Abstract
The medial-olivocochlear (MOC) acoustic reflex is thought to provide frequency-specific feedback that adjusts the gain of cochlear amplification, but little is known about how frequency specific the reflex actually is. We measured human MOC tuning through changes in stimulus frequency otoacoustic emissions (SFOAEs) from 40-dB-SPL tones at probe frequencies (fps) near 0.5, 1.0, and 4.0 kHz. MOC activity was elicited by 60-dB-SPL ipsilateral, contralateral, or bilateral tones or half-octave noise bands, with elicitor frequency (fe) varied in half-octave steps. Tone and noise elicitors produced similar results. At all probe frequencies, SFOAE changes were produced by a wide range of elicitor frequencies with elicitor frequencies near 0.7–2.0 kHz being particularly effective. MOC-induced changes in SFOAE magnitude and SFOAE phase were surprisingly different functions of fe: magnitude inhibition largest for fe close to fp, phase change largest for fe remote from fp. The metric ΔSFOAE, which combines both magnitude and phase changes, provided the best match to reported (cat) MOC neural inhibition. Ipsilateral and contralateral MOC reflexes often showed dramatic differences in plots of MOC effect vs. elicitor frequency, indicating that the contralateral reflex does not give an accurate picture of ipsilateral-reflex properties. These differences in MOC effects appear to imply that ipsilateral and contralateral reflexes have different actions in the cochlea. The implication of these results for MOC function, cochlear mechanics, and the production of SFOAEs are discussed.
Keywords: hearing, cochlear amplifier, masking, tuning curves, otoacoustic emissions
the final link in the control of cochlear mechanical amplification of the descending auditory system is through the medial olivocochlear (MOC) efferent fibers. MOC fibers innervate electro-motile cochlear outer hair cells (OHCs), the cells that amplify basilar-membrane (BM) motion in response to sound (Dallos 1992). The MOC-OHC synapse is unusual in that it is cholinergic with α9-α10 acetylcholine receptors (Elgoyhen et al. 2001). Activation of these receptors allows the entry of calcium into OHCs, which activates Ca2+-activated K+ channels that hyperpolarize the OHCs (Fuchs 2002). The net effect of the MOC synaptic activation is to decrease cochlear amplification and thereby to reduce sound-induced vibration of the BM and organ of Corti (Guinan 2006).
A variety of evidence indicates that MOC fibers continuously adjust cochlear amplification for the current acoustic and behavioral needs. MOC neurons receive major inputs from brainstem reflex circuitry such that MOC neurons to one ear can be activated by sound in either ear. This MOC acoustic reflex provides negative feedback; i.e., background noise excites MOC activity that reduces cochlear amplifier gain that then reduces the response to the noise. This negative feedback produces an antimasking effect because the reduced noise response allows new, information-bearing sounds to be heard by reducing the deleterious effects of the ongoing, adapted neural response to the noise (Winslow and Sachs 1988; Kawase et al. 1993; Jennings et al. 2011). MOC neurons also receive inputs from the descending auditory system, and there is evidence that these descending inputs allow task-oriented modification of MOC activation (Warr 1992; Oatman 1976; Maison et al. 2001). Descending activity allows attention-directed control of MOC efferents (Meric and Collet 1994) and may be involved in learning (Veuillet et al. 2007; de Boer and Thornton 2008; Irving et al. 2011). The interaction between the descending control of MOC firing and the MOC acoustic reflex is poorly understood. It remains to be determined whether descending systems directly activate MOC neurons or are more accurately thought of as modulating the MOC acoustic reflex (see Xiao and Suga 2002; Ota et al. 2004). Finally, at high sound levels MOC feedback reduces acoustic trauma (Rajan 1992; Maison and Liberman 2000). At high sound levels, MOC activation produces only a small reduction of cochlear amplifier gain, but it produces a substantial change in BM response phase, indicating the presence of substantial mechanical changes (Cooper and Guinan 2011). Our understanding of all of these aspects of MOC action is impacted by the degree to which the peripheral MOC system provides frequency-specific feedback to the cochlea.
Anatomical work and single-neuron recordings in animals indicate that the MOC reflex is tonotopically organized, but relatively little is known about the functional tuning of the MOC reflex (Liberman and Brown 1986; Brown 1989). In Lilaonitkul and Guinan (2009a) we showed that, in humans, elicitors over a wide range of frequencies inhibit responses to probe sounds near 1 kHz. Furthermore, the MOC effects were skewed so that elicitor frequencies 0.5–1.0 octave below the probe sound produced larger MOC effects than elicitors 0.5–1.0 octave above. Both the skew and the lack of sharp tuning were surprising because recording and labeling of single MOC fibers in animals showed near tonotopic cochlear innervation and tuning almost as sharp as auditory-nerve fibers (Robertson 1984; Liberman and Brown 1986; Brown 1989). MOC innervation patterns have been interpreted as being consistent with frequency-specific MOC feedback, i.e., a sound that excites a cochlear frequency region would elicit MOC firing that decreases cochlear-amplifier gain specifically in this frequency region (Winslow and Sachs 1987).
The main objective of the present study is to extend our measurements of MOC tuning in humans to other probe frequencies. We cannot assume that the results with 1-kHz probes apply to other frequency regions because data from animals indicate that the innervation, neurochemistry, and physiology of medial efferents vary along the length of the cochlea (Ryugo et al. 2011). In particular, MOC fibers with best frequencies (BFs) below 10 kHz tend to have lower thresholds, higher discharge rates, and shorter latencies (Liberman and Brown 1986; Liberman 1988; Brown 1989). MOC fibers also show differences in their response patterns to ipsilateral vs. contralateral sound (Liberman 1988; Brown 1989). A second objective of our work is to study the tuning properties to ipsilateral, contralateral, and bilateral elicitors, not just to contralateral elicitors as was done in almost all other studies.
Investigations of MOC effects in humans normally use the change in otoacoustic emissions (OAEs) as a metric for MOC effect because OAEs provide noninvasive probes of cochlear amplification. OAEs are sounds in the ear canal attributable to energy from cochlear amplification that has traveled backward through the middle ear. Our previous work (Lilaonitkul and Guinan 2009a; 2009b), and almost all other studies, looked only at the change in OAE magnitudes. However, OAE phase also provides information, and recent work has shown that MOC activation by broad-band noise produces systematic changes in OAE phases and delays (Deeter et al. 2009; Francis and Guinan 2010; Henin et al. 2011). A third objective of the present paper is to analyze the MOC-induced changes in OAE phase. Phase information may produce additional insights into MOC control of the cochlea. Also, because MOC efferents change cochlear mechanical properties, phase information may produce insight into the poorly understood area of cochlear micromechanics.
MATERIALS AND METHODS
Methods overview.
Many of our methods are the same as those in Lilaonitkul and Guinan (2009a). Here we will present methods highlights and details that are different. To measure cochlear mechanical effects produced by sound-evoked MOC activity, we used stimulus frequency OAEs (SFOAEs). SFOAEs are low-level tones in the ear canal that are generated in healthy cochleae in response to tones. We used SFOAEs, instead of other OAEs, because SFOAEs are the most frequency-selective OAE, and the probe sound used to evoke SFOAEs elicits relatively little MOC activity (Guinan et al. 2003). For OAE changes to be interpretable as attributable to only activation of MOC fibers, there must be no activation of the middle-ear-muscles (MEMs), and to rule these out we used the same MEM test as in Lilaonitkul and Guinan (2009a).
The basic paradigm used here was to measure changes in the SFOAE from a tone at a probe frequency (fp) as a narrow-band elicitor sound was stepped in frequency above and below fp. These elicitor frequency (fe) series were done with ipsilateral, contralateral, and bilateral elicitors. Two different metrics were used to quantify the change in the SFOAE because each metric had certain advantages.
Subjects.
Data were used from 8 ears for probe frequencies near 0.5 kHz, 11 ears for probes near 1 kHz, and 3 ears for probes near 4 kHz. Additional details are given in Table 1. All subjects had normal thresholds in both ears, i.e., <20 dB HL in octave steps 0.25–4 kHz. Subjects were rejected if they were unable to stay awake and sit still during the experiment, or if they did not return to complete the study. An ear was accepted only if it passed the MEM test and if the magnitude of the MOC-induced change in the SFOAE elicited by a bilateral, 60-dB-SPL, broad-band noise (BBN) (100 Hz-10 kHz) was greater than 0 dB SPL. For each fp, the same subjects were used for the measurements with tone and noise-band elicitors. To avoid significant magnitude estimation biases from low signal-to-noise ratios (SNRs) (see Backus 2007), data from individual ears were used only if they passed a SNR criterion of 3 (a ratio of 3 equals 9.54 dB) in SFOAE magnitude and in MOC effect (ΔSFOAE magnitude, defined below). The MOC-effect criterion was applied to the maximum ΔSFOAE point in a fe series, rather than to each point individually, to avoid frequency biases, i.e., to allow points to be used even if they showed no response, as long as there was a large response at some frequency in the series. During the measurements of MOC effects, the subjects had no assigned task and were not instructed to pay attention to any aspect of the sounds presented. All experiments followed protocols approved by the Massachusetts Eye and Ear Infirmary and Massachusetts Institute of Technology Human Studies Committees.
Table 1.
