Abstract
Endurance training-induced changes in hemodynamic traits are heritable. However, few genes associated with heart rate training responses have been identified. The purpose of our study was to perform a genome-wide association study to uncover DNA sequence variants associated with submaximal exercise heart rate training responses in the HERITAGE Family Study. Heart rate was measured during steady-state exercise at 50 W (HR50) on 2 separate days before and after a 20-wk endurance training program in 483 white subjects from 99 families. Illumina HumanCNV370-Quad v3.0 BeadChips were genotyped using the Illumina BeadStation 500GX platform. After quality control procedures, 320,000 single-nucleotide polymorphisms (SNPs) were available for the genome-wide association study analyses, which were performed using the MERLIN software package (single-SNP analyses and conditional heritability tests) and standard regression models (multivariate analyses). The strongest associations for HR50 training response adjusted for age, sex, body mass index, and baseline HR50 were detected with SNPs at the YWHAQ locus on chromosome 2p25 (P = 8.1 × 10−7), the RBPMS locus on chromosome 8p12 (P = 3.8 × 10−6), and the CREB1 locus on chromosome 2q34 (P = 1.6 × 10−5). In addition, 37 other SNPs showed P values <9.9 × 10−5. After removal of redundant SNPs, the 10 most significant SNPs explained 35.9% of the ΔHR50 variance in a multivariate regression model. Conditional heritability tests showed that nine of these SNPs (all intragenic) accounted for 100% of the ΔHR50 heritability. Our results indicate that SNPs in nine genes related to cardiomyocyte and neuronal functions, as well as cardiac memory formation, fully account for the heritability of the submaximal heart rate training response.
Keywords: genome-wide association study, genotype
physically active lifestyle is a central contributor to optimal heart health. Physically active individuals have considerably lower risks of cardiovascular disease morbidity and mortality than their sedentary counterparts, and this cardioprotection is usually credited to improvements in traditional cardiovascular disease risk factors. However, regular physical activity also induces beneficial changes in cardiac function. For example, a physically active individual can perform the same amount of physical work with less strain on the heart (as evidenced by lower heart rate and blood pressure during a given work output) than a sedentary individual.
Heart rate is commonly used as a physiological indicator of general cardiac function in medicine and exercise physiology. Heart rate is usually considered a consequence, rather than a cause, of a given (patho)physiological condition. However, data from animal studies suggest that modification of heart rate may also have direct health benefits that are independent of traditional cardiovascular risk factors. In cynomolgus monkeys, lowering of heart rate by sinoatrial node ablation slowed or even prevented high-fat diet-induced atherosclerotic changes in coronary arteries (7) and bifurcation of the carotid artery (6). A mean reduction of 15% in ambulatory heart rate, while other cardiovascular risk factors (blood pressure, serum cholesterol and triglycerides, and body weight) remained unchanged, was associated with 56% smaller lesion area and 53% lower stenosis in coronary arteries, while in carotid bifurcation the values were 62% for lesion area and 50% for stenosis (6, 7).
The most effective nonpharmacological strategy to lower heart rate, at rest and during submaximal physical work, is exercise training. We previously reported in the HERITAGE Family Study that 20 wk of regular exercise training induces an average reduction of 11 beats/min in heart rate measured during steady-state submaximal exercise at 50 W (HR50), while individual HR50 training responses (ΔHR50) ranged from a 12 beat/min increase to a 42 beat/min decrease (32). We also showed previously that ΔHR50 aggregates in families: maximal heritability estimate of age, sex, body mass index, and baseline HR50-adjusted ΔHR50 reached 34% in white HERITAGE families (4). Furthermore, complex segregation analysis supported the hypothesis of a major dominant gene effect on ΔHR50 in the same set of families (3). A genome-wide linkage scan identified a promising quantitative trait locus for ΔHR50 on chromosome 2q34, with an LOD score of 2.10 (28). A fine mapping study of the quantitative trait locus identified cAMP-responsive element-binding protein 1 (CREB1) as the strongest contributor to the linkage signal (23).
While a CREB1 DNA sequence variant explained >4% of the variation in ΔHR50, a considerable portion of the genetic variance remained unaccounted for (23). To identify additional gene loci contributing to ΔHR50 variance, we performed a genome-wide association study (GWAS) with >300,000 single-nucleotide polymorphisms (SNPs). Here we report that nine of these SNPs, including CREB1, accounted for 34% of the total variance in HR50 training response and 100% of ΔHR50 heritability.
MATERIALS AND METHODS
Subjects.
The HERITAGE Family Study design, inclusion criteria, and protocol are described elsewhere (8). Complete training response data were available for 472 white subjects (229 men and 243 women) from 99 nuclear families. All subjects were healthy and sedentary at baseline. Sedentary was defined as no regular physical activity over the previous 6 mo. The study protocol had been approved by the Institutional Review Board at each of the five participating centers of the HERITAGE Family Study consortium. Written informed consent was obtained from each participant.
