Abstract
We use a combination of in vitro whole cell recordings and computer simulations to characterize the cellular and synaptic properties that contribute to processing of auditory stimuli. Using a mouse thalamocortical slice preparation, we record the intrinsic membrane properties and synaptic properties of layer 3/4 regular-spiking (RS) pyramidal neurons and fast-spiking (FS) interneurons in primary auditory cortex (AI). We find that postsynaptic potentials (PSPs) evoked in FS cells are significantly larger and depress more than those evoked in RS cells after thalamic stimulation. We use these data to construct a simple computational model of the auditory thalamocortical circuit and find that the differences between FS and RS cells observed in vitro generate model behavior similar to that observed in vivo. We examine how feedforward inhibition and synaptic depression affect cortical responses to time-varying inputs that mimic sinusoidal amplitude-modulated tones. In the model, the balance of cortical inhibition and thalamic excitation evolves in a manner that depends on modulation frequency (MF) of the stimulus and determines cortical response tuning.
Keywords: primary auditory cortex, synaptic depression, modulation transfer function
neurons in the auditory cortex exhibit diverse response properties even to similar classes of stimuli. While some of these receptive field properties are inherited from the thalamus, it is clear that significant processing occurs within the local cortical circuitry (Creutzfeldt et al. 1980; Linden and Schreiner 2003; Miller et al. 2002; Winer et al. 2005). However, the relative contributions of cellular and synaptic mechanisms to the cortical transformation of thalamic input remain unclear.
Feedforward inhibition (FFI) is a fundamental phenomenon observed in a variety of neural circuits. Thalamic afferents provide the principal ascending input to primary sensory cortices forming synapses directly onto both excitatory and inhibitory neurons (Agmon and Connors 1992; Cruikshank et al. 2007; Gabernet et al. 2005; Hersch and White 1981; Rose and Metherate 2005; Verbny et al. 2006). Fast-spiking (FS) inhibitory interneurons are the primary thalamorecipient inhibitory cell population (Gibson et al. 1999; Markram et al. 2004; Ren et al. 1992; Rose and Metherate 2005; Staiger et al. 1996). FS neurons make powerful synaptic connections onto neighboring excitatory cells to produce robust and reliable FFI (Cruikshank et al. 2007; Gabernet et al. 2005; Wehr and Zador 2003; Zhang et al. 2003). FFI has been postulated to play an important role in a variety of cortical processes including tuning of receptive field properties, shaping the time course of cortical firing, limiting the temporal window for integration of synaptic inputs, producing forward suppression of auditory stimuli, and generating nonmonotonic auditory intensity-tuning curves (Bruno and Simons 2002; de la Rocha et al. 2008; Gabernet et al. 2005; Swadlow 2003; Wehr and Zador 2003, 2005; Wilent and Contreras 2005; Wu et al. 2006).
Both thalamocortical (TC) and intracortical synapses exhibit short-term plasticity, either depressing or facilitating in response to repetitive stimulation. Short-term depression (STD) of TC inputs has been postulated to contribute to adaptation to sensory stimuli, contrast-invariant orientation tuning and cross-orientation suppression in visual cortices, forward suppression of auditory stimuli, and the tuning of temporal response properties to repetitive stimuli (Carandini et al. 2002; Chance et al. 1998; Chung et al. 2002; Eggermont 2002; Wehr and Zador 2005). Because both FFI and STD are time-dependent suppressive processes, they are likely to play key roles in determining neuronal responses to time-varying stimuli such as drifting sine gratings in visual cortex, repetitive whisker stimulation in barrel cortex, and sinusoidally modulated acoustic stimuli in auditory cortex.
In the primary auditory cortex (AI), in vivo intracellular recordings have shown that both depression and FFI are prominent features of the synaptic responses to auditory stimuli (Wehr and Zador 2003, 2005; Zhang et al. 2003). However, the underlying synaptic and intrinsic membrane properties of excitatory and inhibitory thalamorecipient cells remain incompletely characterized. Furthermore, computational models of the auditory TC circuit have not investigated how these dynamic cellular properties shape cortical responses to time-varying auditory stimuli such as sinusoidal amplitude-modulated (sAM) tones.
Here we characterize the intrinsic properties of layer 3/4 regular-spiking (RS) pyramidal neurons and FS interneurons in AI and their synaptic inputs from thalamus. We performed simultaneous whole cell recordings from RS and FS cells in a mouse slice preparation that contains AI, the medial geniculate nucleus (MGv), and interconnecting fibers (Cruikshank et al. 2002). We use these data to construct a simple model of the auditory TC circuit and subsequently examine the model's responses to time-varying inputs that mimic sAM tones. In the model, the dynamics governing FFI and STD cause the balance of cortical inhibition and thalamic excitation to evolve in a manner that depends on modulation frequency (MF) of the stimulus. This interplay between inhibition and excitation determined tuning of responses to stimuli at varying MFs. Furthermore, we show that STD can act to disinhibit cortical responses acting as an activity-dependent gate on powerful FFI. We find that the differences in intrinsic membrane properties and TC inputs between FS and RS cells observed in vitro are crucial for generating model behavior similar to that observed in vivo.
METHODS
Auditory thalamocortical slice preparation.
All animal handling and surgical procedures were reviewed and approved by the New York University Animal Welfare Committee. Auditory TC brain slices were prepared as in Cruikshank et al. (2002). Briefly, Swiss-Webster mice at P14–P20 were decapitated under halothane anesthesia, and the brain was rapidly excised and immersed in ice-cold, oxygenated artificial cerebral spinal fluid (ACSF, in mM: 125 NaCl, 2.5 KCl, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, and 1 MgCl2). Five hundred-micrometer-thick brain slices were cut at an ∼15° angle to the horizontal with a vibratome (Campden Instruments). Slices were incubated at 37°C for ∼30 min and then maintained in oxygenated ACSF at room temperature for >30 min until being transferred to the recording chamber. The primary slice was identified by anatomical landmarks and confirmed with local field potential recordings (Cruikshank et al. 2002).
Whole cell patch-clamp recordings.
Whole cell recordings were obtained at ∼34°C (32–37°C) in a submersion recording chamber continuously perfused with oxygenated ACSF. Slices were visualized on an upright microscope (Olympus BX50WI) with infrared differential interference contrast (IR-DIC) microscopy.
Whole cell patch electrodes (4–15 MΩ) were pulled from borosilicate glass capillaries (World Precision Instruments) and filled with (in mM) 130 potassium gluconate, 5 KCl, 2 MgCl2, 4 MgATP, 0.3 GTP, 10 phosphocreatine, and 10 HEPES (pH 7.3 with KOH). In some experiments, 0.5% biocytin (Sigma) was added to label cells for histological identification. Current-clamp recordings were performed with Cornerstone amplifiers (Dagan), filtered at 3–10 kHz, and digitized at 10 kHz. A Power Macintosh G4 (Apple) was used in conjunction with an ITC-18 D/A and A/D board and IGOR software (Wavemetrics) for stimulus delivery, data acquisition, and data storage for off-line analysis.
Simultaneous whole cell recordings were performed in one FS cell and one to three RS cells separated by less than ∼200 μm and located in lower layer 3 and in layer 4. Cells that lacked prominent apical dendrites and had large, non-pyramidal-shaped somata under IR-DIC were targeted for recordings as presumptive FS cells. Only cells with stable initial resting membrane potentials (< −50 mV), low access resistance (<50 MΩ), and nonspontaneous firing were used. Voltages were not corrected for the junction potential.
Intrinsic membrane and firing properties of cells were characterized by injecting 1-s current steps at varying amplitudes. Action potential (AP) voltage threshold was defined as the membrane potential at the time of the peak of the second derivative of the voltage for a given AP. Current threshold is the smallest current step that evoked an AP. AP amplitude is the difference between the peak AP voltage and the voltage threshold, and AP half-width is the time between half-amplitude voltage crossings. Time to first AP, voltage threshold, and AP half-width are given for the first AP generated by current threshold steps. Adaptation ratio was defined as the ratio of the first interspike interval (ISI) to the average of the last three ISIs for a current injection of 0.5 nA (or the largest current injection delivered < 0.5 nA). The portion of the firing rate vs. current curve between just subthreshold current and the maximum observed firing rate prior to saturation was fit with a line to obtain its slope. A few cells were encountered that exhibited electrophysiological properties consistent with those of non-FS interneurons (low-threshold spiking or burst-spiking nonpyramidal cells); these cells were found infrequently and were excluded from the comparison of RS and FS cells.
Thalamic stimulation protocols.