Summary of subject sex, age, number screened, and number included
| Subjects Included / Subjects Screened |
|||||
|---|---|---|---|---|---|
| Probe Frequency Region, kHz | Male | Female | Number of Ears Included in Study | Subject Age Range, yr | Average Subject Age, yr |
| 0.5 | 1/3 | 4/5 | 8 | 22–31 | 24.4 |
| 1.0 | 3/4 | 4/4 | 11 | 22–33 | 27.5 |
| 4.0 | 0/5 | 3/7 | 3 | 22–40 | 28 |
Acoustic stimuli.
To simultaneously evoke SFOAEs in both ears, a 40-dB-SPL continuous probe tone at a frequency within ±10% of 0.5, 1.0, or 4.0 kHz was presented bilaterally through one earphone of the Etymotic ER10C acoustic assembly in each ear. Although the SFOAE probe stimulus elicits less MOC activity than other OAE probe stimuli, it can have an effect on the results (Lilaonitkul and Guinan 2009a). An fp was selected on each subject that 1) was at least 100 Hz away from any spontaneous OAE (SOAE) with magnitude above −10 dB SPL (to avoid SOAE entrainment and other interactions, e.g., Zwicker and Schloth 1984; Long et al. 1991), and 2) produced the largest ΔSFOAE magnitude to a 60-dB-SPL, contralateral BBN (so that the SNR criteria could be reached with the smallest number of stimulus repeats). No overall differences were found in the normalized ΔSFOAEs from frequencies ±10% of 1 kHz vs. from using 1 kHz (Backus and Guinan 2007).
The SFOAE produced by the probe tone alone, referred to as the baseline SFOAE, was measured by the suppression method (Guinan 1990; Kalluri and Shera 2007). With this method, a brief second tone is presented at a frequency near fp and a level 20 dB, or more, above the fp level. This “suppressor tone” is believed to push OHC stereocilia into saturation regions, which lowers the cochlear amplifier gain at nearby frequencies, thereby suppressing the SFOAE from the probe. Because of their different actions, we distinguish between this “two-tone suppression” and MOC “inhibition”. The baseline SFOAE was taken to be the vector difference of the ear-canal sound pressure from the 40-dB-SPL probe tone with and without a suppressor (a 60-dB-SPL tone at 110 Hz below the fp presented for 0.5 s every 1 s). Suppressor-tone runs were interleaved with fe series runs. To minimize spectral splatter, 5-ms rise/fall cosine ramps were used on both suppressor tones and MOC elicitors. Elicitor and suppressor polarities were alternated across presentations so that averaging would cancel their acoustic waveforms, leaving a residual equal to the induced change in the SFOAE vector. The acoustic output from the two sound sources in each ER10C acoustic assembly were calibrated at the beginning of every data-gathering session and frequently within a session. Noise bursts were made spectrally flat at the probe-tube tip by applying these calibrations.
To elicit MOC activity, a 60-dB-SPL tone or a narrow, half-octave noise band (NBN) was presented ipsilaterally, contralaterally, or bilaterally for 2.5 s. Because the probe tone was presented bilaterally, an ipsilateral elicitor for the right ear was simultaneously a contralateral elicitor for the left ear, which increased the efficiency of data gathering. Preceding the elicitor was a 0.5-s, probe-alone onset period from which the baseline response was measured, and following the elicitor was a 2-s period for recovery, which resulted in a stimulus repetition period of 5 s. The fe will be used to refer to either the frequency of a tone elicitor or the center frequency (on a logarithmic scale) of a NBN elicitor. fes were separated by half-octave steps and ranged from −2 to +3 octaves re. the 0.5-kHz probe, from −2.5 to +2.5 octaves re. the 1-kHz probe, and from −3.5 to +1 octave re. the 4-kHz probe. At each fp, fe series using tones were interleaved with fe series using NBN to allow direct comparison of the results. Within each fe series, the fes were presented in a randomized order.
fe series were done in blocks that averaged 4–10 (always an even number) artifact-free responses at each fe. Responses were rejected as being contaminated by artifacts when the difference between one pair of responses and the next pair exceeded a criterion set for each subject. Stimulus pairs were used because elicitors (or suppressors) were alternated in polarity across stimulus repetitions. Multiple data blocks were vectorially averaged to achieve response averages with N ≥ 50 stimulus repetitions so that the series SNR of 3 was achieved. The averaged waveforms were then heterodyned (heterodyning is equivalent to sending the signal through a lock-in amplifier; for more details see Guinan et al. 2003) to obtain P(t), the magnitude and phase of the fp sound pressure as a function of time over one repetition period. Note that the magnitude and phase are expressed by the single complex time function P(t).
Measurement analysis.
The ear-canal sound pressure, P, is the vector sum of the probe tone and the evoked SFOAE (Fig. 1). An MOC elicitor, or a suppressor tone, can change the SFOAE over time yielding P(t). Changes in the SFOAE, ΔSFOAE(t), were calculated from P(t) by the vector difference between P(t) and Pbaseline, where Pbaseline is the average P(t) before the suppressor or MOC elicitor has any effect [i.e., as the vector average of P(t) between 50–450 ms]. The MOC-induced change is expressed as a fraction of the SFOAE by dividing ΔSFOAE(t) by the magnitude of the baseline SFOAE, which yields the normalized ΔSFOAE(t), ΔSFOAEn(t). The baseline SFOAE was obtained as the difference in sound pressure at fp with and without the suppressor tone.
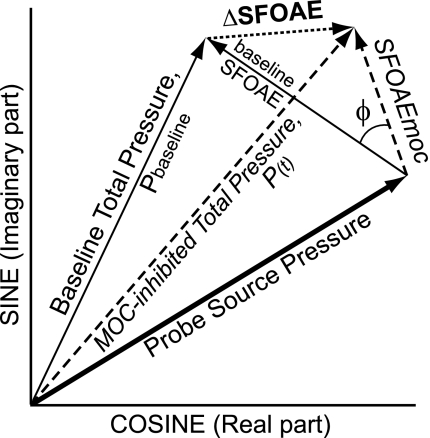
Fig. 1.
Vector diagram of medial-olivocochlear (MOC) effects on sound pressure in the ear canal. The arrows are vectors in the complex plane with length representing magnitude and direction representing phase. Before stimulation, the Probe Source Pressure and the baseline stimulus frequency otoacoustic emission (SFOAE) summate to form the Baseline Total Pressure, Pbaseline. In the presence of an MOC-elicitor, the SFOAE is changed to SFOAEmoc resulting in a new ear-canal sound pressure, the MOC-inhibited Total Pressure, P(t). The change from Pbaseline to P(t) is the change in the SFOAE, ΔSFOAE. A positive angle Φ is a phase advance of SFOAEmoc re. the baseline SFOAE.
In addition to ΔSFOAEn(t), the change in the SFOAE produced by MOC activity was considered a second way, as the SFOAE obtained during MOC inhibition, SFOAEmoc(t) (Fig. 1). SFOAEmoc(t) was obtained from the vector sum of the baseline SFOAE, plus ΔSFOAE(t), as shown in Fig. 1. The SFOAE during MOC inhibition is expressed as a fraction of the SFOAE by dividing SFOAEmoc(t) by the magnitude of the baseline SFOAE, which yields the normalized SFOAEmoc(t), nSFOAEmoc(t).
To obtain MOC-effect metrics from time-varying data, the data were vector averaged within either a 0.1-s “during-elicitor” time window ending 0.05 s before the end of the elicitor, or a 0.1-s “postelicitor” time window starting 0.05 s after the end of the elicitor. The postelicitor window avoids two-tone suppression produced by ipsilateral and bilateral elicitors but at the expense of capturing the MOC effect during its decay. There is no two-tone suppression produced by 60-dB-SPL contralateral elicitors, so we can use a during-elicitor window, which allows us to capture the maximum MOC effect. We also tried a 1-s during-elicitor time window, which increased the signal/noise of the individual measurement. However, because this made almost no difference in the averages across subjects, we kept all of the windows the same 0.1-s duration (the standard deviations in the averages across subjects were principally from different results in different subjects, not poor signal/noise in the individual measurements). The noise floor estimates were obtained by averaging in 0.1-s time windows that ended 0.05 s before the end of the stimulus repetition period.
Determining statistical significance.
To determine the statistical significance of the variations in the magnitudes of ΔSFOAEn and nSFOAEmoc produced by variations in stimulus parameters, we used an n-dimensional ANOVA with, when appropriate, a Bonferroni correction. Statistical significance was accepted at the 0.05 level. ANOVA to test the significance of ΔSFOAEn magnitude variations across fe included data from the whole fe range. To avoid using magnitudes that were too close to the noise floor, ANOVA to determine the significance of magnitude variation across subject, ear, and laterality used a restricted fe range of −2 to 2 octaves re. the 0.5-kHz probe, −2.5 to 2.5 octaves re. the 1-kHz probe, and −1 to 1 octaves re. the 4-kHz probe. To determine whether the ΔSFOAEn magnitudes from fes below fp were larger than those from above fp (i.e., were skewed) to a statistically significant degree, we used a bootstrap technique (Efron and Tibshirani 1993; for details see Lilaonitkul and Guinan, 2009a).