Exercise training program.
Subjects completed a 20-wk endurance training program (3 days/wk for a total of 60 exercise sessions) using Universal Aerobicycles (Cedar Rapids, IA), which were monitored electronically by the FitNet system to maintain the participants' heart rates at levels associated with fixed percentages of their maximal O2 uptake (V̇o2max). The training program started at the heart rate associated with 55% of V̇o2max for 30 min per session and gradually increased to 75% of V̇o2max for 50 min per session during the last 6 wk of training. All training sessions were supervised on site, and adherence to the protocol was strictly monitored (27).
Submaximal exercise test.
Before and after the 20-wk training program, each subject completed two submaximal exercise tests on a cycle ergometer on separate days. Subjects exercised for 8–12 min at an absolute workload of 50 W. Heart rate was monitored throughout the test with an electrocardiogram, and two heart rate values were recorded once steady state had been achieved. The heart rate values used in this study represent in each case the mean of two submaximal tests at 50 W (HR50), before and after training. The exercise test methodology is described elsewhere (33). The reproducibility of the HR50 measurements was very high: 4.7% coefficient of variation and 0.90 intraclass correlation among the subjects used for the GWAS analyses (33).
GWAS SNP genotyping.
Genomic DNA was prepared from immortalized lymphoblastoid cell lines by a commercial DNA extraction kit (Gentra Systems, Minneapolis, MN). GWAS SNPs were genotyped using Illumina HumanCNV370-Quad v3.0 BeadChips on the Illumina BeadStation 500GX platform. The genotype calls were done with Illumina GenomeStudio software, and all samples were called in the same batch to eliminate batch-to-batch variation. All GenomeStudio genotype calls with a GenTrain score <0.885 were checked and confirmed manually. Monomorphic SNPs and SNPs with only one heterozygote, as well as SNPs with >30% missing data, were filtered out with GenomeStudio. Quality control of the GWAS SNP data confirmed all family relationships and found no evidence of DNA sample mix-ups. Of the 334,207 SNPs, only 78 (0.023%) had >10% missing data, and none of the SNPs had a missing rate >25%. Minor allele frequency was <1% for 1,301 SNPs (0.39%). Hardy-Weinberg equilibrium test P values were <10−5 and 10−6 for 55 (0.017%) and 12 (0.0037%) SNPs, respectively. Twelve samples were genotyped in duplicate with 100% reproducibility.
Statistical analyses.
Associations between the GWAS SNPs and ΔHR50 were analyzed using the MERLIN software package (1). The total association model of MERLIN utilizes a variance-components framework to combine phenotypic means model and estimates of additive genetic effect and residual genetic and environmental variances from a variance-covariance matrix into a single likelihood model. The evidence of association is evaluated by maximization of the likelihoods under two conditions: the null hypothesis (L0) restricts the additive genetic effect of the marker locus (βa) to zero, whereas the alternative hypothesis (L1) does not impose restrictions on βa. The quantity of twice the difference of the log likelihoods between the alternative and the null hypotheses {−2[ln(L1) − ln(L0)]} is distributed as χ2 with 1 degree of freedom (difference in number of parameters estimated).
The overall contribution of the most significant GWAS SNPs on ΔHR50 was evaluated using multivariate regression procedures. All GWAS SNPs with P ≤ 9.9 × 10−5 were included. First, a regression model with backward elimination was used to filter out redundant SNPs (mainly due to strong pair-wise linkage disequilibrium). The threshold for keeping the SNPs in the model was P = 0.05. Next, the SNPs that were retained in the final backward-elimination model were analyzed with a multivariate regression model using forward selection.
Whether the most significant SNPs from the regression model contributed to the heritability of the HR50 training response was tested using conditional heritability analysis. If a SNP contributes to the genetic variance (heritability) of a trait, the maximal heritability estimate should decrease when the marker is included as a covariate in the model. Starting with the SNP showing the greatest partial R2, the SNPs were added one at a time in the heritability model until the H2 estimate reached zero [heritability fully accounted for by the covariates (SNPs)]. The conditional heritability analyses were performed with the MERLIN software package (1).