Extracellular electrical stimuli (monophasic, 0.1–0.2 ms) of varying intensities were delivered to MGv via a bipolar stimulating electrode (FHC) and a constant-current stimulus isolation unit (A 360, Axon Instruments). MGv was stimulated with five pulses at 10, 20, and 40 Hz with a recovery pulse 500 ms after the last pulse in each train, generally repeated 10–15 times for each frequency and with a delay of 5 s between each train. Extracellular stimulation intensity was initially at or below the intensity needed to evoke a postsynaptic potential (PSP) in a FS cell and then increased in varying increments (2.5–100 μA) up to 250 μA. Throughout the text, the term “threshold stimulus intensity” or “threshold stimulus” is used to denote the lowest extracellular stimulus intensity delivered to thalamus that reliably evoked a PSP (<50% failures) that had a fixed latency (<1 ms SD) and whose amplitude, latency, and general shape varied only mildly with small increases in stimulus intensity. The PSP elicited in a given cell at the threshold stimulus intensity is referred to as the threshold response or the threshold PSP. Near-threshold stimulus intensities were sampled more finely (at 2.5–10 μA) than higher-intensity stimuli. We do not use the term “minimal stimulation” or “minimal stimulus intensity” because the term as defined in similar previous studies (for example, Cruikshank et al. 2007; Gabernet et al. 2005) has a strict definition of ∼50% failures with finer sampling (≤1 μA) of near-minimal stimulus intensities. The threshold responses in this study are likely similar to those that would be produced with minimal or near-minimal stimulation, but we avoid using the term “minimal” to avoid any potential confusion.
Postsynaptic potential analysis.
PSPs were analyzed with a semiautomated procedure that characterized several PSP properties including successes/failures, amplitude, and latency. Failures were defined as a failure of the membrane potential to rise above a hard threshold (usually 200 μV) from a baseline reference point located between the stimulus artifact and the PSP onset within a given period of time from stimulus onset (∼10 ms). PSP amplitude was taken as the difference between the baseline voltage and the peak membrane potential within a given time window from stimulus onset (usually 25 ms). PSP latency was defined as the intersection point of a line fit through the baseline voltage and a third-order polynomial fit to the PSP rising phase.
Only cells and responses that had identifiable, individual PSPs for each pulse in a train were selected for the analysis of STD. The lowest stimulus intensity that generated PSP responses with the above criteria was used for each cell. A simple parametric model for fitting STD was used following Abbott et al. (1997) and Tsodyks and Markram (1997):
| (1) |
where Rn and Rn+1 are PSP amplitudes of the n and n + 1 stimulus pulse normalized by the PSP amplitude of the first pulse in a train, Df is a multiplicative depressing factor, τrec is the time constant of recovery from depression, and Δt is the interval between nth and (n + 1)th pulses. In this model, when a presynaptic afferent fires, the synaptic efficacy R immediately decreases by the factor Df and then recovers to 1 as exponential with time constant of τrec.
Statistical tests.
Results are reported as means ± SD to emphasize population variability. Paired-sample Student's t-tests or Wilcoxon signed-rank tests were used for comparison of FS-RS cell pairs. Two-sample t-tests and Kolmogorov-Smirnov tests were used to compare the FS and RS cell populations. Statistical tests were performed with functions from the statistics toolbox in MATLAB (The MathWorks).
Firing rate neuronal model.
We constructed a simplified network model consisting of one thalamic and one cortical layer (see Fig. 4). Simulated stimuli were delivered to the thalamic layer composed of 50 cells evenly distributed along a one-dimensional tonotopic axis (de la Rocha et al. 2008). Input to the thalamic layer had a Gaussian profile, with the center cell receiving the largest input and inputs to the other thalamic cells smoothly decreasing farther from the center:
| (2) |
where gTH(Δf) is a Gaussian weighting function on the stimulus, Δf indicates the difference between the stimulus tonotopic frequency and a thalamic cell characteristic frequency (cf), and σTH determines the spread of thalamic activity along the tonotopic axis. Default parameters for the thalamic layer were 50 cells spread over 1 octave and σTH = 0.15 octaves. The thalamic cells had only a transfer function (Eq. 4) and no temporal dynamics; therefore the output of this layer [rTH(f,t)] represented an instantaneous transformed response to a given stimulus. The cortical layer consisted of only two populations of model neurons, one inhibitory (I) and one excitatory (E), that received inputs from the thalamic layer that could be subject to STD (Eq. 6). The E and I population thalamic inputs were also weighted with a Gaussian function (Eq. 2) such that the thalamic cell in the center of the thalamic layer provided the largest input to the cortical cells. Default parameters for cortical populations were σE = σI = 0.05 octaves. The I population provided FFI to the E population with varying synaptic weights (Eq. 5; see Fig. 4B).
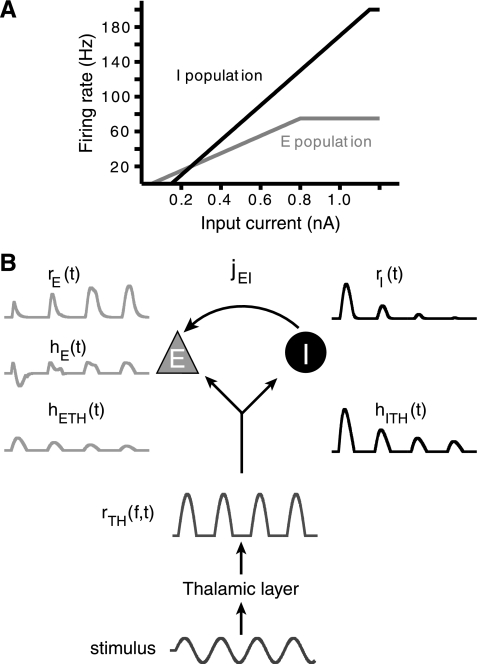
Fig. 4.
Model design. A: transfer functions, ΦE and ΦI, for E (gray) and I (black) populations. ΦE and ΦI are threshold-linear functions. Note the narrow range of current intensities for which the E population firing rate lies above the I population. B: schematic depiction of 2-population feedforward network. Examples are shown next to specific processing stages for a given stimulus. A modeled stimulus (bottom) is delivered to the thalamic populations. This input is transformed by ΦTH into thalamic firing rate rTH(Δf,t). Thalamic activity is summed and then converted by synaptic strength (jETH, jITH in pA/Hz) and scaled by synaptic depression into thalamic currents hETH(t) and hITH(t) (in pA). The I population response, rI(t), is converted by jEI (arrow) into the inhibitory current onto the E population, hEI (not shown). hETH(t) and hEI(t) sum linearly to hE(t) to generate the E population rate response rE(t) (top left).
We chose the firing rate (or rate-cell) model to represent the neuronal activity of each cortical population (de la Rocha et al. 2008; Pinto et al. 1996, 2003; Wilson and Cowan 1972). Neuronal activity is represented by an analog variable rα(t) that evolves following:
| (3) |
The time constant τα determines the time scale over which rα(t) reaches steady state and is related to both synaptic and membrane time scales of biological neurons (Dayan and Abbott 2001). Changes in rα(t) are driven by the total input current hα, in picoamperes, which is converted into an output firing rate in spikes per second by the cell's input/output transfer function, Φα. Φα is analogous to firing rate vs. current curves (f-I curves) obtained experimentally, and rα(t) is most similar to experimentally derived peristimulus time histograms. Φα was modeled as a threshold-linear transfer function with saturation:
| (4) |
where Θα is a current threshold in nanoamperes, Gα is the gain or slope of the transfer function in hertz per nanoampere, and rαmax is the maximum firing rate (see Fig. 4A). The model parameters Θ and G and the rαmax for each population were based on average firing rate vs. current curves obtained during in vitro recordings (Fig. 1E, Table 1; data not shown for thalamic cells). We chose τI < τE to reflect the faster membrane time constants and PSP kinetics observed in FS cells. Default parameters for the thalamic layer were ΘTH = 10 dB, GTH = 2 Hz/dB, and rTHmax = 125. Default parameters for cortical populations were τE = 10 ms, τI = 5 ms, ΘE = 0.05 nA, ΘI = 0.15 nA, GE = 100 Hz/nA, GI = 200 Hz/nA, rEmax = 75 Hz, and rImax = 200 Hz.
Fig. 1.
