Abstract
Attention influences visual processing in striate and extrastriate cortex, which has been extensively studied for spatial-, object-, and feature-based attention. Most studies exploring neural signatures of feature-based attention have trained animals to attend to an object identified by a certain feature and ignore objects/displays identified by a different feature. Little is known about the effects of feature-selective attention, where subjects attend to one stimulus feature domain (e.g., color) of an object while features from different domains (e.g., direction of motion) of the same object are ignored. To study this type of feature-selective attention in area MT in the middle temporal sulcus, we trained macaque monkeys to either attend to and report the direction of motion of a moving sine wave grating (a feature for which MT neurons display strong selectivity) or attend to and report its color (a feature for which MT neurons have very limited selectivity). We hypothesized that neurons would upregulate their firing rate during attend-direction conditions compared with attend-color conditions. We found that feature-selective attention significantly affected 22% of MT neurons. Contrary to our hypothesis, these neurons did not necessarily increase firing rate when animals attended to direction of motion but fell into one of two classes. In one class, attention to color increased the gain of stimulus-induced responses compared with attend-direction conditions. The other class displayed the opposite effects. Feature-selective activity modulations occurred earlier in neurons modulated by attention to color compared with neurons modulated by attention to motion direction. Thus feature-selective attention influences neuronal processing in macaque area MT but often exhibited a mismatch between the preferred stimulus dimension (direction of motion) and the preferred attention dimension (attention to color).
Keywords: color, middle temporal sulcus, motion
attentional modulation of activity in visual areas can be divided into three commonly studied categories (Boynton 2005; Maunsell and Treue 2006; Reynolds and Chelazzi 2004; Seitz and Watanabe 2005; Yantis and Serences 2003). “Space-based attention” refers to attention that is directed at particular regions of the visual field, and its effects are detectable in the responses of neurons with receptive fields (RFs) that correspond to the attended locations. “Object-based attention” refers to a heightening in sensitivity toward specific combinations of features that characterize an attended object (Fallah et al. 2007; Hayden and Gallant 2005, 2009; Patzwahl and Treue 2009; Reynolds et al. 2003; Roelfsema et al. 1998; Saenz et al. 2002). “Feature-based attention” (McAdams and Maunsell 2000) refers to attention directed at a specific stimulus feature (e.g., a specific direction of motion or the orientation of a stimulus) that characterizes an object and makes it distinct from other objects.
In this study we focus on a specific form of feature-based attention that has so far attracted little scrutiny. This form of feature-based attention requires subjects to selectively attend to and report a specific feature of an object (e.g., the direction of motion) and ignore other simultaneously present features of the object (e.g., the color) during some trials, while reporting the color of that object on other trials (and ignoring the direction of motion of the same object). We will refer to this type of attention as “feature-selective attention,” being well aware that this term has been used interchangeably with “feature-based attention” in the literature. We decided to provide a separate label, as feature-selective attention is conceptually distinct from feature-based attention. Feature-based attention allows subjects to use a cued feature to select objects and locations in the environment to attend to, but it does not require them to specifically process a certain feature of an object while simultaneously ignoring other features present in that object. Ignoring, or even suppressing, the irrelevant stimulus dimension is particularly important if the two feature values result in response conflict, e.g., if the behavioral response to the direction of motion requires an upward saccade while the behavioral response to the specific stimulus color would require a downward saccade. Feature-selective attention has been studied in macaque V4 (Mirabella et al. 2007), where macaques attended to and reported either the color or the orientation of a single bar stimulus located in the RF of the recorded neuron. This affected neuronal responses in V4, but, somewhat surprisingly, upmodulation of firing rates occurred even if the preferred neuronal stimulus feature did not match the attended feature. This might be because V4 neurons are often selective for the feature dimensions tested in the study of Mirabella et al. (2007), i.e., V4 neurons are often selective for orientation as well as for color, and the selectivity for both features within the feature map might have contributed to the reported lack of attentional selectivity.
If true, feature-selective attention might have more specific effects in area MT, where it should alter the gain of neuronal firing when attention is focused on direction of motion compared with attention focused on color, as MT neurons are highly selective for the direction of stimulus motion but make very limited contributions to color processing, even if they can exploit chromatic cues for motion signaling (Dobkins and Albright 1994; Gegenfurtner et al. 1994; Riečanský et al. 2005; Seidemann et al. 1999; Thiele et al. 1999b, 2001).
A recent study by Katzner et al. (2009) has investigated the effects of feature-selective attention on neurons in macaque area MT. In this study, animals had to report either a change in motion direction or a change in the color of the dots that comprised the moving stimulus, and ignore changes in the other feature dimension, at a cued location. However, animals were not required to report a specific feature value, e.g., whether motion was upward versus downward or whether the stimulus was green or red. The authors reported that attentional modulation did not differ for the two attention conditions when averaged across the population of neurons, but they did not specifically reveal whether this upmodulation of responses was present for both feature dimensions in all neurons, or whether some neurons were upmodulated when attention was directed at motion while others were upmodulated when attention was directed at the color of the stimulus, even though their Figure 3c hints at the latter possibility.
Fig. 3.
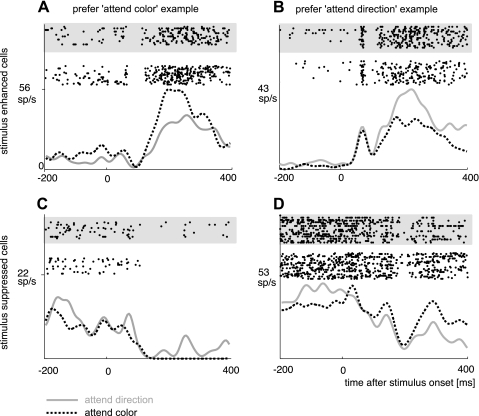
Examples of neurons that showed significant effects of feature-selective attention. A: neuron that showed increased stimulus-driven responses in the attend-color condition. B: neuron that showed increased stimulus-driven responses in the attend-direction condition. C: neuron that showed increased stimulus-driven suppression in the attend-color condition. D: neuron that showed increased stimulus-driven suppression in the attend direction condition. All firing rate differences are significant at P < 0.0032 (Kruskal-Wallis ANOVA). Dashed line and raster plots on white background, attend-color condition; solid gray line and raster plots on gray background, attend-direction condition.
Our study required subjects to engage feature-selective attention, by focusing on either the color or the direction of motion of a stimulus and reporting the color or direction of motion that was present, respectively. The complexity of our task ensured that subjects paid close attention to the relevant feature. It also encouraged them to suppress information regarding the nonrelevant feature, as the behavioral response required by one attention condition was often incompatible with the response required during the other condition. We assumed that this would result in increased neuronal gain when attention was directed to the direction of motion compared with conditions when attention was directed at the color of the stimulus. This could take the form of either multiplicative gain or feature similarity gain, whereby in the latter case increased firing rates would be found when stimuli of preferred direction of motion were attended to and reduced activity level found when stimuli moving in antipreferred direction were attended to. While we found that 22% of MT neurons were significantly affected by feature-selective attention, our initial hypothesis was not supported. Neurons in our study were often upmodulated when attention was directed at the color of the stimulus, the upregulation was largely unrelated to stimulus preference, and the modulation occurred earlier in neurons that were upmodulated when attention was directed to stimulus color than in neurons that were upmodulated by attention to direction of motion.
EXPERIMENTAL METHODS
Standard training and electrophysiological methods were used to record extracellular single-unit activity from area MT. Protocols for all experiments were approved by the Salk Institute Animal Care and Use Committee and by the Regierungspraesidium Arnsberg. They conformed to USDA regulations and NIH guidelines for the humane care and use of laboratory animals and followed published guidelines on the use of animals in research (European Communities Council Directive 86/609/ECC). Details regarding surgical implantation techniques and postsurgical analgesia have been described previously (Thiele et al. 1999a).
