Abstract
The purpose of this study was to investigate the influence of anion size and the contribution of the epithelial sodium channel (ENaC) and the transient receptor potential vanilloid-1 (TRPV1) channel on sodium-taste responses in rat chorda tympani (CT) neurons. We recorded multiunit responses from the severed CT nerve and single-cell responses from intact, narrowly tuned and broadly tuned, salt-sensitive neurons in the geniculate ganglion simultaneously with stimulus-evoked summated potentials to signal when the stimulus contacted the lingual epithelium. Artificial saliva served as the rinse and solvent for all stimuli (0.3 M NH4Cl, 0.5 M sucrose, 0.03–0.5 M NaCl, 0.01 M citric acid, 0.02 M quinine hydrochloride, 0.1 M KCl, and 0.03–0.5 M Na-gluconate). We used the pharmacological antagonist benzamil to assess NaCl responses mediated by ENaC, and SB-366791 and cetylpyridinium chloride to assess responses mediated by TRPV1. CT nerve responses were greater to NaCl than Na-gluconate at each concentration; this was attributed mostly to broadly tuned, acid-generalist neurons that responded with higher frequency and shorter latency to NaCl than Na-gluconate. In contrast, narrowly tuned NaCl-specialist neurons responded more similarly to the two salts, but with subtle differences in temporal pattern. Benzamil reduced CT nerve and single-cell responses only of narrowly tuned neurons to NaCl. Surprisingly, SB-366791 and cetylpyridinium chloride were without effect on CT nerve or single-cell NaCl responses. Collectively, our data demonstrate the critical role that apical ENaCs in fungiform papillae play in processing information about sodium by peripheral gustatory neurons; the role of TRPV1 channels is an enigma.
Keywords: chorda tympani, geniculate ganglion, epithelial sodium channel, transient receptor potential vanilloid-1, electrophysiology
salt-taste detection depends upon two salt-sensing transduction pathways that involve specialized membrane channels on the surface of fungiform taste bud cells in rats. One is the well-documented epithelial sodium channel (ENaC) that is selective for the sodium cation and can be blocked pharmacologically by amiloride. The other is not well understood in the rodent taste system, but has recently been hypothesized to be a variant of the nonspecific-cation channel of the transient receptor potential (TRP) family, TRP vanilloid-1 (TRPV1t) (Lyall et al. 2004), which is broadly receptive to several cations, sodium as well as potassium, calcium, and ammonium. These two salt-sensing pathways in fungiform taste bud cells communicate with two divergent afferent neuron groups in the chorda tympani (CT) nerve. Narrowly tuned NaCl-specialist neurons respond selectively to sodium (and lithium) salts and little, if at all, to other salts or other basic taste stimuli. ENaC antagonists attenuate NaCl responses of NaCl-specialist neurons, but not the NaCl responses of other neuron groups (Breza et al. 2010; Hettinger and Frank 1990; Lundy and Contreras 1999; Ninomiya and Funakoshi 1988). Compelling behavioral studies in rats with amiloride (Spector et al. 1996) and in conditional ENaC knockout mice (Chandrashekar et al. 2010) demonstrate the critical involvement of the ENaC salt-sensing pathway for sodium discrimination. In contrast to NaCl-specialist neurons, broadly tuned neurons of the CT nerve respond to NaCl, KCl, and NH4Cl, as well as to other basic taste stimuli (Contreras and Lundy 2000; Lundy and Contreras 1999). It is possible that TRPV1t may be involved in salt-sensing by broadly tuned neurons, but it has yet to be explored at a single-unit level in rats.
It is generally accepted that the positively charged cation is the active stimulus in salt-taste transduction, but the negatively charged anion also influences the spike discharges of afferent neurons. In fact, the amplitude of the rat chorda-tympani nerve response to sodium was progressively smaller as the size of the anion became larger (Beidler 1954; Elliott and Simon 1990; Ye et al. 1991; 1993). Rehnberg, et al. (1993) showed that this “anion effect” may be a characteristic of the nonselective salt-sensing pathway. They found that narrowly tuned NaCl-best fibers of the hamster chorda-tympani nerve responded with similar spike rate to NaCl and Na acetate, whereas broadly tuned generalist neurons responded at a lower spike rate to Na acetate than to NaCl. The suggestion is that anion size influences the salt-intensity signal conveyed by the nonselective salt-sensing pathway and not the selective salt-sensing pathway, but this also has yet to be tested and determined by single-unit recordings of the rat chorda-tympani nerve.
The rat is the dominant animal model of taste-mediated behaviors, yet, as mentioned above, many questions remain unanswered at a single-cell level. In the present study, we recorded single-cell spike discharges from the geniculate ganglion to a battery of taste solutions representing the four basic taste qualities, and to concentration series of NaCl and Na-gluconate. Single cell responses were examined in detail, especially between 500 and 1,000 ms, when rats discriminate taste quality and intensity (Geran and Spector 2000b; Halpern and Tapper 1971) and longer recording intervals (5 s) for consistency with our laboratory's prior studies (Breza et al. 2007; 2006; Breza et al. 2010; Lundy and Contreras 1999) and those in the literature (Geran and Travers 2006; Smith and Travers 1979; Sollars and Hill 2005). Parallel whole nerve studies were performed to compare our results with previous studies using similar methods. We also used the stimulus-evoked potential [electrogustogram (EGG)] from the tongue to mark the moment when the chemical stimulus contacted the lingual epithelium and constructed a device to measure the electrical conductance property of each chemical stimulus. Accordingly, we used these methods to address three critical issues in salt taste.
Our first purpose was to confirm and extend prior work in the hamster investigating the influence of anion size on single-cell responses of the rat CT nerve. We hypothesized that the anion effect is a unique property of the nonselective pathway, and that the large gluconate anion will reduce the spike rate and increase the spike latency of broadly tuned acid-generalist neurons, but not change responses of NaCl-specialist neurons. Our second purpose was to examine the inhibitory effects of ENaC and TRPV1 antagonists on sodium responses of narrowly tuned NaCl specialists and broadly tuned acid-generalist neurons. Based on whole nerve studies in rats (Lyall et al. 2004; Lyall et al. 2005), we hypothesized that apical TRPV1 channels mediate a portion of the NaCl response in acid-generalist neurons, whereas the remaining signal is paracellular in origin and therefore unaffected by TRPV1 antagonists. Based on the psychophysical evidence of apical ENaCs in sodium-taste-mediated behavior (Geran and Spector 2000b), we hypothesized that NaCl specialists would be more responsive than acid generalists during the first 500–1,000 ms after stimulus contact (phasic-response component). Our third purpose was to analyze the EGG waveform and CT nerve responses to chemical stimulation and compare them to the conductance values of the test solutions as a means to assess how chemical stimuli may interact with the lingual epithelium. We hypothesized that the large-gluconate anion attenuates the EGG signal based on its poor conductance through the paracellular shunts in fungiform taste buds (Ye et al. 1991; 1994; 1993).
METHODS
Animals, Surgery, and Recording Techniques
Animals and surgery.
Adult male Sprague-Dawley rats (Charles River Laboratories; n = 27) weighing 299–450 g were housed individually in plastic cages in a temperature-controlled colony room on a 12:12-h light-dark cycle with lights on at 0700. Rats were anesthetized with urethane (1.7 g/kg body wt), tracheostomized, and secured in a nontraumatic head holder. For CT preparations (n = 17), rats were placed in a custom-made brass head holder, which could be rotated for the nerve dissection. The whole CT nerve was exposed by a mandibular approach, transected proximally, and desheathed for recording. For single-unit experiments, rats (n = 10) were placed in a stereotaxic instrument with blunt ear bars. The geniculate ganglion was exposed with a dorsal approach (Breza et al. 2007; 2006; Breza et al. 2010; Lundy and Contreras 1999). Each rat's tongue was gently extended and held in place by a suture attached to its ventral surface. Body temperature was maintained at 37°C with a custom-made (Paul Hendrick; FSU) heating pad and temperature controller.
Recording Techniques
EGG.
The EGG was recorded in vivo with Ag/AgCl electrodes by means of saline-agar-filled capillary pipettes (diameter 100 μm, 0.15 M NaCl, 0.5% agar). The active electrode (negative polarity) was placed near the receptive field, and an indifferent electrode (positive polarity) made contact with muscle on the neck. The electrode with negative polarity was used as the active electrode so that an increase in conductance (voltage drop) from electrolyte solutions [NH4Cl, NaCl, Na-gluconate, quinine hydrochloride (QHCl), and KCl] resulted in upward deflections (viewing purposes only). The signal was amplified (direct current ×50; A-M Systems, Sequim, WA), digitized with waveform hardware and software (Spike 2; Cambridge Electronic Design, Cambridge, England), and stored on a computer for offline analysis.
CT nerve.
The CT nerve was cut near its entrance into the tympanic bulla and draped over a platinum wire hook (positive polarity), and the entire cavity was then filled with high-quality paraffin oil (VWR) to isolate the signal from ground and maintain nerve integrity. An indifferent electrode (negative polarity) was attached to the skin overlying the cranium with a stainless steel wound clip. Neural activity was differentially amplified [alternating current (AC) ×10,000; A-M Systems, Sequim, WA, bandpass 300–5,000 Hz], observed with an oscilloscope, digitized, and stored as described above.
Geniculate ganglion.
Low-impedance (1.1–1.5 MΩ) glass-insulated tungsten microelectrodes (tip diameter 1 μm) were mounted on a stereotaxic micromanipulator (Siskiyou Design Instruments, Grants Pass, OR) and advanced downward from the dorsal surface of the ganglion. Unit-to-few unit activity was recorded extracellularly (criteria 3:1 signal-to-noise ratio). An indifferent electrode (negative polarity) was attached to the skin overlying the cranium with a stainless steel wound clip. Neural activity was amplified (AC ×10,000; A-M Systems, Sequim, WA, bandpass 300–5,000 Hz), observed with an oscilloscope, digitized, and stored as described above.
