Abstract
Recent evidence shows that brain-derived steroids such as estrogens (“neuroestrogens”) are controlled in a manner very similar to traditional neurotransmitters. The advent of in vivo microdialysis for steroids in songbirds has provided new information about the spatial and temporal dynamics of neuroestrogen changes in a region of the auditory cortex, the caudomedial nidopallium (NCM). Here, experiments using in vivo microdialysis demonstrate that neuroestradiol (E2) fluctuations occur within the auditory NCM during presentation of naturalistic auditory and visual stimuli in males but only to the presentation of auditory stimuli in females. These changes are acute (within 30 min) and appear to be specific to the NCM, because similar treatments elicit no changes in E2 in a nearby mesopallial region or in circulating plasma. Further experiments coupling in vivo steroid microdialysis with extracellular recordings in NCM show that neuroestrogens rapidly boost auditory responses to song stimuli in females, similar to recent observations in males. We also find that the rapid actions of estradiol on auditory responses are fully mimicked by the cell membrane-impermeable estrogen biotinylestradiol, consistent with acute estrogen actions at the neuronal membrane. Thus we conclude that local and acute E2 flux is regulated by convergent multimodal sensory input, and that this regulation appears to be sex-specific. Second, rapid changes in local E2 levels in NCM have consequences for the modulation of auditory processing in females and males. Finally, the rapid actions of neuroestrogens on NCM auditory processing appear to be mediated by a nonclassical, membrane-bound estrogen receptor.
Keywords: aromatase, birdsong, nongenomic action, multimodal signal
the interconnected forebrain nuclei that control singing and song learning in the zebra finch are largely sexually dimorphic. The enlargement of these forebrain nuclei in males compared with females was the first notable sexual dimorphism identified in the vertebrate brain (Nottebohm and Arnold 1976). The mechanisms that give rise to this striking dimorphism appear to be a blend of the actions of steroid hormones like estrogens (Holloway and Clayton 2001) and direct chromosomal effects (Agate et al. 2003). In contrast to the sexual dimorphism of the song pathway(s), the ascending circuits that process auditory signals [i.e., the cochlear nucleus (CN), midbrain mesencephalicus lateralis dorsalis (MLd), thalamic nucleus ovoidalis (Ov), and cortical nuclei Field L, caudomedial nidopallium (NCM), and caudomedial mesopallium (CMM)] are largely similar in size and composition in adult males and females (Bailey and Wade 2005; Braun et al. 1991; Pinaud et al. 2006). In the absence of a pronounced anatomical dimorphism in auditory pathways, auditory processing demands that may differ between males and females could depend on the sex-specific actions of neuromodulators.
Estrogens are a candidate group of intrinsic cortical modulators, since they can be produced within brain circuits independent of the circulation (Remage-Healey et al. 2008; Schlinger and Arnold 1992). Aromatase, the enzyme that synthesizes estrogens, is expressed and active within presynaptic terminals in some neuronal circuits (Naftolin et al. 1996; Peterson et al. 2005; Rohmann et al. 2007; Saldanha et al. 2000). Alongside estrogen production in brain, novel actions have been identified in which estrogens rapidly modulate ion channel receptors on neuronal membranes. Estrogens can therefore act within extremely fast timescales to change the activity of neurons in the hippocampus, hypothalamus, hindbrain, and cortex (Herbison 2009; Kelly and Ronnekleiv 2009; Kuo et al. 2010; McEwen 2002; Meitzen and Mermelstein 2011; Remage-Healey and Bass 2007; Woolley 2007). These findings have bolstered the view that some neural estrogen actions are in fact neuromodulatory rather than classically endocrine (Balthazart and Ball 2006; Garcia-Segura 2008; Remage-Healey et al. 2011b; Saldanha et al. 2011).
A current gap in the understanding of estrogens as neuromodulatory agents is how and whether they fluctuate in brain circuits in the manner of traditional neurochemicals. The vertebrate brain is capable of fast changes in estrogen production, as indicated by rapid shifts in enzymatic activity of the aromatase protein in brain tissue explants in response to social and/or sexual encounters (reviewed in Balthazart et al. 2006; Black et al. 2005; Cornil et al. 2005). A direct method to measure estrogen fluctuations in the forebrain has recently been developed and validated for songbirds (Remage-Healey et al. 2008). This method employs in vivo microdialysis coupled to sensitive ELISAs to allow direct monitoring of changes in neuroestradiol (E2) in awake, behaving animals engaged in behavioral tasks.
Recent experiments using in vivo microdialysis have shown that E2 in the auditory cortex of male zebra finches can change quickly in social contexts. Experiments focused on the secondary auditory cortex (NCM) in adult males have demonstrated that E2 is elevated (<30 min) during brief social interactions with females as well as during acoustic playback of zebra finch songs (Remage-Healey et al. 2008). Furthermore, in vivo microdialysis coupled to extracellular recordings from neurons within the NCM has shown that auditory processing of zebra finch song is enhanced by the local production of neuroestrogens in adult males (Remage-Healey et al. 2010). These latter electrophysiological findings were simultaneously and independently confirmed by Tremere and Pinaud, using slightly different methods in NCM (multiunit array recordings coupled to microinjections; Tremere et al. 2009; Tremere and Pinaud 2011). Together, these studies indicate that estrogens are produced within the auditory cortex during auditory stimulation in males and appear to rapidly enhance the neural processing of auditory signals. The extent to which this neuromodulatory system in auditory NCM is activated in females and/or in other sensory contexts is unclear.
Here, we examine the sex-specificity of endogenous neuroestrogen fluctuations and neurophysiological actions in the zebra finch NCM, employing in vivo microdialysis technology. This study formally tests two hypotheses. First, because social interactions caused local E2 elevation even in the absence of singing by focal males (see Remage-Healey et al. 2008), we tested the hypothesis that either visual or auditory stimulation alone can elicit acute E2 fluctuations in the NCM of adult males and females. Second, because the results of this first experiment showed that auditory stimulation caused rapid elevations in NCM E2 levels in females, we tested the hypothesis that the auditory-evoked activity of NCM neurons in females is modulated by fluctuating neuroestrogens, and that this modulation depends on neuronal membrane-specific actions in NCM.
MATERIALS AND METHODS
Subjects
Adult zebra finches (>130 days posthatch) were taken for this study from breeding colonies at UCLA and University of Massachusetts, Amherst (14 L:10 D light cycle), and all animal care and use protocols were approved by the respective institutional animal care and use committees. In vivo microdialysis for changing neuroestrogen levels has been previously optimized in adult males of this species (Remage-Healey et al. 2008, 2010, 2011a). Animals recover quickly from surgery and are observed engaging in normal social and maintenance behaviors when tethered to the microdialysis apparatus.
Microdialysis
All procedures followed those validated previously, including cannula implantation (Remage-Healey et al. 2008, 2010, 2011a). Birds were anesthetized with equithesin, stabilized in a custom stereotaxic device with a head angle of 45° (Herb Adams Engineering), and kept warm with a heating pad. Lidocaine (2% in ethanol) was injected under the scalp, and feathers were removed to expose the skull area dorsal to NCM. A CMA-7 microdialysis cannula (CMA Microdialysis) was then implanted into NCM and cemented in place with alternating layers of cyanoacrylate and dental cement (Perm Reline/Repair Resin; Coltene-Whaledent). The scalp incision was sealed with cyanoacrylate to the cement, and birds were returned to individual sound-attenuation chambers for postsurgical recovery (∼5 days).