Receptive Field Mapping, Stimulus Properties, and Behavioral Paradigm
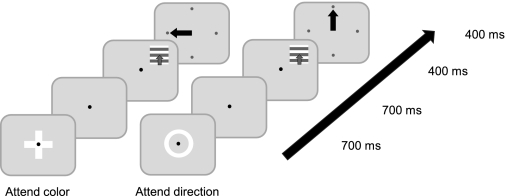
Receptive fields were mapped by the minimum response field methods. Bars were swept across the screen (under computer mouse control) while animals fixated centrally. The outer diameter of the zone from which audible responses could be elicited were taken as the RF size. Most RFs were located at an eccentricity of ∼3–10°, and the RF size (diameter) usually matched the eccentricity of the RF center location, i.e., a RF with a center at 10° eccentricity would usually have a diameter of 10°. The stimuli presented during the main part of the experiment were size matched to these RFs. In the main part of the experiment a single moving, colored sine wave grating stimulus was presented in the RF of the recorded MT neuron. The colored gratings were presented at 10% luminance contrast (relative to a gray background), at a spatial frequency of 0.7 cycles/° and a temporal frequency of 4 Hz on a cathode ray tube (CRT) monitor (100-Hz refresh rate, 800 × 600 pixel resolution). A grating of 0.7 cycles/° and a temporal frequency of 4 Hz results in stimulus speed of 5.7°/s, which is at the lower end of MT cell speed preference (Britten et al. 1993) but usually still resulted in robust responses. The monkey performed a four-alternative-forced-choice discrimination task (Fig. 1), for either direction or color. At the beginning of each trial, monkeys were required to fixate a central fixation spot. The fixation window was 1.5° × 1.5° wide. Eye position was monitored by the scleral search coil technique. Stimulus presentation and behavioral control were performed by custom-written software in monkey C and by means of Cortex 5.95 (http://dally.nimh.nih.gov/) in monkeys T and I. After fixation onset a cue appeared centrally, to indicate whether the animal had to report the color (cue: a cross presented centrally) or the direction of motion (cue: a ring surrounding the fixation spot) of the grating. The cue was presented for 400 ms, after which a gap period of at least 500 ms occurred (for additional details, see below) before the visual stimuli were presented. Attention conditions could either alternate randomly on a trial-by-trial basis (monkey C) or in blocks (monkeys T and I) whereby the attention condition remained constant throughout a block of 15 trials. Blocks were altered in a semirandom manner, such that block alternation was not always AB AB AB… but could be, e.g., AB BA BA AB…. Thus, as the sequence started, a block was randomly allocated, but it was then followed by the block not chosen initially. After that second block was finished, blocks were again randomly allocated, and the second block in that new minisequence was again the block that had not been chosen during the random allocation. This ensured that, e.g., slow but gradual decrease in attention over the course of the experiment would not always result in affecting block B more strongly if block B always followed block A. Effectively the block order was balanced by the randomization process.
Fig. 1.
1) The monkey fixated upon a centrally located red dot. 2) Once fixation was sustained, a cue appeared, indicating whether the relevant feature for the current trial was color or direction. The cue stayed on for 700 ms. 3) A fixation-point-only period lasted 700 ms. 4) This was followed by a 400-ms grating stimulus presentation. The stimulus was positioned within the receptive field (RF) of the recorded neuron. For purposes of illustration, a stimulus that has an upward direction of motion is depicted. 5) After stimulus offset, the monkey was given up to 400 ms to indicate his decision. Correct motor responses were rewarded with juice. In this example figure, the monkey had to make a leftward saccade for the attend-color task (to report the color “red”) and an upward saccade for the attend-direction task (to report the direction “up”).
Visual Stimuli
The grating moved left, up, right, or down within a square aperture. For attend-direction trials, the monkey had to make a behavioral response in the same direction of motion as the stimulus (e.g., leftward choice for leftward motion, rightward choice for rightward motion, etc.). For attend-color trials, red required a leftward, blue an upward, green a rightward, and yellow a downward behavioral response. Four of these 16 feature combinations required the monkey to make a motor response in the same direction, irrespective of whether the task was attend-color or attend-motion (termed “congruent stimuli,” e.g., a red stimulus moving to the left), whereas the remaining 12 combinations required motor movements in different directions, depending on the attention task (“incongruent stimuli,” e.g., a red stimulus moving to the right).
The colors of the stimuli were constructed such that the maximum-intensity red was a pure CRT (RGB) red of 19.1 cd/m2 (X: 0.622, Y: 0.343) modulated through gray of 17.4 cd/m2 (X: 0.302, Y: 0.306), green was a pure CRT (RGB) green of 19.0 cd/m2 (X: 0.282, Y: 0.601) modulated through gray of 17.4 cd/m2 (X: 0.302, Y: 0.306), blue was a mixed CRT (RGB) of blue (13.6 cd/m2) and green (5.6 cd/m2) resulting in an overall luminance of 19.2 cd/m2 (X: 0.161, Y: 0.090) modulated through gray of 17.4 cd/m2 (X: 0.302, Y: 0.306), and yellow was a mixed CRT (RGB) of red (7.9 cd/m2) and green (11.2 cd/m2) resulting in an overall luminance of 19.1 cd/m2 (X: 0.464, Y: 0.465) modulated through gray of 17.4 cd/m2 (X: 0.302, Y: 0.306). The background was a gray of 17.4 cd/m2 (X: 0.302, Y: 0.306). The sinusoidal modulation between colors and gray was performed based on gamma-corrected CRT measurements. All color and luminance measurements were performed with a PR-650 Spectrascan Colorimeter (Photo Research).
For monkeys T and I, all color and direction combinations were presented, encompassing 16 possible stimuli and yielding a total of 32 conditions (16 stimuli × 2 attention-task conditions). For monkey C a smaller subset of stimuli was used: For the first 36 neurons recorded (over the course of 29 recording sessions) 8 of 16 possible stimulus combinations were used (4 congruent: red moving left, blue moving up, green moving right, and yellow moving down; 4 incongruent: red moving right, blue moving down, green moving left, and yellow moving up). During recordings made from the subsequent 48 neurons (over the course of 18 sessions), only incongruent stimuli were presented (all 12 incongruent combinations were used).
The behavioral response consisted of a saccadic eye movement to one of four locations, for monkeys T and I, or a hand movement to one of four touch bars, for monkey C. The touch bars were located within the primate chair and were well removed from the animal's field of view.
One monkey was engaged in a reaction time task (monkey C) in which cue conditions were identical to those described above. However, the stimulus was presented after intervals (randomly chosen for each trial) of 500, 1,000, 1,500, or 2,000 ms after cue offset, and the monkey was allowed to indicate his decision immediately after stimulus onset, by releasing the central touch bar and moving the hand to one of four peripheral touch bars located in front of his abdomen. Regardless of the time at which he executed a motor response, he was required to maintain fixation for a period of 500 ms after contact with a peripheral touch bar. For the other two monkeys, the stimulus was always presented 700 ms after cue offset, for 400 ms, after which four saccade targets appeared. These animals were allowed to make a saccade to the chosen target within 400 ms after saccade target onset.