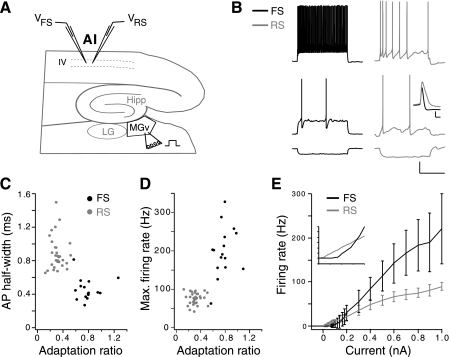
Experimental design and comparison of firing properties of fast-spiking (FS) and regular-spiking (RS) cells. A: schematic illustrating auditory thalamocortical brain slice. Simultaneous recordings were performed in layers 3–4 of primary auditory cortex (AI) in one FS cell with 1–3 RS cells. The ventral division of the medial geniculate nucleus (MGv) was stimulated extracellularly at various intensities with a bipolar stimulating electrode. Hippocampus (Hipp) and lateral geniculate nucleus (LG) are also depicted. B: example RS and FS cell responses to 1-s current step injections at −0.05 nA (bottom), threshold current injection for each cell (middle; 0.12 nA for FS cell, 0.04 nA for RS cell), and ∼2.5× threshold current (top). Scale bars are 20 mV, 500 ms. Inset: the 1st action potentials (APs) in B, middle, aligned to voltage threshold to illustrate the characteristic AP shapes of FS and RS cells. Inset scale bars are 20 mV, 1 ms. C: AP half-width vs. adaptation ratio. D: maximum firing rate vs. adaptation ratio. E: average firing rate vs. current curves for FS and RS cell population. Inset: magnification of curves near threshold. Error bars omitted for clarity. Inset division markings are 5 Hz, 0.05 nA. C–E: RS cells, n = 31; FS cells, n = 16.
Table 1.
Comparison intrinsic membrane properties for FS and RS cells
| FS (n = 16) | RS (n = 31) | |
|---|---|---|
| Input resistance, MΩ* | 163.92 ± 48.10 | 285.10 ± 80.61 |
| Membrane time constant, ms* | 11.83 ± 4.94 | 29.38 ± 10.32 |
| Vrest, mV | −67.88 ± 6.51 | −67.15 ± 7.75 |
| AP threshold, mV* | −30.57 ± 6.24 | −37.27 ± 7.54 |
| Threshold I, nA* | 0.16 ± 0.08 | 0.05 ± 0.03 |
| Number of APs at threshold* | 10.25 ± 10.72 | 3.65 ± 2.50 |
| 1st AP time, ms | 266.13 ± 304.10 | 223.09 ± 237.20 |
| AP half-width, ms* | 0.46 ± 0.13 | 0.89 ± 0.20 |
| Adaptation ratio* | 0.78 ± 0.17 | 0.31 ± 0.09 |
| Max firing rate, Hz* | 199.00 ± 64.31 | 75.71 ± 15.53 |
| FR vs. I slope, Hz/nA* | 273.28 ± 81.56 | 109.90 ± 24.69 |
Values are means ± SD. FS, fast spiking; RS, regular spiking; Vrest, resting membrane potential; AP, action potential; I, current; FR, firing rate.
P < 0.01 by Student's t-test.
Total input current hα was obtained as a linear sum of the individual input currents hXY(t):
| (5) |
jαβ is the synaptic weight of the input onto cell type α from cell type β in units of picoamperes per hertz, e.g., jITH is the synaptic weight of the thalamic cell input onto the I population. rα(f,t) represents the firing rate of all cells of a given type across the tonotopic axis over the stimulus. Because there is only one cortical population of each type, rα(f,t) is simply rα(t) and gα(f − Δf) = 1, so that hαα= jαα × rα(t). Thus jαα converts the rate of the presynaptic cortical population, rα(t), into current. For hαTH, the total current from thalamus, current is summed over all thalamic cell inputs, rTH(f,t), with weights on each thalamic cell determined by gα(Δf) as in Eq. 2. Importantly, the TH input onto the I population was two times stronger than the input onto the E population, i.e., jITH = 2 × jETH, matching the approximately twofold greater TC PSPs seen in FS versus RS cells (Fig. 2, Table 2). Default parameters were jETH = 1 pA/Hz, jITH = 2 pA/Hz, and jEI = −4 pA/Hz.
Fig. 2.
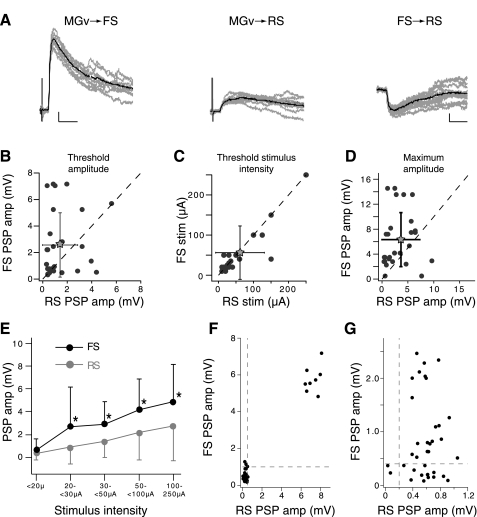
Comparison of FS and RS cell postsynaptic potential (PSP) amplitudes and stimulus intensities. A: threshold PSPs evoked in FS (left) and RS (center) cell pairs after stimulation of the ventral division of the MGv. Scale bars: 1 mV, 10 ms. Right: inhibitory PSP evoked the RS cell after suprathreshold, intracellular current injection in the FS cell. Scale bars: 0.5 mV, 10 ms. B–D: scatterplots of FS vs. RS cell pairs' threshold PSP amplitudes, threshold stimulus intensity, and maximum evoked PSP amplitudes. Threshold amplitude, threshold stimulus intensity, and maximum amplitude were determined independently for each cell in the FS-RS cell pair (n = 28 pairs). Gray stars represent means; error bars are SD. Both threshold and maximal evoked amplitudes were on average larger for FS cells vs. RS cells in the cell pairs. Threshold stimulus intensities were generally similar. E: PSP amplitude vs. stimulus intensity averaged across all cells in a population. The average amplitude of the 1st PSP in a train for a given cell at a given stimulus intensity was grouped according to intensity and averaged across cells. Black asterisks indicate significant difference between the 2 populations for a given stimulus range (P < 0.05, Kolmogorov-Smirnov test). Above 20 μA, FS cells have significantly larger PSP amplitudes than RS cells (n = 17 FS cells, 26 RS cells). F: scatterplot of PSPs elicited in an example RS and FS cell pair for which thalamic stimulation produces perfectly correlated PSP failures and successes. G: example cell pair for which PSP successes and failures were not correlated. Dashed lines in F and G indicate threshold for PSP failure.
Table 2.
Comparison of FS and RS PSP properties
| FS | RS | |
|---|---|---|
| PSP properties for FS-RS cell pairs (n = 28 cell pairs) | ||
| Threshold PSP amplitude, mV* | 2.57 ± 2.42 | 1.42 ± 1.44 |
| Maximum PSP amplitude, mV* | 6.33 ± 4.34 | 3.65 ± 3.65 |
| Threshold stimulus intensity, μA | 56.25 ± 66.63 | 61.25 ± 69.46 |
| FS threshold amp/RS threshold amp | 3.21 ± 3.96 | |
| FS maximum amp/RS maximum amp | 3.51 ± 3.30 | |
| PSP properties for individual cells (FS n = 17, RS n = 26) | ||
| Maximum amp/threshold amp | 4.39 ± 4.18 | 4.93 ± 10.66 |
| Threshold PSP latency, ms | 5.16 ± 1.52 | 5.12 ± 1.44 |
| Threshold PSP jitter, ms | 0.29 ± 0.17 | 0.43 ± 0.32 |
Values are means ± SD. PSP, postsynaptic potential.
P < 0.05 by paired-sample Wilcoxon signed-rank test. Top 3 data rows describe data presented in Fig. 2, C–E.
Stimuli.
Stimuli consisted of a single spectral frequency aligned to the characteristic frequency of the middle cell of the thalamic layer. The amplitude (dB) of this stimulus was sinusoidally modulated at varying frequencies (MF). The gain (G) of the thalamic cells transfer function was in hertz per decibel; decibel and nanoampere are interconvertable, with 1 nA = 100 dB and transformed as described above. These stimuli therefore represent amplitude-modulated (AM) stimuli with 100% modulation at the MF of a single carrier frequency. Fourier transforms were performed on cortical population responses during stimulus presentation. Simulated stimuli were of 1,000-ms duration for the two-population network or 500 ms for the full network model unless otherwise indicated.
STD.
Thalamic STD was modeled with a mean-field version of the model presented in Eq. 1 (Carandini et al. 2002; Chance et al. 1998; Tsodyks et al. 1998):
| (6) |
with rTH(Δf,t) representing the rate of a presynaptic thalamic cell with characteristic frequency Δf and Rα(Δf,t) acting as a weight on that cell such that:
| (7) |
Initially Rα(f,0) = 1; therefore if Dfα = 1 (i.e., no depression) then this variable is constant. Default parameters for depression were τrec E = τrec I = 1 s, DfE = 0.8, and DfI = 0.7.
Simulations.
Equations 2 and 5 were solved numerically in MATLAB (The MathWorks) with the forward Euler method with a fixed time step of 0.1 ms.
RESULTS
Comparison of intrinsic membrane properties of FS and RS cells.