RESULTS
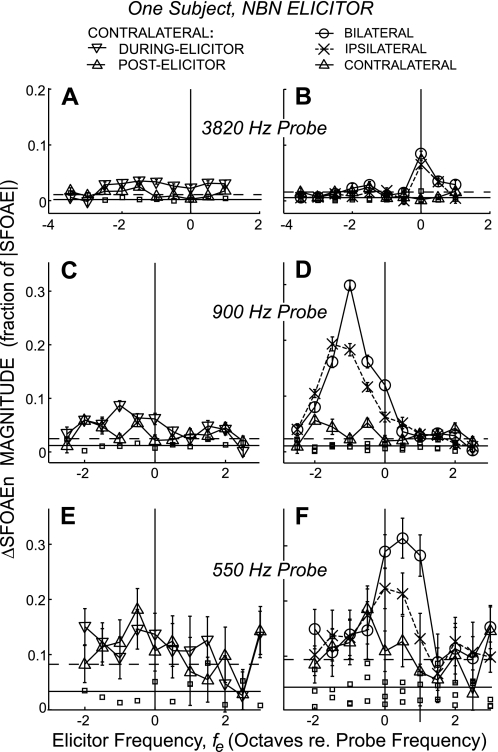
ΔSFOAEn magnitudes for NBN elicitors from a typical subject are shown in Fig. 2. The left panel shows data from contralateral elicitors for both the postelicitor window and from the more accurate, during-elicitor window. The right panel shows the data from all three elicitor lateralities using only the postelicitor window in which data from all three lateralities are comparable. Although details of the MOC effects vary from subject to subject, Fig. 2 illustrates the main trends in the data. In general, MOC effects increased as probe frequency decreased and were largest for bilateral elicitors and smallest for contralateral elicitors. In addition, the patterns of MOC effects vs. elicitor frequency were different across elicitor lateralities and probe frequencies (Fig. 2). These differences will be considered in detail later, with data averaged across subjects. In addition to the MOC effects in Fig. 2, we also show the corresponding MEM-test results (small squares). Note that the MEM-test ΔSFOAEn magnitudes were above the average noise level at many fes, but the values from all of the MEM-test runs are within the statistical variation of the noise level. For all of the stimulus frequencies and levels reported in this study, MEM tests such as this were done and showed no evidence of MEM contractions.
Fig. 2.
An example from a typical subject of ΔSFOAEn magnitudes from narrow-band, half-octave noise (NBN) elicitors as functions of elicitor frequency, and corresponding middle-ear-muscle-test responses (small squares). The elicitors were 60 dB SPL, half-octave NBN, and the probe frequencies were near 0.5 (E and F), 1.0 (C and D), and 4.0 kHz (A and B). Left: ΔSFOAEn magnitudes from contralateral elicitors measured in ▽ = the during-elicitor window (100-ms duration ending 50 ms before elicitor offset), or △ = the postelicitor window (100-ms duration starting 50 ms after elicitor offset). Right: ΔSFOAEn magnitudes to bilateral (○), ipsilateral (X), and contralateral (△, same as at left) elicitors measured in the postelicitor window. Points are mean data and error bars are ±1 SE of the mean from repeated measures. Horizontal lines: solid = noise-floor mean, dashed = 2 SD above the noise mean.
Our standard metric for measuring MOC effects has been ΔSFOAE, as used in Fig. 2, in part because it has the best SNR (Lilaonitkul and Guinan 2009a; 2009b). However, we also want to consider MOC-induced changes in SFOAE phase, and this is better done with a different metric, the MOC-inhibited SFOAE vector, SFOAEmoc (Fig. 1) because SFOAEmoc can be more readily interpreted in terms of underlying changes in cochlear mechanics. We use normalized versions of both metrics so that they are expressed as a fraction of the original SFOAE (see materials and methods). By either metric, the differences between the average left and right ear MOC effects were not significant at any fp, so further analysis was done with the data from both ears combined. ANOVA results to determine whether ear (left vs. right) had a significant effect on the ΔSFOAE magnitudes are as follows: Ptone,0.5kHz = 0.53, PNBN,0.5kHz = 0.90; Ptone,1kHz = 0.55, PNBN,1kHz = 0.60; Ptone,4kHz = 0.49, and PNBN,4kHz = 0.78.
For all fps, significant intersubject variation was observed in the ΔSFOAEn magnitude except that the 4-kHz responses to tone elicitors were statistically indistinguishable across subjects. ANOVA results to determine whether across-subject variation has a significant effect on the ΔSFOAE magnitudes are as follows: Ptone,0.5kHz < 10-3, PNBN,0.5kHz = 0.02, Ptone,1kHz < 10-3, PNBN,1kHz < 10-3, Ptone,4kHz = 0.49, PNBN,4kHz = 1.4x10-3. Examples of the scatter in ΔSFOAEn magnitudes across subjects were shown in Figs. 2 and 3 of Lilaonitkul and Guinan (2009a). Here we focus on, and show in Figs. 3–7, data averaged across all relevant subjects.
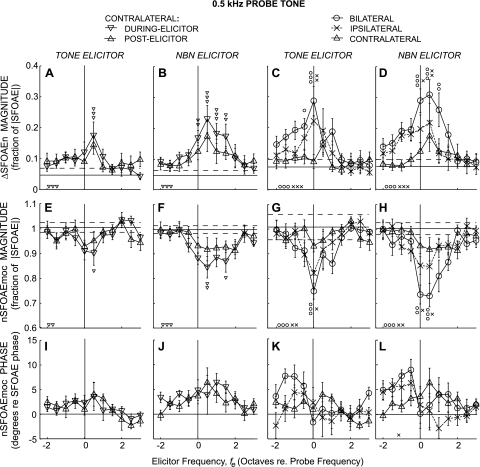
Fig. 3.
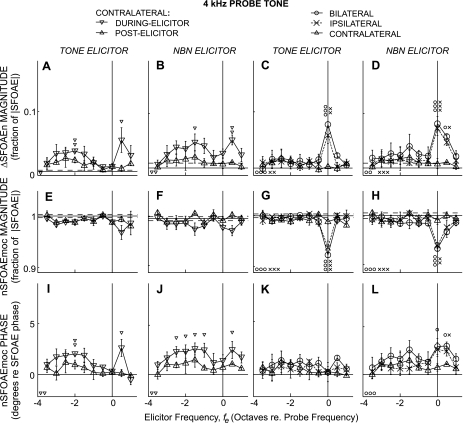
Changes in SFOAEs expressed as ΔSFOAEn magnitude (top, A–D), nSFOAEmoc magnitude (middle, E–H), and nSFOAEmoc phase (bottom, I–L) produced by tone or noise elicitors (labels above columns) as functions of elicitor frequency re. the 0.5-kHz probe frequency. Large symbols show averages across subjects (key at top, same as Fig. 2). Error bars are ±1 SE across subjects. Horizontal lines in A–H: solid = noise-floor mean, dashed = 1 SD from the noise mean. Line in I–L is zero phase. Small symbols directly above or below the large symbols indicate significant differences (Student's t-test with a Bonferroni correction for multiple comparisons) from the control (noise floor). Small symbols at bottom left in each panel indicate that the quantity varied significantly across elicitor frequency by an ANOVA test. For all significances: 1 symbol = P ≤ 0.05, 2 symbols = P ≤ 0.01, 3 symbols = P ≤ 0.001.
Fig. 4.
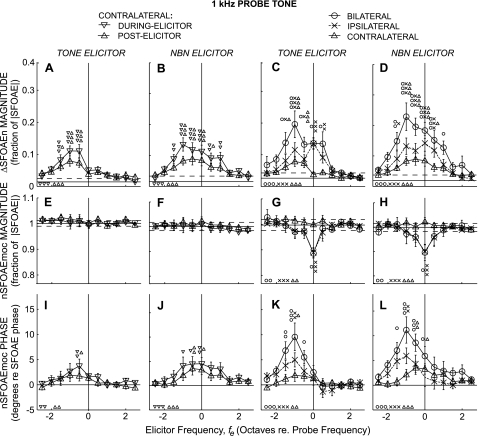
Changes in SFOAEs for 1-kHz probes. Layout and symbols as in Fig. 3.
Fig. 5.
Changes in SFOAEs for 4-kHz probes. Layout and symbols as in Fig. 3.
Fig. 6.
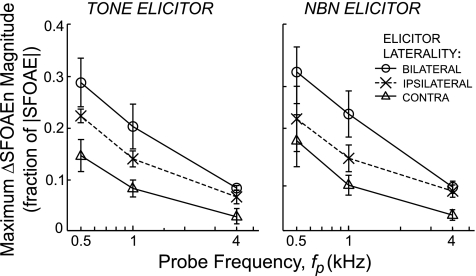
Peak MOC effect is a decaying function of probe frequency for tone and noise elicitors and for all lateralities. Each point shows the largest ΔSFOAEn magnitude (across frequency) from Figs. 3–5.
Fig. 7.
