Abstract
Phase variable restriction-modification (R-M) systems have been identified in a range of pathogenic bacteria. In some it has been demonstrated that the random switching of the mod (DNA methyltransferase) gene mediates the coordinated expression of multiple genes and constitutes a phasevarion (phase variable regulon). ModA of Neisseria and Haemophilus influenzae contain a highly variable, DNA recognition domain (DRD) that defines the target sequence that is modified by methylation and is used to define modA alleles. 18 distinct modA alleles have been identified in H. influenzae and the pathogenic Neisseria. To determine the origin of DRD variability, the 18 modA DRDs were used to search the available databases for similar sequences. Significant matches were identified between several modA alleles and mod gene from distinct bacterial species, indicating one source of the DRD variability was via horizontal gene transfer. Comparison of DRD sequences revealed significant mosaicism, indicating exchange between the Neisseria and H. influenzae modA alleles. Regions of high inter- and intra-allele similarity indicate that some modA alleles had undergone recombination more frequently than others, generating further diversity. Furthermore, the DRD from some modA alleles, such as modA12, have been transferred en bloc to replace the DRD from different modA alleles.
Introduction
Restriction-modification (R-M) systems are ubiquitous in bacteria and are involved in protecting the host cell from the invasion of foreign DNA [1]. R-M systems are comprised of two components, a methyltransferase (Mod) and a restriction endonuclease (Res). Mod catalyses the methylation of host DNA at a specific nucleotide within a defined recognition sequence, allowing for the recognition of self DNA and Res catalyses the cleavage of unmethylated DNA [2]. R-M systems are classified into four groups on the basis of subunit composition, cleavage position, sequence specificity and co-factor requirements [3].
Type I R-M systems are comprised of three subunits, S, M and R, which together form a holoenzyme that performs both methylation and restriction activity. Type I systems cleave DNA at random, often far from their recognition sequences [4],[5]. Type II systems are the most common of the four with most commercially available restriction enzymes belonging to this class [3]. Type II systems consist of two independently acting enzymes for methylation and restriction, each encoded by a separate gene. Type II restriction enzymes cleave DNA at very defined positions within or close to their recognition sequences, making them valuable laboratory tools [6]. Type IV systems are extremely similar to type II systems in that methylation and restriction are catalysed by independently acting enzymes but the restriction endonuclease requires a methyl donor for successful cleavage [3].
Type III systems are complex although they only consist of two subunits, the methyltransferase and restriction endonuclease. Mod can function independently to methylate one strand of DNA within an asymmetric 5–6 bp recognition sequence [7], [8]. Res must form a complex with Mod for restriction activity as Mod contains the DNA recognition domain (DRD) [9]. Res cleaves unmethylated double-stranded DNA outside the recognition sequence, 25–27 base pairs to one side [8].
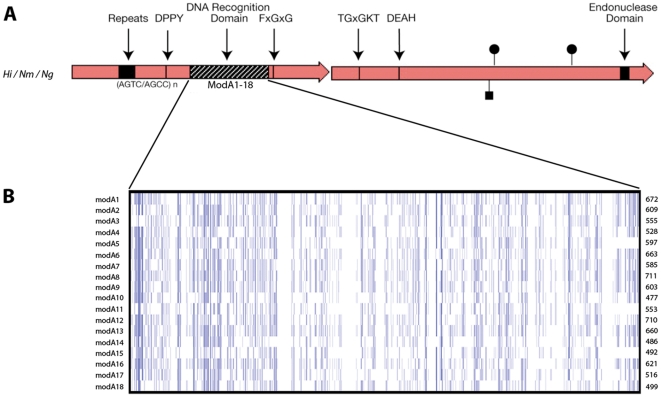
Genetic analysis of type III R-M systems has shown that the res gene of H. influenzae and the pathogenic Neisseria is highly conserved with DEAD box motifs commonly associated with the superfamily II helicases, see Figure 1 [10]. These are the ATP-binding motif, TGxGKT, the ATP-hydrolysis motif, DEAH, and the endonuclease motif, PD(x)17(D/E)xK. The mod gene however, contains conserved 5′ and 3′ regions that flank a highly variable, central region [10], [11]. The conserved regions of the modA gene encode the S-adenosyl-l-methioine (AdoMet) binding pocket, FxGxG, and a catalytic region, DPPY, which accommodates the target adenine in the recognition sequence after it has been flipped out of the double-helix, see Figure 1 [11], [12]. The variable region contains the DRD and dictates the sequence specificity for the methyltransferase [13].
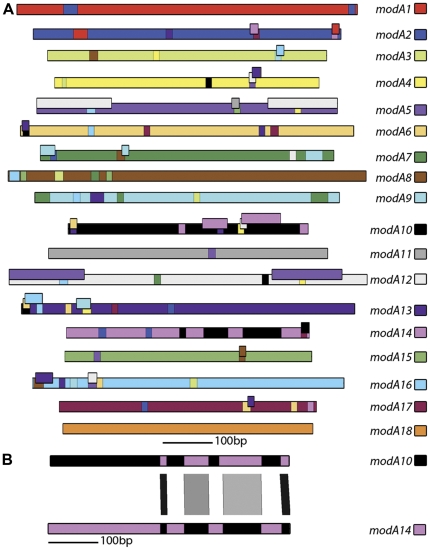
Figure 1. Diagrammatical representation of modA and res genes of H. influenzae, N. meningitidis and N. gonorrhoeae.
