Abstract
Dietary sodium and blood pressure regulation differs between normotensive men and women, an effect which may involve endothelial production of nitric oxide (NO). Therefore, we tested the hypothesis that differences in the NO component of endothelium-dependent vasodilation between low and high dietary sodium intake depend on sex. For 5 days prior to study, healthy adults consumed a controlled low-sodium diet (10 mmol/day, n = 30, mean age ± SE: 30 ± 1 yr, 16 men) or high-sodium diet (400 mmol/day, n = 36, age 23 ± 1 yr, 13 men). Forearm blood flow (FBF, plethysmography) responses to brachial artery administration of acetylcholine (ACh, 4 μg·100 ml tissue−1·min−1) were measured before and after endothelial NO synthase inhibition with NG-monomethyl-l-arginine (l-NMMA, 50 mg bolus + 1 mg/min infusion). The NO component of endothelium-dependent dilation was calculated as the response to ACh before and after l-NMMA accounting for changes in baseline FBF: [(FBF ACh − FBF baseline) − (FBF AChL-NMMA − FBF baselineL-NMMA)]. This value was 5.7 ± 1.3 and 2.5 ± 0.8 ml·100 ml forearm tissue−1·min−1 for the low- and high-sodium diets, respectively (main effect of sodium, P = 0.019). The sodium effect was larger for the men, with values of 7.9 ± 2.0 and 2.2 ± 1.4 for men vs. 3.1 ± 1.3 and 2.7 ± 1.0 ml·100 ml forearm tissue−1·min−1 for the women (P = 0.034, sex-by-sodium interaction). We conclude that the NO component of endothelium-dependent vasodilation is altered by dietary sodium intake based on sex, suggesting that endothelial NO production is sensitive to dietary sodium in healthy young men but not women.
Keywords: vascular endothelial function, nitric oxide, dietary sodium, acetylcholine
dietary sodium intake influences the pathogenesis and treatment of hypertension (12, 18). Endothelial dysfunction—abnormalities in the regulatory functions of vascular endothelium—is one of the earliest detectable stages in the pathophysiology of atherosclerosis, arterial stiffening, and hypertension. Even in healthy individuals, endothelial dysfunction occurs earlier in men than in women (2). A hallmark of endothelial dysfunction is reduced endotheliumdependent vasodilation, which is commonly quantified by the vasodilator response to pharmacological administration of acetylcholine (ACh).
Acetylcholine stimulates muscarinic cholinergic receptors on vascular endothelium to increase free intracellular calcium concentration, leading to mechanisms that promote endothelium-dependent relaxation of the underlying smooth muscle. The binding of calcium to calmodulin activates endothelial nitric oxide synthase (eNOS) to generate production of nitric oxide (NO) (28). Another mechanism is the production of prostacyclin (PGI2) via metabolism of arachidonic acid by cyclooxygenase (1). Additional pathways being elucidated involve endothelium-derived hyperpolarizing factors (EDHF) and activation of calcium-dependent potassium channels (20). The endothelial NO component of ACh-induced vasodilation in vivo is estimated by the difference in ACh vasodilator responsiveness before and after competitive inhibition of eNOS with NG-monomethyl-l-arginine (l-NMMA). Multiple investigations incorporating this experimental paradigm in healthy humans suggest that the contribution of NO to ACh-induced vasodilation ranges from 30% to 50% (7, 9, 11, 20, 23).
One mechanism responsible for endothelial dysfunction is decreased bioavailability of NO. Recent evidence suggests that supplemental salt tablets decrease endothelial NO in healthy, normotensive young men (27). Conversely, blood pressure regulation in normotensive young women is generally insensitive to salt (21). A potential explanation for sex differences in salt sensitivity and vascular function is endothelial NO, but this idea has not been explored. Therefore, the purpose of this study was to test the hypothesis that the endothelial NO component of ACh-evoked forearm vasodilation based on low and high dietary sodium intake is influenced by sex. By measuring the vasodilator response to ACh before and immediately after inhibition of eNOS with l-NMMA, we were able to quantify the effect of high and low dietary sodium intake on the NO component of endothelium-mediated vasodilation.
METHODS
Subjects.