Simultaneous whole cell recordings were made from RS pyramidal cells and FS cells in the lower half of layer 3 and in layer 4. Putative FS and RS cells were identified on the basis of morphology under IR-DIC, by their spiking responses to depolarizing current steps, and (for a subset of cells) by post hoc labeling with biocytin (Connors and Gutnick 1990; McCormick et al. 1985). The defining electrophysiological features of FS cells are high adaptation ratio, narrow AP half-width, high maximum evoked firing rate, short membrane time constant, and deep afterhyperpolarizations with rapid near-linear repolarization (see Fig. 1B, Table 1; Oswald and Reyes 2011). The two populations were generally well separated by AP half-width, adaptation ratio, and maximum firing rate, with some overlap at the extremes for each group (Fig. 1, C and D). Despite having resting membrane potentials similar to RS cells, FS cells needed approximately threefold higher currents to evoke APs because of their low input resistance (Table 1, Fig. 1E). The higher current thresholds and maximum firing rates of FS cells produced a much steeper slope to their firing rate vs. current curve (Fig. 1E, Table 1).
FS cells receive larger-amplitude TC PSPs than RS cells.
FS and RS cells are organized to produce FFI: Both cell types receive monosynaptic connections from auditory thalamus, and FS cells innervate RS cells (Fig. 2A; Rose and Metherate 2005). To compare directly the PSPs evoked by thalamic stimulation in RS and FS cell populations and control for slicing artifacts across preparations, we obtained simultaneous whole cell recordings from an FS cell and one to three RS cells located within 200 μm from each other and electrically stimulated the ventral division of the MGv (Fig. 1A). Stimulation intensity was increased in small increments (2.5–5 μA) until a PSP could be reliably evoked in a given cell; this lowest stimulus intensity for evoking a response is referred to as the threshold stimulus intensity (see methods). Extracellular stimulus intensity was increased gradually to examine compound PSPs evoked when additional thalamic afferents are recruited. FS cells generally had larger-amplitude PSPs than simultaneously recorded RS cells (Fig. 2), although the threshold stimulus intensity for each cell type did not differ significantly on average (Fig. 2C, Table 2).
Several factors may have contributed to the larger PSPs evoked in FS compared with RS cells (Fig. 2). One possibility is that the intensity of stimulation required for activating afferents to FS cells is lower than that for activating RS cells. This is unlikely because 1) threshold stimulation intensities did not differ between the two cell types (Fig. 2C); 2) PSPs evoked with threshold stimulation were larger in FS cells than RS cells (Fig. 2B); and 3) PSPs evoked in FS cells were on average significantly larger than those evoked in RS cells at all stimulus intensities (Fig. 2E) and remained greater even at saturating intensities where maximal PSP amplitudes were reached (Fig. 2D). Another possibility is that the FS cells receive more numerous thalamic afferents than RS cells. If so, increasing stimulus intensities should recruit progressively more fibers onto FS cells (Cruikshank et al. 2007; Gabernet et al. 2005). However, a plot of the PSP amplitude versus stimulus shows that the slope of the curve for FS cells was not substantially steeper than that for RS cells (Fig. 2E). Furthermore, the ratio of the average maximum evoked amplitudes for FS and RS cell pairs (6.33 mV/3.65 mV = 1.8) was similar to the ratio of threshold PSP amplitudes (2.57 mV/1.42 mV = 1.73). Taken together, these observations suggest that single-fiber thalamic PSPs evoked in FS cells are larger, but FS and RS cells receive approximately equal numbers of thalamic inputs.
Since thalamic afferents innervate wide regions of auditory cortex and contact both RS and FS cells with synapses with high probability of release (Cetas et al. 1999; Gil et al. 1999; Hersch and White 1981; Hull et al. 2009; Lee and Sherman 2008; McMullen and de Venecia 1993; Rose and Metherate 2005), simultaneously recorded FS and RS cell pairs were expected to receive divergent input from collaterals of shared thalamic fibers. This would be manifested as a perfect concurrence of PSP successes and failures in each cell at the same threshold stimulation intensity (Cruikshank et al. 2007; Gabernet et al. 2005; Inoue and Imoto 2006). We occasionally observed this phenomenon (Fig. 2F); however, most RS-FS cell pairs differed in their threshold stimulation intensities and did not show perfect correspondence of PSP successes and failures (Fig. 2G; only 4/28 cell pairs appeared to receive single-fiber innervation). These data indicate that either 1) these cells receive input from different TC afferents or 2) FS or RS cells are contacted by collaterals of the same TC fiber that differ in the number of synaptic contacts, probabilities of release, or degree of axon failure according to postsynaptic cell type.
Short-term dynamic properties of TC PSPs.
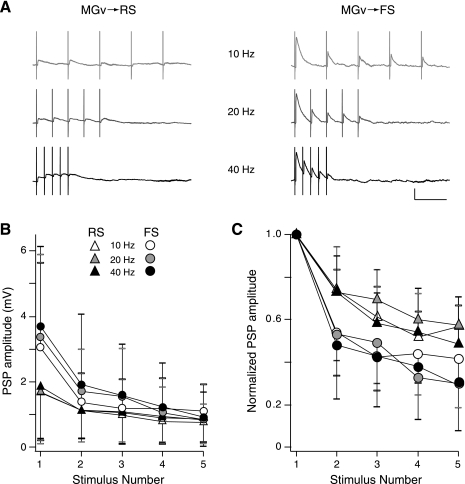
Thalamically evoked PSPs generally exhibit STD (Castro-Alamancos and Connors 1997). Differences in synaptic depression of PSPs in RS and FS cells are likely to determine how each population is recruited by thalamic activity (Gabernet et al. 2005). Repetitive stimulation of MGv with pulse trains at varying frequencies revealed that PSPs evoked in FS cells generally depressed more than those evoked in RS cells (Fig. 3). This greater degree of depression is evident when PSP amplitudes are averaged across cells for each pulse in a train or normalized to the first pulse in the train for each cell and then averaged (Fig. 3, B and C, respectively). Synaptic depression was adequately described with a simple phenomenological model (Eq. 1). As expected, the depression factor (Df) that describes the degree of decrease in response amplitude for each presynaptic event was significantly smaller (indicating greater depression) for FS versus RS cell PSPs [Df = 0.59 ± 0.14 for FS cells (n = 14), Df = 0.77 ± 0.07 for RS cells (n = 14), means ± SD; P < 0.01, 2-sample Kolmogorov-Smirnov test]. However, the time constant governing recovery from depression (τREC) was not significantly different between FS and RS cells [τREC = 2.17 ± 3.32 s for FS cells (n = 14), τREC = 2.45 ± 2.67 s for RS cells (n = 14), means ± SD; P > 0.05].
Fig. 3.
Comparison of thalamocortical (TC) synaptic depression in FS and RS cells. A: example FS-RS cell pair illustrating synaptic depression. Trains of 5 near-threshold intensity stimuli were delivered to MGv at 10, 20, and 40 Hz. Scale bar: 2 mV, 100 ms. B: average PSP amplitudes elicited by repetitive stimulation of MGv. TC PSPs recorded in FS cells are initially larger than RS cells but depress to similar amplitudes, indicating that FS cells show greater synaptic depression. C: average of normalized PSP amplitudes elicited by repetitive stimulation of MGv. Individual cell responses were normalized to the 1st PSP response in a train and then averaged across cells. FS cells exhibited greater synaptic depression at all stimulus frequencies. B and C: 10 Hz: FS cells, n = 14; RS cells, n = 19; 20 Hz: FS, n = 12; RS, n = 16; 40 Hz: FS, n = 10; RS, n = 14. Key refers to both B and C.
Firing rate model of thalamocortical circuit.
The combined effect of the observed intrinsic and synaptic properties of RS and FS cells on TC transformations is difficult to predict: Some of these properties appear to bias the circuit toward recruiting inhibition, while others bias toward excitation. For example, the larger-amplitude PSPs evoked in FS cells coupled with their steeper f/I slope would favor robust recruitment of inhibition, while the lower current thresholds and less depression of thalamic PSPs onto RS cells would favor net excitation. The dynamic balance of inhibition and excitation is particularly difficult to predict for time-varying input.
To gain insights into how network dynamics are affected by cellular and synaptic properties, we constructed a simple two-population cortical network consisting of one excitatory (E, analogous to RS cells) and one inhibitory (I, analogous to FS cells) population (de la Rocha et al. 2008; Pinto et al. 1996, 2003; Wilson and Cowan 1972; see methods and Fig. 4). The two populations were configured as a feedforward circuit in which both E and I populations received thalamic input and the I population inhibited the E population (Fig. 4). This highly simplified feedforward network produced results similar to those obtained with larger networks with recurrent cortical connections and a pseudo-tonotopic organization (data not shown; de la Rocha et al. 2008).
