Abstract
Umami is considered to be the fifth basic taste quality and is elicited by glutamate. The mouse is an ideal rodent model for the study of this taste quality because of evidence that suggests that this species, like humans, may sense umami-tasting compounds as unique from other basic taste qualities. We performed single-unit recording of taste responses in the parabrachial nucleus (PbN) of anesthetized C57BL/6J mice to investigate the central representation of umami taste. A total of 52 taste-responsive neurons (22 sucrose-best, 19 NaCl-best, 5 citric acid-best, and 6 quinine-best) were recorded from stimulation period with a large panel of basic and umami-tasting stimuli. No neuron responded best to monopotassium glutamate (MPG) or inosine 5′-monophosphate (IMP), suggesting convergence of input in the central nervous system. Synergism induced by an MPG-IMP mixture was observed in all sucrose-best and some NaCl-best neurons that possessed strong sensitivity to sucrose. In more than half of sucrose-best neurons, the MPG-IMP mixture evoked stronger responses than those elicited by their best stimulus. Furthermore, hierarchical cluster analysis and multidimensional analysis indicated close similarity between sucrose and the MPG-IMP mixture. These results strongly suggest the mixture tastes sweet to mice, a conclusion consistent with previous findings that show bidirectional generalization of conditioned taste aversion between sucrose and umami mixtures, and suppression of taste responses to both sucrose and mixtures by the antisweet polypeptide gurmarin in the chorda tympani nerve. The distribution pattern of reconstructed recording sites of specific neuron types suggested chemotopic organization in the PbN.
Keywords: umami taste, mouse, amino acid, brainstem
l-glutamate, typically presented as a monosodium salt (MSG), and the derivatives of 5′-ribonucleotides such as inosine 5′-monophosphate (IMP) and guanosine 5′-monophosphate (GMP), elicit a taste described by humans as “umami”, a Japanese word that can be roughly translated as “delicious taste” or “savory taste” (Ikeda 1909; Kawamura and Kare 1987). In humans, umami is thought to be perceived as a unique taste quality different from the prototypical four basic tastes: sweet, salty, sour, and bitter (Yamaguchi 1991).
At least two candidate G protein-coupled receptors for umami taste have been molecularly identified in taste bud cells. These include the heterodimer T1R1/T1R3 and a truncated form of the metabotropic glutamate receptor 4 (taste-mGluR4) (Chaudhari et al. 1996; 2000; Li et al. 2002; Nelson et al. 2002). T1R1/T1R3 has been cloned and characterized in humans and other mammals, including mice. In the oral cavity, mouse T1R1/T1R3 is expressed in taste buds on the anterior and posterior tongue, and palate (Nelson et al. 2001; Kim et al. 2003). Mouse T1R1/T1R3 is activated in vitro by a variety of l-type amino acids, whereas human T1R1/T1R3 specifically responds to l-glutamate (Nelson et al. 2002; Li et al. 2002). Taste-mGluR4 is also widely expressed in taste bud cells in the oral cavity and has been implicated in the taste response to l-glutamate and l-(+)-2-amino-4-phosphonobutryic acid (l-AP4) (Chaudhari et al. 2009).
In rodents, there appear to be species differences in the ability to perceive sapid stimuli classified by humans as umami-tasting as comprising a unique quality. Conditioned taste aversion studies in rats are consistent with the notion that MSG may be perceived as a salty-sweet amalgamation (Yamamoto et al. 1985; 1991; Chaudhari et al. 1996; Stapleton et al. 1999). Physiologically, single chorda tympani (CT) fibers, geniculate ganglion neurons, and central taste neurons responsive to MSG also tend to possess high sensitivity to sucrose and NaCl in the rat (Sato et al. 1970; Yamamoto et al. 1985; Adachi and Aoyama 1991; Nishijo et al. 1991; Breza et al. 2007). In contrast to rats, there is evidence that mice can behaviorally discriminate the taste of MSG from stimuli representing the four traditional basic tastes (Ninomiya and Funakoshi 1989a; Nakashima et al. 2001; Yamamoto et al. 2009; but see Murata et al. 2009). Furthermore, some mouse glossopharyngeal (GL) single fibers were reported to respond best to MSG (Ninomiya and Funakoshi 1989b). Given this concordance between behavioral and neural data, the mouse may be an ideal model rodent species to study the mechanisms of umami taste processing.
One defining characteristic of umami taste in humans is the fact that when glutamate is mixed with 5′-ribonucleotides such as IMP, the intensity of the perceived taste becomes substantially enhanced (Yamaguchi 1967; 1991). This phenomenon, called “synergism” has been observed in a variety of experimental species. In neurophysiological studies in animals, synergism is expressed as an augmentation of the neural taste response to the mixture higher than the sum of each component (Adachi and Aoyama 1991; Ninomiya et al. 1992; Sako and Yamamoto 1999; Sako et al. 2000; 2003). Previous studies demonstrate that synergism occurs in CT fibers that respond robustly to sucrose in both the rat and mouse (Sato et al. 1970; Ninomiya and Funakoshi 1989b). There are only two studies that have investigated the central representation of synergism in rodents. Taste responses of 19 neurons to MSG, IMP, and their mixture were recorded in the nucleus of the solitary tract (NST), the first central taste relay, in the rat (Adachi and Aoyama 1991). Consistent with findings from the previous CT studies (Sato et al. 1970), all of synergistic neurons were highly sensitive to sucrose. Another study tested 41 taste-responsive neurons with MSG, GMP, and their mixture in the parabrachial nucleus (PbN) of awake rats (Nishijo et al. 1991). Although they failed to detect synergism as defined by a criteria described above (Yamamoto et al. 1991; Sako and Yamamoto 1999; Sako et al. 2003) because of the unexpectedly large responses to GMP, it was shown that taste responses to the mixture were significantly greater than those to MSG alone in sweet-sensitive neurons but not others.
In the present study, we investigated the neural representation of umami-tasting stimuli and synergism in the PbN of C57BL/6J mice. This also represents the first investigation into physiological characteristics of taste-responsive neurons in this brainstem area in this species, a key interface between brainstem and forebrain gustatory areas (Tokita et al. 2009; 2010). Neurophysiological investigations into the mouse central taste system have begun only recently, including two recent studies on taste-response properties in neurons in the NST (McCaughey 2007; Lemon and Margolskee 2009).
MATERIALS AND METHODS
Subjects.
A total of 31 adult male C57BL/6J mice (18–30 g, aged 3 to 4 mo) were used. The animals were maintained in a temperature- and humidity-controlled colony room on a 12-h:12-h light/dark cycle (lights on at 0700 h, off at 1900 h) and were given ad libitum access to normal dry pellet (22/5 rodent diet; Harlan Teklad, Madison, WI) and water. This study was approved by the Animal Care and Use Committee at UTHSC, and all experiments were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publications No. 80–23), revised 1996.
Surgery.
Animals were anesthetized with urethane (1 g/kg) followed by supplemental anesthetic as necessary throughout the experiment to maintain deep anesthesia. After tracheotomy was performed to permit respiration, each mouse was fixed in a stereotaxic instrument equipped with nontraumatic ear bars (Stoelting, Wood Dale, IL). The body temperature was maintained at 35°C using a heating pad (Elenco Electronics, Wheeling, IL). The scalp was opened with a midline incision, and the skull was leveled between and lambda by adjusting the bite bar. A hole (∼4.0 mm in diameter, just posterior to the lambda), serving access to the PbN, was drilled through the skull.