RESULTS
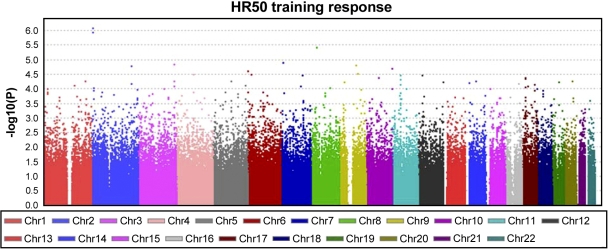
An overview of the ΔHR50 GWAS results across the 22 autosomes is presented in Fig. 1. The strongest single-SNP associations were detected with SNPs rs6432018 (P = 8.1 × 10−7, R2 = 5.8%) and rs12692388 (P = 1.19 × 10−6), located on chromosome 2p25 in the tyrosine 3-monooxygenase/tryptophan 5-monooxygenase-activating protein, θ polypeptide (YWHAQ) gene locus. In addition, SNP rs2979481 (RBPMS locus on chromosome 8p12) showed an association with P = 4.0 × 10−6. Furthermore, 37 additional SNPs were associated with ΔHR50 with P < 9.9 × 10−5 (Table 1).
Fig. 1.
Manhattan plot of heart rate measured during steady-state exercise at 50 W (HR50) training response genome-wide association study (GWAS) results across the 22 autosomes.
Table 1.
Summary of the most significant SNP associations with HR50 training response in the HERITAGE Family Study
| SNP | Chromosomes | Map | Frequency of Common Allele | H2 | P Value | Nearest Gene Locus* |
|---|---|---|---|---|---|---|
| rs10732279 | 1 | 20,177,652 | 0.744 | 4.03 | 0.000097 | PLA2G2A |
| rs857838 | 1 | 157,017,174 | 0.6 | 3.92 | 0.000076 | OR6N2 |
| rs1832544 | 1 | 216,200,160 | 0.615 | 4.15 | 0.000055 | SPATA17 (93 kb) |
| rs6432018 | 2 | 9,639,347 | 0.524 | 5.84 | 8.08 × 10−7 | YWHAQ |
| rs12692388 | 2 | 9,671,920 | 0.509 | 5.65 | 1.19 × 10−6 | YWHAQ |
| rs3791749 | 2 | 10,057,215 | 0.594 | 3.72 | 0.000095 | GRHL1 |
| rs2360969 | 2 | 208,081,241 | 0.557 | 4.17 | 0.000066 | CREB1 |
| rs2253206 | 2 | 208,100,223 | 0.522 | 4.90 | 0.000016 | CREB1 |
| rs9869355 | 3 | 188,388,480 | 0.572 | 3.96 | 0.000080 | RTP1 |
| rs6444210 | 3 | 188,393,353 | 0.552 | 4.01 | 0.000055 | RTP1 |
| rs9872701 | 3 | 188,404,642 | 0.526 | 4.77 | 0.000015 | RTP1 |
| rs1560488 | 4 | 90,444,858 | 0.791 | 4.55 | 0.000033 | GPRIN3 |
| rs6865159 | 5 | 95,473,750 | 0.677 | 3.95 | 0.000053 | MIR538 (33 kb) |
| rs2270895 | 5 | 169,400,821 | 0.551 | 4.09 | 0.000079 | DOCK2 |
| rs9378283 | 6 | 1,166,578 | 0.698 | 4.60 | 0.000024 | FOXQ1 (90 kb) |
| rs909562 | 6 | 16,238,312 | 0.875 | 3.99 | 0.000032 | MYLIP |
| rs12672644 | 7 | 15,096,677 | 0.901 | 4.74 | 0.000012 | TMEM195 (110 kb) |
| rs7792872 | 7 | 105,985,048 | 0.763 | 4.01 | 0.000082 | C7orf74 (105 kb) |
| rs10248479 | 7 | 115,395,591 | 0.876 | 4.40 | 0.000034 | TFEC |
| rs2979481 | 8 | 30,382,328 | 0.645 | 5.22 | 3.75 × 10−6 | RBPMS |
| rs10099863 | 8 | 98,943,869 | 0.834 | 4.02 | 0.000094 | MATN2 (6.5 kb) |
| rs4246861 | 9 | 25,589,995 | 0.785 | 3.84 | 0.000060 | TUSC1 (77 kb) |
| rs615189 | 9 | 86,886,974 | 0.517 | 4.59 | 0.000015 | NTRK2 (58 kb) |
| rs4498613 | 9 | 95,545,460 | 0.808 | 4.31 | 0.000031 | PHF2 (60 kb) |
| rs2252578 | 10 | 65,372,850 | 0.512 | 4.72 | 0.000042 | REEP3 (300 kb) |
| rs7893895 | 10 | 133,084,400 | 0.675 | 4.45 | 0.000021 | TCERG1l (85 kb) |
| rs12789205 | 11 | 43,132,366 | 0.615 | 3.74 | 0.000070 | API5 (160 kb) |
| rs17508783 | 11 | 43,376,486 | 0.751 | 4.17 | 0.000035 | TTC17 |
| rs903514 | 11 | 44,308,970 | 0.581 | 4.06 | 0.000049 | ALX4 |
| rs2140892 | 11 | 73,100,527 | 0.779 | 3.62 | 0.000098 | RAB6A |
| rs7964046 | 12 | 20,169,533 | 0.574 | 4.09 | 0.000035 | PDE3A (240 kb) |
| rs4759659 | 12 | 129,403,241 | 0.538 | 4.02 | 0.000057 | PIWIL1 |
| rs11622895 | 14 | 20,384,840 | 0.624 | 3.97 | 0.000061 | RNASE1 (45 kb) |
| rs2057368 | 14 | 54,373,759 | 0.804 | 3.71 | 0.000056 | GCH1 |
| rs235987 | 16 | 69,806,847 | 0.727 | 3.59 | 0.000068 | HYDIN |
| rs3183702 | 17 | 17,688,014 | 0.642 | 3.72 | 0.000090 | TOM1L2 |
| rs854813 | 17 | 17,944,570 | 0.613 | 4.24 | 0.000042 | DRG2 |
