Abstract
According to traditional models of the basal ganglia-thalamocortical network of connections, dopamine exerts D2-like receptor (D2LR)-mediated effects through actions on striatal neurons that give rise to the “indirect” pathway, secondarily affecting the activity in the internal and external pallidal segments (GPi and GPe, respectively) and the substantia nigra pars reticulata (SNr). However, accumulating evidence from the rodent literature suggests that D2LR activation also directly influences synaptic transmission in these nuclei. To further examine this issue in primates, we combined in vivo electrophysiological recordings and local intracerebral microinjections of drugs with electron microscopic immunocytochemistry to study D2LR-mediated modulation of neuronal activities in GPe, GPi, and SNr of normal and MPTP-treated (parkinsonian) monkeys. D2LR activation with quinpirole increased firing in most GPe neurons, likely due to a reduction of striatopallidal GABAergic inputs. In contrast, local application of quinpirole reduced firing in GPi and SNr, possibly through D2LR-mediated effects on glutamatergic inputs. Injections of the D2LR antagonist sulpiride resulted in effects opposite to those of quinpirole in GPe and GPi. D2 receptor immunoreactivity was most prevalent in putative striatal-like GABAergic terminals and unmyelinated axons in GPe, GPi, and SNr, but a significant proportion of immunoreactive boutons also displayed ultrastructural features of glutamatergic terminals. Postsynaptic labeling was minimal in all nuclei. The D2LR-mediated effects and pattern of distribution of D2 receptor immunoreactivity were maintained in the parkinsonian state. Thus, in addition to their preferential effects on indirect pathway striatal neurons, extrastriatal D2LR activation in GPi and SNr also influences direct pathway elements in the primate basal ganglia under normal and parkinsonian conditions.
Keywords: dopamine, globus pallidus, substantia nigra, parkinsonism
it is generally assumed that dopamine's effects on the basal ganglia circuitry are mediated by its regulation of striatal activity (Albin et al. 1989; Alexander and Crutcher 1990) and that changes in the firing rates and patterns of basal ganglia nuclei in response to lesions of dopaminergic neurons in the substantia nigra pars compacta (SNc) result from abnormalities of striatal outflow (Albin et al. 1989; DeLong 1990). However, the internal and external segments of the globus pallidus (GPi and GPe, respectively) and the substantia nigra pars reticulata (SNr) also receive direct dopaminergic inputs from the SNc. In the pallidum, dopamine is released from axon terminals, which in monkeys originate from a group of SNc neurons that are at least partly different from those giving rise to the nigrostriatal projection (Jan et al. 2000; Smith and Kieval 2000; Smith et al. 1989; Smith and Villalba 2008). In the SNr, dopamine is released from local dendrites of ventral tier SNc neurons (Bjorklund and Lindvall 1975; Cheramy et al. 1981; Cragg and Greenfield 1997; Geffen et al. 1976; Zhou et al. 2009).
Dopamine exerts its actions through D1-like and D2-like receptors (D1LRs, D2LRs). The D2LRs, which are the subject of this study, include D2, D3, and D4 receptors. The actions of these receptors are generally linked with the “indirect” pathways of the basal ganglia, i.e., polysynaptic pathways that connect the striatum to GPi/SNr via GPe, including either direct GPe-GPi/SNr projections or projections via the intercalated subthalamic nucleus (STN). Several studies have, indeed, demonstrated the presence of D2LR binding and D2LR protein expression in the primate GPe (Camps et al. 1989, 1990; Levey et al. 1993; Richfield et al. 1987; Smith and Kieval 2000). Activation of D2LRs was found to reduce GABA release in the rat globus pallidus (GP; homologous to the primate GPe), presumably through presynaptic effects on the striatopallidal projection (Cooper and Stanford 2001; Floran et al. 1997; Querejeta et al. 2001). Functional studies in brain slices suggest that activation of D2LRs may also reduce glutamatergic transmission in the rat GP (Hernandez et al. 2006). Despite changes in striatal D2LR mRNA expression and ligand binding reported in animal models of Parkinson's disease (Bezard et al. 2001), very little is known about functional changes these receptors undergo in the parkinsonian state.
However, despite the relatively low levels of D2LR protein or mRNA in the primate GPi (or entopeduncular nucleus in rodents) and SNr compared with GPe (or rodent GP, e.g., Camps et al. 1989; Gnanalingham et al. 1993; Gurevich and Joyce 1999; Levey et al. 1993), recent in vitro electrophysiological studies have suggested that D2LR activation can directly modulate SNr activity through presynaptic effects on the excitatory subthalamonigral projection in rats (Ibanez-Sandoval et al. 2006), thus supporting extrastriatal D2LR actions independent of the indirect pathway.
These rodent data, therefore, suggest that activation of extrastriatal D2LR may not only influence the GABAergic striatopallidal transmission in GPe but also may directly modulate basal ganglia outflow through regulation of glutamatergic transmission in GPi and SNr. To characterize the functional significance and anatomical substrate of these in vitro observations on the primate basal ganglia circuitry, the present study examined the electrophysiological effects of local activation of extrastriatal D2LRs and the ultrastructural distribution of D2 receptor immunoreactivity in GPe, GPi, and SNr in monkeys. In addition to normal animals, we also examined whether the distribution of D2 receptors and the effects of D2LR agonists persisted in the parkinsonian state, using monkeys that were rendered parkinsonian by injections of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). These studies in parkinsonian animals are important because they may help us to identify extrastriatal sites at which some of the clinically most commonly used antiparkinsonian agents may act.
MATERIALS AND METHODS
Electrophysiological Studies
Animals, surgical procedures, and MPTP treatment.
Two rhesus monkeys (Macaca mulatta, 7–9 kg, monkeys W and X) were used for the electrophysiological experiments. The animals were housed under conditions of protected contact housing, with ad libitum access to food and water. All experiments were performed in accordance with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals” and the United States Public Health Service policy on humane care and use of laboratory animals (Garber et al. 2010). All studies were approved by the Institutional Animal Care and Use Committee and the Chemical Safety Committee of Emory University. Before surgery and recording procedures, the animals were trained to sit in a primate chair and to permit handling by the experimenter.
After completion of the conditioning procedures, three stainless steel chambers for chronic access (Crist Instruments, Hagerstown, MD; inner chamber diameter 16 mm) were stereotactically placed over trephine holes under aseptic conditions and isoflurane anesthesia (1–3%). Two chambers were directed at the pallidum on either side of the brain, using an angle of 40° from the vertical in the coronal plane (A = 12, L = 12, D = 4). A third chamber, aimed at the right SN, was placed 25° posterior to the vertical in the sagittal plane (A = 10, L = 6, D = −3). The chambers were affixed to the skull with dental acrylic. Metal head holders were also embedded into the acrylic cap to permit head stabilization during the recording procedures. The animals were allowed to recover for 1 wk after the surgery. All subsequent recordings were carried out while the animals were awake and seated quietly in a primate chair with their heads restrained, free to move their body and limbs but not engaged in specific behavioral tasks.
After completion of the recording experiments in the normal state, the animals were rendered parkinsonian through administration of MPTP. Monkey W received 26 weekly injections of MPTP (MPTP-HCl; 0.5 mg/kg im, total 13 mg/kg; Sigma-Aldrich, St. Louis, MO), whereas monkey X received 5 weekly injections of the toxin (3 injections of 0.8 mg/kg im, 1 injection of 0.6 mg/kg im, and 1 injection of 0.2 mg/kg im, total 3.2 mg/kg) before they developed stable motor signs of parkinsonism. To assess the degree and stability of the MPTP-induced motor disability, we carried out 15-min observation runs, during which spontaneous arm (left and right) and leg (left and right) movements, trunk rotations (right and left), and head movements (right and left rotations) were counted by an observer who pressed keys on a computer keyboard at the occurrence of such movements. The animal's spontaneous movements were also scored by an automated system of counting beam breaks in a standard monkey cage equipped with infrared beams. In addition, we examined video records of the animals' behavior, which was scored on a nine-item scale (rating bradykinesia, freezing, extremity posture, trunk posture, the presence and severity of tremor, the frequency of arm movements, finger dexterity, home cage activity, and balance, each on a 3-point scale).
Mapping procedure.
As the first step of these experiments, the boundaries of GPi, GPe, and SNr within the recording chambers were delineated using extracellular electrophysiological recording methods with tungsten microelectrodes (FHC, Bowdoinham, ME; Z = 0.5–1.0 MΩ at 1 kHz). All probes used in this study were lowered past the dura into the brain with a microdrive (MO-95B; Narishige, Tokyo, Japan) using a 20-gauge guide tube. The anterior-posterior and mediolateral target coordinates were determined using the microdrive's X-Y stage, and the depth of the tip of the electrode was determined by using a linear potentiometer that was coupled to the Z-axis of the microdrive. The electrical neuronal signal was amplified (DAM-80 amplifier; WPI, Sarasota, FL), filtered (400–10,000 Hz; Krohn-Hite model 3700 filter, Brockton, MA), displayed on a digital oscilloscope (DL1640; Yokogawa, Tokyo, Japan), and made audible using an audio amplifier. During the electrophysiological mapping sessions and the subsequent recording-injection experiments, neurons in GPi, GPe, and SNr were easily identified by their discharge characteristics, which differ substantially from those of surrounding structures.
Recording-injection experiments.