A. The methyltransferase (modA) genes, and restriction endonuclease (res) genes, with the repeat regions that mediate phase variation. The DNA recognition domain is represented by the striped box [14]. B. The variable regions for each of the 18 modA alleles in the multiple sequence alignment were aligned in ClustalW and visualised with JalView using the overview feature. The nucleotides are represented as vertical bars coloured according to consensus identity (dark blue >80% identity; light blue >50% identity; white <50% identity or gap). The mod alleles are as follows: modA1 Hi R3327 (126508350); modA2 Hi 723 (126508378); modA3 Hi R3366 (126508386); modA4 Hi 3579 (126508396); modA5 Hi 1268 (126508406); modA6 Hi C1626 (126508412); modA7 Hi R3265 (126508420); modA8 Hi C505 (126508426); modA9 Hi 1209 (126508430); modA10 Hi R3157 (126508438); modA11 Nm BZ83 (257124056); modA12 Nm 129E (257124138); modA13 Ng 1291 (26506021); modA14 Hi R1527 (126508442); modA15 Hi R3570 (126508452); modA16 Hi ATCC9007 (126508448); modA17 Hi ATCC9833 (126508446); modA18 Nm NGE28 (257124039). As each mod allele is a different length, the number of base pairs is indicated to the right of the nucleotide alignment.
Sequence analysis of the type III mod genes of pathogenic bacteria has identified simple tandem repeats that allow phase variable expression [14]. Phase variation is the reversible, high-frequency, on/off switching of gene expression and is often mediated by simple tandem repeats in the open reading frame or promoter region of genes encoding surface expressed virulence determinants, for review see Moxon et al. [15]. Phase variation results in the presence or absence of certain surface components and a phenotypically diverse population [16]. Methyltransferases are not surface expressed, nor are they involved in the biosynthesis of a cell surface structure, and so they represent a unique class of phase variable genes. There are several examples of phase variable type III R-M systems in host adapted bacterial pathogens, such as Haemophilus influenzae [17], Neisseria gonorrhoeae and Neisseria meningitidis [18], [19], Helicobacter pylori [20], Pastuerella haemolytica [21] and Moraxella catarrhalis [22], for review see Srikhanta et al. [23]. The identification of phase variable R-M systems has indicated a possible role in gene regulation through differential methylation of the genome. The modA gene of H. influenzae [24] and both pathogenic Neisseria species [19] has been shown to phase vary, and to coordinate the expression of multiple genes important in pathogenesis, constituting a phase variable regulon, “phasevarion”.
The central region defines the modA allele as it contains the DNA recognition domain (DRD) that is responsible for DNA recognition sequence specificity. Diversity in this region has been reported [25], and further analysis on a set of 59 capsulated (typeable) and non-typeable H. influenzae strains defined a set of 15 distinct modA alleles (modA1–10 and modA13–17) and three additional modA alleles in the pathogenic Neisseria (modA11–12 and modA18) [14]. By definition, mod alleles exhibit high conservation of the DRD sequence (>95% nucleotide identity) within groups, but very little similarity between groups. To date, all reported modA DRD sequences have been classified as belonging to one of these 18 mod alleles. The modA alleles of H. influenzae and pathogenic Neisseria are essentially the same gene with evidence of horizontal transfer between the two species [26]. Some of the alleles are common to H. influenzae and Neisseria, such as modA13 and modA15, while others are only associated with one, for example modA11–12 are Neisseria specific [27]. Interestingly, most N. meningitidis strains have a second phase variable methyltransferase, modB [19] and a third, modD, has been recently identified in the hypervirulent N. meningitidis clonal complex 41/44 [28].
In the current study we undertake comprehensive bioinformatics analysis of the 18 available modA alleles in order to better understand the mechanisms giving rise to DRD sequence diversity.
Materials and Methods
Sequences
Nucleotide and amino acid sequences for the 18 modA alleles were obtained from GenBank. Each of the modA sequences had been previously described in one of three previous studies: A) 112 modA sequences encompassing the DRD domain, and at least 330 nt upstream and 450 nt downstream [19]; B) 22 sequences, 1188 nt to 5262 nt in length, encompassing part of modA 5′ and DRD up to the entire mod/res region [25]; and C) 54 modA DRD sequences, 477 nt to 711 nt in length [14]. Inter-allele comparisons were carried out with representative sequences chosen from the numerically dominant variant for each DRD allele: modA1 Hi R3327 (126508350); modA2 Hi 723 (126508378); modA3 Hi R3366 (126508386); modA4 Hi 3579 (126508396); modA5 Hi 1268 (126508406); modA6 Hi C1626 (126508412); modA7 Hi R3265 (126508420); modA8 Hi C505 (126508426); modA9 Hi 1209 (126508430); modA10 Hi R3157 (126508438); modA11 Nm BZ83 (257124056); modA12 Nm 129E (257124138); modA13 Ng 1291 (26506021); modA14 Hi R1527 (126508442); modA15 Hi R3570 (126508452); modA16 Hi ATCC9007 (126508448); modA17 Hi ATCC9833 (126508446); modA18 Nm NGE28 (257124039). Additionally, 77 N. meningitidis modA12 sequences from dataset B [19], were used in an intra-allele comparative analysis.
Sequence Analysis
Inter-allele comparisons were carried out using all-versus-all BLASTn and BLASTp comparisons of representative mod DRD alleles using unfiltered stand-alone BLAST (version 2.2.18). To identify homologous DRD sequences, the amino acid sequence for each representative modA DRD allele was used as the query sequence for tBLASTn searches of the nucleotide databases at NCBI, including the whole-genome-shotgun and environmental databases. Mod and res loci from complete and unfinished genomes were identified by using the H. influenzae KW20 modA1 4.6 kb locus to query the GenBank nucleotide database and Whole Genome Shotgun database, respectively. Matches were further analysed using Artemis Comparison Tool [29] to retrieve the entire mod and res sequences and determine if either were pseudogenes. The modA alleles for 38 genome-derived mod sequences were determined by unfiltered stand-alone BLASTn against a custom database consisting of representative modA DRD allele nucleotide sequences. Unless otherwise stated, all BLAST searches were carried out with default parameters. Amino acid and nucleotide sequences were aligned using ClustalX 2.0.11 with default parameters. Alignments were viewed and edited using Jalview 2.4.0.b2 [30]. Sequence alignments were analysed for evidence of recombination using PhiPack [31]. Phylogenetic analyses were carried out using the PHYLIP package [32]. Sequence alignments used in Figures 1, 4 and 6 are provided in Data S1.
