Abstract
We tested the hypothesis that adrenergic and nonadrenergic receptor responsiveness and protein expression would be altered with advancing age. Young (n = 6; 22 ± 1 mo; mean ± SE) and old (n = 6; 118 ± 9 mo) beagles were instrumented with flow probes and an indwelling catheter for continuous measurement of external iliac blood flow and arterial blood pressure. Vascular conductance (VC) was calculated as hindlimb blood flow/mean arterial pressure. Selective agonists for α-1, α-2, neuropeptide-Y (NPY), and purinergic (P2X) receptors were infused at rest and during treadmill running at moderate (2.5 mph) and heavy (4 mph with 2.5% grade) exercise intensities. Feed arteries were dissected from gracilis muscles, and α-1D, α-1B, α-2A, P2X-4, P2X-1, and NPY-Y1 receptor protein expression was determined. Phenylephrine produced similar decreases (P > 0.05) in VC in young and old beagles at rest (young: −62 ± 5%; old: −59 ± 5%) and during moderate (young: −67 ± 5%; old: −62 ± 4%) and heavy (young: −54 ± 4%; old: −49 ± 3%) exercise. Clonidine caused similar (P > 0.05) decreases in VC in old compared with young dogs at rest (young: −59 ± 8%; old: −70 ± 6%) and during moderate (young: −52 ± 6%; old: −47 ± 5%)- and heavy (young: −42 ± 5%; old: −43 ± 5%)-intensity exercise. NPY infusion resulted in a similar decline in VC in young and old beagles at rest (young: −40 ± 7%; old: −39 ± 9%) and during moderate (young: −47 ± 6%; old: −40 ± 6%)- and heavy (young: −40 ± 3%; old: −38 ± 4%)-intensity exercise. α-β-Methylene-ATP also produced similar decreases in VC in young and old beagles at rest (young: −36 ± 6%; old: −40 ± 8%) and during exercise at moderate (young: −42 ± 5%; old: −40 ± 9%) and heavy (young: −47 ± 5%; old: −42 ± 8%) intensities. α-1B receptor protein expression was elevated (P < 0.05) in old compared with young dogs, whereas there were no age-related differences in α-1D or α-2A receptor expression and nonadrenergic P2X-4, P2X-1, and NPY-Y1 receptor expression. The present findings indicate that postsynaptic adrenergic and nonadrenergic receptor responsiveness was not altered by advancing age. Moreover, the expression of adrenergic and nonadrenergic receptors in skeletal-muscle feed arteries was largely unaffected by aging.
Keywords: blood flow, exercise, sympathetic, vasoconstriction
sympathetic vasoconstriction is mediated by the neurotransmitter norepinephrine (NE) and sympathetic cotransmitters, including the nonadrenergic neurotransmitters neuropeptide-Y (NPY) and ATP (11). NE binds to postsynaptic α-1 and α-2 adrenergic receptors, whereas NPY and ATP bind to NPY-Y1 and purinergic (P2X) receptors, respectively, to produce vasoconstriction (3a, 5, 6, 8, 11, 39). Indeed, sympathetic nerve activity has been shown to produce adrenergic and nonadrenergic receptor-mediated tonic vasoconstriction in young and old humans and animals at rest and during exercise (3a, 4, 6, 7, 7a, 13, 40).
It is generally accepted that aging is associated with an increase in resting muscle sympathetic nerve activity (MSNA) (17, 41). Despite this chronic elevation of MSNA, the available scientific literature related to the effect of aging on the neural control of skeletal muscle vascular tone at rest and during exercise is contradictory. For example, in the human forearm, nonselective α-adrenergic receptor blockade produced a smaller increase in resting blood flow in old compared with young adults (16), suggesting that tonic α-adrenergic receptor-mediated vasoconstriction was reduced with age. In contrast, in the leg, nonselective α-adrenergic receptor blockade elicited greater increases in resting blood flow and vascular conductance (VC) in older adults, suggesting that tonic α-adrenergic receptor-mediated vasoconstriction was elevated with age (19). We recently demonstrated that the magnitude of basal nonadrenergic receptor-mediated tonic vasoconstriction in the skeletal muscle vascular bed was altered with aging, with P2X receptor-mediated constriction being elevated and NPY-mediated constriction reduced in old compared with young dogs, whereas adrenergic receptor-mediated constriction was unaffected (13). In contrast, during dynamic exercise, both adrenergic and nonadrenergic receptor-mediated vasoconstriction was similar in old and young dogs (13).
An alteration in the magnitude of tonic vasoconstriction with aging may be mediated by presynaptic and/or postsynaptic factors. Changes in the magnitude and/or pattern of sympathetic outflow and neurotransmitter synthesis and release may alter the amount and relative proportions of neurotransmitters available (3, 21, 22, 30, 32, 37, 39, 43, 44). Additionally, the responsiveness and density of postsynaptic receptors may alter the amount of constriction, independent of changes in sympathetic activity.
Thus further investigation of the effect of aging on postsynaptic mechanisms of vasoconstriction is warranted. Current evidence regarding the effect of aging on postsynaptic receptor responsiveness is limited to studies of α-adrenergic receptors and has produced conflicting results with reports that α-adrenergic receptor responsiveness is unchanged (1, 12, 35), increased (20, 46), or even decreased (15, 42) with aging. The effect of aging on postsynaptic, nonadrenergic sympathetic receptor responsiveness has yet to be investigated. Additionally, the influence of aging on the expression/density of postsynaptic sympathetic adrenergic and nonadrenergic receptors in the skeletal-muscle vasculature is unknown.
Therefore, the purpose of the present study was to investigate the effect of aging on postsynaptic adrenergic and nonadrenergic receptor responsiveness and protein expression in resting and contracting skeletal muscles. We hypothesized that aging would be associated with increased responsiveness and expression of adrenergic (α-1 and α-2) and nonadrenergic (NPY and P2X) receptors.
METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin and were conducted in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Animals. Young (n = 6; 22 ± 1 mo) and old (n = 6; 118 ± 9 mo) beagles were selected for their willingness to run on a motorized treadmill and were chronically instrumented in a series of three sterile surgeries. These animals were used in a previous investigation (13). Each animal underwent a series of three sterile surgeries. For each surgery, anesthesia was induced with thiopental sodium (25 mg/kg; Gensia Sicor Pharmaceuticals, Irvine, CA), after which, animals were intubated with a cuffed endotracheal tube, and a surgical level of anesthesia was maintained through mechanical ventilation with 1.5% isoflurane (Halocarbon Products, River Edge, NJ) and 98.5% O2. Animals were given an analgesic for pain management (buprenorphrine hydrochloride, 0.015 mg/kg; Reckitt & Colman, Kingston-upon-Hull, UK) and received antibiotics for 10 days, (cefazolin sodium, 500 mg twice/day; Apothecon, Princeton, NJ) postoperatively. During the first surgery, the carotid arteries were placed in neck-skin tubes so that arterial blood pressure could be measured continuously via percutaneous cannulation. In the second, ultrasonic transit-time flow probes (4–6 mm; Transonic Systems, Ithica, NY) were placed around the external iliac of each hindlimb for measurement of skeletal muscle blood flow. Flow probe cables were tunneled under the skin to the back and externalized. Animals were given 2 wk to recover from flow probe implantation. In the final surgery, a heparinized catheter (0.045 in. OD; 0.015 in. ID; 60 cm length; Data Science International, St. Paul, MN) was implanted in a side branch and advanced into the femoral artery of one hindlimb. The catheter was tunneled under the skin to the back, externalized, and used for infusion of experimental drugs. The catheter was flushed daily with saline and filled with a heparin lock (100 IU heparin/ml in 50% dextrose solution) to maintain patency. Dogs were given 2 days to recover from the final surgery before any experimental procedures were performed.
Laboratory temperature was maintained below 20°C for all experiments to minimize changes in body temperature during the exercise sessions. For each experiment, the dog was brought to the laboratory and rested in a semirestrictive sling while the flow probes were connected to a flowmeter (Transonic Systems), and a 20-gauge intravascular catheter (Insyte, Becton Dickinson, Sandy, UT) was inserted retrogradely into the lumen of one carotid artery and attached to a solid-state pressure transducer (Abbott Laboratories, Abbott Park, IL) for measurement of arterial pressure.
To investigate the effect of aging on adrenergic and nonadrenergic receptor responsiveness at rest and during exercise, four series of investigations (series 1–4) were completed.
Series 1 and 2.
To investigate the effect of aging on the responsiveness of α-1 and α-2 receptors, flow-adjusted doses of selective α-1 [phenylephrine (PE), 0.1 μg/ml/min; series 1] and α-2 (clonidine, 1 μg/ml/min; series 2) agonists were infused into the experimental hindlimb, while the animals rested quietly and while the dogs ran on a motorized treadmill at a moderate (2.5 mph) and heavy (4 mph and 2.5% grade) exercise intensity.
Series 3 and 4.
To investigate the effect of aging on the responsiveness of NPY-Y1 and P2X receptors, flow-adjusted doses of selective NPY-Y1 ([Leu31,Pro34] NPY, 1 μg/ml/min; series 3) and P2X (α,β-methylene ATP, 1 μg/ml/min; series 4) agonists were infused into the experimental hindlimb while the animals rested quietly and while the dogs ran on a motorized treadmill at moderate (2.5 mph) and heavy (4 mph and 2.5% grade) exercise intensities.
Pharmacological infusions.
Each agonist was infused at rest and at each exercise intensity on a separate day in random order; thus each animal was brought to the laboratory on six occasions to complete series 1 and 2 and six additional occasions to complete series 3 and 4. Infusions during exercise took place after ∼5 min of treadmill running. Small volumes of each drug (<1 ml) were infused, followed by a 3-ml saline flush. Each infusion was ∼2–3 s in duration. Saline infusions were completed to determine the effect of the infusion vehicle in each animal at rest and during exercise. Vehicle infusions had no effect on hindlimb blood flow or systemic hemodynamics.
Arterial blood pressure, external iliac blood flow, and rectal temperature were recorded at 100 Hz directly to a computer with a PowerLab data acquisition system (ADInstruments, Castle Hill, Australia). Data were analyzed offline to calculate the absolute and relative change in mean arterial pressure (MAP), experimental and contralateral (control) limb iliac blood flow, and iliac VC (iliac blood flow/MAP) in response to intra-arterial infusions. For each agonist infusion, a 30-s average, immediately prior to each drug infusion (see Tables 1–4), was used for comparison with the postinfusion response to calculate the magnitude of response for each variable. After drug infusion, all variables were averaged over 1-s intervals, and the nadir 1-s average for VC was chosen as the peak response. The percent change in VC was calculated as [(preinfusion VC − postinfusion VC)/(preinfusion VC)] × 100.
Table 1.