The activities of E and I populations were generated with simple rate models (de la Rocha et al. 2008; Pinto et al. 1996, 2003; Wilson and Cowan 1972). The model parameters were adjusted to preserve differences in RS and FS cells observed experimentally such as their f-I curves, strength of thalamic input, and degree of thalamic STD (Table 1; see methods for details). The auditory stimuli delivered to the thalamic layer were sinusoids of different MFs and are analogous to AM tones (see methods). The effects of FFI and thalamic STD on E population responses [rE(t)] to dynamic stimuli were evaluated in isolation as well as concurrently.
Effect of inhibition on model E population responses.
To examine the effect of FFI on E cell rate, sinusoidal stimuli of varying MFs were delivered to the thalamic layer without synaptic depression while varying the strength of the I-to-E connection, jEI (Fig. 5). With strong inhibition (jEI = −4 pA/Hz), the response of the E cells [rE(t)] decreased in both amplitude and duration and was phase shifted relative to the stimulus (Fig. 5). These effects were greater for low than for high MFs (Fig. 5, B and C). In addition, the effect of FFI varied between initial and steady-state responses for higher MFs (see below).
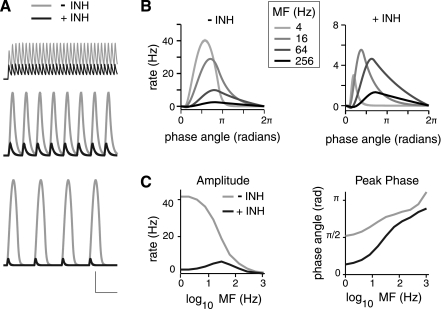
Fig. 5.
Effect of inhibition on rE(t) for stimuli of varying modulation frequencies (MFs). A: example E population responses to stimuli of different MF in the absence (gray) and presence (black) of inhibition (INH). MFs for each row from bottom to top are 4 Hz, 16 Hz, and 64 Hz. Peak amplitude of stimulus was 50 dB, and jEI = −4 pA/Hz for the case with inhibition. Scale bars are 10 Hz, 200 ms for MF = 4 Hz; 10 Hz, 100 ms for MF = 16 and 64 Hz. B: comparison of cycle of rE(t) at steady state for stimuli of varying MFs with (right) and without (left) inhibition. Each cycle of the rE(t) is plotted vs. phase angle for each MF. Steady-state responses are taken as the cycle beginning at 500 ms after stimulus onset. The value of rE(t) at the start of the cycle (phase angle = 0) has been subtracted for each MF. Responses are taken from the example in A. C, left: peak rE(t) amplitude plotted vs. MF with and without inhibition. rE(t) amplitudes are measured from the steady-state cycle beginning 500 ms after stimulus onset. Amplitude is taken as the maximum minus minimum rE(t) for the cycle. Right: phase of the rE(t) peak of the steady-state cycle is plotted against MF with and without inhibition.
To quantify the E population activity at varying MFs, the Fourier transform of rE(t) was calculated. The amplitudes of the frequency-modulated component of rE(t) corresponding to MF (F1) and the unmodulated component (F0) were calculated and compared across the different conditions (Fig. 6, A and B, respectively; Preuss and Muller-Preuss 1990). The F0 component is the mean firing rate during a stimulus for a given MF. The F1 component measures how faithfully rE(t) tracks MF. As expected for a passive leaky integrator (Eq. 3), the F0 component (Fig. 6A) without FFI (i.e., jEI = 0) is flat across frequencies, while the F1 component (Fig. 6B) is low pass with the cutoff frequency determined by the E population time constant, τE (data not shown). Increasing the inhibitory synaptic strength not only decreased the magnitude of F0 and F1 as expected but also altered their tuning properties (Fig. 6): The F0 tuning became increasingly high pass, while the F1 tuning became band pass with increasing jEI (Fig. 6). Thus both the F0 and F1 components showed greater suppression at lower MF.
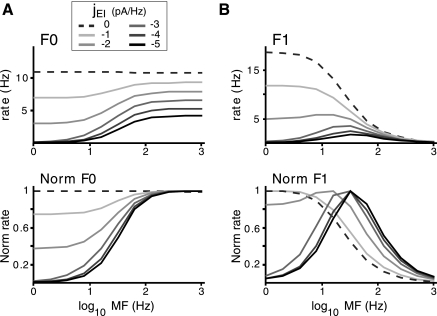
Fig. 6.
Effect of varying levels of inhibition on the E population tuning. A, top: mean rE(t) (F0) vs. MF for varying inhibitory synaptic strengths, jEI. Bottom: F0 tuning curves normalized to peak response for each jEI. Increasing inhibition makes the F0 vs. MF tuning more high pass. B, top: magnitude of the Fourier transform of the rE(t) at the stimulus frequency (F1) vs. MF. Bottom, F1 tuning curves normalized to the peak response for each jEI. Increasing inhibition makes the F1 vs. frequency tuning increasingly band pass. Peak amplitude of stimulus was 50 dB in all cases.
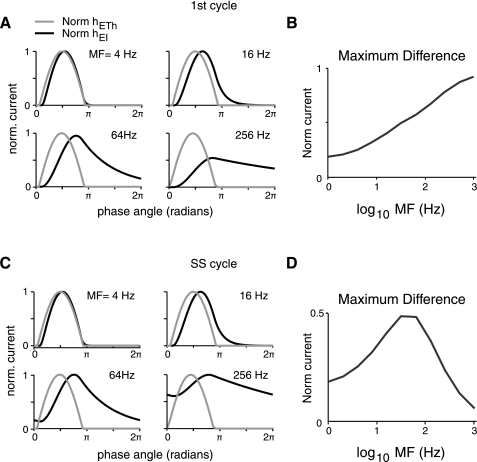
The changes that occur in the E population responses with MF are due to the interplay between excitatory inputs from thalamus [hETH(t)] and inhibitory inputs [hEI(t)] (Fig. 7). Figure 7A shows these currents for the first cycle of the stimulus, each normalized to its respective peak. With increasing MF, the excitatory thalamic input does not change appreciably (Fig. 7A), while inhibitory input increases proportionately with the I cell population firing rate [rI(t), data not shown]. The I population time constant (τI, Eq. 3) produces low-pass filtering of the stimulus (Fig. 7A). At low MFs, the I population response tracks the thalamic input (e.g., MF = 4 Hz). Consequently, there is considerable temporal overlap between the excitatory [hETH(t)] and inhibitory [hEI(t)] currents onto the E population, producing net cancellation (Fig. 7A). With increasing MF, the temporal overlap decreases as the slower inhibition can no longer track the excitatory input from thalamus (e.g., MF = 64 Hz; Fig. 7A). These effects can be seen by calculating the difference between the normalized thalamic and inhibitory currents at each MF (Fig. 7B). The maximum difference between these currents increases monotonically with MF, indicating that FFI, for the first cycle, acts as a high-pass filter (Fig. 7B). The suppression of E population responses to low-MF stimuli with increasing inhibition (Fig. 6) is primarily due to this high-pass filtering of thalamic inputs by FFI.
Fig. 7.
Tuning of synaptic currents differs for onset vs. steady-state response. hETH(t) and hEI(t) were normalized to their maximums for a given stimulus MF. Normalized hEI(t) is positive, although all nonnormalized values are negative. A: normalized synaptic currents elicited for the 1st cycle of modulated stimuli plotted vs. phase angle. As MF increases, there is an increasing delay in hEI(t) relative to hETH(t). B: maximum of the difference of the normalized 1st cycle currents plotted vs. MF. Inhibition makes the network high pass for peak current. C: normalized synaptic currents for 1 cycle at steady-state (SS) response of modulated stimuli vs. phase angle. Measurements are taken from the cycle starting 500 ms after stimulus onset. At higher MFs, hEI(t) summates temporally and remains elevated during the duration of the cycle. D: maximum of the difference of the normalized steady-state currents vs. MF. Temporal summation of hEI(t) and inhibition makes the network band pass with respect to peak current for repetitive stimuli.
The temporal interactions of excitation and inhibition differ later in the stimulus (Fig. 7, C and D). With sufficient time, the firing rates of E and I cell populations reach a steady state at which responses to each subsequent stimulus cycle are identical. The absolute time and number of cycles it takes to reach steady state vary across MF. At low MFs, responses decay to zero during each stimulus cycle and there is no change in synaptic currents or responses over time (e.g., MF = 4 Hz; Fig. 7, A and C). At high MFs, slowly changing inhibitory currents do not decay to zero between stimulus cycles and summate such that the peak-to-trough amplitude decreases (compare normalized currents for MF = 64 and 256 Hz in Fig. 7, A and C). The inhibitory current eventually reaches a relatively constant level, and there is no longer a time window in which thalamic excitation is unopposed by inhibition (Fig. 7, A and C). Consequently, the peak normalized synaptic current for high MF at steady state (Fig. 7D) is smaller than that for the first cycle (Fig. 7B), resulting in the changes in maximum rE(t) over the course of the stimulus. Unlike the first cycle, the peak normalized current at steady state increases to a maximum (MF = 32 Hz in Fig. 7D) and then decreases, indicating band-pass filtering (Fig. 7D).
