Abstract
Assessment of locomotion following exposure of animals to noxious or painful stimuli can offer significant insights into underlying mechanisms of injury and the effectiveness of various treatments. We developed a novel method to track the movement of mice in two dimensions using computer vision and neural network algorithms. By using this system we demonstrated that mice exposed to chlorine (Cl2) gas developed impaired locomotion and increased immobility for up to 9 h postexposure. Postexposure administration of buprenorphine, a common analgesic agent, increased locomotion and decreased immobility times in Cl2- but not air-exposed mice, most likely by decreasing Cl2-induced pain. This method can be adapted to assess the effectiveness of various therapies following exposure to a variety of chemical and behavioral noxious stimuli.
Keywords: ethograms, buprenorphine, pain, total distance covered, mobility
chlorine (Cl2), is the ninth largest produced chemical by volume in the United States and is widely used for pulp bleaching, pharmaceutical manufacture, and maintenance of pathogen-free swimming pools (23, 27). Human exposures during industrial accidents or acts of terrorism may result in the development of acute lung injury (ALI) necessitating treatment with mechanical ventilation and supplemental oxygen (25, 26). We have shown that mice and rats exposed to 400–600 ppm of Cl2 develop respiratory distress, hypoxemia, airway hyperreactivity, decreased vectorial sodium transport across distal lung epithelia, and non-cardiogenic edema (13, 21, 22, 28, 29). Humans exposed to Cl2 report severe pain in their exposed tissues and airways presumably because of the stimulation of transient receptor potential ankyrin 1 (TRPA1) channels in airway sensory neurons and tissue damage by secondary reactive intermediates (2, 3, 26). Postexposure administration of analgesic agents to those affected by first responders may be of significant benefit. Pain is a key factor in the recognition of the severity of injury in both humans and animals exposed to noxious stimuli (16, 24). Reductions in locomotion and physical inactivity are often observed in animals experiencing pain, and these events may be reversed by the administration of analgesics (16, 24). Currently, a variety of commercial systems are used to assess locomotion changes as a pain-depressed behavior in rodents (5, 7, 8, 11, 17).
The purpose of the present study was to quantify changes in locomotion of mice exposed to sublethal concentrations of Cl2 and subsequently returned to room air, before and following the administration of the opioid analgesic buprenorphine (16). We used the video camera of an iPhone to track the movement of mice in two dimensions before and at various intervals postexposure to Cl2 gas and developed highly efficient algorithms utilizing neural network (NN) theory to compute times of activity and inactivity, total distance covered (TDC), and the mean velocity at 2-min intervals for up to 9 h postexposure. This system represents a low-cost but highly efficient alternative to the commercially available hardware and software packages currently available to study locomotion and can be adapted to a variety of experimental conditions.
MATERIALS AND METHODS
Animals.
C57BL/6 male mice, weighing 20–25 g, were purchased from Charles River Laboratories (Wilmington, MA). They were housed in groups of three in regular mouse cages (Maxi-Miser #9, floor area of 435.7 cm2, Thoren Caging) under a 12:12-h light/dark cycle, in a temperature controlled environment in the animal facilities of the University of Alabama, and were acclimated for 4 days before experimental procedures. Access to food and water were ad libitum. All procedures described in this manuscript were approved by the University of Alabama at Birmingham IACUC.
Experimental procedures.
Six mice at a time were placed inside a custom-made glass chamber (Specialty Glass) and exposed to 400 ppm Cl2 for 30 min as described previously (21, 29). The Cl2 concentrations in the chamber were monitored with a Cl2 detector (Interscan, model RM34–1000m). After each exposure the chamber was vented with compressed air for 2–3 min and the mice were returned to their cages, breathing room air for up to 9 h. Shortly (5–10 m) after being returned to room air, mice were injected intraperitoneally once with 0.05 mg/kg body wt buprenorphine (Buprenex, Wellness Pharmacy, University of Alabama Hospital, Birmingham, AL; vial concentration 0.3 mg/ml; diluted to 0.005 mg/ml; volume injected 0.01 ml/g) or an equal volume of pyrogen-free normal saline (0.01 ml/g; Hospira, Lake Forest, IL). Their movement in the cage was recorded for 2-min intervals before and at 0.1, 3, 6, and 9 h post-Cl2 exposure. These measurements were repeated with air breathing mice. Peripheral oxygen saturation was assessed periodically with a MouseOx pulse oximeter equipped with a neck collar according to manufacturer instructions (STARR Lifesciences.)
Video capture and acquisition.
