Abstract
Epilepsy is the most common neurological disorder in dogs, with an incidence ranging from 0.5% to up to 20% in particular breeds. Canine epilepsy can be etiologically defined as idiopathic or symptomatic. Epileptic seizures may be classified as focal with or without secondary generalization, or as primary generalized. Nine genes have been identified for symptomatic (storage diseases) and one for idiopathic epilepsy in different breeds. However, the genetic background of common canine epilepsies remains unknown. We have studied the clinical and genetic background of epilepsy in Belgian Shepherds. We collected 159 cases and 148 controls and confirmed the presence of epilepsy through epilepsy questionnaires and clinical examinations. The MRI was normal while interictal EEG revealed abnormalities and variable foci in the clinically examined affected dogs. A genome-wide association study using Affymetrix 50K SNP arrays in 40 cases and 44 controls mapped the epilepsy locus on CFA37, which was replicated in an independent cohort (81 cases and 88 controls; combined p = 9.70×10−10, OR = 3.3). Fine mapping study defined a ∼1 Mb region including 12 genes of which none are known epilepsy genes or encode ion channels. Exonic sequencing was performed for two candidate genes, KLF7 and ADAM23. No variation was found in KLF7 but a highly-associated non-synonymous variant, G1203A (R387H) was present in the ADAM23 gene (p = 3.7×10−8, OR = 3.9 for homozygosity). Homozygosity for a two-SNP haplotype within the ADAM23 gene conferred the highest risk for epilepsy (p = 6.28×10−11, OR = 7.4). ADAM23 interacts with known epilepsy proteins LGI1 and LGI2. However, our data suggests that the ADAM23 variant is a polymorphism and we have initiated a targeted re-sequencing study across the locus to identify the causative mutation. It would establish the affected breed as a novel therapeutic model, help to develop a DNA test for breeding purposes and introduce a novel candidate gene for human idiopathic epilepsies.
Introduction
Epilepsy is one of the most common neurological diseases affecting 1–3% of the human population [1]. Epilepsy refers to a group of chronic neurological symptoms characterized by recurrent unprovoked seizures. Seizures are transient symptoms of abnormal, excessive or synchronous neuronal activity in the brain and can be classified into two major types: focal-onset and primarily generalized. In focal-onset seizures, the synchronized activity is restricted to a single part of the cortex, and may or may not subsequently spread to recruit the thalamocortical pathways and result in secondary generalization. Focal motor seizures may be characterized by elementary motor events, which consist of a single type of stereotyped contraction of a muscle or group of muscles or by autonomic features or paroxysms of behavioral signs probably corresponding to disturbance of higher cerebral activity in humans known as psychic seizures [2]. In generalized seizures, the thalamocortical circuitry is involved in the attack and results in synchronized firing of neurons brain-wide, unconsciousness and often tonic-clonic seizures. In humans, epileptic syndromes are defined by such phenotypic criteria as age of onset, survival, type of electroencephalographic (EEG) abnormalities, seizure characteristics, and the type of stimulus that induces seizures [3]–[7]. A majority of epilepsies have a suspected polygenic background. However, only a few risk genes are known to date, and a large number of genes contributing to human epilepsy still remain to be identified [8].
Epilepsy is also the most common chronic neurological disorder in dogs, and has been identified by breeders as one of the top three diseases of concern. Canine epilepsy can be classified either as idiopathic (genetic) or symptomatic (structural/metabolic) according to recent ILAE recommendations in humans [9]. Epileptic seizures are classified according to initial clinical signs either as focal or generalized seizures [9], [10]. Additionally, focal seizures may become secondarily generalized.
The prevalence of epilepsy in purebred dogs is estimated to range from 0.5% to 1%. However, in some breeds there is a strong suspicion of an underlying genetic factor as there is an accumulation of epileptic individuals within families with an incidence as high as 20% [4], [11]–[18]. Moreover, the majority of the pedigree studies suggest a polygenic mode of inheritance.
Genetic homogeneity within dog breeds and heterogeneity across breeds, together with the recent advent of genome-wide mapping tools with high resolution provide a powerful approach for gene mapping of both simple and complex traits in dogs [19]–[22]. Naturally occurring spontaneous canine epilepsies resemble clinically human epilepsies and provide exciting models to further understand the genetics and the etiopathologies of seizure disorders. The first canine symptomatic epilepsy gene, NHLRC1, was found in the Miniature Wirehaired Dachshund presenting canine Lafora disease [23]. This was followed by eight genes related to particular forms of neuronal ceroid lipofuscinoses (NCLs) [24]–[31]. Most of these known canine progressive myoclonus epilepsy (PME) genes are orthologues of the corresponding human syndromes and two new NCL candidate genes, ARSG and ATP13A2, have been identified for human NCLs [24], [27].
The first canine IE mutation in the LGI2 gene was recently identified in Lagotto Romagnolo dogs with focal remitting juvenile epilepsy [32], [33]. Despite efforts using either candidate gene [34] or low-resolution genome wide approaches [35], [36], the genetic background of many focal and generalized epilepsies remains largely unknown.
As part of our larger ongoing program to tackle the genetics of canine epilepsies (www.eurolupa.org), we have developed further resources to map the IE genes in Belgian Shepherds (BS) suffering from epilepsy dominated by focal seizures with or without secondary generalization [18]. Epileptic seizures vary from mild to intractable and typically have an onset around 3 years of age in this breed [18], [37]. Various pedigree analyses have suggested different modes of inheritance from simple recessive to polygenic with a major gene or a gene with incomplete penetrance [7], [18], [35], [36]. A recent microsatellite-based genome wide linkage scan with 366 dogs including 74 cases identified six tentative loci on four chromosomes, although none of them reached a genome-wide significance [36]. This could indicate genetic or phenotypic heterogeneity of epilepsy in BS. However, due to lack of power and resolution these results are not conclusive.
We have performed clinical characterizations including EEG recordings and a high-resolution genome-wide association study (GWAS) in a case-control cohort of BS dogs to identify IE loci. We successfully mapped a locus at CFA37 and defined a ∼1 Mb region containing novel candidate IE genes. This study establishes the first locus for the most common forms of seizures in dogs.
Results
Summary of the epilepsy cases collected for the investigation
To identify the genetic cause of IE in Belgian Shepherds (BS) we collected altogether 307 samples including 159 cases and 148 controls collected in Finland, Denmark and USA. Characterization of the Finnish study cohort was based on clinical examination of selected dogs and analysis of the owner-filled epilepsy questionnaires. To describe the Finnish cohort we analyzed 94 questionnaires from epileptic dogs as summarized in the Table S1. The vast majority of the dogs with only questionnaire data (78%) had also been diagnosed with epilepsy by a practicing veterinarian. The epileptic dogs showed a highly variable age of onset ranging from 3 months to 9 years with a mean at 3.3 years. The median seizure frequency was 5.25 per year with some dogs having less than one seizure per year and others having up to 10 seizures per day. The epileptic dogs had experienced on average 10 seizures (range 2–100) and one third presented clustered seizures (more than one seizure in a day). The typical duration of seizure was 2–4 minutes although ranging from 0.5–60 min. Almost half of the owners (42.7%) were able to identify phenomenology preceding convulsions as a sign of focal seizure activity. The typical clinical signs included restlessness, seeking of the owner's attention, drooling and nausea, which suggest a focal onset. Secondary generalization of focal seizures was commonly characterized by stiffening of limbs and neck, muscle fasciculation, tremor, drooling, staring, falling, tonic-clonic convulsions and urination. One third of the dogs did not react to owners' calls indicating a severely impaired consciousness. Postictal recovery time varied from minutes to hours.
The seizure types of the dogs were defined based on the seizure description. The majority of the dogs (37%) showed focal seizures with secondary generalization, one third of the dogs (34%) showed generalized seizures with unknown onset, 18% showed primarily generalized seizures and 7% of the dogs seizures were focal without secondary generalization. The seizure type of three dogs remained unclassified.