Stimulus Delivery and Stimulation Protocols
Solutions were presented to the tongue at a constant flow rate (50 μl/s) and temperature (35 ± 0.3°C) by an air-pressurized, 32-channel commercial, fluid-delivery system (16 channels for the present study) and heated perfusion cube (OctaFlow; ALA Scientific Instruments, Farmingdale, NY), respectively. The flow rate, temperature, and rinse composition were essentially identical to our recent geniculate ganglion study (Breza et al. 2010), except that we reduced the dead volume in the delivery tube from 250 to 25 μl. This resulted in a more abrupt change in stimulus quality and concentration from rinse to stimulus without introducing tactile or thermal artifacts, as switching between solutions reservoirs containing artificial saliva (AS) did not affect EGG (Table 1) or CT nerve (see Fig. 2) responses. All solutions and pharmacological reagents were reagent grade and purchased from VWR International or Sigma Aldrich. AS (0.015 M NaCl, 0.022 M KCl, 0.003 M CaCl2, and 0.0006 MgCl2; pH 5.8 ± 0.2) served as the rinse solution and solvent for all stimuli. We tested CT-nerve responses to 5-s applications of 0.3 M NH4Cl, 0.5 M sucrose, 0.03–0.5 M NaCl, 0.01 M citric acid, 0.02 M QHCl, 0.1 M KCl, 0.03–0.5 M Na-gluconate, 1 and 5 μM benzamil HCl + 0.1 M NaCl, 1 and 5 μM SB-366701 + 0.1 M NaCl, 0.1% DMSO (vehicle for SB-366791) + 0.1 M NaCl, and to 0.2 and 2 mM of cetylpyridinium chloride (CPC) + 0.1 M NaCl. CPC is water soluble and has been shown to modulate the amiloride-insensitive component of sodium taste (DeSimone et al. 2001) and, therefore, was used in addition to SB-366791 for control purposes (solubility). Benzamil and SB-366791 were used to access the contribution of ENaC and TRPV1 channels, respectively. SB-366791 is a lipophilic compound and, therefore, was dissolved in 100% DMSO (10 mM stock solution), and then diluted to a final concentration of 1 and 5 μM immediately before use. The final concentration of DMSO for both SB-366791 solutions was 0.1%. Additionally, we tested CT-nerve responses to 60-s applications (tonic responses) of 0.1 M NaCl, 1 and 5 μM benzamil + 0.1 M NaCl, 1 and 5 μM SB-366701 + 0.1 M NaCl, and to 0.2 and 2 mM of CPC + 0.1 M NaCl, 5 μM benzamil + 1 or 5 μM SB-366701 + 0.1 M NaCl, and to 5 μM benzamil + 0.2 or 2 mM of CPC + 0.1 M NaCl for consistency with experiments in the literature (DeSimone et al. 2001; Lyall et al. 2004; Lyall et al. 2005). Because benzamil, SB-366791, and CPC are light sensitive, we wrapped reservoirs containing these compounds in aluminum foil throughout the duration of the experiment. Stock solutions of benzamil and SB-366791 were stored at −20°C.
Table 1.
Conductance of test solutions (dissolved in artificial saliva) and AUC measurements for 5-s EGG responses
| Solution | Conductance, mS/cm | EGG, AUC |
|---|---|---|
| Artificial saliva | 4.85 | −0.01 ± 0.02 |
| 0.5 M sucrose | 3.25 | −1.22 ± 0.13 |
| 0.01 M citric acid | 6.13 | −3.28 ± 1.10 |
| 0.02 M QHCl | 5.99 | 3.18 ± 0.31 |
| 0.1 M KCl | 10.87 | 8.94 ± 0.74 |
| 0.03 M NaCl | 6.67 | 3.36 ± 0.42 |
| 0.1 M NaCl | 10.20 | 7.79 ± 0.52 |
| 0.3 M NaCl | 13.89 | 11.05 ± 0.94 |
| 0.5 M NaCl | 16.13 | 12.24 ± 0.80 |
| 0.03 M Na-gluconate | 5.85 | 0.75 ± 0.10 |
| 0.1 M Na-gluconate | 7.81 | 1.45 ± 0.24 |
| 0.3 M Na-gluconate | 11.36 | 2.53 ± 0.41 |
| 0.5 M Na-gluconate | 14.29 | 2.65 ± 0.50 |
| 0.3 M NH4Cl | 18.18 | 11.36 ± 0.99 |
The area under the curve (AUC) measurement for artificial saliva was derived from switching between two-stimulus channels containing artificial saliva (tactile control). High conducting solutions produce large electrogustogram (EGG) responses (larger voltage drop), but this alone does not account for EGG magnitude or polarity, as is the case for Na-gluconate and citric acid, respectively. QHCl, quinine hydrochloride.
Fig. 2.
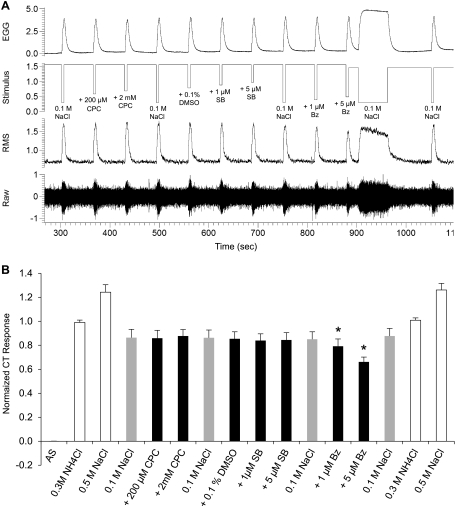
Raw electrophysiological traces from the CT nerve (A) and average responses (B; n = 7) to 0.1 M NaCl, 0.1 M NaCl mixed with cetylpyridinium chloride (CPC; 200 μM and 2 mM), SB-366791 (SB; 1 and 5 μM), 0.1% DMSO, and benzamil (Bz; 1 and 5 μM). Data were normalized to the average NH4Cl response. Comparative stimuli (0.3 M NH4Cl and 0.5 M NaCl) are shown in white, 0.1 M NaCl applications are shown in gray, and 0.1 M NaCl mixed with pharmacological antagonists of epithelial sodium channel (ENaC) and transient receptor potential vanilloid-1 (TRPV1) are shown in black. Notice that switching rinse channels containing artificial saliva (AS) had no impact on CT nerve responses. Following the 5-s application of 5 μM Bz, 0.1 M NaCl was applied for 60 s to ensure that all Bz was washed from the receptor surface before the final 5-s application of 0.1 M NaCl. *Significantly different from 0.1 M NaCl responses.
We tested each single neuron's response to 5 s of stimulation with the following stimuli: 0.5 M sucrose, 0.03–0.5 M NaCl, 0.01 M citric acid, 0.02 M QHCl, 0.1 M KCl, 0.03–0.5 M Na-gluconate, 1 μM benzamil HCl + 0.1 M NaCl, and 1 μM SB-366701 + 0.1 M NaCl. Each stimulus was presented three to nine times and averaged within cells. The standard stimuli (0.5 M sucrose, 0.1 M NaCl, 0.01 M citric acid, 0.02 M QHCl, and 0.1 M KCl), which were used to categorize geniculate ganglion neurons previously (Breza et al. 2010), were applied twice at the beginning and twice at the end of the protocol to validate the stability of the recording.
Electrical Conductance
We measured the conductance property of each test solution and AS by a custom-made solution-conductance device to compare EGG voltage deflections with solution conductance (FSU; Paul Hendrick, Fred Fletcher, and Dr. Te Tang). The device was constructed out of a Plexiglas block, with an area bored out in the center for fluid placement. The fluid reservoir was 1 cm wide, 3.333 cm long, and 3.333 cm deep. Stainless steel electrode plates (1 cm wide and 3.333 cm high) were embedded at each end of the reservoir. Exactly 10 ml of solution filled the reservoir, raising the fluid level to the top of the two electrode plates. A variac was plugged into terminals on one end of the chamber and was used to apply 100 volts (AC) across the solution between the electrodes. The output of the variac was modified with a 100 KΩ resistor, wired in series, so that only 1 mA of current was constantly applied through the chamber. A multimeter (Fluke 85 III, Everett, WA) was plugged into terminals on the end of the chamber, and the voltage of the solution was measured. The chamber was designed in accordance with solution-volume resistance: R = l/(σ × A), where R is the resistance, l is the length of the chamber, σ is the conductivity of the solution, and A is the area of the electrodes. With these factors in mind, the chamber and circuitry were designed so that 10 ml of a standard-conductivity solution (1 mS/cm; Ricca Chemical, Arlington, TX) read as 1 V across the two electrode plates; the electrical conductance of each test stimulus was relative to the conductance of the standard-conductivity solution. Since the supplied voltage and current from the variac were known and constant, the voltage output across the electrodes was related to the resistance of the solution (increasing solution concentration decreases voltage); solution conductance could then be solved for via Ohm's law because conductance = the reciprocal of resistance. Results of solution conductance are shown in Table 1.
Data Analysis
CT nerve and EGG.
A 5-s period of baseline activity immediately before each stimulus was used to calculate the area under the curve (AUC) for the integrated 5-s response during stimulation by way of vertical cursors in Spike 2. The onset of the EGG waveform (change in potential from baseline) was used to mark the time when the stimulus first contacts the lingual epithelium (change in potential from baseline) and start of analysis. These results of the EGG were used to compare relative magnitudes of the EGG waveforms with solution conductances (Table 1). Consistent responses (criteria ± 10%) to standards (0.3 M NH4Cl and 0.5 M NaCl) at the beginning and end of the protocol were indicators of nerve integrity and recording stability (see Figs. 1 and 2). Whole nerve responses were normalized to the average response to 0.3 M NH4Cl at the beginning and end of the protocol. For 60-s applications, the AUC of the tonic-neural response was calculated during the last 30 s of each stimulus (steady-state response) and was subtracted from baseline activity 30 s before the first stimulus by means of vertical cursors. The AUC during the last 30 s of the response was calculated from the change in voltage from the stimulus channel because the voltage of the EGG was virtually unaffected during the switch from 0.1 M NaCl alone to 0.1 M NaCl + drug (see Fig. 3). Data were normalized to the average 0.1 M NaCl response (beginning, middle, and end of protocol). Average responses to 0.1 M NaCl at the beginning, middle, and end of the protocol are shown in Fig. 3 to show the stability of the recordings.