Prior to an experimental session, the microdialysis probe, swivel, and FEP tubing were rinsed with 70% ethanol and ddH2O and prefilled with artificial cerebrospinal fluid (aCSF; in mM: 199 NaCl, 26.2 NaHCO3, 2.5 KCl, 1.0 MgSO4, 2.5 CaCl2, and 11.0 glucose, with 1% bovine serum albumin; pH = 7.4). Microdialysis probes were implanted at least 12 h prior to an experimental sampling session to allow behavioral adaptation and for implantation-induced phenomena to subside. A CMA-7 microdialysis probe that had been prefilled with aCSF at a flow rate of 2.0 μl/min (Harvard Apparatus 22 syringe pump) was implanted, and birds were connected to the microdialysis system. All samples in this study were collected at 30-min intervals. At the end of each sampling period each sample was immediately sealed and stored at −80°C. A previously optimized ELISA protocol (Remage-Healey et al. 2008) using a commercially available ELISA kit for 17β-estradiol (E2; Cayman Chemical) was used to determine fluctuating levels in plasma and dialysate samples in response to visual and acoustic stimuli. At the end of each experimental session birds were euthanized via isoflurane overdose and perfused through the heart with 0.9% PBS followed by 4% paraformaldehyde. Cannulas and probe were removed after perfusion to preserve the implantation site. Brains were cryoprotected and sectioned at 40- to 50-μm thickness, and sections were mounted onto gel-subbed Superfrost slides (Fisher Scientific), air dried, stained with thionin, and photographed under light microscope to verify probe placement. Histological analysis confirmed that probes were within the NCM region for all birds in this study (with the exception of mesopallium control subjects, see below).
Auditory Stimulation
Previous study of male zebra finches showed that acoustic playback of male song specifically induced a rapid and robust elevation in E2 levels within NCM (Remage-Healey et al. 2008). Because the NCM of females also expresses aromatase (Peterson et al. 2005; Rohmann et al. 2007), we therefore tested whether the acute effects of song on NCM E2 levels were sex-specific. Dialyzed females were housed individually in sound-attenuation chambers, and dialysate was collected for two 30-min silent periods prior to playback to establish baseline conditions (“pre1” and “pre2”). Dialysate was then collected for 30 min from females exposed to acoustic playback of either male song (1-min recording of intermittent male song from 3 individual males, repeatedly looped 30×; n = 8) or white noise (1-min intermittent white noise pulses matched to the duration and interstimulus interval of male song, repeatedly looped 30×; n = 7). Stimuli were band-pass filtered (1–12 kHz, CoolEdit Pro; identical to procedures in Remage-Healey et al. 2008) and were played back through a calibrated speaker inside each chamber at ∼70 dB. After the playback period dialysate was again collected for two 30-min silent periods (“post1” and “post2”). All trials were videotaped through a one-way glass partition, and behaviors were monitored off-line. Blood samples were drawn from a subset of females (n = 6) at the end of pre1 and “male song” playback periods, respectively, to determine effects of song on circulating E2 levels.
Visual Stimulation
Auditory playback elicits acute changes in NCM E2 levels in both males (Remage-Healey et al. 2008) and females (present study). In addition, social interactions with females, accompanied by singing in some but not all cases by the focal male, caused acute elevations in NCM E2 levels in males (Remage-Healey et al. 2008). Because of the potential multimodal (i.e., visual and auditory) input to NCM (see Avey et al. 2005; Kruse et al. 2004), we therefore tested whether visual stimuli alone can elicit acute changes in NCM E2 levels in both male and female adult zebra finches, using in vivo microdialysis. The sampling design of these experiments mirrored the above design for acoustic playbacks. A thin-film transistor (TFT) LCD monitor (refresh rate > 75 Hz; Dell) was inserted into the bird's chamber prior to lights-on, and an opaque barrier was placed on the monitor to restrict the viewing range to a 4-in. × 4-in. window at the center of the screen. Video playback of conspecific and heterospecific stimuli using TFT displays elicits prosocial behaviors in both male and female zebra finches (i.e., social vocalizations and beak wiping; Campbell and Hauber 2010; Galoch and Bischof 2007), indicating that this paradigm reasonably approximates natural social stimulation. Dialysate was collected for two 30-min periods to establish baseline (pre1 and pre2), during which the TFT display broadcasted a constant black background. During the video playback period, dialysate was collected for 30 min while one of the following video clips (Sony PMB 5.0 software) was presented per experimental subject: 1) one female zebra finch expressing prosocial behaviors (n = 7 female microdialysis subjects; n = 11 male microdialysis subjects); 2) one actively courting male zebra finch (n = 8 female microdialysis subjects; n = 7 male microdialysis subjects); 3) one courting male Bengalese finch (n = 5 male microdialysis subjects). Each video stimulus consisted of 5-min clips of behaving animals interspersed with 1–2 min of constant black background, and video stimuli were repeatedly broadcast until the end of the 30-min trial. After the video playback period, dialysate was again collected for two 30-min periods (post1 and post2), during which a constant black background was broadcast through the TFT display. No auditory playback occurred during video presentations. All trials were videotaped through a one-way glass partition, and behaviors were monitored off-line. All subjects were observed visually scanning the TFT display during video playbacks. Importantly, 7 of 11 microdialysis males who were exposed to female visual stimuli engaged in limited courtship singing, consistent with earlier work showing that visual stimulation elicits the full complement of courtship singing behavior (Galoch and Bischof 2007). The effects of video presentation on calling and singing behaviors by the microdialyzed males were analyzed to assess the potential contribution of auditory self-stimulation on NCM E2 levels.
Mesopallium Control Experiment
The spatial specificity of changing NCM E2 levels was determined in a separate set of n = 6 female subjects each for both visual and auditory playback treatments (as above). Cannulas were surgically implanted and directed toward the mesopallium (lacking in aromatase) anterior to NCM and Field L. Histological analyses of all control birds confirmed that probes were targeted to mesopallium in the vicinity of CMM, and in some cases the probe lesion extended slightly ventral into the underlying anterior nidopallium (in no case was the probe sampling from NCM in these mesopallium control birds).
Electrophysiology
The auditory playback study with females showed that E2 levels are specifically elevated in the NCM of females during song playback. It has been recently established that both exogenous and endogenous neuroestrogens can exert rapid actions on the firing frequency of NCM neurons (Remage-Healey et al. 2010; Tremere et al. 2009; Tremere and Pinaud 2011). These combined observations led to the prediction that estrogens exert rapid modulation of auditory processing in the NCM of females. This hypothesis was tested by using unilateral multiunit extracellular recordings in NCM combined with ipsilateral reverse microdialysis (retrodialysis) of the predominant neuroestrogen E2 (30 μg/ml; n = 7), the abundant neuroandrogen 5β-dihydrotestosterone (5β-DHT, 30 μg/ml; n = 3), or the estrogen synthesis inhibitor fadrozole (FAD, 100 μM; n = 3).