We used a combined recording-injection probe (Kliem and Wichmann 2004) to examine the effects of D2LR-selective ligands on the neuronal activity in GPi, GPe, and SNr. The system consists of a standard polyimide-coated tungsten microelectrode alongside a section of fused silica tubing (inner diameter 40 μm, outer diameter 102 μm; Polymicro Technologies, Phoenix, AZ). Unlike the original device (Kliem and Wichmann 2004), the device used in the present experiments did not have a protective polyimide sleeve. The device design was changed to minimize the probe's diameter and reduce the tissue damage produced by penetrations. A 10-mm section of 23-gauge stainless steel tube was placed over the proximal end of the fused silica and glued in place with epoxy, to provide a leak-free connection via Tygon tubing (inner diameter 0.020 in.; Saint-Gobain, Akron, OH) to a 1-ml gastight syringe (CMA). During the experiments, the syringe was driven by a remotely controlled infusion pump (CMA/102) for pressure infusion of submicroliter quantities of drug or vehicle solutions.
Artificial cerebrospinal fluid [aCSF, consisting of (in mM) 143 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, and 1 Na2HPO4, pH 7.2–7.4] was used for drug solutions and for control experiments. The selective D2LR agonist (4aR-trans)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g] [LY 17555 or (−)-quinpirole hydrochloride; 5 μg/μl; Tocris Bioscience, Ellisville, MO] was dissolved in aCSF and the pH adjusted to 7.3 ± 0.1. Quinpirole binds with significantly higher affinity to D2LRs than to D1LRs, serotonin, or α2-adrenergic receptors (Millan et al. 2002; Seeman and Van Tol 1994) and has a fivefold higher affinity for D2 receptors than for D3 or D4 receptors (Seeman and Van Tol 1994). The selective D2LR antagonist (S)-(−)-5-aminosulfonyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide [(S)-(−)-sulpiride; Tocris Bioscience] was dissolved in hydrochloric acid (0.1 N). The sulpiride solution was subsequently diluted in aCSF to the final concentration (10 μg/μl), and the pH was adjusted to 7.3 ± 0.1 with sodium hydroxide. Sulpiride is highly specific for D2LRs (Seeman and Van Tol 1994; Shin et al. 2003; Wang and Pickel 2002). This agent has very low affinity for D4 receptors, even at high concentrations (Seeman and Van Tol 1994; Shin et al. 2003; Wang and Pickel 2002). All solutions were filtered before infusion (pore size 0.2 μm; Fisher Scientific, Hampton, NH).
Before the start of the experiments, the syringes were filled with aCSF or drug solution in control or drug effect experiments, respectively. To test the integrity of the recording-injection system, the system was flushed (at 20 μl/min) for 30 s. The pump was then switched off, and the probe was inserted into the brain. Once a neuron was isolated with sufficient recording quality and the recording was stable for at least 120 s, we began the data collection with a recording of the neuron's baseline activity for at least 60 s, followed by recording during the infusion [0.15 μl/min for 200 s (0.5 μl)] and for at least 240 s thereafter.
The amplified neuronal signal was collected to computer disk using a data acquisition interface (Power1401 with Spike2 software; CED, Cambridge, UK) for off-line analysis. Each animal underwent several recording-injection experiments (in GPe, GPi, and SNr; in the normal state and post-MPTP states). Individual experiments were separated by at least 24 h. In most experiments, single injections were carried out, although in a few instances a second injection was done, spatially separated from the previous injection by at least 1 mm and temporally by at least 45 min. In all cases, the injections were done at least 500 μm away from the border of the structure under study (GPe, GPi, or SNr) to make sure that the direct drug effects were local. In our experience, drug effects with the injection volume used in this study rarely extend beyond a 200- to 300-μm distance from the injection site (see also Kita et al. 2004) and generally last for <10 min. At the end of each experimental session, the probe was removed from the brain and the integrity of the recording-injection system tested by flushing the system.
Termination of the experiment.
After completion of the recording experiments, the animals were killed with an overdose of pentobarbital sodium (100 mg/kg iv) and then transcardially perfused with oxygenated Ringer solution followed by 2 liters of fixative [4% paraformaldehyde, 0.1% glutaraldehyde in phosphate buffer (PB); 0.1 M, pH 7.2]. The brains were then removed from the skull and cut into 10-mm-thick blocks containing GPe, GPi, or SNr. The tissue blocks were further cut into 60-μm-thick sections with a freezing microtome and stained with cresyl violet to verify electrode locations. Other sections were immunostained for tyrosine hydroxylase (TH), mounted on slides, and analyzed at the light microscopic level to confirm the loss of dopaminergic neurons in the SNc. Unbiased stereological cell counts of SNc neurons were used to quantify the loss of midbrain dopamine neurons in these animals. The unbiased stereological estimation of the number of dopamine neurons was achieved using the optical fractionator principle (StereoInvestigator; MicroBrightField, Williston, VT), a stereological approach that combines the optical dissector with a fractionator sampling scheme. The random systematic sampling of counting areas was performed using the Leica DMR microscope. We first took low-power micrographs (×1.25) of TH-immunostained ventral midbrain sections and manually delineated the borders of the SNc (Masilamoni et al. 2010). Counts of TH-positive cells were generated using a ×100 oil-immersion objective. To perform unbiased stereology, counting frames (65 × 65 μm) were randomly placed by the stereology software within the chosen region of interest. The software also controlled the position of the X-Y stage of the microscope so that the entire brain region could be scanned by successively meandering between counting frames. On average, 12 sections were analyzed and ∼300 cells counted per animal. The software calculated the estimated total number of cells in each region of interest per hemisphere.
Data analysis.
We only included recordings from cells in both monkeys that were confirmed to be in the target structures (GPi, GPe, and SNr) based on the probe coordinates during recording sessions, by the firing properties of recorded neurons, and through postmortem histological analyses. A template-matching spike-sorting routine with subsequent principal component analysis (Spike2) was used to identify spikes and measure interspike intervals (ISIs) during off-line analysis. For each neuron, ISI distribution histograms and autocorrelogram were carefully examined for evidence of possible erroneous sampling of multiple cells or inclusion of noise in the data. In such cases, the records were re-sorted or discarded.
All subsequent steps of the analysis were done with custom-written algorithms in the Matlab software environment (The MathWorks, Natick, MA). The same analysis methods have been used in our previous studies (for instance, Kliem et al. 2007, 2010; Soares et al. 2004). ISIs were binned in 1-s intervals to generate second-by-second readouts of the discharge rates and subsequently smoothed using a sliding 21-point moving average. The median of discharge rates in the 60-s segment of data before the delivery of drugs was defined as the neuron's baseline activity. An injection was considered effective if the frequency of the recorded neuron was outside of a 10th and 90th percentile window of baseline discharge rates for at least 60 s, with an effect onset within 240 s from the beginning of the drug injection. The “effect epoch” was defined as a 60-s time period that contained 30 s of data before and 30 s after the maximum-effect point. This data segment was used for analyzing the effects of drug injection. In most cases, drug effects lasted much longer than 60 s, usually until the end of the recording. For neurons in which the injection had no effect on the firing rate, a 60-s data segment starting 140 s after the beginning of the injection was used for the analysis. Although “drug effects” can also be defined by other measures of neuronal activity (for instance, coefficients of variation), the use of these measures in previous studies from our laboratory (Galvan et al. 2010, 2011) did not provide evidence that this approach is superior to the more straightforward measurement of firing rate changes used in the present study.
For individual experiments, the median discharge rate of the effect epoch was statistically compared with the median discharge rate of the baseline period. In each case, the median discharge rate during the effect epoch was also expressed as a percentage of the baseline discharge rate. Medians, as well as 25th and 75th percentiles of these percentages, are reported in the results. In addition, burst indexes were calculated for the baseline and effect periods. For this, bursts were detected using the Poisson “surprise” method developed by Legendy and Salcman (1985). A surprise value of 3 was used (Aldridge and Gilman 1991; Wichmann and Soares 2006), and burst indexes were calculated as the ratio between the number of spikes in bursts and the total number of spikes in the record. In addition, changes in oscillatory firing characteristics of the target structures were assessed with power spectral methods, as described in our previous publication (Soares et al. 2004). The algorithm was written in Matlab (Welch's method, mean detrending). For each neuron, the raw spectra were integrated in ranges of 1–3, 3–8, 8–13, 13–30, and 30–100 Hz and normalized to the total power in the spectrum to study changes in the sub-beta-, beta-, and gamma-band ranges. Previous studies in parkinsonian patients and monkeys have suggested that dopamine loss favors the generation of beta-band oscillations and that (systemic) restoration of dopaminergic transmission results in increased gamma-band power in neural signals (Brown et al. 2001; see also review by Gatev et al. 2006).
For within-subject comparisons of the frequency values in the baseline period with those in the drug effect epoch, nonparametric paired comparison statistics (Wilcoxon signed-rank test) were used.
Anatomical Studies
Animals.