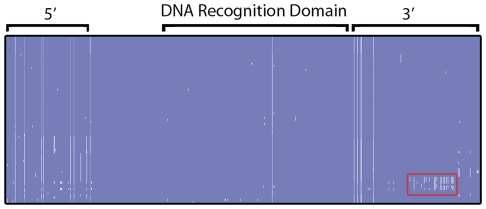
Figure 4. Alignment profile for 77 N. meningitidis modA12 alleles and flanking regions.
The DRD variable domain, 330 nt upstream and 450 nt downstream were aligned using ClustalW and visualized using Jalview. The nucleotides are represented as vertical bars coloured according to consensus identity (dark blue >90% identity; light blue >50% identity; white <50% identity). Highlighted in red is a block of sequence indicative of recombination, where some sequences are identical to equivalent regions of H. influenzae PittEE, modA6.
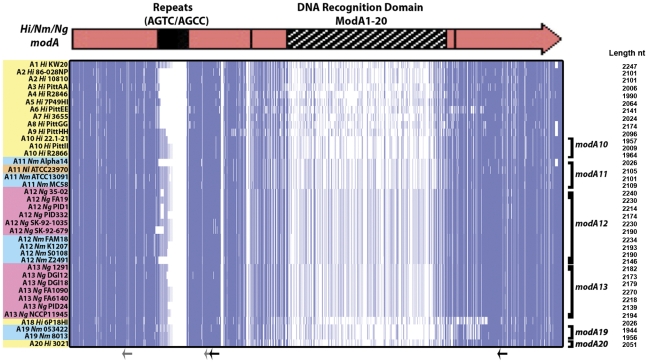
Figure 6. Multiple nucleotide sequence alignment of complete modA sequence from available genome sequences.
The modA genes from H. influenzae (yellow), N. gonorrhoeae (pink), N. meningitidis (blue) and N. lactamica (orange) isolates were aligned. Nucleotides are represented as vertical bars (dark blue >80% identity; light blue >50% identity; white <50% identity or gap). Strain names are indicated on the left of the nucleotide alignment and major modA alleles represented here are indicated on the right. Arrows indicate the position of Neisserial uptake sequence 5′-GCCGTCTGAA-3′ (grey are imperfect matches, 8/10 in majority of strains; black arrows are perfect matches). Sequences were aligned in ClustalW and displayed using JalView (jalview.org).
Results
Detailed analysis of the diversity of the DRD domain that defines modA alleles
Representatives of all 18 modA alleles were compared by multiple sequence alignment. Figure 1 illustrates the diversity seen throughout the DRD of the modA alleles at the nucleotide level with respect to the nucleotide sequence and length of the DRD. The closest modA DRD domains share 38% amino acid identity, whereas the most distant share 29%. To probe the origin of DRD diversity and the relative contribution of intra- and inter-species recombination mechanisms we undertook a detailed analysis of all available modA alleles.
Source of modA Diversity: Inter-species recombination
Our previous work [14] has identified homology between particular modA DRDs and DNA methyltransferases from unrelated bacterial species suggesting acquisition of new DRDs en-bloc via horizontal gene transfer. To further explore the source of extant modA alleles, BLAST searches using all available modA DRD regions were carried out. We used the amino acid sequences for the DRD of each modA allele to undertake tBLASTn searches of NCBI nucleotide databases to identify likely source genomes. Ignoring self-matches, all matches with >50% amino acid identity (E-value <0.001) identified in the WGS division of Genbank are outlined in table 1. Notably, some of the most significant matches were to other upper respiratory tract bacterial species, for example Legionella pneumophila and Moraxella catarrhalis. As previously stated, the amino acid sequence identity of the DRD between known modA alleles (i.e. ModA1–ModA18) is >29%, therefore the identification of matches >50% between the modA DRD and non-related bacterial species required further investigation. In all cases the match was with a predicted adenine specific type III R-M system methyltransferase. Matches between modA4 and Helicobacter sp. [20] and modA5 and Moraxella catarrhalis [22] are with methyltransferases that are predicted phase variable, however the repeat regions mediating phase variation are different.
Table 1. Significant matches identified with tBLASTn.
| modA Allelea | Matchb | GenBank Accessionc | % Idd | % Simd | Length of Matche |
| modA1 | Fusobacterium sp. 3_1_33 cont1.59 | ACQE01000059.1 | 52 | 72 | 228 |
| modA2 | Enhydrobacter aerosaccus SK60 contig00039 | ACYI01000007.1 | 72 | 83 | 210 |
| modA4 | Helicobacter acinonychis str. Sheeba | AM260522 | 57 | 72 | 176 |
| Helicobacter pylori G27 | CP001173 | 56 | 71 | 176 | |
| Helicobacter pylori P12 | CP001217 | 56 | 72 | 176 | |
| Helicobacter pylori Shi470 | CP001072 | 56 | 71 | 176 | |
| Helicobacter pylori 26695 | AE000511 | 55 | 70 | 176 | |
| modA5 | Haemophilus parasuis SH0165 | CP001321 | 69 | 81 | 199 |
| Fusobacterium sp. 4_1_13 cont1.41 | ACDE01000041.1 | 68 | 79 | 199 | |
| Clostridium perfringens CPE str. F4969 gcontig_1106202596928 | ABDX01000002.1 | 57 | 76 | 200 | |
| Stenotrophomonas sp. SKA14 ctg_1108481805199 | ACDV01000027.1 | 50 | 69 | 200 | |
| Moraxella catarrhalis | AY049057.1 | 73 | 87 | 200 | |
| modA6 | Helicobacter pylori B128 contig00164 | ABSY01000012.1 | 66 | 80 | 220 |
| modA7 | Lactobacillus jensenii 269-3 contig00058 | ACOY01000053.1 | 53 | 69 | 196 |
| Lactobacillus jensenii 1153 cont1.15 | ABWG01000015.1 | 53 | 69 | 196 | |
| modA15 | Legionella pneumophila str. Paris | CR628336 | 69 | 83 | 164 |
As dictated by the mod variable region.