Effect of thalamic synaptic depression.
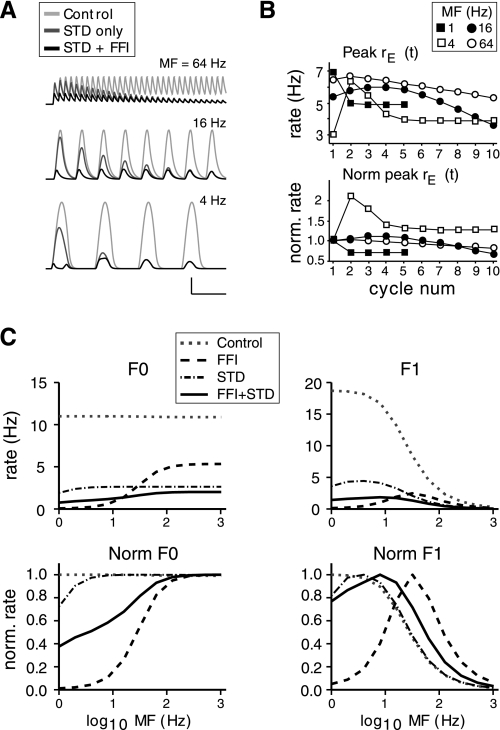
PSPs evoked with thalamic stimulation depressed more in FS cells than in RS cells (Fig. 3). To determine how this differential depression might affect the balance of inhibition and excitation during a time-varying stimulus, short-term synaptic depression was incorporated into the model and FFI was removed. In general, depression of thalamic inputs decreased the excitatory drive and suppressed rE(t) at all MFs (Fig. 8, A and C). At low MFs, stimuli vary slowly enough that STD reduces response amplitudes for the initial cycle of the stimulus and causes a phase shift of responses (Fig. 8A). At high MFs, STD does not substantially affect the first cycle of the stimuli because the time constant for depression (Eq. 5) is relatively long compared with a given stimulus period. As a result, depression accumulates gradually over the stimulus (Fig. 8A). In these ways, synaptic depression acts similarly to inhibition but on a slower time scale.
Fig. 8.
Effect of thalamic synaptic depression on model responses. A: comparison of rE(t) for stimuli of varying MF with thalamic input without depression [short-term depression (STD)], with depression only, and with depression + inhibition [feedforward inhibition (FFI)]. Control (light gray traces) is the default condition without depression or inhibition. Depression-only traces (dark gray) are without inhibition. Peak amplitude of stimulus was 50 dB, and jEI = −4 pA/Hz for the case with inhibition (black traces). For rows from bottom to top, MF is 4, 16, and 64 Hz. Scale bars are 10 Hz, 200 ms for MF = 4 Hz; 10 Hz, 100 ms for MF = 16 and 64 Hz. B: peak rE(t) plotted vs. stimulus cycle for condition with depression and inhibition. Top: peak rE(t) for first 5–10 stimulus cycles for varying MFs. Bottom: peak rE(t) responses normalized to 1st cycle amplitude. The change in peak rE(t) over time varies according to MF. 1 Hz and 4 Hz responses are taken from a 5-s stimulus. jEI = −4 pA/Hz. C: F0 and F1 tuning for various conditions. Top: F0 and F1 plotted vs. MF for the indicated conditions. Bottom: F0 and F1 tuning curves normalized to peak response for each condition. Peak amplitude of stimulus was 50 dB and jEI = −4 pA/Hz for the conditions including inhibition.
When FFI is reincorporated into the model, the overall effect of depression is disinhibition of the E cell population (Fig. 8C). Compared with STD alone, the E population rate is preferentially suppressed at low MFs, though the decrease is less than with inhibition only (Fig. 8C; i.e., the solid black traces are intermediate between the dot-dashed and dashed traces at low MFs). Disinhibition occurs because the I cell population has thalamic inputs that depress more than those for the E cell population (DfI < DfE, Eq. 5) as well as a higher threshold (ΘI) for firing than the E cell population. Consequently, depression leads to a greater decrease in I cell population firing and the E cell population is disinhibited. If the E and I populations are identical, disinhibition is not observed (data not shown).
Diverse patterns of E cell activity emerge as a consequence of the temporal evolution of disinhibition and depend on MF and the time constants of inhibition and depression (Fig. 8B). Disinhibition changes the peak amplitude of E population activity at successive stimulus cycles (Fig. 8B): Depending on MF, peak rE(t) could decrease monotonically (1 Hz) or nonmonotonically (4 Hz) with stimulus cycle.
DISCUSSION
We used a combination of experiments and simulations to characterize the cellular and synaptic properties that contribute to processing of auditory stimuli. Using whole cell recordings, we demonstrate for the first time in auditory cortex significant differences in the synaptic properties of RS pyramidal cells and FS interneurons. We used these experimental data to constrain a simple computational model in order to examine how FFI and synaptic dynamics affect cortical responses. We found that both FFI and synaptic depression alter the tuning of RS cells to time-varying stimuli that mimic sAM tones.
Comparison of FS and RS cells.
The PSPs evoked in FS cells were significantly larger and depressed more than those evoked in RS cells after stimulation of MGv. These observations differ somewhat from previous studies using similar auditory TC slice preparations (Rose and Metherate 2005; Verbny et al. 2006; but see Lee and Sherman 2008 for qualitatively similar results). One study did not find a significant difference in threshold PSP amplitudes or a consistent difference in STD recorded in FS and RS cells (Rose and Metherate 2005). Part of the discrepancy might be due to the fact that in that study (Rose and Metherate 2005) the sample was smaller (n = 5 FS cells), the RS and FS cells were often recorded in separate slice preparations, and the mice were younger (P14–P16) and of a different genetic strain. In combination, these factors may have obscured differences between the two already variable cell populations (Table 2). Another previous in vitro study observed smaller PSP amplitudes in thalamorecipient interneurons compared with pyramidal cells (Verbny et al. 2006). However, that study did not specifically target FS cells in layers 3 and 4 and therefore may have overlooked this interneuron subpopulation known to receive particularly strong input from thalamus in other systems (Beierlein et al. 2003; Cruikshank et al. 2007; Gabernet et al. 2005; Markram et al. 2004; Ren et al. 1992; Staiger et al. 1996). In our model, FFI is reliably recruited because FS cells receive large-amplitude TC PSPs. This study represents the first in vitro evidence for larger-amplitude TC PSPs in FS cells in auditory cortex. As such, these data may present a physiological mechanism for the strong FFI seen in vivo and are similar to what has been documented in somatosensory cortex (Cruikshank et al. 2007; Gabernet et al. 2005; Inoue and Imoto 2006; Wehr and Zador 2003; Zhang et al. 2003).
The differences in PSP properties of RS and FS cells are unlikely to reflect bias or variability in experimental conditions (e.g., slicing procedure, stimulus intensity, electrode placement, etc.). These possibilities were effectively eliminated since FS and RS cells were recorded simultaneously and were located within 200 μm of one another; therefore both cell types should have been affected equally by experimental variables. Because experiments were performed in current rather than voltage clamp, it is possible that there may have been an inhibitory component of the threshold PSPs observed in RS cells that contributed to their smaller amplitude compared with FS cells. However, this appears unlikely for the following reasons: 1) the intensity used was the lowest to achieve a response in the RS cells; 2) we generally did not observe spiking of the FS cells at these lower intensities (data not shown); and 3) when an inhibitory component was present at suprathreshold stimulus intensities, it was generally readily apparent (data not shown, but see de la Rocha et al. 2008 for examples). Finally, differences in PSP properties between FS cells and RS cells observed here accord well with previous in vitro data on these cell types as well as data correlating initial PSP amplitude and Df (Beierlein et al. 2003; Cruikshank et al. 2007; Gabernet et al. 2005).
Simulations.
To assess the functional implications of thalamic synaptic dynamics and the FS-RS cell interactions, we constructed a model that incorporated the experimental data. To simplify analysis, we used a firing rate model (Wilson and Cowan 1972) rather than a spiking neuronal model. Rate models have fewer variables, which facilitates the interpretation of changes in model parameters on network behavior. The output of rate models may be viewed as the pooled response of a population of spiking neurons (Pinto et al. 1996) and is not intended to reproduce exactly the fine time scales of the spiking behavior of neurons. Another simplification we employed was to implement thalamic activity in response to AM stimuli as a smooth, rectified sinusoid that mirrored the stimulus (Eq. 4); thalamic activity in vivo shows more complex temporal profiles as well as diverse response tuning (Bartlett and Wang 2007; Creutzfeldt et al. 1980; Miller et al. 2002; Preuss and Muller-Preuss 1990). Nonetheless, the model provides insight into the qualitative effects that the modeled synaptic and intrinsic properties may have on cortical responses.