Fig. 1.
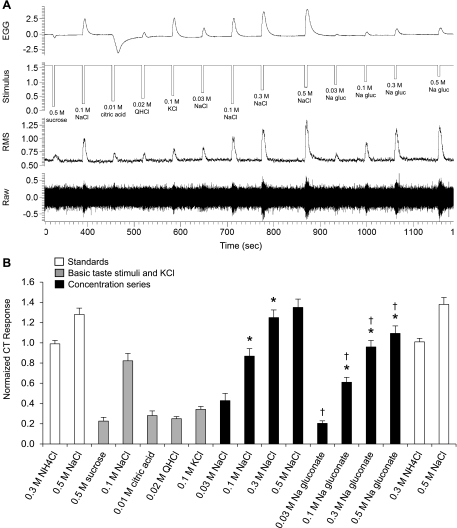
Raw electrophysiological traces from the chorda tympani (CT) nerve (A) and average responses (B; n = 6) to 0.3 M NH4Cl, 0.5 M sucrose, 0.03–0.5 M NaCl, 0.01 M citric acid, 0.02 M quinine hydrochloride (QHCl), 0.1 M KCl, and 0.03–0.5 M Na-gluconate. Data are normalized to the average NH4Cl response. Comparative stimuli (0.3 M NH4Cl and 0.5 M NaCl) are shown in white, standard taste stimuli are shown in gray, and sodium salt concentration response functions are shown in black. *Significantly different from the proceeding concentration; †significantly different from NaCl at an equimolar concentration. EGG, electrogustogram; RMS, root mean square.
Fig. 3.
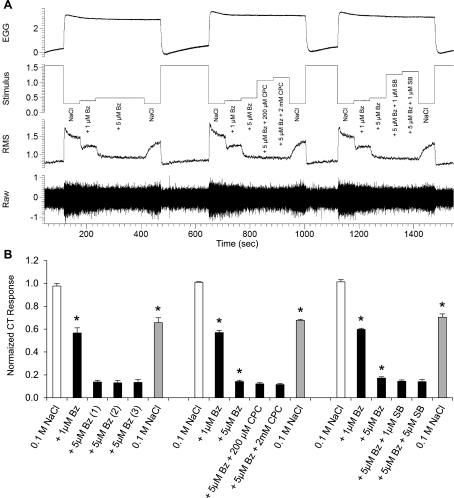
Raw electrophysiological traces from the CT nerve (A) and average responses (B; n = 4) to 0.1 M NaCl, or 0.1 M NaCl mixed with pharmacological antagonists of ENaC and TRPV1. NaCl 0.1 M was presented alone for 60 s following drug delivery to demonstrate the prolonged, competitive, antagonistic effects of Bz and to ensure its removal from the receptor surface. To measure the efficacy of TRPV1 antagonists on 0.1 M NaCl responses, 5 μM Bz were presented in combination with CPC (200 μM and 2 mM) or SB (1 and 5 μM). Data were normalized to the average 0.1 M NaCl response. Responses to 0.1 M NaCl after AS rinse are shown in white, 0.1 M NaCl mixed with pharmacological antagonists of ENaC and TRPV1 are shown in black, and 0.1 M NaCl applications following drug adaptation are shown in grey (recovery responses). *Significantly different from the proceeding stimulus.
Geniculate ganglion.
Spike templates were formed by amplitude and waveform shape. Spontaneous firing rate for each neuron was calculated as the average number of spikes/100 ms during the 5 s immediately before each stimulus. Response frequency was calculated as the difference between the spontaneous firing rate immediately before stimulation and the average number of spikes/100 ms occurring during a full 5-s period of chemical stimulation. Multiple applications of the same stimulus (baseline and stimulus) were averaged within each cell to produce an average response to that chemical stimulus. We used a hierarchal cluster analysis [Pearson's product-moment correlation coefficient (r − 1) and the average-linking method between subjects (Statistica; StatSoft, Tulsa, OK)] to categorize neurons by their average responses to 0.5 M sucrose, 0.03–0.5 M NaCl, 0.01 M citric acid, 0.02 M QHCl, 0.1 M KCl, 0.03–0.5 M Na-gluconate, 1 μM benzamil HCl + 0.1 M NaCl, 1 μM SB-366701 + 0.1 M NaCl, and the 0.1 M NaCl recovery responses following each drug.
Responses to the four basic taste stimuli and 0.1 M KCl were used to determine the breadth of tuning (H) for each neuron, calculated as H = −K ∑ pi log pi, where K is a scaling constant (1.43 for 5 stimuli) (Geran and Travers 2006), and pi is the proportion of the response to individual stimuli to which the neuron responded against the total responses to all of the stimuli (Smith and Travers 1979). H values range from 0 to 1: 0 corresponds to neurons that responded to only one stimulus, and 1 corresponds to neurons that responded equally to all the stimuli. Thus H values provide a quantitative measure of breadth of tuning. Because the entropy measure is unable to deal with negative proportions, absolute values were used for inhibitory responses; inhibitory responses were generally infrequent and of low magnitude. Taking the absolute value of inhibitory responses was the original method of dealing with inhibitory responses as discussed in Smith and Travers (1979). The results of entropy were compared with the noise-to-signal ratio (N/S), as described previously (Spector and Travers 2005). Briefly, the N/S was defined as the product of the response to the second-best stimulus and the reciprocal of the response to the best stimulus (N/S = 2nd best stimulus × 1/best stimulus). Effect size, using the Glass's d method [d = (x̄2 − x̄1)/SD x̄1] was calculated for each neuron's average response to a chemical stimulus over the full 5-s period and was used to indicate the response magnitude for each neuron and as a neuron group. By convention, the effect sizes of 0.2, 0.5, and 0.8-∞ are used to indicate small, medium, and large effects (Cohen 1992) and are, therefore, used here to indicate the size of the effect (difference in response from baseline). The results from the N/S and Glass's d were compared with the breadth of tuning results shown in Table 2.
Table 2.
Breadth of tuning, noise-to-signal ratios, and effect sizes to 5-s applications with the standard stimuli
| Glass's d |
|||||||
|---|---|---|---|---|---|---|---|
| Neuron Type | H, 5 Stimuli | N/S, 2 Stimuli | Sucrose | NaCl | Citric acid | QHCl | KCl |
| NaCl specialist | 0.41 ± 0.05 | 0.15 ± 0.04 | 1.32 ± 0.33 | 20.78 ± 6.16 | 0.00 ± 0.10 | 0.28 ± 0.15 | 1.01 ± 0.39 |
| Acid generalist | 0.90 ± 0.01 | 0.64 ± 0.03 | 1.34 ± 0.25 | 6.32 ± 0.75 | 6.34 ± 1.06 | 2.90 ± 0.55 | 2.79 ± 0.34 |
Values are means ± SE. Breadth of tuning is indicated by H values for each neuron group, whereas noise-to-signal ratios are indicated by N/S values, and effects sizes are indicated by Glass's d for each of the standard stimuli.
Spikes/100 ms (relative to baseline) were calculated for each neuron's average response to a chemical stimulus 500 ms and 1 s after stimulus onset and averaged for each neuron group for detailed analysis of spike rate during behaviorally relevant time frames (Halpern and Tapper 1971). This method was also used to assess the effect of 1 μM benzamil and 1 μM SB-366791 on 0.1 M NaCl responses, in NaCl-specialist and acid-generalist neurons. Here we calculated spikes/100 ms (relative to baseline) for each second following stimulus onset to validate when the antagonist effectively attenuated 0.1 M NaCl responses. Effects sizes (Glass's d) for each 100-ms bin following stimulus onset were calculated for each neuron's average response to a chemical stimulus and were averaged within each neuron group to estimate response latency and response magnitude (peak response) for comparison with our recent geniculate ganglion study (Breza et al. 2010). Here, we used the medium size effect (0.5) to determine response latency. The effect size needed to stay at or above 0.5 for at least five 100-ms bins for response latency is to be determined.
Statistics.
Further statistical analyses were conducted with appropriate analysis of variance (ANOVA; Statistica; StatSoft, Tulsa, OK) within and between subjects. One-way repeated-measures ANOVAs were used to evaluate the effect of stimulus quality and intensity, and drug on the integrated CT nerve response (5 and 60 s), and from 5-s responses to all stimuli within neuron group. EGG responses were averaged and presented (Table 1); however, statistical analyses were not performed on EGG waveforms because, at this juncture, the exact nature of these signals are still ambiguous (amplitude, shape, polarity, etc.) and, therefore, are used primarily as a marker of stimulus onset and validation of stimulus delivery (see below). Two-way ANOVAs were used to compare spikes/100 ms (500 ms and 1 s) for NaCl (0.03–0.5 M) and Na-gluconate (0.03–0.5 M), within and between neuron groups (stimulus × group). Two-way repeated-measures ANOVAs were used to compare the spikes/100 ms (1-s intervals) for NaCl with or without drug within neuron type (stimulus × time). Significant main effects or interactions (P < 0.05) of ANOVAs were further examined with Fisher's least significant difference method. Graphic and tabular data are presented as group means ± SE.