Protocol 1 (low sodium) and protocol 2 (high sodium) were conducted in accordance with the Declaration of Helsinki and were approved by the Mayo Institutional Review Board. Both protocols were subsets of a larger investigation focusing on adrenergic receptor gene variation, dietary sodium intake, and cardiovascular control. Subjects were recruited based on common single-nucleotide polymorphisms in the beta-2 adrenergic receptor gene (Arg16/Gly) to explore the influence of gene variation on forearm beta-2 adrenergic receptor-mediated vasodilation. Measures in this report include forearm vasodilator responses to agents not mediated by adrenergic receptors (ACh and nitroprusside, NTP). In protocol 1, a separate analysis has been published without respect to sex (8), and for the present analysis vasodilator responses to ACh and NTP were available from 14 women and 16 men. From protocol 2, similar data were available for 23 women and 13 men. Genotype breakdown was not different between sexes and protocol groups, and no subject participated in both protocols.
Each participant gave written informed consent. Men were under age 40, women were under age 50 and premenopausal, and neither had a history of tobacco use or any acute or chronic disorder associated with alterations in cardiovascular structure or function. Enrollment screening included a physical examination and resting blood pressure which was measured by manual random zero sphygmomanometry in protocol 1 and automated oscillometry in protocol 2. Females were required to have a negative pregnancy test and were studied during the low-hormone phase of the menstrual cycle or oral contraceptives to minimize the influence of progesterone and high levels of estrogen on renal and systemic parameters (21).
Diets.
In protocol 1, subjects consumed a 5-day prestudy diet containing 10 mmol of sodium (0.23 g Na; 0.6 g salt) per day from the Mayo CTSA Clinical Research Unit (CRU) (8). Food items provided constant daily amounts of protein (1.4 g·kg body wt−1·day−1), potassium (100 mmol/day), and calcium (1,100 mg/ day). The caloric content of the diet was adjusted using the Harris Benedict equation to maintain constant body weight, and no more than 35% of calories were provided by fat. On day 5, a 24-h urine collection was obtained for measurement of sodium, potassium, and creatinine excretion. On the morning of study subjects remained fasting until the study measurements were completed.
In protocol 2, subjects consumed a 5-day prestudy diet in the exact fashion as protocol 1 except subjects received 400 mmol of sodium per day (9.2 g Na; 23 g salt). Similarly, a 24-h urine collection was obtained and subjects fasted until completion of the study.
Measurements, analysis, and statistics.
Subjects were positioned supine and a 20-gauge brachial arterial catheter was placed, under local anesthesia, in the nondominant arm for blood pressure (BP) measurement and drug infusions. FBF was measured with venous occlusion plethysmography as previously described (8). After baseline recording of FBF for 2 min, ACh was administered at a rate 4.0 μg·100 ml forearm tissue−1·min−1 for at least 2 min until steady-state FBF was reached. After 10 min and return of FBF to baseline levels, a bolus dose of l-NMMA (50 mg) was infused over 10 min, followed by a “maintenance” dose of l-NMMA (1 mg/min). The dose of l-NMMA was consistent with previous forearm investigations of eNOS inhibition (5–7, 9, 20, 27). Because l-NMMA is a competitive inhibitor of eNOS and the measurement of NO inhibition in intact human regional models is problematic, the complete inhibition of eNOS in this study can only be inferred based on the large dose of l-NMMA administered into the forearm relative to systemic infusions (3, 13, 25). Importantly, the dose of ACh was based on forearm volume, and was identical in the dose and time infused immediately before and after l-NMMA infusion. The baseline recording and ACh infusion was repeated in the presence of l-NMMA. To determine an effect of dietary sodium on endothelium-independent vasodilation, NTP was administered for 2 min at 1.0 μg·100 ml tissue−1·min−1 both before and after the l-NMMA. Subject characteristics were summarized by calculating means and standard error of the mean (SE) for continuous variables and proportions for categorical variables, then compared between low- and high-sodium study cohorts using ANOVA for continuous variables, and Fisher's exact test for categorical traits. Subsequent analyses were performed to assess whether sodium effects were dependent on sex, with explanatory variables including sodium, sex, and the sodium-by-sex interaction. Based on a previous report that sodium loading attenuates endothelial NO in men (27), analyses of sodium were conducted using one-tailed tests.