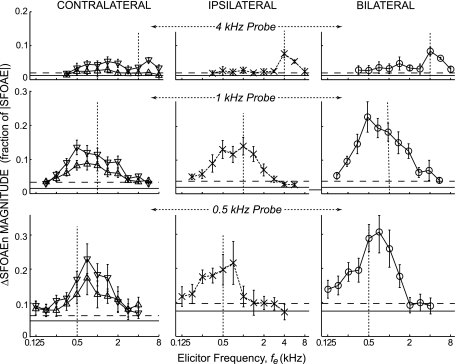
Comparison of MOC effects across probe frequencies on the same absolute-frequency axis. Shown are ΔSFOAEn magnitudes from NBN elicitors (from B and D of Figs. 3–5). Symbols as in Fig. 3.
Probe frequencies near 0.5 kHz.
MOC effects near 0.5 kHz elicited by tone and NBN elicitors and averaged across subjects are shown in Fig. 3. First, we consider ΔSFOAEn magnitude (Fig. 3, top) and then nSFOAEmoc magnitude and phase (Fig. 3, middle and bottom). ANOVA showed that ΔSFOAEn magnitude varied significantly with elicitor frequency for bilateral and ipsilateral elicitors (In Figs. 3–5, ANOVA results are shown by the small symbols at the bottom left of each panel). For contralateral elicitors, ANOVA showed MOC effects that varied significantly with fe when analyzed in the during-elicitor window but not in the postelicitor window by which time much of the MOC effect had decayed into the noise floor. Individual ΔSFOAEn magnitude points that were significantly different from the noise floor are marked by small symbols above the points.
ΔSFOAEn magnitude varied significantly with elicitor laterality (ANOVA: P < 10−3). Bilateral elicitors yielded a greater ΔSFOAEn magnitude than the corresponding ipsilateral or contralateral elicitor, when the bilateral ΔSFOAEn magnitude was statistically significant. Ipsilateral ΔSFOAEn magnitudes ranged from greater than the contralateral ΔSFOAEn magnitude, to not statistically different, implying that the relative magnitude of ipsilateral to contralateral ΔSFOAEn is dependent on fe.
Changes in the ΔSFOAEn magnitude patterns from NBNs were qualitatively similar to those from tones, except that for NBNs large MOC effects occurred over a wider fe range, making the NBN ΔSFOAEn magnitude pattern appear less peaky. The greatest ΔSFOAEn magnitude for ipsilateral and bilateral elicitors was for fe at fp (tone elicitors) or for fe a half-octave above fp (noise elicitors), but for contralateral elicitors was for fe half-octave above fp for both tone and noise elicitors (Fig. 3, A–D).
Many of the ΔSFOAEn magnitude vs. fe patterns appear skewed. Significant skews, with fes above fp being most effective, were found for NBN elicitors presented bilaterally and contralaterally, but not ipsilaterally (Table 2). For all three elicitor lateralities, significant NBN-elicited ΔSFOAEn magnitudes were observed over a broad fe range, from fp to 1.0–1.5 octaves above fp.
Table 2.
Summary of asymmetry measures (skewness) for ΔSFOAEn magnitudes for bilateral, ipsilateral, and contralateral, 60-dB-SPL tone and NBN elicitors
| Bilateral |
Ipsilateral |
Contralateral (Postelicitor) |
Contralateral (During Elicitor) |
|||||
|---|---|---|---|---|---|---|---|---|
| Probe Tone Frequency | Tone | NBN | Tone | NBN | Tone | NBN | Tone | NBN |
| ∼0.5 kHz [-2 to 2 oct] | ||||||||
| Pearson's Skewness Factor, b1 | 0.12 | −0.18 | 0.01 | −0.08 | 0.08 | −0.27 | −0.07 | −0.37 |
| Pb1 | 0.06 | 0.97 | 0.43 | 0.19 | 0.19 | 0.98 | 0.78 | 0.99 |
| ∼1.0 kHz [-2.5 to 2.5 oct] | ||||||||
| Pearson's Skewness Factor, b1 | 0.41 | 0.29 | 0.25 | 0.19 | 0.38 | 0.19 | 0.53 | 0.23 |
| Pb1 | < 10−3 | < 10−3 | 4x10−3 | 0.03 | < 10−3 | < 10−3 | 0.00 | 6.60x10−3 |
Asymmetry was quantified by the skewness factor where 0 signifies perfect symmetry about the mean, positive values indicate a response bias toward lower frequencies (left of the mean), and negative values indicate a bias toward higher frequencies. The significance, Pb1, of the skewness factors being different from 0 was tested using a bootstrapped distribution under the null hypothesis of symmetry. Statistically significant values (bold in the table) were taken as P ≤ 0.05 for positive b1 and as P > 0.95 for negative b1. The data are shown in Figures 3–4. SFOAEn, normalized stimulus frequency otoacoustic emission; NBN, narrow-band noise.
MOC effects considered as the MOC-inhibited SFOAEn, nSFOAEmoc, are shown in the second and third rows of Fig. 3. Significant nSFOAEmoc magnitude inhibition was observed for all elicitor lateralities at some fe. Compared with tones, NBNs elicited inhibition over a wider range of fes (Fig. 3, E–H). Inhibition of nSFOAEmoc magnitude by ipsilateral and bilateral tone elicitors was greatest for tones centered at fp (Fig. 3G). In contrast, inhibition by contralateral tones and by NBN of all lateralities was greatest a half-octave above fp (Fig. 3, E, F, and H).
The MOC effects on SFOAEn phase, as shown by nSFOAEmoc phase (Fig. 3, bottom) were not statistically significant. However, because the tone and NBN experiments yielded roughly similar patterns in phase-change vs. fe (Fig. 3, I–L) and these are independent data sets, it seems likely that the observed patterns are due to MOC effects and not simply a result of noise.
Probe frequencies near 1 kHz.
MOC effects near 1 kHz averaged across subjects are shown in Fig. 4. Many aspects of the 1-kHz results are similar to those at 0.5 kHz (e.g., ΔSFOAEn magnitudes from bilateral elicitors were generally larger than those from ipsilateral and contralateral elicitors). At 1 kHz, ΔSFOAEn magnitude varied significantly with elicitor frequency for all elicitor lateralities (ANOVA: P < 10−3). One difference in the results at 1 kHz compared with 0.5 kHz is that the ΔSFOAEn magnitude skew is in the opposite direction. At 1 kHz, significant skews, with fes below fp being most effective, were found for both tone and NBN elicitors of all lateralities (Table 2).
Perhaps the largest differences between the 1.0- and 0.5-kHz results are in nSFOAEmoc. At 1 kHz, the patterns of change vs. fe were substantially different in nSFOAEmoc magnitude compared with ΔSFOAEn magnitude (Fig. 4, middle vs. top). In particular, the changes in nSFOAEmoc magnitude from contralateral elicitors were remarkably small (Fig. 3, E and F). In addition, the changes in nSFOAEmoc magnitude from ipsilateral and bilateral elicitors showed dips at fe = fp that were sharper than those found at 0.5 kHz. Finally, there were statistically significant variations across fe in nSFOAEmoc phase for tone and noise elicitors of all lateralities, and these showed very different patterns vs. fe than nSFOAEmoc magnitude (Fig. 3, middle vs. bottom).
Probe frequencies near 4 kHz.
MOC effects near 4 kHz averaged across subjects are shown in Fig. 5. The 4-kHz results were substantially different from those at 0.5 and 1.0 kHz, especially in ΔSFOAEn magnitude and nSFOAEmoc phase. At 4 kHz, as before, ΔSFOAEn magnitude varied significantly with elicitor frequency for all elicitor lateralities.
Similar to 0.5 and 1.0 kHz, near 4 kHz the ΔSFOAEn magnitudes from bilateral elicitors were generally larger than those from ipsilateral and contralateral elicitors. The ratio of ΔSFOAEn magnitudes from ipsilateral vs. contralateral elicitors varied with fe (e.g., for fe = fp, ΔSFOAEn magnitude was much larger for ipsilateral compared with contralateral elicitors, but for fes near two octaves below fp, they were approximately equal). For low-frequency contralateral elicitors, statistically significant changes were found only when measuring in the during-elicitor window (Fig. 5, left) although, as noted earlier, the similarity of the tone and NBN data, which were from independent measurements, helps to give confidence in the statistical significance of the data. Because there were not enough data for fes above fp, the test for asymmetry was not carried out at 4 kHz. Because the calculation of skew about the probe requires data over a symmetric range above and below the probe frequency, using only the available symmetric range would exclude the broad low-frequency region one octave or more below the probe in which elicitors evoke significant effects on ΔSFOAEn.
The pattern of ΔSFOAEn magnitude vs. fe varied across elicitor lateralities for 4-kHz probes. The largest ΔSFOAEn magnitude was at fe = fp for bilateral and ipsilateral elicitors, but was a half-octave above fp for contralateral elicitors (Fig. 5, A–D). All elicitor lateralities showed a pattern of ΔSFOAEn magnitude vs. fe that had an area of sensitivity at fes an octave or more below fp. For contralateral fes, the ratio of the during-elicitor to the postelicitor ΔSFOAEn magnitudes was larger at 4 kHz than for 0.5- and 1.0-kHz probes (Figs. 3–5, left), suggesting that the MOC effect decays (or changes in some way) faster at 4 kHz than at 0.5 and 1.0 kHz.