Just prior to a recording session (2 m each), one mouse at a time was transferred from its home cage to an identical video capture cage with new bedding (video capture cage). A smartphone (iPhone 4, Apple), capable of capturing high definition video (HD, 720p, 1,280×720 pixels) at 30 frames/s, was mounted on a vertical plane 48 cm above the bottom of the cage. Its optical field included the whole floor area of 435.7 cm2. The video capturing sessions took place before exposure to Cl2, shortly after exposure (within 5 m), and at 3, 6, and 9 h postexposure while the mice were breathing room air. The video capture duration was necessitated by digital storage requirements and reasonable processing analysis times while at the same time allowing us to reach definitive conclusions to the questions posed. Similar recording times have been used in a number of behavioral studies (6). Mice were injected with buprenorphine or saline at the conclusion of the first postexposure video recording session and had time to recover from the injection prior to the next recording. After the video acquisition session, the captured video was transferred to a computer for analysis.
Video transformation and conversions.
Captured video was converted from MOV into AVI format. We identified the bottom of the video capture cage by using video cropping. We also resized the video frames to 160×120 pixels to optimize the processing time with MATLAB (see Video processing with MATLAB). The audio channel was removed to reduce the video size. These tasks were performed using open-source video editing software (Virtual Dub 1.9.11, http://www.virtualdub.org/).
Video processing with MATLAB. We used a series of algorithms, which we developed in MATLAB R2011a (Mathworks, Natick, MA) and gathered under the ARES (Advanced Rodent Effort Sequencer) using our open-source software suite. We used the SIMULINK toolbox of MATLAB to construct the algorithms to obtain the coordinates of the mouse positions over time. By using blob analysis, we visualized the mouse position in each frame by generating a green box surrounding the mouse; a red cross represented its centroid (the geometric center of the body shape after extracting the tail by using computer vision morphometric transformations in MATLAB) and a blue line with a variable length represented its instantaneous velocity vector (Figs. 1 and 2). The algorithm for the blob analysis was based on blob video detection after transforming the video to frame images with intensity color map and applying Otsu's threshold detection to each video frame. Otsu's method determines the threshold by splitting the histogram of the input image so that the variance for each of the pixel groups is minimized (18). The coordinates of the centroid in each video frame were stored in a variable (named simout) for postprocessing. We used a 200 ms temporal resolution for processing and storing the centroids in each frame for optimum resolution and data processing speeds. All algorithms are available as open-source software and can be run within the MATLAB program.
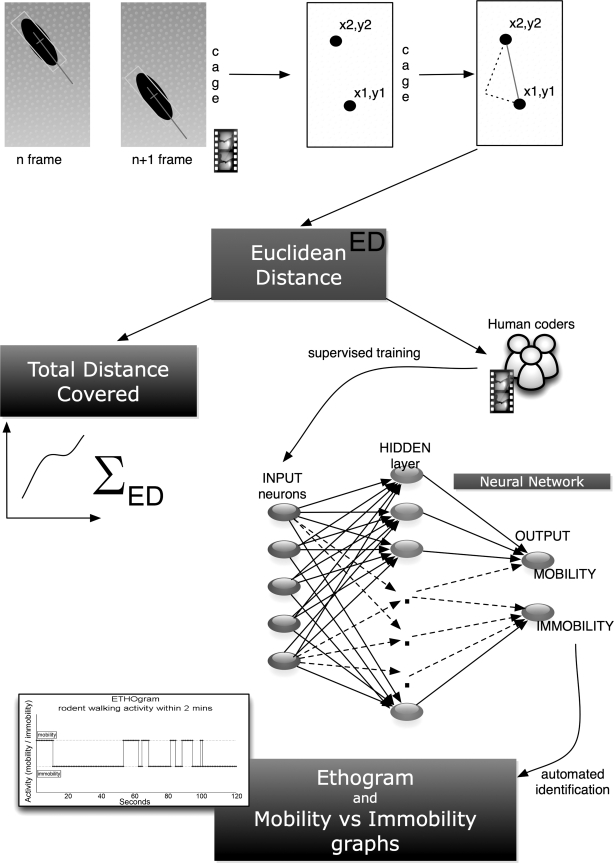
Fig. 1.
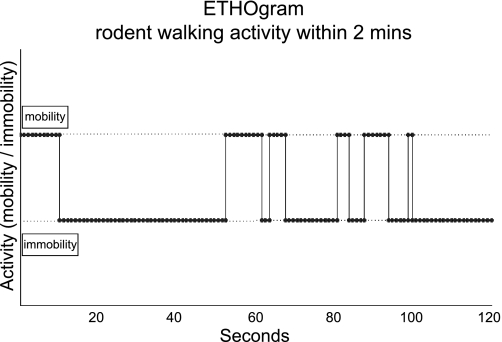
Calculation of total distance covered (TDC), ethograms and mobility/immobility graphs. Figure demonstrates the calculation of Euclidian distance (ED) and how it is derived from the video session acquisition of the frames and the centroid of the mouse coordinates in the cage. In addition, the development of the neural network (NN) for automated detection of immobility events in the obtained video is described along with the creation of ethograms used in behavioral studies to plot the behavioral patterns of a test subject over time. In this case, we tracked mobility vs. immobility events.