All electrophysiology procedures followed a protocol previously established for males (Remage-Healey et al. 2010). For craniotomy surgery to expose NCM, birds were anesthetized with 20% urethane via intramuscular injections (3 × 30-μl injections over 2-h period). Lidocaine (2% in ethanol) was injected under the scalp, and feathers were removed to expose the skull area dorsal to NCM. A midline incision and craniotomy exposed the point of origin, and the location of NCM was stereotaxically marked with a marking pipette. A stainless steel headpost (Herb Adams Engineering) was attached to the rostral skull via dental cement/cyanoacrylate. The dura mater was carefully dissected over the NCM region. The bird was transferred to a custom headpost stage (Herb Adams Engineering) inside a sound-attenuation chamber and kept warm with a heating pad (FHC Neurocraft). A CMA-7 microdialysis probe prefilled with aCSF (as above) was advanced into NCM with a motorized micromanipulator (Warner Instruments) equipped with a custom mounting clip. During a 60-min stabilization period dialysate was collected to allow implantation-induced phenomena (i.e., neurochemical release and excitability due to probe implantation) to subside. A hydraulic micromanipulator (Narishige) was then used to advance a carbon fiber electrode (Carbostar-1, Kation Scientific) into NCM immediately adjacent to the retrodialysis probe. Neuronal activity was band-pass filtered (0.3–5 kHz), digitized at 20 kHz (Micro 1401, Cambridge Electronic Design), and stored on a computer with Spike 2 software. Auditory-responsive NCM neurons were located with playback of filtered, band-passed white noise stimuli.
To test for steroid-dependent auditory modulation, experimental subjects were exposed to four acoustic stimuli: two distinct conspecific songs (CON1, CON2), a conspecific song played in reverse (REV), and white noise (WN). All stimuli were band-pass filtered (0.5–10 kHz) and were presented in randomized order at an interstimulus interval of 10 ± 2 s at ∼70 dB SPL via an audio amplifier connected to a speaker inside the recording chamber. In contrast to the triple song used for microdialysis (above), song stimuli used in the electrophysiology experiment were of brief duration (∼2 s) to facilitate analysis of changes in auditory-evoked activity of NCM neurons. Electrophysiology experiments were divided into three successive 30-min playback periods, such that each playback stimulus was presented 20 times within a 15-min block (after an initial 15-min period to allow drug washin or washout, for a total treatment period of 30 min). During playbacks and recordings aCSF was retrodialyzed for the first 30 min to determine baseline responsiveness, followed by 30-min retrodialysis of E2, 5β-DHT, or FAD, followed by a 30-min washout period of aCSF alone. Each animal was treated with one compound exclusively. All recordings were analyzed off-line by experimenters blind to treatment condition. At the conclusion of each experimental session, the NCM recording site was lesioned (+10 μA for 8 s) for verification of probe and electrode placement. All recordings in this study were histologically confirmed to be within the NCM.
Bilateral Recordings
A separate set of adult females (n = 3) were used for dual-hemisphere recordings and retrodialysis. Surgery, recordings, and playback experiments were conducted as above, with the exception that craniotomy exposed the NCM in both left and right hemispheres. Extracellular electrodes were advanced into each NCM hemisphere, and a microdialysis probe was advanced into the left NCM. After a 60-min stabilization period and baseline aCSF dialysis/playback recordings as above, biotinylated estradiol (E2-biotin; Steraloids E1360-100; 60 μg/ml in aCSF) was retrodialyzed for 30 min for playback/recordings. E2-biotin is a membrane-impermeable conjugate that has been demonstrated to act specifically at membrane estrogen binding sites (Dewing et al. 2007; Germain et al. 1993) and was used to test for membrane-specific effects of E2.
Electrophysiology Analyses
The voltage threshold for detecting multiunit responses in NCM was set by the experimenter to exclude low-amplitude events (i.e., spikes with peak amplitude-to-background noise ratio less than ∼2:1), following established protocols (Coleman et al. 2007; Remage-Healey et al. 2010). For each recording session threshold levels were maintained for the entire duration of recordings, so that all sampling periods per experiment (e.g., aCSF, E2, washout) were analyzed with the same voltage threshold. Suprathreshold multiunit activity was then used to compute average response strength (RS) for each 30-min recording. For each playback event, the mean firing rate for 2 s immediately prior to playback stimulation was subtracted from the mean firing rate for 2 s during auditory stimulation, resulting in RS. The RS metric therefore accounts for baseline firing rate, and average RS was computed for the entire 20 presentations for each stimulus during each treatment period. Because NCM neurons can habituate to repeated presentation of song stimuli (Chew et al. 1995) the root mean square (RMS) amplitude of NCM multiunit activity was analyzed for all treatment periods. The slope of the decay in RMS amplitude over 20 stimulus presentations is an indication of auditory habituation (Chew et al. 1995; Phan et al. 2006) and was therefore analyzed for all treatment periods for changes in auditory-evoked activity (RMS for 2 s prior to the stimulus subtracted from RMS during the 2-s auditory stimulus).
Statistical Analyses
Statistical analyses were carried out off-line with Statview 4.5 (Abacus) and R (GNU project). Both dialysate samples and multiunit RS measures (see below) were analyzed with multivariate repeated-measures ANOVAs for within-subject measurements over time with successive sampling periods grouped as a single dependent variable. ANOVAs for dialysate measures were computed for within- and between-subject variability for the treatment period and the preceding sampling period only (e.g., 30 min playback and 30 min pre). Results for all samples including pre1 and pre2 and post1 and post2 are presented in figures to illustrate time-dependent variability. When overall F-tests were significant for independent treatment effects they were followed by appropriate Wilcoxon signed rank post hoc tests (WSRTs) for the effects of treatment (e.g., 30 min male song playback vs. 30 min pre) for a given playback stimulus (e.g., male song, REV, etc.). WSRTs are not robust to sample sizes < 5 (Sokal and Rohlf 1981), so for the electrophysiology experiment with FAD, data were combined from all playback groups for each treatment period with a pooled WSRT (i.e., CON, REV, WN grouped for aCSF vs. FAD). The same procedure for a pooled WSRT for all playback groups was computed for the electrophysiology experiment with E2-biotin.
RESULTS
No Sex Difference in Baseline NCM Neuroestrogen Levels
A subset of in vivo microdialysate samples were collected from the NCM of both males and females and run simultaneously in the same set of ELISA plates. Analysis of these samples provided the opportunity to test for potential sex differences in neuroestradiol (E2) levels at baseline (i.e., in the absence of any experimental manipulation) in vivo, as predicted by sex differences in the expression of aromatase fibers and synaptic terminals in NCM (Peterson et al. 2005; Rohmann et al. 2007; Saldanha et al. 2000) and reported sex differences in gonadal E2 secretion (Adkins-Regan et al. 1990; Agate et al. 2003; Naguib et al. 2004). In contrast to the circulation, we did not detect a significant sex difference in dialysate E2 levels from NCM at baseline (males: 13.95 ± 1.68 pg/ml; n = 12; females: 12.29 ± 3.14 pg/ml; n = 10; mean ± SE; P = 0.65 for unpaired t-test), indicating that baseline levels in NCM are not different between adult males and females. Instead, the presence of sex differences in synaptic aromatase in NCM may be associated with rapid fluctuations in neuroestrogens in the NCM that are sex-specific, as presented below.