RESULTS
CT and EGG: Standard Taste Stimuli and Sodium Salts
Shown in Fig. 1 are the typical responses from one CT nerve recording and the average responses from six male rats to 5-s applications of the standard taste stimuli, 0.3 M NH4Cl, and to a NaCl and Na-gluconate concentration series (0.03–0.5 M). CT nerve response magnitude varied by taste stimulus [F(16,80) = 98.56, P < 0.001]. The responses to 0.1 M NaCl were significantly greater (all P values < 0.001) than those to KCl and the other standard stimuli; KCl responses were equal to sucrose, citric acid, and QHCl responses. Responses to NaCl increased with each concentration (P < 0.001), except from 0.3–0.5 M; whereas responses to Na-gluconate increased for all stimulus concentrations (all P values < 0.05). Responses to NaCl were greater than those to Na-gluconate at equimolar concentrations (all P values < 0.001). Responses to 0.5 M NaCl and 0.3 M NH4Cl at the beginning and end of the protocol did not change statistically, demonstrating response stability over the recording interval. Notably, each test solution elicited a unique, but concentration-dependent EGG waveform reflective, in part, of the conductive/resistive properties of the solution (Table 1) and indicative of controlled delivery of test solutions to the tongue. Interestingly, citric acid and Na-gluconate produced EGG waveforms that could not be explained by the solution conductance alone (see Table 1): other factors are involved. For example, although citric acid is more conductive than AS alone, citric acid produced EGG deflections that were the opposite polarity from other conducting solutions; and NaCl produced EGG deflections that were 4.4–5.4× greater than those to equimolar concentrations of Na-gluconate, despite the fact that measures of solution conductance were similar.
CT: Ion Channel Antagonists
Shown in Fig. 2 are the typical responses from one CT nerve recording and the average responses of seven male rats to short-duration 5-s applications of 0.1 M NaCl, with and without ion channel antagonists, as well as the average responses to 0.3 M NH4Cl and 0.5 M NaCl at the beginning and end of the protocol, and to AS (tactile control). CT nerve response magnitude varied by taste stimulus [F(14,84) = 30.66, P < 0.001]. Five-second responses to 0.1 M NaCl were unaffected by DMSO (SB-366791 vehicle) or TRPV1 channel antagonists. In contrast, benzamil suppression of 0.1 M NaCl responses was dose dependent: 7% with 1 μM (P < 0.05) and 24% with 5 μM (P < 0.001). Switching fluid delivery between two channels containing AS had no discernible effect on CT nerve (Fig. 2) or EGG response magnitudes (Table 1), indicating the reliability of the stimulus delivery system to present stimuli at controlled flow rate. Responses to 0.5 M NaCl and 0.3 M NH4Cl at the beginning and end of the protocol did not change statistically, demonstrating response stability over the recording interval. Notably, the 0.1 M NaCl (with or without drug) elicited consistent EGG waveforms, indicating controlled delivery of test solutions to the tongue.
Shown in Fig. 3 are the typical responses from one CT nerve recording and the average responses of four male rats to long-duration 60-s applications of 0.1 M NaCl, with or without ion channel antagonists. Benzamil suppression of 0.1 M NaCl tonic responses [F(17,51) = 327.28, P < 0.001] was greater with 60-s application and dose-dependent 44% with 1 μM (P < 0.001) and 86% with 5 μM (P < 0.001). Even with longer duration application, the addition of TRPV1 antagonists CPC (200 μM and 0.2 M) or SB-366791 (1 μM and 5 μM) was without effect on tonic NaCl responses.
Geniculate Ganglion: Basic Firing Characteristics
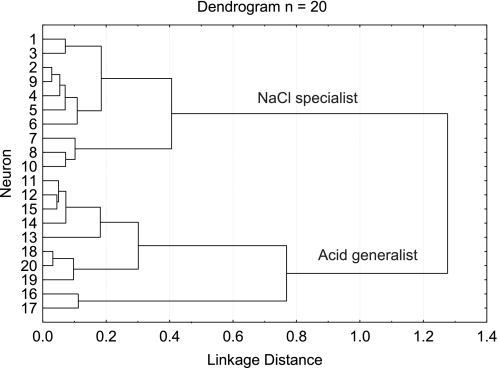
Hierarchical cluster analysis grouped neurons (n = 20) on the basis of their responses to all test stimuli (see methods); results from this analysis are shown in the dendrogram (Fig. 4). Analysis of agglomeration by means of a scree plot (data not shown) indicated that an abrupt upward deflection occurred at 0.41, which separated the neurons into two main groups (NaCl specialists and acid generalists). Two neurons (Fig. 4; 16 and 17) fell outside the acid-generalist cluster, but they closely resembled neurons within the cluster and, therefore, were included in the group. In fact, the only difference between those two neurons and the other eight in the cluster was the proportion of their citric acid response to other stimuli, which was more robust than that of the other neurons in the group.
Fig. 4.
Dendrogram of the results from the hierarchical cluster analysis.
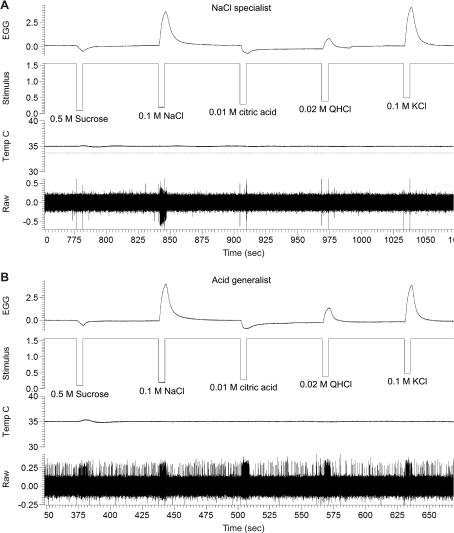
Shown in Fig. 5 is the typical response profile of a NaCl-specialist and an acid-generalist neuron to 5-s stimulation with the standard stimuli. As shown, the NaCl-specialist neuron responds selectively to NaCl in a background of low spontaneous firing rate, whereas the acid-generalist neuron responds broadly to all five standard stimuli in a background of high spontaneous firing rate, consistent with our laboratory's recent studies (Breza et al. 2010; Lawhern et al. 2011). Notably, each test solution elicited a unique and consistent EGG waveform on 2 separate recording days, indicative of controlled delivery of test solutions to the tongue.
Fig. 5.
Raw electrophysiological traces from a NaCl specialist (A) and from an acid generalist (B) in response to 0.5 M sucrose, 0.1 M NaCl, 0.01 M citric acid, 0.02 M QHCl, and 0.1 M KCl.
NaCl Specialists and Acid Generalists: Response Profiles to Standard Stimuli, ENaC and TRPV1 Antagonists
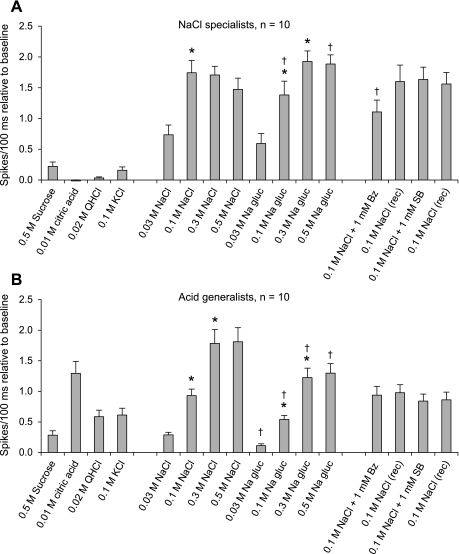
Shown in Fig. 6 are the average 5-s responses of NaCl-specialist and acid-generalist neurons. NaCl specialists responded robustly to 0.1 M NaCl and little if at all to the other standard stimuli [F(15,135) = 43.37, P < 0.001] and were, therefore, narrowly tuned, with a low N/S (Table 2). Although there was no overall difference in NaCl and Na-gluconate responses, there was a different pattern of response across concentration. Responses to NaCl increased from 0.03 to 0.1 M (P < 0.001), but 0.1 M responses were similar to 0.3 and 0.5 M NaCl; responses to Na-gluconate increased between 0.03 and 0.3 M, but 0.3 and 0.5 M responses were similar. Responses to 0.1 M NaCl were greater than those to 0.1 M Na-gluconate (P < 0.05), but responses to 0.5 M NaCl were less than those to 0.5 M Na-gluconate (P < 0.01). The ENaC blocker benzamil attenuated responses to 0.1 M NaCl by 37% (P < 0.001), while the TRPV1 antagonist SB-366791 had no effect.
Fig. 6.
Average responses to all test stimuli (5-s presentations relative to baseline) from NaCl specialists (A) and acid generalists (B). *Significantly different from the proceeding concentration; †significantly different from NaCl at an equimolar concentration.
Acid generalists responded broadly to test stimuli [F(15,135) = 30.46, P < 0.001]. Responses to citric acid were significantly greater than those to other standard stimuli representing the basic taste qualities (P values < 0.01): the order of stimulus effectiveness was citric acid > 0.1 M NaCl > QHCl = KCl > sucrose (significant P values ≤ 0.02). Responses to NaCl and Na-gluconate increased from 0.03–0.3 M (all P values ≤ 0.001), but responses to NaCl were greater than those to Na-gluconate from 0.1–0.5 M (all P values < 0.01). Neither benzamil nor SB-366791 had any effect on 0.1 M NaCl responses.