Fig. 2.
Snapshots of the ARES software running under MATLAB. A: snapshot from original video showing the mouse in its cage. B: mouse with the surrounding green bounding box, the red cross representing the centroid used to record its position in the cage, and the blue line representing the instantaneous velocity at the starting point of the centroid. C: blob analysis after Otsu shape-based image thresholding and morphometric processing to extract the tail from the processed frames.
Calculation of TDC.
The Euclidean Distance (ED) covered by the mouse within 200 ms was calculated from two sets of coordinates representing the rodent position in a two dimensional (x,y) plane at the bottom of the cage derived from the centroid. The TDC was the sum of the EDs for 2 min (Fig. 1). We developed another algorithm in MATLAB, part of the ARES software, to extract the EDs and TDCs from the obtained simout variable for each video tracking session. To generate plots of total distance covered by the mouse over a 2-min period, we plotted the centroid coordinates in a 160×120 matrix representing the bottom of the cage. Each point is shown as a colored dot and indicates the mouse instantaneous velocity at this time point (Fig. 3). We also used the TDC values obtained at each time point to construct a plot of the mean TDC of each experimental group (treated with buprenorphine or vehicle) for different times postexposure (Fig. 4A).
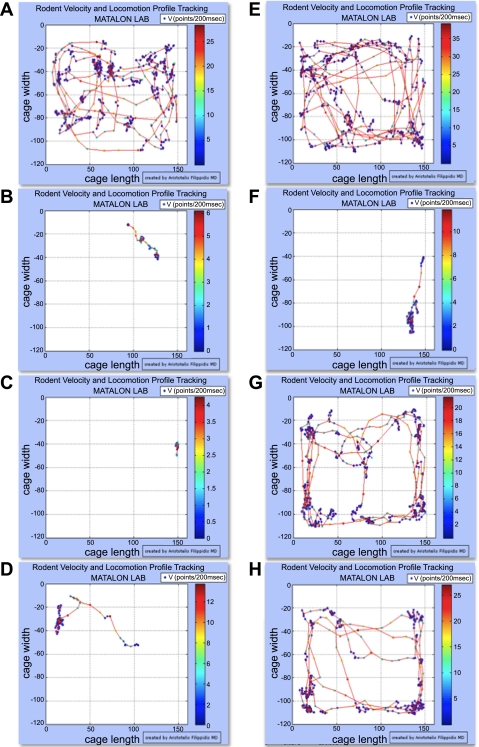
Fig. 3.
Traveled distances (track paths) and instantaneous velocities [V (points/200 ms)] of mice during 2-m intervals before and following exposure to Cl2. A: red lines, TDC covered within a 2-min period by a C57BL/6 mouse breathing air. Color dots represent instantaneous velocities during a 200-ms period according to the color scale on the right. The mouse was then exposed to Cl2 (400 ppm for 30 m) and returned to room air. B: ∼5 m post Cl2 exposure. After video tracking, the mouse received an intraperitoneal injection of saline (0.01 ml/g). C: 6 h post Cl2 exposure. D: 9 h post Cl2 exposure. E–H: same as in A–D but the mouse was injected with buprenorphine (0.05 mg/kg as described in materials and methods) immediately after F. Additional details are shown in materials and methods. Notice the much higher TDC post buprenorphine treatment.
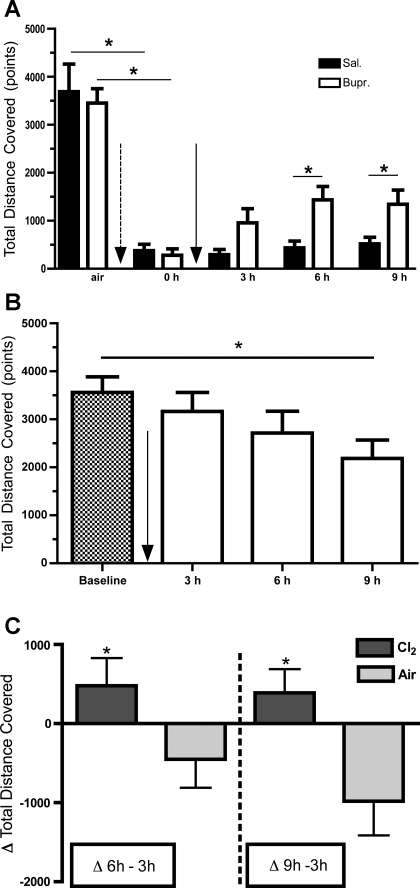
Fig. 4.