| rs854762 | 17 | 17,949,827 | 0.611 | 4.20 | 0.000045 | DRG2 |
| rs938298 | 17 | 30,711,529 | 0.789 | 3.89 | 0.000084 | SLFN11 |
| rs8069419 | 17 | 66,505,986 | 0.795 | 3.80 | 0.000072 | LOC100131241 (14 kb) |
| rs1885831 | 20 | 40,836,321 | 0.885 | 4.16 | 0.000055 | PTPRT |
A genome-wide association study was conducted to determine the most significant (P < 9.9 × 10−5) single-nucleotide polymorphisms (SNPs) associated with heart rate during steady-state exercise at 50 W (HR50).
Values in parentheses indicate distance between the SNP and the nearest gene locus; if no distance is given, the SNP is located within the gene.
Next, all SNPs with P < 9.9 × 10−5 were included in a multivariate regression analysis. In addition, SNP rs2253206, which is missing from the CNV370K array, was included in the regression models; we previously showed that this SNP, which is located in the CREB1 gene locus, is strongly associated with the HR50 training response (23). After removal of redundant SNPs using the backward-elimination regression model, 30 SNPs were retained and analyzed with a multivariate regression model with forward selection. In the final model, six SNPs each explained ≥3% of the variance in ΔHR50 (range 3–6%), while another four SNPs contributed 2–3% each (Table 2). The full model of 10 SNPs explained 35.9% of the variance in the HR50 training response.
Table 2.
Results of the final HR50 training response regression model and conditional heritability analysis
| Regression Model |
||||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Chromosomes | Map | Frequency of Common Allele | Nearest Gene Locus* | Partial R2 | R2 model | P value | Remaining Heritability, % |
| rs2979481 | 8 | 30,382,328 | 0.645 | RBPMS | 0.0605 | 0.0605 | 8.1 × 10−8 | 24.6 |
| rs6432018 | 2 | 9,639,347 | 0.524 | YWHAQ | 0.0457 | 0.1062 | 5.0 × 10−7 | 20.0 |
| rs2253206 | 2 | 208,100,223 | 0.522 | CREB1 | 0.0447 | 0.1509 | 2.2 × 10−6 | 14.8 |
| rs1560488 | 4 | 90,444,858 | 0.791 | GPRIN3 | 0.0423 | 0.1932 | 2.4 × 10−6 | 8.6 |
| rs10248479 | 7 | 115,395,591 | 0.876 | TFEC | 0.0333 | 0.2264 | 1.1 × 10−5 | 6.8 |
| rs857838 | 1 | 157,017,174 | 0.60 | OR6N2 | 0.0302 | 0.2566 | 1.8 × 10−5 | 5.0 |
| rs909562 | 6 | 16,238,312 | 0.875 | MYLIP | 0.0296 | 0.2861 | 1.7 × 10−5 | 0.7 |
| rs4759659 | 12 | 129,403,241 | 0.538 | PIWIL1 | 0.0276 | 0.3138 | 2.3 × 10−5 | 1.6 |
| rs2057368 | 14 | 54,373,759 | 0.804 | GCH1 | 0.0238 | 0.3375 | 6.3 × 10−5 | 0 |
| rs4498613 | 9 | 95,545,460 | 0.808 | PHF2 (60 kb) | 0.0218 | 0.3593 | 0.0001 | NA |
Remaining heritability, estimate when a given SNP (plus preceding SNPs) is included as covariate(s) in the MERLIN heritability model; N/A, not applicable.
Values in parentheses indicate distance between the SNP and the nearest gene locus; if no distance is given, the SNP is located within the gene.
Given that the final regression model R2 of 35.9% is very close to the ΔHR50 maximal heritability estimate of 34%, we used conditional heritability analysis to determine if these 10 SNPs truly account for the genetic variance of ΔHR50 in white HERITAGE families. The SNPs were added to the MERLIN heritability model as covariates one at a time, starting with the most significant one, and the previously added SNPs were retained in the model when new variants were included. This procedure was repeated until the heritability estimate reached 0%. As shown in Table 2, nine SNPs were required to reduce the maximal heritability estimate to 0%. That is, these nine SNPs were able to account for all the heritability of ΔHR50 in white HERITAGE families.