RESULTS
As shown in Table 1, the age of the low-sodium cohort was greater, prediet screening SBP was lower, and prediet screening DBP tended to be higher than the high-sodium cohort. Because prediet blood pressure was measured manually by random zero sphygmomanometry in protocol 1, and by computer-automated oscillometric cuff in protocol 2, the screening blood pressure discrepancies were likely an effect of the screening method of measurement because the prediet MAP values were similar between groups. Furthermore, compared with before the diet, resting HR or BP was not affected by diet in either cohort. As expected, there was a significant effect of dietary sodium intake on the 24-h urine volume and sodium excretion. Potassium excretion was increased in the low- vs. high-sodium cohort. Interestingly, this was dependent on sex, as potassium excretion in men was 103 ± 4.3 and 76 ± 5 mmol in the low- and high-sodium cohorts, respectively (P < 0.001). Potassium excretion in women was 77.5 ± 6 and 77.4 ± 5 mmol in the low- and high-sodium cohorts (P = 0.99; P = 0.02, sex-by-sodium interaction).
Table 1.
Subject characteristics
| Dietary Condition |
|||
|---|---|---|---|
| Characteristic | Low Sodium (n = 30) | High Sodium (n = 36) | P value |
| Sex, F/M | 14/16 | 23/13 | 0.2 |
| Age | 30 ± 1 | 23 ± 1 | <0.001 |
| BMI, kg/m2 | 23 ± 0.5 | 24 ± 0.5 | 0.6 |
| Height, cm | 173 ± 2 | 174 ± 1 | 0.9 |
| Screening wt, kg | 70 ± 2 | 72 ± 2 | 0.6 |
| Δwt, kg | −0.2 ± 0.1 | 0.5 ± 0.3 | 0.01 |
| Screening HR, beats/min | 67 ± 1 | 68 ± 2 | 0.5 |
| Δ HR, beats/min | 0.7 ± 2 | −0.6 ± 2 | 0.7 |
| Screening SBP, mmHg | 105 ± 2 | 121 ± 2 | <0.001 |
| Δ SBP, mmHg | −4 ± 2 | −4 ± 2 | 0.5 |
| Screening DBP, mmHg | 70 ± 1 | 67 ± 1 | 0.1 |
| Δ DBP, mmHg | −0.2 ± 2 | 0.1 ± 2 | 0.4 |
| Screening MAP, mmHg | 82 ± 2 | 85 ± 1 | 0.1 |
| Δ MAP, mmHg | −2 ± 1 | −1 ± 1 | 0.4 |
| 24-h urine volume, ml | 1841 ± 128 | 2535 ± 291 | 0.02 |
| 24-h Na+ excretion, mmol | 31 ± 3 | 311 ± 18 | <0.001 |
| 24-h K+ excretion, mmol | 91 ± 4 | 77 ± 4 | 0.008 |
| 24-h Cr excretion, mmol | 1698 ± 83 | 1538 ± 105 | 0.12 |
Values are means ± SE. Hemodynamic values were recorded during a screening visit prior to beginning the 5-day diet. Following the diet, the changes in vital signs from the screen visit are listed as Δ. Urinary indexes were obtained from a 24-h urine collection on the final day of the diets. BMI, body mass index; HR, heart rate, SBP, systolic blood pressure; DBP, diastolic blood pressure.
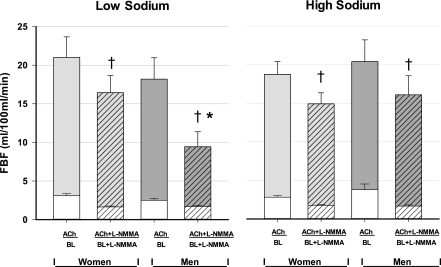
For all subjects in the low-sodium cohort, ACh increased FBF from 2.8 ± 0.2 at baseline to 19.5 ± 1.9 ml·100 ml forearm tissue−1·min−1 (P < 0.001). l-NMMA decreased resting FBF to 1.7 ± 0.1 (P < 0.001 from baseline); then ACh increased FBF to 12.7 ± 1.6 (P < 0.001). l-NMMA produced a significant reduction of the vasodilator response to ACh (P < 0.001, pre- vs. post-l-NMMA). For the high-sodium cohort, ACh increased FBF from 3.2 ± 0.3 at baseline to 19.3 ± 1.4 ml·100 ml forearm tissue−1·min−1 (P < 0.001). l-NMMA decreased resting FBF to 1.7 ± 0.1 (P < 0.001 from baseline); then ACh increased FBF to 15.3 ± 1.3 ml·100 ml forearm tissue−1·min−1 (P < 0.001). l-NMMA produced a significant reduction of the vasodilator response to ACh (P < 0.001, pre- vs. post-l-NMMA). These values were further delineated by sex and depicted in Fig. 1.