A: TDC within a 2-m period before and following exposure to Cl2 and treatment with either buprenorphine or saline. Following return to room after exposure to Cl2 (400 ppm for 30 min; dotted arrow), mice were injected intraperitoneally with either buprenorphine (0.05 mg/kg) or saline (solid arrow). The y-axis shows the TDC [means ± SE (n = 6 mice per group)] for a 2-min period. This was done by measuring the ED of 2 centroids within 2 consecutive frames (200 ms apart) and then summing these distances in a 2-min period. 0 h refers to the first recording session, within 5 min postexposure. *P < 0.05 compared with the corresponding buprenorphine value of the same interval. One-sided Wilcoxon matched-pairs signed rank test was used for comparison of pretreatment groups. Repeated measures 2-way ANOVA was used for post-treatment groups followed by individual Bonferroni post hoc at individual sampling times. B: TDC within a 2-min period before (baseline) and following injection of buprenorphine in air breathing mice. Mice breathed air instead of Cl2. Solid arrow signifies the time of buprenorphine injection. The y-axis shows TDC (means ± SE; n = 6) in a 2-min period. One-way ANOVA followed by individual comparisons with Bonferroni post hoc t-tests. A significant reduction in TDC was identified only after 9 h postbuprenorphine injection compared with baseline values. *P < 0.05 compared with baseline. C: ΔTDC for Cl2 or air breathing mice treated with buprenorphine. The y-axis shows the difference in TDC at 6 or 9 h postbuprenorphine administration from the corresponding 3-h interval. Values are means ± SE; n = 6. *P < 0.05 compared with the corresponding value in the same time interval (1-sided, unpaired t-test). Dark bars, Cl2; shaded bars, air.
NN design and analysis.
Human coders were instructed to define immobility (physical inactivity) events if there was no change of the centroid coordinates within 1 s (the limit of resolution of the human coders). They noted the exact time at which they identified the end or the start of a movement within 12 video clips provided in a shared drive. To conceal the time point of the video capture and the assignment of the mouse in a particular group, the video clips were gathered randomly in a blinded fashion and numbered. The coders provided the first author (A.F.) with notes concerning immobility time frames within the videos they watched. They did not discuss their results with each other. The performance of the NN was validated with confusion matrices and performance plots.
We used the simout variable obtained for each video capture session and extracted the sites labeled as immobile by the human coders. We then pooled signals containing EDs for 200 ms by sequentially appending them and refer to this pooled signal as immobility signal, which was then rearranged to a matrix of five ED values per 1 s. This is because each ED value was logged with a 200-ms resolution, and the resolution of optical recognition of immobility events by the human coders was 1 s.
Construction of the NN.
We used the NN Toolbox in MATLAB R2011a to construct a neural network for pattern recognition. Because we wanted to identify immobility events, we provided the NN with the immobility signal, which had the combined observations of two human coders. Within the immobility signal 70% of the data were used for training and 15% to validate that the network was generalizing and to stop training prior to over-fitting. The last 15% of the data were used as a completely independent test of network generalization.
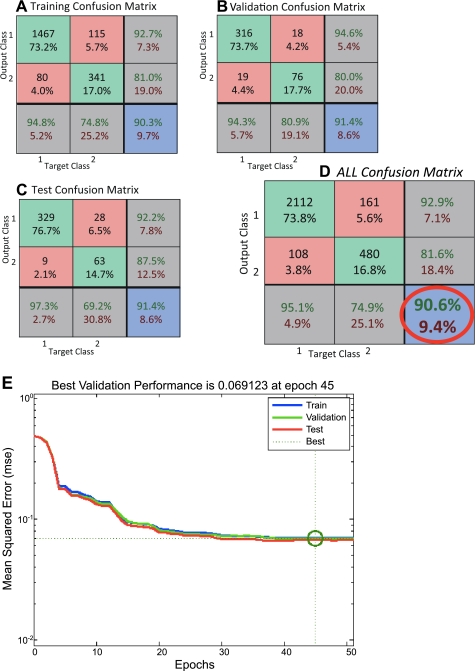
A two-layer feed-forward network, with sigmoid hidden and output neurons was used, which is a good classifier for pattern recognition problems(9). The architecture of the NN had 5 input neurons, 20 neurons at a hidden intermediate layer, and 2 output neurons related to the immobility status of the time frame processed (1 s). The output was classified as immobile or mobile. The network was trained with a scaled conjugate gradient back-propagation algorithm, which is appropriate for pattern recognition problems (15). The performance of the NN reached a 90.6% overall accuracy for recognizing immobility events as can be seen in the confusion matrix used for validation (Fig. 5A) and achieved its best performance in epoch 45 of training (Fig. 5B).