The two-population model lacked recurrent cortical connections and associated synaptic dynamics largely because of the absence of sufficient data generated in these experiments to constrain these parameters. However, the effects that inhibition, thalamic synaptic depression, and the differences in E and I population parameters had on the model behavior are expected to extend to any system (including spiking models or real neurons) in which the general relationships among the elements remain similar (de la Rocha et al. 2008; Pinto et al. 1996). Indeed, simulations that used a spiking neuronal model with synaptic conductances or that included recurrent E-to-E, E-to-I, and I-to-I connections and the associated synaptic dynamics produced qualitatively similar results for a relatively large parameter space provided that overall network activity generated sufficiently strong FFI to modify E cell responses (data not shown). One potential mechanism of reducing FFI that was not included in the model is powerful mutual inhibition between I cells. However, previous in vitro experiments in auditory cortex indicate that thalamorecipient FS cells are less affected by FFI than RS cells (de la Rocha et al. 2008), indicating that mutual inhibition has relatively modest effects on these cells. Furthermore, recurrent excitation between RS cells is weak (Oswald and Reyes 2008), and so recurrent E connections are unlikely to overwhelm the inhibitory drive. Nonetheless, we cannot exclude the possibility that additional excitatory or inhibitory drive arising from complex cortical circuit interactions or from outside AI may alter the balance of inhibition and excitation in thalamorecipient cells.
Similar results were also obtained with simulations using a large recurrent network with multiple cortical populations (data not shown; see de la Rocha et al. 2008 for methods). In these simulations, E and I populations had different characteristic frequencies arranged tonotopically and stimuli were delivered to a confined central region of the network. This model was used to examine the effects of FFI and STD on the spatial spread of excitation and inhibition about the central region. The essential effects on tuning of tonotopic responses are largely similar to those observed by de la Rocha et al. (2008). In particular, increasing the strength of inhibition or decreasing excitation provided by thalamic input via STD sharpened receptive field tuning. On the other hand, decreasing inhibition via disinhibition broadened the modeled receptive field over time.
In previous experiments using a similar model, we explored the effects of FFI on cortical processing of static stimuli (de la Rocha et al. 2008). Here we show that an additional effect of FFI is to alter the response properties of the E population to time-varying stimuli. When time-varying stimuli of different MFs were delivered to the network with strong FFI, the E population response became high pass for F0 amplitude and band pass for F1 amplitude as a result of selective suppression of responses at low MFs (Fig. 6). This change in tuning resulted from cortical mechanisms alone, specifically delayed inhibition. The model therefore provides a simple cellular mechanism as the basis of receptive field differences between thalamus and cortex. Synaptic depression tempered the effects of FFI: Depression of thalamic input acts to disinhibit E population firing by reducing the synaptic currents that drive FFI (Fig. 8). The effect of disinhibition was qualitatively similar for a range of jEI: The degree of disinhibition was correlated with the strength of jEI and the attendant suppression of E population responses by FFI (data not shown). Disinhibition can cause the E cell population activity to increase over the course of the stimulus (Fig. 8B), suggesting that the enhancement of cortical responses observed in vivo may be due to disinhibition rather than facilitation of thalamic inputs (Fig. 8, A and B; Bartlett and Wang 2005; Brosch and Schreiner 2000; Wehr and Zador 2005).
In the model, disinhibition produces a relatively modest absolute effect on response amplitude in the E population (Fig. 8). However, the firing rates of AI cells in vivo in the anesthetized animal are often quite low (DeWeese et al. 2003), and therefore small-magnitude changes in firing rate can produce relatively large effects on response tuning. As shown in Fig. 8, C and D, the modest absolute changes in E population responses caused by disinhibition produce a rather dramatic change in tuning of E responses from band pass to more low pass when the responses are normalized to their peak amplitude. One reason that we chose to normalize the tuning curves was precisely to highlight the relative changes in tuning under the different simulated conditions.
The synaptic depression measured in vitro strongly suppressed thalamic input and in our model resulted in disinhibition of cortical responses. However, synaptic depression observed in vivo may differ from that observed in vitro for a variety of reasons including effects of age, spontaneous activity, and presence of neuromodulators (Castro-Alamancos and Connors 1997). If the degree of depression is mild, depression will have a less profound effect on cell activity and disinhibition will be less prominent (data not shown).
Comparison to in vivo studies in auditory cortex.
In general, the in vitro and modeling results are in accord with those obtained in vivo with acoustic stimuli. The large PSPs evoked in FS cells (Fig. 2, Table 2) may provide the substrate for powerful FFI in vivo (Wehr and Zador 2003; Zhang et al. 2003). Moreover, the time constant of recovery from depression of PSPs that we observe in vitro is similar to that documented in vivo (Asari and Zador 2009; Wehr and Zador 2005). In addition, a substantial fraction of cells recorded in vivo respond to the second click of a pair with greater suppression of inhibitory than excitatory conductances as predicted by our model (Fig. 8; Wehr and Zador 2005). Finally, several in vivo studies have shown that auditory cortical neurons respond to paired stimuli with long-lasting response enhancement that develops over time (Bartlett and Wang 2005; Brosch et al. 1999; Brosch and Schreiner 2000), similar to the effects of disinhibition predicted by the model (Fig. 8).
The stimuli used in our simulations are analogous to sAM tones and were chosen for several reasons. In most sensory systems, natural stimuli are often time varying rather than transient or constant. Moreover, in the auditory system, amplitude modulation envelopes are key components of biologically relevant natural sounds such as species-specific vocalizations. sAM tones are also expected to fully engage the dynamics imparted to the network by FFI and synaptic depression. Finally, the response properties of auditory cortical neurons to AM stimuli are well characterized.
Temporal coding of repetitive acoustic stimuli is often evaluated with the use of modulation transfer functions (MTFs), which assess the cell responses to repetitive stimuli. We calculated the frequency modulated (F1) and unmodulated (F0) components of E cell responses to compare to temporal and rate modulation transfer functions (tMTFs and rMTFs), respectively, obtained from in vivo recordings (Liang et al. 2002; Miller et al. 2002; Preuss and Muller-Preuss 1990). The model predicts that band-pass tMTFs and high-pass rMTFs, similar to what is observed for many cells in vivo (Liang et al. 2002; Miller et al. 2002), are a product of strong FFI (Fig. 6). Cells that have low-pass tMTF (Eggermont 2002; Liang et al. 2002; Miller et al. 2002) are predicted to result from low levels of FFI (Fig. 6); such limited inhibition could be due to weak inhibitory synaptic strength, low levels of I population activity, spontaneous firing in the thalamic population, or disinhibition produced by thalamic synaptic depression.
The model makes further testable predictions of the mechanisms that underlie the tuning of cortical responses. For cells whose inputs are dominated by FFI, the model predicts that neural responses will be more transient than tonic, be phase advanced for a periodic stimuli, show nonmonotonic rate-level functions (data not shown; see de la Rocha et al. 2008), exhibit band-pass tMTF and high-pass rMTF, respond more robustly to rapidly changing stimuli, and exhibit changes in tuning over repetitions of a stimulus. The model also provides a possible explanation for the increased responsiveness of auditory cortical cells observed in awake versus anesthetized animals. In the awake animal, there is likely to be sufficient spontaneous thalamic activity to produce chronic depression of TC and inhibitory synapses, thus reducing FFI and allowing for more tonic responses to auditory stimuli (Wang et al. 2005).
One of the best-documented TC transformations in the auditory system is the decrease in the high-frequency cutoff of temporal MTFs between thalamus and cortex (Creutzfeldt et al. 1980; Miller et al. 2002). To assess the contribution of cortical mechanisms to the MTF, we explored the effect of thalamic inputs with untuned tMTFs (similar results were found with tuned thalamic tTMF; data not shown). The modeling results provide evidence that FFI and synaptic depression play a major role in determining the high-frequency cutoff of cortical cells' tMTFs. This finding is in line with in vivo studies demonstrating that cortical tMTF is independent of the tuning of their thalamic inputs (Creutzfeldt et al. 1980; Miller et al. 2002).
The significant heterogeneity in MTF tuning of neurons recorded in vivo (Liang et al. 2002; Wang et al. 2008) is postulated to reflect differences in the tuning of thalamic afferents onto these cells. On the other hand, the simulations indicate that such heterogeneity may reflect differences in the levels of inhibition or synaptic depression that drive cortical responses. Both FFI and synaptic depression can be altered by neuromodulation and long-term synaptic plasticity (Gil et al. 1997; Kruglikov and Rudy 2008; Maffei et al. 2004), thereby providing a potential mechanism for adaptive tuning of cortical responses (Kilgard and Merzenich 1998).