Test solutions.
Taste stimuli used were 0.5 M sucrose, 0.1 M sodium chloride (NaCl), 0.01 M citric acid, 0.01 M quinine hydrochloride (QHCl), 0.1 M potassium chloride (KCl), 0.003, 0.01, 0.03, and 0.1 M monopotassium glutamate (MPG), 0.0003, 0.0001, 0.003, and 0.01 M IMP, mixtures of each concentration of MPG and IMP, 0.2 M l-alanine, 0.2 M l-serine, and 0.2 M l-lysine. In the present study, MPG was used instead of MSG to more clearly show synergism by precluding the taste effect of the sodium ion (Yamamoto et al. 1991; Sako et al. 2003). All stimuli were prepared from reagent-grade chemicals and dissolved in distilled water. Taste solutions were maintained at room temperature during testing.
Electrophysiological recording.
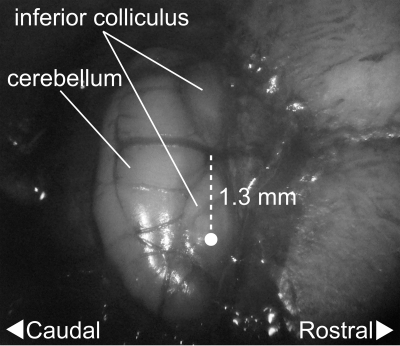
The taste-responsive zone of the PbN was visually identified using the inferior colliculus, just posterior to the transverse sinus on the dorsal surface of the exposed tissue, as a landmark. Unlike other rodents such as rats and hamsters, vertical access to the PbN was possible in mice. Preliminary experiments showed that the PbN taste-responsive neurons were most often found 0 ± 0.2 mm anterior or posterior to the boundary of the inferior colliculus and cerebellum, 1.3 ± 0.1 mm lateral to the midline, and 2.9 ± 0.2 mm ventral to the surface of the inferior colliculus (Fig. 1). In the present study, bregma was not used as the reference point. The glass-insulated tungsten microelectrode (impedance = 1–8 MΩ at 1 kHz; FHC, Bowdoinham, ME) was advanced through the inferior colliculus into the PbN with a micromanipulator (SM-191; Narishige, Tokyo, Japan). Neuronal activity was amplified and monitored with a computer-aided data-acquisition and analysis system (CED 1401, Spike2 version 4.01; Cambridge Electronic Design, Cambridge, UK).
Fig. 1.
Digital photograph of the exposed dorsal surface of the inferior colliculus and cerebellum of the C57BL/6 mouse. The taste-responsive zone of the parabrachial nucleus (PbN) was most often found 1.3 ± 0.1 mm lateral to the midline and 2.9 ± 0.2 mm ventral to the surface of the inferior colliculus (indicated by a circle).
A mixture of the four basic tastants (0.5 M sucrose, 0.3 M NaCl, 0.02 M citric acid, and 0.02 M QHCl) was used as a search stimulus. After isolating a single unit in the PbN, taste stimuli were presented at room temperature (23–24°C). The tongue was pulled out using a string glued to its ventral surface to make it easier to stimulate posterior (GL-innervated) taste buds. The oral cavity was stimulated with a method modified from our previous studies with rats (Tokita et al. 2004; Shimura et al. 2002). Fluid stimuli were delivered through a length of intraorally inserted slender tubing (PE 100), with the end positioned ∼2 mm above the dorsal anterior tongue. During delivery, fluid could be seen engaging both the tongue and palate, and preliminary experiments using methylene blue dye suggested that this method reliably bathes the entire oral cavity. Five milliliters of each taste stimulus were presented at a rate of 0.5 ml/s, delivered under mild pressure from a 5-ml syringe. Each stimulus trial consisted of a 10-s rinse of distilled water, 10-s stimulus, and 10-s rinse of distilled water, all presented at the same rate. Gustatory stimuli and water were cleared from the delivery tubing by air pressure. Stimulus onset could also be determined by a response artifact that occurred when the stimulus first contacts the tongue (Bradley and Mistretta 1980). When taste-evoked neural activity persisted after the 10-s poststimulus rinse of distilled water, we continued the water rinse until the activity returned to the prestimulus level. At least 90 s were allowed to elapse between stimuli to avoid the effects of adaptation.
Data analysis.
A neuron was considered to be taste responsive if the neural activity evoked by a taste stimulus increased or decreased ≥2 SD from the mean of its spontaneous activity. All data analyses were based on neural activity quantified in 10-s samples. Spontaneous activity and responses to prestimulus water were calculated from multiple samples. The spontaneous rate was determined during the periods just before the prestimulus water rinse. Water and taste neural responses were calculated during the first 10-s period after the onset of stimulation with prestimulus water or a taste solution. The net response rate, obtained by subtracting the immediately preceding raw water responses from the raw taste responses, was used for data analyses. Each neuron was classified into sucrose (S)-best, NaCl (N)-best, citric acid (C)-best, or QHCl (Q)-best categories based on which of the prototypical taste stimuli evoked the greatest net response.
With the use of adjusted response data, a breadth-of-response measure was derived for each neuron from the formula for entropy (Smith and Travers 1979)
where Pi represents the response to each of the four basic taste stimuli. The constant K = 1.661 for four stimuli. Values of entropy (H) close to zero indicate sensitivity to a single stimulus (narrow tuning); values close to 1.0 indicate sensitivity to all four stimuli (broad tuning). In the present study entropy was obtained using the excitatory components of responses to four basic taste stimuli.
The synergistic ratio was calculated by a formula: magnitude of response to mixture/sum of magnitudes of responses to individual components (0.1 M MPG and 0.01 M IMP) in the mixture. If the magnitude of response to the mixture was negative, then the synergistic ratio was judged as zero. In theory, a ratio >1.0 would be classified as synergistic. However, it is possible for the ratio to slightly exceed (or fall under) 1.0 in cells with small magnitude nonsynergistic responses. A previously published criterion of 1.2 (Ninomiya and Funakoshi 1989b) corresponded to a breakpoint in our own data, with just 3 of 27 nonsynergistic cells possessing ratios greater than 1 but less than 1.15; the first clearly synergistic neuron possessed a ratio of 1.36. The veracity of the synergistic response was typically (but not in all cases) confirmed with multiple trials with 0.1 M MPG, 0.01 M IMP, and their mixture but for consistency ratios were only computed from the first presentation of these stimuli. Throughout the article, we refer to neurons as either being synergistic or nonsynergistic. Because the criterion for classifying neurons into these categories is arbitrary, this is not meant to be a descriptor of absolute biological significance but rather shorthand for neurons that possess or do not possess a synergistic response to the mixture.
Poststimulus time histograms (PSTHs) consisting of 0.1-s bins over 10 s after stimulus onset were constructed on the basis of net responses to 0.1 M MPG, 0.01 M IMP, and their mixture to reveal temporal patterns of mixture synergism.