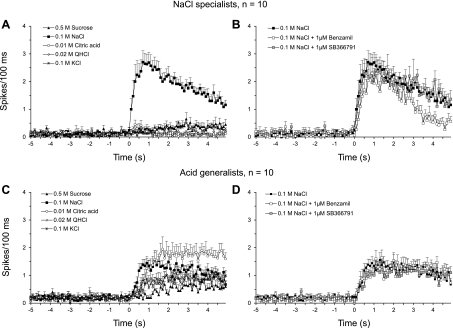
Shown in Fig. 7 are average-response profiles of NaCl specialists and acid generalists to the standard stimuli (5-s applications) and to specific ENaC and TRPV1 antagonists, averaged in 100-ms intervals. NaCl specialists responded robustly to 0.1 M NaCl and weakly at best to other taste stimuli. Consequently, the breadth of tuning for NaCl specialists was narrow over 5 s, as was the N/S, and the effect size for 0.1 M NaCl was 20× greater than to the other standard taste stimuli (see Table 2). The NaCl-specialists group responded to 0.1 M NaCl with a short latency and spike rate peaked before 1 s after stimulus onset (see Table 3). The effect sizes for other taste stimuli did not reach or stay at 0.5 for more than 100 ms throughout the entire 5-s stimulation, indicating that other taste stimuli have weak effects on spike frequency in narrowly tuned NaCl-specialist neurons.
Fig. 7.
Average response profiles to 0.5 M sucrose, 0.1 M NaCl, 0.01 M citric acid, 0.02 M QHCl, and 0.1 M KCl from NaCl specialists (A) and acid generalists (C), and to 0.1 M NaCl alone or mixed with 1 μM Bz or 1 μM SB from NaCl specialists (B) and acid generalists (D) in 100-ms bins. Vertical line indicates stimulus onset (time 0).
Table 3.
Response latencies and peak-response frequencies of NaCl specialists and acid generalist to test stimuli as measured by Glass's d
| Stimulus | Response Latency (NaCl Specialists) | Peak Response (NaCl Specialists) | Response Latency (Acid Generalists) | Peak Response (Acid Generalists) |
|---|---|---|---|---|
| 0.5 M sucrose | ||||
| Range, ms | N/A | 2,900–2,999 | 1,600–1,699 | 2,300–2,399 |
| d | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.8 ± 0.2 | |
| 0.01 M citric acid | ||||
| Range, ms | N/A | 300–399 | 600–699 | 1,700–1,799 |
| d | 0.4 ± 0.2 | 0.5 ± 0.2 | 1.8 ± 0.4 | |
| 0.02 M QHCl | ||||
| Range, ms | N/A | 600–699 | 600–699 | 1,900–1,999 |
| d | 0.3 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.2 | |
| 0.1 M KCl | ||||
| Range, ms | N/A | 2,500–2,599 | 700–799 | 1,900–1,999 |
| d | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.2 | |
| 0.03 M NaCl | ||||
| Range, ms | 100–199 | 600–699 | 1,300–1,399 | 1,600–1,699 |
| d | 0.5 ± 0.2 | 1.6 ± 0.3 | 0.6 ± 0.1 | 0.8 ± 0.2 |
| 0.1 M NaCl | ||||
| Range, ms | 100–199 | 700–799 | 300–399 | 1,300–1,399 |
| d | 1.1 ± 0.4 | 2.9 ± 0.4 | 0.5 ± 0.1 | 1.5 ± 0.3 |
| 0.3 M NaCl | ||||
| Range, ms | 0–99 | 800–899 | 200–299 | 1,400–1,499 |
| d | 0.6 ± 0.2 | 3.3 ± 0.4 | 0.5 ± 0.2 | 2.6 ± 0.4 |
| 0.5 M NaCl | ||||
| Range, ms | 0–99 | 1,000–1,099 | 100–199 | 1,600–1,699 |
| d | 0.7 ± 0.3 | 3.5 ± 0.3 | 0.5 ± 0.2 | 2.4 ± 0.3 |
| 0.03 M Na-gluconate | ||||
| Range, ms | 200–299 | 1,000–1,099 | N/A | 2,400–2,499 |
| d | 0.7 ± 0.4 | 1.3 ± 0.3 | 0.5 ± 0.1 | |
| 0.1 M Na–gluconate | ||||
| Range, ms | 100–199 | 1,300–1,399 | 500–599 | 2,500–2,599 |
| d | 0.8 ± 0.4 | 2.3 ± 0.4 | 0.5 ± 0.2 | 1.1 ± 0.1 |
| 0.3 M Na gluconate | ||||
| Range | 100–199 | 700–799 | 300–399 | 1,300–1,399 |
| d | 1.3 ± 0.6 | 3.4 ± 0.4 | 0.5 ± 0.2 | 2.0 ± 0.3 |
| 0.5 M Na-gluconate | ||||
| Range, ms | 100–199 | 700–799 | 300–399 | 1,500–1,599 |
| d | 0.9 ± 0.5 | 3.6 ± 0.5 | 0.5 ± 0.2 | 2.4 ± 0.3 |
| 1 μM benzamil | ||||
| Range, ms | 200–299 | 900–999 | 300–399 | 1,400–1,499 |
| d | 0.6 ± 0.3 | 2.6 ± 0.5 | 0.5 ± 0.1 | 1.5 ± 0.3 |
| 1 μM SB-366791 | ||||
| Range, ms | 100–199 | 500–599 | 300–399 | 1,100–1,199 |
| d | 0.7 ± 0.3 | 2.3 ± 0.5 | 0.6 ± 0.2 | 1.4 ± 0.2 |
Acid generalists responded robustly to 0.01 M citric acid and 0.1 M NaCl, moderately to 0.02 M QHCl and 0.1 M KCl, and weakly to 0.5 M sucrose over the 5-s stimulus duration. Consequently, the breadth of tuning for acid generalists was broad, as was the N/S, and the effect sizes for citric acid and 0.1 M NaCl were two to six times greater than those to the other standard taste stimuli (see Table 2). Effect sizes for 0.01 M citric acid and 0.1 M NaCl over the 5-s period were virtually identical, as were effect sizes for 0.02 M QHCl and 0.1 M KCl (Table 2). There were, however, remarkable differences in responses latencies and peak responses between stimuli, which are largely in agreement with our recent investigation (Breza et al. 2010). The response latency to 0.1 M NaCl was longer than those to NaCl specialists, and spike frequency peaked later. The response latency to citric acid and to QHCl was identical, but spike frequency to citric acid peaked earlier than QHCl. KCl had a slightly longer latency than citric acid and QHCl, but peaked at the same time as QHCl. The response latency to sucrose was longer; spike frequency peaked late and oscillated around the peak for the remainder of the 5-s application.
For NaCl-specialist neurons, there were significant main effects for drug [F(2,18) = 45.79, P < 0.001] and time [F(4,36) = 2.81, P < 0.05], and a significant drug × time interaction [F(8,72) = 2.81, P < 0.01] on response frequency. As shown in Fig. 7, benzamil significantly inhibited NaCl response frequency by 37%. This inhibition was evident at response onset and increased progressively over time. For example, benzamil increased the average response latency by 100 ms and delayed the peak response by 200 ms (see Table 3). By the end of the 5-s stimulation period, the average response to NaCl with benzamil was about near baseline. In contrast, SB-366791 had no effect on NaCl responses by NaCl-specialist neurons. For acid generalists, there were no significant effects of drug or time on response frequency. Neither benzamil nor SB-366791 influenced latency and peak responses to NaCl as shown in Table 3 and in Fig. 7.
NaCl Specialists and Acid Generalists: Concentration Response Profiles to Sodium Salts
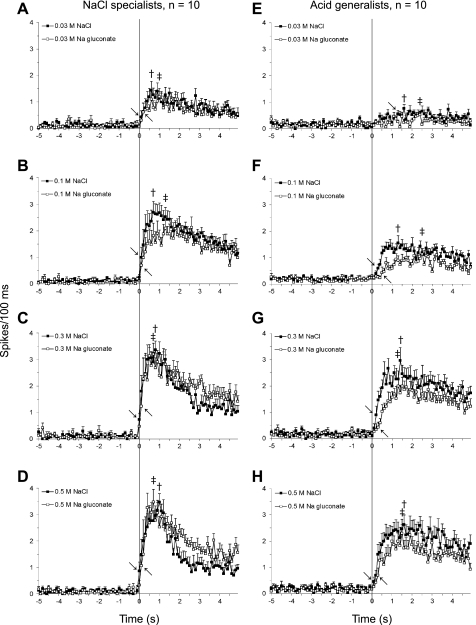
Figure 8 shows the average response patterns (100-ms bins) of NaCl specialists (left) and acid generalists (right) to four concentrations of NaCl and Na-gluconate. Overall, spike rates were greater and response latencies shorter for NaCl specialists than for acid generalists (see Table 3). As a rule for both neuron groups, response frequency increased and response latency decreased with increasing stimulus concentration.
Fig. 8.
Average concentration responses profiles (0.03–0.5 M) of NaCl specialists (A–D) and acid generalists (E–H) to NaCl and to Na-gluconate in 100-ms bins. Vertical line indicates stimulus onset (time 0), → indicates response latency to NaCl, whereas ← indicates response latency to Na-gluconate. †Peak responses to NaCl; ‡peak responses to Na-gluconate.
Although the average 5-s responses by NaCl-specialist neurons were similar to both salts (see Fig. 6), there were notable differences in the temporal response pattern (Fig. 8) as reflected by latency and peak response measures (see Table 3). As indicated above, response latency decreased as concentration increased for both sodium salts; however, the average response latencies were shorter to NaCl than to Na-gluconate at all concentrations, except 0.1 M. Peak responses also differed between the two salts, with earlier NaCl peaks for the two weakest concentrations, but earlier Na-gluconate peaks for the two strongest concentrations.
Similar to NaCl specialists, the response latency of acid-generalist neurons decreased as concentration increased for the sodium salts, but was always shorter to NaCl than to Na-gluconate. Peak responses occurred earlier for all NaCl concentrations, except for the strongest concentration, where the peak response to 0.5 M gluconate occurred earlier than that to NaCl. As a group, acid generalists were weakly responsive, at best, to 0.03 M NaCl and completely unresponsive to 0.03 M Na-gluconate because the effect size was never at or above 0.5 for more than 100 ms.