Baseline hemodynamics and vascular response to intra-arterial infusion of α-1 receptor agonist
| MAP (mmHg) |
Control Limb Blood Flow (ml/min) |
Experimental Limb Blood Flow (ml/min) |
Control Limb Conductance (ml · min−1 · mmHg−1) |
Experimental Limb Conductance (ml · min−1 · mmHg−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 118 ± 6 | 122 ± 8 | 65 ± 11 | 74 ± 18 | 72 ± 9 | 26 ± 3* | 0.53 ± 0.09 | 0.59 ± 0.16 | 0.62 ± 0.09 | 0.22 ± 0.03* |
| 2.5 mph | 130 ± 3 | 135 ± 4 | 227 ± 35 | 244 ± 35 | 220 ± 35 | 82 ± 24* | 1.73 ± 0.25 | 1.78 ± 0.22 | 1.68 ± 0.25 | 0.59 ± 0.16* | |
| 4 mph 2.5% grade | 137 ± 4 | 137 ± 4 | 290 ± 41 | 299 ± 47 | 268 ± 34 | 123 ± 16* | 2.14 ± 0.34 | 2.19 ± 0.36 | 1.97 ± 0.27 | 0.90 ± 0.12* | |
| Old | Rest | 122 ± 11 | 123 ± 11 | 60 ± 14 | 68 ± 14 | 59 ± 9 | 19 ± 5* | 0.51 ± 0.08 | 0.57 ± 0.09 | 0.50 ± 0.09 | 0.18 ± 0.02* |
| 2.5 mph | 141 ± 8 | 144 ± 10 | 238 ± 19 | 240 ± 23 | 207 ± 15 | 81 ± 14* | 1.79 ± 0.23 | 1.80 ± 0.25 | 1.50 ± 0.17 | 0.60 ± 0.14* | |
| 4 mph 2.5% grade | 159 ± 9 | 164 ± 12 | 318 ± 40 | 313 ± 39 | 270 ± 22 | 136 ± 13* | 2.16 ± 0.43 | 2.13 ± 0.45 | 1.70 ± 0.09 | 0.83 ± 0.05* | |
Values are mean ± SE. MAP, mean arterial pressure; Pre/Post, prior to/after each drug infusion.
Significant difference from Pre value, P < 0.05.
Table 2.
Baseline hemodynamics and vascular response to intra-arterial infusion of α-2 receptor agonist
| MAP (mmHg) |
Control Limb Blood Flow (ml/min) |
Experimental Limb Blood Flow (ml/min) |
Control Limb Conductance (ml · min−1 · mmHg−1) |
Experimental Limb Conductance (ml · min−1 · mmHg−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 117 ± 6 | 120 ± 4 | 75 ± 12 | 82 ± 14 | 88 ± 14 | 36 ± 9* | 0.65 ± 0.08 | 0.69 ± 0.10 | 0.74 ± 0.10 | 0.30 ± 0.07* |
| 2.5 mph | 132 ± 5 | 134 ± 5 | 200 ± 24 | 230 ± 26 | 191 ± 23 | 95 ± 18* | 1.50 ± 0.14 | 1.72 ± 0.19 | 1.44 ± 0.14 | 0.69 ± 0.11* | |
| 4 mph 2.5% grade | 134 ± 7 | 139 ± 8 | 283 ± 51 | 292 ± 56 | 285 ± 44 | 172 ± 37* | 2.09 ± 0.36 | 2.12 ± 0.43 | 2.13 ± 0.30 | 1.24 ± 0.25* | |
| Old | Rest | 133 ± 13 | 133 ± 13 | 64 ± 12 | 63 ± 13 | 79 ± 18 | 20 ± 4* | 0.50 ± 0.08 | 0.48 ± 0.08 | 0.60 ± 0.12 | 0.15 ± 0.03* |
| 2.5 mph | 142 ± 6 | 142 ± 7 | 254 ± 29 | 257 ± 27 | 220 ± 23 | 122 ± 11* | 1.84 ± 0.27 | 1.85 ± 0.26 | 1.58 ± 0.20 | 0.87 ± 0.09* | |
| 4 mph 2.5% grade | 158 ± 6 | 159 ± 7 | 331 ± 24 | 324 ± 34 | 285 ± 32 | 148 ± 6* | 2.51 ± 0.32 | 2.56 ± 0.40 | 1.81 ± 0.19 | 0.93 ± 0.07* | |
Values are mean ± SE.
Significant difference from Pre value, P < 0.05.
Table 3.
Baseline hemodynamics and vascular response to intra-arterial infusion of NPY-Y1 receptor agonist
| MAP (mmHg) |
Control Limb Blood Flow (ml/min) |
Experimental Limb Blood Flow (ml/min) |
Control Limb Conductance (ml · min−1 · mmHg−1) |
Experimental Limb Conductance (ml · min−1 · mmHg−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 119 ± 4 | 121 ± 4 | 55 ± 11 | 53 ± 9 | 59 ± 6 | 34 ± 3* | 0.47 ± 0.09 | 0.45 ± 0.08 | 0.49 ± 0.04 | 0.29 ± 0.03* |
| 2.5 mph | 127 ± 5 | 134 ± 6 | 192 ± 26 | 198 ± 21 | 210 ± 23 | 115 ± 13* | 1.51 ± 0.21 | 1.50 ± 0.17 | 1.66 ± 0.17 | 0.85 ± 0.07* | |
| 4 mph 2.5% grade | 135 ± 5 | 144 ± 6 | 262 ± 31 | 235 ± 19 | 256 ± 32 | 161 ± 16* | 1.96 ± 0.27 | 1.65 ± 0.20 | 1.92 ± 0.27 | 1.13 ± 0.12* | |
| Old | Rest | 124 ± 11 | 128 ± 13 | 50 ± 14 | 59 ± 9 | 53 ± 3 | 30 ± 4* | 0.40 ± 0.11 | 0.48 ± 0.08 | 0.43 ± 0.03 | 0.25 ± 0.04* |
| 2.5 mph | 149 ± 10 | 156 ± 11 | 241 ± 26 | 240 ± 25 | 208 ± 19 | 135 ± 17* | 1.74 ± 0.29 | 1.65 ± 0.27 | 1.42 ± 0.17 | 0.88 ± 0.13* | |
| 4 mph 2.5% grade | 152 ± 5 | 157 ± 4 | 301 ± 24 | 311 ± 23 | 255 ± 45 | 161 ± 23* | 1.98 ± 0.19 | 1.98 ± 0.13 | 1.68 ± 0.28 | 1.03 ± 0.12* | |
Values are mean ± SE. NPY-Y1, neuropeptide-Y1.