The pattern of nSFOAEmoc magnitude vs. fe for 4-kHz probes was generally similar to the pattern for 1 kHz (a dip at fe = fp for ipsilateral and bilateral elicitors, and little effect for contralateral elicitors), but for nSFOAEmoc phase, the patterns were quite different (Figs. 4, 5). For 4-kHz probes, ipsilateral and bilateral elicitors produced significant phase changes in nSFOAEmoc only for NBN with fe at and above fp. In contrast, contralateral tone and NBN elicitors produced significant phase leads in nSFOAEmoc for fes above and far below fp (Fig. 5, bottom).
Strength of MOC effects (ΔSFOAEn magnitudes) as a function of probe frequency.
To compare the MOC strength across probe frequencies we used ΔSFOAEn magnitude, both because it has the best SNR and because it is best at matching neural MOC effects (see discussion). For each fp, elicitor laterality, and elicitor type, we used the highest point in ΔSFOAEn magnitude vs. fe (Figs. 3–5D). The MOC-effect strength decreased as a function of fp for all lateralities, and the patterns for tone and NBN elicitors were similar (Fig. 6). The MOC-effect strength was largest for bilateral elicitors followed by ipsilateral elicitors and then contralateral elicitors.
DISCUSSION
Summary of principal findings and discussion overview.
For all three probe frequencies, MOC tuning showed little frequency specificity in that tones and noise over a wide range of frequencies (e.g., an octave, or more, away from the probe frequency) elicited significant MOC effects (Figs. 3–5). If elicitor frequencies are considered relative to the probe frequencies, then the reflex activation pattern was skewed in opposite directions for 0.5-kHz probes (fes > fp produced more effect) than for 1-kHz probes (fes < fp produced more effect). However, when looked at in absolute frequency, the activation patterns for 0.5- and 1.0-kHz probes were more similar (Fig. 7). For 4=kHz probes, elicitors at or a half-octave above fp produced the largest effects, but there was also a long tail of contralateral elicitors being effective at low fes that overlapped with the fes that were most effective for 0.5- and 1.0-kHz probes (Fig. 7). Overall, elicitors in a range ∼0.5–2.0 kHz appear to be particularly effective in eliciting MOC activity that affects all of the probe-frequency regions.
ΔSFOAEn, the metric used in the above summary, has been our preferred way to measure MOC effects because it has the best SNR (Lilaonitkul and Guinan 2009a; 2009b). However, to show phase changes in a way that can be more easily related to what is happening in the cochlea, we also used the metric, nSFOAEmoc, and this showed surprisingly different frequency patterns, in several ways. First, for ipsilateral and bilateral elicitors, nSFOAEmoc magnitude vs. fe almost always showed MOC inhibition peaked at fp (Figs. 3–5). In contrast, for contralateral elicitors, nSFOAEmoc magnitudes vs. fe plots showed small changes for 1- and 4-kHz probes, and a broad, moderate-amplitude MOC inhibition for 0.5-kHz probes (Figs. 3–5). These small changes in nSFOAEmoc magnitudes for contralateral elicitors seem perplexing at first because our measured ΔSFOAEn, and results from many other studies, show substantial MOC effects elicited by contralateral sound. Part of the explanation is that the present results used narrow-band contralateral sound, whereas most previous studies used broad-band sound, and MOC effects are small when elicitor bandwidth is small (Maison et al. 2000; Lilaonitkul and Guinan 2009b). A second explanation, expanded later, is that MOC-induced phase changes may play a more important role in OAE measurements than is generally appreciated. Although the phase changes were seldom more than 10°, a 10° phase change is not negligible and has a bigger effect on ΔSFOAEn than a 10% magnitude change (e.g., Fig. 4, C, G, and K).
Another surprising feature of the nSFOAEmoc data is that across fe very different patterns were found in magnitude vs. phase (Figs. 3–5). As noted above, nSFOAEmoc magnitude vs. fe functions typically showed peak inhibition for elicitors near fp. In contrast, nSFOAEmoc phase functions typically had their largest values at fes away from fp. What the differences in fe patterns tell us about MOC action in the cochlea will be considered later. Before doing that, because we have two metrics for MOC effect (ΔSFOAEn and nSFOAEmoc) that produce different-looking results, we need to consider which gives the better picture of the MOC effect from a functional point of view. We will start by comparing our results to previous measurements of MOC tuning in humans, all of which used OAE metrics for measuring MOC effects. However, the MOC effect that ultimately counts is the effect on cochlear neural responses. The frequency tuning of MOC effects on auditory-nerve responses has been measured only in animals, and our review of these data will point to a way of evaluating the comparison of ΔSFOAEn vs. nSFOAEmoc.
Finally, we note one reassuring aspect of our results. The tuning patterns obtained using tone and NBN elicitors were very similar (Figs. 3–5). The main difference is that, compared with tones, NBN elicitors produced MOC effects that were slightly larger, and broader across fe. Because these measurements were from independent data sets, they serve to confirm the reproducibility of our measurements and to show that narrow-band elicitors produce similar MOC effects no matter whether they are tones or noise bands.
Methodological considerations.
Our methods are basically the same as those of Lilaonitkul and Guinan (2009a), and they have all of the shortcomings noted there. We used a 40-dB-SPL bilateral probe tone, and this may have influenced our results. In the tests presented with that paper, we showed that the results did change somewhat when a bilateral probe was used instead of a unilateral (ipsilateral) probe, but we found that this produced no change in the skewness of MOC effects vs. fe for 1-kHz probes. In a subject in whom both 20-dB and 40-dB probes were used, we found larger responses with the 20-dB probe and slightly different MOC effects vs. fe. To do tests at specific probe frequencies, some probe sound must be used, and ours arguably influences the results the least of any in the literature. SOAEs do not require any probe sound, but sufficiently large SOAEs only occur at idiosyncratic frequencies and in a small fraction of subjects. Although the amplitudes of the MOC effects may have been larger if the probe tone had a lower SPL, or was monaural, this would have made already long measurements more time consuming.
We have primarily shown averages of MOC effects across subjects, but at 0.5 and 1.0 kHz individual subjects showed relatively heterogeneous response patterns (e.g., compare the Fig. 2 data from one subject to the average data in Figs. 3–5; also see Lilaonitkul and Guinan 2009a, Figs. 2–3). For 4-kHz probes there was much less scatter across subjects. The MOC effect at 4 kHz was very small, and this was the main cause of a weakness of the present study, namely that there were only three subjects measured at 4 kHz. The SNR of MOC effects on SFOAEs depends on the magnitude of the raw ΔSFOAE (ΔSFOAE before normalization by SFOAE magnitude) and the magnitude of the background noise, which is mostly microphone noise. The noise and ΔSFOAE both decrease as fp increases, such that the best SNR is at 1 kHz and the worst is at 4 kHz. Because of this, it is difficult to find subjects with SNRs at 4 kHz that are good enough for these measurements. The fact that the three subjects whom we successfully tested at 4 kHz all had relatively similar response patterns (note the small error bars in Fig. 5) helps to ameliorate this weakness.
Comparison with prior work on MOC tuning from OAEs in humans.
Useful data on MOC frequency tuning in humans is provided by two previous studies (Veuillet at al. 1991; Chery-Croze et al. 1993). These used OAE reduction as the metric of MOC effects [distortion-product OAEs (DPOAEs) in Chery-Croze et al., and transient-evoked OAEs (TEOAEs) in Veuillet at al. 1991]. At first glace, OAE reduction seems to correspond best to our metric, nSFOAEmoc magnitude. However, DPOAE reduction is a complex statistic because the DPOAE is made up of two components (Kalluri and Shera 2001), and these components are affected differently by MOC activity (Abdala et al. 2009; Henin et al. 2011). Because the two components sum vectorially, a change either in magnitude or phase of one component can change the overall DPOAE magnitude. Thus, in its sensitivity to phase changes, the change in DPOAE magnitude may be more similar to ΔSFOAEn magnitude than to nSFOAEmoc magnitude. In contrast, MOC-induced phase changes seem unlikely to have a major influence on TEOAE amplitudes. Despite the differences in MOC-effect metrics, the results in these two studies are similar to ours in that they found that MOC tuning was broad. Although they summarize their results as showing that the largest MOC effects were for noise bands with fe near fp, both studies show individual cases where the most effective fe was not at fp.
Comparison with prior work on MOC tuning from neural responses in animals.