Data Analysis
Data analysis was carried out with custom-written MATLAB functions. Behavioral performance (percentage correct/incorrect) was calculated for each recording session for each of the three monkeys. In our subsequent examinations of spiking activity, we only included data from trials during which the monkey made a correct behavioral response, and from neurons for which data from a minimum of correct 10 trials were available for each condition (median: 20, range: 10 to 35). From the single-cell spike activity recorded during presentations of stimulus motion in each of four possible directions, we determined the preferred direction of stimulus motion and calculated a directional index (DI), DI = 1 − (AND/APD), where APD is the average activity to stimuli moving in the preferred direction (PD, the stimulus direction with the highest activity) and AND is the average activity elicited from stimulus movement in the opposite direction (null direction, ND). In line with established criteria (e.g., Britten et al. 1992, 1993), we categorized neurons as directionally selective (DS) for DIs ≥ 0.5 or non-DS for DIs < 0.5. Only the results from DS neurons are reported in this article. We analyzed modulations in firing rate due to attention condition during the response period, by comparing activity levels of each cell under the two attention task conditions (attend to color or attend to direction of motion). For all monkeys the response period lasted from 40 to 400 ms after stimulus onset (note that monkey C was required to maintain fixation throughout this period, although he was allowed to release his touch bar before the period ended). We performed a nonparametric ANOVA for each neuron, and each stimulus presented (with attention and time as factors), and used the false discovery rate (FDR) to account for multiple comparisons (Benjamini and Hochberg 1995). We controlled for FDR to be at or below a value of q = 0.05, by first ordering our P values, P(1) ≤ P(2) ≤P(3) ≤ … ≤ P(n), where n was the number of stimulus conditions (tests). We then accepted a threshold value of p(r), where r was the largest i such that p(i) ≤ (i/n) × q. Thus, if we had 16 conditions, and one P value was smaller than 0.003125, another P value was smaller than 2 × 0.003125, while all the remaining P values were larger than 3 × 0.003125, then the conditions with the two smallest P values were accepted as showing significant effects of feature-selective attention. Our value for n was 16 when four colors and four directions were used (16 possible stimulus combinations); it was 8 when only 8 of 16 possible stimulus combinations were used and 12 when 12 of 16 possible stimulus combinations were used. Once attention-modulated neurons were identified, we determined the specific stimulus combination(s) that was accompanied by effects of feature-selective attention. We refer to these specific stimulus combinations as “attention-modulated stimuli.” For each attention-modulated response, we determined the activity for attend-direction and attend-color trials. From these we calculated an attention modulation index (AMIsig) for each attended stimulus and neuron, as a measure of the neuron's preference for a particular attention condition; AMIsig = (PF − NF)/(PF + NF), where PF and NF are the average activities across conditions involving the preferred attention features and nonpreferred attention features, respectively. An index value of 0 is obtained when no preference exists for the PF, while a value of 1 indicates an absolute preference for the PF, over the NF.
We analyzed the time course of the neuronal response to determine the point at which attention started to have a significant effect at the population level. For each neuron, receiver operator characteristic (ROC) values (Green and Swets 1966) were calculated for each attention-modulated stimulus condition within a sliding time window of 80 ms from 40 to 400 ms, spaced 10 ms apart (70 ms overlap between adjacent time bins, yielding 37 bins), after stimulus onset (Britten et al. 1992; Thiele et al. 1999a; Vogels et al. 1989). The ROC values provide a measure of how well an ideal observer could tell from the single-trial neuronal response whether attention was directed toward direction of motion or to the stimulus color. We combined ROC values across neurons that favored a particular attention condition (i.e., neurons that were more modulated by attention to color vs. neurons that were more modulated by attention to direction of motion) and identified the time bins in which the ROC values diverged significantly from 0.5 with a Wilcoxon signed-rank test.
Variability of Visual Response
Attention might alter firing rate variability, as well as average firing rates. Effects of feature-selective attention on rate variability were examined by determining the variability of firing from trial to trial. Variability of firing from trial to trial was analyzed by calculating the Fano factor (FF) = variance/mean, where the variance was measured across trials, in units of (spikes/epoch)2, and the mean was in units of spikes/epoch. To account for differences in variability between transient and sustained components of the response, activity was examined in two separate epochs for each trial, spanning 50 to 150 ms, then 150 to 400 ms, from stimulus onset. Only neurons that had stimulus-evoked firing rates of at least 5 spikes/s were included in this analysis.
Attention Modulation Indices
To investigate what the effects of feature-selective attention were on the population of cells, we calculated an attention modulation index (AMIpop) for all neurons, regardless of whether their response was significantly modulated. We calculated the AMIpop based on the mean activity of the neuron during stimulus presentation, AMIpop = (RC − RD)/(RC + RD), where RC is the response during the attend-color condition and RD is the response during the attend-direction condition. For neurons that underwent stimulus-evoked suppression, we calculated the AMIpop based on the absolute difference between the spontaneous and stimulus-evoked responses.
We subdivided the AMIpop data from all neurons into three groups, according to their directional tuning preferences. For each stimulus condition, we identified 1) neurons with PDs in the direction of stimulus motion, 2) neurons with PDs in a direction opposite to stimulus motion, and 3) neurons with PDs 90° clockwise or counterclockwise from stimulus motion.
We repeated this analysis, using data only from neurons that underwent significant feature-attention modulation AMIsig, i.e., using data from trials where attention-modulated stimuli (i.e., those eliciting significant modulation) were presented.
Effects of Congruency
Up to this point, our analysis was carried out separately for each stimulus condition, without pooling trials across stimulus conditions. Attention might exert different effects depending on whether the stimulus presented was congruent or incongruent, i.e., whether the motor response differed for a specific stimulus depending on the attention condition (incongruent) or whether the motor response was the same irrespective of the attention condition (congruent). To determine whether congruency had an effect on the strength of attentional modulation, we pooled across trials with congruent stimuli and, separately, trials with incongruent stimuli. We performed an ANOVA to identify attention-modulated neurons, running two separate analyses for each neuron, using either only congruent or only incongruent trials. We identified neurons in which a main effect of attention was present (regardless of the specific stimulus used), as well as neurons that showed an interaction between attention and stimulus. Finally, we compared the proportions of attention-modulated neurons that were identified using congruent trial data only with those identified using incongruent trials only.
Neuronal Activity and Its Relation to Behavioral Responses
Choice probabilities.
To determine the relation between neuronal activity and behavioral choice we compared the activity elicited by a given stimulus when the choice was in preferred direction of the neuron and when it was in antipreferred direction of the neuron. We used data from two monkeys (monkeys T and I). For each directionally selective neuron, we used stimuli that were incongruent (i.e., the 2 attention conditions required different choices), and stimulus motion was in PD (yielding choices in PD for one attention condition and choices in ND for the other attention condition) or stimulus motion was in ND (yielding choices in ND for one attention condition and choices in PD for the other attention condition). For each of the two stimuli, we examined the activity elicited from 50 to 400 ms. Thus we obtained two response distributions from which to calculate the ROC curves, where the area under the curve corresponded to choice probabilities (CPs) of a single neuron (see Britten et al. 1996 or Thiele et al. 1999a for additional detail). Pooling across neurons yielded a CP distribution for stimulus motion in PD and another distribution for stimulus motion in ND. We used a permutation test (Britten et al. 1996) to determine whether or not CPs were significant (P < 0.05, 2-sided test). Here we randomly assigned the data recorded during both choice (attention) conditions to one of two response distributions and recalculated bootstrapped CPs (random with replacement, 200 iterations).
Translation of attended visual features into behaviorally relevant categories.
In a separate analysis, we investigated whether the directions in which motor responses were made could modulate MT responses, independent of feature attention conditions and direction of stimulus motion. For example, if a red stimulus moving to the right was presented, then during an attend-color trial the monkey had to make a behavioral response to the left, but during an attend-direction trial he had to make a response to the right. Behaviorally modulated neurons would display significantly and consistently higher activity with one particular response direction, compared with the other response directions, irrespective of the stimulus or attention condition. Note that this is different from the above-mentioned CPs, as CPs explicitly compare choice in relation to preferred versus antipreferred directions of the neuron, while the analysis performed here disregards neuronal stimulus preference and determines “motor/choice” preference. Using incongruent trials alone, we grouped trials according to the behavioral response direction (leftward, upward, rightward, or downward hand or eye movements), disregarding stimulus and feature attention conditions. The activity for each neuron was analyzed within six time epochs for monkeys T and I, spanning −50 to 490 ms relative to stimulus onset, while it spanned four time epochs for monkey C (−30 to 310 ms after stimulus onset). For monkeys T and I, the epochs spanned −50 to 40, 40 to 130, 130 to 220, 220 to 310, 310 to 400, and 400 to 490 ms, relative to stimulus onset, for epochs 1–6, respectively. For monkey C, the epochs spanned −50 to 30, 40 to 130, 130 to 220, and 220 to 310 ms, relative to stimulus onset, for epochs 1–4, respectively, and we only included trials where his reaction time exceeded the analysis period. For each of the four possible behavioral response directions, the task imposed three incongruent stimulus combinations and two attention conditions, resulting in six stimulus/attention conditions. We performed a two-way ANOVA with behavioral response direction and attention condition as factors. For neurons to be considered significantly behaviorally modulated, a significant main effect of behavioral motor response direction (P < 0.05) had to be present and, in addition, there had to be no significant interaction between behavioral response and attention condition.