Repeated-measure ANOVA was performed to examine concentration-dependent responses to MPG, IMP, and their mixture in synergistic and nonsynergistic neurons (concentration × neuron type). Post hoc comparisons were performed using a Bonferroni correction. To examine the influence of location of recording site on synergistic and nonsynergistic neurons in the PbN, Fisher's exact probability test was used. On the basis of taste-response profiles, several multivariate analyses were performed. For cluster analysis, we used the Pearson product-moment correlation coefficients between response profiles of the neurons and the unweighted pair-group average method. Multidimensional analysis was then executed using an Euclidean distance model to generate a three-dimensional taste space.
All statistical analyses described above were performed using a general statistics package (Statistica version 6; StatSoft, Tulsa, OK). The statistical rejection criterion for all tests was set at P < 0.05.
Histology.
At the end of the last experimental session, a small electrolytic lesion (20 μA for 20 s, electrode positive) was made at the final recording site in the PbN. The mice were then injected (ip) with 0.5 ml of 25% urethane and perfused transcardially with phosphate-buffered saline and 10% formalin. The brains were removed and placed in 10% formalin and then transferred to a 30% buffered sucrose solution and stored at 4°C for at least 5 days. Coronal sections of 40-μm thickness were serially cut using a freezing microtome and then stained with cresyl violet. The location of each recording site was histologically verified and reconstructed.
RESULTS
Basic characteristics.
A total of 52 taste-responsive neurons were isolated and recorded from the PbN while all taste stimuli were presented. All the neurons showed excitatory activity to at least one of the four basic taste stimuli (sucrose, NaCl, citric acid, or QHCl). The mean spontaneous firing rate (spikes/s) was 0.68 ± 0.09 (range: 0.0–3.36). The mean breadth of tuning value across all neurons was 0.59 ± 0.04 (range: 0.0–0.95). Two N-best neurons and one Q-best neuron had a value of 0 (i.e., these neurons were exclusively activated by only 1 of the 4 basic tastes).
Response profiles and classification.
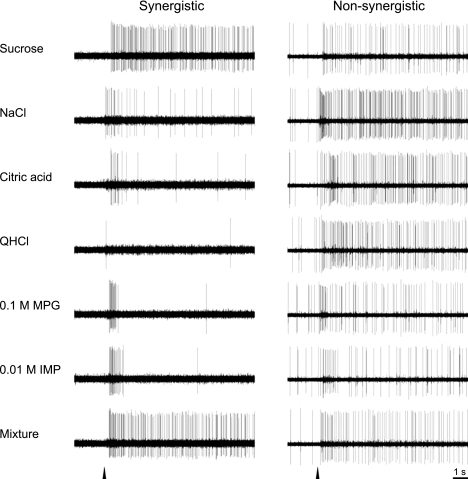
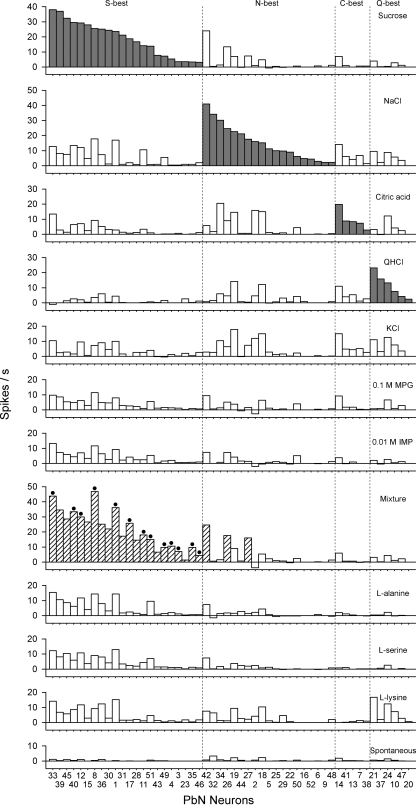
On the basis of their largest net response to the four standard taste stimuli as well as MPG and IMP, we classified the 52 PbN neurons as follows: 22 S-best, 19 N-best, 5 C-best, and 6 Q-best. No neuron responded best to 0.1 M MPG or 0.01 M IMP or any of the other nonstandard stimuli except the MPG-IMP mixture (see below). Neurons were also classified as synergistic (n = 25) or nonsynergistic (n = 27). Figure 2 shows examples of taste responses in synergistic and nonsynergistic neurons: an S-best neuron showed a marked enhancement in response to the mixture of 0.1 M MPG and 0.01 M IMP, whereas an N-best neuron did not. Figure 3 displays the total gustatory net response profiles for all neurons. Taste neurons were ordered by best-stimulus category and within categories by response magnitude. S-best neurons are on the left, followed by N-, C-, and Q-best neurons (gray bars). Synergistic responses to MPG-IMP mixture are indicated by crosshatched bars, and black dots are associated in cases where the mixture responses exceeded those to the best stimulus. S- and N-best neurons predominated in the dataset, at 42 and 37% of all neurons, respectively. Of the 25 neurons showing a synergistic response, 22 were S-best neurons (100% of this type), and the remaining 3 were N-best neurons whose second most effective stimulus was sucrose. All of the neurons whose strongest responses were evoked by the mixture were S-best.
Fig. 2.
Examples of responses of synergistic and nonsynergistic neurons to taste stimuli. A marked augmentation of taste responses to the mixture of monopotassium glutamate (MPG) and inosine 5′-monophosphate (IMP) was observed in the synergistic but not in the nonsynergistic neuron. Arrowheads indicate stimulus onset. QHCl, quinine hydrochloride.
Fig. 3.
Response profiles of PbN taste neurons. Taste neurons were grouped into best-stimulus categories (shaded bars) and arranged within those categories in descending order of response magnitude to the best-stimulus [n = 52; 22 sucrose (S)-best; 19 NaCl (N)-best; 5 citric acid (C)-best; 6 QHCl (Q)-best]. Taste responses are presented as net responses (i.e., responses to stimulus − responses to water). The hatched bars indicate synergistic responses to the mixture of MPG and IMP. Filled circles are associated with hatched bars when the responses evoked by the mixture of MPG and IMP were stronger than those evoked by classical 4 basic taste stimuli.
Characteristics of synergism.
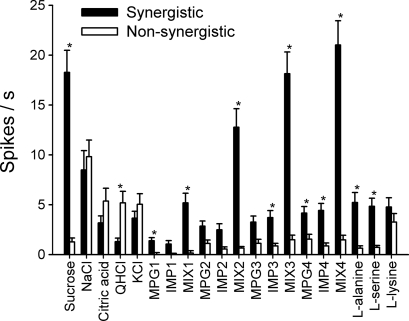
Mean net taste responses to taste stimuli in synergistic and nonsynergistic neurons are shown in Fig. 4. A two-way ANOVA with repeated measures (neuron type × stimulus) revealed a significant main effect of neuron type [F (1,50) = 23.3, P < 0.01] and stimulus [F (19,950) = 38.9, P < 0.01] as well as a neuron type × stimulus interaction [F (19,950) = 40.3, P < 0.01]. Taste responses of synergistic neurons to sucrose, 0.003 and 0.1 M MPG, 0.003 and 0.01 M IMP, all concentrations of the mixture, l-alanine, and l-lysine were significantly higher than those of nonsynergistic neurons (P < 0.05). QHCl was the only stimulus that elicited significantly more responses in nonsynergistic neurons than in synergistic neurons (P < 0.05). A t-test revealed no significant difference in the mean breadth of tuning values between synergistic (0.60 ± 0.04, range: 0.28–0.94) and nonsynergistic neurons (0.59 ± 0.04, range: 0.0–0.95).