Brain tissue from six adult rhesus monkeys (3 normal, 3 parkinsonian animals), different from those used in the electrophysiological experiments, was used to study the distribution of D2 receptor expression in GPe, GPi, and SNr. Three animals were rendered parkinsonian with injections of MPTP. One of the animals received systemic injections of MPTP (0.3–0.8 mg/kg im per week), whereas the two other monkeys received intracarotid injections of MPTP (0.5 mg/kg) followed by a series of intramuscular injections (0.2–0.3 mg/kg per week) until they reached a parkinsonian state that remained stable for a minimum of 6 wk before euthanasia. The total amount of MPTP injected per animal ranged between 12 and 36.5 mg/kg. Parkinsonian motor signs were documented through observations as mentioned above. In the two animals that received intracarotid MPTP infusions, we used the data from the computer-assisted behavioral scoring system to quantify hemiparkinsonism, forming ratios of the movement scores from the contralateral and ipsilateral to the injection. Only the tissue from the ipsilateral side of the intracarotid MPTP injection was used for electron microscopic (EM) analysis.
Tissue preparation and histology.
Animals were deeply anesthetized with an overdose of pentobarbital sodium (100 mg/kg iv) and perfused with a mixture of paraformaldehyde (4%) and glutaraldehyde (0.1%). Tissue blocks were cut in 60-μm-thick sections with a vibrating microtome and processed for light microscopy (LM) or EM immunohistochemistry to visualize D2 receptor immunoreactivity (see below). Before immunohistochemical processing, sections prepared for EM were rinsed in phosphate-buffered saline (PBS; 0.01 M, pH 7.4), incubated in 1% sodium borohydride solution in PBS (20 min), rinsed in PBS, treated with a cryoprotectant solution (EM only), frozen at −80°C, thawed, and rinsed again in PBS.
Primary antisera.
Commercially available polyclonal antibodies to the D2 receptor (AB5084P; Millipore, Temecula, CA) were used at a 1:1,000 concentration of the stock 1 mg/ml solution. The antibody was raised against a 28-amino acid peptide sequence of the third intracellular loop of the human D2 receptor, which is shared by both the long and short forms of the receptor. No significant homology to other dopamine receptors (D1, D3–D5) has been reported for this peptide. Western blots using human brain tissue identified a specific band at ∼50 kDa, and the peptide has been previously characterized in neuroblastoma cells and with ultrastructural studies in rodent tissue (Lei et al. 2004; Macey et al. 2004; Mengual and Pickel 2002). Omission of the primary antiserum resulted in a loss of immunoreactivity in the monkey tissue used in the present study.
Immunoperoxidase procedure.
After sodium borohydride treatment, sections were placed in cryoprotectant solution for 20 min (0.05 M PB, pH 7.4, 25% sucrose, 10% glycerol), frozen at −80°C for 20 min, returned to a decreasing gradient of cryoprotectant solutions, and rinsed in PBS. Sections were then preincubated for 1 h at room temperature in PBS containing 10% normal goat serum and 1% bovine serum albumin, followed by incubation in the primary antibody solution containing 1% normal goat serum, 1% bovine serum albumin, and the D2 receptor antibodies (1:1,000; Millipore) for 48 h at 4°C. This was followed by three rinses in PBS and then incubation in secondary biotinylated goat anti-rabbit IgGs (1:200; Vector Laboratories, Burlingame, CA) for 90 min. The sections were then rinsed again in PBS and incubated for 90 min with the avidin-biotin peroxidase complex at a dilution of 1:100 (Vector Laboratories). Sections were then washed in PBS and Tris buffer (50 mM, pH 7.6) and transferred to a solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich), 1 mM imidazole (Fisher Scientific, Norcross, GA), and 0.005% hydrogen peroxide in Tris buffer for 10 min. Sections were then rinsed in PB (0.1 M, pH 7.4) and treated with 1% OsO4 for 20 min. The tissue was then returned to PB and dehydrated with increasing concentrations of ethanol. With exposure to 70% ethanol, 1% uranyl acetate was added to the solution and the tissue was incubated for 35 min to increase the contrast of the tissue in the EM studies. After dehydration, sections were treated with propylene oxide and embedded in epoxy resin for 12 h (Durcupan ACM; Fluka, Buchs, Switzerland), mounted onto slides, and placed in a 60°C oven for 48 h. Samples of GPe, GPi, and SNr were mounted onto resin blocks with cyanoacrylate ester and cut into 60-nm-thick ultrathin sections with an ultramicrotome (Leica Ultracut T2; Nussloch, Germany). These sections were collected on Pioloform-coated copper grids, stained with lead citrate for 5 min to enhance tissue contrast, and examined with a Zeiss electron microscope. Electron micrographs were taken and saved with a charge-coupled device camera (DualView 300W; Gatan, Pleasanton, CA) controlled by DigitalMicrograph software (version 3.10.1; Gatan).
Data analysis.
Data were collected from 17 blocks of tissue, i.e., 1 block/animal in each brain region (GPe, GPi, SNr) from 3 control and 3 MPTP-treated parkinsonian monkeys. Because of poor ultrastructural preservation, the SNr tissue from only two of the three MPTP-treated animals could be used. In each animal, ultrathin sections from the surface of tissue blocks were scanned, and ∼100 electron micrographs of every randomly encountered immunoreactive element from each of the brain regions were digitized at ×25,000. The total analyzed tissue surface was 15,939 μm2 for GPe in control animals and 15,745 μm2 in MPTP-treated animals; 14,007 μm2 for GPi in control animals and 14,103 μm2 in MPTP-treated animals; and 15,939 μm2 for SNr in control animals and 8,887 μm2 in MPTP-treated animals. The labeled elements were categorized as dendrites, spines, axons, axon terminals, or glia on the basis of ultrastructural features described by Peters et al. (1991).
To avoid misinterpretation of false negative neural elements due to limited access of antibodies to their antigenic sites, the analysis of labeling in this study was solely focused on immunoreactive elements. To assess the relative distribution of D2 receptor labeling across neuronal elements, the relative percentages of labeled elements in each category was calculated by dividing the number of specific labeled elements by the total number of all labeled elements examined in one monkey. The average percentages of each category of immunoreactive structures were then calculated for the normal and parkinsonian monkeys. The relative percentages of labeled terminals forming either asymmetric or symmetric synapses were calculated by dividing the number of terminals forming a specific type of synapse by the total number of labeled terminals in each animal. Terminals that did not form clear synapses in the examined plane of sections were discarded from this analysis but were included to calculate the overall proportion of specific D2-immunoreactive elements.
The data are expressed as means ± SD. Some of the digitally acquired electron micrographs were adjusted for brightness or contrast (DigitalMicrograph or Adobe Photoshop 8.0).
RESULTS
MPTP Treatment Effects
The MPTP-treated animals used in the electrophysiological studies showed stable parkinsonian motor signs during the time of the recordings. Monkey W reached a parkinsonism rating score of 13 on our 27-point behavioral observation scale (see materials and methods), whereas monkey X reached a score of 10. No significant recovery occurred over the course of the post-MPTP state. The animals used for the anatomical experiments were treated with either systemic MPTP infusions or intracarotid MPTP. The animal treated with systemic injections reached a score of 12 on the parkinsonian rating scale. The degree of parkinsonism in the animals treated with intracarotid MPTP infusions was assessed with observations of arm and leg movements, which were subsequently used to calculate ratios of the amount of movement contralateral to the treated and untreated sides. Results from these animals documented significant reductions of movements in the arm and leg contralateral to the side of the intracarotid MPTP administration. In both animals, the ratio of limb movements on the affected/nonaffected side ranged from 0.8–0.95 in the normal state to 0.1–0.28 in the stable parkinsonian condition.
Postmortem TH immunohistochemistry studies in the animals used for the anatomical and electrophysiological studies showed an almost complete loss of TH immunoreactivity in the dorsal striatum and significant depletion in the number of TH-immunoreactive neurons in the SNc. Stereological counts of TH-positive neurons in the two monkeys used in electrophysiological experiments revealed that monkey W had 31,522 cells in the SNc and 17,050 dopaminergic cells in the ventral tegmental area (VTA), whereas monkey X had 49,861 cells in the SNc and 34,387 neurons in the VTA. In comparison, an untreated (nonparkinsonian) animal had 201,605 neurons in the SNc and 57,580 neurons in the VTA. Thus these results demonstrate that the MPTP induced between 75 and 85% loss of neurons in the SNc and 40–70% dopaminergic cell loss in the VTA. In these animals, the average firing rates of GPi neurons changed from 41.7 ± 20.2 spikes/s (n = 20) to 66.6 ± 44.7 spikes/s (n = 9). In GPe and SNr neurons, firing rates remained unchanged (GPe: normal, 55.6 ± 19.2 spikes/s, n = 21; MPTP, 63.5 ± 26.8 spikes/s, n = 8; SNr: normal, 42.4 ± 19.7 spikes/s, n = 18; MPTP, 42.7 ± 25.3 spikes/s, n = 8). None of these changes was significant. Although stereological cell counts of midbrain dopaminergic neurons were not performed in the three monkeys used for anatomical studies, the extent of TH labeling in the SNc of these animals was reduced to a level comparable to those used in the electrophysiological studies (data not shown).