Color Tuning
Previous studies have reported significant S-cone inputs to area MT (Seidemann et al. 1999). To determine color tuning within our sample of neurons, we examined neuronal responses to stimulus color, pooling 128 directionally selective neurons across monkeys T and I, to whom the full set of 16 possible stimulus combinations (4 colors and 4 directions of motion) were presented. We performed a three-way ANOVA (with attention, color of stimulus, and direction of stimulus as factors) on activity elicited during the period of 40 to 400 ms from stimulus onset. Cells that showed a significant main effect of color (P < 0.05) were classified as exhibiting color tuning.
To determine the color tuning preferences across the subpopulation of 128 neurons, we calculated the average activity elicited by each stimulus color and plotted the vectors in a x-y coordinate system. A color-dependent activity vector was calculated for each neuron, according to the equations
where AR is visually evoked activity to red stimuli, AG to green, AB to blue, and AY to yellow stimuli. A strongly color-tuned cell would have a nonnormalized tuning vector length of maximally 1, while cells that show little preference for specific colors would have a vector length of closer to 0. To determine whether the distribution of vectors was clustered toward specific color dimensions (i.e., whether the distribution of vector angles was clustered), we calculated the magnitude of the mean vector across neurons as
and performed a Rayleigh test to determine whether the distribution was significantly different from random.
RESULTS
Behavioral Performance
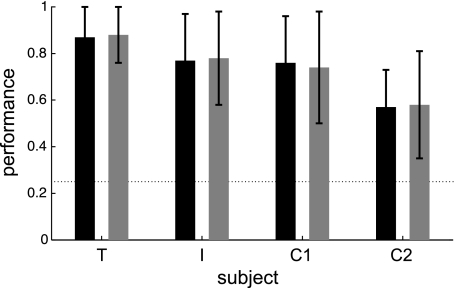
Given the task difficulty, all three monkeys performed the task considerably well (Fig. 2). Two of the monkeys (T and I) were trained on the full set of possible stimuli, with 16 combinations of color and direction, and achieved average performance levels of 0.88 ± 0.13 (monkey T, 87 sessions) and 0.78 ± 0.20 (monkey I, 17 sessions). Performance at chance level would equal 0.25. Monkey C was engaged in a slightly more demanding version of the task, in which the attention-task condition varied randomly from trial to trial (rather than remaining the same throughout a block of trials) and the period from cue offset to stimulus onset was variable. However, he was presented with a smaller set of stimulus combinations, initially with just 8 of 16 possible combinations, then with 12 for later sessions. For earlier sessions, half of his stimuli were congruent and half were incongruent, whereas for subsequent sessions, all of his stimuli were incongruent. He achieved levels of 0.75 (n = 29, SD 0.22) averaged across the first set of sessions and 0.57 (n = 18, SD 0.20) averaged across the second set of sessions.
Fig. 2.
Average performance of each subject was well above chance level (0.25, dotted line). Attend-color and attend-direction conditions are shown in black and gray separately for the different monkeys (indicated by the letters on the x-axis). Two sets of data are shown for monkey C, because one set was recorded with and the other without congruent stimulus conditions. Error bars indicate 1 SD.
One monkey (T) performed the direction discrimination task slightly better compared with the color discrimination task (attend-color mean correct = 0.87, SD 0.13, attend-direction mean correct= 0.88, SD 0.12; P = 0.0072, 2-sample t-test). No significant difference in performance between attention conditions was found for the other two monkeys [monkey I: attend-color mean = 0.77, SD 0.20, attend-direction mean = 0.78, SD 0.20 (P = 0.64); monkey C earlier sessions: attend-color mean = 0.76, SD 0.20, attend-direction mean = 0.74, SD 0.24 (P = 0.47); monkey C later sessions: attend-color mean = 0.57, SD 0.16, attend-direction mean = 0.58, SD 0.23 (P = 0.78)].
Acting on the Cue
The attention condition varied from one block of trials to the next; thus the relevant feature changed multiple times within each session. To determine how well monkeys heeded the cue, we examined performance in two subjects (T and I), looking only at the first trial of each block, as those were the trials during which the cue stimulus differed from that shown in preceding trials, excluding trials where the monkey broke fixation prematurely. Average performance was good across all such trials (monkey T: mean = 0.77, SD 0.07, n = 25; monkey I: mean = 0.77, SD 0.08, n = 10), and average performance was similar between attend-color (T: mean = 0.77, SD 0.11; I: mean = 0.71, SD 0.14) and attend-direction (T: mean = 0.77, SD 0.11; I: mean = 0.84, SD 0.11) trials. As monkey C engaged in a task in which the cue varied randomly from trial to trial, he was required to heed the cue on every trial in order to perform above chance performance. However, his performance was not as good as the performance of monkeys I and T. This indicates that monkey C heeded the cue only partially.
Congruency
For “congruent” stimulus combinations (e.g., a red grating moving to the left) the required behavioral response did not vary with attention condition, whereas for “incongruent” stimuli (e.g., a red grating moving to the right) the behavioral response could be in either of two directions, depending on the attention condition. To compare the effects of stimulus congruency on task difficulty, we analyzed performance separately for these two stimulus conditions for two subjects (monkeys T and I). In both monkeys, average performance across trials with congruent stimuli was significantly higher than that for incongruent trials [monkey T: congruent mean = 0.98, SD 0.06, incongruent mean = 0.70, SD 0.10 (P < 0.0001); monkey I: congruent mean = 0.99, SD 0.02, incongruent mean = 0.70, SD 0.12 (P < 0.0001, 2-sample t-test)]. When performance was compared between attention conditions (attend direction vs. attend color) for congruent trials, no effects of attention were found in either monkey [monkey T: attend-color mean = 0.96, SD 0.10, attend-direction mean = 1.00, SD 0.00 (P = 0.21); monkey I: attend-color mean = 0.98, SD 0.03, attend-direction mean = 1.00, SD 0.00 (P = 0.36)]. However, for incongruent trials we found significantly better performance under attend-direction conditions, compared with attend-color, for one of the monkeys [monkey T: attend-color mean = 0.70, SD 0.03, attend-direction mean = 0.70, SD 0.03 (P = 0.88); monkey I: attend-color mean = 0.61, SD 0.16, attend-direction mean = 0.78, SD 0.16 (P = 0.035, 2-sample t-test)].
Reaction Times
Monkey C was engaged in a reaction time task in which the grating appeared after randomly chosen intervals of 500, 1,000, 1,500, or 2,000 ms. We analyzed reaction times for this monkey, subdividing the entire response period (from stimulus onset onwards) into the “reaction time (RT) period” (from stimulus onset to initiation of motor response) and the “movement time” (from motor response initiation to termination by touching the peripheral touch bar). The RTs were stimulus dependent and varied across conditions, whereas the movement time remained relatively constant across all trials, indicating that the subject made his behavioral response decision prior to initiating the motor response. Average movement time was 149 ms across all trials (SD 74, n = 14,610), with no difference between attention or congruency conditions. A mean RT was calculated for each attention condition, based on RTs obtained across sessions. Significantly lower RTs occurred for attend-color compared with attend-direction trials for the earlier 18 sessions where only 8 stimulus conditions were presented, half of which were congruent and half of which were incongruent (RT direction: 353 ms, SD 55 ms, n = 3,578; RT color: 349 ms, SD 57 ms, n = 3,622; P = 0.0003, 2-sample t-test). On the other hand, significantly lower RTs occurred for attend-direction compared with attend-color trials for the second lot of 27 sessions, where 12 incongruent stimulus conditions were presented (mean RT direction: 328 ms, SD 39 ms, n = 3,704; RT color: 342 ms, SD 40 ms, n = 4,093; P < 0.001, 2-sample t-test). Subsequently, we compared RTs between attention conditions, using only trials with congruent or incongruent stimuli. There was no difference between attention conditions for congruent stimuli (RT direction congruent: 345 ms, SD 52 ms, n = 1,757; RT color congruent: 344 ms, SD 55 ms, n = 1,723; P = 0.7, 2-sample t-test). There was, however, a significant difference between attention conditions when the comparison was performed for incongruent stimuli (RT direction incongruent: 338 ms, SD 47 ms, n = 5,318; RT color incongruent: 345 ms, SD 46 ms, n = 5,812; P < 0.001, 2-sample t-test).