Fig. 4.
Net taste responses of synergistic (shaded bar) and nonsynergistic neurons (open bars) to all taste stimuli used in the present study. Concentration series of umami substances: 0.1 M MPG and 0.01 M IMP; 0.03 M MPG and 0.003 M IMP; 0.01 M MPG and 0.0001 M IMP; 0.003 M MPG and 0.0003 M IMP. *P < 0.05.
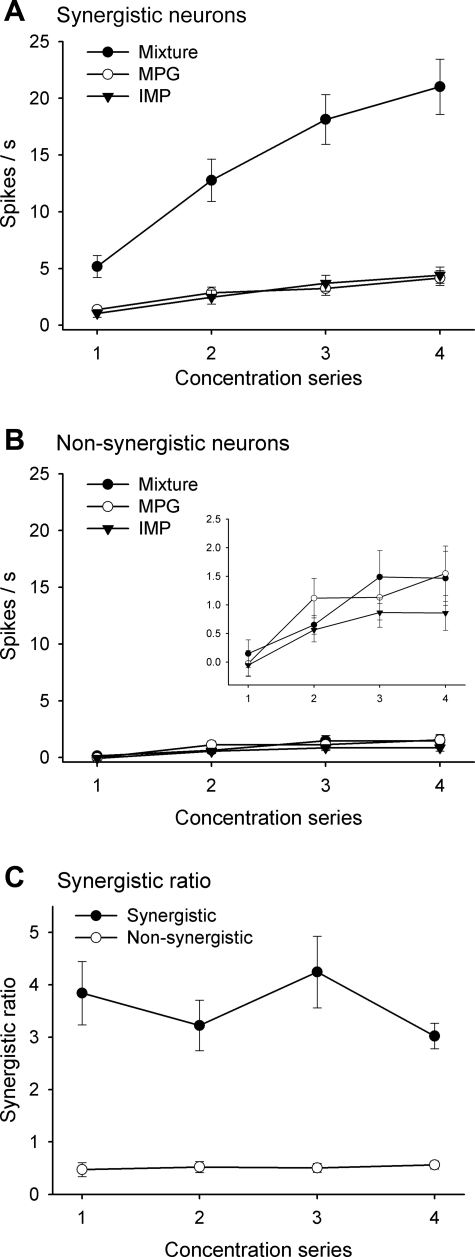
We analyzed the concentration dependency of the neural response to MPG, IMP, and their mixture and calculated synergism ratios (net response to mixture/net response to MPG + net response to IMP) in all neurons (Fig. 5). Data were analyzed with a series of one-way ANOVAs followed by multiple comparisons. There was a significant main effect of concentration on the responses of synergistic neurons to both the individual stimuli and their mixture [Fs (1,3) > 18.8, Ps < 0.01]. Direct comparisons (Bonferroni-corrected t) at each concentration step indicate the mixture response was greater than the sum of the MPG and IMP responses (P < 0.013). There was also a main effect of concentration on the responses of nonsynergistic neurons to these stimuli [Fs (1,3) > 3.7, Ps < 0.05]. However, the mixture response was not significantly greater than the sum of the individual responses. In Fig. 5C, synergism ratios at each concentration series in both types of neurons are compared. A two-way ANOVA with repeated measures (neuron type × concentration) revealed a significant main effect of neuron type [F (1,50) = 81.8, P < 0.01] but not concentration [F (3,150) = 1.7, P = 0.18].
Fig. 5.
Net taste responses of synergistic (A) and nonsynergistic neurons (B) to concentration series of MPG, IMP, and their mixture solutions. A blown-up inset is shown in B. Synergistic ratios derived from taste responses are shown in C. Concentration series: 0.1 M MPG and 0.01 M IMP; 0.03 M MPG and 0.003 M IMP; 0.01 M MPG and 0.0001 M IMP; 0.003 M MPG and 0.0003 M IMP.
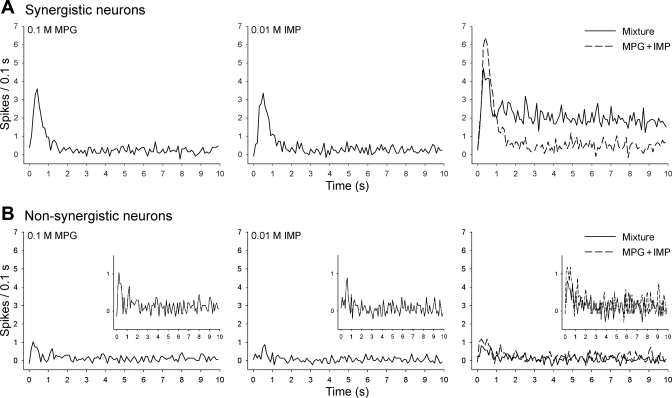
Figure 6 shows the time course of net responses to 0.1 M MPG, 0.01 M IMP, and the mixture in the form of PSTHs consisting of 0.1-s bins. In synergistic neurons, the first 1-s phasic response to the mixture was even smaller than the sum of the MPG and IMP responses. However, about 1 s after the onset of the stimulus, the mixture starts to evoke stronger responses than each component does. In contrast, in nonsynergistic neurons, such a dynamic temporal pattern of responding was not observed.
Fig. 6.
Poststimulus histograms showing temporal net responses of synergistic (A) and nonsynergistic neurons (B) to 0.1 M MPG, 0.01 M IMP, and their mixture. Insets in B show magnification of scale. Synergistic responses occurred about 1 s after the stimulus onset. Time bins = 0.1 s.
Across-neuron representation of taste.
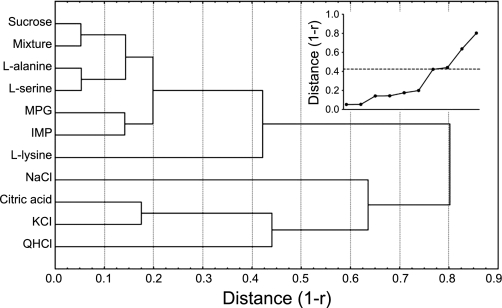
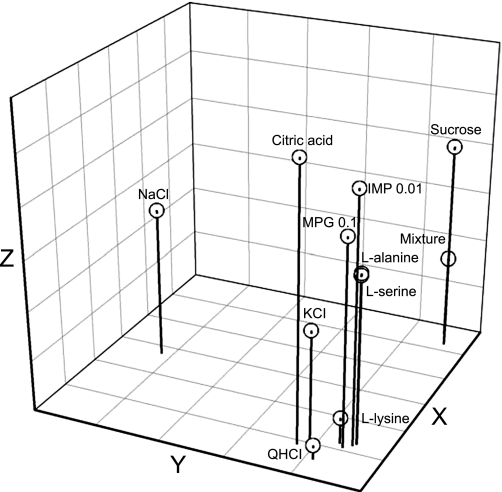
We used a hierarchical cluster analysis derived from Pearson product-moment correlations to categorize taste stimuli (Fig. 7). It is obvious from the dendogram that sucrose, MPG, IMP, and amino acid stimuli were clustered together. Moreover, the MPG-IMP mixture was more closely clustered with sweet stimuli such as sucrose, l-alanine, and l-serine. A three-dimensional “taste space” showing relative stimulus similarity is presented in Fig. 8. The four basic stimuli (sucrose, NaCl, citric acid, and QHCl) occupied separate positions in the taste space. Amino acids, MPG, and IMP were tightly clustered. Only l-lysine, which evoked noticeable responses in Q-best neurons (Fig. 3), was positioned close to QHCl. Consistent with the results of the hierarchical cluster analysis, sucrose and the MPG-IMP mixture were positioned very close to one another.