In the Finnish study cohort, 48 dogs out of 94 dogs (51%) were on anti-epileptic medication. Based on the 33 response reports, anti-epileptic medication was effective and prevented seizures in 18 (55%), halved the frequency in 10 (30%) and decreased it in 4 dogs (12%). Only one dog (3%) did not respond to medication. Seizure medication consisted mainly of Phenobarbital (88%) or potassium bromide (10%).
Clinical studies
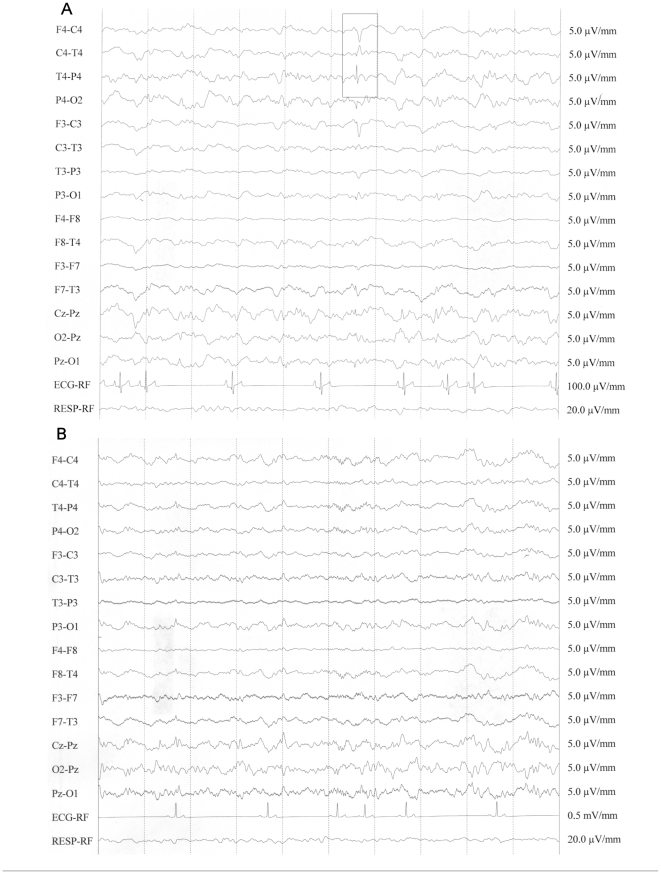
Clinical examinations were performed on 17 Finnish cases and 4 controls ( Table 1 ). All examined dogs were normal with regards to neurological examination, MRI, blood and CSF tests. These results exclude possible external causes of epilepsy and further support the presence of IE in the breed. At the visual examination of the EEG recordings, all dogs exhibited high-voltage low-frequency background activity. Background activity was superimposed with spindles or focal beta bursts in control dogs and in 2 dogs with epilepsy (dog 4 and dog 9). The standard descriptions used in human neurophysiology were adapted to describe all the EEG patterns [38]. Paroxysmal activity was observed in epileptic dogs, and it was characterized by sharp waves, spikes, and spike-and-slow-wave complexes in variable derivations ( Table 1 , Fig. 1 ). Two of the dogs exhibited midline spikes (dog 30 and dog 33), one had volley of sharp waves in centro-temporal-posterior right derivations (dog 9) and the other epileptic dogs exhibited spikes and spike-and-wave complexes in variable derivations. BETS and sleep spindles in healthy and epileptic dogs under medetomidine sedation were described previously [39]. Beta bursts are very similar to sleep spindles. They differ in having higher frequency, longer duration, and do not begin and end abruptly [40]. Both of these transients occur in the frontal, central, and parietal derivations. In humans, midline spike is supposed to represent epileptiform activity of uncertain clinical relevance [41], [42]. Spikes and spike-and-slow-wave complexes are considered as specific findings in many human epileptic syndromes [42], [43]. These findings were the only interictal abnormal EEG patterns detected in the dogs with epilepsy, suggesting a variable focal paroxysmal discharge.
Table 1. Summary of clinical examinations performed on 17 epileptic and 4 healthy Finnish BS dogs.
| Dog ID | variation/sex | age | age of seizure onset | overall clinical examination | neurologic examination | blood chemistry | MRI | CSF | EEG activity | Regions where EEG activity were detected | Seizure type based on questionnaire1 | diagnosis2 |
| Dog 4 | ter/female | 13 y | 2 y | norm | norm | norm | norm | norm | focal | Left anterior derivations | CFG | IE |
| Dog 6 | gro/male | 6 y | 5,5 y | norm | norm | norm | norm | norm | NA | CFG | IE | |
| Dog 7 | gro/female | 4 y | 3 y | norm | norm | norm | norm | norm | NA | CFG | IE | |
| Dog 8 | gro/male | 6 y | 2 y | norm | norm | norm | norm | norm | NA | CF | IE | |
| Dog 9 | gro/female | 7 y | 3 y | norm | norm | norm | norm | norm | focal | Central (entire right hemisphere) | CFG | IE |
| Dog 11 | gro/female | 8 y | 7 y | norm | norm | norm | norm | norm | NA | CFG | IE | |
| Dog 12 | gro/female | 6 y | 5 y | norm | norm | norm | norm | norm | NA | CFG | IE | |
| Dog 13 | gro/female | 4 y | 3 y | norm | norm | norm | norm | norm | focal | Right temporal posterior derivations | CFG | IE |
| Dog 17 | ter/male | 5 y | 2,5 y | norm | norm | norm | norm | norm | NA | CFG | IE | |
| Dog 18 | ter/female | 4 y | 0,5 y | norm | norm | norm | norm | norm | NA | CFG | IE | |
| Dog 20 | ter/male | 8 y | 5 y | norm | norm | norm | norm | norm | NA | GUO | IE | |
| Dog 22 | ter/male | 3 y | 2,5 y | norm | norm | norm | norm | norm | focal | Right and left posterior derivations | CFG | IE |
| Dog 26 | ter/female | 3 y | 2 y | norm | norm | norm | norm | norm | focal | Right central and posterior derivations | CFG | IE |
| Dog 27 | ter/male | 3 y | 2 y | norm | norm | norm | norm | norm | NA | CFG | IE | |
| Dog 30 | ter/female | 4 y | 2,5 y | norm | norm | norm | norm | norm | focal | Midline | CF | IE |
| Dog 33 | gro/female | 5 y | 1,5 y | norm | norm | norm | norm | norm | focal | Midline | CFG | IE |
| Dog 34 | gro/male | 6 y | 3,5 y | norm | norm | norm | norm | norm | NA | NA | IE | |
| Dog 1C | ter/female | 3,8 y | norm | norm | norm | norm | norm | NA | healthy | |||
| Dog 2C | ter/male | 6,9 y | norm | norm | norm | norm | norm | NA | healthy | |||
| Dog 3C | mal/female | 7,5 y | norm | norm | norm | norm | norm | NA | healthy | |||
| Dog 4C | gro/female | 6,3 y | norm | norm | norm | norm | norm | norm | healthy |
CFG = complex focal generalized, CF = complex focal, GUO = generalized with unknown onset, NA = not available.
IE = idiopathic epilepsy.
Figure 1. An example of interictal EEG recording for an epileptic (A) and for a healthy (B) BS dog.
The epileptic and healthy dogs correspond to dogs 26 and 4C in Table 1, respectively. The Epileptic dog shows spike and slow waves in right central and posterior derivations. The control dog exhibits a high-voltage low-frequency background activity. Background activity is superimposed with focal beta bursts in frontal derivation. The EEG pattern is consistent with the sedation protocol used. Bipolar montage, time constant = 0.3 s; high filter 70 Hz; notch filter inserted.
The Danish epilepsy cases were investigated with interview questionnaires, clinical and neurological examination and para-clinical tests and has been characterized with respect to clinical epilepsy phenotype and semiology reported in previous publications [7], [18]. In general the clinical epilepsy phenotype displayed by the Finnish and the Danish cohorts were similar.