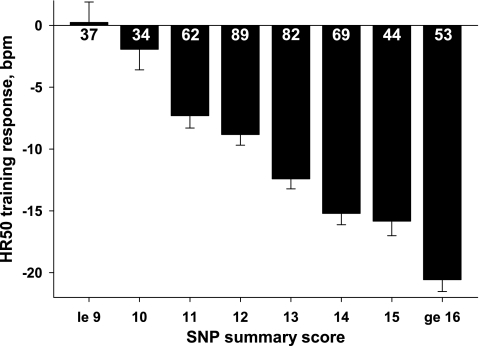
To illustrate the combined contribution of the 10 SNPs with a partial R2 >2%, a SNP summary score was constructed. Each SNP was recoded on the basis of the number of alleles associated with a favorable HR50 training response: homozygote = 2, heterozygote = 1, homozygote for nonfavorable allele = 0. The summary score was derived by summing the 10 recoded SNPs. The age, sex, baseline body mass index, and baseline HR50-adjusted training responses across the summary score categories are shown in Fig. 2. Subjects with a summary score ≤9 (8% of the subjects) showed no improvements in HR50, while HR50 decreased >20 beats/min in those with a summary score ≥16 (11% of the subjects).
Fig. 2.
Exercise training-induced changes in HR50 (adjusted for age, sex, body mass index, and baseline HR50) across 8 GWAS single-nucleotide polymorphism (SNP) summary score categories in white subjects in the HERITAGE Family Study. Number of subjects within each category is indicated inside or below each bar.
DISCUSSION
The main novel finding of the present study is that nine SNPs account for the heritability of the HR50 training response in white subjects of the HERITAGE Family Study. The contribution of the individual SNPs varied from 2% to 6%, and collectively these SNPs explained ∼36% of the variance in regular physical activity-induced changes in HR50.
The strongest evidence of association with ΔHR50 was found with SNPs located in the YWHAQ locus. YWHAQ (also known as 14-3-3τ/θ) is a member of the 14-3-3 family of signaling proteins involved in apoptosis, cell proliferation and metabolism, and check point control pathways (12). This highly conserved protein family is found in plants and mammals, and the human protein is 99% identical to the mouse and rat orthologs. YWHAQ is expressed in the heart and neurons (19). Mice carrying one copy of the cardiac-specific disrupted YWHAQ gene have been shown to be vulnerable to experimental myocardial infarction and to develop pathological ventricular remodeling with increased cardiomyocyte apoptosis (17), a phenotype very similar to that observed in mice with cardiac double-negative mutation of the 14-3-3 η (YWHAH) isoform (25, 30, 36). The η and ε (YWHAE) isoforms of 14-3-3 have been shown to interact with cardiac ion channels. The YWHAH isoform has been indicated to act as a cofactor of the cardiac Na+ channel, thereby regulating cardiac Na+ current (2), whereas the YWHAE isoform modulates cardiac function by amplifying and prolonging β-adrenergic stimulation of rapid outward K+ currents produced by the K+ channel hERG (9, 16, 29). However, it is unknown if the YWHAQ isoform has similar functions in regulation of myocardial contractility, although interactome studies have shown that YWHAQ does interact (as does YWHAE) with cardiac Na+/Ca2+ exchanger (SLC8A1), which is responsible for returning the cardiomyocyte to its resting state following excitation (22).
An interesting feature of our results is that the SNPs showing the strongest associations with the HR50 training response were located within a gene or in the immediate vicinity (<5 kb) of a gene. While none of the genes could be characterized as traditional heart rate candidate genes (e.g., adrenergic receptors), they do have physiological functions that are relevant to cardiovascular phenotypes. Four of the top nine SNPs are expressed in the heart and are related to neuron growth and degeneration. RBPMS has been shown to play a central role in nervous system development in several organisms, and, in Drosophila melanogaster, mutations in the RBPMS ortholog couch potato gene have been shown to cause various neurological abnormalities (13). In humans, RBPMS interacts with several ataxia-related proteins (e.g., ataxin 1) (18). YWHAQ plays a role in peripheral nerve injury and nerve regeneration, and its expression is upregulated in spinal cords of amyotrophic lateral sclerosis patients (19). GPRIN3 encodes a homolog of the G protein-regulated inducer of neurite outgrowth (15), while overexpression of MYLIP has been shown to inhibit nerve growth factor-driven neurite outgrowth in neuronal PC-12 cells (20). In addition, MYLIP has been shown to induce LDL receptor degradation by ubiquitination (35) and, thereby, affect plasma LDL-cholesterol levels (31, 35). Furthermore, CREB1 is a central component of memory formation in the heart (cardiac memory, i.e., altered electrocardiogram T waveform after ventricular pacing or arrhythmia) (21), as well as in the brain (24, 34). Finally, GCH1 encodes a rate-limiting enzyme of tetrahydrobiopterin synthesis. Tetrahydrobiopterin is an essential cofactor of nitric oxide synthases, and GCH1 mutations have been associated with dopamine-sensitive, as well as exercise-induced, dystonies (5, 11).