Significant matches from the microbial genome, non-redundant, or environmental databases.
GenBank accession number for each significant match.
Identity and similarity values correspond to the entire mod variable region.
Length of match in amino acids DRD lengths: modA4 176aa, modA5 199aa, modA6 221aa, modA7 196aa, modA15 164aa.
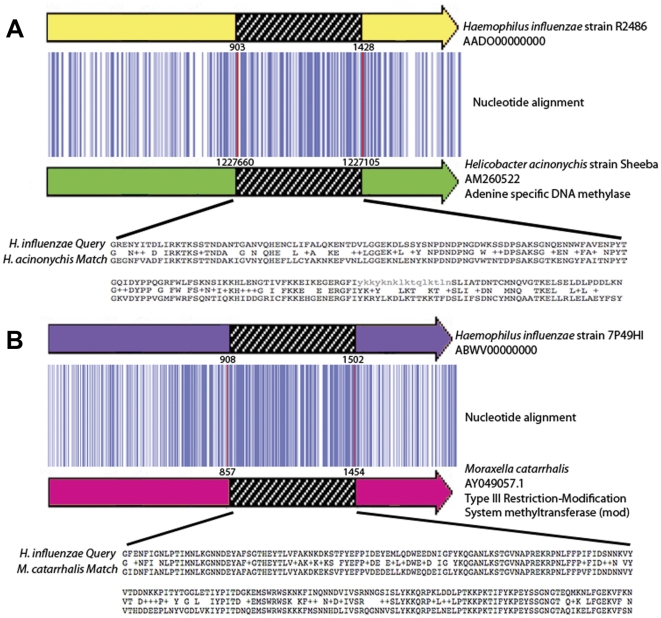
The local alignments identified by BLAST were extended using the corresponding complete sequences to determine the actual length of the match and determine if the alignment extended beyond the boundaries of the modA DRD. The matches between the modA4 and Helicobacter acinonychis Hac_1417 and modA5 and Moraxella catarrhalis McaRII are shown in figure 2. In both cases it appears that the nucleotide identity extends beyond the DRD boundaries, which are highlighted with vertical red lines (Figure 2), suggesting that the conserved sequence flanking the variable region could have provided a long homologous area for recombination to occur.
Figure 2. Diagrammatical representation of significant matches.
A. Diagrammatical representation of the H. influenzae and Helicobacter acinonychis mod alignment. The tBLASTn local alignment identified between the DNA recognition domain of modA4 and Helicobacter acinonychis was further extended to include the entire modA gene of H. influenzae. A nucleotide alignment was completed to include the regions flanking the DRD. The yellow arrow represents entire modA gene and the green arrow the match in H. acinonychis. B. Diagrammatical representation of the H. influenzae and Moraxella catarrhalis mod alignment. The tBLASTn local alignment identified between the DNA recognition domain of modA5 and Moraxella catarrhalis was further extended to include the entire modA gene of H. influenzae. A nucleotide alignment was completed to include the regions flanking the DRD. The purple arrow represents the entire modA gene in H. influenzae and the pink arrow the match in M. catarrhalis. The nucleotides are represented as vertical blue bars (dark blue >80% identity; light blue >50% identity; white >50% identity or gap). Strain and accession numbers that define the matches are shown to the left. The position of nucleotide that the match occurs is indicated above the coloured arrows and beside the amino acid alignment.
Source of modA Diversity: Inter-allele recombination
To examine the contribution of recombination between the DRDs of modA genes within the same species to overall modA allele diversity, we undertook all-versus-all BLASTn searches using the 18 representative modA sequences. Matches between the alleles identified with a length greater than 13 nucleotides were mapped onto the corresponding allele and coloured according to the scheme established in Fox et al. [14]. The number of reciprocal exchanges identified was a clear indication that the modA alleles have recombined in the past. Most interestingly, new relationships between the modA alleles were identified, and some alleles were found to have undergone recombination much more readily than others, indicating possible selection. Figure 3B shows the close relationship identified between modA10 and modA14. The DRDs show evidence of recombination with long stretches of nucleotide identity (>80%) between the two alleles. Sequences within the same modA allele share very high identity over the DRD region (>95%) suggesting that recombination is not presently contributing to the diversity. Indeed, the high conservation suggests that the DRD is moving as a unit. To investigate this further we undertook an analysis of a particular modA allele where a large number of sequences are available.
Figure 3. Diagrammatical representation of the 18 modA alleles of H. influenzae and pathogenic Neisseria.
A. The 18 modA DNA recognition domains are shown as coloured lines and the colour scheme established in Fox et al. (14) was used here for each representative allele: 1, H. influenzae R3327; 2, H. influenzae 723; 3, H. influenzae R3366; 4, H. influenzae R3579; 5, H. influenzae 1268; 6, H. influenzae C1626; 7, H. influenzae R3265; 8, H. influenzae C505; 9, H. influenzae 1209; 10, H. influenzae R3157; 11, N. meningitidis BZ83; 12, N. gonorrhoeae 01DO64; 13, H. influenzae R3023; 14, H. influenzae R1527; 15, H. influenzae R3570; 16, H. influenzae ATCC9007; 17, H. influenzae ATCC9833; 18, N. meningitidis NGE28. Each modA DNA recognition domain has been assigned a unique colour to permit visual identification of possible recombination events between different modA DNA recognition domains. BLASTn matches longer than 13 nucleotides and >80% identity between the 18 mod alleles were mapped onto the corresponding allele in the appropriate colour. Table S1 contains the nucleotide coordinates for each BLASTn match. B. Diagrammatical representation of the tBLASTn match between modA10 and modA14. Horizontal bars represent the modA alleles 10 and 14 described in panel A, and are coloured according to the same scheme. The vertical bars between the coloured lines indicate the nucleotide match according to BLAST, ranging from light grey (>80% nt identity) to black (100% nt identity).