GWAS, replication and fine mapping
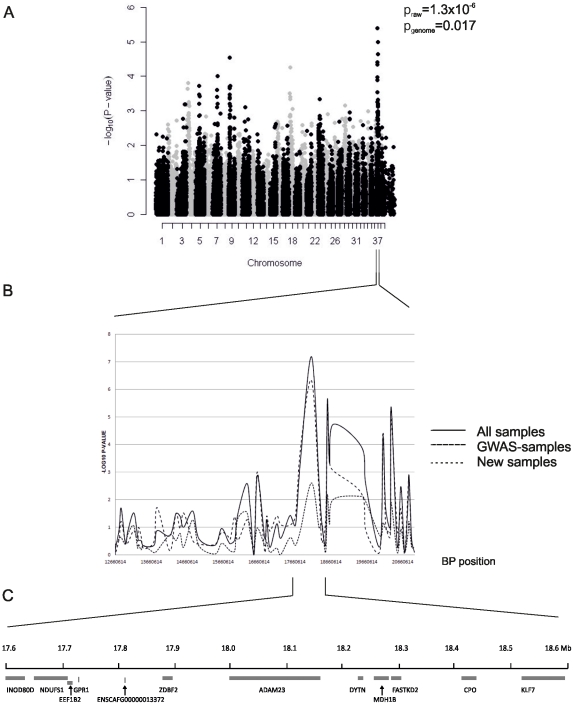
To map the epilepsy genes we performed a GWAS with 40 cases and 44 seizure-free controls (27 cases and 27 controls from Finland and 13 cases and 17 controls from Denmark). The controls were >7 years old and country- and variant-matched to the cases. A significant association was detected around SNPs on CFA37 with the best SNP BICF2P890779 at 18,123,961 bp (praw = 1.3×10−6, pgenome = 0.017) ( Fig. 2A ). Other putative loci were found on chromosomes 3, 4, 9, 18, and 23 although not at a genome-wide significant level ( Fig. 2A ).
Figure 2. A genome-wide association study reveals a locus at CFA37.
Genomic control –adjusted p-values are shown (A). Fine-mapping of a 8.3 Mb region with 96 additional SNPs on chromosome 37 defines a 1 Mb associated region (B). The region showing strongest association with epilepsy (17,525,804–18,623,591 bp) contains 12 genes including two neuronal candidates, ADAM23 and KLF7 (C).
As a replication study we genotyped the SNP BICF2P890779 showing the strongest association with IE at CFA37 in an independent sample cohort of 81 cases and 88 controls (p = 3.7×10−5, OR = 2.6, 95% CI 1.6–4.1). A combined analysis of all genotyped samples including the original GWAS and the replication cohorts yielded a p-value of 9.7×10−10 and OR = 3.3 (95% CI 2.2–4.9) ( Table 2 ). Homozygosity with respect to the allele A increased the risk of epilepsy by 5.4-fold (95% CI 3.1–9.3, p = 6.8×10−10). The frequency of the AA genotype was 0.62 among cases and 0.24 among controls. The nominal association signals on chromosomes 3, 4, 9, 18, and 23 were followed up by replication of the best-associated SNPs in each locus using 54 cases and 62 controls. None of these loci showed evidence for association in the replication cohort ( Table 2 ).
Table 2. The summary of the results in GWAS, replication and combined datasets.
| GWA1 | Replication2 | Combined3 | ||||||||||||||
| Chromosome | SNP | Position | F_A4 | F_U5 | Praw | PGC | Pgenome | OR | F_A4 | F_U5 | Praw | OR | F_A4 | F_U5 | Praw | OR |
| 3 | BICF2P397912 | 82766251 | 0.60 | 0.33 | 3.91E-4 | 0.001 | 0.96 | 3.11 | 0.49 | 0.40 | 0.14 | 1.41 | 0.51 | 0.39 | 0.014 | 1.65 |
| 4 | TIGRP2P58276 | 13088720 | 0.43 | 0.16 | 1.39E-4 | 5.25E-4 | 0.76 | 3.91 | 0.39 | 0.29 | 0.07 | 1.55 | 0.40 | 0.26 | 0.004 | 1.86 |
| 9 | BICF2P1288768 | 18597315 | 0.43 | 0.47 | 4.71E-4 | 0.001 | 0.98 | 0.31 | 0.43 | 0.47 | 0.53 | 0.86 | 0.38 | 0.47 | 0.078 | 0.69 |
| 18 | TIGRP2P239410 | 15846321 | 0.15 | 0.47 | 1.68E-5 | 8.95E-5 | 0.18 | 0.21 | 0.30 | 0.39 | 0.10 | 0.67 | 0.27 | 0.43 | 0.001 | 0.49 |
| 23 | BICF2G630382382 | 22138085 | 0.20 | 0.48 | 1.60E-4 | 5.91E-4 | 0.79 | 0.27 | 0.25 | 0.31 | 0.22 | 0.73 | 0.24 | 0.37 | 0.005 | 0.53 |
| 37 | BICF2P890779 | 18123961 | 0.53 | 0.18 | 1.34E-6 | 1.09E-5 | 0.02 | 5.41 | 0.72 | 0.50 | 3.72E-05 | 2.59 | 0.76 | 0.49 | 9.70E-10 | 3.29 |
GWAS dataset: 40 cases, 44 controls.
Replication dataset: chromosomes 3, 4, 9, 18, 23: 54 cases, 62 controls; chromosome 37: 81 cases, 88 controls.
Combined dataset: chromosomes 3, 4, 9, 18, 23: 94 cases, 106 controls; chromosome 37: 116 cases, 130 controls.
Frequency of the minor allele (based on GWAS controls) in affected individuals.
Frequency of the minor allele (based on GWAS controls) in unaffected individuals.
To fine map the associated locus we genotyped 83 BS cases and 99 BS controls with 96 SNPs from a 8.3 Mb region at CFA37 (12,660,614–20,989,289 bp). Fine mapping defined a ∼1 Mb region with the strongest association to a SNP BICF2P890779 at 18,123,961 bp (praw = 6.6×10−8, p1000×perm = 1.0×10−3) ( Fig. 2B ).
Candidate gene sequencing
The associated 1 Mb region at CFA37 contains 12 genes of which two, ADAM23 and KLF7, have functions in neuronal systems ( Fig. 2C ). Mutations in ADAM23 have not been found in epileptic patients, but it interacts with LGI1, a gene associated with familial temporal lobe epilepsy-1 (ETL1) in human [44] and with LGI2, which is the causative gene for benign focal epilepsy in dogs [33]. KLF7 is a neuronal transcription factor, which is required for neuronal morphogenesis and axon guidance in selected regions of the brain [45]. Sequence analysis of KLF7 did not reveal any coding variants. Screening of the ADAM23 exons revealed a non-synonymous variant in exon 12 (G1203A according to predicted mRNA XM_844759) at 18,113,688 bp causing an amino acid change (R387H according to XP_849852.1) in four affected dogs. To further investigate the frequency of the variant, we genotyped a total of 159 cases and 148 controls. The risk allele A frequency was 72% in the cases compared to 49% in the controls (p = 3.1×10−9, OR = 2.7, 95% CI: 1.9–3.8). Homozygosity for the A allele increased the risk (p = 3.7×10−8, OR = 3.9, 95% CI: 2.4–6.4). However, 22% of controls were also homozygous. Comparison of frequency of the R387H variant in different epilepsy types did not show enrichment to specific seizures (data not shown). This variation was also screened in three epileptic dogs from 38 other breeds (altogether 114 epileptic dogs), and we found that the homozygous AA genotype was present in some of the three affected dogs in altogether 12 breeds (19% of tested dogs) (Barbet, Beagle, Border Collie, Dachshund, Dalmatian, Golden Retriever, Irish Water Spaniel, Miniature Pinscher, Petit Basset Griffon Vendeen, Miniature Poodle, Rottweiler and Whippet). In 15 breeds, the risk allele was present in heterozygous form in some of the individuals (32%). Furthermore, Panther and PolyPhen-2 programs predicted that R387H is not pathogenic. Overall, these results suggest that the observed variation is not a causative mutation but rather a polymorphism, which is likely in the vicinity of the actual predisposing mutation. Indeed, we identified the highest risk of epilepsy among individuals homozygous for the haplotype composed of the risk-conferring alleles of the G1203A and BICF2P890779 variants (p = 6.28×10−11 OR = 7.4, 95% CI: 3.9–14.0) which are in strong linkage disequilibrium (LD) with each other (D′ = 0.87). This suggests that the functional variant lies within this haplotype block but is neither one of the two SNPs.