Fig. 5.
A–D: confusion matrices and performance plots obtained for the NN vs. the human coders. Overall performance and validation of the network can be identified within the red circle. NN was able to identify 90.6% of the events of immobility in agreement with the human coders. In each one of the confusion matrices, the top left green square corresponds to true positive (TP) incidents in absolute numbers and the percentage of TP incidents in total incidents used. Central green square corresponds to true negative (TN) incidents in absolute numbers and the percentage of TN incidents in total incidents used. Top red square corresponds to false positive (FP) incidents in absolute numbers and the percentage of FP incidents in total incidents used (describing statistical type 1 error). Middle left red square corresponds to false negative (FN) incidents in absolute numbers and the percentage of FN incidents in total incidents used (describing statistical type 2 error). Top right gray square with green letters describes the positive predictive value [TP/(TP+FP)]×100 and with the red letters the [FP/(TP+FP)]×100. Middle right gray square with green letters describes the negative predictive value [TN/(TN+FN)]×100 and with the red letters the [FN/(TN+FN)]×100. Bottom left gray square with green letters describes the sensitivity [TP/(TP+FN)]×100 and with the red letters the [FN/(TP+FN)]×100. Bottom middle gray square with green letters describes the specificity [TN/(TN+FP)]×100 and with the red letters the [FN/(TN+FP)]×100. Finally, the bottom right blue square with green letters describes the correctly classified incidents [(TP+TN)/total incidents used]×100, and with the red letters the incorrectly classified incidents [(FP+FN)/total incidents used]×100. Glossary of terms used: incidents were the signal data used as an input for the NN (immobility or mobility signals); total incidents were the total number of signals used for the construction of each confusion matrix; target class refers to the known immobility signals (target class 1) and the known mobility signals (target class 2) that the NN was supplied with; output class refers to the NN's classification response to the input data denoting as output class 1 the classification of immobile and as output class 2 the classification of mobile; TP were the incidents that were immobile and were classified as immobile; TN were the incidents that were mobile and were classified as mobile; FP were the incidents that were mobile and were classified as immobile; FN were the incidents that were immobile and were classified as mobile. E: performance plot of the NN. y-Axis demonstrates the mean squared error where the lower the error the better the performance. x-Axis demonstrates the epochs, which represent a step at the training of the NN. The best results were achieved at epoch 45 in our NN.
Using the NN to identify immobility times-ethogram plots.
On the basis of the constructed NN, we designed a MATLAB algorithm that uses as input a tracked sequence of ED values obtained after a video tracking session for one mouse and generates the number of seconds within this video that the animal was immobile (immobility seconds). The algorithm also plots ethograms (events of a tracked behavior over a specific time frame) of mobility or immobility within 2 min of tracked video (Figs. 6 and 7). Currently the MATLAB software suite is required to run the ARES software we developed.
Fig. 6.
Example of an ethogram. Plot of an observed behavior where mobility and immobility events are tracked for a two min period. ARES can create such plots by using the NN structure to automatically label the mice behavior in a video track. Top line level is used for events (dots) of mobility whereas the bottom line level is used to plot events of immobility.
Fig. 7.
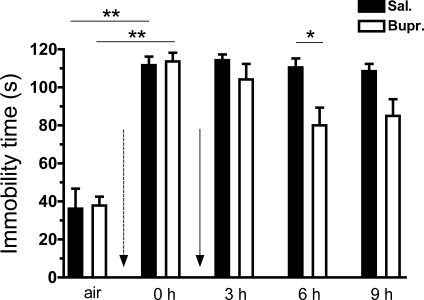
Immobility times calculated by the NN within a 2-min period before and following exposure to Cl2 and treatment with either buprenorphine or saline. We used the NN developed for the ARES software to identify immobility times in different groups. Following return to room after exposure to Cl2 (400 ppm for 30 min; dotted arrow), mice were injected intraperitoneally with either buprenorphine (0.05 mg/kg) or saline (solid arrow). y-Axis shows the immobility times in s [means ± SE (n = 6 mice per group)] for a 2-min period. Comparisons were done pre-exposure (air) vs. mice immediately postexposure to Cl2 (0 h, within 15 min). Dotted arrow signifies return to room after Cl2 exposure before the treatments with saline or buprenorphine. Solid arrow signifies the administration of treatments. Black bars refer to the saline injected mice and white bars to the buprenorphine-injected mice. **P < 0.01 (Mann-Whitney test, Kolmogorov-Smirnov test for normality). *P < 0.05 (2-way repeated-measures ANOVA to compare means for 3, 6, and 9 h postexposure followed by post hoc analysis with the t-test).