Source of modA Diversity: Intra-allele recombination
Despite evidence for recombination between modA alleles, it is apparent from previous studies and our own analysis that the DRD domains are highly conserved amongst members of the same modA allele [14], [19]. To further examine how diversity arises within modA alleles nucleotide alignments were completed with 77 available sequences for the N. meningitidis modA12 DRD alleles and their flanking regions [19] (Figure 4). The DRD was remarkably well conserved across all the analysed modA12 sequences (0.2% nt diversity, across 895 nt of sequence) (Table 2). In contrast, the intra-allele variability was found in the regions flanking the DRD domain (Table 2). Throughout the entire alignment, the majority of nucleotide substitutions gave rise to non-synonymous substitutions suggesting that some codons may be subject to positive selection, as previously described [25]. Interestingly, the alignments showed discrete blocks of sequence divergence, mostly confined to the 3′ region of the modA gene, that were suggestive of recombination, rather than selection (Figure 4). A closer examination of these sequences indicate that these blocks match the nucleotide sequence of other modA alleles; for example, a short segment in the 3′ end of some modA12 alleles was observed to be a perfect match to the 3′ end of the modA6 alleles (Figure 4). To formally test for recombination we used PhiTest [31] which implements a pair-wise homoplasy test. There was good evidence for recombination in the 3′ region, but not the DRD or 5′ region of the modA12 alleles (Figure 4), consistent with the modA6 observation. To examine if similar evolutionary processes were shaping the diversity of other modA groups we wanted to take advantage of the availability of entire modA loci as part of complete and unfinished genome projects.
Table 2. Sequence analysis of N. meningitidis modA12 genes.
| GC-content | Diversity | Phi | Recomb? | Ns sitesa | ||
| modA12_5prime | 330 nt | 40% | 2.40% | 3.70E-01 | N | 6 |
| modA12_DRD | 895 nt | 28% | 0.20% | 9.38E-01 | N | 4 |
| modA12_3prime | 450 nt | 40% | 1.50% | 1.89E-29 | Y | 10 |
Ns = Non-synonymous sites with more than 2 sequences contain a Non-synonymous substitution.
DRD acquisitions en bloc between pathogenic Neisseria
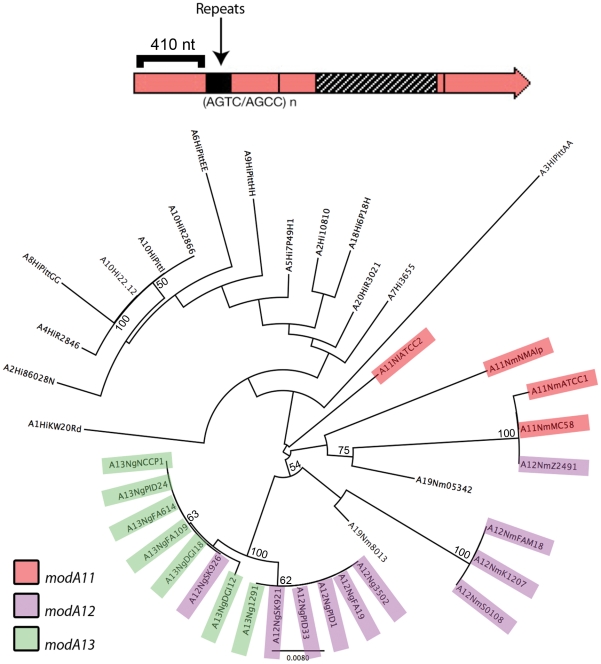
The sequence conservation between the DRD of each modA allele is >95% at the nucleotide level (Table S2). To determine if the conserved regions flanking each DRD contained evidence of previous recombination, a phylogenetic analysis was undertaken using the available H. influenzae, N. meningitidis and N. gonorrhoeae genomes. BLASTn searches with the H. influenzae Rd 4.6 kb modA1 region identified matches in 38 complete or unfinished genomes. Subsequent analysis enabled the identification of mod and res genes in most cases (several genomes contained frame-shifts in mod or res, or suspected repeat-tract “OFF” variants, Table S3). For each sequence, the 5′ region (between the ATG start codon and the beginning of the tetranucleotide repeats) were retrieved and aligned. A phylogram of this alignment shows that the 5′ regions of modA tend to cluster with their species of origin, rather than with their cognate modA DRD allele (Figure 5). Identical 5′ modA regions are found to be associated with modA11 and modA12 alleles in N. meningitidis MC58 and Z2491, respectively; whereas the 5′ region of modA12 from several N. gonorrhoeae strains are identical to that found in a modA13 allele from N. gonorrhoeae 12291 (Figure 5). A similar observation can be made for the 5′ regions of modA4 and modA10 alleles from H. influenzae, which are near-identical. This result is consistent with other findings e.g. modA4 from H. influenzae and N. gonorrhoeae. Therefore, the modA alleles are in a constant flux, retaining a high degree of conservation within DRD domains that are able to recombine into different modA “back-bones” and overwrite the existing allele by homologous replacement.
Figure 5. An unrooted phylogram shows the relationship between 5′ regions of modA genes (ATG to repeat, 410 nt).
Phylogram of the 410 nt modA 5′ nucleotide sequence produced using the Neighbor-joining algorithm, as implemented in PHYLIP [32]. Taxa that do not cluster according to their cognate mod allele are highlighted: N. meningitidis and N. gonorrhoeae modA11 (red), modA12 (purple) and modA13 (green). Bootstrap values from 1000 replications are shown as percentages for nodes with more than 50% support. Scale bar shows distances in nucleotide substitutions per site.