Functions of the remaining four genes are less well known, and available data are not sufficient to support or rule out their involvement in heart rate-related mechanisms. Transcription factor EC (TFEC) encodes a protein that belongs to a microphthalmia family of basic helix-loop-helix leucine zipper transcription factors (37). It has been shown to be a transcriptional activator of the nonmuscle myosin II heavy chain-A gene (10). PIWI-like 1 (PIWIL1) gene belongs to a PIWI subfamily of Argonaute proteins, which are involved in stem cell self-renewal, RNA silencing, and translational regulation (26), while PHD finger protein 2 (PHF2) is a plant homeodomain finger gene that functions as a transcriptional regulator of eukaryotic gene expression (14). Finally, OR6N2 encodes an olfactory receptor.
Our results may have physiological, as well as clinical and public health, implications, provided they can be confirmed in subsequent studies. These advances would illuminate the biology of heart rate regulation in response to exercise and perhaps other stressors. They would lead to the definition of molecular pathways and mechanisms by which these genes modify heart rate adaptation to regular exercise and could potentially uncover novel therapeutic targets for individuals who experience brady- or tachycardia. Moreover, heart rate is commonly used in exercise prescription to guide initial intensity level and to monitor progress in response to an exercise program, with an expectation that heart rate at a given work load will decrease substantially with exposure to regular exercise. However, in an individual with no or only a few alleles associated with a positive heart rate training response, chances are that the decrease in exercise heart rate will not occur. In this case, the physician and fitness specialist may conclude falsely that the participant was not compliant with the requirements of the program, or the specialist may assume that the exercise prescription was insufficient and may try to increase the exercise training load. In both cases, the specialist would be wrong. These misconceptions could be prevented by use of appropriate genetic markers to identify a priori the exercise heart rate high and low responders, which would result in a more individualized and more efficacious exercise prescription. In the future, this rationale could be extended to public health recommendations, as more personalized exercise recommendations could be developed as detailed information of one's genome through whole-genome sequencing applications becomes common.
Our study has several strengths, such as highly standardized submaximal exercise heart rate phenotypes, a fully controlled exercise training program with excellent compliance, and high-quality GWAS SNP data genotyped in a single laboratory, as well as a family study design that allowed us to directly examine the heritability of the HR50 training response. While our findings are new, several additional steps are necessary before one can claim with confidence that the SNPs and genes identified here are truly predictive of submaximal exercise heart rate training responses. The biggest challenge is to replicate these findings in other comparable cohorts; however, studies with similar exercise program and phenotype measurements with equal or larger sample size and similar ethnicity simply do not exist. Also, we cannot rule out the possibility that there are additional DNA sequence variants (e.g., rare or common SNPs, insertions or deletions, and copy number variants) not captured by the CNV370K array that may have even greater effect(s) on ΔHR50 than the SNPs reported here. Finally, it remains to be seen if the contribution of the SNPs and genes reported here is similar for ΔHR50 in other types of exercise training programs (e.g., greater intensity and exercise session frequency and resistance or interval training) or programs of longer duration. These questions remain to be explored in future studies.
In summary, our data indicate that multiple genes contribute to genetic variation in the adaptation of submaximal exercise heart rate to regular physical activity: in white HERITAGE families, nine SNPs accounted for 34% of the total variance in the HR50 training response and 100% of the ΔHR50 heritability. The genes identified by our GWAS have potential relevance to myocardial and neuronal function. However, additional studies are needed to confirm these findings and to fully understand the functional significance of these genes and DNA sequence variants and their role in the cardiac responsiveness to regular exercise.
GRANTS
The HERITAGE Family Study is supported by National Heart, Lung, and Blood Institute Grant HL-45670 (T. Rankinen, Principal Investigator). C. Bouchard is partially supported by the John W. Barton, Sr., Endowed Chair in Genetics and Nutrition.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.R., D.C.R., and C.B. are responsible for design of the study; T.R. performed GWAS genotyping; T.R., Y.J.S., M.A.S., T.K.R., and D.C.R. formulated the data analysis plan; T.R., D.C.R., and D.C. collected the data; T.R., Y.J.S., M.A.S., T.K.R., D.C.R., and C.B. analyzed the data; T.R., Y.J.S., M.A.S., T.K.R. D.C.R., and C.B. interpreted the results; T.R. drafted the manuscript; T.R., Y.J.S., M.A.S., D.C.R., and C.B. edited the manuscript; T.R. approved the final version of the manuscript.