The MOC effect that is functionally significant is the effect on cochlear neural responses, not OAEs. Unfortunately, the correspondence between MOC effects on neural and OAE responses is not known for any OAE. Measurements in guinea pigs and cats indicate that the MOC inhibition of DPOAEs is correlated with the prevention of acoustic trauma but does not accurately predict neural inhibition (Puria et al. 1996; Maison and Liberman 2000). We can, however, bypass OAEs and look directly at the effect of MOC activity on responses of single auditory-nerve (AN) fibers. Warren and Liberman (1989) recorded from AN fibers in lightly anesthetized cats and studied the effects of MOC activity elicited by contralateral sound. Fibers were excited by an ipsilateral tone at the fiber characteristic frequency (CF), and firing rate was measured as a contralateral tone was swept in frequency. From the results, they determined the frequency of the contralateral tone that produced the greatest inhibition. For AN fibers with CFs <1 kHz, the most effective inhibitory frequency was typically above CF, often by approximately a half-octave. For AN fibers with CFs ∼1–7 kHz, the most effective inhibitory frequency was typically below CF, often by approximately a half-octave. For AN fibers with CFs >7 kHz, the most effective inhibitory frequencies were all close to CF. In comparing these data to our human data, the tone at fiber CF plays the role of the probe tone and the contralateral sound is the MOC elicitor. Also, to compare to humans, the cat data need to be shifted down in frequency by approximately one octave because the auditory range is lower in humans than in cats. With this in mind, the human pattern of ΔSFOAEn magnitude, in which the most effective fe is above fp for 0.5-kHz probes, below fp for 1 kHz probes and close to fp for 4-kHz probes, corresponds well to the pattern of neural inhibition from contralateral elicitors in cats. In contrast, the patterns in nSFOAEmoc magnitude or phase do not correspond (at least at 0.5 and 1 kHz). For ipsilateral and bilateral elicitors at 4 kHz, the MOC effect vs. fe patterns are similar for both magnitude metrics, so these 4-kHz data do not help in deciding which is best. Overall, ΔSFOAEn magnitude, which combines the magnitude and phase changes of nSFOAEmoc (Fig. 1), appears to correspond best to the pattern of MOC effects in cat neural responses and is therefore the preferred SFOAE metric for indicating the functionally significant MOC effect.
How are nSFOAEmoc magnitude and phase changes produced?
Although ΔSFOAEn may be a better index of functionally significant MOC effects, nSFOAEmoc is a direct representation of the MOC inhibited SFOAE. Because of this, we expect that the underlying MOC-induced cochlear changes will be easier to understand in terms of nSFOAEmoc than in terms of ΔSFOAEn. One of the main things to understand is the surprising finding that nSFOAEmoc magnitude and phase show different patterns as functions of fe. Although these patterns superficially resemble the patterns of amplitude and phase changes in a filter frequency response when the filter is made less sharp, the measurement here is fundamentally different. The frequency that is being changed is that of the MOC elicitor, whereas the probe frequency (which corresponds to the input frequency to the filter) is constant in each plot. What is the MOC system doing in the cochlea to produce the nSFOAEmoc vs. fe patterns? Before setting out to understand the origin of these patterns, we will review two important background issues: how MOC effects are produced, and how SFOAEs are generated.
MOC effects are produced by two mechanisms: “fast” and “slow” (Sridhar et al. 1995; Cooper and Guinan 2003), but our measured MOC effects are due almost entirely to MOC fast effects. MOC fast effects work on a 100-ms time scale, decrease cochlear amplifier gain, and produce a phase advance in BM motion. In contrast, MOC slow effects work on a tens-of-seconds time scale, decrease cochlear amplifier gain, and produce a phase lag in BM motion (Cooper and Guinan 2003). Our paradigm compares SFOAEs in time periods that are 2.5 s apart, so it measures MOC fast effects and selects against MOC slow effects. Considering this, and because MOC slow effects have only been observed at high CFs and are very small in humans (Sridhar et al. 1995; Zhao and Dhar 2011), we will only consider explanations of our results that involve MOC fast effects.
SFOAEs are produced by coherent reflection of the traveling wave by small cochlear irregularities (Zweig and Shera 1995). Irregularities all along the cochlea produce reflections, but the largest contributions come from near the peak where the traveling wave is largest. In the peak region, the traveling-wave phase changes rapidly with position along the cochlea, so reflections from different places have different phases. In the peak region these reflected wavelets interfere and mostly cancel each other. However, some wavelets are in phase in the ear canal at a latency determined by the slope of the phase vs. position of the traveling wave, and these wavelets add coherently to produce the SFOAE. As shown by BM measurements and scaling symmetry, the phase of the traveling wave from a tone changes more rapidly at more apical reflection positions, with the result that wavelets from more apical positions arrive at the ear canal with longer delays than wavelets from more basal positions. Thus, although the overall SFOAE has a single measured latency, reflections from more apical (or more basal) places relative to the peak region produce SFOAE components with longer (or shorter) latencies.
After these reviews of background material, the first issue we consider is where along the length of the cochlea the MOC-induced changes that produce the nSFOAEmoc change are taking place. Are the changes near the peak of the fp traveling wave (the fp cochlear place), or elsewhere? According to the longstanding idea that MOC fibers have narrow tuning curves and innervate a cochlear region with a best frequency similar to the MOC-fiber best frequency (Robertson 1984; Liberman and Brown 1986; Brown 1989), an elicitor only activates MOC fibers with synapses on OHCs near the fe cochlear region along the cochlea. With this hypothesis, the only OHC changes are in the fe cochlear region, so any observed change in fp SFOAEs must be produced by OHCs near the fe cochlear place even if this fe cochlear place is far from the fp cochlear place. An alternate hypothesis is that the longstanding idea is wrong and changes in fp SFOAEs are produced by OHC changes near the fp cochlear place, even when fe is far in frequency from fp.
To decide where along the cochlea elicitors change SFOAEs, we first consider cases in which fe is a half-octave, or more, away from fp. For fe < fp, the fe cochlear region is apical to the fp cochlear region, and the fp traveling wave never reaches the fe region. This means that MOC synapses in the fe region cannot affect fp SFOAEs, so, presumably, for fe < fp, the elicitor must have its effect on fp SFOAEs from synapses on OHCs that are near the fp region, i.e., close enough to the fp region to change the cochlear amplification of fp. Note that fe < fp is the direction of the low-frequency fe extension of MOC-effect tuning for 1-and 4-kHz probes (Figs. 4, 5, and 7). The above analysis indicates that, for this circumstance, the change in the SFOAE from the low-frequency elicitor must be produced by OHCs near the higher-frequency, more basal fp place in the cochlea.
For changes in the opposite direction, i.e., for fe > fp, the conclusion is less compelling, but it still appears that the important MOC effects are on OHCs in a cochlear region near the fp place. For fe > fp, the fe cochlear region is basal to the fp cochlear region and the fp traveling wave goes through the fe region. For fe an octave or more above fp, the basilar membrane impedance is dominated by stiffness, at least in the basal half of the cochlear (de Boer and Nuttall 1999), and MOC synapses on OHCs in this region would not be expected to have much effect on the traveling wave. Considering that cochlear amplification of the fp response is thought to take place slightly basal to the fp cochlear place (e.g., de Boer and Nuttall 1999; Shera 2007), MOC effects on OHCs within this amplification region are likely to have an influence on fp SFOAEs. From this it appears that most, if not all, of the elicitor-induced cochlear changes that affect the fp SFOAE occur near, or slightly basal to, the fp cochlear place.
How do narrow-band elicitors at fes far from fp activate synapses on OHCs near the fp place? Perhaps the simplest way is if MOC fibers have wide tuning in awake humans (as opposed to the narrow tuning found in anesthetized cats and guinea pigs). With this hypothesis, MOC fibers that innervate the fp place are excited by elicitors with fes at distant frequencies. Another possibility would be that MOC fibers have narrow tuning curves, but an individual MOC fiber innervates a long extent of the cochlea. MOC fibers that innervate OHCs over 20% of cochlear length have not been found in cats and guinea pigs but were occasionally found in mice and rats (Robertson and Gummer 1985; Liberman and Brown 1986; Brown 1987; 1989; Brown et al. 1991; Warr and Boche 2003) and could possibly be present in humans. Another intriguing possibility is that the spread of MOC effect comes about not from the direct MOC innervation of OHCs but from the MOC innervation of Type II auditory afferents, which then change cochlear mechanical properties via Type II reciprocal synapses on OHCs (Thiers et al. 2002; 2008). Of these possibilities, a widening of MOC-fiber tuning seems the most likely, but more work is needed on this issue.
Next we consider how synapses on OHCs near the fp place produce the changes in nSFOAEmoc. MOC inhibition of cochlear amplification is expected to produce a decrease in nSFOAEmoc magnitude. In fact, the narrow peak of MOC reduction in nSFOAEmoc magnitude from ipsilateral and bilateral elicitors on 1- and 4-kHz SFOAEs is what one would expect from MOC fibers with narrow tuning curves acting in a cochlear region with tuning similar to the MOC-fiber tuning. For 0.5-kHz probes, the inhibition of nSFOAEmoc magnitude from ipsilateral and bilateral elicitors is wider than for 1- and 4-kHz probes, but perhaps that is because the traveling wave is amplified over a wider cochlear region at low frequencies (Shera 2007).
Understanding how the nSFOAEmoc phase changes come about is not straightforward. First, note that the predominant phase change is a phase advance and MOC stimulation produces a phase advance in basilar membrane motion (Guinan and Cooper 2003; 2008). This phase advance may come about because cochlear amplification is accompanied by a phase delay, so that reducing cochlear amplification produces a phase advance. However, this does not explain why the phase advance is much greater for fe < fp and why it is less, or a phase lag, for fe > fp. One mechanism that might produce this phase change is a shift in the traveling wave. With this hypothesis, when the fe place is apical to the fp place, the MOC effect is greatest at the most apical part of the traveling wave, the part that produces SFOAE wavelets with the longest latency. Reducing the amplitude of the apical part of the traveling wave might, therefore, reduce the overall delay of the SFOAE, i.e., produce a phase advance. Similarly, when the fe place is basal to the fp place, the greatest reduction of the traveling wave would be in the region producing shorter-latency wavelets than those at the peak, so reducing the traveling wave amplitude in this region might increase the overall SFOAE latency. Another way of stating this “traveling wave shift” hypothesis is that activating synapses on OHCs that are predominantly apical (or basal) to the peak of the traveling wave shifts the envelope of the traveling wave away from this inhibited region, thereby producing a change in the SFOAE latency, which for a tone is equivalent to a shift in phase. Although this is a plausible mechanism to account for the MOC-induced phase changes in nSFOAEmoc, more evidence is needed. A first step might be an exploration in a cochlear model of the effect of focused MOC inhibition on SFOAEs.