Effects of D2LR Activation or Blockade
We examined the effects of local microinjection of the selective D2LR agonist quinpirole on the activity of 9 GPe, 10 GPi, and 9 SNr neurons in the normal state, whereas 8 GPe, 9 GPi, and 8 SNr neurons were examined in the post-MPTP state. We also examined the effects of the D2LR antagonist sulpiride on the activity of 12 GPe, 10 GPi, and 9 SNr neurons in the normal state (the effects of this agent were not assessed in the dopamine-depleted state). Control perfusions of aCSF revealed no significant effects on the rate and pattern of activity of 3 GPe, 1 GPi, and 2 SNr neurons (data not shown). Note that the number of aCSF infusions was kept to a minimum to limit the amount of tissue damage in the animals. We have done similar aCSF infusions in several other studies using the same recording-injection methods and also have not found significant effects (Galvan et al., 2005, 2010; Kliem et al. 2007, 2010). We systematically covered the entire extent of the structures to be recorded (GPe, GPi, and SNr) in a 0.5 × 0.5-mm grid. Therefore, the cells presented in this study come from putative motor and nonmotor territories of these basal ganglia structures. None of the microinjections used in this study had observable behavioral effects. However, it should be noted that the reported studies were not designed to assess behavioral changes, because the volumes of drug injections were very small and covered only a regionally specific sector of each structure (see also above).
Effect of D2LR activation or blockade on discharge rates.
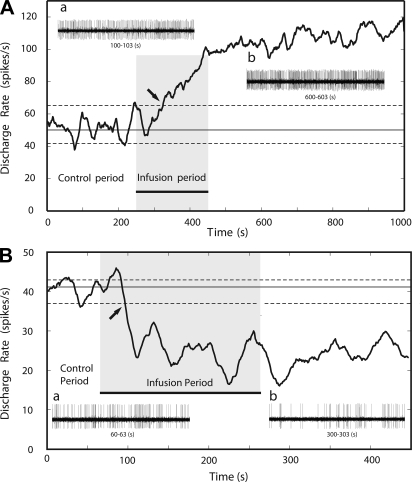
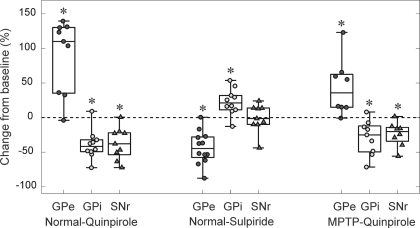
The example in Fig. 1 illustrates the spiking response of a GPe neuron, recorded in the normal state, to the local infusion of the D2LR agonist quinpirole. Figure 1 shows drug-induced changes in firing rate. Before infusion of quinpirole, the cell discharged at a median rate of ∼50 spikes/s, indicated by the solid horizontal line, with dashed lines indicating the 10th and 90th percentile values of the preinjection firing rate estimates. Infusion of quinpirole doubled the cell's firing rate. As shown on the left side of Fig. 2, similar changes in firing rates were seen in most GPe neurons. Figure 2 also demonstrates that most of the recorded neurons in GPi or SNr responded to quinpirole infusion with a significant decrease in their neuronal discharge rates.
Fig. 1.
Effect of D2-like receptor (D2LR) activation on pallidal discharge rates. Examples are shown of the effect of quinpirole on the neuronal discharge rate of a neuron from the internal (GPi; A) and external segment of the globus pallidus (GPe; B). Approximately 0.5 μl of quinpirole was infused at the rate of 0.15 μl/min for 200 s during the infusion period (gray shading). The solid horizontal line represents the median discharge rate during the preinjection baseline period; dashed lines indicate 10th and 90th percentiles. Infusions were considered effective if the postinfusion discharge rate was outside of the 10th and 90th percentiles (the start of this period is marked by the arrow) for a minimum duration of 60 s. Insets a and b show 3 s of neuronal discharge during the pre- and postinjection periods, respectively.
Fig. 2.
Effects of the infusion of D2LR ligands on the discharge rates of GPe, GPi, and substantia nigra pars reticulata (SNr) neurons in the normal and post-MPTP states. For each cell in an experimental group, the median discharge rate during the effect epoch was compared with the baseline rate. Changes in the discharge rate are expressed as a percentage change from baseline. Each box plot illustrates the median and the 25th and 75th percentiles (box) and minima and maxima (error bars). *P < 0.05 (Wilcoxon signed-rank test).
The firing rate responses of GPe and GPi neurons to the infusion of the D2LR antagonist sulpiride were opposite to the responses to D2LR activation. The box plots in the middle of Fig. 2 show that most GPe neurons responded with a significant decrease to sulpiride infusions, whereas most GPi neurons showed a significant increase. The infusion of sulpiride into the SNr had no significant effect on the firing rate.
In the parkinsonian state, we examined only the effects of the D2LR agonist quinpirole and found that the principal effects of this drug on discharge rates were maintained. As documented on the right side of Fig. 2, neurons in GPe responded to local quinpirole infusions with an increase in discharge, whereas neurons in GPi and SNr responded with a reduction in discharge, similar to those in the normal state.
Effect of D2LR activation or blockade on oscillatory and burst firing properties.
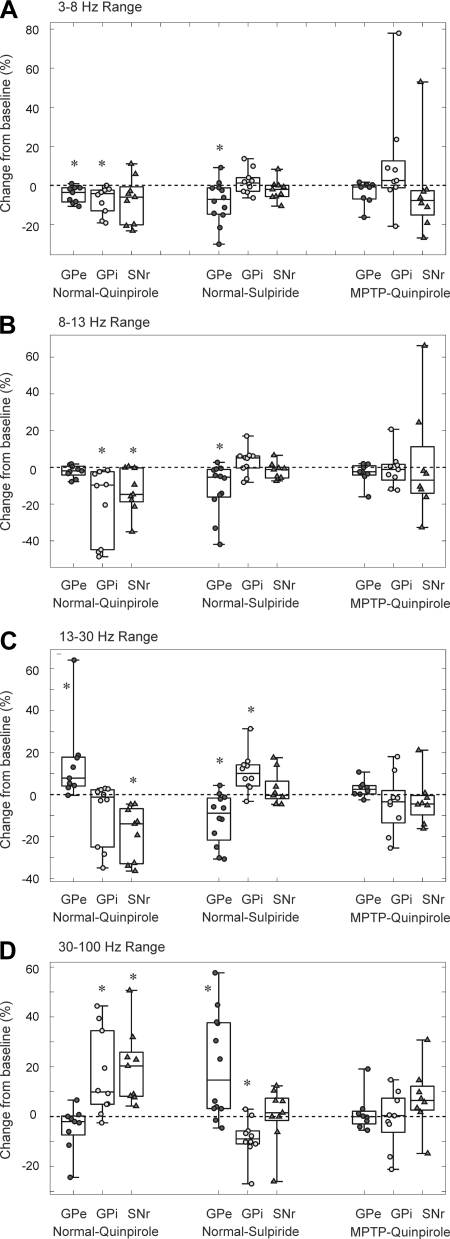
The spike sequences used to generate firing rate readouts were also used to describe features of the neuronal firing pattern, including power spectra and burst firing. Similar to the analysis of firing rates, the analysis of power spectral properties of neuronal firing in GPe, GPi, and SNr also revealed a dichotomy between the drug infusion responses of GPe vs. GPi and SNr neurons. The results of this analysis are summarized in Fig. 3. In GPe, quinpirole infusion resulted in significant decreases in the proportional spectral power in the ranges of 1–3 and 3–8 Hz and an increase in the range of 13–30 Hz. No changes were seen in the other frequency bands. In GPi, the drug reduced power in the ranges of 3–8 and 8–13 Hz and increased it in the range of 30–100 Hz. In the SNr, quinpirole reduced the spectral power in the ranges of 8–13 and 13–30 Hz and increased it in the range of 30–100 Hz. Sulpiride infusions into GPe reduced spectral power in the ranges of 3–8, 8–13, and 13–30 Hz and increased it in the range 30–100 Hz. In GPi, the drug increased the spectral power in the band range of 13–30 Hz and reduced it in the frequency range of 30–100 Hz. No significant changes were observed in the power spectral distribution in the SNr after sulpiride infusions. In the MPTP-treated state, no changes were observed in the power spectral distribution of pallidal and SNr neurons after quinpirole infusions.
Fig. 3.
Effects of the infusion of D2LR ligands on the discharge pattern of GPe, GPi, and SNr neurons in normal and post-MPTP states. Changes in integrated power spectra in the range of 3–8 (A), 8–13 (B), 13–30 (C), and 30–100 Hz (D) are expressed as a percentage change of median value during effect epoch from baseline for each cell in an experimental group. For each experimental group, individual data points are depicted along with box plots. Each box plot illustrates the median and the 25th and 75th percentiles (box) and minima and maxima (error bars). *P < 0.05 (Wilcoxon signed-rank test).
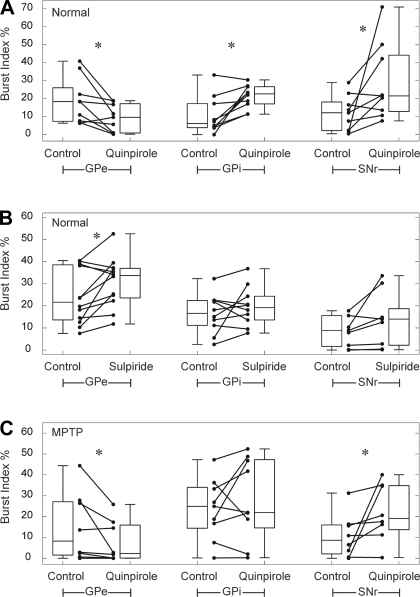
We also examined the effects of quinpirole and sulpiride on indexes of burst firing, as detected by analyzing the spike time series with the Poisson surprise method (Fig. 4). Local administration of quinpirole in the normal state significantly reduced burst firing in GPe, whereas a significant increase in bursting occurred in GPi and SNr (Fig. 4A). Sulpiride infusions (Fig. 4B) had the opposite effect on GPe activity (significantly increased bursting) but had mixed effects on GPi and SNr activity, which were not significant. In the MPTP-treated state, the effects of quinpirole infusions were maintained in GPe and SNr (Fig. 4C), whereas the effect on GPi cells was not significant.