Statistical analysis.
The statistical analysis was performed using Graphpad Prism 5.03 for Microsoft Windows (GraphPad Software, La Jolla, CA). The level of statistical significance was defined as P < 0.05. Normality tests were performed with the Kolmogorov-Smirnov test (Dallal-Wilkinson-Lillie for P value) and, depending on the outcome, data were analyzed with parametric or a nonparametric tests. Values are represented as means ± SE. Statistical significance among group means (saline vs. buprenorphine) for various times for exposure was tested using two-way ANOVA followed by Bonferroni post-test. One-way ANOVA with Bonferroni' s post-test was used to analyze the effect of buprenorphine on the locomotion patterns of mice that were exposed to air instead of Cl2.
RESULTS
The ARES algorithms accomplished the following tasks: 1) tracked the movement of the mouse within its cage (Fig. 2); 2) calculated the TDC within a 2-m period (Fig. 4A); 3) plotted the position of the mouse in a two-dimensional plane (Fig. 3); 4) used an automated NN based system (trained by 2 human coders) for the identification of mobility vs. immobility events (Figs. 6 and 7); and 5) constructed graphs depicting mouse's physical activity via locomotion detection (ethograms of movement or no movement) for each video capture session (Figs. 1 and 7). This technique is based on the calculation of the ED between two serial frames containing the position of the mouse (Fig. 1). The TDC for a 2-m period was calculated by adding the EDs acquired every 200 ms. The centroids of the rodent's position (the geometric center of rodent's shape) were plotted over time in a two-dimensional plane track-path representing the cage bottom (Fig. 3). A NN approach was used to assess whether the mice were moving or remained stationary (immobility and mobility status) and achieved 90.6% overall accuracy in correctly identifying mobility/immobility status of the rodent compared with the pooled decisions of the human coders (Figs. 1 and 5). Thus the ARES suite and MATLAB commands can be used to automatically track events of motion and create ethograms (Figs. 6 and 7).
Mice exposed to Cl2 (400 ppm for 30 min) and returned to room air exhibited depressed locomotion as shown by marked decreases of TDC (Fig. 4A) and increased immobility times (Fig. 7) lasting throughout the duration of our measurements (9 h postexposure). A single injection of buprenorphine shortly on return to room increased TDC (Fig. 4A) and decreased immobility times (Fig. 7). These results indicate that the Cl2-exposed saline-treated mice were more immobile and covered less distance at 6 h compared with the buprenorphine ones, whereas they were almost equally immobile but covering less distance at 9 h. In contrast, injection of buprenorphine in air breathing mice resulted in either no change (up to 6 h) or decreased TDC at 9 h postexposure (Fig. 4B). To highlight the differential effects of buprenorphine in Cl2 and air breathing animals we calculated the difference in TDC at 6 and 9 h compared with 3-h postinjection. These data are shown in Fig. 4C. As can be seen, buprenorphine increased TDC in Cl2 exposed but decreased TDC in air breathing mice.
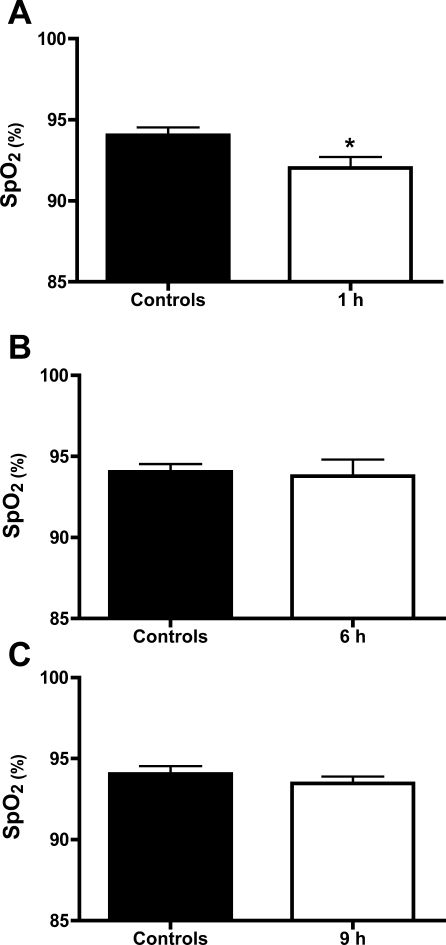
Buprenorphine is administered routinely to patients with chronic pain. Measurements of peripheral oxygen saturation showed that although mice were hypoxic shortly after exposure to Cl2, they had near normal levels at 6 h and 9 h postexposure (Fig. 8). These data suggest that pain and not hypoxia contributed to decreased locomotion and thus physical inactivity. This difference can also be observed by comparison of the corresponding track paths (Fig. 3, A–D, saline group and E–H, buprenorphine group).