Auditory Playback Elevates NCM Neuroestrogens in Females
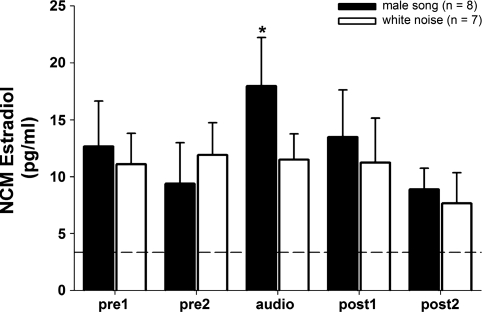
Aromatase is expressed in both neuronal cell bodies and presynaptic terminals in the NCM of adult male and female zebra finches (Peterson et al. 2005), and adult males exhibit acute elevation in NCM E2 levels in response to 30 min of song playback (Remage-Healey et al. 2008). Because NCM is a site of auditory processing of complex stimuli in both sexes, we tested the hypothesis that auditory activation of NCM in females also elicits acute changes in local E2 levels. Playback of auditory stimuli to awake, behaving microdialyzed females caused significant changes in local E2 levels within NCM (Fig. 1; 2-way repeated-measures ANOVA; playback effect: F = 10.497, P = 0.007; stimulus effect: F = 0.191, P = 0.669; playback × stimulus interaction: F = 12.719; P = 0.004). Post hoc analysis revealed that local E2 levels were elevated during the 30-min audio playback period of male song (P = 0.036; relative to the pre2 period, within subject) but not during white noise playback (P = 0.866; relative to the pre2 period, within subject). Plasma E2 levels in a separate set of n = 6 females were unchanged after male song playback (pre playback: 33.35 ± 8.94 pg/ml; post playback: 29.79 ± 11.40 pg/ml), suggesting that circulating E2 does not covary with fast changes in E2 levels measured with in vivo microdialysis within NCM (for similar results of NCM vs. plasma E2 levels in male zebra finches, see Remage-Healey et al. 2008). Therefore, similar to adult males, an acute rise in NCM E2 levels occurs in females during 30 min of exposure to conspecific song stimuli.
Fig. 1.
Rapid elevation in caudomedial nidopallium (NCM) neuroestradiol (E2) levels in female subjects exposed to song but not white noise stimuli. Each time point represents E2 levels collected over 30-min periods. *P < 0.05 for the “audio” period vs. the “pre2” period for the group exposed to male song only. Dotted line indicates average background ELISA reading for artificial cerebrospinal fluid (aCSF) alone, prior to microdialysis.
Neither Visual Nor Auditory Stimulation Alters Neuroestrogens in Mesopallium of Females
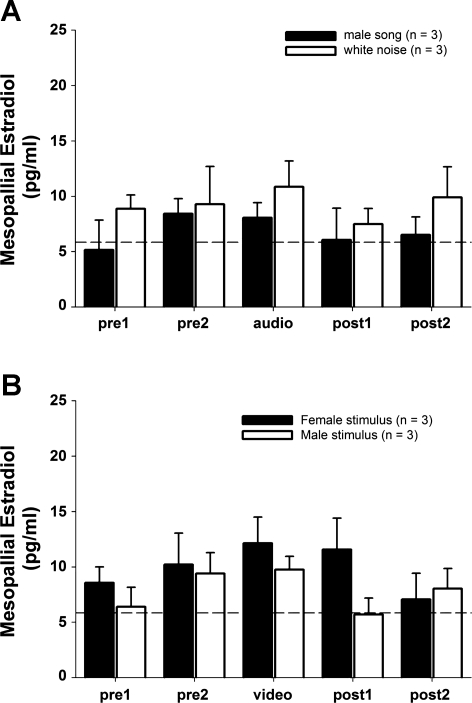
The spatial specificity of changing E2 levels in NCM observed above for females was tested in a new group of females with probes directed at the mesopallium anterior to NCM (n = 6). Auditory playback of either male song or white noise caused no significant changes in local E2 levels within the anterior mesopallium (Fig. 2A; 2-way repeated-measures ANOVA; playback effect: F = 1.231, P = 0.337; stimulus effect: F = 1.232, P = 0.329). Similarly, visual presentation of either conspecific males or females (as described below) caused no significant changes in local E2 levels within the anterior mesopallium (Fig. 2B; 2-way repeated-measures ANOVA; presentation effect: F = 1.065, P = 0.360; stimulus effect: F = 0.385, P = 0.569). Therefore, the acute changes in local E2 levels observed within NCM of females in response to male song playback were not accompanied by similar changes in a nearby mesopallial region, indicating that neuroestrogen changes in response to auditory or visual stimuli are specific to NCM.
Fig. 2.
No significant changes in neuroestradiol levels in a control mesopallium region in females exposed to auditory (A) or visual (B) stimuli. Each time point represents E2 levels collected over 30-min periods. Dotted line indicates average background ELISA reading for aCSF alone, prior to microdialysis.
Visual Playback Elevates NCM Neuroestrogens in Males but Not Females
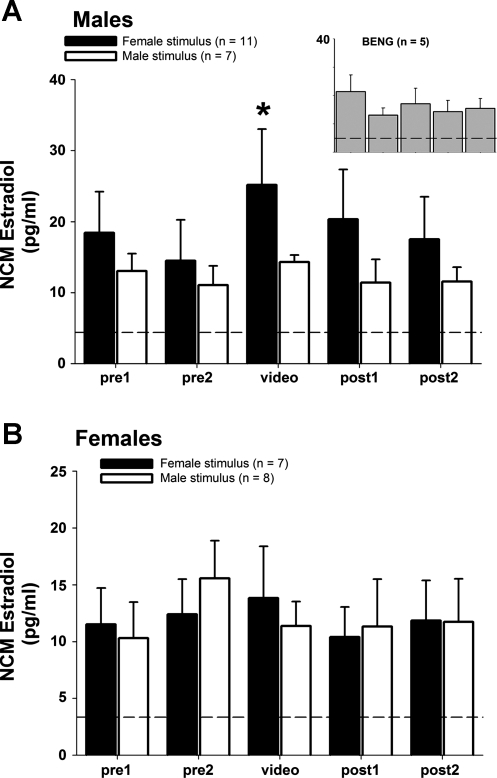
Local E2 levels in NCM of males are acutely elevated during social interactions with females, and in some males this response occurs in the absence of courtship singing (Remage-Healey et al. 2008). We reasoned therefore that a visual stimulus of a conspecific alone could elicit changes in local E2 levels within NCM. We tested this hypothesis in both male and female adult zebra finches with in vivo microdialysis. In males, video playback of conspecific stimuli caused significant changes in local E2 levels within NCM (Fig. 3A; 2-way repeated-measures ANOVA; presentation effect: F = 5.203, P = 0.034; stimulus effect: F = 1.258, P = 0.278). Post hoc analysis revealed that local E2 levels were elevated in response to female video playback (P = 0.013; relative to pre2 period, within subject) but not in response to male video playback (P = 0.236; relative to pre2 period, within subject). During the video presentation of females, 7 of 11 males vocalized to the stimulus, including courtship song. Because auditory activation of NCM with song stimuli can elicit robust increases in NCM E2 levels in males (Remage-Healey et al. 2008), we tested for the effects of self-stimulation on changing E2 levels. Vocal behavior during trials was analyzed for total vocalizations (number and duration) and for the occurrence and duration of primary vocalization subtypes (tets, long calls, songs, chirps; categories after Zann 1996). There was no significant difference between singing and nonsinging males in NCM E2 levels during the 30-min female video trial (unpaired t-test: t = 0.63, df = 9, P = 0.54). Variation in individual vocalization data and E2 levels was also tested with linear regression. We analyzed E2 levels both during the female video stimulation for each male and as a function of the transition in E2 levels from baseline to determine the extent of changes in E2 levels that are accounted for by vocal behavior of the microdialyzed male (self-stimulation). All regression tests for the effects of vocalizations (tets, long calls, songs, chirps) on E2 levels during video playback were nonsignificant (all P > 0.20), indicating that any direct effect of self-stimulation (auditory) on NCM E2 levels was negligible.
Fig. 3.
A: rapid elevation in NCM neuroestradiol levels in male subjects exposed to female, but not male, conspecific visual stimuli. Each time point represents E2 levels collected over 30-min periods; *P < 0.05 for the “video” period vs. the pre2 period for the group exposed to female video stimulus only. Inset: no significant change in response to heterospecific visual stimulus (Bengalese finch, BENG). B: no significant changes in NCM E2 levels in female subjects exposed to either female or male conspecific visual stimuli. Dotted lines indicate average background ELISA reading for aCSF alone, prior to microdialysis.