We investigated ADAM23 as a candidate gene also through RNA expression studies. Expression level of ADAM23 was compared between three healthy and three epileptic Belgian Shepherds from Denmark. All dogs were clinically examined. A difference in expression level between the two groups of dogs was not observed.
Association of the CFA37 locus in other IE breeds
As part of our ongoing clinical and genetic studies on epilepsies, we have established well-characterized sample cohorts for many breeds presenting IE. To test whether the identified epilepsy locus at CFA37 associates with epilepsy in other breeds, we genotyped the best associated BS SNP (BICF2P890779) in an epilepsy cohort of 303 cases and 316 controls including samples from Lagotto Romagnolo, Miniature Pinscher, Kromfohrländer, Whippet, Border Terrier, Schipperke, Finnish Spitz and Finnish Lapphund ( Table 3 ). Almost all tested Finnish Spitz and Schipperke dogs were homozygous for the A-allele and therefore no association could be calculated in these two breeds. Kromfohrländers (p = 0.003) and Whippets (p = 0.02) showed a tentative association ( Table 3 ). These results in both breeds need to be confirmed in a larger sample cohort with additional markers before further conclusions.
Table 3. Association of ADAM23 intronic SNP (BICF2P890779 at 18,123,961 bp) with epilepsy in 9 different breeds.
| Breed | N cases+N controls | F_A1 (allele A) | F_U2 (allele A) | P | OR |
| Belgian Shepherd | 116+130 | 0.76 | 0.49 | 9.70E-10 | 3.3 |
| Kromfohrländer | 23+18 | 0.93 | 0.67 | 3.44E-03 | 6.5 |
| Whippet | 24+26 | 0.81 | 0.60 | 0.018 | 2.9 |
| Finnish Spitz | 62+81 | 1.00 | 0.98 | 0.13 | NA |
| Lagotto Romagnolo | 23+23 | 0.35 | 0.48 | 0.20 | 0.6 |
| Miniature Pinscher | 22+20 | 0.89 | 0.80 | 0.27 | 2.0 |
| Border Terrier | 42+40 | 0.93 | 0.89 | 0.36 | 1.6 |
| Schipperke | 63+48 | 0.99 | 1.00 | 0.38 | NA |
| Finnish Lapphund | 44+60 | 0.79 | 0.82 | 0.58 | 0.8 |
Frequency of the minor allele (based on GWAS controls) in affected individuals.
Frequency of the minor allele (based on GWAS controls) in unaffected individuals.
Frequencies of allele A are shown for each breed. P-values<0.05 are bolded.
Discussion
We describe here the second IE locus in dogs. A locus at CFA37 predisposes Belgian Shepherds to focal epilepsy with seizures originating from multiple cerebral lobes and without any detectable cerebral lesions on MRI studies. The first canine IE mutation was described in the Lagotto Romagnolos. This mutation causes a breed-specific focal epilepsy with remission [33], whereas BS dogs suffer from seizures that are commonly seen across breeds. Therefore our results may suggest a genetic locus for the most common forms of IE in dogs.
The clinically examined BS dogs had normal blood biochemistry, CSF, MRI and neurological examination and symptomatic epilepsy and seizures of extracranial origin was therefore not suspected. In the cases where interictal EEG was performed we detected paroxysmal activity originating from different cerebral lobes. The most common seizure type was a focal-onset with secondary generalization. The seizures in the minority of the dogs remained focal and some dogs had primarily generalized seizures. The fact that only half of the affected dogs received anti-epileptic medication suggests that epilepsy in the BS has a relatively mild course. Our study cohort included samples from several countries including a previously described cohort from Denmark [7], [18]. The onset and clinical features in different populations are similar. There are some differences in proportion of seizure types which may arise from the fact that a focal seizure onset may be challenging to observe and describe retrospectively by the owners. This is most likely the explanation why seizure distributions differ in the Danish and Finnish cohorts.
The CFA37 locus identified in this study is syntenic with the region on human chromosome 2q33 (206.8–208.2 Mb). Overlapping interstitial deletions in 2q24–31 have been described in many human epilepsies often associated with other developmental defects [46]. Our locus is close to these deletions but not syntenic. In addition, our clinical characterizations indicate that epileptic BS dogs present only a seizure disorder without developmental abnormalities. Another type of human epilepsy called familial partial epilepsy with variable foci (FPEVF) has also been mapped to 2q [47]. FPEVF is an autosomal dominant epilepsy with incomplete penetrance and characterized by epileptic seizures originating from different cerebral lobes [47], [48]. Affected individuals respond well to antiepileptic drugs and have no brain lesions. Causative mutations have not been found for FPEVF [47]–[51]. Although the clinical features in our dogs resemble the characteristics of human FPEVF, the most significant region in dogs is ∼10 Mb from the strongest association signal in human patients. Besides human, an overlapping epilepsy region has been found in WAG/Rij rats representing a model for human childhood absence epilepsy [52]. However, the syntenic region in rats covers an extensive region of the chromosome with many possible candidate genes.
Previous epidemiological studies have demonstrated a high prevalence of IE in the BS breed and various inheritance models have been suggested [7], [18], [35]–[37]. We found a major locus at CFA37 overlapping a previous tentative QTL [36], but with only a modest disease risk suggesting that still other susceptibility loci exist. Alternatively, the mildest focal seizures may have been missed and it is therefore possible that some controls are actually cases, which would result in the underestimation of the disease risk. This assumption is supported by a genealogical study performed in an extended family of dogs investigated over several years [7]. Our GWAS was performed in a relatively small sample cohort and with the older version of SNP chip arrays including ∼50,000 markers. It is possible that additional loci could be discovered with a larger sample size and higher resolution available today.
The 1 Mb region showing the strongest association includes 12 genes of which none encode ion channels commonly mutated in human IEs or other known epilepsy genes [53]. We screened two genes, ADAM23 and KLF7 for coding and splice site mutations. ADAM23 represents an excellent candidate gene. It encodes a member of the disintegrin and metalloprotease domain (ADAM) family and belongs to a neuronal subfamily of ADAMs together with ADAM22 and ADAM11 [54]. ADAM23 binds two epilepsy-associated proteins, LGI1 and LGI2. LGI1 is mutated in familial temporal lobe epilepsy-1 (ETL1) in humans, and LGI2 is mutated in benign familial juvenile epilepsy (BFJE) in Lagotto Romagnolo dogs [33], [44]. The truncating mutations of LGI1 or LGI2 prevent their secretion and interaction with the ADAM23 complexes. The LGI1-ADAM23 complex is involved in the stimulation of neurite outgrowth and dendritic arborisation [55]. ADAM23 containing complex plays a role in pulling together both pre- and post-synaptic membranes, stabilizing and strengthening synaptic neurotransmission [56]. Furthermore, homozygous removal of Adam23 from mice results in a seizure disorder and even heterozygous mice have lowered seizure thresholds [56]. We identified only a single non-synonymous variant from ADAM23 gene, R387H, which is in strong LD with the associated intronic marker identified in the GWAS. The coding variant is highly associated and increases the epilepsy risk by 4-fold. However, given that the homozygous risk allele is common (22%) in controls, present frequently in 27 other breeds, and unlikely pathogenic, these results suggest that it is a polymorphism rather than a causative mutation. Based on our haplotype association analysis, the causative mutation is likely located in the same haplotype block tagged by these two variants. The fact that there were no changes in the expression of ADAM23 in epileptic dogs suggests that the possible disease causing variant, if present in ADAM23 at all, does not affect its transcript levels in the brain.