Response Magnitude During 500–1,000 ms: Phasic Responses
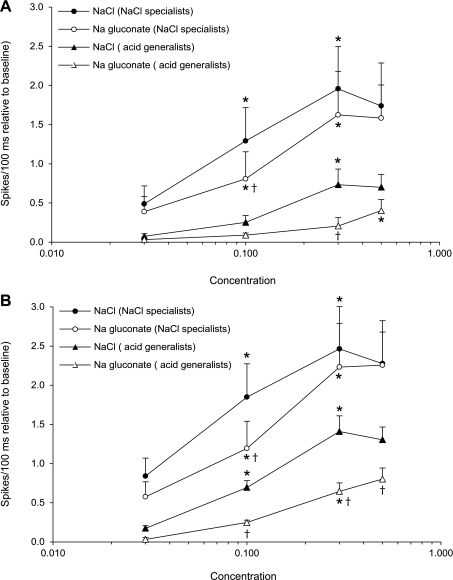
Figure 9 is a plot of the average responses (spikes/100 ms) occurring over the first 500 and first 1,000 ms after stimulus onset by NaCl-specialist and acid-generalist neurons. Over the first 500 ms, there were significant main effects for stimulus [F(7,126) = 13.46, P < 0.001] and neuron group [F(1, 18) = 5.67, P < 0.05], and a significant stimulus × group interaction [F(7,126) = 2.81, P < 0.01]. The plot shows that NaCl specialists increased their response to NaCl and Na-gluconate as concentration increased from 0.03 to 0.3 M (all P values < 0.05). NaCl specialists responded similarly to 0.03, 0.3, and 0.5 M NaCl and Na-gluconate, but responded significantly greater to 0.1 M NaCl than to 0.1 M Na-gluconate (P < 0.05). NaCl specialists responded more to NaCl and Na-gluconate than did acid generalists (P values < 0.05) across all concentrations, except 0.03 M. Acid generalists increased their response to NaCl as concentration increased from 0.1 to 0.3 M (P < 0.05) and increased their response to Na-gluconate as concentration increased from 0.3 to 0.5 M (P ≤ 0.05), but were virtually unresponsive to 0.03–0.1 M NaCl and 0.03–0.3 M Na-gluconate. Furthermore, acid generalist's responses to 0.3 M NaCl were greater than those to 0.3 M Na-gluconate (P < 0.01), but responses to both salts were equal at 0.5 M.
Fig. 9.
Spikes/100 ms (relative to baseline) to NaCl and Na-gluconate in half-log increments (0.03–0.5) in NaCl specialists and acid generalists for the first 500 ms (A) and 1 s after stimulus onset (B). *Significantly different from the proceeding concentration; †significantly different from NaCl at an equimolar concentration.
Over the first 1,000 ms, there were significant main effects for stimulus [F(7,126) = 43.83, P < 0.001] and neuron group [F(1, 18) = 10.70, P < 0.01], and a significant stimulus × group interaction [F(7,126) = 3.77, P < 0.001]. For NaCl-specialist neurons, the addition of 500 ms to the response measure had little impact beyond that already described above, whereas, for acid generalists, the effect of adding 500 ms was pronounced. For NaCl specialists, response frequency increased as concentration increased from 0.03 to 0.3 M (all P values < 0.01) to both sodium salts. NaCl specialists responded similarly to 0.03, 0.3, and 0.5 M NaCl and Na-gluconate, but significantly more to 0.1 M NaCl (P < 0.001) than to 0.1 M Na-gluconate. Acid generalist's responses to NaCl and Na-gluconate increased with concentration. Responses to NaCl increased with increasing stimulus concentration from 0.03 to 0.3 M (P values < 0.01), whereas responses to Na-gluconate were equal at 0.03 and 0.1 M, but both were less than 0.3 and 0.5 M (P values < 0.01). At the lowest concentration (0.03 M), responses to NaCl and Na-gluconate were equal because acid generalists were unresponsive to both salts at this concentration during the first second; however, responses to NaCl were greater than those to Na-gluconate at 0.1–0.5 M (P values ≤ 0.01). Overall, NaCl specialists responded more to 0.03–0.5 M NaCl and Na-gluconate than did acid generalists at each concentration (all P values < 0.05).
DISCUSSION
The purpose of our research was to determine how two well-known salt-sensing pathways, one that processes information specific to sodium, and the other that processes information broadly to a range of cation salts, code information about taste quality and intensity. Our research focused on the spike discharge patterns that occurred early after stimulation, because this is the time when rats make critical decisions to either ingest or avoid a taste solution (Halpern and Tapper 1971). We explored two major issues concerning salt taste: what are the receptor mechanisms, and how does anion size influence neural responses. First, we hypothesized that sodium-specific pathway would use ENaC exclusively, while the nonselective pathway would use TRPVt, to detect sodium solutions. With fine-grained analysis of the temporal response features of both neuron groups to pharmacological antagonism of ENaC or TRPV1t, we confirmed the former and refuted the latter. Second, we hypothesized that anion size would reduce responses of the nonselective pathway, but not the sodium-specific pathway. We found that anion size had a large effect on the nonselective pathway, but also influenced responses of the selective pathway as well. Additionally, we showed that the EGG is not only a reliable measure of stimulus onset, but also is an informative measure for taste solution interactions with the lingual epithelium. These findings will be addressed in detail below: first with emphasis on the overall effects of test stimuli on the lingual epithelial potential (EGG) and integrated CT nerve responses, and second on the effects of test stimuli on two well-known sodium-responsive neuron types, NaCl specialists and acid generalists.
EGG and CT Nerve Responses
Examination of the relationship among conductance, EGG, and CT nerve responses provides a useful heuristic for understanding how each chemical stimulus interacts with the lingual epithelium. NaCl-, KCl-, and NH4Cl-rated high electrical conductance (Table 1) elicited concentration-dependent EGG and CT nerve responses (Fig. 1). The conductance values for 0.1 M NaCl and 0.1 M KCl were similar as were their EGG responses in terms of waveform amplitude, polarity, and shape. As we have seen previously (Breza et al. 2010), sucrose elicited a small downward EGG deflection of opposite polarity to ionic solutions (NaCl, KCl, QHCl). In the present study, we found that the conductance of 0.5 M sucrose mixed in AS was less than that of AS alone (Table 1). Therefore, the downward EGG deflection to sucrose most likely reflected a decrease in conductance from baseline AS. This indicates that a large portion of the EGG response to sucrose is electrochemical in nature. Although the polarity and magnitude of EGG waveforms of sucrose, NaCl, and KCl appear to reflect only the electrochemical properties of solutions (i.e., conductivity/resistivity), Na-gluconate and citric acid did not follow this pattern: other factors may be involved. The conductance values for 0.01 M citric acid, 0.02 M QHCl, and 0.03 M Na-gluconate were similar (Table 1), but the EGG response to QHCl was more than three times larger than the EGG response to Na-gluconate, and citric acid produced a large EGG response that was of opposite polarity to the ionic solutions (Table 1). In fact, the EGG response to citric acid often persisted after stimulus offset, indicating a lag in recovery of the lingual epithelium, perhaps due to a reduction in the pH of cells in the lingual epithelium (Lyall et al. 2001), and ongoing studies are addressing this issue.
The CT nerve responded more to NaCl than to Na-gluconate, consistent with previous studies showing large anion inhibition of CT nerve responses to sodium solutions (Beidler, 1954; Ye et al. 1991). This difference in response magnitude between the two salts was much greater for the EGG than the CT nerve; the EGG response to Na-gluconate was much reduced. The EGG results are consistent with measurements of lingual-transepithelial conductance (Ye et al. 1991; 1993; 1994). Chloride is highly permeable through the paracellular pathway, facilitating diffusion of positively charged ions to receptor sites below the epithelial surface (Ye et al. 1991). Gluconate is about 5.5 times larger than chloride and, therefore, poorly permeable through the paracellular pathway. Consequently, gluconate compromised sodium diffusion through tight junctions and onto receptor sites deep within the epithelium (Elliott and Simon 1990; Ye et al. 1991; 1993).
The conductance values for NaCl were modestly higher than those for equimolar concentrations of Na-gluconate (Table 1), but this difference was too minor to account for the large difference in EGG and CT response magnitudes to the two salts. The difference in EGG and CT signals also cannot be due to a difference in sodium concentration (dissociation), as both salts are highly soluble in water. It is highly probable that the impermeable gluconate anion caused a small voltage change across the lingual epithelium, leading to a small EGG response. The small voltage change to Na-gluconate is the result of lessened electroneutral driving force for sodium diffusion to paracellular-receptor sites. If sodium transduction occurs mostly at the apical membrane of taste receptor cells, then it makes sense that CT nerve responses to sodium would be less affected by anion size than the EGG.
Sodium Transduction Mechanisms: Apical and Nonapical Transduction Sites
It is generally accepted that salt taste detection depends upon two salt-sensing pathways that involve specialized membrane channels on taste bud cells of rats. In the present study, we found that short, 5-s applications of the potent, but selective, ENaC antagonist, benzamil, significantly reduced CT nerve responses to 0.1 M NaCl in a dose-dependent manner (Fig. 2). Prolonged benzamil exposure again in a dose-dependent manner dramatically reduced 0.1 M NaCl responses (Fig. 3). In fact, 60–180 s of exposure to the highest concentration of benzamil (5 μM benzamil) reduced the tonic response of 0.1 M NaCl by nearly 86%, consistent with the notion that ENaC is the principle ion channel for the transduction of sodium in rats (Heck et al. 1984). These findings are consistent with the results of previous studies showing that 100 μM amiloride reduced by ∼75% the CT nerve response to 0.1 M NaCl at room temperature (Heck et al. 1984; Ye et al. 1993). Benzamil is a large, hydrophilic molecule and, therefore, is impermeable to tight junctions. The fact that benzamil had an immediate effect on NaCl responses suggests that it inhibited ENaC channels on the apical membrane of taste receptor cells.