Analysis of modA Alleles in Available Genome Sequences
A multiple nucleotide alignment of all modA gene sequences identified in table S3 was generated (Figure 6). Although several new strains containing the modA12 and modA13 alleles were identified, no representatives of modA14–17 were found in the new genome sequence projects. Three modA genes were found to have central regions with no significant matches at the nucleotide level to known modA DRD alleles. We propose that the DRD regions define new modA alleles called modA19 (N. meningitidis 053422 and N. meningitidis 8013) and modA20 (H. influenzae R3021), respectively.
As previously shown (Figure 4), the data in figure 6 indicates recombination events of large fragments that encompass the entire DRD region; this is particularly obvious in the 3′ regions of the modA6, modA18 and modA19 alleles. In the case of modA19, the high conservation within the DRD, coupled with the relatively non-conserved sequence at either end suggests independent homologous recombination events of the entire DRD region from an unknown source into different modA alleles. This suggests that the same DRD allele should be found within modA genes with less identity in their flanking regions. The identification of Neisserial specific uptake sequences (nUSS), 5′-GCCGTCTGAA-3′, flanking the variable domain in all the aligned genome sequences in this study and H. influenzae specific uptake sequences (hUSS) in a previous study [26], both from pathogenic Neisseria and H. influenzae, indicates that the DRD would be preferentially transformed into these species following cell death, lysis, then natural transformation of random genomic fragments. nUSS and hUSS represent a well characterised feature used to enhance transformation efficiency [33] and constitute a predicted 1% of the genome [34]. The association of the nUSS with the modA DRD is consistent with the hypothesis that the variable domain is frequently transferred between H. influenzae, N. gonorrhoeae and N. meningitidis. The comparison of the relative GC content is also a good indicator that a specific region of DNA originates outside its current organism. The calculated GC content of the DRD of the analysed modA12 sequences is significantly lower than either the 5′ or 3′ regions of the modA gene, see table 2, providing further evidence of acquisition via horizontal gene transfer.
Discussion
Investigating the origins of the distinct modA phasevarions in Haemophilus influenzae and the pathogenic Neisseria forms the basis of this study. The modA alleles in Haemophilus and pathogenic Neisseria contain conserved 5′ and 3′ coding regions, which flank a highly variable central region. The origins of the diversity of the central, variable region, the DRD, have been previously investigated by Bayliss et al. (25), although this analysis only identified 13 modA alleles as fewer strains were examined. A further 5 distinct modA alleles have been determined through sequencing of modA genes in H. influenzae [14] and N. gonorrhoeae and N. meningitidis [19], therefore we undertook a new analysis with all known 18 modA alleles and took advantage of the increase in publicly available bacterial genome sequences in recent years. Our sequence analysis of the publicly available genomes led to the discovery of an additional 2 modA alleles, which we designate modA19 and modA20.
The diversity seen between the 20 modA alleles has been generated by both small and large scale recombination between species and strains at various evolutionary time-points. The first source of novel DRD sequences that we investigated were other bacterial species. All available bacterial genomic sequences located in the NCBI databases were searched using tBLASTn to identify the initial matches between other bacterial species and the modA DRD. In all cases the most significant matches were with putative adenine-specific type III R-M methyltransferases. There have been over 500 putative type III methyltransferases identified but only a small number of these have been completely characterised [35] and so there is little experimental data available for the modA DRD matches. However, the modA4 matches with H. pylori are homologs of the methyltransferase, JHP1296 or modH1, which has been shown to phase vary [20]. The switching of expression of the H. pylori methyltransferase has been demonstrated with phase-variable lacZ expression [20], indicating that this system may possibly be shifting from its traditional role in host genome protection to one of gene regulation. Additionally, modA5 matched a putative phase variable methyltransferase in M. catarrhalis [22]. The M. catarrhalis mod was predicted to phase vary based on the presence of tetranucleotide repeats in the open reading frame of a type III R-M system [22]. The identification of phase variable methyltransferases in other host-adapted pathogens indicated that this system of gene regulation, the phasevarion, could be important for host adaption and immune system evasion.
Early in the evolution of modA, diversity was generated by the transfer of short segments of the DRD between different H. influenzae and Neisseria modA genes. There are known elements associated with the modA genes that have been established as allowing the transfer to occur, namely H. influenzae and Neisserial specific uptake sequences and remnants of an integrase [26]. Our analysis revealed that there were a number of segments found within the DRD that displayed a high sequence similarity to other modA alleles, and it is proposed that these segments have been transferred between the different modA alleles. It is hypothesised that recombination between different modA DRDs affects the sequence specificity recognised by the modA allele and therefore can play a role in the fitness of the organism to an evolving host environment by introducing new regulatory patterns.
Within the same modA allele, strains were found to differ in ≤5% of their nucleotide sequence [14], [25]. The modA12 allele was chosen for an in depth analysis of intra-allele variability as it was the most highly represented group in available modA sequences and has recently been reported as a functional phasevarion [19]. The majority of variation was found outside of the established DRD boundaries suggesting strong selective pressure to maintain the modA12 DRD sequence in these strains. Indeed, the modA12 DRD sequences examined here exhibited only 0.2% diversity within the DRD itself. Bayliss et al. [25] reported that the presence of stretches of homologous sequence between the DRDs of modA alleles indicated that it was a dynamic area that was in a constant flux. Notably, that analysis used only a handful of representative sequences. In contrast, recent recombination between the DRDs of different modA alleles to generate diversity does not hold with our analysis of 77 modA12 sequences. Instead the modA12 sequences show evidence of other modA alleles in their 5′ and 3′ regions, indicating that the entire modA12 DRD had “over written” a previous allele. We propose that the intra-allele sequence shuffling occurred early in the acquisition of the DRD by either H. influenzae or Neisseria and that there is a strong selection to maintain the current sequence of the modA12 DRD.