The next question is how the above mechanisms for producing the changes in nSFOAEmoc can also produce neural inhibition with a frequency pattern like that found by Warren and Liberman (1989) or like ΔSFOAEn magnitude. The short answer is: “We don't know”. However, some relevant observations can be made. The largest differences between the nSFOAEmoc magnitude and phase patterns are for 0.5- and 1.0- kHz probes, frequencies that are in the apical half of the cochlea. There is evidence that in the apical region two separate sources contribute to SFOAEs (Shera et al. 2008; 2010). Interference between the resulting two SFOAE components will then shape the SFOAE amplitude and phase changes. Presuming the two sources have different organ-of-Corti vibration patterns, they will interfere in different ways at different places; e.g., how they mix to produce SFOAEs may be different from how they mix to drive inner hair cell stereocilia. Interference between two vibration patterns provides a mechanism by which a phase change seen in SFOAEs may correspond to an amplitude reduction in AN responses. Although there is no direct evidence for this hypothesis, there is ample evidence that AN excitation in the apex is due to the interaction of multiple drives (Gifford and Guinan 1983; Lin and Guinan 2000; 2004; Guinan et al. 2005). Considering this, it seems reasonable to hypothesize that the multiple drives evident in AN responses are also involved in producing MOC effects on SFOAEs. Although much needs to be learned, it seems possible that an explanation of how the MOC system produces seemingly divergent effects in SFOAE amplitudes, SFOAE phases, and AN responses may be due to the interaction of multiple vibrational components along the lines described above.
Ipsilateral vs. contralateral MOC reflex effects.
Another interesting aspect of our data is the considerable difference between the nSFOAEmoc vs. fe patterns produced by ipsilateral compared with contralateral elicitors (Figs. 3–5). At 1 and 4 kHz, nSFOAEmoc magnitude was barely changed by contralateral elicitors at all fes but was significantly decreased by ipsilateral and bilateral elicitors for fe = fp. At 0.5 kHz, the ipsilateral and contralateral patterns of change vs. fe were similar for nSFOAEmoc magnitudes but were different for nSFOAEmoc phases; i.e., nSFOAEmoc phase peaked at fe < fp for ipsilateral elicitors but peaked at fe > fp for contralateral elicitors (a direction that is not explained by the traveling-wave-shift hypothesis given above).
These differences in ipsi compared with contra reflexes appear to imply that the MOC fibers that mediate ipsi and contra reflexes have different actions in the cochlea. Although the ratio of ipsi-reflex to contra-reflex MOC innervation may vary along the length of the cochlea (e.g., Guinan et al. 1984), there is no known mechanism by which a difference in the number of fibers activated, or their activation amplitude, would lead to a phase change in one case (the contra reflex) and an amplitude change in another case (the ipsi reflex). Differences in central activation can explain ipsi/contra differences in the strength of MOC effects, but the production of magnitude changes vs. phase changes appears to require some anatomical difference in the cochlea. No substantial differences have been found in the cochlear structures innervated by ipsi compared with contra MOC neurons in the cochlea, but that cannot be ruled out; e.g., perhaps contra fibers innervate Deiter cells but ipsi fibers do not (Warr and Boche 2003). There might be a difference in the way ipsi and contra MOC fibers innervate OHCs or innervate Type II afferent fibers that have reciprocal synapses on OHCs (Thiers et al. 2002; 2008). Whatever their origin, the differences in the MOC effects of ipsi and contra MOC reflexes are substantial (Figs. 3–5).
The ipsi-contra differences in nSFOAEmoc patterns across fe suggest that the ipsi and contra MOC reflexes have different functions. The ipsilateral reflex produces “closed loop” feedback, but the contralateral reflex does not, i.e., the ipsilateral reflex reduces the cochlear response that is the reflex input, whereas the contralateral reflex does not change the input that drives it. Nearly all papers on the MOC reflexes have used only contralateral sound and assumed that ipsilateral sound would produce similar effects, except perhaps stronger. The present results serve as a cautionary note that this may not be true in more ways than just in the amplitude of the effects.
What functions might be served by the observed pattern of MOC tuning?
Considerable evidence indicates that one of the main functions of MOC activity is to reduce masking (reviewed by Guinan 2011). For reducing cochlear masking, what counts is the MOC reduction of the cochlear-amplifier gain seen by an AN fiber. Although noise components outside the amplified frequency region of an AN fiber may mask responses from that AN fiber by excitation and inner hair cell adaptation, changing cochlear amplifier gain will not change this because these noise components are outside of the amplified frequency region of the fiber. Thus, for antimasking, MOC feedback in a particular cochlear frequency region would be expected to have tuning similar to the tuning of the cochlear amplifier as seen by an AN fiber in that region. Cochlear amplifier gain, in guinea pigs and chinchillas, appears to extend from the local best frequency to about a half-octave lower in the base and about one octave lower in the apex (de Boer and Nuttall 1999; Shera 2007). Because humans have much sharper cochlear tuning than small animals (Shera et al. 2002; 2010), the frequency region that produces cochlear-amplifier gain might be much narrower in humans. With this in mind, the very broad patterns of MOC activation for 0.5- and 1.0-kHz probes (Figs. 3 and 4) are difficult to explain in terms of antimasking from the removal of AN excitation. Even more difficult to explain in terms of cochlear antimasking is the long tail of low-frequency elicitor sensitivity seen at 4 kHz (Fig. 5).
If MOC inhibition does not reduce direct energetic masking in the cochlea, could it reduce cochlear masking less directly by reducing two-tone suppression? A high-side suppressor at a suppressor frequency (Fs), which is slightly higher than the probe frequency, fp, may have its peak amplitude in the amplification region for fp (which is basal to the fp cochlear place). Thus, if the MOC activation reduces the amplitude of Fs, it can reduce the two-tone suppression of fp produced by Fs. This extends the region in which a reduction of cochlear-amplifier gain may have a beneficial effect to higher frequencies (i.e., basally), perhaps by approximately a half-octave, from the cochlear region with gain at fp. There is no corresponding benefit for a low-side suppressor (one with Fs < fp). Thus there does not appear to be a way in which MOC inhibition of OHCs apical to the fp place would aid the cochlear response at the fp place.
If the wide pattern of MOC activation cannot be explained by providing frequency-focused cochlear unmasking, perhaps it is explained by reducing informational masking in the central nervous system. In our experiments the subjects were not doing any task; i.e., they were listening passively, so why informational masking would play a role in these experiments is not clear. Perhaps focused MOC effects are only produced when there is attention-directed behavior and that during passive listening MOC activation is broad to anticipate and prevent informational masking. The nonfocused aspect of our passive listening condition may be one reason why the pattern of MOC effects at 0.5 and 1.0 kHz varied considerably across subjects.
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grants RO1 DC-005977 and P30 DC-05029.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.L. and J.J.G. conception and design of research; W.L. performed experiments; W.L. analyzed data; W.L. and J.J.G. interpreted results of experiments; W.L. and J.J.G. prepared figures; W.L. and J.J.G. drafted manuscript; W.L. and J.J.G. edited and revised manuscript; W.L. and J.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. L. D. Braida, Dr. M. C. Brown, Dr. Oded Ghitza, Dr. M. C. Liberman, Dr. S. F. Maison, Dr. W. T. Peake, and Dr. C. A. Shera for comments on an earlier version of the manuscript.
