic resonance investigation of the synthetic deoxyribonucleic acid decamer d(ATATCGATAT)2. Biochemistry. 1983 Dec 6;22(25):5943–5951. doi: 10.1021/bi00294a038. [DOI] [PubMed] [Google Scholar]
- Frechet D., Cheng D. M., Kan L. S., Ts'o P. O. Nuclear Overhauser effect as a tool for the complete assignment of nonexchangeable proton resonances in short deoxyribonucleic acid helices. Biochemistry. 1983 Oct 25;22(22):5194–5200. doi: 10.1021/bi00291a020. [DOI] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Hare D., Shapiro L., Patel D. J. Wobble dG X dT pairing in right-handed DNA: solution conformation of the d(C-G-T-G-A-A-T-T-C-G-C-G) duplex deduced from distance geometry analysis of nuclear Overhauser effect spectra. Biochemistry. 1986 Nov 18;25(23):7445–7456. doi: 10.1021/bi00371a029. [DOI] [PubMed] [Google Scholar]
- Hunter W. N., Brown T., Anand N. N., Kennard O. Structure of an adenine-cytosine base pair in DNA and its implications for mismatch repair. Nature. 1986 Apr 10;320(6062):552–555. doi: 10.1038/320552a0. [DOI] [PubMed] [Google Scholar]
- Hunter W. N., Brown T., Kneale G., Anand N. N., Rabinovich D., Kennard O. The structure of guanosine-thymidine mismatches in B-DNA at 2.5-A resolution. J Biol Chem. 1987 Jul 25;262(21):9962–9970. doi: 10.2210/pdb113d/pdb. [DOI] [PubMed] [Google Scholar]
- Lindahl T. Irreversible heat inactivation of transfer ribonucleic acids. J Biol Chem. 1967 Apr 25;242(8):1970–1973. [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Privé G. G., Heinemann U., Chandrasegaran S., Kan L. S., Kopka M. L., Dickerson R. E. Helix geometry, hydration, and G.A mismatch in a B-DNA decamer. Science. 1987 Oct 23;238(4826):498–504. doi: 10.1126/science.3310237. [DOI] [PubMed] [Google Scholar]
- Quignard E., Fazakerley G. V., van der Marel G., van Boom J. H., Guschlbauer W. Comparison of the conformation of an oligonucleotide containing a central G-T base pair with the non-mismatch sequence by proton NMR. Nucleic Acids Res. 1987 Apr 24;15(8):3397–3409. doi: 10.1093/nar/15.8.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUSTER H., SCHRAMM G. Bestimmung der biologisch wirksamen Einheit in der Ribosenucleinsäure des Tabakmosaikvirus auf chemischem Wege. Z Naturforsch B. 1958 Nov;13B(11):697–704. [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Klein R. S. The deamination of cytidine and cytosine by acidic buffer solutions. Mutagenic implications. Biochemistry. 1966 Jul;5(7):2358–2362. doi: 10.1021/bi00871a026. [DOI] [PubMed] [Google Scholar]
- Sowers L. C., Eritja R., Kaplan B., Goodman M. F., Fazakerly G. V. Equilibrium between a wobble and ionized base pair formed between fluorouracil and guanine in DNA as studied by proton and fluorine NMR. J Biol Chem. 1988 Oct 15;263(29):14794–14801. [PubMed] [Google Scholar]
- Sowers L. C., Goodman M. F., Eritja R., Kaplan B., Fazakerley G. V. Ionized and wobble base-pairing for bromouracil-guanine in equilibrium under physiological conditions. A nuclear magnetic resonance study on an oligonucleotide containing a bromouracil-guanine base-pair as a function of pH. J Mol Biol. 1989 Jan 20;205(2):437–447. doi: 10.1016/0022-2836(89)90353-7. [DOI] [PubMed] [Google Scholar]
- Wang R. Y., Kuo K. C., Gehrke C. W., Huang L. H., Ehrlich M. Heat- and alkali-induced deamination of 5-methylcytosine and cytosine residues in DNA. Biochim Biophys Acta. 1982 Jun 30;697(3):371–377. doi: 10.1016/0167-4781(82)90101-4. [DOI] [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. In vitro correction of G.T mispairs to G.C pairs in nuclear extracts from human cells. Nature. 1989 May 18;339(6221):234–236. doi: 10.1038/339234a0. [DOI] [PubMed] [Google Scholar]