Fig. 7.
Dendrograms showing clustering of 11 stimuli based on the response profile. Screen plot shows the major breakpoint. Cluster distance on the abscissa indicates similarity between stimuli.
Fig. 8.
Distribution of 11 taste stimuli in a 3-dimensional taste space resulting from multidimensional scaling.
Histology.
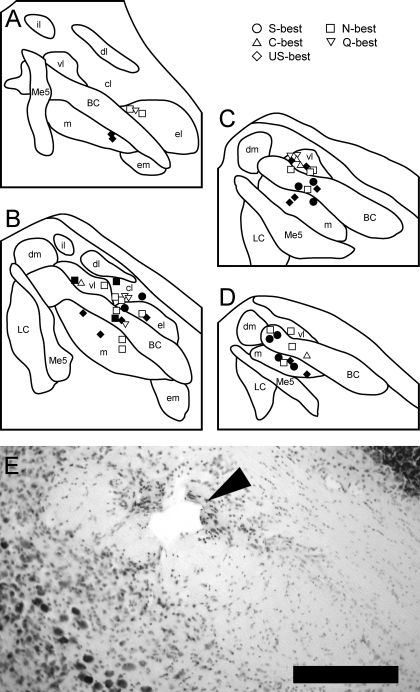
On the basis of stereotaxic coordinates of recording sites and the marking lesions made in the final recording sites, the locations of all 52 taste-responsive PbN neurons were successfully reconstructed. Of these 52 locations, 31 were directly identified by the marking lesions. In most cases (16 out of 21) the units whose recording sites were inferred from stereotaxic coordinates were recorded within the same track where the final unit recordings, i.e., the marking lesions, were made. Figure 9 shows recording site plots in rostrocaudally arranged PbN schema at four different levels (Fig. 9, A–D), as well as a representative photomicrograph of a Nissl-stained section of the PbN with a marking lesion (Fig. 9E). Recording sites were located in the medial, central lateral, ventral lateral, and external lateral subnuclei as well as in the brachium conjunctivum (waist subnucleus). No gustatory neurons were isolated in other areas such as dorsal medial, external medial, dorsal lateral, or internal lateral subnuclei. The distribution of recording sites in terms of neuron type based on both best stimulus and synergism are summarized in Tables 1 and 2. Recording sites were classified into medial (medial subnucleus), waist, and lateral divisions (central lateral, ventral lateral, and external lateral subnuclei). The proportion of best neurons in the medial and lateral divisions was significantly different, with a greater amount of sweet-best neurons found medially (Fisher's exact probability test, P < 0.01). A similar effect was found for neurons classified as either synergistic or nonsynergistic, with more neurons possessing synergism found medially.
Fig. 9.
Anatomical reconstruction of 52 recording sites in the PbN. A–D: sections A–D are arranged rostral to caudal and +50, −100, −250, and −400 μm separated from the caudal end of the cuneiform nucleus, respectively. Filled symbols, synergistic units; open symbols, nonsynergistic units. Umani-sucrose (US)-best neurons are S-best neurons for which responses evoked by the mixture of MPG and IMP were stronger than those evoked by sucrose. BC, brachium conjunctivum; cl, central lateral subnucleus; dm, dorsal medial subnucleus; dl, dorsal lateral subnucleus; el, external lateral subnucleus; il, internal lateral subnucleus; em, external lateral subnucleus; LC, locus coeruleus; m, medial subnucleus; Me5, mesencephalic trigeminal nucleus; vl, ventral lateral subnucleus. E: photomicrograph of a cresyl violet-stained section in the PbN. Marking lesion in the ventral lateral subnucleus is indicated by an arrowhead. Scale bar = 250 μm.
Table 1.
Location of recording site of neurons in terms of best stimulus
| Recording Site |
||||
|---|---|---|---|---|
| Neuron Type | Medial | BC | Lateral | Total |
| S-best | 11 | 6 | 5 | 22 |
| N-best | 3 | 6 | 10 | 19 |
| C-best | 0 | 1 | 4 | 5 |
| Q-best | 0 | 1 | 5 | 6 |
| Total | 14 | 14 | 24 | 52 |
S-best, sucrose-best; N-best; NaCl-best; C-best, citric acid-best; Q-best, quinine hydrochloride-best; BC, brachium conjunctivum.
Table 2.
Location of recording site of neurons in terms of synergism
| Recording site |
||||
|---|---|---|---|---|
| Neuron Type | Medial | BC | Lateral | Total |
| Synergistic | 11 | 7 | 7 | 25 |
| Nonsynergistic | 3 | 7 | 17 | 27 |
| Total | 14 | 14 | 24 | 52 |
DISCUSSION
In the present study, we demonstrate for the first time the basic neurophysiological properties of mouse gustatory PbN neurons, including taste-evoked responsiveness to a wide range of concentration series of umami-tasting stimuli. Methodical reconstruction of recording sites also sheds light on the functional anatomical organization of the mouse PbN. Considering the fact that the mouse is recognized as an excellent rodent model for investigating amino acid taste (Ninomiya and Funakoshi 1989a; 1989b), the present data contribute to the study of the mechanisms thorough which umami-tasting stimuli and umami synergism are represented in the mammalian brain.
Spontaneous firing rate and breadth of tuning.
Mean spontaneous firing rates of taste-activated PbN neurons in the present study (0.68 ± 0.09) were low compared with those reported in previous studies on PbN and NST using rats, hamster, and mice under anesthesia. In two recent mouse NST studies, taste-activated neurons showed higher mean spontaneous rates (5.0 ± 0.5 and 2.0 ± 0.1, respectively) (McCaughey 2007; Lemon and Margolskee 2009). The reason for the difference is not totally apparent, but anesthesia may suppress spontaneous activity by blocking descending excitatory inputs to the PbN; spontaneous firing rates of PbN neurons in awake behaving rats were found to be much higher than those recorded under anesthesia (e.g., Kovacs and Hajnal 2008; Nishijo and Norgren 1997). Moreover, decerebration at the level of the superior colliculus decreased PbN spontaneous firing rates in rats (Tokita et al. 2004). The mouse PbN receives dense projections from taste-related structures in the forebrain (Tokita et al. 2009). Finally it should be also noted that the anesthetic used might have an influence on spontaneous neural activity. However, our spontaneous firing rate is still lower than those in other studies recording from the NST or PbN using the same anesthetic, urethane (Adachi and Aoyama 1991; Boughter and Smith 1998; Lemon and Margolskee 2009; Cho and Li 2008; Shimura et al. 1997; 2002; Tokita et al. 2004). Another potential limitation of urethane is that it causes hyperglycemia (Reinert 1964), which suppresses neural responses to sapid sugars (Giza and Scott 1983).