In summary this study has characterised the diversity between the 20 modA alleles and examined origins of the significant diversity seen in the modA DRD domain. We have shown that several DRD regions of modA alleles found in pathogenic Neisseria and H. influenzae were acquired en-bloc from type III R-M systems from unrelated bacterial species. Subsequent diversity is generated by recombination between modA alleles via frequent horizontal transfer between and within these two species; in some cases this has involved the transfer of the entire DRD region. We find remarkable conservation within any given modA DRD, even when they are found in different genetic contexts. Accordingly, modA alleles should be defined only by the DRD region itself, and exclude the flanking 5′ and 3′ regions that often bare the scars of multiple recombination events. Our previous work on the pathogenic Neisseria has shown that distinct modA alleles recognised distinct DNA targets sites and thus regulate distinct regulons of genes with key roles in model systems of disease [19]. Amid the high frequency exchange and recombination driving the generation of this diverse set of modA alleles we observed selection for maintenance the modA12 allele in N. meningitidis. This indicates a strong selective pressure and benefit conferred by presence of the modA12 phasevarion in these N. meningitidis strains. The modA12 allele is the modA allele most frequently observed in N. meningitidis disease isolates (78.5%; [19]). The wide dissemination of the modA12 allele suggests that the ability to alter modA sequence specificity and the concomitant phasevarion in a single step may be key to the survival of some pathogenic strains. The original source of DRDs sequence diversity in restriction systems was undoubtedly generated by phage resistance as a dominant selective pressure [36]. However, in the case of modA alleles constituting phasevarion regulatory systems in H. influenzae [14], the pathogenic Neisseria [19], and also modH phasevarions of H. pylori [37], the mod genes are frequently associated with an inactive res (restriction) component of the Type III system, indicating selection for particular mod alleles observed in these studies is likely to be driven by the advantage conferred by the gene regulation phenotype, not by a restriction related function.
Supporting Information
Significant BLASTn matches between modA DNA recognition domains of modA alleles in H. influenzae and the pathogenic Neisseria .
(DOCX)
Within-allele diversity of DRD sequences.
(DOC)
modA alleles identified from available genomes.
(DOCX)
(ZIP)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Health and Medical Research Council (Australia) Program Grant 565526 and Australian Research Council Discovery Grant DP110101058 to MPJ, National Health and Medical Research Council (Australia) Training Fellowship to YNS. SAB is the recipient of an Australian Research Council Fellowship (project DP0881347). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bickle TA, Kruger DH. Biology of DNA restriction. Microbiol Rev. 1993;57:434–450. doi: 10.1128/mr.57.2.434-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer HW. DNA restriction and modification mechanisms in bacteria. Annu Rev Microbiol. 1971;25:153–176. doi: 10.1146/annurev.mi.25.100171.001101. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, et al. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 2003;31:1805–1812. doi: 10.1093/nar/gkg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray NE. Type I restriction systems: sophisticated molecular machines (a legacy of Bertani and Weigle). Microbiol Mol Biol Rev. 2000;64:412–434. doi: 10.1128/mmbr.64.2.412-434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Woude MW, Baumler AJ. Phase and antigenic variation in bacteria. Clin Microbiol Rev. 2004;17:581–611, table of contents. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pingoud A, Fuxreiter M, Pingoud V, Wende W. Type II restriction endonucleases: structure and mechanism. Cell Mol Life Sci. 2005;62:685–707. doi: 10.1007/s00018-004-4513-1. [DOI] [PubMed] [Google Scholar]
- 7.Hadi SM, Bachi B, Iida S, Bickle TA. DNA restriction–modification enzymes of phage P1 and plasmid p15B. Subunit functions and structural homologies. J Mol Biol. 1983;165:19–34. doi: 10.1016/s0022-2836(83)80240-x. [DOI] [PubMed] [Google Scholar]
- 8.Meisel A, Bickle TA, Kruger DH, Schroeder C. Type III restriction enzymes need two inversely oriented recognition sites for DNA cleavage. Nature. 1992;355:467–469. doi: 10.1038/355467a0. [DOI] [PubMed] [Google Scholar]
- 9.Meisel A, Mackeldanz P, Bickle TA, Kruger DH, Schroeder C. Type III restriction endonucleases translocate DNA in a reaction driven by recognition site-specific ATP hydrolysis. EMBO J. 1995;14:2958–2966. doi: 10.1002/j.1460-2075.1995.tb07296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha S, Ahmad I, Reddy YV, Krishnamurthy V, Rao DN. Functional analysis of conserved motifs in type III restriction-modification enzymes. Biol Chem. 1998;379:511–517. doi: 10.1515/bchm.1998.379.4-5.511. [DOI] [PubMed] [Google Scholar]
- 11.Timinskas A, Butkus V, Janulaitis A. Sequence motifs characteristic for DNA [cytosine-N4] and DNA [adenine-N6] methyltransferases. Classification of all DNA methyltransferases. Gene. 1995;157:3–11. doi: 10.1016/0378-1119(94)00783-o. [DOI] [PubMed] [Google Scholar]
- 12.Malone T, Blumenthal RM, Cheng X. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J Mol Biol. 1995;253:618–632. doi: 10.1006/jmbi.1995.0577. [DOI] [PubMed] [Google Scholar]
- 13.Humbelin M, Suri B, Rao DN, Hornby DP, Eberle H, et al. Type III DNA restriction and modification systems EcoP1 and EcoP15. Nucleotide sequence of the EcoP1 operon, the EcoP15 mod gene and some EcoP1 mod mutants. J Mol Biol. 1988;200:23–29. doi: 10.1016/0022-2836(88)90330-0. [DOI] [PubMed] [Google Scholar]