To investigate the role of TRPV1 channels in salt taste, we used CPC, a water-soluble TRPV1 modulator (DeSimone et al. 2001) and SB-366791, a highly specific but lipophilic TRPV1 antagonist, previously shown to inhibit the amiloride-insensitive component of the CT nerve response to NaCl (Lyall et al. 2004; Lyall et al. 2005). Surprisingly, we were unable to confirm the earlier findings, despite the fact that we used the same effective doses of CPC as used in the DeSimone (2001) study, and a 5 μM dose of SB-366791 that was five times stronger than the most effective dose used in the Lyall et al. (2004 and 2005) investigations. There is a difference in stimulation methods between the present and earlier studies that may account for the discrepant findings. We delivered solutions topically over a freely exposed tongue at a constant slow flow rate. This method of stimulation and pharmacological antagonism is extremely effective when receptors are located on the apical membrane of taste cells. Thus the present results show clearly that TRPV1 channels are not located on the apical membrane of taste receptor cells; however, it is possible that TRPV1 receptors are located along the basal membranes of taste-receptor cells or on fibers below tight junctions, but high-molecular-weight antagonists, like CPC and SB-366791, cannot penetrate the tight junction barrier to exert an effect. This latter point is consistent with the anion effect on sodium responses in acid generalists, as discussed in detail below. In the earlier studies (DeSimone et al. 2001; Lyall et al. 2004; Lyall et al. 2005), the tongue was encased into a Lucite chamber and sealed in place by vacuum while stimulus solutions were flushed into the chamber at high flow rate. The combination of negative pressure over the tongue and higher stimulus velocity may have increased the permeability of TRPV1 antagonists to receptor sites below tight junctions. Consequently, it was only with this stimulation procedure that TRPV1 antagonists suppressed rat CT nerve responses. Taken altogether, we cannot provide evidence for a functional role of TRPV1 channels in salt taste detection, nor can we discount it entirely either.
Response Profiles of NaCl-Specialist and Acid-Generalist Neurons
We recorded from two neuron classes distinguished by their unique response patterns to the basic taste stimuli, KCl, Na-gluconate, and benzamil. NaCl specialists responded almost exclusively to sodium solutions, and the responses to NaCl were significantly attenuated by benzamil, consistent with our laboratory's prior studies of the geniculate ganglion (Breza et al. 2007; 2006; Breza et al. 2010; Lundy and Contreras 1999) and single fiber studies in the rat (Frank et al. 1983) and hamster (Hettinger and Frank 1990) CT nerve. In fact, NaCl-specialist neurons were so narrowly tuned that the effect size for their response to NaCl was 20× greater than the effect sizes to the other standard stimuli. The N/S and Glass's d were more accurate predictors of breadth of tuning, since some inhibitory responses increased the entropy measure, and N/S do not take into account responses to all stimuli. When averaged over the entire 5-s period, NaCl specialists responded robustly to NaCl and Na-gluconate across a range of concentrations. Although NaCl-specialist neurons distinguished NaCl from Na-gluconate by subtle differences in temporal pattern, the overall 5-s response rate was similar for the two salts.
An important difference between the present and our prior study (Breza et al. 2010) was a change in our stimulus delivery system from the former, providing a graded change in stimulus concentration (due to 10-fold higher dead volume level in the delivery tube when switching from rinse to stimulus) to the present providing an abrupt change. This had a profound influence mostly on the phasic portion of stimulus-evoked neural response across a range of concentrations (Fig. 8), but also influenced the average 5-s response, as well (Fig. 6). In our previous study, the spike discharge rate to NaCl peaked around 2 s and remained at a relatively high rate with little decay throughout the 5-s stimulus period. In the present study, the spike discharge rate to NaCl peaked early (less than 1 s), especially to the higher concentrations, and adapted to a progressively slower rate over the 5-s stimulus interval (Fig. 8). In the present study, the 5-s response measure lacked the sensitivity to discriminate the three strongest NaCl concentrations, whereas the immediate phasic response rate (Figs. 8 and 9) and response latency (Table 3) changed more predictably with stimulus concentration. As NaCl concentration increased, phasic response rate increased, and response latency decreased. This is consistent with whole nerve experiments (Bealer 1978) showing that the phasic portion of the CT nerve response was responsible for coding NaCl intensity information. Furthermore, benzamil's suppression of NaCl-specialist responses to NaCl was immediate, dose dependent, and increased with time. This was evident by a reduction in phasic response rate and an increase in response latency (Breza et al. 2010), reflecting the importance of the ENaC to immediate NaCl detection. The present results match well with psychophysical results, demonstrating that rats can discriminate differences in NaCl concentration with just five licks (<1 s) of the solution (Geran and Spector 2000b).
Consistent with our laboratory's prior studies (Breza et al. 2010; Lundy and Contreras 1999), acid generalists responded best to citric acid over 5 s, second best to NaCl, and less to QHCl, and KCl. Our acid-generalist neurons are analogous to H units identified in single-fiber experiments in the rat (Frank et al. 1983; Ninomiya and Funakoshi 1988) and hamster CT (Hettinger and Frank 1990; Rehnberg et al. 1993). Like NaCl specialists, acid generalists responded with increased spike rate and decreased response latency as sodium concentration increased. As noted in our earlier paper (Breza et al. 2010), acid generalists are an intriguing neuron group because they responded with a shorter latency and higher spike rate over the first second of stimulation to NaCl than to citric acid. Based on temporal response pattern, acid generalists are chameleon-like as they are first NaCl generalists and then later acid generalists. While we do not yet understand their function, we could not appreciate acid generalists' unique response characteristics without a means to mark stimulus onset.
In contrast to NaCl specialists, acid-generalist neurons responded with lower phasic spike rate, longer latency, and high threshold to NaCl and Na-gluconate. This contrasts with an earlier report (Frank et al., 1983) showing that H units had a lower NaCl threshold than NaCl specialists. In the earlier study, room temperature distilled water served as the rinse and solvent for the stimuli, while in the present study body temperature AS served as the rinse and solvent. This is important because the salt composition of AS elevates the spontaneous firing rate of acid-generalist neurons, but does not influence that of NaCl specialists (Breza et al. 2010). Consequently, when using AS as the rinse, acid generalists become unresponsive to sodium concentrations below adapting salivary sodium concentration and have a higher sodium threshold. The present study showing that NaCl specialists have a lower sodium threshold is consistent with detection thresholds in rats (Geran and Spector 2000b) and intake tests in conditional ENaC knockout mice (Chandrashekar et al. 2010).
In the present study, sodium response frequency in acid generalists was profoundly attenuated by the large anion gluconate, consistent with single-fiber recordings with Na-acetate in hamsters (Rehnberg et al. 1993). Our findings that Na-gluconate decreased response frequency and increased response latency are consistent with human psychophysical findings that large anions decrease stimulus intensity and increase reaction times (Delwiche et al. 1999; van der Klaauw and Smith 1995).
Neither benzamil nor SB-366791, both of which are impermeable to tight junctions, influenced NaCl responses of acid-generalist neurons. We expected benzamil to be ineffective, but anticipated that the TRPV1 antagonist would suppress a portion of NaCl responses consistent with prior whole nerve studies (DeSimone et al. 2001; Lyall et al. 2004; Lyall et al. 2005) that suggested a variant of the TRPV1 channel was involved, at least in part, in NaCl transduction. As noted earlier, TRPV1 receptors may be located below tight junctions and inaccessible with our stimulation method. Since large-anion inhibition is a special feature of acid-generalist neurons, it is likely that these neurons are in synaptic contact with taste cells below their tight junction barrier where they can be activated through TRPV1 or some other mechanism.
As far as we know, there are no studies to support the involvement of TRPV1 on taste-mediated behavior like there is for ENaC (e.g., Chandrashekar et al. 2010; Spector et al. 1996). In fact, it has been shown that TRPV1 knockout and wild-type mice have equal detection thresholds for NaCl (Ruiz et al. 2006; Treesukosol et al. 2007). Furthermore, it has been shown that the increased licking responses to NaCl solutions by potassium-deprived rats were unaffected by TRPV1 antagonists, SB-366791 and ruthenium red (Guenthner et al. 2008). Behaviorally, amiloride is an excellent antagonist because it is tasteless to rats (Markison and Spector 1995), while TRPV1 antagonists like CPC taste bitter to rats (St. John and Hallagan 2005), and SB-366791, a lipophilic molecule, must be dissolved in DMSO. Thus there seems to be some roadblocks in the way of gaining insight into transduction mechanism for the nonselective salt-sensing pathway involving acid-generalist neurons.
NaCl-specialists and Acid-generalist Pathways: Implications for Behavior
Most electrophysiological studies of taste deliver taste solutions to the tongue for 10–120 s to ensure sufficient impulse data for analysis. This long measurement period has its advantages, especially when studying the effects of competitive antagonists on neural responses, as shown in Fig. 3. Conscious rats, however, can recognize NaCl solutions within 600 ms after stimulus contact (Halpern and Tapper 1971). Similarly, animals can discriminate visual stimuli within 500–700 ms, with discrimination occurring more rapidly as stimulus intensity increased (Blough 1977). It is logical to expect that the underlying neural process for rapid taste quality and intensity discrimination must be in temporal registry with the behavior. In the present study, the most obvious response measures more in temporal registry with behavioral discrimination are response latency (Breza et al. 2010; Lawhern et al. 2011) and phasic response frequency (Bealer 1978). For the ENaC salt-sensing pathway, NaCl-specialist neurons responded to NaCl and Na-gluconate with concentration-dependent response latency and phasic response frequency components during the first 500-ms measurement period. Moreover, we found that benzamil increased NaCl response latency and reduced phasic response rate. Importantly, these effects of an ENaC antagonist on neural responses are consistent with psychophysical results showing that amiloride raised NaCl detection thresholds and shifted the response function to suprathreshold NaCl concentrations to the right (Geran and Spector 2000a). In essence, rats behaved as if amiloride made NaCl solutions taste less intense over a broad range of concentrations.