REFERENCES
- 1. Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30: 97–101, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Allouis M, Le Bouffant F, Wilders R, Peroz D, Schott JJ, Noireaud J, Le Marec H, Merot J, Escande D, Baro I. 14-3-3 is a regulator of the cardiac voltage-gated sodium channel Nav1.5. Circ Res 98: 1538–1546, 2006 [DOI] [PubMed] [Google Scholar]
- 3. An P, Borecki IB, Rankinen T, Perusse L, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Evidence of major genes for exercise heart rate and blood pressure at baseline and in response to 20 weeks of endurance training: the HERITAGE Family Study. Int J Sports Med 24: 492–498, 2003 [DOI] [PubMed] [Google Scholar]
- 4. An P, Perusse L, Rankinen T, Borecki IB, Gagnon J, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Familial aggregation of exercise heart rate and blood pressure in response to 20 weeks of endurance training: the HERITAGE Family Study. Int J Sports Med 24: 57–62, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bardien S, Keyser R, Lombard D, du Plessis M, Human H, Carr J. Novel non-sense GCH1 mutation in a South African family diagnosed with dopa-responsive dystonia. Eur J Neurol 17: 510–512, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Beere PA, Glagov S, Zarins CK. Experimental atherosclerosis at the carotid bifurcation of the cynomolgus monkey. Localization, compensatory enlargement, and the sparing effect of lowered heart rate. Arterioscler Thromb 12: 1245–1253, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science 226: 180–182, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE Family Study. Aims, design, and measurement protocol. Med Sci Sports Exerc 27: 721–729, 1995 [PubMed] [Google Scholar]
- 9. Choe CU, Schulze-Bahr E, Neu A, Xu J, Zhu ZI, Sauter K, Bahring R, Priori S, Guicheney P, Monnig G, Neapolitano C, Heidemann J, Clancy CE, Pongs O, Isbrandt D. C-terminal HERG (LQT2) mutations disrupt IKr channel regulation through 14-3-3ε. Hum Mol Genet 15: 2888–2902, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Chung MC, Kim HK, Kawamoto S. TFEC can function as a transcriptional activator of the nonmuscle myosin II heavy chain-A gene in transfected cells. Biochemistry 40: 8887–8897, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Dale RC, Melchers A, Fung VS, Grattan-Smith P, Houlden H, Earl J. Familial paroxysmal exercise-induced dystonia: atypical presentation of autosomal dominant GTP-cyclohydrolase 1 deficiency. Dev Med Child Neurol 52: 583–586, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, regulation. Annu Rev Pharmacol Toxicol 40: 617–647, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Glasscock E, Tanouye MA. Drosophila couch potato mutants exhibit complex neurological abnormalities including epilepsy phenotypes. Genetics 169: 2137–2149, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasenpusch-Theil K, Chadwick BP, Theil T, Heath SK, Wilkinson DG, Frischauf AM. PHF2, a novel PHD finger gene located on human chromosome 9q22. Mamm Genome 10: 294–298, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Iida N, Kozasa T. Identification and biochemical analysis of GRIN1 and GRIN2. Methods Enzymol 390: 475–483, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kagan A, Melman YF, Krumerman A, McDonald TV. 14-3-3 amplifies and prolongs adrenergic stimulation of HERG K+ channel activity. EMBO J 21: 1889–1898, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau JM, Jin X, Ren J, Avery J, DeBosch BJ, Treskov I, Lupu TS, Kovacs A, Weinheimer C, Muslin AJ. The 14-3-3τ phosphoserine-binding protein is required for cardiomyocyte survival. Mol Cell Biol 27: 1455–1466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim J, Hao T, Shaw C, Patel AJ, Szabo G, Rual JF, Fisk CJ, Li N, Smolyar A, Hill DE, Barabasi AL, Vidal M, Zoghbi HY. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell 125: 801–814, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Malaspina A, Kaushik N, de Belleroche J. A 14-3-3 mRNA is up-regulated in amyotrophic lateral sclerosis spinal cord. J Neurochem 75: 2511–2520, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Olsson PA, Bornhauser BC, Korhonen L, Lindholm D. Neuronal expression of the ERM-like protein MIR in rat brain and its localization to human chromosome 6. Biochem Biophys Res Commun 279: 879–883, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Patberg KW, Shvilkin A, Plotnikov AN, Chandra P, Josephson ME, Rosen MR. Cardiac memory: mechanisms and clinical implications. Heart Rhythm 2: 