Comparison to studies performed in somatosensory cortex.
In general, the in vitro and modeling results reported here agree with similar studies in the somatosensory cortex (Beierlein et al. 2003; Cruikshank et al. 2007; Gabernet et al. 2005; Pinto et al. 1996, 2003). In vitro studies in somatosensory TC slices have demonstrated that FS cells receive larger-amplitude TC inputs that exhibit greater STD compared with RS cells (Beierlein et al. 2003; Cruikshank et al. 2007; Gabernet et al. 2005). In vivo and modeling studies of somatosensory cortex have also demonstrated that FFI can act as a high-pass filter on thalamic inputs (Fig. 7, A and B; Pinto et al. 1996, 2003).
One potentially important difference between the auditory and somatosensory systems is that the amplitudes of TC PSPs onto FS and RS cells reported here are smaller and more variable than those generally reported in somatosensory slices (Beierlein et al. 2003; Cruikshank et al. 2007; Gabernet et al. 2005). These differences may reflect variation in slice connectivity, slice health, or stimulation conditions between studies and/or slice preparations or more significantly reflect genuine divergence in TC processing across the two modalities. The heterogeneous and complex spectrotemporal receptive fields of auditory cortical neurons (Miller et al. 2002) may reflect the diversity of thalamic input strength to both RS and FS cells. Nonetheless, the differences in the biophysical and synaptic properties of RS and FS cells appear to represent a cardinal feature of primary sensory cortices. Consequently, the modeling results describing the relationship of cellular properties to the tuning of response properties to dynamic stimuli are likely to generalize across modalities.
GRANTS
This work was supported in part by National Institute on Deafness and Other Communication Disorders Grants F31 DC-007025 to M. L. Schiff and R01 DC-005787-08 to A. D. Reyes.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.L.S. and A.D.R. conception and design of research; M.L.S. performed experiments; M.L.S. analyzed data; M.L.S. and A.D.R. interpreted results of experiments; M.L.S. and A.D.R. prepared figures; M.L.S. drafted manuscript; M.L.S. and A.D.R. edited and revised manuscript; M.L.S. and A.D.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. de la Rocha, P. Jercog, and E. Goldberg for helpful comments on earlier versions of the manuscript.
REFERENCES
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997 [DOI] [PubMed] [Google Scholar]
- Agmon A, Connors BW. Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. J Neurosci 12: 319–329, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asari H, Zador AM. Long-lasting context dependence constrains neural encoding models in rodent auditory cortex. J Neurophysiol 102: 2638–2656, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Long-lasting modulation by stimulus context in primate auditory cortex. J Neurophysiol 94: 83–104, 2005 [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol 97: 1005–1017, 2007 [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 [DOI] [PubMed] [Google Scholar]
- Brosch M, Schreiner CE. Sequence sensitivity of neurons in cat primary auditory cortex. Cereb Cortex 10: 1155–1167, 2000 [DOI] [PubMed] [Google Scholar]
- Brosch M, Schulz A, Scheich H. Processing of sound sequences in macaque auditory cortex: response enhancement. J Neurophysiol 82: 1542–1559, 1999 [DOI] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 22: 10966–10975, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci 22: 10053–10065, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Thalamocortical synapses. Prog Neurobiol 51: 581–606, 1997 [DOI] [PubMed] [Google Scholar]
- Cetas JS, de Venecia RK, McMullen NT. Thalamocortical afferents of Lorente de No: medial geniculate axons that project to primary auditory cortex have collateral branches to layer I. Brain Res 830: 203–208, 1999 [DOI] [PubMed] [Google Scholar]
- Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. J Neurosci 18: 4785–4799, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 34: 437–446, 2002 [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 99–104, 1990 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Hellweg FC, Schreiner C. Thalamocortical transformation of responses to complex auditory stimuli. Exp Brain Res 39: 87–104, 1980 [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007 [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol 87: 361–384, 2002 [DOI] [PubMed] [Google Scholar]
- Dayan P, Abbott LF. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. Cambridge, MA: MIT Press, 2001 [Google Scholar]
- DeWeese MR, Wehr M, Zador AM. Binary spiking in auditory cortex. J Neurosci 23: 7940–7949, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rocha J, Marchetti C, Schiff M, Reyes AD. Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition. J Neurosci 28: 9151–9163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. Temporal modulation transfer functions in cat primary auditory cortex: separating stimulus effects from neural mechanisms. J Neurophysiol 87: 305–321, 2002 [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48: 315–327, 2005 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19: 679–686, 1997 [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron 23: 385–397, 1999 [DOI] [PubMed] [Google Scholar]
- Hersch SM, White EL. Thalamocortical synapses involving identified neurons in mouse primary somatosensory cortex: a terminal degeneration and golgi/EM study. J Comp Neurol 195: 253–263, 1981 [DOI] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci 29: 9127–9136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Imoto K. Feedforward inhibitory connections from multiple thalamic cells to multiple regular-spiking cells in layer 4 of the somatosensory cortex. J Neurophysiol 96: 1746–1754, 2006 [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci 1: 727–731, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron 58: 911–924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol 100: 317–326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol 87: 2237–2261, 2002 [DOI] [PubMed] [Google Scholar]
- Linden JF, Schreiner CE. Columnar transformations in auditory cortex? A comparison to visual and somatosensory cortices. Cereb Cortex 13: 83–89, 2003 [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci 7: 1353–1359, 2004 [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985 [DOI] [PubMed] [Google Scholar]
- McMullen NT, de Venecia RK. Thalamocortical patches in auditory neocortex. Brain Res 620: 317–322, 1993 [DOI] [PubMed] [Google Scholar]
- Metherate R, Aramakis VB. Intrinsic electrophysiology of neurons in thalamorecipient layers of developing rat auditory cortex. Brain Res Dev Brain Res 115: 131–144, 1999 [DOI] [PubMed] [Google Scholar]
- Miller LM, Escabi MA, Read HL, Schreiner CE. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. J Neurophysiol 87: 516–527, 2002 [DOI] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol 99: 2998–3008, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Development of inhibitory timescales in auditory cortex. Cereb Cortex 2: 1351–1361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DJ, Brumberg JC, Simons DJ, Ermentrout GB. A quantitative population model of whisker barrels: re-examining the Wilson-Cowan equations. J Comput Neurosci 3: 247–264, 1996 [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Hartings JA, Brumberg JC, Simons DJ. Cortical damping: analysis of thalamocortical response transformations in rodent barrel cortex. Cereb Cortex 13: 33–44, 2003 [DOI] [PubMed] [Google Scholar]
- Preuss A, Muller-Preuss P. Processing of amplitude modulated sounds in the medial geniculate body of squirrel monkeys. Exp Brain Res 79: 207–211, 1990 [DOI] [PubMed] [Google Scholar]
- Ren JQ, Aika Y, Heizmann CW, Kosaka T. Quantitative analysis of neurons and glial cells in the rat somatosensory cortex, with special reference to GABAergic neurons and parvalbumin-containing neurons. Exp Brain Res 92: 1–14, 1992 [DOI] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol 94: 2019–2030, 2005 [DOI] [PubMed] [Google Scholar]
- Staiger JF, Zilles K, Freund TF. Distribution of GABAergic elements postsynaptic to ventroposteromedial thalamic projections in layer IV of rat barrel cortex. Eur J Neurosci 8: 2273–2285, 1996 [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex 13: 25–32, 2003 [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Pawelzik K, Markram H. Neural networks with dynamic synapses. Neural Comput 10: 821–835, 1998 [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA 94: 719–723, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbny YI, Erdelyi F, Szabo G, Banks MI. Properties of a population of GABAergic cells in murine auditory cortex weakly excited by thalamic stimulation. J Neurophysiol 96: 3194–3208, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D, Bartlett E. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience 154: 294–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature 435: 341–346, 2005 [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron 47: 437–445, 2005 [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci 8: 1364–1370, 2005 [DOI] [PubMed] [Google Scholar]
- Wilson HR, Cowan JD. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J 12: 1–24, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Miller LM, Lee CC, Schreiner CE. Auditory thalamocortical transformation: structure and function. Trends Neurosci 28: 255–263, 2005 [DOI] [PubMed] [Google Scholar]
- Wu GK, Li P, Tao HW, Zhang LI. Nonmonotonic synaptic excitation and imbalanced inhibition underlying cortical intensity tuning. Neuron 52: 705–715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature 424: 201–205, 2003 [DOI] [PubMed] [Google Scholar]