Although relatively low, the mean breadth of tuning values (0.59 ± 0.04) found in the present study fall within the range of those obtained in previous studies in the PbN in rat and hamster (Van Buskirk and Smith 1981; Nishijo and Norgren 1997; Cho and Li 2008; Geran and Travers 2009; for review see Spector and Travers 2005). This value is much higher than those in the single CT fibers of mice, which range from 0.22 to 0.41 (Ninomiya et al. 1982; 1984). The increase in breadth of responsiveness in the PbN likely reflects convergence of input at the central level. It should be noted that three neurons, two N- and one Q-best, with entropy values = 0 were recorded in the present study. All of these neurons showed very weak responsiveness to their best stimuli (2.2, 2.4, and 4.4 spikes/s).
Taste response profiles.
The proportion of cells in each best-stimulus category was characterized by S- and N-best units dominating nearly 80% of data (Fig. 3), which is generally consistent with the previous NST data from C57BL/6J mice (McCaughey 2007; Lemon and Margolskee 2009). Compared with previous reports with other rodents on the PbN or NST, the proportion of S-best and Q-best neurons appear to be larger in the present study (e.g., Boughter and Smith 1998; Shimura et al. 1997; Tokita et al. 2004). These results are plausible considering the findings that C57BL/6J mice have strong neural and behavioral sensitivity to sweeteners (Inoue et al. 2004), and the mouse CT nerve appears to have more Q-best fibers than do other rodents (Ninomiya et al. 1982; 1984; Ninomiya and Funakoshi 1989b). It should be noted that our stimulus delivery method may have optimally stimulated the anterior tongue and not other taste bud-bearing regions, although preliminary results with methylene blue dye showed that stimulation bathed almost all of the gustatory receptor fields innervated by the three primary gustatory nerves.
Representation of umami taste in the mouse PbN.
The mouse has been recognized as a good animal model to study the neural or behavioral response to sapid stimuli characterized by humans as umami-tasting. Like humans, this species appears to be able to perceive these stimuli as possessing a distinct taste quality in behavioral experiments (Ninomiya and Funakoshi 1989a; Nakashima et al. 2001; Yamamoto et al. 2009). Neural evidence supporting this idea is the existence of MSG-best GL fibers found in the mouse (Ninomiya and Funakoshi 1989b). However, we did not find MPG-best neurons in the PbN in the present study.
Although there is in general a paucity of taste electrophysiology studies focusing on umami taste mechanisms in the central nervous system, many previous studies in rodents include at least MSG as a taste stimulus. Several studies on the NST, PbN, or gustatory cortex report at least some MSG-best neurons (Nishijo et al. 1991; Nakamura and Norgren 1993; 1995; Nishijo and Norgren 1997; Kovacs and Hajnal 2008), whereas others do not (Adachi and Aoyama 1991; Shimura et al. 2002; Verhagen et al. 2005; Kang and Lundy 2010). These MSG-best neurons often possess high sensitivity to NaCl or sucrose, whereas those found in the GL nerve did not. In the recent study by Lemon and Margolskee (2009) using anesthetized mice, some MSG-best neurons were found in the NST of both wild-type and T1R3 knockout mice; however, the concentration of MSG was very high (0.5 M), and in the MSG-best neurons the response to NaCl was large (Lemon and Margolskee 2009). Therefore, the response of neurons that seemingly are most strongly activated by MSG might merely reflect their sensitivity to other components than glutamate. It should be noted that a recent report showed the existence of S-best and MPG-best mouse CT fibers, each of which can be further divided into two subcategories that do or do not possess a synergistic response to an umami stimulus mixture (Yasumatsu et al. 2011). Some mouse single fungiform taste cells are also known to respond best and specifically to MSG (Yoshida et al. 2009). Therefore, at the taste cell and fiber levels, there is support for a specific coding of umami-tasting stimuli in the mouse. As it is highly unlikely that we failed to stimulate the anterior tongue (innervated by the CT), this discrepancy is possibly explained by a greater convergence of input in the PbN.
Response properties of synergistic and nonsynergistic neurons in the PbN.
All of the neurons possessing a synergistic taste response in the present study were S-best or N-best and were strongly activated by sucrose (Figs. 2 and 3). Moreover, these neurons had much stronger responses to sucrose than did those that did not possess a synergistic response (Fig. 4). Synergistic neurons possessed significantly stronger responses to the mixture at all concentrations and also tended to respond more strongly to MPG and IMP alone at high concentrations. In contrast, responses to QHCl were significantly higher in nonsynergistic than in synergistic neurons. In accordance with this, synergistic neurons also possessed stronger responses to the sweet-tasting amino acids, l-alanine and l-serine, but not to bitter-tasting l-lysine. Collectively, these results suggest that synergistic neurons are more sensitive to sweet and preferred stimuli and less sensitive to aversive taste stimuli. The results are also compatible with rat and mouse CT single-fiber studies showing a strong correlation between sensitivity to sweet-tasting stimuli and umami synergism (Sato et al. 1970; Ninomiya and Funakoshi 1989b).
Both synergistic and nonsynergistic neurons showed concentration-dependent responses to MPG, IMP, and their mixture, but this effect was clearer in synergistic neurons (Fig. 5, A and B). However, we did not find concentration dependency of the ratio itself (Fig. 5C). This result is inconsistent with a previous rat whole-nerve study showing that the degree of synergism in the CT and greater superficial petrosal nerves is highest when lower concentration of umami-tasting substances are used (Sako et al. 2000). It is not clear whether this discrepancy reflects species difference or different methodology (e.g., MSG vs. MPG, whole nerve vs. single unit recording).
Time course analysis revealed that synergistic responses to the mixture occur about 1 s after the onset of stimulation (Fig. 6). Between 0 and 1 s, the initial phasic response occurred, which is likely dependent to some degree on gustatory transduction of the sodium and potassium ions contained in MPG and IMP. During this interval, the sum of MPG and IMP responses are greater than those elicited by the mixture. This suggests that IMP requires about 1 s to exert its allosteric effect on the receptor.
Umami synergism: a sweet taste to mice?
In our dataset, we found an extremely close correspondence between synergism (but not necessarily the individual stimuli) and sweet-tasting stimuli among the gustatory neural responses of PbN neurons in mice. Our results of hierarchical cluster analysis and multidimensional analysis also showed close grouping of sucrose and the mixture among all stimuli (Figs. 7 and 8). Although it has recently been shown that synergism occurs by activation of the Venus flytrap domain of T1R1, where MSG and IMP bind cooperatively (Zhang et al. 2008), it is still unclear how the mixture stimulates the sweet pathway.
l-glutamate, but not sweet stimuli such as sucrose or artificial sweeteners, activates the T1R1/T1R3 receptor heterodimer in mice; sweet-tasting compounds instead activate the T1R2/T1R3 heterodimer (Zhao et al. 2003). However, several lines of evidence suggest that the T1R1/T1R3 receptor is simply a sweet-taste receptor that is activated by l-type amino acids. First, the T1R1/T1R3 heterodimer, and indeed the T1R3 subunit, appears to be necessary and sufficient for the synergistic response (Zhao et al. 2003). Lingual treatment with the sweet-taste-suppressing polypeptide gurmarin diminishes physiological and behavioral responses to both sweet-tasting compounds and an umami mixture (Nakashima et al. 2001; Yamamoto et al. 1991; Ninomiya et al. 2000; Sako et al. 2003). Second, there is a high degree of overlap of T1R receptor subunits, including T1R1 and T1R2, in taste buds of the anterior and posterior tongue (Kim et al. 2003), supporting the idea of a single “sweet” taste receptor cell type, much in the way there is a bitter taste receptor type that may possess more than one type of T2R bitter receptor (e.g., Chandrashekar et al. 2000; 2006).