Fig. 8.
Peripheral oxygen saturations post Cl2 exposure. Mice were exposed either in Cl2 (400 ppm for 30 min; white bars) or air (solid bars). Oxygen saturations were measured noninvasively as mentioned in materials and methods at various times post Cl2 exposure. Values are means ± SE (n = 6 for each group). Comparisons were made based on Kolmogorov-Smirnov normality test results using either one-sided unpaired t-test (A and C) or one-sided Mann-Whitney exact test (B). *P = 0.0241.
DISCUSSION
Locomotion studies provide an essential behavioral index in studies of pain-depressed behavioral physiology and injury-severity models. A reduction in locomotion in animal models is often related to either severe pain or injury whereas a reversal in this reduction produced by analgesics can provide critical translational data about the efficacy of potentially analgesic drugs (16, 24).
Currently, there are numerous methods to measure locomotion in rodents (5, 7, 8, 12, 17, 20). Most of these methods rely on expensive equipment, such as custom-made animal cages that differ from their home cage environment, special video capture equipment, and beam crossing sensors. Software packages can be expensive because they are tailored to the specific features of the hardware. Finally, the data obtained from such devices provide a rather limited quantification of the behavior actually taking place, e.g., number of grid or beam crossings.
The novelty of our approach lies in its use of a home cage automated detection approach, a smartphone, and algorithms written in MATLAB, ensuring that locomotion is assessed in an environment (video capture cage) that is identical to its home cage. This is very important because transferring rodents to a new and unfamiliar test environment will drastically alter behavioral patterns (19) and thus introduce significant errors. Additionally, instead of static sensors (infrared or laser beams) we track each mouse continuously with an iPhone camera and a trained NN that identifies events of immobility vs. mobility (physical inactivity vs. activity). Neural networks allow unsupervised tagging of video sessions by computer software trained to distinguish mobility-locomotion vs. immobility events and time of occurrence. It is known for years in the behavioral field that labeling and tagging videos is a time-consuming task that requires trained personnel. In the current implementation of the algorithm, the NN reached a high level of performance (Fig. 5, A and B) and can be used as an artificial intelligence aid to tag videos tracking locomotion of rodent from a top-two-dimension view using our algorithms. The NN acts as an add-on to simplify the process of unsupervised tagging. This automated approach is highly accurate, objective, and highly reproducible.
The speed, frequency, and duration of locomotion have been thought to represent pain-depressed behavior or injury severity (16, 24). By using our method we were able to identify for the first time that inhalation of sublethal concentrations of Cl2 (400 ppm for 30 min), known to result in pain through activation of TRPA1 receptors and tissue injury (2–4, 10, 28) lead to a significant reduction of locomotion and thus induces physical inactivity (Figs. 4 and 7). Postexposure administration of buprenorphine, a common analgesic, led to improved locomotion and fewer events of immobility, compared with Cl2-exposed, vehicle-treated mice. In contrast, in our studies, buprenorphine had either no effect or decreased locomotion (at 9 h postadministration) in air breathing mice. These results indicate that the salutatory effect of buprenorphine on locomotion was most likely attributable to Cl2-induced pain alleviation. In previous studies, investigators reported a transient increase in locomotion in rats and mice within 1 h of buprenorphine administration (1, 11). In addition, Liles and Flecknell (14) found that buprenorphine administration in rats not experiencing pain increased locomotion transiently at 5 h postadministration. However, the authors noted that different results may be expected in animals experiencing pain. Thus buprenorphine administration in Cl2-exposed humans experiencing pain may be of significant value by increasing their locomotion, thus allowing them to move away from the epicenter of Cl2 release. In addition, assessment of locomotion with our method may establish an objective clinical biomarker, which will be useful in assessing the severity of injury and effectiveness of various treatments.
One limitation of our method is the inability of smartphone cameras to film in the dark. Thus it is not possible to assess locomotion at night without additional lighting (which may alter locomotion) or an integrated infrared device. In addition, the short focal distance (f = 3.85 mm) of the iPhone camera lens may introduce optical distortion in the captured images and thus contribute to measurement error of the mouse location. However, all measurements were performed with the same device, mounted at 48 cm above the floor of the cage and focused on the center of the floor. Any systematic errors induced in the measured parameters are the same for every video capture.