Significant difference from Pre value, P < 0.05.
Table 4.
Baseline hemodynamics and vascular response to intra-arterial infusion of P2X receptor agonist
| MAP (mmHg) |
Control Limb Blood Flow (ml/min) |
Experimental Limb Blood Flow (ml/min) |
Control Limb Conductance (ml · min−1 · mmHg−1) |
Experimental Limb Conductance (ml · min−1 · mmHg−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 113 ± 2 | 115 ± 4 | 71 ± 4 | 81 ± 12 | 102 ± 12 | 61 ± 6* | 0.63 ± 0.03 | 0.73 ± 0.10 | 0.89 ± 0.09 | 0.54 ± 0.06* |
| 2.5 mph | 127 ± 4 | 130 ± 3 | 199 ± 27 | 195 ± 23 | 197 ± 24 | 113 ± 15* | 1.61 ± 0.28 | 1.52 ± 0.21 | 1.59 ± 0.25 | 0.87 ± 0.12* | |
| 4 mph 2.5% grade | 139 ± 6 | 143 ± 5 | 254 ± 33 | 258 ± 29 | 251 ± 32 | 141 ± 26* | 1.78 ± 0.23 | 1.77 ± 0.19 | 1.81 ± 0.23 | 0.98 ± 0.17* | |
| Old | Rest | 130 ± 10 | 134 ± 10 | 73 ± 14 | 71 ± 11 | 84 ± 23 | 48 ± 11* | 0.57 ± 0.10 | 0.54 ± 0.08 | 0.67 ± 0.18 | 0.37 ± 0.09* |
| 2.5 mph | 150 ± 10 | 155 ± 12 | 256 ± 16 | 263 ± 16 | 209 ± 23 | 132 ± 25* | 1.80 ± 0.16 | 1.81 ± 0.14 | 1.39 ± 0.15 | 0.85 ± 0.19* | |
| 4 mph 2.5% grade | 159 ± 7 | 167 ± 9 | 308 ± 70 | 309 ± 61 | 222 ± 18 | 137 ± 29* | 1.93 ± 0.58 | 1.85 ± 0.59 | 1.40 ± 0.11 | 0.81 ± 0.13* | |
Values are mean ± SE. P2X, purinergic.
Significant difference from Pre value, P < 0.05.
Following the completion of all conscious experiments, dogs were anesthetized. The gracilis muscles were dissected free from one hindlimb and placed in a chilled buffer solution. With the aid of a dissection microscope, feed arteries from young (n = 5) and older (n = 5) dogs were isolated and placed in liquid N2 and stored at −80°C. Arteries were homogenized in 150 μl buffer [50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 5:1,000 PMSF (200 nM), 5:1,000 Na3VO4 (200 nM), and 5:1,000 NaF (200 nM)]. The homogenates were centrifuged at 45,000 rpm for 30 min at 4°C. The supernatants were collected and used for analysis. Protein quantity and quality were determined using the Experion semiautomated electrophoresis system (Bio-Rad Laboratories, Hercules, CA). Denatured protein for each sample (4 μl) was loaded into an Experion Pro260 chip (Bio-Rad Laboratories). Equivalent amounts of protein were separated by gel electrophoresis (10% Tris·HCl; Criterion Precast Gel, Bio-Rad Laboratories). Proteins were transferred onto a nitrocellulose membrane (0.2 μm; Schleicher & Schuell BioScience, Keene, NH) by semidry electroblotting (Trans-Blot SD, Bio-Rad Laboratories) at 15 V for 1 h. The nitrocellulose membrane was soaked in 10 mM Tris·HCl containing 5% nonfat dry milk (Bio-Rad Laboratories) and 0.7% polyoxyethylene-sorbitan monolaurate (Tween 20), pH = 7.2, overnight at 4°C to block nonspecific sites. The membranes were then incubated with the P2X and NPY-Y1-purified goat polyclonal antibodies [1:300 dilution in Tris-buffered saline (TBS) with 5% nonfat dry milk and 0.1% Tween 20 (Santa Cruz Biotechnology, Santa Cruz, CA)] for 2 h at room temperature. Positive controls for α-adrenoceptor antibody subtypes were conducted using rat heart extracts. β-Actin antiserum was used for internal control (1:300 dilution in TBS with 5% nonfat dry milk and 0.1% Tween 20; Sigma-Aldrich, St. Louis, MO). Blots were washed and incubated with donkey anti-goat IgG-horseradish peroxidase secondary antibody (1:3,000 dilution; Santa Cruz Biotechnology) for 1 h at room temperature. Immunoreactivity was visualized with an enhanced chemiluminescence Western-blotting detection kit (Amersham Biosciences, Little Chalfont, UK). Quantitative assessment of band densities was performed by scanning densitometry.
The responsiveness of each receptor type was analyzed separately using two-way (age × exercise intensity) repeated-measures ANOVA. Receptor density data were analyzed by unpaired t-test. Where significant F ratios were found, Tukey's post hoc analysis was performed. All data are presented as means ± SE. A P value of <0.05 was considered statistically significant.
RESULTS
Series 1: effect of aging on skeletal-muscle α-1-adrenergic receptor responsiveness.