Behaviorally, evidence suggests that mice can discriminate MSG vs. sucrose although blocking the sodium ion of MSG with amiloride makes the discrimination more difficult (Delay et al. 2006). Mice also can discriminate (although with some difficulty) sucrose from l-serine and l-threonine (Dotson and Spector 2007), which supposedly selectively activate the T1R1/T1R3 receptor. If activation of T1R1/T1R3 leads to sweet taste perception in mice, how can mice correctly discriminate between umami- and sweet-tasting stimuli (see also Ninomiya and Funakoshi 1989a)? The most parsimonious explanation is that the distinct taste of glutamate stimuli is encoded by another receptor, likely taste mGluR4. This receptor is strongly expressed in taste buds on the posterior tongue and is activated by l-glutamate and the agonist l-AP4 (Chaudhari et al. 2000). Moreover, the MSG-evoked response in the GL nerve was not affected by knockout of T1R3 (although the CT response is affected; see Damak et al. 2003), and the GL lacks gurmarin sensitivity to sweet- or umami-tasting stimuli (Ninomiya et al. 1997; Ninomiya et al. 2000). Finally, as mentioned above, Ninomiya and Funakoshi (1989b) reported the existence of MSG-best GL fibers in the mouse that shows strong synergism. A logical extension of our present work would be to gauge the contribution of lingual mGluR receptors to the central taste response via pharmacological block by an antagonist such as MAP4 or cyclopropyl-4-phosphonophenylglycin (Sako and Yamamoto 1999; Nakashima et al. 2012). Recently, Nakashima et al. (2012) demonstrated that cyclopropyl-4-phosphonophenylglycin reduced behavioral taste responses in mice when mixed with MSG or MPG. If our hypothesis is correct, this manipulation may also severely disrupt the ability of mice to discriminate between sweet- and umami-tasting stimuli.
Distribution of recording sites in the PbN.
Recording sites in the PbN were distributed in the medial and lateral part of the PbN and also within the brachium conjunctivum (Fig. 9 and Tables 1 and 2). In terms of subnuclei (Hashimoto et al. 2009; Tokita et al. 2010), recordings were made in the medial, central lateral, ventral lateral, and external lateral subnuclei. These results are generally in agreement with previous neurophysiological studies done in the rat and hamster PbN (Norgren and Pfaffmann 1975; Van Buskirk and Smith 1981; Ogawa et al. 1984; 1987; Halsell and Frank 1991; Halsell and Travers 1997; Shimura et al. 1997; Tokita et al. 2004; Geran and Travers 2009). On the basis of the distribution pattern of recording sites of specific neuron types, some of these studied suggest the existence of chemotopy in the PbN (Van Buskirk and Smith 1981; Ogawa et al. 1984; 1987; Yamamoto et al. 1994; Shimura et al. 1997; Tokita et al. 2004; but see Geran and Travers 2009). One consistent finding of these studies is that more neurons responding best to acid stimuli appear to be preferentially located in the lateral PbN. We also found more C-best neurons in the lateral PbN. Interestingly, we also found that all Q-best neurons, few of which were recorded from in the studies cited above, were also distributed in the lateral PbN. Furthermore, we found that synergistic neurons were preferentially located in the medial PbN, whereas nonsynergistic neurons were often found in the lateral PbN. Taken together, these results may imply that neurons in the medial and lateral PbN tend to be responsive to preferred and aversive taste stimuli, respectively, although this hypothesis appears to require further confirmation.
GRANTS
This research was supported by the Ajinomoto Amino Acid Research Program and NIH grant DC000353 to J. Boughter, Jr.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.T., T.Y., and J.D.B.J. conception and design of research; K.T. performed experiments; K.T. and J.D.B.J. analyzed data; K.T., T.Y., and J.D.B.J. interpreted results of experiments; K.T. and J.D.B.J. prepared figures; K.T. and J.D.B.J. drafted manuscript; K.T., T.Y., and J.D.B.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Matthew Ennis for technical support, Drs. Noritaka Sako, Keiko Yasumatsu, and Steven St. John for valuable comments, and Drs. Robert Lundy, Stuart McCaughey, Thomas Scott, and Justus Verhagen for providing information on MSG taste responses in their previous studies.
REFERENCES
- Adachi A, Aoyama M. Neuronal responses of the nucleus tractus solitarius to oral stimulation with umami substances. Physiol Behav 49: 935–941, 1991 [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, Smith DV. Amiloride blocks acid responses in NaCl-best gustatory neurons of the hamster solitary nucleus. J Neurophysiol 80: 1362–1372, 1998 [DOI] [PubMed] [Google Scholar]
- Bradley RM, Mistretta CM. Developmental changes in neurophysiological taste responses from the medulla in sheep. Brain Res 191: 21–34, 1980 [DOI] [PubMed] [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Monosodium glutamate but not linoleic acid differentially activates gustatory neurons in the rat geniculate ganglion. Chem Senses 32: 833–846, 2007 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 444: 288–294, 2006 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell 100: 703–711, 2000 [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 3: 113–119, 2000 [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Pereira E, Roper S. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr 90: 738S-–742S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci 16: 3817–3826, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YK, Li CS. Gustatory neural circuitry in the hamster brain stem. J Neurophysiol 100: 1007–1019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee R. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003 [DOI] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses 31: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: implications for the neural coding of T1R ligands. J Neurosci 27: 11242–11253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol 101: 1598–1612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza BK, Ackroff K, McCaughey SA, Sclafani A, Scott TR. Preference conditioning alters taste responses in the nucleus of the solitary tract of the rat. Am J Physiol Regul Integr Comp Physiol 273: R1230–R1240, 1997 [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR. Blood glucose selectively affects taste-evoked activity in rat nucleus tractus solitarius. Physiol Behav 31: 643–650, 1983 [PubMed] [Google Scholar]
- Halsell CB, Frank ME. Mapping study of the parabrachial taste-responsive area for the anterior tongue in the golden hamster. J Comp Neurol 306: 708–722, 1991 [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol 78: 920–938, 1997 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Obata K, Ogawa H. Characterization of parabrachial subnuclei in mice with regard to salt tastants: possible independence of taste relay from visceral processing. Chem Senses 34: 253–267, 2009 [DOI] [PubMed] [Google Scholar]
- Ikeda K. On a new seasoning. J Tokyo Chem Soc Jpn 27: 279–284, 1909 [Google Scholar]
- Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J Neurosci 24: 2296–2303, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Lundy RF. Amygdalofugal influence on processing of taste information in the nucleus of the solitary tract of the rat. J Neurophysiol 104: 726–741, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 312: 500–506, 2003 [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Kare MR. Umami, a Basic Taste. New York: Marcel Dekker, 1987 [Google Scholar]
- Kosar E, Schwartz GJ. Cortical unit responses to chemical stimulation of the oral cavity in the rat. Brain Res 513: 212–224, 1990 [DOI] [PubMed] [Google Scholar]