Fig. 4.
Effects of the infusion of D2LR ligands on burst indexes of GPe, GPi, and SNr neurons in normal (A and B) and post-MPTP states (C). For each experimental group, burst indexes of individual neurons (circles) during baseline period (control) and the effect epoch (quinpirole or sulpiride) are shown. The box plots represent the median and the 25th and 75th percentiles (box) and the minima and maxima of the burst index distribution (error bars). *P < 0.05 (Wilcoxon signed-rank test).
D2 Receptor Immunohistochemistry
At the light microscopic level, the overall pattern of D2 receptor immunoreactivity in the GPe, GPi, and SNr of normal monkeys was similar to that previously described in primates and nonprimate species using receptor binding or immunocytochemical approaches, i.e., the GPe neuropil displayed strong diffuse immunoreactivity interspersed with rare lightly labeled cell bodies. The GPi showed a more lightly labeled neuropil without perikaryal staining. In the SNr, dendrites and cell bodies of strongly labeled SNc dopaminergic neurons were seen (see Smith and Kieval 2000; Smith and Villalba 2008). There was no apparent difference in this pattern of labeling between normal and MPTP-treated monkeys in GPe and GPi, except that the intrusion of the SNr by D2-containing SNc neurons was not as prominent in the parkinsonian state because of the severe MPTP-induced dopaminergic cell loss in these animals (data not shown).
GPe.
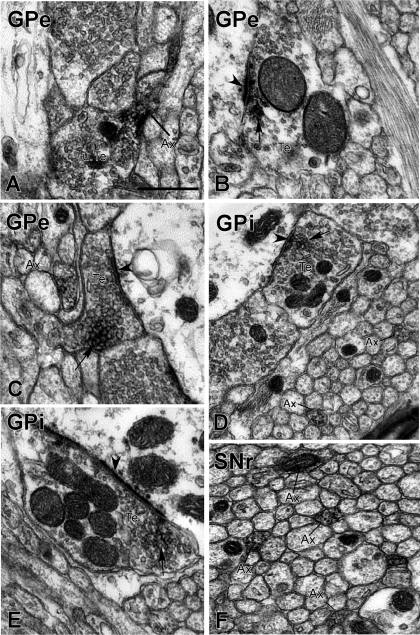
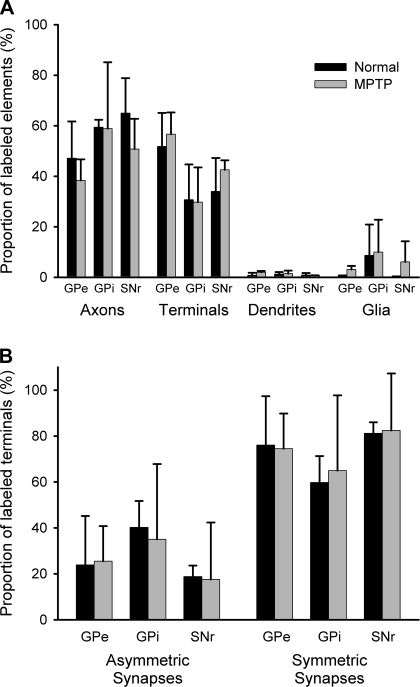
At the EM level, the D2 receptor labeling was almost exclusively presynaptic with more than half of the labeling seen in axon terminals in control (51.8 ± 13.3%; n = 3, mean ± SD) and MPTP-treated monkeys (56.6 ± 8.6%, n = 3). Most immunoreactive terminals whose synaptic junctions could be identified (i.e., ∼60% of all labeled terminals) formed symmetric synapses and displayed the ultrastructural features of putative GABAergic boutons from the striatum (Smith et al. 1998) in normal and dopamine-depleted conditions (normal animals: 76.1 ± 21.3%; MPTP-treated animals: 74.5 ± 15.3% of total D2-labeled terminals with clear synaptic junctions). A significantly smaller proportion of labeled terminals formed asymmetric synapses in normal (23.9 ± 21.3%) and MPTP-treated animals (25.5 ± 15.3%). Unmyelinated axons were the next largest proportion of labeled elements, accounting for 47.1 ± 14.6% of immunoreactive structures in normal monkeys and 38.4 ± 8.3% of labeled structures in MPTP-treated monkeys. Postsynaptic labeling represented a much smaller proportion (<5%) of labeled structures in normal and MPTP-treated monkeys. The proportion of immunoreactive glial processes was small (0.4 ± 0.3% in normal animals and 3.0 ± 1.4% in MPTP-treated animals; see also Figs. 5 and 6).
Fig. 5.
Electron micrographs showing examples of D2 receptor-immunoreactive elements in GPe (A–C), GPi (D and E), and SNr (F) of normal and MPTP-treated monkeys. A: a D2 receptor-immunoreactive preterminal unmyelinated axonal segment (Ax) attached to an unlabeled axon terminal (u.Te) in GPe. B: a D2 receptor-immunoreactive axon terminal (Te) forming an asymmetric axodendritic synapse in GPe. C: a D2 receptor-immunoreactive terminal forming a symmetric synapse with an unlabeled dendrite in GPe. An immunoreactive unmyelinated axon is visible in the neuropil. D: a D2 receptor-immunoreactive terminal forming a symmetric synapse and numerous positive unmyelinated axons in GPi. E: a D2 receptor-containing terminal forming an asymmetric axodendritic synapse in GPi. F: numerous D2 receptor-immunoreactive unmyelinated axons in SNr. In all panels, arrows indicate the peroxidase deposit in labeled terminals and arrowheads point at symmetric or asymmetric synapses. Scale bar in A, 0.5 μm (valid for all panels).
Fig. 6.
A: histograms comparing the relative proportions of D2 receptor-immunoreactive elements in GPe, GPi, and SNr between normal and MPTP-treated monkeys. The total number of D2 receptor-immunopositive elements examined in controls and MPTP-treated animals was 953 and 858 in GPe, 677 and 657 in GPi, and 1,051 and 564 in SNr, respectively. B: comparisons of the relative abundance of D2 receptor-containing terminals forming symmetric or asymmetric synapses in GPe, GPi, and SNr in normal and parkinsonian monkeys. The total number of terminals forming identifiable synapses in controls and MPTP-treated monkeys was 271 and 237 in GPe, 88 and 107 in GPi, and 173 and 115 in SNr, respectively. Vertical columns and error bars represent means ± SD.
GPi.
As shown in Figs. 5 and 6, D2 receptor labeling was almost exclusively presynaptic in GPi tissue from normal and MPTP-treated monkeys. In both normal and MPTP-treated animals, almost 60% (normal animals: 59.4 ± 2.9%; MPTP-treated animals: 58.8 ± 26.3%) of labeled structures were small unmyelinated axons. Terminals represented the next largest proportion of immunoreactive elements (normal animals: 30.7 ± 14.0%; MPTP-treated animals: 29.7 ± 13.8%). Most of the terminals with identifiable synaptic junctions formed symmetric synapses (normal animals: 59.8 ± 11.5%; MPTP-treated animals: 65.0 ± 32.8%). A smaller proportion of labeled terminals was associated with asymmetric synaptic specializations and displayed the ultrastructural features of putative glutamatergic boutons in both groups of animals (normal animals: 40.2 ± 11.5%; MPTP-treated animals: 35.0 ± 32.8%).
Glial processes accounted for ∼10% of the labeled elements in normal and MPTP-treated monkeys (normal animals: 8.6 ± 12.3%; MPTP-treated animals: 10.0 ± 12.8%). Postsynaptic dendritic labeling was not significant.
SNr.
Tissue samples from the SNr displayed a pattern of labeling similar to that in GPi (Figs. 5 and 6). D2 receptor labeling was almost exclusively presynaptic in normal and MPTP-treated animals. As in GPi, the largest proportion of labeled elements were unmyelinated axons (normal animals: 65.0 ± 13.8%, n = 3; MPTP-treated animals: 50.7 ± 12.0%, n = 2). Axon terminals were the second most abundant immunoreactive structures, accounting for 34.0 ± 13.2% of labeled structures in normal animals and 42.5 ± 3.8% of immunoreactive elements in MPTP-treated animals. The majority of immunoreactive terminals with identifiable synaptic specializations had the ultrastructural features of putative GABAergic boutons and formed symmetric synapses (normal animals: 81.2 ± 4.8%, n = 3; MPTP-treated animals: 82.5 ± 24.8%, n = 2). A smaller proportion of labeled terminals were identified as putative glutamatergic boutons with clear asymmetric synapses in both normal (18.8 ± 4.8%) and MPTP-treated animals (17.5 ± 24.8%). As in GPe and GPi, glial and postsynaptic labeling was rare in both groups of monkeys.