Fig. 1.
Mean forearm blood flow (FBF) values at baseline (BL) and in response to acetylcholine (ACh) before and after inhibition of endothelial nitric oxide synthase with l-NMMA. The response to ACh before l-NMMA was not affected by dietary sodium in either sex. l-NMMA significantly reduced baseline FBF (BL + l-NMMA) in both sexes and both sodium diets. l-NMMA blunted the FBF response to ACh (ACh + l-NMMA) in all conditions (†P < 0.001), with a greater blunting in men in the low-sodium cohort (*P = 0.034, sex-by-sodium interaction). Error bars denote standard error of the mean (SE).
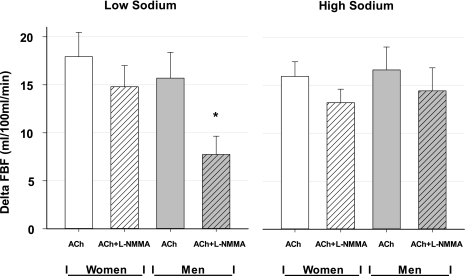
We quantified the NO component of ACh-evoked vasodilation, accounting for the shift in baseline FBF caused by l-NMMA within each sodium condition, using the following formula: [(FBF ACh − FBF baseline) − (FBF AChL-NMMA − FBF baselineL-NMMA)]. For all subjects pooled across sodium conditions, this value was 5.7 ± 1.3 and 2.5 ± 0.8 ml·100 ml forearm tissue−1·min−1 in the low- and high-sodium diets, respectively (main effect of sodium, P = 0.019). However, the sodium effect was larger for the men, with values of 7.9 ± 2.0 and 2.2 ± 1.4 for men vs. 3.1 ± 1.3 and 2.7 ± 1.0 ml·100 ml forearm tissue−1·min−1 for the women (P = 0.034, sex-by-sodium interaction). As shown in Fig. 2, for men in the low-sodium condition, l-NMMA caused a significant blunting in the vasodilator response to ACh compared with men in the high-sodium diet, or compared with women in either diet. Finally, infusions of ACh and l-NMMA into the brachial artery did not evoke a systemic effect on hemodynamics, as HR and BP were unaffected by the forearm infusions in either dietary sodium group (data not shown).
Fig. 2.
Mean change in forearm blood flow (FBF) from baseline values immediately preceding ACh infusion before and after concomitant infusion with l-NMMA, in women and men in the low-sodium (left panel) and high-sodium (right panel) cohorts. l-NMMA caused a significant decrease in the FBF response to ACh in both sexes and diets. *The NO component of ACh-induced vasodilation was significant for a sex-by-sodium interaction (P = 0.034). Error bars denote standard error of the mean (SE).
For endothelium-independent vasodilation in the low-sodium cohort, NTP increased FBF from 2.1 ± 0.1 to 15.1 ± 0.9; after l-NMMA, NTP increased FBF from 2.1 ± 0.1 to 15.9 ± 0.9 ml·100 ml forearm tissue−1·min−1. In the high sodium cohort NTP increased FBF from 2.2 ± 0.2 to 15.8 ± 0.8; after l-NMMA NTP increased FBF from 1.7 ± 0.1 to 16.2 ± 0.9 ml·100 ml forearm tissue−1·min−1 (P < 0.001 for all). There was no evidence to suggest that the responses to NTP were dependent on dietary sodium or sex.
DISCUSSION
This is the first report to compare endothelium-dependent vasodilation in healthy normotensive young men and women following a controlled 5-day diet consisting of either low or high dietary sodium. There were three major findings. First, the significant vasodilator response to ACh (before eNOS inhibition with l-NMMA) was not affected by dietary sodium in either sex. Second, following inhibition of eNOS, the magnitude of the reduction in the vasodilator response to ACh was significantly affected by diet in the men but not the women. Compared with the low-sodium diet, high dietary sodium reduced the ability of l-NMMA to blunt the vasodilator response, indicating that the NO component of ACh-mediated vasodilation was reduced after high sodium intake in the men. Finally, there were no sex differences in the endothelial NO component of vasodilation after high sodium intake. Therefore, the overall vasodilator response to ACh is not affected by extremes of dietary sodium in this healthy population, but there are sex differences in the mechanisms responsible for how vasodilation is achieved that are influenced by dietary sodium.