In microdialyzed females, in contrast to male subjects, video playback of conspecific stimuli caused no changes in local E2 levels within NCM, in response to either male or female video stimuli (Fig. 3B; 2-way repeated-measures ANOVA; presentation effect: F = 0.738, P = 0.406; stimulus effect: F = 0.007, P = 0.935). Similarly, video playback of a control heterospecific stimulus (Bengalese finch) to male microdialysis subjects did not cause changes in local E2 levels (Fig. 3A, inset; F = 1.241, P = 0.333). Therefore, these results indicate that males exhibit an acute elevation of E2 levels in NCM specifically in response to visual exposure to conspecific females, while female NCM E2 levels are unresponsive to these same stimuli.
Electrophysiology
Neuroestrogens rapidly modulate auditory responsiveness in NCM of females.
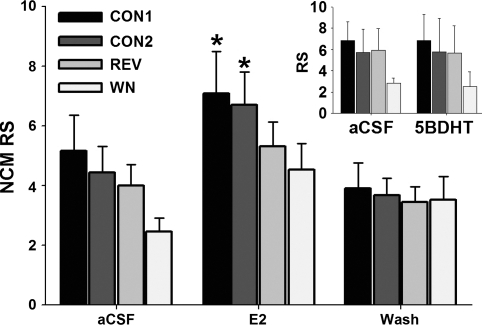
Because E2 levels are acutely elevated in the NCM of females during auditory playback (above), we reasoned that neuroestrogens rapidly modulate auditory-evoked activity of neurons within NCM. This hypothesis was tested in a new group of females with combined extracellular electrophysiology and retrodialysis of E2, 5β-DHT, or FAD. In anesthetized females, both up- and downregulation of local neuroestrogen levels produced rapid changes in NCM response strength (RS; see materials and methods). Retrodialysis of E2 caused a significant change in NCM auditory-evoked RS (Fig. 4; 2-way repeated-measures ANOVA; E2 treatment effect: F = 19.996, P < 0.0001; playback stimulus effect: F = 1.128, P = 0.357), and post hoc analysis revealed that RS was significantly elevated during 30 min of E2 treatment (relative to the aCSF period) for both CON1 (P = 0.028) and CON2 (P = 0.042) playback stimuli. RS during playback of REV (P = 0.091) and WN (P = 0.063) stimuli were not significantly elevated but showed similar overall increases in response to E2.
Fig. 4.
Auditory-evoked activity of NCM neurons is rapidly increased in the presence of E2 in adult females (RS = response strength; see text for derivation). CON1, CON2, conspecific songs 1 and 2; REV, conspecific song played in reverse; WN, white noise. *P < 0.05 for E2 vs. aCSF comparison for the CON1 and CON2 groups only. Inset: no effect of the abundant neurosteroid 5β-dihydrotestosterone (5β-DHT) on NCM auditory processing.
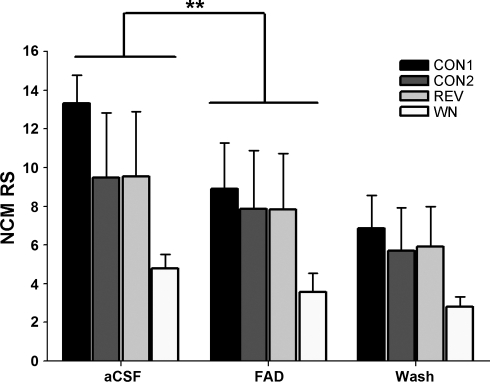
Similarly, when endogenous neuroestrogen levels were suppressed with 30-min treatment with the aromatase inhibitor FAD, RS was significantly and rapidly decreased to all playback stimuli (Fig. 5; 2-way repeated-measures ANOVA; FAD treatment effect: F = 36.247, P < 0.0001; playback stimulus effect: F = 1.287, P = 0.343; pooled WSRT: P = 0.0037 for FAD vs. aCSF for all playback stimuli). We note that a pooled post hoc test should be interpreted with caution, and that an overall downward trend in RS that occurred for all treatments in the FAD experiment is suggestive of response habituation to repeated presentation of the same auditory stimuli. However, detailed analysis showed that neural habituation was minimal for all stimuli and treatment groups (see below). In summary, upregulation of local neuroestrogen levels with E2 retrodialysis caused rapid increases in NCM auditory responsiveness, while downregulation of local neuroestrogen levels with FAD retrodialysis was associated with rapid decreases in NCM auditory responsiveness.
Fig. 5.
Local aromatase inhibition causes a rapid decline in RS in NCM to auditory stimuli in adult females. Retrodialysis of the aromatase inhibitor fadrozole (FAD) into NCM rapidly suppresses RS. **P < 0.005 for the pooled Wilcoxon signed rank post hoc test (WSRT) for FAD vs. aCSF.
In contrast to the rapid actions of neuroestrogens, there was no effect of a brain-derived androgen similarly derived from testosterone (5β-DHT) on RS to any playback stimuli (Fig. 4, inset; 2-way repeated-measures ANOVA; 5β-DHT treatment effect: F = 0.036, P = 0.854; playback stimulus effect: F = 0.658, P = 0.601). This indicates that the observed acute effects of E2 are steroid-specific. Importantly, analysis of neuronal habituation to repeated presentation of auditory stimuli revealed a shallow habituation rate (as measured by the slope of RMS amplitude vs. stimulus iteration for each sampling period; all slopes < 0.0128). Negligible habituation to the auditory stimuli presented in this study was expected based on the recording site (ventral NCM) and behavioral state (anesthetized; see Chew et al. 1995; Phan et al. 2006) of our study animals (see also Remage-Healey et al. 2010 for similar findings with males). Also, habituation rate did not significantly change in the presence of E2, 5β-DHT, or FAD (all P > 0.38 for 1-way repeated-measures F-tests). Similarly, baseline firing rate (i.e., in the absence of auditory playback stimulation) was not significantly altered by E2, 5β-DHT, or FAD treatments (all P > 0.54 for repeated-measures F-tests). Therefore, the rapid actions of neuroestrogens on NCM neurons were restricted to the modulation of auditory-evoked activity.
Lateralized, Rapid Actions of a Membrane-Impermeable Estrogen
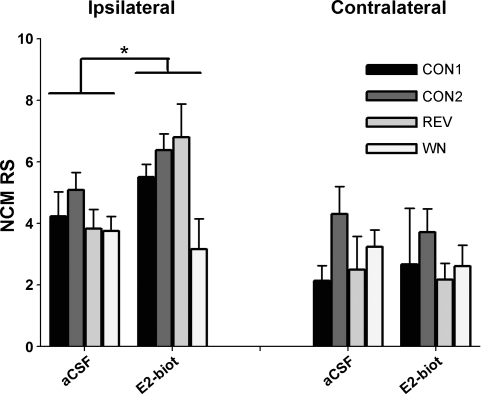
To test for possible rapid E2 actions at putative neuronal membrane receptors, membrane-impermeable E2-biotin was retrodialyzed unilaterally into NCM in a separate set of anesthetized females. Bilateral electrode placement into each NCM hemisphere allowed recordings from one electrode ipsilateral and one contralateral to the site of local E2-biotin delivery. Results of this experiment showed a significant effect of E2-biotin on RS (Fig. 6; 3-way repeated-measures ANOVA; hemisphere effect: F = 15.796, P = 0.001; E2-biotin treatment effect: F = 3.354, P = 0.085; playback stimulus effect: F = 1.869, P = 0.175) and a significant E2-biotin treatment × hemisphere interaction (F = 7.773, P = 0.013). The pooled WSRT for all treatments revealed that RS was rapidly elevated during E2-biotin treatment relative to the aCSF period only in the ipsilateral NCM (P = 0.018) and not the contralateral hemisphere NCM (P = 0.272). Thus rapid E2 effects observed in females were fully mimicked by a membrane-impermeable biotinylated E2 and were limited to the hemisphere NCM that was ipsilateral to the E2-biotin delivery site.