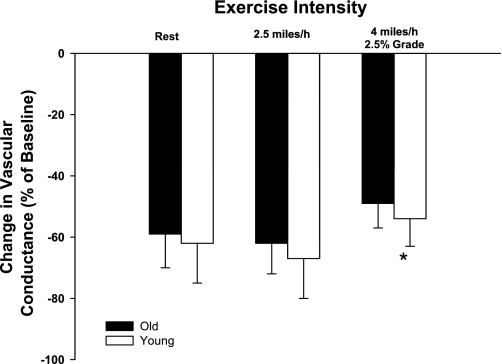
Baseline hemodynamics and responses to intra-arterial infusions of PE are presented in Table 1. Control and experimental limb blood flow and VC increased (P < 0.05) in an exercise intensity-dependent manner. Control and experimental limb blood flow and VC were not different between young and old dogs prior to the infusion of PE at rest and during exercise at moderate and heavy intensities (Table 1). Intra-arterial infusions of PE caused similar decreases (P > 0.05) in experimental limb VC at rest and during moderate-intensity exercise in young and old dogs (Fig. 1). The vascular response to infusion of PE was reduced (i.e., sympatholysis) during heavy- compared with moderate-intensity exercise and at rest. No interaction was observed between age and exercise intensity, indicating that the amount of sympatholysis was similar between old and young dogs.
Fig. 1.
Percent change in experimental limb iliac vascular conductance (VC) in response to intra-arterial infusion of the selective α-1 adrenergic receptor agonist phenylephrine (PE). Values are means (SE). *Main effect of exercise intensity; P < 0.05.
Series 2: effect of aging on skeletal-muscle α-2 adrenergic receptor responsiveness.
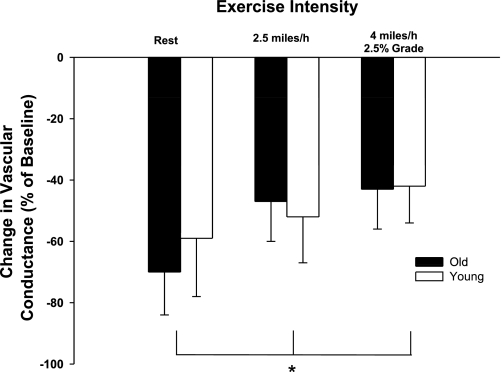
Baseline hemodynamics and responses to intra-arterial infusions of clonidine are presented in Table 2. Control and experimental limb blood flow and VC increased (P < 0.05) in an exercise intensity-dependent manner. Control and experimental limb blood flow and VC were not different between young and old dogs prior to the infusion of clonidine at rest and during moderate- and heavy-intensity exercise (Table 2). Intra-arterial infusions of clonidine caused similar decreases (P > 0.05) in experimental limb VC at rest and during exercise in young and old dogs (Fig. 2). The vascular response to infusion of clonidine was reduced (i.e., sympatholysis) during exercise at moderate and heavy intensities compared with rest. No interaction was observed between age and exercise intensity, indicating that the amount of sympatholysis was similar between old and young dogs.
Fig. 2.
Percent change in experimental limb iliac VC in response to intra-arterial infusion of the selective α-2 adrenergic receptor agonist clonidine. Values are means (SE). *Main effect of exercise intensity; P < 0.05.
Series 3: effect of aging on skeletal-muscle NPY-Y1 receptor responsiveness.
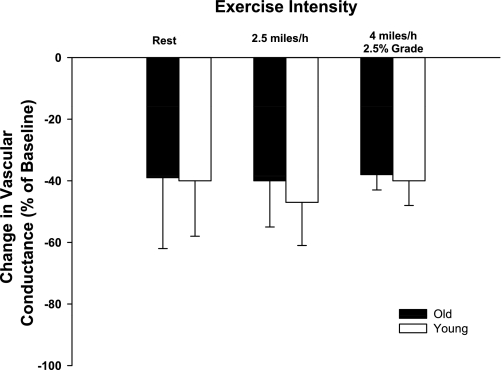
Baseline hemodynamics and responses to intra-arterial infusions of [Leu31, Pro34] NPY are presented in Table 3. Control and experimental limb blood flow and VC increased (P < 0.05) in an exercise intensity-dependent manner. Control and experimental limb blood flow and VC were not different between young and old dogs prior to the infusion of [Leu31,Pro34] NPY at rest and during moderate- and heavy-intensity exercise (Table 3). Intra-arterial infusions of NPY caused similar decreases (P > 0.05) in experimental limb VC of young and old dogs at rest and during exercise (Fig. 3).
Fig. 3.
Percent change in experimental limb iliac VC in response to intra-arterial infusion of the selective neuropeptide-Y1 (NPY-Y1) receptor agonist [Leu31, Pro34] NPY. Values are means (SE).
Series 4: effect of aging on skeletal-muscle P2X receptor responsiveness.
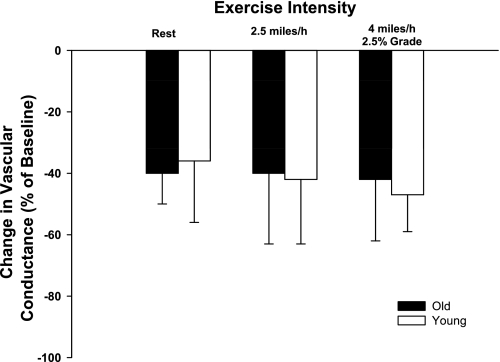
Baseline hemodynamics and responses to intra-arterial infusions of α,β-methylene ATP are presented in Table 4. Control and experimental limb blood flow and VC increased (P < 0.05) in an exercise intensity-dependent manner. Control and experimental limb blood flow and VC were not different between young and old dogs prior to the infusion of α,β-methylene ATP at rest and during moderate- and heavy-intensity exercise (Table 4). Intra-arterial infusions of α,β-methylene ATP caused similar decreases (P > 0.05) in experimental limb VC of young and old dogs at rest and during exercise (Fig. 4).
Fig. 4.
Percent change in experimental limb iliac VC in response to intra-arterial infusion of the selective purinergic (P2X) receptor agonist α, βmethylene ATP. Values are means (SE).
Effect of aging on adrenergic and nonadrenergic receptor protein expression.