Basic Tuning Properties
We recorded from a total of 265 neurons from 3 monkeys in the task. With the calculated direction index (see experimental methods) 212 neurons were classified as directionally selective (individual ratios were monkey T: 109/153 DS neurons; monkey I: 19/22 DS neurons; monkey C: 84/90 DS neurons).
The stimuli used varied systematically in color, which allowed determination of whether evidence for color tuning was present in our sample. The analysis was restricted to directionally selective cells tested under all four color and direction of motion conditions (n = 128). A main effect of color was identified in 78 of 128 neurons (60.9%), of which almost all (77/78) also showed a significant interaction between color and direction of motion. Thus, for the vast majority of neurons, effects of stimulus color on activity could be partially, if not wholly, accounted for by the direction tuning preferences of the neuron.
Significance of color tuning for individual cells was assessed by calculating a three-way ANOVA with attention, color of stimulus, and direction of stimulus as factors on activity elicited during the period of 40–400 ms from stimulus onset. A main effect of color was identified in 78 of 128 cells (60.9%), of which almost all (77/78) also showed a significant interaction between color and direction of motion. Thus, for the vast majority of cells, the effects of stimulus color on activity could be partially, if not wholly, accounted for by the direction tuning preferences of the neuron.
Color tuning preferences across the subpopulation of 128 neurons were determined by calculating the average activity elicited by each stimulus color, plotting the vectors in a x-y coordinate system, and determining whether the distribution of vectors differed significantly from a random distribution. For the population of directionally selective neurons we found values of R = 0.0429 and Z = 0.236 (Z < 2.990, n = 128, P = 0.813) and for the subpopulation of color-modulated neurons R = 0.0830 and Z = 0.538 (Z < 2.986, n = 78, P = 0.591). Thus the distribution of vector was not significantly different from a random distribution, indicating that across the MT subpopulations there was no systematic preference for one color over another. Overall, our results confirm that strict color tuning in MT neurons is minimal, if not absent.
Feature Attention Modulation in Directionally Selective Neurons
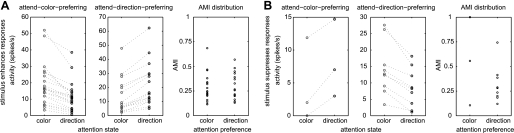
Neurons identified as DS (212 of 265 recorded neurons) were pooled across the three subjects (individual ratios were monkey T: 109/153 DS neurons, monkey I: 19/22 DS neurons, monkey C: 84/90 DS neurons). Of these, 47 neurons (22.2%) were modulated by feature-selective attention during the presentation of at least one particular stimulus (e.g., a red grating moving to the left), which we termed the “attention-modulated stimulus” (Table 1). The 47 neurons fell into three categories. Category 1 included neurons for which feature-selective attention effects occurred exclusively upon presentation of stimuli that elicited an excitatory response (relative to spontaneous rate, 35 neurons). Category 2 included neurons for which feature-selective attention was elicited during a stimulus-evoked suppression of activity (relative to spontaneous rate, 10 neurons). Category 3 included neurons that showed feature-selective attention effects during either stimulus-evoked excitation or suppression (depending on the stimulus presented, i.e., motion in preferred or antipreferred direction, 2 neurons). Of the 35 feature-selective attention-modulated neurons that fell into category 1, 20 neurons had a significantly higher response during attend-color trials for at least one attention-modulated stimulus (“attend-color-preferring, enhanced” neurons; nonparametric ANOVA, P < 0.05, FDR corrected, see Fig. 3A for an example), while 15 neurons had a significantly higher response during attend-direction trials for at least one attention-modulated stimulus (“attend-direction-preferring, enhanced” neurons, see Fig. 3B for an example). Eight of 10 neurons that fell into category 2 (stimulus-induced suppression) showed significantly more inhibition for the attend-direction condition (therefore classified as “attend-direction-preferring, suppressed” neurons; see Fig. 3D for an example), while 2 of the 10 neurons showed significantly stronger inhibition for the attend-color condition (therefore classified as “attend-color-preferring, suppressed” neurons; see Fig. 3C for an example). Of the two neurons that formed category 3, one was attend-color-preferring, while the other was attend-direction-preferring.
Table 1.
Direction-selective attention-modulated neurons separated according to whether attention occurred for stimulus-driven response enhancement or suppression and according to direction of stimulus motion relative to preferred
| Attention Task Modulation | Type of Modulation | Direction Preference | |||
|---|---|---|---|---|---|
| Attend-direction-preferring | 24 | Enhancement | 15 | PD only | 5 |
| Non-PD only | 8 | ||||
| PD and non-PD | 2 | ||||
| Suppression | 8 | PD only | 0 | ||
| Non-PD only | 6 | ||||
| PD and non-PD | 2 | ||||
| Enhancement and suppression | 1 | PD only | 0 | ||
| Non-PD only | 0 | ||||
| PD and non-PD | 1 | ||||
| Attend-color-preferring | 23 | Enhancement | 20 | PD only | 3 |
| Non-PD only | 13 | ||||
| PD and non-PD | 4 | ||||
| Suppression | 2 | PD only | 1 | ||
| Non-PD only | 1 | ||||
| PD and non-PD | 0 | ||||
| Enhancement and suppression | 1 | PD only | 0 | ||
| Non-PD only | 1 | ||||
| PD and non-PD | 0 | ||||
Values are numbers of direction-selective attention-modulated neurons, separated according to whether attention occurred for stimulus-driven response enhancement or suppression and separated according to the direction of stimulus motion relative to preferred. PD, attention-modulated stimuli that coincided with preferred direction; non-PD, attention-modulated stimuli that did not coincide with preferred direction.
Thus it should be noted that the attention condition preference assigned to each neuron depended on two factors: the difference in the amount of stimulus-evoked activity between attention conditions and the direction of modulatory effects (i.e., excitation or suppression of activity). No neurons were identified as being both attend-color-preferring and attend-direction-preferring (i.e., with an attend-direction preference for a particular stimulus combination and an attend-color preference for another stimulus); thus significantly modulated cells fell strictly into one of the two attentional classes.
Direction Preferences of Attention-Modulated Neurons
We examined the direction selectivity of the feature attention-modulated neurons to see whether modulations occurred during stimulus motion in preferred or nonpreferred directions (Table 1). We found that of the 15 attend-direction-preferring neurons that were enhanced in activity, 5 were enhanced only when motion was in PD, 8 were enhanced only when motion was in directions other than the PD, and 2 were enhanced during both PD and non-PD conditions. Of the 8 attend-direction-preferring neurons that were suppressed, 6 were suppressed only when motion was in directions other than PD and 2 were suppressed during both PD and non-PD conditions. The attend-direction-preferring neurons that were both excited and suppressed displayed these effects during both PD and non-PD conditions. Of the 20 attend-color-preferring neurons that were only enhanced, 3 were enhanced only when motion was in PD, 13 were enhanced only when motion was in directions other than PD, and 4 were enhanced during both PD and non-PD conditions. Of the 2 attend-color-preferring neurons that were only suppressed, 1 was suppressed only when motion was in PD (note that this was a neuron that exhibited direction-selective suppression, i.e., a moving stimulus never enhanced the activity relative to spontaneous firing), while the other was suppressed only when motion was in directions other than PD. The attend-color-preferring neurons that were both excited and suppressed displayed these effects only when motion was in directions other than PD. Overall, 10 of 24 attend-direction-preferring neurons were modulated in activity when motion was in PD, and 5 of these 10 were also modulated during motion in directions other than PD. Eight of 23 attend-color-preferring neurons were modulated in activity when motion was in PD, and 4 of these 10 were also modulated during motion in directions other than PD.
Attentional Modulation at the Population Level
As detailed in the previous section, a neuron significantly affected by feature-selective attention showed these effects of attention only for specific stimulus combinations (termed “attention-modulated stimuli”). We calculated the average activity across trials during which an attention-modulated stimulus was present. If more than one stimulus was capable of eliciting feature-selective attention, we averaged the responses across attention-modulated stimuli for that neuron. We then normalized each neuron's activity to the maximum firing rate that this neuron exhibited, for any attention-modulated stimuli. We did so separately for conditions in which the stimulus enhanced the activity and conditions in which it reduced the activity. Normalized average activity levels were calculated across neurons separately for each of the four groups of attention-modulated neurons (attend-color-preferring and attend-direction-preferring, further subdivided according to enhancement or suppression of stimulus-evoked activity).