- Kovacs P, Hajnal A. Altered pontine taste processing in a rat model of obesity. J Neurophysiol 100: 2145–2157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH, Margolskee RF. Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101: 2459–2471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692–4696, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey SA. Taste-evoked responses to sweeteners in the nucleus of the solitary tract differ between C57BL/6ByJ and 129P3/J mice. J Neurosci 27: 35–45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Beauchamp GK, Bachmanov AA. Taste perception of monosodium glutamate and inosine monophosphate by 129P3/J and C57BL/6ByJ mice. Physiol Behav 98: 481–488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. Taste responses of neurons in the nucleus of the solitary tract of awake rats: an extended stimulus array. J Neurophysiol 70: 879–891, 1993 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. Sodium-deficient diet reduces gustatory activity in the nucleus of the solitary tract of behaving rats. Am J Physiol Regul Integr Comp Physiol 269: R647–R661, 1995 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Eddy MC, Katsukawa H, Delay ER, Ninomiya Y. Behavioral responses to glutamate receptor agonists and antagonists implicate the involvement of brain-expressed mGluR4 and mGluR1 in taste transduction for umami in mice. Physiol Behav 105: 709–719, 2012 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Katsukawa H, Sasamoto K, Ninomiya Y. Behavioral taste similarities and differences among monosodium l-glutamate and glutamate receptor agonists in C57BL mice. J Nutr Sci Vitaminol (Tokyo) 47: 161–166, 2001 [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002 [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Behavioral discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol 92A: 365–370, 1989a [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Peripheral neural basis for behavioral discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol 92A: 371–376, 1989b [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Inoue M, Imoto T, Nakashima K. Lack of gurmarin sensitivity of sweet taste receptors innervated by the glossopharyngeal nerve in C57BL mice. Am J Physiol Regul Integr Comp Physiol 272: R1002–R1006, 1997 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kurenuma S, Nomura T, Uebayashi H, Kawamura H. Taste synergism between monosodium glutamate and 5'-ribonucleotide in mice. Comp Biochem Physiol A Comp Physiol 101A: 97–102, 1992 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Mizukoshi T, Higashi T, Katsukawa H, Funakoshi M. Gustatory neural responses in three different strains of mice. Brain Res 302: 305–314, 1984 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr 130: 950S-–953S, 2000 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Tonosaki K, Funakoshi M. Gustatory neural response in the mouse. Brain Res 244: 370–373, 1982 [DOI] [PubMed] [Google Scholar]
- Nishijo H, Norgren R. Parabrachial neural coding of taste stimuli in awake rats. J Neurophysiol 78: 2254–2268, 1997 [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Norgren R. Parabrachial gustatory neural responses to monosodium glutamate ingested by awake rats. Physiol Behav 49: 965–971, 1991 [DOI] [PubMed] [Google Scholar]
- Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res 91: 99–117, 1975 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Hayama T, Ito S. Location and taste responses of parabrachio-thalamic relay neurons in rats. Exp Neurol 83: 507–517, 1984 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Hayama T, Ito S. Response properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Exp Brain Res 68: 449–457, 1987 [DOI] [PubMed] [Google Scholar]
- Reinert H. Urethane hyperglycemia and hypothalamic activation. Nature 204: 889–891, 1964 [DOI] [PubMed] [Google Scholar]
- Sako N, Harada S, Yamamoto T. Gustatory information of umami substances in three major taste nerves. Physiol Behav 71: 193–198, 2000 [DOI] [PubMed] [Google Scholar]
- Sako N, Tokita K, Sugimura T, Yamamoto T. Synergistic responses of the chorda tympani to mixtures of umami and sweet substances in rats. Chem Senses 28: 261–266, 2003 [DOI] [PubMed] [Google Scholar]
- Sako N, Yamamoto T. Analyses of taste nerve responses with special reference to possible receptor mechanisms of umami taste in the rat. Neurosci Lett 261: 109–112, 1999 [DOI] [PubMed] [Google Scholar]
- Sato M, Yamashita S, Ogawa H. Potentiation of gustatory response to monosodium glutamate in rat chorda tympani fibers by addition of 5'-ribonucleotides. Jpn J Physiol 20: 444–464, 1970 [DOI] [PubMed] [Google Scholar]
- Shimura T, Tanaka H, Yamamoto T. Salient responsiveness of parabrachial neurons to the conditioned stimulus after the acquisition of taste aversion learning in rats. Neuroscience 81: 239–247, 1997 [DOI] [PubMed] [Google Scholar]
- Shimura T, Tokita K, Yamamoto T. Parabrachial unit activities after the acquisition of conditioned taste aversion to a nonpreferred HCl solution in rats. Chem Senses 27: 153–158, 2002 [DOI] [PubMed] [Google Scholar]
- Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses Flav 4: 215–229, 1979 [Google Scholar]
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4: 143–191, 2005 [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Roper SD, Delay ER. The taste of monosodium glutamate (MSG), L-aspartic acid, and N-methyl-d-aspartate (NMDA) in rats: are NMDA receptors involved in MSG taste? Chem Senses 24: 449–457, 1999 [DOI] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience 161: 475–488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience 171: 351–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Karádi Z, Shimura T, Yamamoto T. Centrifugal inputs modulate taste aversion learning associated parabrachial neuronal activities. J Neurophysiol 92: 265–279, 2004 [DOI] [PubMed] [Google Scholar]
- Van Buskirk RL, Smith DV. Taste sensitivity of hamster parabrachial pontine neurons. J Neurophysiol 45: 144–171, 1981 [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Giza BK, Scott TR. Effect of amiloride on gustatory responses in the ventroposteromedial nucleus of the thalamus in rats. J Neurophysiol 93: 157–166, 2005 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. The synergistic effect of monosodium glutamate and disodium 5'-inosinate. J Food Sci 32: 473–478, 1967 [Google Scholar]
- Yamaguchi S. Basic properties of umami and effects on humans. Physiol Behav 49: 833–841, 1991 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Asai K. Studies on responses of cortical taste neurons to umami substances. In: Umami, a Basic Taste, edited by Kawamura Y, Kare MR. New York, NY: Marcel Dekker, 1987 [Google Scholar]
- Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav 49: 919–925, 1991 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sakai N, Ozaki N. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol Behav 56: 1197–1202, 1994 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Watanabe U, Fujimoto M, Sako N. Taste preference and nerve response to 5'-inosine monophosphate are enhanced by glutathione in mice. Chem Senses 34: 809–818, 2009 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. III. Neural and behavioral measures compared. J Neurophysiol 53: 1370–1386, 1985 [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. Umani taste in mice uses multiple receptors and transduction pathways. J Physiol. First published December 19, 2011; doi:10.1113/jphysiol.2011.211920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol 587, 4425–4439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA 105: 20930–20934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003 [DOI] [PubMed] [Google Scholar]