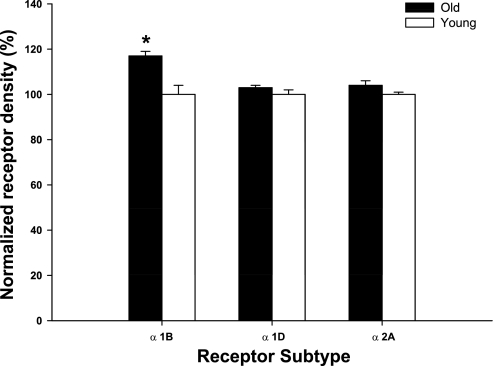
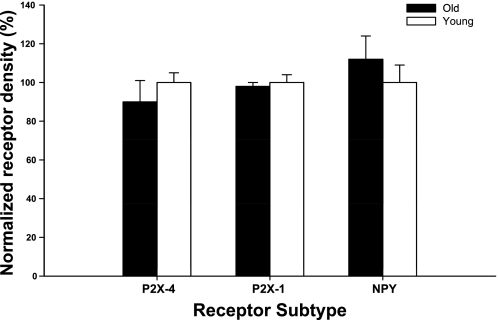
Western blot analysis was performed to examine gracilis-muscle feed artery receptor protein expression. Receptor protein densities in young and old dogs were normalized to young dog values. β-Actin content was used as an internal control, and no difference was evident between groups (data not shown). α-1B receptor protein expression was elevated significantly in old compared with young dogs, whereas there were no age-related differences in α-1D or α-2 receptor expression (Fig. 5) and nonadrenergic P2X-4, P2X-1, and NPY-Y1 receptor expression (Fig. 6).
Fig. 5.
α-Adrenergic (α-1B, α-1D, and α-2A) receptor protein expression in gracilis feed arteries in young and old animals. Values are normalized to receptor protein density in young. Values are means (SE). *Significant difference between young and old dogs; P < 0.05.
Fig. 6.
P2X-4 and P2X-1 and NPY-Y1 (NPY) receptor protein expression in gracilis feed arteries in young and old animals. Values are normalized to receptor protein density in young. Values are means (SE).
DISCUSSION
The purpose of this study was to investigate the effect of aging on postsynaptic adrenergic and nonadrenergic receptor protein expression and receptor responsiveness in resting and contracting skeletal muscles. The primary finding from the present study was that aging does not alter the responsiveness of postsynaptic adrenergic and nonadrenergic receptors in the skeletal-muscle vasculature of the canine hindlimb at rest or during exercise. Previous studies have relied on pharmacological approaches alone. In the current study, postsynaptic receptor protein expression was also evaluated to provide a more complete picture. α-1D, α-2A, P2X-4, P2X-1, and NPY-Y1 receptor protein expression was similar between young and old canines. α-1B receptor protein expression was significantly greater in old compared with young beagles, although this difference did not result in an enhanced response to exogenous receptor stimulation.
Infusion of selective α-1 and α-2 agonists produced similar decreases in skeletal-muscle vasoconstriction at rest and during exercise in old and young beagles, suggesting that α-adrenergic receptor responsiveness was not affected by aging. Previous studies of the effect of aging on adrenergic receptor responsiveness have produced conflicting findings. In aged animals, studies have reported reduced (15), unchanged (1, 12, 35), or increased (20) α-adrenergic receptor responsiveness. Most recently, Donato et al. (20) reported increased NE-mediated vasoconstriction in first-order arterioles isolated from the soleus muscle of old rats, whereas vascular responsiveness of vessels isolated from the gastrocnemius muscle was not affected by aging. In humans, Koch et al. (33) reported a larger reduction in VC in contracting skeletal muscle with cold pressor-induced reflex sympathetic vasoconstriction in old compared with young men. The authors (33) interpreted these findings as an increase in receptor responsiveness to sympathetic stimulation. Consistent with this interpretation, a larger decrease in VC during forearm contractions was observed in old compared with young men in response to infusion of tyramine (which evokes endogenous NE release) and α-1 and α-2 agonists (PE and clonidine) (18). In another study, PE infusion resulted in a smaller decrease in VC under basal conditions, whereas there was a larger decrease in VC in old compared with young adults during a single-leg, knee-extension exercise at 40% and 60% of maximum work rate (46). However, when these subjects performed knee-extension exercise at a similar work rate and oxygen consumption, the magnitude of vasoconstriction produced in response to PE infusion did not differ between young and old adults (46).
The magnitude of vasoconstriction produced in response to the infusion of selective α-1 and α-2 adrenergic receptor agonists declined during exercise in both young and old beagles in the present study. These results are consistent with previous findings from our laboratory. In young humans and animals, it is well established that a number of substances released from either the active muscle or the vascular endothelium can blunt the adrenergic receptor-mediated vascular response to sympathetic stimulation (4, 14, 31, 38). Dinenno et al. (18) have reported that older adults exhibit a reduced ability to blunt α-adrenergic receptor-mediated vasoconstriction during forearm contractions. In contrast, Wray et al. (46) recently argued that the ability of the older adult to inhibit the effects of sympathetic outflow does not diminish as a function of age, evidenced by a similar vascular response to PE infusion in old and young adults exercising at the same absolute work rate. Wray et al. (46) suggested that the ability of old and young adults to inhibit sympathetic vasoconstriction was a function of the local metabolic rate. The present findings of similar sympathetic restraint and vascular responsiveness to agonist stimulation in old and young dogs at rest and during exercise at the same absolute work rates support this interpretation.
To our knowledge, this is the first study to investigate the effect of aging on nonadrenergic receptor responsiveness in resting and contracting skeletal muscle. The responsiveness of P2X and NPY-Y1 receptors was not different between young and old dogs at rest. The maintained NPY-Y1 receptor responsiveness at rest in old dogs suggests that our previous observations of diminished NPY-Y1 and augmented P2X receptor-mediated tonic vasoconstriction may be due to an age-associated alteration in release of NPY and ATP from sympathetic nerves under basal conditions.