Attend-color-preferring neurons.
For attend-color-preferring neurons that underwent a stimulus-evoked enhancement in activity (Fig. 4A), the average activity during attend-direction trials was 11.3 spikes/s (SE 2.0), while that during attend-color trials was 18.7 (SE 2.8), corresponding to an attention modulation effect of 7.4 spikes/s (SE 1.1), with an average AMIsig of 0.29 (SE 0.03). The average size of the difference in spike activity between the preferred and the nonpreferred attention condition, as a percentage of activity in the preferred attention condition, was 43.9% (SE 3.5%). For attend-color-preferring neurons that underwent a stimulus-evoked activity suppression (Fig. 4D), the average activity during attend-direction trials was 8.2 spikes/s (SE 3.4), while that during attend-color trials was 4.6 (SE 3.7), corresponding to an attention modulation effect of 3.6 spikes/s (SE 0.7), with an average AMIsig of −0.55 (SE 0.26). The average size of the difference in spike activity between the preferred and nonpreferred attention conditions, as a percentage of activity in the preferred attention condition, was 63.5% (SE 23.7%). The stimulus-evoked activity under the two attention conditions is displayed for each significantly modulated neuron in Fig. 5. Attend-color-preferring neurons are shown in Fig. 5, A and B, left.
Fig. 4.
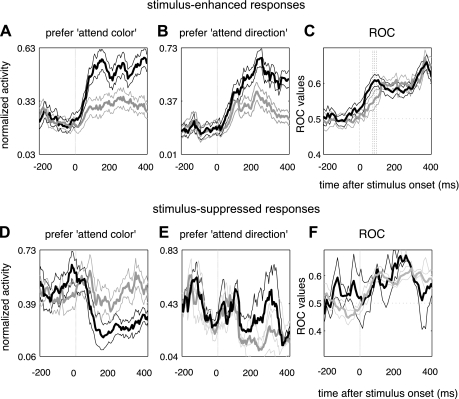
Normalized activity for attend-color-preferring and attend-direction-preferring neurons and conditions in which stimuli enhanced and suppressed neuronal responses. A–C: conditions in which stimulus presentation resulted in increased neuronal responses. D–F: conditions in which stimulus presentation resulted in reduced neuronal responses. A and D: normalized activity for attend-color-preferring neurons. B and E: normalized activity for attend-direction-preferring neurons. For plots of normalized activity, time bins are 5 ms in width and activity is smoothed with a half-Gaussian, with a σ value of 6 ms. C and F: time-resolved receiver operator characteristic (ROC) values for attend-color- and attend-direction-preferring neurons. In C, ROC values diverge from 0.5 earlier for attend-color-preferring than for attend-direction-preferring neurons. Log10(1/P) values are shown at bottom of each subpanel, to indicate if and when the respective comparisons are significant [P = 0.05, log10(1/α) shown by dotted horizontal line].
Fig. 5.
Size of feature-selective attention influences on single neurons. A: effect for stimuli that induced response enhancement in MT neurons. Left and center: absolute change in firing rate, averaged over the time period of 50–400 ms after stimulus onset for the sample of attend-color- and attend-direction-preferring neurons. Right: respective modulation indices [attention modulation index (AMI)] for these neurons. B: effect for stimuli that induced response suppression in MT neurons. Left and center: absolute change in firing rate, averaged over the time period of 50–400 ms after stimulus onset for the sample of attend-color- and attend-direction-preferring neurons. Right: respective modulation indices for these neurons.
Attend-direction-preferring neurons.
For attend-direction-preferring neurons that underwent a stimulus-evoked enhancement in activity (Fig. 4B), the average activity during attend-direction trials was 22.1 spikes/s (SE 3.9), while that during attend-color trials was 13.6 (SE 3.2), corresponding to an attention modulation effect of 8.5 spikes/s (SE 1.0), with an average AMIsig of 0.30 (SE 0.03). The average size of the difference in spike activity between the preferred and nonpreferred attention conditions, as a percentage of activity in the preferred attention condition, was 44.6% (SE 3.9%). For attend-direction-preferring neurons that underwent a stimulus-evoked suppression in activity (Fig. 4E), the average activity during attend-direction trials was 8.4 spikes/s (SE 2.0), while that during attend-color trials was 14.2 (SE 2.7), corresponding to an attention modulation effect of 5.8 spikes/s (SE 1.0), with an average AMIsig of −0.32 (SE 0.06). The stimulus-evoked activity under the two attention conditions is displayed for each significantly modulated neuron in Fig. 5. Attend-direction-preferring neurons are shown in Fig. 5, A and B, center.
Time Course of Attention Modulation
To identify time points when attention exerted its strongest influence, ROC values were calculated for each neuron that was significantly affected by attention, spanning the period from −50 to 400 ms, relative to stimulus onset, in bins of 80-ms width spaced 10 ms apart. ROC values were combined across neurons but kept separate for each of the two groups of attention-modulated neurons and for the stimulus-suppressed and -enhanced responses (Fig. 4, C and F).
A Wilcoxon signed-rank test was performed on attend-color- and attend-direction-preferring neurons to identify the first bins during which ROC values for each group of neurons diverged significantly from 0.5. For attend-color-preferring neurons where stimuli enhanced neuronal responses, the bins in which ROC values were significantly different from 0.5 started when the analysis bin was centered on 25 ms, up to that centered on 465 ms. For attend-direction preferring neurons where stimuli enhanced neuronal responses, the first significant bin occurred at 65 ms to 435 ms. Figure 4, A and B, show values of log10(1/P) for the two populations of neurons, while Fig. 4C shows the respective population ROC curves. Note that because 80-ms bin widths were used to calculate ROC values this method only gives an idea of whether the onset of attentional modulation differs between the two groups of neurons and does not yield an exact time of attentional modulation onset for each group (as the time point of 25 ms would be too early for this). The corresponding graphs for the subpopulation of neurons that underwent suppression are shown in Fig. 4, D–F.
On the basis of the ROC curves, we observed that the color-preferring group of neurons appeared to undergo modulations in activity slightly earlier than the direction-preferring group (Fig. 4C). To check whether the time course of modulation differed significantly between the two groups of attention-modulated neurons, we examined differences in firing rate during presentations of attention-modulated stimuli. For each neuron, we calculated the difference in activity between attention conditions in bins of 40 ms spaced 10 ms apart, spanning 40–90 ms from stimulus onset, as diffn = RPF − RNF, where n is the number of the time bin, RPF is the size of the response to the preferred feature, and RNF is the size of the response to the nonpreferred feature. Values of diffn were pooled across neurons within each group of color-preferring or direction-preferring neurons, and sets of pooled values were compared between the two groups for each bin, with a rank sum test. We applied a Bonferroni correction to adjust the α-level for the number of bins examined (P < 0.05/6 = 0.0083). Attentional modulations differed significantly between attend-direction-preferring and attend-color-preferring groups during the bins centered on 75–95 ms after stimulus onset. For neurons that underwent suppression due to feature-selective attention, no difference was observed for any bins, possibly because only a few neurons were included under this category.
To summarize, attend-color-preferring neurons that underwent enhancements in stimulus-evoked activity showed attentional modulation earlier than attend-direction-preferring neurons.
Variability of Visual Response
Previous studies have demonstrated that attention can increase response reliability (Mitchell et al. 2007), in addition to altering firing rates. To investigate the effects of feature-selective attention on the variability of neuronal responses we calculated the Fano factor (FF). The FF identifies changes in firing rate variation across trials. The analyses were performed for 1) our entire data set of directionally selective neurons, 2) significantly modulated neurons, but across the entire set of stimuli, and 3) significantly modulated neurons, but only for “attention-modulated stimuli,” i.e., those where significant effects of feature selective attention were found.