REFERENCES
- Abdala C, Mishra SK, Williams TL. Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex. J Acoust Soc Am 125: 1584–1594, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus BC. Bias due to noise in otoacoustic emission measurements. J Acoust Soc Am 121: 1588–1603, 2007 [DOI] [PubMed] [Google Scholar]
- Backus BC, Guinan JJ., Jr Measurement of the Distribution of Medial Olivocochlear Acoustic Reflex Strengths Across Normal-Hearing Individuals via Otoacoustic Emissions. J Assoc Res Otolaryngol 8: 484–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol 260: 605–618, 1987 [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40: 93–110, 1989 [DOI] [PubMed] [Google Scholar]
- Brown MC. Cochlear projections of single medial olivocochlear (MOC) axons in the guinea pig. Asso Res Otolaryngol Abstr 25: 310, 2002 [Google Scholar]
- Brown MC, Pierce S, Berglund AM. Cochlear-nucleus branches of thick (medial) olivocochlear fibers in the mouse: A cochleotopic projection. J Comp Neurol 303: 300–315, 1991 [DOI] [PubMed] [Google Scholar]
- Chéry-Croze A, Moulin A, Collet L. Effect of contralateral sound stimulation on the distortion product 2f1-f2 in humans: Evidence of a frequency specificity. Hear Res 68: 53–58, 1993 [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ., Jr Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J Physiol 548: 307–312, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ., Jr Efferent insights into cochlear mechanics. In: What Fire is in Mine Ears: Progress in Auditory Biomechanics, edited by Shera CA, Olson ES. Melville, New York: American Institute of Physics, 2011, p. 396–402 [Google Scholar]
- Dallos P. The active cochlea. J Neurosci 12: 4575–4585, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer E, Nuttall AL. The “inverse problem” solved for a three-dimensional model of the cochlea. III. Brushing-up the solution method. J Acoust Soc Am 105: 3410–3420, 1999 [DOI] [PubMed] [Google Scholar]
- de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci 28: 4929–4937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeter R, Abel R, Calandruccio L, Dhar S. Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions. J Acoust Soc Am 126: 2413–2424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993, p. 436 [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. Alpha10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 98: 3501–3506 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ., Jr Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: Implications for cochlear filter bandwidths. Hear Res 267: 36–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. The synaptic physiology of cochlear hair cells. Audiol Neurootol 7: 40–44, 2002 [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ., Jr Effects of crossed-olivocochlear-bundle stimulation on cat auditory nerve fiber responses to tones. J Acoust Soc Am 74: 115–123, 1983 [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res 29: 179–194, 1987 [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Changes in stimulus frequency otoacoustic emissions produced by two-tone suppression and efferent stimulation in cats. In: Mechanics and Biophysics of Hearing, edited by Dallos P, Geisler CD, Matthews JW, Steele CR. Madison, Wisconsin: Springer Verlag, 1990, p. 170–177 [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27: 589–607, 2006 [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Physiology of the Medial and Lateral Olivocochlear Systems. In: Auditory and Vestibular Efferents, edited by Ryugo DK, Popper AN, Fay RR. New York: Springer Science+Business Media, 2011, p. 39–81 [Google Scholar]
- Guinan JJ, Jr, Cooper NP. Fast effects of efferent stimulation on basilar membrane motion. In: The Biophysics of the Cochlea: Molecules to Models, edited by Gummer AW, Dalhoff E, Nowotny M, Scherer MP. Singapore: World Scientific, 2003, p. 245–251 [Google Scholar]
- Guinan JJ, Jr, Cooper NP. Medial olivocochlear efferent inhibition of basilar-membrane responses to clicks: evidence for two modes of cochlear mechanical excitation. J Acoust Soc Am 124: 1080–1092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Warr WB, Norris BE. Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. J Comp Neurol 226: 21–27, 1984 [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol 4: 521–540, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Lin T, Cheng H. Medial-olivocochlear-efferent inhibition of the first peak of auditory-nerve responses: Evidence for a new motion within the cochlea. J Acoust Soc Am 118: 2421–2433, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin S, Thompson S, Abdelrazeq S, Long GR. Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation. J Acoust Soc Am 129: 2068, 2011 [DOI] [PubMed] [Google Scholar]
- Irving S, Moore DR, Liberman MC, Sumner CJ. Olivocochlear efferent control in sound localization and experience-dependent learning. J Neurosci 31: 2493–2501, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SG, Heinz MG, Strickland EA. Evaluating adaptation and olivocochlear efferent feedback as potential explanations of psychophysical overshoot. J Assoc Res Otolaryngol 12: 345–360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Distortion-product source unmixing: a test of the two-mechanism model for DPOAE generation. J Acoust Soc Am 109: .622–637, 2001 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am 121: 2097–2110, 2007 [DOI] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, Liberman MC. Anti-masking effects of the olivocochlear reflex, II: Enhancement of auditory-nerve response to masked tones. J Neurophysiol 70: 2533–2549, 1993 [DOI] [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: monaural vs. binaural stimulation and the effects of noise. J Neurophysiol 60: 1779–1798, 1988 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 24: 17–36, 1986 [DOI] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol 101: 1394–1406, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol 10: 459–470, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Guinan JJ., Jr Auditory-nerve-fiber responses to high-level clicks: interference patterns indicate that excitation is due to the combination of multiple drives. J Acoust Soc Am 107: 2615–2630, 2000 [DOI] [PubMed] [Google Scholar]
- Lin T, Guinan JJ., Jr Time-frequency analysis of auditory-nerve-fiber and basilar-membrane click responses reveal glide irregularities and non-characteristic-frequency skirts. J Acoust Soc Am 116: 405–416, 2004 [DOI] [PubMed] [Google Scholar]
- Long GR, Tubis A, Jones KL. Modeling synchronization and suppression of spontaneous otoacoustic emissions using Van der Pol oscillators: effects of aspirin administration. J Acoust Soc Am 89: 1201–1212, 1991 [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci 20: 4701–4707, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Andeol G, Gallego S, Collet L. Activation of medial olivocochlear efferent system in humans: influence of stimulus bandwidth. Hear Res 140: 111–125, 2000 [DOI] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Collet L. Influence of focused auditory attention on cochlear activity in humans. Psychophysiology 38: 35–40, 2001 [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in the mouse: immunocytochemical maps, crossed vs. uncrossed contributions, and transmitter colocalization. J Comp Neurol 455: 406–416, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric C, Collet L. Differential effects of visual attention on spontaneous and evoked otoacoustic emissions. Int J Psychophysiol 17: 281–289, 1994 [DOI] [PubMed] [Google Scholar]
- Oatman LC. Effects of visual attention on the intensity of auditory evoked potentials. Exp Neurol 51: 41–53, 1976 [DOI] [PubMed] [Google Scholar]
- Ota Y, Oliver DL, Dolan DF. Frequency-specific effects on cochlear responses during activation of the inferior colliculus in the Guinea pig. J Neurophysiol 91: 2185–2193, 2004 [DOI] [PubMed] [Google Scholar]
- Puria S, Guinan JJ, Jr, Liberman MC. Olivocochlear reflex assays: Effects of contralateral sound on compound action potentials vs ear-canal distortion products. J Acoust Soc Am 99: 500–507, 1996 [DOI] [PubMed] [Google Scholar]
- Rajan R. Protective functions of the efferent pathways to the mammalian cochlea: A Review. In: Noise-Induced Hearing Loss, edited by Dancer A., Henderson D., Salvi R. J., Hamernik RP. St. Louis: Mosby Year Book, 1992, p. 429–444 [Google Scholar]
- Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res 15: 113–121, 1984 [DOI] [PubMed] [Google Scholar]
- Robertson D, Gummer M. Physiological and morphological characterization of efferent neurons in the guinea pig cochlea. Hear Res 20: 63–77, 1985 [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Popper AN, Fay RR. Auditory and Vestibular Efferents. New York: Springer Science+Business Media, 2011, p. 359 [Google Scholar]
- Schrott-Fischer A, Egg G, Kong WJ, Renard N, Eybalin M. Immunocytochemical detection of choline acetyltransferase in the human organ of Corti. Hear Res 78: 149–157, 1994 [DOI] [PubMed] [Google Scholar]
- Shera CA. Laser amplification with a twist: traveling-wave propagation and gain functions from throughout the cochlea. J Acoust Soc Am 122: 2738–2758, 2007 [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Jr, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci USA 99: 3318–3323, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Tubis A, Talmadge CL. Testing coherent reflection in chinchilla. J Acoust Soc Am 123: 3851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Jr, Oxenham AJ. Otoacoustic estimation of cochlear tuning: validation in the chinchilla. J Assoc Res Otolaryngol 11: 343–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of olivocochlear stimulation on cochlear potentials in the guinea pig. J Neurosci 15: 3667–3678, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers FA, Burgess BJ, Nadol JB. Reciprocal innervation of outer hair cells in a human infant. J Assoc Res Otolaryngol 3: 269–278, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers FA, Nadol JB, Jr, Liberman MC. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J Assoc Res Otolaryngol 9: 477–489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: Dependence on stimulus variables. J Neurophysiol 65: 724–735, 1991 [DOI] [PubMed] [Google Scholar]
- Veuillet E, Magnan A, Ecalle J, Thai-Van H, Collet L. Auditory processing disorder in children with reading disabilities: effect of audiovisual training. Brain 130: 2915–2928, 2007 [DOI] [PubMed] [Google Scholar]
- Warr WB. Organization of olivocochlear efferent systems in mammals. In: Mammalian Auditory Pathway: Neuroanatomy, edited by Webster DB, Popper AN, Fay RR. NY: Springer-Verlag, 1992, p. 410–448 [Google Scholar]
- Warr WB, Boche JE. Diversity of axonal ramifications belonging to single lateral and medial olivocochlear neurons. Exp Brain Res 153: 499–513, 2003 [DOI] [PubMed] [Google Scholar]
- Warren EH, III, Liberman MC. Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables. Hear Res 37: 105–122, 1989 [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol 57: 1002–1021, 1987 [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of crossed olivocochlear bundle. Hear Res 35: 165–190, 1988 [DOI] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat Neurosci 5: 57–63, 2002 [DOI] [PubMed] [Google Scholar]
- Zhao W, Dhar S. Fast and slow effects of medial olivocochlear efferent activity in humans. PLos One 6: e18725, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig G, Shera CA. The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am 98: 2018–2047, 1995 [DOI] [PubMed] [Google Scholar]
- Zwicker E, Schloth E. Interrelation of different oto-acoustic emissions. J Acoust Soc Am 75: 1148–1154, 1984 [DOI] [PubMed] [Google Scholar]