1376–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Pulina MV, Rizzuto R, Brini M, Carafoli E. Inhibitory interaction of the plasma membrane Na+/Ca2+ exchangers with the 14-3-3 proteins. J Biol Chem 281: 19645–19654, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Rankinen T, Argyropoulos G, Rice T, Rao DC, Bouchard C. CREB1 is a strong genetic predictor of the variation in exercise heart rate response to regular exercise: the HERITAGE Family Study. Circ Cardiovasc Genet 3: 294–299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosen MR, Cohen IS. Cardiac memory—new insights into molecular mechanisms. J Physiol 570: 209–218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sari FR, Watanabe K, Widyantoro B, Thandavarayan RA, Harima M, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Partial inactivation of cardiac 14-3-3 protein in vivo elicits endoplasmic reticulum stress (ERS) and activates ERS-initiated apoptosis in ERS-induced mice. Cell Physiol Biochem 26: 167–178 [DOI] [PubMed] [Google Scholar]
- 26. Sharma AK, Nelson MC, Brandt JE, Wessman M, Mahmud N, Weller KP, Hoffman R. Human CD34+ stem cells express the hiwi gene, a human homologue of the Drosophila gene piwi. Blood 97: 426–434, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Skinner JS, Wilmore KM, Krasnoff JB, Jaskolski A, Jaskolska A, Gagnon J, Province MA, Leon AS, Rao DC, Wilmore JH, Bouchard C. Adaptation to a standardized training program and changes in fitness in a large, heterogeneous population: the HERITAGE Family Study. Med Sci Sports Exerc 32: 157–161, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Spielmann N, Leon AS, Rao DC, Rice T, Skinner JS, Rankinen T, Bouchard C. Genome-wide linkage scan for submaximal exercise heart rate in the HERITAGE Family Study. Am J Physiol Heart Circ Physiol 293: H3366–H3371, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Tutor AS, Delpon E, Caballero R, Gomez R, Nunez L, Vaquero M, Tamargo J, Mayor F, Jr, Penela P. Association of 14-3-3 proteins to β1-adrenergic receptors modulates Kv11.1 K+ channel activity in recombinant systems. Mol Biol Cell 17: 4666–4674, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe K, Ma M, Hirabayashi K, Gurusamy N, Veeraveedu PT, Prakash P, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Swimming stress in DN 14-3-3 mice triggers maladaptive cardiac remodeling: role of p38 MAPK. Am J Physiol Heart Circ Physiol 292: H1269–H1277, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, Aulchenko YS, Zhang W, Yuan X, Lim N, Luan J, Ashford S, Wheeler E, Young EH, Hadley D, Thompson JR, Braund PS, Johnson T, Struchalin M, Surakka I, Luben R, Khaw KT, Rodwell SA, Loos RJ, Boekholdt SM, Inouye M, Deloukas P, Elliott P, Schlessinger D, Sanna S, Scuteri A, Jackson A, Mohlke KL, Tuomilehto J, Roberts R, Stewart A, Kesaniemi YA, Mahley RW, Grundy SM, McArdle W, Cardon L, Waeber G, Vollenweider P, Chambers JC, Boehnke M, Abecasis GR, Salomaa V, Jarvelin MR, Ruokonen A, Barroso I, Epstein SE, Hakonarson HH, Rader DJ, Reilly MP, Witteman JC, Hall AS, Samani NJ, Strachan DP, Barter P, van Duijn CM, Kooner JS, Peltonen L, Wareham NJ, McPherson R, Mooser V, Sandhu MS. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30: 2264–2276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilmore JH, Stanforth PR, Gagnon J, Rice T, Mandel S, Leon AS, Rao DC, Skinner JS, Bouchard C. Heart rate and blood pressure changes with endurance training: the HERITAGE Family Study. Med Sci Sports Exerc 33: 107–116, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Wilmore JH, Stanforth PR, Turley KR, Gagnon J, Daw EW, Leon AS, Rao DC, Skinner JS, Bouchard C. Reproducibility of cardiovascular, respiratory, and metabolic responses to submaximal exercise: the HERITAGE Family Study. Med Sci Sports Exerc 30: 259–265, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Wu H, Zhou Y, Xiong ZQ. Transducer of regulated CREB and late phase long-term synaptic potentiation. FEBS J 274: 3218–3223, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325: 100–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang S, Ren J, Zhang CE, Treskov I, Wang Y, Muslin AJ. Role of 14-3-3-mediated p38 mitogen-activated protein kinase inhibition in cardiac myocyte survival. Circ Res 93: 1026–1028, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Zhao GQ, Zhao Q, Zhou X, Mattei MG, de Crombrugghe B. TFEC, a basic helix-loop-helix protein, forms heterodimers with TFE3 and inhibits TFE3-dependent transcription activation. Mol Cell Biol 13: 4505–4512, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]