DISCUSSION
Our data suggest that the functional effects of extrastriatal D2LRs are not exclusive to the GPe and the indirect pathway of the basal ganglia but also directly influence, in an opposite manner, basal ganglia output neurons in GPi and SNr, and that D2LRs on GPe and GPi neurons are subject to an endogenous dopaminergic tone. In line with these observations, our electron microscopic immunocytochemical studies showed that D2LRs are expressed presynaptically not only in putative GABAergic axons and terminals in the GPe but also in GABAergic and glutamatergic terminals and unmyelinated axons in GPi and SNr. These studies, specifically the dopamine receptor antagonist experiments, may help us to understand the significance of extrastriatal dopamine release toward the regulation of the normal firing patterns of basal ganglia output neurons.
The function and localization of D2LR in GPe, GPi, and SNr were very similar in normal and parkinsonian animals, suggesting that D2LRs remain functional in the dopamine-depleted state. The results add to our knowledge of the pathophysiology of parkinsonism, demonstrating that loss of dopaminergic transmission in extrastriatal basal ganglia nuclei may contribute to firing abnormalities in these structures (and presumably, the entire basal ganglia-thalamocortical loop system). Most of the quinpirole-induced changes in firing rates and patterns in GPe, GPi, and SNr support the notion that some of the known antiparkinsonian effects of D2LR agonists may result from actions of these drugs at extrastriatal sites in the basal ganglia.
Technical Considerations
The interpretation of the functional data presented in this study relies on the specificity and limitations of in vivo local drug application methods to assess receptor-mediated effects on electrophysiological changes of neuronal activity. The effects of the locally injected drugs, as performed in the present study, are generally considered to be limited to a sphere of 0.5–1 mm in diameter around the tip of the injection device. Such estimates are supported by various sets of experimental data gathered from different brain regions. For instance, microinjections of muscimol (up to 1 μl) into the monkey STN result in topographically organized behavioral responses that depend on the location of the injection within the dorsolateral tier of the STN (Baron et al. 2002; Wichmann et al. 1994). However, given that different drugs (and drug concentrations) and different tissue barriers may lead to differences in tissue diffusion, it is not possible to state with certainty the actual spread of effective drug concentrations in a given experiment. However, we feel confident that the volumes and concentrations of drug used in the present experiments were small enough to avoid significant diffusion and contamination of neighboring structures. On the other hand, because of the close proximity of SNc neurons and the tight intermingling of their dendrites with SNr neurons (Arsenault et al. 1988), we cannot rule out the possibility that some of the physiological effects elicited by drug application in the SNr of normal monkeys were mediated in part by activation of D2LRs on SNc neurons. However, the fact that the effects of the agonist on SNr activity were similar before and after the treatment with MPTP (in which SNc dopaminergic dendrites and cell bodies were largely wiped out) suggests that most of the observed physiological responses recorded in SNr neurons were not mediated through the SNc.
Another technical issue related to the microinjection technique used in the present study is the limited relevance of such an approach to examine behavioral changes induced by the injected drugs. To elicit behavioral responses through intracerebral drug deliveries, larger drug volumes and dosages than those used in our study would be required. For this reason, our findings cannot provide any relevant information about the possible D2-mediated behavioral effects in GPe, GPi, and SNr.
Effects of D2LR Ligands on Firing Rates
GPe.
In agreement with previous findings in rats (Querejeta et al. 2001), we found that microinjections of quinpirole increased firing, whereas sulpiride injections reduced discharge rates in most GPe neurons. Our ultrastructural data were consistent with previous anatomical evidence for presynaptic D2LRs on striatopallidal fibers in the primate GPe and the rodent GP (Smith and Kieval 2000; Smith and Villalba 2008). In the rat GP, there is functional evidence that D2LR activation reduces GABAergic transmission (Cooper and Stanford 2001; Floran et al. 1997; Watanabe et al. 2009). In light of these findings, the most likely substrate for the increased activity of GPe neurons in response to quinpirole in our experiments is a presynaptic D2-receptor mediated reduction of GABA release from striatal afferents and local axon collaterals. Although electrophysiological studies have shown that individual striatal medium spiny neurons have a very low discharge rate, at least in the normal state (see, e.g., Liang et al. 2008), striatopallidal GABAergic projections (as well as GABA release from local axon collaterals) may be sufficient to maintain a substantial tissue level of GABA regulated by presynaptic D2LRs.
Electrophysiological experiments in rodent brain slice preparations have suggested that activation of presynaptic D2LRs may also lower glutamate release in GP (Hernandez et al. 2006). In agreement with these observations, our EM data showed that presynaptic D2 receptors are expressed in a subset of putatively glutamatergic terminals that form asymmetric synapses in the monkey GPe and that some GPe neurons respond to D2LR activation with decreased firing, perhaps mediated by a reduction of glutamate release. Although we did not identify the exact source of the D2 receptor-containing boutons, it is likely that most of them originated from the STN (Smith et al. 1998). In GPe, reductions in firing rates in response to D2LR activation were outnumbered by increases, likely because of the greater number of D2LRs on GABAergic striatopallidal afferents than glutamatergic terminals. As described below, these results are in sharp contrast to those in GPi and SNr, where D2LR activation lead to decreases in firing, most easily explained as the result of predominant activation of presynaptic inhibitory D2LRs on glutamatergic terminals.
In addition to these heteroreceptor-mediated effects, quinpirole may also have affected dopamine release via actions on dopaminergic nigropallidal terminals (Rommelfanger and Wichmann 2010; Smith and Kieval 2000). However, the fact that the quinpirole effects were similar in the normal and parkinsonian state, despite the severe degeneration of the nigrofugal dopaminergic system in parkinsonian animals, suggests that the D2-mediated autoreceptor effects were probably not as prominent as the aforementioned heteroreceptor actions toward the regulation of pallidal neuronal activity.
Quinpirole may also have acted at postsynaptic locations. D2 and D3 receptor mRNAs have been described in the human GPe (Murray et al. 1994), although a more recent study was inconclusive in measuring a detectable level of D3 receptor mRNA in the GPe of squirrel monkeys (a new world monkey species, Quik et al. 2000). In rats, mRNA for D2 receptors has been reported in GP neurons projecting to the striatum (Hoover and Marshall 2004; Marshall et al. 2001), but a very low level of postsynaptic D2 receptor protein labeling has been detected (Khan et al. 1998; Yung et al. 1995), which is consistent with the low level of postsynaptic D2 receptor immunoreactivity we detected in the monkey GPe. D4 receptors are also expressed in rat and monkey GPe neurons (Ariano et al. 1997; Mauger et al. 1998; Mrzljak et al. 1996). In rats, D4 receptor activation reduces GABAergic inhibitory postsynaptic currents (Shin et al. 2003) and glutamatergic excitatory postsynaptic currents (Hernandez et al. 2006), suggesting that quinpirole effects on GPe neurons recorded in our study could have been mediated via D4 receptors activation. However, this is unlikely given the much higher affinity of quinpirole for D2 over D4 receptors.
The fact that blocking D2LRs with sulpiride affected the physiological activity of GPe neurons indicates that endogenous dopamine occupies D2LRs in GPe under normal conditions, as suspected based on previous evidence for the presence of dopamine in the monkey and human GPe (Pifl et al. 1990; Whone et al. 2003). The present study is the first evidence for a functional role of endogenous dopamine at D2LRs in the monkey GPe.
GPi and SNr.
Our results demonstrate that local microinjection of quinpirole reduces firing in most GPi and SNr neurons. These observations are consistent with electrophysiological studies in rat brain slices showing that activation of D2LRs reduces subthalamonigral glutamatergic transmission (Ibanez-Sandoval et al. 2006). In agreement with these data, we found a significant proportion of D2 receptor immunoreactivity associated with putative glutamatergic terminals in the monkey GPi and SNr. Thus the most likely explanation for decreased activity of basal ganglia output neurons in response to quinpirole is that the drug reduced excitation of GPi and SNr neurons via downregulation of glutamate release from STN afferents. Other effects of the drug may have arisen from reducing GABA release from collaterals of the striato-GPe projections (Kawaguchi et al. 1990; Levesque and Parent 2005). Our ultrastructural data, indeed, revealed D2 receptor immunoreactivity in a subset of putative GABAergic terminals that ultrastructurally resembled striatal terminal boutons in the monkey GPi and SNr. However, because most GPi and SNr neurons decreased their activity in response to quinpirole infusion, we suggest that the presynaptic D2 receptor-mediated effect on glutamatergic transmission most likely predominates in GPi and SNr.
As for the effects on GPe neurons, one cannot rule out the possibility that some of the quinpirole-mediated changes of GPi activity may have involved putative presynaptic D2 autoreceptors on nigropallidal axons. However, as argued above for the D2LR agonist effects in GPe, the quinpirole effects in GPi were not altered by the dopamine depletion in parkinsonian monkeys, thereby suggesting that autoreceptor effects likely played a small, if any, role in the D2LR-mediated effects on GPi neurons.
Most GPi neurons increased their spontaneous firing rate in response to sulpiride microinjections, suggesting the presence of an endogenous tone of dopamine (Pifl et al. 1990), most likely released from terminals of the nigropallidal projection (Jan et al. 2000; Parent et al. 1990; Smith et al. 1989). Because the effects in the SNr did not reach statistical significance, the activation of D2LRs in the SNr by endogenous dopamine may not be as strong as in GPi. The same conclusion was reached in our recent study of the effects of D1LR blockade in GPi and SNr (Kliem et al. 2007).