Fig. 6.
The neuronal membrane-impermeable estrogen conjugate biotinylestradiol (E2-biot) recapitulates the rapid actions of E2 on auditory-evoked activity of NCM neurons (RS) in adult females. This effect is restricted to the NCM hemisphere ipsilateral to the retrodialysis delivery probe. This is consistent with an acute mode of E2 acting via a putative membrane-bound receptor. *P < 0.05 for the pooled WSRT for E2-biot vs. aCSF.
DISCUSSION
Motor circuits devoted to vocalization present some of the most striking sexual dimorphisms found in the brain of vertebrates, with males typically expressing motor nuclei manyfold larger than females (see, e.g., Kelley and Bass 2010; Nottebohm and Arnold 1976). By contrast, the circuits that process sensory input, including the auditory NCM of songbirds, are largely similar in structure and composition between the sexes. Even still, sensory regions may be differentially modulated in males versus females by neurochemicals to aid in sex-specific neural and behavioral tasks. Neuroestradiol may be one such neuromodulator because of its unique chemical properties, its presence at neuronal synapses, and its direct association with sex differences in nervous system structure and function (Balthazart and Ball 2006; Remage-Healey et al. 2011b; Saldanha et al. 2011; Sisneros et al. 2004).
Here, we present evidence that neuroestrogen fluctuations and actions in the auditory cortical nucleus NCM of zebra finches exhibit several properties that are similar between adult males and females, with one notable exception in the context of visual stimulation. Prior work had established that in males neuroestradiol (E2) levels are locally elevated within NCM when subjects hear song but not control white noise (Remage-Healey et al. 2008). In this study, this property of NCM is now extended to females, which exhibit the same acute auditory selectivity for song versus white noise in terms of rapid E2 elevation. Second, prior work had established that E2 rapidly boosts (while inhibition of neuroestrogen production rapidly suppresses) the auditory-evoked firing rate of NCM neurons in males (Remage-Healey et al. 2010; see also pooled data for both males and females in Tremere et al. 2009). This property of NCM is also now formally extended to females in this study. Moreover, the same direction of the effects of neuroestrogen up- and downregulation on the auditory-evoked firing rate of NCM neurons is observed in females as in males. Also, as in males, the rapid effect of E2 on NCM neurons is steroid-specific, because a steroid product similarly derived from testosterone in the brain, 5β-DHT, caused no significant changes in neurophysiological activity. Together this evidence therefore indicates that rapid neuroestrogen fluctuation in NCM (as measured by microdialysis) has a primary role in auditory processing of song in a rapid, neuromodulatory manner (as measured by electrophysiology), regardless of sex. These results further reinforce the primacy that neuroestrogens have in the modulation of auditory information in the songbird brain.
Source of Estrogens Measured in NCM in Females
All of the females used in these studies were gonadally intact, and therefore some of the rapid elevations in E2 measured within NCM in response to male song playback could be of ovarian origin. Indeed, circulating sex steroids secreted from the gonads or other steroidogenic glands can shift rapidly in response to changes in the social environment and/or auditory cues (Harding 1981; Hirschenhauser and Oliveira 2006; Remage-Healey and Bass 2005; Wingfield 1985). Moreover, in female zebra finches systemic E2 levels are elevated in response to repeated playback of male song, but this was observed over a period of 5 days of exposure (Tchernichovski et al. 1998). Two results from this study are consistent with rapid (<30 min) fluctuations observed in NCM that are intrinsic to NCM and independent of circulating E2. First, plasma E2 levels were unchanged in females that were exposed to 30 min of male song playback relative to preplayback. Second, E2 levels in the mesopallium near NCM were unchanged during male song playback. These findings are similar to observations in males, in which both plasma E2 levels and E2 levels outside NCM remained unchanged during male song playback (Remage-Healey et al. 2008). Together, these results are consistent with a local, rapid modulation of neuroestrogen levels intrinsic to the NCM region of both male and female zebra finches.
Aromatase expression (both somal and presynaptic) is virtually absent in the mesopallium of female zebra finches (Saldanha et al. 2000), which is consistent with the present observations of generally lower baseline E2 levels and lack of acute changes in E2 in the mesopallium, compared with NCM. E2 levels in the mesopallium therefore originate in either the circulation or nearby brain regions or via a combination of the two. By contrast, the NCM of females appears to be synthesizing neuroestrogens locally and acutely (independent of the circulation), just as has been observed in males. One major difference between males and females, however, is that circulating androgen levels are low in females (Agate et al. 2003; Naguib et al. 2004) and therefore are not as likely to provide a sustainable supply of aromatizable substrate to the female NCM (in contrast to males, for whom circulating androgens are abundant). Therefore, in contrast to the traditional view that the female vertebrate brain is thought to respond exclusively to estrogens synthesized in the ovary, the results of this study lead us to the hypothesis that both neuroandrogens and neuroestrogens originate within the female NCM itself. Although this hypothesis awaits an explicit experimental test, the critical enzymes for neuroandrogen production are expressed in the zebra finch CNS (London et al. 2003, 2006) and can be rapidly regulated in the songbird brain (Pradhan et al. 2010; Soma et al. 2004). How these androgen synthetic enzymes coordinate with aromatase in the functional synthesis of neuroestrogens remains an intriguing and active area of research.
Rapid Electrophysiological Actions of Neuroestrogens
Previous work in this species has shown that estrogens modulate auditory-evoked activity of NCM neurons on a fast timescale (i.e., within 5–30 min; Remage-Healey et al. 2010; Tremere et al. 2009). This rapid action is confirmed here for females, and is inconsistent with a “classical” genomic mechanism of estrogen action, which depends on gene transcription events and is typically manifest within hours. A multitude of alternative, “nonclassical” modes of estrogen action have been documented in the vertebrate brain, including recruitment of signaling cascades via actions at the neuronal membrane (Meitzen and Mermelstein 2011; Moenter and Chu 2012; Roepke et al. 2011). The present study shows that the rapidity of the actions of estradiol in the NCM can be accounted for by putatively membrane-specific actions. Specifically, the membrane-impermeable conjugate E2-biotin fully mimicked the rapid actions of unconjugated E2 itself on NCM neuronal activity. The receptor-mediated mechanism for this effect is unclear. Although evidence for extranuclear estrogen receptors is scant thus far for zebra finches, extranuclear/membrane expression of estrogen receptors appears to be a conserved feature in the vertebrate lineage (Beyer et al. 2003; Milner et al. 2001; Mitterling et al. 2010; Montague et al. 2008; Thomas et al. 2005). Recent in vitro evidence indicates that estradiol in NCM can rapidly alter presynaptic inhibitory potentials (Tremere et al. 2009). The present results implicate a neuronal membrane receptor in NCM that may be coupled to this or a similarly acute mechanism (see, e.g., Dewing et al. 2007).