Endothelium-dependent vasodilation involves several mechanisms. ACh evokes increases in endothelial generation of NO, EDHF, and PGI2, and each component is likely modulated by dietary sodium (17). In male Sprague-Dawley rats fed a high-salt diet (8%) for 4 wk vs. those fed a low-salt diet (0.4%), mesenteric vessels displayed a similar vasodilator response to ACh but the mechanism appeared to shift from endothelium-derived NO in the low-salt condition to endothelium-derived EDHF in the high-salt condition (24). Moreover, in mice lacking the PGI2 receptor gene, sodium loading increased blood pressure and increased the urinary excretion of prostacyclin metabolites (29). Taken together, these animal models suggest that sodium loading causes a reduction in endothelial NO with a compensatory increase in EDHF and PGI2, which appears consistent with our findings in men.
A recent randomized double-blind crossover study of 16 healthy normotensive young men receiving a 5-day course of placebo or salt tablets (200 mmol/day) demonstrated that the forearm vasodilator response to brachial arterial administration of ACh was blunted after salt loading; moreover, the vasoconstrictor response to incremental doses of l-NMMA was less pronounced after salt loading, indicating a reduction in endothelial levels of NO (27). Our data are consistent with a reduction in endothelial NO, but we were unable to demonstrate an effect of dietary sodium on the vasodilator response to ACh (before eNOS inhibition), which may be due to protocol differences such as controlled dietary potassium intake (19), and may also represent the aforementioned redundant components of ACh-evoked vasodilation.
Sex differences in our findings may be interpreted in several ways. First, men may have a greater capacity to increase NO bioavailability during sodium restriction compared with high sodium intake, suggesting that improvements in endothelial NO from dietary sodium restriction may be achieved to a greater extent in men than women. Second, endothelial NO in healthy women may be less responsive to extremes of dietary sodium intake. Taken together, this would suggest that women have alternative means of endothelium-dependent vasodilation that are less dependent on NO compared with men. Indeed, serum NO metabolite levels are reportedly greater in young healthy men than women (10), supporting the idea of sex differences in NO regulation.
An additional interpretation may relate to the sex differences in potassium excretion. Dietary sodium restriction activates the renin-angiotensin system, which increases potassium excretion compared with sodium loading (22). This effect was seen in men only, with a significantly elevated potassium excretion during low sodium compared with men in high sodium or in women of either dietary cohort. Normotensive men have higher renin activity than normotensive women at any age (16). Whether potassium balance directly affected endothelial NO in men during low sodium is speculative, as linear regression analysis revealed no suggestive correlation between urinary potassium excretion and the NO component of endothelium-dependent vasodilation.
The Dietary Approaches to Stop Hypertension (DASH) eating plan includes a recommended daily sodium intake of 1.5 to 2.3 g per day. Recently, Hodson and colleagues administered a 30-day DASH diet to 14 middle-aged adults (8 men, 6 women) and found a reduction in BP but no change in brachial artery flow-mediated dilation compared with matched controls (14). While that study may have been underpowered to detect a diet effect flow-mediated dilation (FMD) in both sexes, our findings suggest that exploration of a sex-by-sodium interaction specifically targeting the contribution of NO to vasodilation may be necessary to provide mechanistic insight into the reduction in BP following reduced dietary sodium regimens such as DASH. Importantly, in our cohorts resting BP was not changed after sodium loading or restriction, an effect that was likely due to the shorter duration of the study diet or the notion that subjects were young and free of disorders or lifestyle habits that affect cardiovascular and renal function. Furthermore, potassium supplementation to levels similar to what we provided our subjects has been shown to essentially eliminate salt sensitivity of blood pressure in normotensive black and white men (19). Conversely, a low-sodium diet improves markers of endothelial function in normotensive overweight and obese normotensive adults (4) and hypertensive adults (15), whereas high-sodium diet impairs vascular function (pulse wave velocity) in hypertensive adults (26). Few if any studies have addressed sex differences in these physiological markers.
The limitations of this report center on the comparison of data obtained in a present cohort (high sodium) with data from a separate cohort (low sodium) using similar methodology except for dietary sodium intake. However, the present findings clearly raise important questions about the interaction of dietary sodium, sex, and endothelial function that warrant detailed, prospective trials within individuals of both sexes to further elucidate how dietary sodium alters vascular function in humans.
Conclusions.