In conclusion, we developed an automated system to track rodent locomotion by video recording of their movements with the camera of a smartphone and analyzing the records with NN algorithms. By using this technique we clearly distinguished mobility vs. immobility events and demonstrated that mice exposed to Cl2 in concentrations likely to be encountered in the vicinity of industrial accidents and acts of terrorism develop decreased locomotion even 9 h postexposure. Furthermore, a single injection of buprenorphine, an analgesic commonly prescribed to patients for the management of chronic pain, increased mobility significantly. The algorithms we developed can easily be adapted to assess locomotion in a variety of settings. Thus our novel approach provides an objective way to assess injury severity or treatment efficiency attributable to an intervention in laboratory animals and could be a valuable tool in translational, behavioral physiology, and veterinary applications.
GRANTS
This work was funded by the National Institute of Environmental Health Sciences Grants 5U54ES017218 and 5U01ES015676 to S. Matalon and a European Respiratory Society Fellowship (LTRF58-2010) to S. G. Zarogiannis.
DISCLOSURES
S. Matalon was a consultant for Angion (Long Island, NY). In the past, S. Matalon received research funding from Sepracor, to test the efficacy of BROVANA in the amelioration of Cl2-induced lung injury and attended a research conference sponsored by Sepracor to report the results of these studies.
AUTHOR CONTRIBUTIONS
Author contributions: A.S.F., T.J.N., and S.M. conception and design of research; A.S.F., S.G.Z., and A.R. performed experiments; A.S.F., S.G.Z., and S.M. analyzed data; A.S.F. and S.M. interpreted results of experiments; A.S.F. and S.M. prepared figures; A.S.F., S.G.Z., and S.M. drafted manuscript; A.S.F., S.G.Z., A.R., T.J.N., and S.M. edited and revised manuscript; A.S.F., S.G.Z., A.R., T.J.N., and S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Asta Jurkuvenaite, Dr. Ahmed Lazrak, and Ms. Solana Fernandez for their contribution on the development of the neural network (coding section) and Ms. Gloria Y. Son for her editorial assistance.
REFERENCES
- 1. Akay T, Fouad K, Pearson KG. New technique for drug application to the spinal cord of walking mice. J Neurosci Methods 171: 39–47, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology 23: 360–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J 23: 1102–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118: 1899–1910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dell'Omo G, Vannoni E, Vyssotski AL, Di Bari MA, Nonno R, Agrimi U, Lipp HP. Early behavioural changes in mice infected with BSE and scrapie: automated home cage monitoring reveals prion strain differences. Eur J Neurosci 16: 735–742, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Dutra-Filho CS, Wannmacher CM, Pires RF, Gus G, Kalil AM, Wajner M. Reduced locomotor activity of rats made histidinemic by injection of histidine. J Nutr 119: 1223–1227, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Galsworthy MJ, Amrein I, Kuptsov PA, Poletaeva II, Zinn P, Rau A, Vyssotski A, Lipp HP. A comparison of wild-caught wood mice and bank voles in the Intellicage: assessing exploration, daily activity patterns and place learning paradigms. Behav Brain Res 157: 211–217, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH. A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci USA 105: 20575–20582, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hagan MT, Demuth HB, Beale MH. Neural Network Design. Boston: PWS, 1996 [Google Scholar]
- 10. Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, Patel RP. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol 45: 419–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson HC, Griffin IJ, Nutt DJ. Buprenorphine-cocaine interactions in mice: effect on locomotor activity and hole-dipping behaviour. J Pharm Pharmacol 45: 636–640, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Jackson WS, Tallaksen-Greene SJ, Albin RL, Detloff PJ. Nucleocytoplasmic transport signals affect the age at onset of abnormalities in knock-in mice expressing polyglutamine within an ectopic protein context. Hum Mol Genet 12: 1621–1629, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 295: L733–L743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liles JH, Flecknell PA. The effects of buprenorphine, nalbuphine and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movement in rats. Lab Anim Care 26: 180–189, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Moller MF. A scaled conjugate gradient algorithm for fast supervised learning. Neural Networks 6, 525–533, 1993 [Google Scholar]
- 16. Negus SS, Bilsky EJ, Do Carmo GP, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol Biol 617: 79–91, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput 33: 398–414, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Otsu NA. Threshold selection method from gray-level histograms. IEEE Trans Sys, Man Cyber 9: 62–66, 1979 [Google Scholar]
- 19. Richter SH, Garner JP, Wurbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat Methods 6: 257–261, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Rudenko O, Tkach V, Berezin V, Bock E. Detection of early behavioral markers of Huntington's disease in R6/2 mice employing an automated social home cage. Behav Brain Res 203: 188–199, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a β2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol 45: 88–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 285: 9716–9728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 299: L289–L300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci 85: 309–315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van SD, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 27: 1–7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7: 257–263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 7: 278–283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 300: L362–L369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol 45: 386–392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]