KLF7 belongs to a large family of KLF transcription factors. KLF7 is the only family member with a neuronally restricted expression during development. KLF7 is required for neuronal morphogenesis and axon guidance in hippocampus, olfactory bulbs and cortex [45], [57]. We could not find any variants in the coding regions of KLF7. The identified locus contains also three other candidate genes, DYTN, NDUFS1 and FASTKD2, that function in the CNS or have been associated with neuronal phenotypes. DYTN is poorly characterized but expressed in the CNS [58]. NDUFS1 is a core component of the mitochondrial complex I system, and mutations in this system has been associated with neurodegenerative disorders [59]. FASTKD2 is a cytochrome oxidase deficiency related gene and its mutations cause various neurological phenotypes including convulsions [60]. However, the phenotypes related to the latter two mitochondrial genes or systems are not restricted to CNS but affect other organs as well.
There are several possibilities where the actual predisposing mutation may be located. First, mutation may still lie in the regulatory regions of the ADAM23 or KLF7 genes. Second, mutation is present in the other candidate genes not screened yet. Third, we focused here only on the 1 Mb region showing strongest association, while our genotype data indicates a remarkable signal outside the most significant locus. This ∼2 Mb region contains also several candidate genes. To identify the causative variant we have initiated a targeted re-sequencing project to capture a 4 Mb locus on CFA37.
As part of our larger canine epilepsy research program we have collected samples from IE dogs in several breeds. To test the association of the CFA37 locus in other breeds, we screened the ADAM23 intronic variant in eight additional breeds. We used Lagotto Romagnolos as a control breed in the study since we recently identified the causative mutation in the LGI2 gene on CFA3 [33]. Our across breed analysis found some evidence for association in Kromfohrländer and Whippet breeds. However, a single marker association in a small sample cohort should be interpreted cautiously and confirmed with replication using more samples and markers.
The high prevalence of IE among BS dogs causes a severe health issue in the breed [37]. Although many dogs respond well to treatments, still almost every fifth epileptic dog is euthanized within three years after the onset [18]. There is a clear need for genetic counseling and for the development of marker-assisted breeding programs. Our study identifies a significant risk allele for IE. However, since the majority (75%) of the unaffected dogs also carries one or two copies of the risk allele, it cannot be used for efficient diagnostics. The identification of the causative mutation remains as an important task to improve breeding plans, to reveal a new candidate gene for human IEs, to identify novel IE pathways, and to establish the breed as a large therapeutic animal model for IEs. This study makes a breakthrough by mapping a novel IE locus and paves the way towards the discovery of the first mutation in the most common seizure type in dogs.
Materials and Methods
Study cohort
A cohort of Belgian Shepherd dogs including 159 epileptic cases and 148 unaffected controls collected in Finland (178 dogs), Denmark (65 dogs) and USA (64 dogs) was used in this study. The Finnish cohort included mainly Finnish dogs (64%) but also dogs from Sweden, Poland, Australia, Switzerland, Austria, Germany and the Netherlands. The Danish cohort has been described previously by Berendt et al. [7], [18] and the US cohort by Oberbauer et al. [35], [36] and the Finnish cohort in this paper (Table 1, Table S1). All study cohorts were collected through Breed Clubs, breeders and owners and epilepsy diagnoses were based on questionnaires, telephone interviews and clinical, neurological and para-clinical examinations on selection of dogs. The clinical characterization of the Finnish cohort was based on clinical examination on 17 affected dogs and 4 healthy controls from Finland and detailed owner-filled epilepsy questionnaires from 94 dogs (http://koirangeenit.fi/Tiedostot/EpilepsyQuestionnaire.doc). Epilepsy questionnaire requested information about the age of onset, the number, duration and frequency of seizures, anti-epileptic medication, and typical characteristics of the ictal, pre- and post-ictal phases of seizures.
Inclusion criteria for the case in all cohorts required that the dog had experienced at least two seizures. The age of onset was reported by the owners and was not used as exclusion criterion due to possible inaccuracies. The average age of onset was between 3.1 to 4.1 years in different cohorts (ranging from 3 months to 9 years in the Finnish cohort, from 1.5 years to 11 years in the Danish cohort and from 2 years to 5 years in the US cohort). None of the epileptic dogs were known to be affected by other diseases. The control dogs had no history of seizures and were over 7 years of age in all cohorts.
The cohort in the GWAS included selected dogs from the Finnish and Danish cohorts. The GWAS cohort did not include first degree relatives. The replication cohort was independent from GWAS and included samples collected in Finland, Denmark and USA. Fine mapping cohort contains samples collected in Finland and Denmark. It includes the GWAS samples and overlaps with replication study cohort (50 cases and 59 controls of the fine mapping study were included in the replication). The controls were matched to the cases according to country of origin and breed variant.
The Finnish Kennel Club's breeding database, KoiraNet, was utilized for Finnish pedigrees. EDTA-blood samples were collected for each dog with the owner's consent in the genetic analyses and genomic DNA was extracted using a commercially available kit (Puregene, Gentra Systems, Minneapolis, MN). We have a valid ethical permit (ESLH-2009-07827/Ym-23, expiring Oct 2012).
Clinical studies
Clinical studies included clinical and neurological examination, blood and in selected cases CSF, MRI, EEG tests and were performed at the Referral Animal Neurology Hospital Aisti, Vantaa, Finland and Department of Small Animal Clinical Sciences, University of Copenhagen, Denmark. Blood examination included complete blood count and serum biochemistry (sodium, potassium, calcium, phosphorus, magnesium, glucose, total protein, albumin, globulin, cholesterol, blood urea nitrogen, creatinine, total bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and creatine kinase). MRI examinations of Finnish dogs were performed as described previously [61]. CSF samples were collected from the cerebellomedullary cistern after MRI examination. Total cell count, cytology and protein concentration were evaluated.
EEG in Finnish dogs was performed under medetomidine sedation (0.04 mg/kg IM). An additional 0.02 mg/kg of medetomidine was given IM if the dog was not ready for examination 15 minutes after the initial injection. EEG examinations were performed in a quiet, darkened room. Dogs were placed in sternal recumbency and electrodes were placed transcutaneously in a 14 channel montage, modified from a 17-channel montage as described previously [62]. To assure good electrical contact with the electrodes, the scalp was defatted by rubbing vigorously with ethyl alcohol. Referential and bipolar montages (F7, F3, F4, F8, T3, C3, Cz, C4, T4, P3, Pz, P4, O1, O2 ;F4-C4, C4-T4, T4-P4, P4-O2, F3-C3, C3-T3, T3-P3, P3-O1, F4-F8, F8-T4, F3-F7, F7-T3, Cz-Pz, O2-Pz, Pz-O1; odd number = left hemisphere; even number = right hemisphere) were used. The acquisition parameters to record bio-electrical activity were set as follows: sensitivity = 5 µV/mm; time constant = 0.3 s; high filter (Hf) = 70 Hz; notch filter inserted; reference: on the bridge of the nose; ground: caudally to the external occipital protuberance; electrode impedance <3 KΩ; sampling rate 256 Hz. Sixteen EEG needles (thirty-gauge 15 mm monopolar stainless steel needle electrodes, Bionen S.a.S., Italy) were used as active, reference, and ground electrodes. No local infiltration of lidocaine was performed around electrode placement sites. Electrocardiogram and respiratory rates were recorded via polygraphic electrodes (EKG: sensitivity = 70 µV/mm, time constant = 0.1 s, Hf = 30 Hz; respiration - CHEST - : sensitivity = 20 µV/mm, time constant = 0.3 s, Hf = 30 Hz) connected to alligator clips (thin cable for bridge electrode, Bionen S.a.S., Firenze, Italy) and to a respiratory effort system (thoracic respiratory transducer, Bionen S.a.S., Firenze, Italy). EEG recording started when electrode placement was completed, and the total recording time was 20 min, including calibration and the initial impedance check. The EEG data were stored in the acquisition station (Halley Galileo, EBNeuro, Firenze, Italy) for later analysis.