Sex-Specific Effects of Visual vs. Auditory Stimulation
In contrast to the results with auditory stimulation, only males responded to playback of visual stimuli in this study, and the response was restricted to an elevation in NCM E2 levels to the presentation of conspecific females. We considered the possibility that the focal males' vocalizations in response to the visual stimulus directly caused elevations in their NCM E2 levels (i.e., “self-stimulation”; Cheng 1986; Cheng et al. 1998). Our analysis, however, showed that no aspect of the focal male's behavior, including his vocalizations, accounted for the elevation in NCM E2 levels in response to female video playback. This is consistent with an earlier finding in which NCM E2 was acutely elevated in males during social interactions with females, regardless of the presence or amount of singing by the focal male (Remage-Healey et al. 2008). Therefore, at least one aspect of social interactions appears to involve a direct, visually evoked neuroestrogen elevation in the NCM of male (but not female) zebra finches.
This striking sex difference in NCM E2 fluctuations most likely reflects the sensory-specific demands of social interactions in adult male versus female zebra finches. Given that males court females using both auditory (song) and visual (plumage and postural elements of courtship displays) modalities, it is rather surprising that NCM E2 levels were elevated in females only during auditory playback of male song. Although we presented females with naturalistic video stimuli of actively courting males, we may not have fully captured crucial visual elements of courtship that occur during live social interactions that could lead to changes in NCM neuroestrogens in females, and this possibility awaits further investigation. The present findings predict further that visually evoked neuroestrogen changes in the NCM should also occur in males of other songbird species, especially those in which visual and auditory displays are used in territorial disputes, such as the song sparrow (see, e.g., Pradhan et al. 2010; Wingfield 1985).
Rapid elevations in E2 in the NCM of males in response to female video playback (and during social interactions with live females) are suggestive of a modulatory transition to a “prepared” state for auditory processing demands that are likely to ensue. “Preparatory” hormonal changes occur in the general circulation in response to visual cues in the early stages of a social encounter (e.g., plasma androgens: Antunes and Oliveira 2009) and thus may similarly occur within discrete brain circuits to provide local modulatory events. According to this model, when a male zebra finch begins to engage in social interactions with a female, NCM estrogens are elevated rapidly upon visual contact. Thus enhanced auditory gain provided by estrogens within NCM does not require acoustic stimulation per se and appears to be driven by multisensory input in males. Resulting E2 elevation could therefore aid in several neural tasks that occur in NCM during social interactions, such as song memory and retrieval (Gobes and Bolhuis 2007), attentional shifts to song and discrimination among auditory stimuli (Stripling et al. 1997; Terleph et al. 2008; Voss et al. 2007), and/or synaptic plasticity associated with novel individuals and stimuli (Chew et al. 1995; Jarvis et al. 1995). In this context, the salience of incoming auditory stimuli may be thus shaped by preparatory neuroestrogen elevations in response to visual stimuli (for similar findings with the immediate-early gene ZENK, see also Kruse et al. 2004).
Visual Input to NCM
Visual stimuli, especially those of conspecifics during social interactions, can exert powerful influences over an animal's physiology and behavior (Adkins-Regan and Leung 2006; Chen and Fernald 2011; Wingfield and Wada 1989). Although the songbird NCM is considered an auditory region, several previous studies have reported that visual stimuli can evoke or alter responses in NCM, as measured by rapid changes in expression of the immediate-early gene ZENK (Avey et al. 2005; see also George et al. 2006; Hara et al. 2009; Kruse et al. 2004). Among vertebrates, multisensory integration is perhaps best understood for the overlap between visual and auditory modalities (see, e.g., Cohen 2009; Winkowski and Knudsen 2007). Visual input can activate units in auditory cortex in monkeys (Brosch et al. 2005; Kayser et al. 2010), and the existence of true “unisensory” cortex (such as the purely auditory NCM) is in question (Stein and Stanford 2008). To our knowledge, there are no known direct projections from primary visual pathways into NCM, increasing the likelihood that visual information is integrated in regions afferent to the NCM, such as the thalamus or midbrain (see, e.g., George et al. 2011; Winkowski and Knudsen 2007). Although the role of visual input into NCM remains to be fully accounted for, it is noteworthy that the adjacent caudolateral nidopallium (NCL) receives visual input in pigeons (Kirsch et al. 2009; Leutgeb et al. 1996), and a region of dorsocaudal nidopallium activated during courtship receives visual projections in zebra finches (Sadananda et al. 2007; Wild 1994).
Multimodal integration, sensory enhancement, and attentional shifts are proposed to depend upon neuromodulator systems that can alter or tune neuronal activity to meet different behavioral demands. The modulators that mediate multisensory integration and attentional shifts are typically biogenic amines or other neurotransmitters such as thalamocortical acetylcholine (Ding et al. 2010; Parikh et al. 2007). The present work suggests that neuroestrogens can participate in multisensory integration in auditory cortex, either as an independent modulator or in concert with amines or amino acid transmitters associated with auditory processing in the NCM (Maney et al. 2001; Ribeiro and Mello 2000; Sockman and Salvante 2008; Vyas et al. 2008). The role of neuroestrogens as modulators of sensory processing is just emerging (see, e.g., Jeong et al. 2011; Remage-Healey et al. 2010), and the interdependence of multimodal processing and brain-derived steroid production and action provides an exciting new avenue of future research.
GRANTS
This research is supported by National Institute of Neurological Disorders and Stroke Grant K99/R00 NS-066179 and National Institute of Mental Health Grant 061994.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.R.-H. and B.A.S. conception and design of research; L.R.-H., S.M.D., and A.C. performed experiments; L.R.-H. and A.C. analyzed data; L.R.-H. interpreted results of experiments; L.R.-H. prepared figures; L.R.-H. drafted manuscript; L.R.-H. and B.A.S. edited and revised manuscript; L.R.-H., S.M.D., A.C., and B.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Melissa Coleman for technical advice, Randi Oyama, Jenna McHale, Sonja Smith, David Jeon, and Ashley Paon for technical support, and the laboratories of Art Arnold, David Glanzman, Frank Krasne, and Stephanie White for expertise and resource sharing.