In summary, this is the first report of a sex-by-dietary sodium interaction with regard to the NO component of forearm vasodilator mechanisms in healthy young men and women. From a population standpoint, men have higher blood pressure than age-matched premenopausal women, putting men at greater risk of cardiovascular and renal disease earlier in life. Furthermore, blood pressure in young normotensive women is generally less sensitive to salt (21). In this context, our findings raise important new questions about sex differences in salt sensitivity and vascular phenotypes in healthy young adults.
GRANTS
This work was supported by National Institutes of Health Grants U01-HL-54464, HL-63328, M01-RR-00585, CTSA-RR-024150, NCRR-K23-17520, and HL-89331.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.H.E., M.J.J., S.T.T., and W.T.N. conception and design of research; J.H.E., L.R.G., S.L.K., M.J.J., and W.T.N. performed experiments; J.H.E., L.R.G., and S.L.K. analyzed data; J.H.E., L.R.G., S.L.K., M.J.J., S.T.T., and W.T.N. interpreted results of experiments; J.H.E., L.R.G., and S.L.K. prepared figures; J.H.E. and L.R.G. drafted manuscript; J.H.E., M.J.J., S.T.T., and W.T.N. edited and revised manuscript; J.H.E., L.R.G., S.L.K., M.J.J., S.T.T., and W.T.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the study volunteers for their participation. We also thank Jean Knutson, Rachel Elvebak, Christopher Johnson, Pam Engrav, Karen Krucker, and Shelly Roberts for their technical assistance.
This work was presented orally in “Endothelium, Vascular Tone, and Nitric Oxide I” at American Heart Association Scientific Sessions, November 2011.
REFERENCES
- 1. Bulut D, Liaghat S, Hanefeld C, Koll R, Miebach T, Mugge A. Selective cyclo-oxygenase-2 inhibition with parecoxib acutely impairs endothelium-dependent vasodilatation in patients with essential hypertension. J Hypertens 21: 1663–1667, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol 291: H1378–H1383, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89: 485–490, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Dietz NM, Engelke KA, Samuel TT, Fix RT, Joyner MJ. Evidence for nitric oxide-mediated sympathetic forearm vasodilatation in humans. J Physiol 498: 531–540, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol 92: 2019–2025, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Eisenach JH, Schroeder DR, Pike TL, Johnson CP, Schrage WG, Snyder EM, Johnson BD, Garovic VD, Turner ST, Joyner MJ. Dietary sodium restriction and beta2-adrenergic receptor polymorphism modulate cardiovascular function in humans. J Physiol 574: 955–965, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garovic VD, Joyner MJ, Dietz NM, Boerwinkle E, Turner ST. Beta(2)-adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J Physiol 546: 583–589, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghasemi A, Zahedi Asl S, Mehrabi Y, Saadat N, Azizi F. Serum nitric oxide metabolite levels in a general healthy population: relation to sex and age. Life Sci 83: 326–331, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Gilligan DM, Guetta V, Panza JA, Garcia CE, Quyyumi AA, Cannon RO., 3rd Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation 90: 35–41, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol 89: 1830–1836, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Hodson L, Harnden KE, Roberts R, Dennis AL, Frayn KN. Does the DASH diet lower blood pressure by altering peripheral vascular function? J Hum Hypertens 24: 312–319, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovas Dis 3: 347–356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. James GD, Sealey JE, Muller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl 4: S387–S389, 1986 [PubMed] [Google Scholar]
- 17. Katusic ZS. Back to the salt mines—endothelial dysfunction in hypertension and compensatory role of endothelium-derived hyperpolarizing factor (EDHF). J Physiol 543: 1, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Morris RC, Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt sensitivity: effects of race and dietary potassium. Hypertension 33: 18–23, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation 123: 2244–2253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens 17: 994–1001, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension 54: 475–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol 98: 1251–1257, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Sofola OA, Knill A, Hainsworth R, Drinkhill M. Change in endothelial function in mesenteric arteries of Sprague-Dawley rats fed a high salt diet. J Physiol 543: 255–260, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation 89: 2035–2040, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, Mann JI, Walker RJ. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr 91: 557–564, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 997–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Watanabe H, Katoh T, Eiro M, Iwamoto M, Ushikubi F, Narumiya S, Watanabe T. Effects of salt loading on blood pressure in mice lacking the prostanoid receptor gene. Circ J 69: 124–126, 2005 [DOI] [PubMed] [Google Scholar]