Genome-wide association analysis and fine-mapping
To map the epilepsy locus, 40 cases and 44 controls were genotyped using the Affymetrix Canine Genome 2.0 Array platinum set (Affymetrix, Santa Clara, CA, USA) containing 49,663 SNP markers. The genotyping was performed as a part of the LUPA project at the Centre National de Génotypage, Paris, France. The case-control association analysis was performed with PLINK v1.07 [63] with the criteria of MAF <0.05, call rate >75% and <25% of missing genotypes in individual dogs. After applying these filters 43,378 SNPs remained in the analysis for all dogs. Genome-wide significance was ascertained through 100 000 random permutations of the epilepsy phenotype. The genomic inflation factor for the population was 1.13 and the association p-values were adjusted for it.
Fine mapping and replication was performed with 96 selected SNPs from a 8.3 Mb region (12,660,614–20,989,289 bp) on CFA37. The SNP density was 1 SNP/100 Kb. All base pair positions mentioned in this article are based on CanFam 2.0. Genotyping was performed using the Sequenom (San Diego, CA, USA) iPLEX methodology at the Centre of Integrated Genomic Medical Research, University of Manchester, UK. A total of 201 samples were genotyped including samples from Finland (54 cases, 63 controls) Denmark (32 cases, 28 controls), Sweden (11 cases and 13 controls) and Middle Europe. After quality control (MAF <0.05, SNP call rate >0.75, individual call rate >0.75), a total of 58 SNPs and 83 cases and 99 controls were included in the association analysis with PLINK v1.07 using a single-marker association analysis and haplotype sliding window analysis with 3–5 markers at a time [63].
To confirm the nominal associations in the other chromosomes single SNPs on chromosomes 3 (BICF2P397912 at 82,766,251 bp), 4 (TIGRP2P58276 at 13,088,720 bp), 9 (BICF2P1288768 at 18,597,315 bp), 18 (TIGRP2P239410 at 15,846,321 bp) and 23 (BICF2G630382382 at 22,138,085 bp) were genotyped in 54 Belgian Shepherd cases and 62 controls in addition to the samples genotyped in the GWAS. The genotyping was performed using Custom Taqman SNP Genotyping Assays (Applied Biosystems by Life Technologies Corporation, Carlsbad, CA, USA). The polymerase chain reactions were performed according to the standard protocol provided by the manufacturer in 10 µl reaction volume, and run and analyzed using the Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA, USA). The SNP genotype data was analyzed for association using PLINK v1.07 [63]. All the markers were in HWE (p>0.05) and the genotyping call rates ranged from 96–100%.
Association in other breeds
A SNP located at 18,123,961 bp (BICF2P890779) on CFA37 was screened in 8 other breeds with epilepsy including Lagotto Romagnolo (23 cases, 23 controls), Miniature Pinscher (22 cases, 20 controls), Kromfohrländer (34 cases, 20 controls), Whippet (24 cases, 26 controls), Border Terrier (42 cases, 20 controls), Schipperke (63 cases, 48 controls), Finnish Spitz (62 cases, 81controls) and Finnish Lapphund (44 cases, 60 controls). The genotyping was performed using Custom Taqman SNP Genotyping Assays (Applied Biosystems by Life Technologies Corporation, Carlsbad, CA, USA) according to the standard protocol provided by the manufacturer in 4 µl reaction volume, and run and analyzed using the Applied Biosystems 7900HT Fast Real-Time PCR System (Foster City, CA, USA). The SNP genotype data was analyzed for association using PLINK v1.07. The genotyping call rates within breeds ranged from 91–100%, and there was no deviation from HWE in any of the breeds (p>0.05).
Sequencing and mutation analysis
Exons and splice junctions were amplified by PCR with ADAM23-specific primers available upon request. The PCR products were purified with ExoSAP-IT kit (USB Corporation, Cleveland, Ohio) and sequenced with an ABI Prism 3730xl DNA analyzer (Applied Biosystems, Foster City, CA). The exon 12 variant (R387H) was sequenced in 155 BS cases and 111 controls and in three epileptic dogs in 38 other breeds. Pathogenicity of the mutation was predicted by programs Panther [64] and PolyPhen-2 [65].
Gene expression analysis
Expression level of ADAM23 was analyzed in three healthy Belgian Shepherds and three Belgian Shepherds with epilepsy. Samples from cortex cerebrum and cerebellum were taken immediately after euthanasia and snap-frozen in liquid nitrogen. Messenger RNA extraction, cDNA synthesis and quantitative PCR were performed as previously described [66]. RNA quality was ascertained and the RQI number determined by analysis on an Experion System (Bio-Rad, Hercules, CA, USA). Two primer sets for qPCR were designed; set 1: forward primer 5′-CCTGGCAGATGAAGACAACA, reverse primer 5′- GAGCCAAAGGCTTCAATCTG; set 2: forward primer 5′- AGCCACCTGCATCTGTGATT, reverse primer 5′- GTGCCCCCAAGGACAATAG. The gene RPL4 was used as a reference gene in the expression level analysis. Expression data were analyzed using REST v2.0.7 [67].
Supporting Information
Summary of the clinical features of 94 epileptic Belgian Shepherds collected through the owner-filled epilepsy questionnaires.
(XLS)
Acknowledgments
Ranja Eklund, Sini Karjalainen, Minna Virta, Heljä Marjamäki and Janelle Belanger are thanked for excellent technical assistance, Anna But for statistical help, Katarina Truvé and Andrea Short for helping to set up a fine mapping study, Joanna Wyszynska and Monica Isenegger for BS samples. We thank all the dog owners, breeders and breed clubs who participated in this study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the European Commission (FP7-LUPA, GA-201370), the Academy of Finland, the Sigrid Juselius Foundation, the Morris Animal Foundation, the Canine Health Foundation, Biocentrum Helsinki, the Jane and Aatos Erkko Foundation, the University of Helsinki Research Funds, The Finnish Belgian Shepherd Breed Club, Folkhälsan Research Foundation, and the Danish Kennel Club. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Engel J., Jr Intractable epilepsy: Definition and neurobiology. Epilepsia. 2001;42(Suppl 6):3. doi: 10.1046/j.1528-1157.2001.0420s6003.x. [DOI] [PubMed] [Google Scholar]
- 2.Berendt M, Gredal H, Alving J. Characteristics and phenomenology of epileptic partial seizures in dogs: Similarities with human seizure semiology. Epilepsy Res. 2004;61(1–3):167–173. doi: 10.1016/j.eplepsyres.2004.07.009. 10.1016/j.eplepsyres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Sander JW, O'Donoghue MF. Epilepsy: Getting the diagnosis right. BMJ. 1997;314(7075):158–159. doi: 10.1136/bmj.314.7075.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casal ML, Munuve RM, Janis MA, Werner P, Henthorn PS. Epilepsy in irish wolfhounds. J Vet Intern Med. 2006;20(1):131–135. doi: 10.1892/0891-6640(2006)20[131:eiiw]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berendt M, Gredal H, Ersboll AK, Alving J. Premature death, risk factors, and life patterns in dogs with epilepsy. J Vet Intern Med. 2007;21(4):754–759. doi: 10.1892/0891-6640(2007)21[754:pdrfal]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Hulsmeyer V, Zimmermann R, Brauer C, Sauter-Louis C, Fischer A. Epilepsy in border collies: Clinical manifestation, outcome, and mode of inheritance. J Vet Intern Med. 2010;24(1):171–178. doi: 10.1111/j.1939-1676.2009.0438.x. [DOI] [PubMed] [Google Scholar]