In contrast to NaCl specialists, acid generalists were much slower to react to sodium solutions (Table 3) and were unresponsive to 0.03–0.1 M NaCl and 0.03–0.3 M Na-gluconate during the first 500 ms and were still unresponsive to 0.03–0.1 M Na-gluconate during the first second. If the phasic portion of the neural response is responsible for coding stimulus intensity (Bealer 1978), then acid-generalist neurons are primarily responsive to hypertonic concentrations of cationic salt solutions, beginning around 0.3 M for NaCl and 0.5 M for Na-gluconate. At this juncture, it appears that the sodium-selective pathway is critical for sodium discrimination across a broad range of concentrations, whereas the nonselective cation pathway is important for detecting hypertonic concentrations of any stripe, whether they are sodium, potassium, calcium, ammonium, etc. The profound impact of anion size on response latency is consistent with reaction time data from human subjects who report that sodium solutions taste less intense (van der Klaauw and Smith 1995), and they recognize the salt-taste quality of sodium solutions more slowly (Delwiche et al. 1999) when the accompanying anion is large instead of small (van der Klaauw and Smith 1995). These findings relate directly to human health and disease, especially salt-induced hypertension, because processed foods consist of large anion-containing organic sodium salts (James et al. 1987) that serve as food stabilizers and preservatives. Consequently, people who subsist on a diet rich in processed foods are less able to recognize its sodium level and may compensate by adding even more salt to their diet, thereby further increasing the risk for hypertension and stroke (Gleiberman 1973; James et al. 1987; Mancilha-Carvalho et al. 1989; Page 1976).
Conclusions
There are two salt-sensing pathways involved in salt taste detection in the rat. The sodium-specific pathway involves ENaC located on the apical membrane of type I taste receptor cells of fungiform papillae. These receptor cells communicate directly with narrowly tuned NaCl-specialist neurons that respond quickly and selectively to a broad range of NaCl concentrations. Information about NaCl taste quality and intensity seems to be coded immediately, as reflected by systematic, concentration-dependent changes in phasic response frequency and response latency that temporally matches with taste recognition by rats. The nonselective cation pathway involves a yet unknown receptor process most likely located on the basal membrane of taste-receptor cells or on afferent fibers below the tight junction barrier, preventing access by large anion molecules and pharmacological antagonists. Nonselective cation receptor cells communicate with acid-generalist neurons that respond broadly to a range of cations, including sodium as well as to other basic tastes, but especially acids. Large anions, such as gluconate, suppress salt taste signaling by the nonselective pathway by a reduction in response frequency and an augmentation in response latency. This characteristic of anion suppression of salt taste by the nonselective pathway parallels human psychophysical studies, showing that large anions reduce salt taste perception and may enhance the intake of processed foods containing large-anion salts that serve as preservatives and food stabilizers. Since salt-taste in humans seems to possess a nonselective cation pathway (Ossebaard and Smith 1996; 1995), which is suppressed by large anions such as gluconate, there is a pressing need to solve the riddle of how the nonselective pathway detects salt.
GRANTS
Two National Institutes of Health grants from the National Institute on Deafness and Communication Disorders, RO1DC-004785 (R. J. Contreras) and 1F31DC-009920 (J. M. Breza) supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.B. and R.J.C. conception and design of research; J.M.B. performed experiments; J.M.B. analyzed data; J.M.B. and R.J.C. interpreted results of experiments; J.M.B. prepared figures; J.M.B. and R.J.C. drafted manuscript; J.M.B. and R.J.C. edited and revised manuscript; J.M.B. and R.J.C. approved final version of manuscript.
ACNOWLEDGMENTS
We thank Paul Hendrick, Fred Fletcher and Dr. Te Tang for excellent technical support and Dr. Chris Schatschneider for advice on statistical analyses. A portion of this work was presented at the Association for Chemoreception Sciences, in Sarasota, FL, 2010.
Data presented here were part of J. M. Breza's dissertation.
REFERENCES
- Bealer SL. Intensity coding in the transient portion of the rat chorda tympani response. J Comp Physiol Psychol 92: 185–195, 1978 [DOI] [PubMed] [Google Scholar]
- Beidler LM. A theory of taste stimulation. J Gen Physiol 38: 133–139, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough DS, Blough PM. Animal psychophysics. In: Handbook of Operant Behavior, edited by Honig WK, Staddon JER. Englewood Cliffs, NJ: Prentice-Hall, 1977 [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Monosodium glutamate but not linoleic acid differentially activates gustatory neurons in the rat geniculate ganglion. Chem Senses 32: 833–846, 2007 [DOI] [PubMed] [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006 [DOI] [PubMed] [Google Scholar]
- Breza JM, Nikonov AA, Contreras RJ. Response latency to lingual taste stimulation distinguishes neuron types within the geniculate ganglion. J Neurophysiol 103: 1771–1784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull 112: 155–159, 1992 [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Lundy RF. Gustatory neuron types in the periphery: a functional perspective. Physiol Behav 69: 41–52, 2000 [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Halpern BP, Desimone JA. Anion size of sodium salts and simple taste reaction times. Physiol Behav 66: 27–32, 1999 [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V, Heck GL, Phan TH, Alam RI, Feldman GM, Buch RM. A novel pharmacological probe links the amiloride-insensitive NaCl, KCl, and NH(4)Cl chorda tympani taste responses. J Neurophysiol 86: 2638–2641, 2001 [DOI] [PubMed] [Google Scholar]
- Elliott EJ, Simon SA. The anion in salt taste: a possible role for paracellular pathways. Brain Res 535: 9–17, 1990 [DOI] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol 50: 941–960, 1983 [DOI] [PubMed] [Google Scholar]
- Geran LC, Spector AC. Amiloride increases sodium chloride taste detection threshold in rats. Behav Neurosci 114: 623–634, 2000a [DOI] [PubMed] [Google Scholar]
- Geran LC, Spector AC. Sodium taste detectability in rats is independent of anion size: the psychophysical characteristics of the transcellular sodium taste transduction pathway. Behav Neurosci 114: 1229–1238, 2000b [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006 [DOI] [PubMed] [Google Scholar]
- Gleiberman L. Blood pressure and dietary salt in human populations. Ecol Food Nutr 2: 143–156, 1973 [Google Scholar]
- Guenthner CJ, McCaughey SA, Tordoff MG, Baird JP. Licking for taste solutions by potassium-deprived rats: specificity and mechanisms. Physiol Behav 93: 937–946, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BP, Tapper DN. Taste stimuli: quality coding time. Science 171: 1256–1258, 1971 [DOI] [PubMed] [Google Scholar]
- Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223: 403–405, 1984 [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res 513: 24–34, 1990 [DOI] [PubMed] [Google Scholar]
- James WP, Ralph A, Sanchez-Castillo CP. The dominance of salt in manufactured food in the sodium intake of affluent societies. Lancet 1: 426–429, 1987 [DOI] [PubMed] [Google Scholar]
- Lawhern V, Nikonov AA, Wu W, Contreras RJ. Spike rate and spike timing contributions to coding taste quality information in rat periphery. Front Integr Neurosci 5: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol 82: 2970–2988, 1999 [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, DeSimone JA. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol 281: C1005–C1013, 2001 [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558: 147–159, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Desimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses 30, Suppl 1: i42–i43, 2005 [DOI] [PubMed] [Google Scholar]
- Mancilha-Carvalho JJ, de Oliveira R, Esposito RJ. Blood pressure and electrolyte excretion in the Yanomamo Indians, an isolated population. J Hum Hypertens 3: 309–314, 1989 [PubMed] [Google Scholar]
- Markison S, Spector AC. Amiloride is an ineffective conditioned stimulus in taste aversion learning. Chem Senses 20: 559–563, 1995 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res 451: 319–325, 1988 [DOI] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Amiloride suppresses the sourness of NaCl and LiCl. Physiol Behav 60: 1317–1322, 1996 [DOI] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: implications for Na+ receptor mechanisms. Chem Senses 20: 37–46, 1995 [DOI] [PubMed] [Google Scholar]
- Page LB. Epidemiologic evidence on the etiology of human hypertension and its possible prevention. Am Heart J 91: 527–534, 1976 [DOI] [PubMed] [Google Scholar]
- Rehnberg BG, MacKinnon BI, Hettinger TP, Frank ME. Anion modulation of taste responses in sodium-sensitive neurons of the hamster chorda tympani nerve. J Gen Physiol 101: 453–465, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz C, Gutknecht S, Delay E, Kinnamon S. Detection of NaCl and KCl in TRPV1 knockout mice. Chem Senses 31: 813–820, 2006 [DOI] [PubMed] [Google Scholar]
- Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses Flav 4: 215–229, 1979 [Google Scholar]
- Sollars SI, Hill DL. In vivo recordings from rat geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones. J Physiol 564: 877–893, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Guagliardo NA, St. John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci 16: 8115–8122, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4: 143–191, 2005 [DOI] [PubMed] [Google Scholar]
- St. John SJ, Hallagan LD. Psychophysical investigations of cetylpyridinium chloride in rats: its inherent taste and modifying effects on salt taste. Behav Neurosci 119: 265–279, 2005 [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol 292: R1799–R1809, 2007 [DOI] [PubMed] [Google Scholar]
- van der Klaauw NJ, Smith DV. Taste quality profiles for fifteen organic and inorganic salts. Physiol Behav 58: 295–306, 1995 [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. The anion paradox in sodium taste reception: resolution by voltage-clamp studies. Science 254: 724–726, 1991 [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. Effects of voltage perturbation of the lingual receptive field on chorda tympani responses to Na+ and K+ salts in the rat: implications for gustatory transduction. J Gen Physiol 104: 885–907, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. Voltage dependence of the rat chorda tympani response to Na+ salts: implications for the functional organization of taste receptor cells. J Neurophysiol 70: 167–178, 1993 [DOI] [PubMed] [Google Scholar]