During exercise, nonadrenergic receptor responsiveness was not different between young and old beagles. In addition, the magnitude of vasoconstriction produced in response to the infusion of selective nonadrenergic receptor agonists was similar in resting and contracting muscle. Previous work from our laboratory has shown that both P2X and NPY-Y1 receptor responsiveness to exogenous stimulation is attenuated from rest to exercise (5, 8). However, the heaviest work rate (4 mph and 2.5% grade) in the present study is considerably less intense than the work rate (6 mph and 10% grade) that induced attenuation in our previous publication, and it is conceivable that nonadrenergic receptors may be more resistant to sympatholysis and the work rates used in the present study were not sufficient to attenuate nonadrenergic receptor responsiveness.
The vascular response to infusion of selective agonists may be impacted by age-associated changes in receptor protein expression. In the present study, α-1D and α-2A receptor protein expression was not affected by aging, whereas α-1B receptor protein expression was elevated in gracilis-muscle feed arteries from old compared with young beagles. This finding suggests that aging differentially affects the expression of vascular receptors, and the overall effect is an increased expression of adrenergic receptors. The similar vascular response to PE suggests that an age-related increase in α-1B receptor protein expression may offset any age-associated decline in the responsiveness of individual receptors. Additionally, the similar vascular response to infusion of clonidine in combination with a similar expression of α-2 receptors suggests that aging does not diminish downstream signaling involved in α-2 adrenergic receptor-mediated vasoconstriction.
An age-related decline in P2X (subtype 1) receptor density in rat tail (45) and cerebral arteries (34) has been reported. However, to date, no studies of the effects of aging on nonadrenergic receptor protein expression in the skeletal-muscle vascular bed have been reported. The present findings indicate that P2X and NPY-Y1 receptor density is not altered in a representative vessel from the skeletal-muscle vascular bed of the aged canine. It is not surprising that nonadrenergic receptor responsiveness is similar between young and old dogs, given the fact that nonadrenergic receptor protein expression was similar between the two groups. However, in our previous investigation, we demonstrated that tonic P2X receptor-mediated vasoconstriction was elevated, and NPY receptor-mediated vasoconstriction diminished in old compared with young dogs at rest (13). The similarities in P2X and NPY receptor expression suggest that the previous finding must be explained by an increased release of ATP and a decreased release of NPY from sympathetic nerves, respectively, under resting conditions.
Experimental considerations.
A major strength of the present experimental approach is the ability to study basic physiological mechanisms of vascular control in conscious, dynamically exercising animals by delivering selective agonists to a discrete vascular bed without altering blood pressure or blood flow in other vascular beds. The studies used a canine model, which has been used for previous studies of aging and cardiovascular function (13, 23–29). Due to the long lifespan of this experimental model, the experimental design was necessarily cross-sectional. It should also be recognized that infusion of pharmacological agents into the entire hindlimb does not allow determination of the vascular response between individual muscles and the relative contribution of different segments of the vascular tree to the hindlimb vascular response. Behnke et al. (2) have reported that the low-oxidative white gastrocnemius muscle has a greater sympathetic tone and elevated α-adrenergic receptor responsiveness compared with highly oxidative red gastrocnemius and soleus muscle. Additionally, Musch et al. (36) have shown that old and young rats running at the same absolute intensity have similar hindlimb blood flows but an altered distribution of hindlimb limb flow toward type II muscles in aged animals. These findings highlight the potential for differential vascular control in different vascular beds and segments of the vascular tree, which could not be investigated with the present experimental approach. The receptor protein expression data reflect the effect of aging on a single vessel segment (feed artery) in a single muscle (gracilis). It is conceivable that the expression of vascular receptors may differ in different segments of the vascular tree and in different muscles. The determination of receptor expression by Western blot does not allow differentiation between receptors that are membrane bound and those in the cytoplasm. Thus it must be acknowledged that the receptor expression data may not be reflective of the amount of membrane-bound “functional” receptors that were available to bind the neurotransmitter in the young and old dogs.
In conclusion, the present findings indicate that postsynaptic adrenergic and nonadrenergic receptor responsiveness is not altered by advancing age. Moreover, the expression of adrenergic and nonadrenergic receptors in skeletal muscle feed arteries is largely unaffected by aging.
GRANTS
Support for this project was provided by the Natural Sciences and Engineering Research Council of Canada, National Heart, Lung, and Blood Institute, and the Medical Research Service of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.S.D., P.S.C., S.M., and J.B.B. conception and design of research; D.S.D., M.M.A., J.D.T., U.D.D., and J.B.B. performed experiments; D.S.D., J.D.T., U.D.D., and J.B.B. analyzed data; D.S.D., P.S.C., and J.B.B. interpreted results of experiments; D.S.D. prepared figures; D.S.D. drafted manuscript; D.S.D., P.S.C., S.M., M.M.A., H.A.K., J.D.T., U.D.D., and J.B.B. edited and revised manuscript; D.S.D., P.S.C., S.M., M.M.A., H.A.K., J.D.T., U.D.D., and J.B.B. approved final version of manuscript.