- 7.Berendt M, Gullov CH, Fredholm M. Focal epilepsy in the belgian shepherd: Evidence for simple mendelian inheritance. J Small Anim Pract. 2009;50(12):655–661. doi: 10.1111/j.1748-5827.2009.00849.x. doi: 10.1111/j.1748-5827.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 8.Pal DK, Strug LJ, Greenberg DA. Evaluating candidate genes in common epilepsies and the nature of evidence. Epilepsia. 2008;49(3):386–392. doi: 10.1111/j.1528-1167.2007.01416.x. doi: 10.1111/j.1528-1167.2007.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 10.Licht BG, Licht MH, Harper KM, Lin S, Curtin JJ, et al. Clinical presentations of naturally occurring canine seizures: Similarities to human seizures. Epilepsy Behav. 2002;3(5):460–470. doi: 10.1016/s1525-5050(02)00523-1. [DOI] [PubMed] [Google Scholar]
- 11.Bielfelt SW, Redman HC, McClellan RO. Sire- and sex-related differences in rates of epileptiform seizures in a purebred beagle dog colony. Am J Vet Res. 1971;32(12):2039–2048. [PubMed] [Google Scholar]
- 12.Knowles K. Idiopathic epilepsy. Clin Tech Small Anim Pract. 1998;13(3):144–151. doi: 10.1016/S1096-2867(98)80035-2. doi: 10.1016/S1096-2867(98)80035-2. [DOI] [PubMed] [Google Scholar]
- 13.Patterson EE, Armstrong PJ, O'Brien DP, Roberts MC, Johnson GS, et al. Clinical description and mode of inheritance of idiopathic epilepsy in english springer spaniels. J Am Vet Med Assoc. 2005;226(1):54–58. doi: 10.2460/javma.2005.226.54. [DOI] [PubMed] [Google Scholar]
- 14.Patterson EE, Mickelson JR, Da Y, Roberts MC, McVey AS, et al. Clinical characteristics and inheritance of idiopathic epilepsy in vizslas. J Vet Intern Med. 2003;17(3):319–325. doi: 10.1111/j.1939-1676.2003.tb02455.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz-Porsche D. Seizures. In: Braund KG, editor. Clinical Syndromes in Veterinary Neurology. St Louis, MO: Mosby; 1994. pp. 234–251. [Google Scholar]
- 16.Jaggy A, Faissler D, Gaillard C, Srenk P, Graber H. Genetic aspects of idiopathic epilepsy in labrador retrievers. J Small Anim Pract. 1998;39(6):275–280. doi: 10.1111/j.1748-5827.1998.tb03650.x. [DOI] [PubMed] [Google Scholar]
- 17.Kathmann I, Jaggy A, Busato A, Bartschi M, Gaillard C. Clinical and genetic investigations of idiopathic epilepsy in the bernese mountain dog. J Small Anim Pract. 1999;40(7):319–325. doi: 10.1111/j.1748-5827.1999.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 18.Berendt M, Gullov CH, Christensen SL, Gudmundsdottir H, Gredal H, et al. Prevalence and characteristics of epilepsy in the belgian shepherd variants groenendael and tervueren born in denmark 1995–2004. Acta Vet Scand. 2008;50:51. doi: 10.1186/1751-0147-50-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drogemuller C, Karlsson EK, Hytonen MK, Perloski M, Dolf G, et al. A mutation in hairless dogs implicates FOXI3 in ectodermal development. Science. 2008;321(5895):1462. doi: 10.1126/science.1162525. 10.1126/science.1162525. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson EK, Baranowska I, Wade CM, Salmon Hillbertz NH, Zody MC, et al. Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet. 2007;39(11):1321–1328. doi: 10.1038/ng.2007.10. doi: 10.1038/ng.2007.10. [DOI] [PubMed] [Google Scholar]
- 21.Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438(7069):803–819. doi: 10.1038/nature04338. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 22.Wilbe M, Jokinen P, Truve K, Seppala EH, Karlsson EK, et al. Genome-wide association mapping identifies multiple loci for a canine SLE-related disease complex. Nat Genet. 2010;42(3):250–254. doi: 10.1038/ng.525. doi: 10.1038/ng.525. [DOI] [PubMed] [Google Scholar]
- 23.Lohi H, Young EJ, Fitzmaurice SN, Rusbridge C, Chan EM, et al. Expanded repeat in canine epilepsy. Science. 2005;307(5706):81. doi: 10.1126/science.1102832. [DOI] [PubMed] [Google Scholar]
- 24.Abitbol M, Thibaud JL, Olby NJ, Hitte C, Puech JP, et al. A canine arylsulfatase G (ARSG) mutation leading to a sulfatase deficiency is associated with neuronal ceroid lipofuscinosis. Proc Natl Acad Sci U S A. 2010;107(33):14775–14780. doi: 10.1073/pnas.0914206107. doi: 10.1073/pnas.0914206107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awano T, Katz ML, O'Brien DP, Sohar I, Lobel P, et al. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006;89(3):254–260. doi: 10.1016/j.ymgme.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Awano T, Katz ML, O'Brien DP, Taylor JF, Evans J, et al. A mutation in the cathepsin D gene (CTSD) in american bulldogs with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006;87(4):341–348. doi: 10.1016/j.ymgme.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Farias FH, Zeng R, Johnson GS, Wininger FA, Taylor JF, et al. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in tibetan terriers. Neurobiol Dis. 2011;42(3):468–474. doi: 10.1016/j.nbd.2011.02.009. doi: 10.1016/j.nbd.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Melville SA, Wilson CL, Chiang CS, Studdert VP, Lingaas F, et al. A mutation in canine CLN5 causes neuronal ceroid lipofuscinosis in border collie dogs. Genomics. 2005;86(3):287–294. doi: 10.1016/j.ygeno.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Katz ML, Farias FH, Sanders DN, Zeng R, Khan S, et al. A missense mutation in canine CLN6 in an australian shepherd with neuronal ceroid lipofuscinosis. J Biomed Biotechnol. 2011;2011:198042. doi: 10.1155/2011/198042. doi: 10.1155/2011/198042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz ML, Khan S, Awano T, Shahid SA, Siakotos AN, et al. A mutation in the CLN8 gene in english setter dogs with neuronal ceroid-lipofuscinosis. Biochem Biophys Res Commun. 2005;327(2):541–547. doi: 10.1016/j.bbrc.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 31.Sanders DN, Farias FH, Johnson GS, Chiang V, Cook JR, et al. A mutation in canine PPT1 causes early onset neuronal ceroid lipofuscinosis in a dachshund. Mol Genet Metab. 2010;100(4):349–356. doi: 10.1016/j.ymgme.2010.04.009. doi: 10.1016/j.ymgme.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jokinen TS, Metsahonkala L, Bergamasco L, Viitmaa R, Syrja P, et al. Benign familial juvenile epilepsy in lagotto romagnolo dogs. J Vet Intern Med. 2007;21(3):464–471. doi: 10.1892/0891-6640(2007)21[464:bfjeil]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Seppala EH, Jokinen TS, Fukata M, Fukata Y, Webster MT, et al. LGI2 truncation causes a remitting focal epilepsy in dogs. PLoS Genet. 2011;7(7):e1002194. doi: 10.1371/journal.pgen.1002194. doi: 10.1371/journal.pgen.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekenstedt KJ, Patterson EE, Minor KM, Mickelson JR. Candidate genes for idiopathic epilepsy in four dog breeds. BMC Genet. 2011;12:38. doi: 10.1186/1471-2156-12-38. doi: 10.1186/1471-2156-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberbauer AM, Grossman DI, Irion DN, Schaffer AL, Eggleston ML, et al. The genetics of epilepsy in the belgian tervuren and sheepdog. J Hered. 2003;94(1):57–63. doi: 10.1093/jhered/esg010. [DOI] [PubMed] [Google Scholar]
- 36.Oberbauer AM, Belanger JM, Grossman DI, Regan KR, Famula TR. Genome-wide linkage scan for loci associated with epilepsy in belgian shepherd dogs. BMC Genet. 2010;11:35. doi: 10.1186/1471-2156-11-35. doi: 10.1186/1471-2156-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Famula TR, Oberbauer AM, Brown KN. Heritability of epileptic seizures in the belgian tervueren. J Small Anim Pract. 1997;38(8):349–352. doi: 10.1111/j.1748-5827.1997.tb03483.x. [DOI] [PubMed] [Google Scholar]