The FF during the early response period was significantly smaller than during the late response period (P < 0.001, paired t-test). The FF during the early response period was significantly smaller during attention to direction of motion compared with attention to stimulus color (P = 0.0078, paired t-test) if pooled across all stimulus conditions. A more detailed analysis related to direction of motion relative to preferred revealed that feature-selective attention usually resulted in smaller FFs when attention was assigned to direction of motion, but the effects were only significant (FDR corrected) when motion direction was 90° to preferred or opposite to preferred. FF was significantly smaller for attend-direction conditions than attend-color conditions when stimulus motion was 90° off the preferred direction (P = 0.0004, paired t-test); conversely, FF was significantly smaller for attend-color conditions (compared with attend-direction conditions) when stimulus motion was in the null direction (P = 0.0086, paired t-test). The FF during the late response period was also smaller for attention to direction of motion, but the effect was not significant (P = 0.105, paired t-test) when pooled across all stimulus conditions. A detailed analysis of the late response period revealed that a weak trend for significant differences was present for nonpreferred directions of motion (P = 0.06 for stimuli moving 90° relative to preferred and P = 0.03 for stimuli moving in null direction, signed-rank test) whereby FFs were smaller for attend-direction conditions (note that neither P value is significant after FDR correction). No significant differences were found when stimuli moved in the preferred direction (P = 0.129, early response, P = 0.208 late response, paired t-test).
To summarize, attention to direction of motion often changed the response variability of MT cells, provided the stimulus motion direction did not coincide with the preferred direction of the neurons. Attention to direction of motion under these conditions usually decreased response variability; the effects were more profound for the early response period, but the effects were not significant for all comparisons.
Subpopulation-Specific Effects of Attention Modulation
We found that 22.2% of directionally selective neurons showed significant effects of feature-selective attention. The finding that not all of the recorded neurons were influenced by feature-selective attention is not surprising, as MT neurons are responsive to a variety of different features, not solely to color or direction of motion, and equally not all MT neurons show effects of spatial attention in their response, despite the fact that many do undergo modulations (see, e.g., Treue and Maunsell 1996). Within the significantly affected sample, neurons could show higher activity when attention was directed at color than when it was directed at direction of motion (or vice versa), but the degree of modulation appeared to vary along a continuum. Despite this apparent variation along a continuum, MT cells could still form distinct subpopulations, which largely fall into either a “prefer-attend-color” or a “prefer-attend-direction” category, which our statistical analysis might have failed to detect because of the limited trial number that could be obtained for any given cell. If there were these distinct subpopulations, then an analysis of attention modulation index across the entire population of cells could reveal a bimodal distribution, one mode corresponding to “prefer-attend-color” and the other to “prefer-attend-direction” subpopulations. Frankly, we did not expect to find this but still calculated an attention modulation index for all our neurons (AMIpop); we calculated it across our significantly modulated neurons, irrespective of the stimulus (AMImod), and we restricted the calculation to attention-modulated stimuli only (AMIsig). We found that the distributions of AMIpop were mostly unimodal and centered at zero, regardless of the stimulus presented or the direction tuning preferences of the neurons, while not surprisingly AMIsig was bimodal (significantly different from unimodal, P < 0.05, Hartigan's dip test). Thus effects of feature-selective attention do not subdivide the entire sample of MT neurons into distributions with multiple modes.
Congruent and Incongruent Stimuli and Attention Modulation
Thus far, we examined data collected within each of the stimulus conditions, without specific regard for whether stimuli were congruent or incongruent (i.e., whether the behavioral response required was the same or differed between attention conditions for any given stimulus). Reaction times and performance data in our monkeys suggested that attentional demand was higher on incongruent trials, and thus attentional modulation might have been stronger for those trials. To analyze possible effects of congruency on attentional modulation, we performed our analysis for feature-attention-modulated neurons separately for congruent and incongruent trials, pooling data across 4 (number of congruent stimuli) and 12 (number of incongruent stimuli) stimulus conditions, respectively. Doing so, we found 12 neurons that were significantly modulated by attention when congruent trials were analyzed exclusively (7 attend-color-preferring neurons, 5 attend-direction-preferring neurons), and we found 22 neurons that were significantly modulated by feature-selective attention when incongruent trials were analyzed exclusively (10 attend-color-preferring neurons, 12 attend-direction-preferring neurons). Neuronal identities largely overlapped with those identified as being affected by feature-selective attention when congruent and incongruent trials were analyzed together. In total, 30 attention-modulated neurons were identified by this method.
We categorized neurons according to the congruency of the stimuli that elicited feature-selective attention effects. Table 2 provides a summary of the number of significantly modulated neurons. Some neurons were modulated exclusively during presentation of congruent stimuli and others only during presentation of incongruent stimuli. A total of 5 neurons had modulations of activity in the presence of congruent and incongruent stimuli (marked by asterisks in Table 2). Grouping of neurons in this manner, based on stimulus congruency, results in very few neurons per category, so we did not attempt to analyze feature-selective attention effects for each group. Note, furthermore, that monkey C (n = 84 cells total) was presented with only a subset of the 16 possible stimuli (8 conditions were used for the first 29 recording sessions, and 12 conditions were used for the following 18). Thus, for a substantial number of neurons, the fraction of trials during which congruent and incongruent stimuli were presented was not directly proportional to the mathematical ratio of possible congruent to incongruent stimulus combinations. Therefore, the tally of neurons shown in Table 2 does not convey information about the efficacy of congruent versus incongruent stimuli in eliciting feature-selective attention effects.
Table 2.
Significantly modulated neurons grouped according to whether they were attend-color-preferring or attend-motion-preferring, whether attentional modulation occurred for stimuli that enhanced or suppressed the response, and whether attentional effects occurred for congruent or incongruent stimuli (or both)
| Attention Task Modulation | Type of Modulation | Congruency of Condition | |||
|---|---|---|---|---|---|
| Attend-direction-preferring | 24 | Enhancement | 15 | Congruent only | 2 |
| Incongruent only | 11 | ||||
| Congruent and incongruent | 2* | ||||
| Suppression | 8 | Congruent only | 1 | ||
| Incongruent only | 6 | ||||
| Congruent and incongruent | 1* | ||||
| Enhancement and suppression | 1 | Congruent only | 0 | ||
| Incongruent only | 1 | ||||
| Congruent and incongruent | 0 | ||||
| Attend-color-preferring | 23 | Enhancement | 20 | Congruent only | 1 |
| Incongruent only | 17 | ||||
| Congruent and incongruent | 2* | ||||
| Suppression | 2 | Congruent only | 0 | ||
| Incongruent only | 2 | ||||
| Congruent and incongruent | 0 | ||||
| Enhancement and suppression | 1 | Congruent only | 0 | ||
| Incongruent only | 1 | ||||
| Congruent and incongruent | 0 | ||||
Values are numbers of significantly modulated neurons, grouped according to whether they were attend-color-preferring or attend-motion-preferring, according to whether attentional modulation occurred for stimuli that enhanced the response or suppressed the response, and according to whether attentional effects occurred for congruent or incongruent stimuli (or both*). Note that the numbers of neurons shown do not convey information about the efficacy of congruent vs. incongruent stimuli in eliciting feature-selective attention effects, as the ratio of congruent to incongruent stimulus conditions presented varied between monkeys and sessions.
To determine whether incongruent stimuli yielded attentional modulation more often than congruent stimuli, a χ2-test with Yates correction was performed that rejected the null hypothesis (P > 0.05). We thus conclude that congruent stimuli were as likely to result in significant attentional modulation as incongruent stimuli.
Response Modulation in Relation to Behavior
Translation of attended visual features into behaviorally relevant categories.
A related study performed in area V4 has demonstrated that a significant subset of neurons modulate their response in relation to the behavioral decision, not the attention dimension. To determine whether similar effects occur in macaque area MT, we analyzed neuronal activity from incongruent trials in relation to the monkey's choice, irrespective of the attentional dimension. In monkey T, a small minority of neurons (3/128) exhibited effects due to behavioral response direction; no such neurons were found in monkeys I and C. A two-way ANOVA demonstrated a significant main effect of movement direction in these three neurons and detected no influence of attention condition. This behavioral response effect was only evident during the epoch immediately preceding the motor response, from 400 to 490 ms after stimulus onset, and was absent from the other five epochs (see experimental methods for details).
Choice probabilities.