REFERENCES
- 1. Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561: 535–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Behnke BJ, Armstrong RB, Delp MD. Adrenergic control of vascular resistance varies in muscles composed of different fiber types: influence of the vascular endothelium. Am J Physiol Regul Integr Comp Physiol 301: R783–R790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradley E, Law A, Bell D, Johnson CD. Effects of varying impulse number on cotransmitter contributions to sympathetic vasoconstriction in rat tail artery. Am J Physiol Heart Circ Physiol 284: H2007–H2014, 2003 [DOI] [PubMed] [Google Scholar]
- 3a. Buckwalter JB, Clifford PS. alpha-Adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol Heart Circ Physiol 277: H33–H39, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev 29: 159–163, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Buckwalter JB, Hamann JJ, Clifford PS. Neuropeptide Y1 receptor vasoconstriction in exercising canine skeletal muscles. J Appl Physiol 99: 2115–2120, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Buckwalter JB, Hamann JJ, Clifford PS. Vasoconstriction in active skeletal muscles: a potential role for P2X purinergic receptors? J Appl Physiol 95: 953–959, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Buckwalter JB, Hamann JJ, Kluess HA, Clifford PS. Vasoconstriction in exercising skeletal muscles: a potential role for neuropeptide Y? Am J Physiol Heart Circ Physiol 287: H144–H149, 2004 [DOI] [PubMed] [Google Scholar]
- 7a. Buckwalter JB, Mueller PJ, Clifford PS. Sympathetic vasoconstriction in active skeletal muscles during dynamic exercise. J Appl Physiol 83: 1575–1580, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Do P2X purinergic receptors regulate skeletal muscle blood flow during exercise? Am J Physiol Heart Circ Physiol 286: H633–H639, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol 49: 1–30, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Cook JJ, Wailgum TD, Vasthare US, Mayrovitz HN, Tuma RF. Age-related alterations in the arterial microvasculature of skeletal muscle. J Gerontol 47: B83–B88, 1992 [DOI] [PubMed] [Google Scholar]
- 13. DeLorey DS, Buckwalter JB, Mittelstadt SW, Anton MM, Kluess HA, Clifford PS. Is tonic sympathetic vasoconstriction increased in the skeletal muscle vasculature of aged canines? Am J Physiol Regul Integr Comp Physiol 299: R1342–R1349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeLorey DS, Wang SS, Shoemaker JK. Evidence for sympatholysis at the onset of forearm exercise. J Appl Physiol 93: 555–560, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Delp MD, Brown M, Laughlin MH, Hasser EM. Rat aortic vasoreactivity is altered by old age and hindlimb unloading. J Appl Physiol 78: 2079–2086, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Dinenno FA, Joyner MJ. alpha-Adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536: 977–983, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol 579: 115–125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elam M, Sverrisdottir YB, Rundqvist B, McKenzie D, Wallin BG, Macefield VG. Pathological sympathoexcitation: how is it achieved? Acta Physiol Scand 177: 405–411, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Flavahan NA, Vanhoutte PM. Sympathetic purinergic vasoconstriction and thermosensitivity in a canine cutaneous vein. J Pharmacol Exp Ther 239: 784–789, 1986 [PubMed] [Google Scholar]
- 23. Haidet GC. alpha-Adrenergic-mediated reflex responses to induced muscular contraction are changed with age in dogs. Am J Physiol Heart Circ Physiol 265: H1899–H1908, 1993 [DOI] [PubMed] [Google Scholar]
- 23a. Haidet GC. Dynamic exercise in senescent beagles: oxygen consumption and hemodynamic responses. Am J Physiol Heart Circ Physiol 257: H1428–H1437, 1989 [DOI] [PubMed] [Google Scholar]
- 23b. Haidet GC. Effect of age on cardiovascular responses to static muscular contraction in beagles. J Appl Physiol 73: 2320–2327, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Haidet GC. Effects of age on beta-adrenergic-mediated reflex responses to induced muscular contraction in beagles. Mech Ageing Dev 68: 89–104, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Haidet GC, Parsons D. Reduced exercise capacity in senescent beagles: an evaluation of the periphery. Am J Physiol Heart Circ Physiol 260: H173–H182, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Haidet GC, Wennberg PW, Finkelstein SM, Morgan DJ. Effects of aging per se on arterial stiffness: systemic and regional compliance in beagles. Am Heart J 132: 319–327, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Haidet GC, Wennberg PW, Rector TS. Aging and vasoreactivity: in vivo responses in the beagle hindlimb. Am J Physiol Heart Circ Physiol 268: H92–H99, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Haniuda K, Nakane T, Chiba S. Different contributions of ATP and noradrenaline to neurotransmission in the isolated canine intermediate auricular artery. Eur J Pharmacol 333: 163–168, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Hansen J, Sander M, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiol Scand 168: 489–503, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Johnson CD, Coney AM, Marshall JM. Roles of norepinephrine and ATP in sympathetically evoked vasoconstriction in rat tail and hindlimb in vivo. Am J Physiol Heart Circ Physiol 281: H2432–H2440, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol 551: 337–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miao LY, Tang JP, Esposito DP, Zhang JH. Age-related changes in P2 receptor mRNA of rat cerebral arteries. Exp Gerontol 37: 67–79, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Pernow J, Schwieler J, Kahan T, Hjemdahl P, Oberle J, Wallin BG, Lundberg JM. Influence of sympathetic discharge pattern on norepinephrine and neuropeptide Y release. Am J Physiol Heart Circ Physiol 257: H866–H872, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962 [DOI] [PubMed] [Google Scholar]
- 39. Ren LM, Burnstock G. Prominent sympathetic purinergic vasoconstriction in the rabbit splenic artery: potentiation by 2,2′-pyridylisatogen tosylate. Br J Pharmacol 120: 530–536, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rowell LB. Human Cardiovascular Control. New York: Oxford University Press, 1993 [Google Scholar]
- 41. Seals DR, Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol 287: H1895–H1905, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanaka K, Yang XP, Chiba S. Purinergic and adrenergic cotransmission in canine isolated and perfused gastroepiploic arteries. Clin Exp Pharmacol Physiol 30: 678–683, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Tarasova O, Sjoblom-Widfeldt N, Nilsson H. Transmitter characteristics of cutaneous, renal and skeletal muscle small arteries in the rat. Acta Physiol Scand 177: 157–166, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Wallace A, Knight GE, Cowen T, Burnstock G. Changes in purinergic signalling in developing and ageing rat tail artery: importance for temperature control. Neuropharmacology 50: 191–208, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Wray DW, Nishiyama SK, Richardson RS. Role of {alpha}1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]