- 38.Noachtar S, Binnie C, Ebersole J, Mauguiere F, Sakamoto A, et al. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. the international federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:21–41. [PubMed] [Google Scholar]
- 39.Jeserevics J, Viitmaa R, Cizinauskas S, Sainio K, Jokinen TS, et al. Electroencephalography findings in healthy and finnish spitz dogs with epilepsy: Visual and background quantitative analysis. J Vet Intern Med. 2007;21(6):1299–1306. doi: 10.1892/06-285.1. [DOI] [PubMed] [Google Scholar]
- 40.Stern JM, Engel J. Atlas of EEG patterns. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. Beta activity. pp. 93–94. [Google Scholar]
- 41.Jabbari B, Russo MB, Russo ML. Electroencephalogram of asymptomatic adult subjects. Clin Neurophysiol. 2000;111(1):102–105. doi: 10.1016/s1388-2457(99)00189-3. [DOI] [PubMed] [Google Scholar]
- 42.Stern JM, Engel J. Atlas of EEG patterns. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. Interictal epileptiform discharges. pp. 161–165. [Google Scholar]
- 43.Pillai J, Sperling MR. Interictal EEG and the diagnosis of epilepsy. Epilepsia. 2006;47(Suppl 1):14–22. doi: 10.1111/j.1528-1167.2006.00654.x. doi: 10.1111/j.1528-1167.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 44.Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30(3):335–341. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laub F, Lei L, Sumiyoshi H, Kajimura D, Dragomir C, et al. Transcription factor KLF7 is important for neuronal morphogenesis in selected regions of the nervous system. Mol Cell Biol. 2005;25(13):5699–5711. doi: 10.1128/MCB.25.13.5699-5711.2005. doi: 10.1128/MCB.25.13.5699-5711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pescucci C, Caselli R, Grosso S, Mencarelli MA, Mari F, et al. 2q24-Q31 deletion: Report of a case and review of the literature. Eur J Med Genet. 2007;50(1):21–32. doi: 10.1016/j.ejmg.2006.09.001. doi: 10.1016/j.ejmg.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Scheffer IE, Phillips HA, O'Brien CE, Saling MM, Wrennall JA, et al. Familial partial epilepsy with variable foci: A new partial epilepsy syndrome with suggestion of linkage to chromosome 2. Ann Neurol. 1998;44(6):890–899. doi: 10.1002/ana.410440607. doi: 10.1002/ana.410440607. [DOI] [PubMed] [Google Scholar]
- 48.Xiong L, Labuda M, Li DS, Hudson TJ, Desbiens R, et al. Mapping of a gene determining familial partial epilepsy with variable foci to chromosome 22q11–q12. Am J Hum Genet. 1999;65(6):1698–1710. doi: 10.1086/302649. doi: 10.1086/302649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callenbach PM, van den Maagdenberg AM, Hottenga JJ, van den Boogerd EH, de Coo RF, et al. Familial partial epilepsy with variable foci in a dutch family: Clinical characteristics and confirmation of linkage to chromosome 22q. Epilepsia. 2003;44(10):1298–1305. doi: 10.1046/j.1528-1157.2003.62302.x. [DOI] [PubMed] [Google Scholar]
- 50.Berkovic SF, Serratosa JM, Phillips HA, Xiong L, Andermann E, et al. Familial partial epilepsy with variable foci: Clinical features and linkage to chromosome 22q12. Epilepsia. 2004;45(9):1054–1060. doi: 10.1111/j.0013-9580.2004.30502.x. doi: 10.1111/j.0013-9580.2004.30502.x. [DOI] [PubMed] [Google Scholar]
- 51.Morales-Corraliza J, Gomez-Garre P, Sanz R, Diaz-Otero F, Gutierrez-Delicado E, et al. Familial partial epilepsy with variable foci: A new family with suggestion of linkage to chromosome 22q12. Epilepsia. 2010;51(9):1910–1914. doi: 10.1111/j.1528-1167.2010.02680.x. doi: 10.1111/j.1528-1167.2010.02680.x. [DOI] [PubMed] [Google Scholar]
- 52.Gauguier D, van Luijtelaar G, Bihoreau MT, Wilder SP, Godfrey RF, et al. Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG/Rij rat. Epilepsia. 2004;45(8):908–915. doi: 10.1111/j.0013-9580.2004.13104.x. doi: 10.1111/j.0013-9580.2004.13104.x. [DOI] [PubMed] [Google Scholar]
- 53.Turnbull J, Lohi H, Kearney JA, Rouleau GA, Delgado-Escueta AV, et al. Sacred disease secrets revealed: The genetics of human epilepsy. Hum Mol Genet. 2005;14(17):2491–2500. doi: 10.1093/hmg/ddi250. doi: 10.1093/hmg/ddi250. [DOI] [PubMed] [Google Scholar]
- 54.Goldsmith AP, Gossage SJ, ffrench-Constant C. ADAM23 is a cell-surface glycoprotein expressed by central nervous system neurons. J Neurosci Res. 2004;78(5):647–658. doi: 10.1002/jnr.20320. [DOI] [PubMed] [Google Scholar]
- 55.Owuor K, Harel NY, Englot DJ, Hisama F, Blumenfeld H, et al. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci. 2009;42(4):448–457. doi: 10.1016/j.mcn.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukata Y, Lovero KL, Iwanaga T, Watanabe A, Yokoi N, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci U S A. 2010;107(8):3799–3804. doi: 10.1073/pnas.0914537107. doi: 10.1073/pnas.0914537107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caiazzo M, Colucci-D'Amato L, Volpicelli F, Speranza L, Petrone C, et al. Kruppel-like factor 7 is required for olfactory bulb dopaminergic neuron development. Exp Cell Res. 2011;317(4):464–473. doi: 10.1016/j.yexcr.2010.11.006. doi: 10.1016/j.yexcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Jin H, Tan S, Hermanowski J, Bohm S, Pacheco S, et al. The dystrotelin, dystrophin and dystrobrevin superfamily: New paralogues and old isoforms. BMC Genomics. 2007;8:19. doi: 10.1186/1471-2164-8-19. doi: 10.1186/1471-2164-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoefs SJ, Skjeldal OH, Rodenburg RJ, Nedregaard B, van Kaauwen EP, et al. Novel mutations in the NDUFS1 gene cause low residual activities in human complex I deficiencies. Mol Genet Metab. 2010;100(3):251–256. doi: 10.1016/j.ymgme.2010.03.015. doi: 10.1016/j.ymgme.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Ghezzi D, Saada A, D'Adamo P, Fernandez-Vizarra E, Gasparini P, et al. FASTKD2 nonsense mutation in an infantile mitochondrial encephalomyopathy associated with cytochrome c oxidase deficiency. Am J Hum Genet. 2008;83(3):415–423. doi: 10.1016/j.ajhg.2008.08.009. doi: 10.1016/j.ajhg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viitmaa R, Cizinauskas S, Bergamasco LA, Kuusela E, Pascoe P, et al. Magnetic resonance imaging findings in finnish spitz dogs with focal epilepsy. J Vet Intern Med. 2006;20(2):305–310. doi: 10.1892/0891-6640(2006)20[305:mrifif]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 62.Bergamasco L, Accatino A, Priano L, Neiger-Aeschbacher G, Cizinauskas S, et al. Quantitative electroencephalographic findings in beagles anaesthetized with propofol. Vet J. 2003;166(1):58–66. doi: 10.1016/s1090-0233(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 63.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas PD, Kejariwal A, Guo N, Mi H, Campbell MJ, et al. Applications for protein sequence-function evolution data: MRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 2006;34(Web Server issue):W645–50. doi: 10.1093/nar/gkl229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nygard AB, Cirera S, Gilchrist MJ, Gorodkin J, Jorgensen CB, et al. A study of alternative splicing in the pig. BMC Res Notes. 2010;3:123. doi: 10.1186/1756-0500-3-123. doi: 10.1186/1756-0500-3-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the clinical features of 94 epileptic Belgian Shepherds collected through the owner-filled epilepsy questionnaires.
(XLS)