REFERENCES
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata). Gen Comp Endocrinol 78: 93–109, 1990 [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Leung CH. Hormonal and social modulation of cloacal muscle activity in female Japanese quail. Physiol Behav 87: 82–87, 2006 [DOI] [PubMed] [Google Scholar]
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci USA 100: 4873–4878, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes RA, Oliveira RF. Hormonal anticipation of territorial challenges in cichlid fish. Proc Natl Acad Sci USA 106: 15985–15989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey MT, Phillmore LS, MacDougall-Shackleton SA. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav Brain Res 165: 247–253, 2005 [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. FOS and ZENK responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav Brain Res 162: 108–115, 2005 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball G. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29: 241–249, 2006 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil C, Taziaux M, Charlier T, Baillien M, Ball G. Rapid changes in production and behavioral action of estrogens. Neuroscience 138: 783–791, 2006 [DOI] [PubMed] [Google Scholar]
- Beyer C, Pawlak J, Karolczak M. Membrane receptors for oestrogen in the brain. J Neurochem 87: 545–550, 2003 [DOI] [PubMed] [Google Scholar]
- Black MP, Balthazart J, Baillien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, Lythrypnus dalli. Proc Biol Sci 272: 2435–2440, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Scheich H, Heizmann CW, Hunziker W. Parvalbumin and calbindin-D28k immunoreactivity as developmental markers of auditory and vocal motor nuclei of the zebra finch. Neuroscience 40: 853–869, 1991 [DOI] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci 25: 6797–6806, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DLM, Hauber ME. Behavioural correlates of female zebra finch (Taeniopygia guttata) responses to multimodal species recognition cues. Ethol Ecol Evol 22: 167–181, 2010 [Google Scholar]
- Chen CC, Fernald RD. Visual information alone changes behavior and physiology during social interactions in a cichlid fish (Astatotilapia burtoni). PLoS One 6: e20313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MF. Female cooing promotes ovarian development in ring doves. Physiol Behav 37: 371–374, 1986 [DOI] [PubMed] [Google Scholar]
- Cheng MF, Peng JP, Johnson P. Hypothalamic neurons preferentially respond to female nest coo stimulation: demonstration of direct acoustic stimulation of luteinizing hormone release. J Neurosci 18: 5477–5489, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require 2 periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA 92: 3406–3410, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE. Multimodal activity in the parietal cortex. Hear Res 258: 100–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci 27: 10024–10036, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology 146: 3809–3820, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci 27: 9294–9300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67: 294–307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galoch Z, Bischof H. Behavioural responses to video playbacks by zebra finch males. Behav Processes 74: 21–26, 2007 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol 20: 705–712, 2008 [DOI] [PubMed] [Google Scholar]
- George I, Hara E, Hessler NA. Behavioral and neural lateralization of vision in courtship singing of the zebra finch. J Neurobiol 66: 1164–1173, 2006 [DOI] [PubMed] [Google Scholar]
- George I, Richard JP, Cousillas H, Hausberger M. No need to talk, I know you: familiarity influences early multisensory integration in a songbird's brain. Front Behav Neurosci 4: 193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain PS, Metezeau P, Tiefenauer LX, Kiefer H, Ratinaud MH, Habrioux G. Use of a biotinyl-estradiol derivative to demonstrate estradiol-membrane binding sites on adherent human breast cancer MCF-7 cells. Anticancer Res 13: 2347–2353, 1993 [PubMed] [Google Scholar]
- Gobes SMH, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Curr Biol 17: 789–793, 2007 [DOI] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Assessing visual requirements for social context-dependent activation of the songbird song system. Proc Biol Sci 276: 279–289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CF. Social modulation of circulating hormone levels in the male. Am Zool 21: 223–231, 1981 [Google Scholar]
- Herbison AE. Rapid actions of oestrogen on gonadotropin-releasing hormone neurons; from fantasy to physiology? J Physiol 587: 5025–5030, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav 71: 265–277, 2006 [Google Scholar]
- Holloway CC, Clayton DE. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci 4: 170–175, 2001 [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Mello CV, Nottebohm F. Associative learning and stimulus novelty influence the song-induced expression of an immediate early gene in the canary forebrain. Learn Mem 2: 62–80, 1995 [DOI] [PubMed] [Google Scholar]
- Jeong JK, Tremere LA, Burrows K, Majewska AK, Pinaud R. The mouse primary visual cortex is a site of production and sensitivity to estrogens. PLoS One 6: e20400, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Logothetis NK, Panzeri S. Visual enhancement of the information representation in auditory cortex. Curr Biol 20: 19–24, 2010 [DOI] [PubMed] [Google Scholar]
- Kelley DB, Bass AH. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Curr Opin Neurobiol 20: 748–753, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol 308: 17–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch JA, Vlachos I, Hausmann M, Rose J, Yim MY, Aertsen A, Gunturkun O. Neuronal encoding of meaning: establishing category-selective response patterns in the avian “prefrontal cortex.” Behav Brain Res 198: 214–223, 2009 [DOI] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Context-specific habituation of the zenk gene response to song in adult zebra finches. Neurobiol Learn Mem 82: 99–108, 2004 [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci 30: 12950–12957, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Husband S, Riters LV, Shimizu T, Bingman VP. Telencephalic afferents to the caudolateral neostriatum of the pigeon. Brain Res 730: 173–181, 1996 [DOI] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J Comp Neurol 467: 496–508, 2003 [DOI] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology 147: 5975–5987, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett 311: 189–192, 2001 [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res 57: 357–384, 2002 [DOI] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat 42: 236–241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol 429: 355–371, 2001 [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol 518: 2729–2743, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, Chu Z. Rapid nongenomic effects of oestradiol on gonadotrophin-releasing hormone neurones. J Neuroendocrinol 24: 117–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol 20: 893–903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63: 149–155, 1996 [DOI] [PubMed] [Google Scholar]
- Naguib M, Riebel K, Marzal A, Gil D. Nestling immunocompetence and testosterone covary with brood size in a songbird. Proc Biol Sci 271: 833–838, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of songbird brain. Science 194: 211–213, 1976 [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56: 141–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci 272: 2089–2096, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA 103: 1088–1093, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J Neurobiol 66: 182–195, 2006 [DOI] [PubMed] [Google Scholar]
- Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav 57: 381–389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci 27: 1114–1122, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Horm Behav 47: 297–305, 2005 [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA 107: 3852–3857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci 31: 10034–10038, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11: 1327–1334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, Schlinger BA. Estradiol synthesis and action at the synapse: evidence for “synaptocrine” signaling. Front Neuroendocr Sci 2: 28, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV. Gene expression and synaptic plasticity in the auditory forebrain of songbirds. Learn Mem 7: 235–243, 2000 [DOI] [PubMed] [Google Scholar]
- Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci 16: 1560–1573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol 67: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- Sadananda M, Korte S, Bischof H. Afferentation of a caudal forebrain area activated during courtship behavior: a tracing study in the zebra finch (Taeniopygia guttata). Brain Res 1184: 108–120, 2007 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev 32: 532–549, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol 423: 619–630, 2000 [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci USA 89: 7650–7653, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science 305: 404–407, 2004 [DOI] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev Neurobiol 68: 656–668, 2008 [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Wilcoxon signed rank test. In: Biometry, edited by Wilson J. New York: Freeman, 1981, p. 440–444 [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology 145: 1668–1677, 2004 [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci 9: 255–266, 2008 [DOI] [PubMed] [Google Scholar]
- Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. J Neurosci 17: 3883–3893, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Schwabl H, Nottebohm F. Context determines the sex appeal of male zebra finch song. Anim Behav 55: 1003–1010, 1998 [DOI] [PubMed] [Google Scholar]
- Terleph TA, Lu K, Vicario DS. Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS ONE 3: e2854, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146: 624–632, 2005 [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci 29: 5949–5963, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci 31: 3271–3289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss HU, Tabelow K, Polzehl J, Tchernichovski O, Maul KK, Salgado-Commissariat D, Ballon D, Helekar SA. Functional MRI of the zebra finch brain during song stimulation suggests a lateralized response topography. Proc Natl Acad Sci USA 104: 10667–10672, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Harding C, McGowan J, Snare R, Bogdan D. Noradrenergic neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), treatment eliminates estrogenic effects on song responsiveness in female zebra finches (Taeniopygia guttata). Behav Neurosci 122: 1148–1157, 2008 [DOI] [PubMed] [Google Scholar]
- Wild JM. Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. J Comp Neurol 349: 512–535, 1994 [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Short-term changes in plasma levels of hormones during establishment and defense of a breeding territory in male song sparrows, Melospiza melodia. Horm Behav 19: 174–187, 1985 [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Wada M. Changes in plasma levels of testosterone during male-male interactions in the song sparrow, Melospiza melodia: time course and specificity of response. J Comp Physiol A 166: 189–194, 1989 [Google Scholar]
- Winkowski DE, Knudsen EI. Top-down control of multimodal sensitivity in the barn owl optic tectum. J Neurosci 27: 13279–13291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47: 657–680, 2007 [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch : a synthesis of field and laboratory studies. In: The Zebra Finch. Oxford, UK: Oxford Univ. Press, 1996 [Google Scholar]