Effects of D2LR-Mediated Changes on Firing Patterns
D2LR ligands strongly affected the firing patterns of GPe, GPi, and SNr neurons. Quinpirole reduced oscillatory power in GPe in the range of 1–8 Hz but increased it in the range of 13–30 Hz. For unclear reasons, sulpiride had similar effects on oscillatory activities. The findings in GPi and SNr were more consistent, demonstrating quinpirole-induced reductions in the range of 8–30 Hz and an increase in the range of 30–100 Hz, as well as a sulpiride-induced increase in oscillatory activity in the range of 13–30 Hz and a reduction in the range of 30–100 Hz. The findings in GPi and SNr are in line with current hypotheses stating that increased beta-band activity in STN and GPi may be correlated with the dopamine-depleted state, whereas dopamine receptor activation may increase gamma-band activity in these nuclei (see for review, Gatev et al. 2006; Hammond et al. 2007). We also found that quinpirole injections lead to a reduction of the proportion of spikes in bursts in GPe and to an increase in GPi and SNr. D2LR blockade had the opposite effects on bursting in GPe, but not in GPi and SNr.
The effects of systemic administration of dopamine receptor agonists on pallidal and nigral firing patterns have been previously studied in rodents (e.g., Nakanishi et al. 1985; Ruskin et al. 2003; Waszczak et al. 2002), and the mechanisms that underlie oscillatory activities in GPe and SNr have been explored at the levels of single neurons and neuronal networks through recording and modeling studies (see for review, Gatev et al. 2006). Rhythmic activities in GPe and SNr are known to be dependent on the balance between excitatory and inhibitory inputs (Ibanez-Sandoval et al. 2007; Nakanishi et al. 1985; Plenz and Kitai 1999; Terman et al. 2002). Important cellular features that may be modulated by local dopamine release and contribute to oscillatory and bursting activities in GPe and SNr are low-threshold calcium and hyperpolarization-activated cation currents (Cooper and Stanford 2000; Deng et al. 2007; Ibanez-Sandoval et al. 2007; Jiang et al. 1993; Keja et al. 1992; Lledo et al. 1990; Nambu and Llinas 1994).
Effects of Extrastriatal D2LR Activation in the Parkinsonian State
The pharmacological effects of D2LR activation and the ultrastructural distribution of D2 receptors in the parkinsonian state were similar to those in the normal state. Earlier studies in monkeys and other species also suggested that D2LR binding in the pallidum and SNr does not change significantly with MPTP treatment (Betarbet and Greenamyre 2004; Gnanalingham et al. 1993; Hurley et al. 1996), contrasting with the evidence that striatal expression of mRNA levels for D2LRs, presumably in medium spiny neurons, is increased in dopamine-depleted monkeys (Chefer et al. 2008; Herrero et al. 1996; Morissette et al. 1996). Given that these neurons project predominately to GPe, one could expect an increased expression of D2 receptor immunoreactivity along striatopallidal axons and terminals. However, due to inherent limitations of pre-embedding immunocytochemical approaches, our findings do not allow us to make firm statements about changes in the absolute number of D2 receptor-labeled terminals and axons in the GPe of normal vs. parkinsonian monkeys. Thus, although the overall pattern of distribution of immunoreactive elements is comparable between the two states, we cannot rule out the possibility that there might be an upregulation of presynaptic labeling in the parkinsonian condition. Alternatively, an increased mRNA expression at the cell body level does not necessarily imply that the resultant changes in protein expression will be reflected in all components of the targeted neurons.
The lack of change in the pallidum may also be attributed to the fact that the nigropallidal dopaminergic projection is not as severely affected as the nigrostriatal system in Parkinson's disease and animal models of parkinsonism (Jan et al. 2000; Pifl et al. 1990). It has even been suggested that the lack of changes in receptor distribution may be part of a compensatory response to maintain normal pallidal outflow in early stages of Parkinson's disease (Whone et al. 2003). It is also interesting to note that neither the distribution nor the effects of D1LR activation differs between the normal and parkinsonian states in GPi and SNr (Kliem et al. 2010).
The persistence of extrastriatal D2LR-mediated effects in parkinsonian monkeys suggests that dopamine receptor agonist therapy may mediate some of its effects through extrastriatal basal ganglia target sites. Most of the D2LR-mediated effects reported in the present study, such as reductions in overall firing rates and lowered oscillatory beta-band activities, may be therapeutically beneficial in Parkinson's disease. Future development of local dopamine delivery methods that may allow selective restoration of dopaminergic transmission at extrastriatal sites may help to reduce motor and cognitive side effects of dopaminergic drugs that are thought to originate in the striatum.
GRANTS
This work was supported by National Institutes of Health Grants R01 NS049474 (to T. Wichmann), R01 NS071074 (to T. Wichmann), RR-000165 (to Yerkes National Primate Research Center) and T32 DA15040 (to Dr. Michael Kuhar, Principal Investigator).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.H.-N., K.S.R., Y.S., and T.W. conception and design of research; A.H.-N., K.S.R., G.J.M., Y.S., and T.W. performed experiments; A.H.-N., K.S.R., Y.S., and T.W. analyzed data; A.H.-N., K.S.R., Y.S., and T.W. interpreted results of experiments; A.H.-N., K.S.R., Y.S., and T.W. prepared figures; A.H.-N., K.S.R., Y.S., and T.W. drafted manuscript; A.H.-N., K.S.R., Y.S., and T.W. edited and revised manuscript; A.H.-N., K.S.R., Y.S., and T.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The electrophysiological experiments and analyses were achieved by A. Hadipour-Niktarash, whereas K. S. Rommelfanger was in charge of the anatomical studies. We gratefully acknowledge the expert technical assistance of Drs. Yuxian Ma and Cindy Hu with the electrophysiological studies. Thanks are also due to Susan Jenkins and Jean-Francois Pare for help with the anatomical experiments.
REFERENCES
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989 [DOI] [PubMed] [Google Scholar]
- Aldridge JW, Gilman S. The temporal structure of spike trains in the primate basal ganglia: afferent regulation of bursting demonstrated with precentral cerebral cortical ablation. Brain Res 543: 123–138, 1991 [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271, 1990 [DOI] [PubMed] [Google Scholar]
- Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR. Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res 752: 26–34, 1997 [DOI] [PubMed] [Google Scholar]
- Arsenault MY, Parent A, Seguela P, Descarries L. Distribution and morphological characteristics of dopamine-immunoreactive neurons in the midbrain of the squirrel monkey (Saimiri sciureus). J Comp Neurol 267: 489–506, 1988 [DOI] [PubMed] [Google Scholar]
- Baron MS, Wichmann T, Ma D, DeLong MR. Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci 22: 592–599, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Greenamyre JT. Regulation of dopamine receptor and neuropeptide expression in the basal ganglia of monkeys treated with MPTP. Exp Neurol 189: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Neurosci 21: 6853–6861, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res 83: 531–537, 1975 [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci 21: 1033–1038, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Cortes R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience 28: 275–290, 1989 [DOI] [PubMed] [Google Scholar]
- Camps M, Kelly PH, Palacios JM. Autoradiographic localization of dopamine D 1 and D 2 receptors in the brain of several mammalian species. J Neural Transm Gen Sect 80: 105–127, 1990 [DOI] [PubMed] [Google Scholar]
- Chefer SI, Kimes AS, Matochik JA, Horti AG, Kurian V, Shumway D, Domino EF, London ED, Mukhin AG. Estimation of D2-like receptor occupancy by dopamine in the putamen of hemiparkinsonian monkeys. Neuropsychopharmacology 33: 270–278, 2008 [DOI] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature 289: 537–542, 1981 [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM. Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABAA IPSCs in vitro. Neuropharmacology 41: 62–71, 2001 [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM. Electrophysiological and morphological characteristics of three subtypes of rat globus pallidus neurone in vitro. J Physiol 527: 291–304, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci 17: 5738–5746, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990 [DOI] [PubMed] [Google Scholar]
- Deng P, Zhang Y, Xu ZC. Involvement of Ih in dopamine modulation of tonic firing in striatal cholinergic interneurons. J Neurosci 27: 3148–3156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floran B, Floran L, Sierra A, Aceves J. D2 receptor-mediated inhibition of GABA release by endogenous dopamine in the rat globus pallidus. Neurosci Lett 237: 1–4, 1997 [DOI] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and function of GABA transporters in the globus pallidus of parkinsonian monkeys. Exp Neurol 223: 505–515, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and pharmacological modulation of GABA-B receptors in the globus pallidus of parkinsonian monkeys. Exp Neurol 229: 429–439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidment NT, Ackerson LC, Smith Y, Wichmann T. GABAergic modulation of the activity of globus pallidus neurons in primates: in vivo analysis of the functions of GABA receptors and GABA transporters. J Neurophysiol 94: 990–1000, 2005 [DOI] [PubMed] [Google Scholar]
- Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, Quimby FW, Turner PV, Wood GA, Würbel H. Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, DC: National Academies Press, 2010 [Google Scholar]
- Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord 21: 1566–1577, 2006 [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature 260: 258–260, 1976 [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Smith LA, Hunter AJ, Jenner P, Marsden CD. Alterations in striatal and extrastriatal D-1 and D-2 dopamine receptors in the MPTP-treated common marmoset: an autoradiographic study. Synapse 14: 184–194, 1993 [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20: 60–80, 1999 [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007 [DOI] [PubMed] [Google Scholar]
- Hernandez A, Ibanez-Sandoval O, Sierra A, Valdiosera R, Tapia D, Anaya V, Galarraga E, Bargas J, Aceves J. Control of the subthalamic innervation of the rat globus pallidus by D2/3 and D4 dopamine receptors. J Neurophysiol 96: 2877–2888, 2006 [DOI] [PubMed] [Google Scholar]
- Herrero MT, Augood SJ, Asensi H, Hirsch EC, Agid Y, Obeso JA, Emson PC. Effects of l-DOPA-therapy on dopamine D2 receptor mRNA expression in the striatum of MPTP-intoxicated parkinsonian monkeys. Brain Res Mol Brain Res 42: 149–155, 1996 [DOI] [PubMed] [Google Scholar]
- Hoover BR, Marshall JF. Molecular, chemical, and anatomical characterization of globus pallidus dopamine D2 receptor mRNA-containing neurons. Synapse 52: 100–113, 2004 [DOI] [PubMed] [Google Scholar]
- Hurley MJ, Jolkkonen J, Stubbs CM, Jenner P, Marsden CD. Dopamine D3 receptors in the basal ganglia of the common marmoset and following MPTP and l-DOPA treatment. Brain Res 709: 259–264, 1996 [DOI] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Carrillo-Reid L, Galarraga E, Tapia D, Mendoza E, Gomora JC, Aceves J, Bargas J. Bursting in substantia nigra pars reticulata neurons in vitro: possible relevance for Parkinson disease. J Neurophysiol 98: 2311–2323, 2007 [DOI] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Hernandez A, Floran B, Galarraga E, Tapia D, Valdiosera R, Erlij D, Aceves J, Bargas J. Control of the subthalamic innervation of substantia nigra pars reticulata by D-1 and D-2 dopamine receptors. J Neurophysiol 95: 1800–1811, 2006 [DOI] [PubMed] [Google Scholar]
- Jan C, Francois C, Tande D, Yelnik J, Tremblay L, Agid Y, Hirsch E. Dopaminergic innervation of the pallidum in the normal state, in MPTP-treated monkeys and in parkinsonian patients. Eur J Neurosci 12: 4525–4535, 2000 [PubMed] [Google Scholar]
- Jiang ZG, Pessia M, North RA. Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurones. J Physiol 462: 753–764, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci 10: 3421–3438, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keja JA, Stoof JC, Kits KS. Dopamine D2 receptor stimulation differentially affects voltage-activated calcium channels in rat pituitary melanotropic cells. J Physiol 450: 409–435, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A. Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol 402: 353–371, 1998 [DOI] [PubMed] [Google Scholar]
- Kita H, Nambu A, Kaneda K, Tachibana Y, Takada M. Role of ionotropic glutamatergic and GABAergic inputs on the firing activity of neurons in the external pallidum in awake monkeys. J Neurophysiol 92: 3069–3084, 2004 [DOI] [PubMed] [Google Scholar]
- Kliem MA, Maidment NT, Ackerson LC, Chen S, Smith Y, Wichmann T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol 98: 1489–1500, 2007 [DOI] [PubMed] [Google Scholar]
- Kliem MA, Pare JF, Khan ZU, Wichmann T, Smith Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur J Neurosci 31: 836–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliem MA, Wichmann T. A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods 138: 45–49, 2004 [DOI] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985 [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci 24: 8289–8299, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci USA 102: 11888–11893, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA 90: 8861–8865, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci 28: 7537–7547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Legendre P, Israel JM, Vincent JD. Dopamine inhibits two characterized voltage-dependent calcium currents in identified rat lactotroph cells. Endocrinology 127: 990–1001, 1990 [DOI] [PubMed] [Google Scholar]
- Macey TA, Gurevich VV, Neve KA. Preferential Interaction between the dopamine D2 receptor and Arrestin2 in neostriatal neurons. Mol Pharmacol 66: 1635–1642, 2004 [DOI] [PubMed] [Google Scholar]
- Marshall JF, Henry BL, Billings LM, Hoover BR. The role of the globus pallidus D2 subfamily of dopamine receptors in pallidal immediate early gene expression. Neuroscience 105: 365–378, 2001 [DOI] [PubMed] [Google Scholar]
- Masilamoni G, Votaw J, Howell L, Villalba RM, Goodman M, Voll RJ, Stehouwer J, Wichmann T, Smith Y. 18F-FECNT: validation as PET dopamine transporter ligand in parkinsonism. Exp Neurol 226: 265–273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauger C, Sivan B, Brockhaus M, Fuchs S, Civelli O, Monsma F., Jr Development and characterization of antibodies directed against the mouse D4 dopamine receptor. Eur J Neurosci 10: 529–537, 1998 [DOI] [PubMed] [Google Scholar]
- Mengual E, Pickel VM. Ultrastructural immunocytochemical localization of the dopamine D2 receptor and tyrosine hydroxylase in the rat ventral pallidum. Synapse 43: 151–162, 2002 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther 303: 791–804, 2002 [DOI] [PubMed] [Google Scholar]
- Morissette M, Goulet M, Calon F, Falardeau P, Blanchet PJ, Bedard PJ, Di Paolo T. Changes of D1 and D2 dopamine receptor mRNA in the brains of monkeys lesioned with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: correction with chronic administration of l-3,4-dihydroxyphenylalanine. Mol Pharmacol 50: 1073–1079, 1996 [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 381: 245–248, 1996 [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA 91: 11271–11275, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Hori N, Kastuda N. Neostriatal evoked inhibition and effects of dopamine on globus pallidal neurons in rat slice preparations. Brain Res 358: 282–286, 1985 [DOI] [PubMed] [Google Scholar]
- Nambu A, Llinas R. Electrophysiology of globus pallidus neurons in vitro. J Neurophysiol 72: 1127–1139, 1994 [DOI] [PubMed] [Google Scholar]
- Parent A, Lavoie B, Smith Y, Bedard P. The dopaminergic nigropallidal projection in primates: distinct cellular origin and relative sparing in MPTP-treated monkeys. Adv Neurol 53: 111–116, 1990 [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HF. The Fine Structure of the Nervous System-Neurons and Their Supporting Cells. New York: Oxford University Press, 1991 [Google Scholar]
- Pifl C, Bertel O, GS, Hornykiewicz O. Extrastriatal dopamine in symptomatic and asymptomatic Rhesus monkeys treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Neurochem Int 17: 263–270, 1990 [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai S. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 400: 677–682, 1999 [DOI] [PubMed] [Google Scholar]
- Querejeta E, Delgado A, Valdiosera R, Erlij D, Aceves J. Intrapallidal D2 dopamine receptors control globus pallidus neuron activity in the rat. Neurosci Lett 300: 79–82, 2001 [DOI] [PubMed] [Google Scholar]
- Quik M, Police S, He L, Di Monte DA, Langston JW. Expression of D3 receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience 98: 263–273, 2000 [DOI] [PubMed] [Google Scholar]
- Richfield EK, Young AB, Penney JB. Comparative distribution of dopamine D-1 and D-2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. J Comp Neurol 262: 446–463, 1987 [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T. Extrastriatal dopaminergic circuits of the Basal Ganglia. Front Neuroanat 4: 139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Tierney PL, Walters JR. Correlated multisecond oscillations in firing rate in the basal ganglia: modulation by dopamine and the subthalamic nucleus. Neuroscience 117: 427–438, 2003 [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci 15: 264–270, 1994 [DOI] [PubMed] [Google Scholar]
- Shin RM, Masuda M, Miura M, Sano H, Shirasawa T, Song WJ, Kobayashi K, Aosaki T. Dopamine D4 receptor-induced postsynaptic inhibition of GABAergic currents in mouse globus pallidus neurons. J Neurosci 23: 11662–11672, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86: 353–387, 1998 [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci 23: S28–S33, 2000 [DOI] [PubMed] [Google Scholar]
- Smith Y, Lavoie B, Dumas J, Parent A. Evidence for a distinct nigropallidal dopaminergic projection in the squirrel monkey. Brain Res 482: 381–386, 1989 [DOI] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord 23, Suppl 3: S534–S547, 2008 [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of MPTP-induced parkinsonism and lesions of the external pallidal segment. J Neurosci 24: 6417–6426, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman D, Rubin JE, Yew AC, Wilson CJ. Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci 22: 2963–2976, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol 442: 392–404, 2002 [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Martin LP, Finlay HE, Zahr N, Stellar JR. Effects of individual and concurrent stimulation of striatal D1 and D2 dopamine receptors on electrophysiological and behavioral output from rat basal ganglia. J Pharmacol Exp Ther 300: 850–861, 2002 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kita T, Kita H. Presynaptic actions of D2-like receptors in the rat cortico-striato-globus pallidus disynaptic connection in vitro. J Neurophysiol 101: 665–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whone AL, Moore RY, Piccini PP, Brooks DJ. Plasticity of the nigropallidal pathway in Parkinson's disease. Ann Neurol 53: 206–213, 2003 [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 72: 521–530, 1994 [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol 95: 2120–2133, 2006 [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65: 709–730, 1995 [DOI] [PubMed] [Google Scholar]
- Zhou FW, Jin Y, Matta SG, Xu M, Zhou FM. An ultra-short dopamine pathway regulates basal ganglia output. J Neurosci 29: 10424–10435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]