- 14.Fox KL, Dowideit SJ, Erwin AL, Srikhanta YN, Smith AL, et al. Haemophilus influenzae phasevarions have evolved from type III DNA restriction systems into epigenetic regulators of gene expression. Nucleic Acids Res. 2007;35:5242–5252. doi: 10.1093/nar/gkm571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moxon R, Bayliss C, Hood D. Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Annu Rev Genet. 2006;40:307–333. doi: 10.1146/annurev.genet.40.110405.090442. [DOI] [PubMed] [Google Scholar]
- 16.Moxon ER, Thaler DS. Microbial genetics. The tinkerer's evolving tool-box. Nature. 1997;387:659, 661–652. doi: 10.1038/42607. [DOI] [PubMed] [Google Scholar]
- 17.De Bolle X, Bayliss CD, Field D, van de Ven T, Saunders NJ, et al. The length of a tetranucleotide repeat tract in Haemophilus influenzae determines the phase variation rate of a gene with homology to type III DNA methyltransferases. Mol Microbiol. 2000;35:211–222. doi: 10.1046/j.1365-2958.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 18.Saunders NJ, Hood DW, Moxon ER. Bacterial evolution: bacteria play pass the gene. Curr Biol. 1999;9:R180–183. doi: 10.1016/s0960-9822(99)80108-0. [DOI] [PubMed] [Google Scholar]
- 19.Srikhanta YN, Dowideit SJ, Edwards JL, Falsetta ML, Wu HJ, et al. Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS Pathog. 2009;5:e1000400. doi: 10.1371/journal.ppat.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vries N, Duinsbergen D, Kuipers EJ, Pot RG, Wiesenekker P, et al. Transcriptional phase variation of a type III restriction-modification system in Helicobacter pylori. J Bacteriol. 2002;184:6615–6623. doi: 10.1128/JB.184.23.6615-6623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan KA, Lo RY. Characterization of a CACAG pentanucleotide repeat in Pasteurella haemolytica and its possible role in modulation of a novel type III restriction-modification system. Nucleic Acids Res. 1999;27:1505–1511. doi: 10.1093/nar/27.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seib KL, Peak IR, Jennings MP. Phase variable restriction-modification systems in Moraxella catarrhalis. FEMS Immunol Med Microbiol. 2002;32:159–165. doi: 10.1111/j.1574-695X.2002.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Srikhanta YN, Fox KL, Jennings MP. The phasevarion: phase variation of type III DNA methyltransferases controls coordinated switching in multiple genes. Nat Rev Microbiol. 2010;8:196–206. doi: 10.1038/nrmicro2283. [DOI] [PubMed] [Google Scholar]
- 24.Srikhanta YN, Maguire TL, Stacey KJ, Grimmond SM, Jennings MP. The phasevarion: a genetic system controlling coordinated, random switching of expression of multiple genes. Proc Natl Acad Sci U S A. 2005;102:5547–5551. doi: 10.1073/pnas.0501169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayliss CD, Callaghan MJ, Moxon ER. High allelic diversity in the methyltransferase gene of a phase variable type III restriction-modification system has implications for the fitness of Haemophilus influenzae. Nucleic Acids Res. 2006;34:4046–4059. doi: 10.1093/nar/gkl568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroll JS, Wilks KE, Farrant JL, Langford PR. Natural genetic exchange between Haemophilus and Neisseria: intergeneric transfer of chromosomal genes between major human pathogens. Proc Natl Acad Sci U S A. 1998;95:12381–12385. doi: 10.1073/pnas.95.21.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox KL, Srikhanta YN, Jennings MP. Phase variable type III restriction-modification systems of host-adapted bacterial pathogens. Mol Microbiol. 2007;65:1375–1379. doi: 10.1111/j.1365-2958.2007.05873.x. [DOI] [PubMed] [Google Scholar]
- 28.Seib KL, Pigozzi E, Muzzi A, Gawthorne JA, Delany I, et al. A novel epigenetic regulator associated with the hypervirulent Neisseria meningitidis clonal complex 41/44. FASEB J. 25:3622–3633. doi: 10.1096/fj.11-183590. [DOI] [PubMed] [Google Scholar]
- 29.Carver T, Berriman M, Tivey A, Patel C, Bohme U, et al. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24:2672–2676. doi: 10.1093/bioinformatics/btn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruen TC, Philippe H, Bryant D. A simple and robust statistical test for detecting the presence of recombination. Genetics. 2006;172:2665–2681. doi: 10.1534/genetics.105.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felsenstein J. An alternating least squares approach to inferring phylogenies from pairwise distances. Syst Biol. 1997;46:101–111. doi: 10.1093/sysbio/46.1.101. [DOI] [PubMed] [Google Scholar]
- 33.Goodman SD, Scocca JJ. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambur OH, Frye SA, Tonjum T. New functional identity for the DNA uptake sequence in transformation and its presence in transcriptional terminators. J Bacteriol. 2007;189:2077–2085. doi: 10.1128/JB.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts RJ, Vincze T, Posfai J, Macelis D. REBASE–a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res. 2010;38:D234–236. doi: 10.1093/nar/gkp874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoskisson PA, Smith MC. Hypervariation and phase variation in the bacteriophage ‘resistome’. Curr Opin Microbiol. 2007;10:396–400. doi: 10.1016/j.mib.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Srikhanta YN, Gorrell RJ, Steen JA, Gawthorne JA, Kwok T, et al. Phasevarion mediated epigenetic gene regulation in Helicobacter pylori. PLoS One. 2011;6:e27569. doi: 10.1371/journal.pone.0027569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Significant BLASTn matches between modA DNA recognition domains of modA alleles in H. influenzae and the pathogenic Neisseria .
(DOCX)
Within-allele diversity of DRD sequences.
(DOC)
modA alleles identified from available genomes.
(DOCX)
(ZIP)