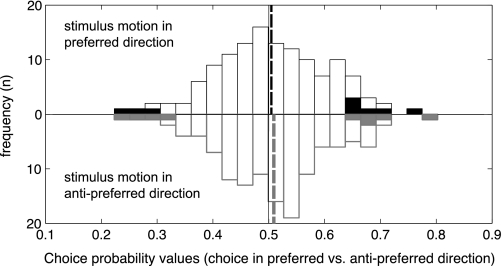
For each direction-selective neuron, we determined the activity distribution when a stimulus moved in the preferred direction and the monkey's choice was in the preferred direction versus the distribution when the stimulus moved in the preferred direction and the choice was in null direction [note that this would still be a correct decision—the choice was in the null direction as the attentional condition (attend color) demanded so]. We did the same for stimuli moving in null (antipreferred) directions. The ensuing CPs yielded two distributions, which are shown in Fig. 6 (stimulus motion in the PD is shown as positive on the y-axis; stimulus motion in the ND is shown as negative on the y-axis). Neither of the two CP distributions had a median significantly different from 0.5 (PD distribution: P = 0.6940, ND distribution: P = 0.2776, signed-rank test). Also, the two CP distributions did not differ significantly from one another (PD vs. ND distributions: P = 0.6693, signed-rank test).
Fig. 6.
Distribution of choice probabilities. Choice probabilities calculated from responses of direction-selective neurons. Positive y-axis (black bars) shows choice probabilities when stimuli moved in preferred direction of the neurons and negative y-axis (gray bars) choice probabilities when stimuli moved in antipreferred direction of the neurons. Filled bars denote significant choice probabilities.
On the whole, our findings suggest that, given the task conditions employed, area MT contains neurons that change their activity according to the feature dimension attended to, while modulation of activity based on the subject's impending behavioral decision is very rare.
DISCUSSION
Feature-selective attention modulated single-unit activity in macaque visual area MT when subjects attended selectively to stimulus features (color vs. motion direction) and were required to report the currently relevant feature value. When we examined average activity levels within the two subpopulations of neurons that responded preferentially to one attention condition over the other, we found that these feature-selective effects appeared significantly earlier when subjects attended to the color of a moving grating than when they attended to its direction of motion. Furthermore, feature-selective attention modulations of single-neuron activity emerged in the presence of motion in a variety of directions, and not necessarily in the neuron's preferred direction. The attention state of the animal also altered the variability of visually evoked responses. We found limited evidence for the existence of firing rate modulations due to behavioral choice in area MT when correct but opposite behavioral responses to stimuli moving in the same direction were compared under the two attend conditions.
Feature-Selective Attention in the Visual System
A number of imaging studies have investigated effects of feature-selective attention in specific parts of the visual cortex by requiring subjects to attend to a particular dimension such as color or direction of motion. These reports suggest that attention-induced BOLD activity modulations were consistent with the known functional specialization of an area (Beauchamp et al. 1997; Chawla et al. 1999; Liu et al. 2003; McMains et al. 2007). Feature-selective modulations have been detected in the left inferior parietal lobule (including the human analog of area MT and MST) while subjects attended to speed of motion instead of object shape or color during PET functional mapping (Corbetta et al. 1991). Chawla et al. (1999) observed modulations in stimulus-evoked as well as baseline hemodynamic activity when human subjects attended to color or to motion. In V4, a color-processing region, the attend-color condition elicited higher responses than the attend-motion condition, whereas in V5, a motion-processing area, the opposite pattern occurred (Chawla et al. 1999). McMains et al. (2007) performed a fMRI “feature competition” study, in which human subjects attended either to color or to motion, and found similar results. Attention modulation was stronger for attend-color than for attend-motion in area TEO, whereas modulation was stronger for attend-motion in area MT (McMains et al. 2007). An earlier study by Beauchamp et al. found the same phenomenon in hMT+ (the human middle temporal complex), with higher activity across the region during attend-direction, compared with attend-color, blocks (Beauchamp et al. 1997).
Feature-Selective Attention in Electrophysiology
The above studies identified large-scale effects of feature-selective attention, in which a preference for one modality over another was seen across an entire functional region (e.g., higher activation during attend-motion in area MT but during attend-color in V4). However, electrophysiology data from Mirabella et al. (2007) indicate that opposing effects of feature-selective attention do exist simultaneously in V4 at the level of single neurons. In their study, 42 of the 136 neurons recorded displayed significant effects of feature-selective attention, with approximately half of these neurons responding preferentially under attend-color and the other half to attend-orientation conditions. Thus the proportion of cells affected by feature-selective attention in the study of Mirabella et al (2007) is slightly larger than what we found, but the differences are modest (22% vs. 31%). Attention modulated the response size by ∼21% in their study, which is not as large as the 45.7% change that we report here. Importantly, they also did not find a systematic relationship between the attended feature and the neuronal selectivity. Neurons that were color selective did not become more selective for color during the color task than during the orientation task, or vice versa (Mirabella et al. 2007). This lack of a match between neuronal feature selectivity and its feature-selective attentional modulation might have been due to the fact that both attended features are strongly represented in V4 neurons, i.e., V4 contains neurons that are orientation- and/or color-selective. In area MT color is a feature that is not explicitly represented, although MT neurons can exploit chromatic cues for motion signaling (Croner and Albright 1999; Dobkins and Albright 1994; Thiele et al. 1999b, 2001). Given this high selectivity for direction of motion in MT neurons, we reasoned that feature-selective attention might be much more pronounced when attention was directed to direction of motion compared with stimulus color. Contrary to our prediction, we found neurons within area MT that were preferentially modulated during attend-color trials, and their number was even higher than the number of neurons that preferred attend-motion. In almost all cases of feature-selective attention modulation, the effects were seen for one (or a few) specific stimulus combinations (e.g., for a red, rightward-moving stimulus rather than for a blue, downward-moving one). Despite the strongly motion-attuned nature of MT neurons, we found that feature attention modulation did not occur solely (or even preferentially) in the presence of the neuron's preferred motion direction but often occurred during motion in directions different from the neuron's preferred. This was the case for color-preferring as well as direction-preferring neurons. For a small number of neurons (4/47), responses underwent feature attention modulations in the presence of motion in preferred as well as nonpreferred directions, while for the rest of the neurons (43/47), feature attention effects occurred either within the quadrant containing the preferred direction or outside of it, but not both.
Mirabella et al. (2007) found, in their study on feature-selective attention in V4, that effects of attention modulation surfaced in a larger number of neurons when their analysis only examined trials with incongruent stimuli compared with when they only observed trials with congruent stimuli. It is possible that incongruent trials required an animal to focus attention specifically on the dimension of relevance and ignore the other, nonrelevant dimension, making the effect of feature-selective attention more robust (Mirabella et al. 2007). Our findings in area MT, based on the same strategy of dividing data into congruent and incongruent batches, also returned a higher number of attention-modulated neurons for the incongruent-only analysis than for the congruent-only analysis, although in our study the number of attention-modulated neurons differed by a factor of two, which is still much lower than the sixfold increase seen by Mirabella et al. (2007), and the difference in our study was not significant.
Behavioral Choice and the Visual System
Neuronal activity that reflects a subject's behavioral choice, or the perceptual interpretation rather than the visual properties of stimuli, have been reported in area V2 (Nienborg and Cumming 2009), V4 (Mirabella et al. 2007), and MT (Britten et al. 1996; Dodd et al. 2001; Krug 2004; Krug et al. 2004; Thiele et al. 1999a; Thiele and Hoffmann 1996, 2008). Previous examinations of the time course of behavioral choice modulations showed that these effects emerge somewhat later than the primary visual response and that often the size of these modulations reaches its peak just before the subject indicates its behavioral decision. Mirabella et al. (2007) identified such behavioral response modulations in 44 of their 152 (28.9%) V4 neurons. They found that representations of the direction in which the impending behavioral response would be made appeared at ∼200 ms after stimulus onset and grew progressively larger, amounting to a change of 35.5% in activity, during the epoch preceding motor response execution. We found little evidence for such effects in area MT (only 2.4% of neurons recorded). Basically, the results of Mirabella et al. (2007) suggest that the late response of these V4 neurons reflects a form of behavioral response categorization/classification. The failure to find a significant number of neurons with such response-mapping properties in area MT is in line with previous reports that show little involvement of area MT in stimulus classification/categorization (Freedman and Assad 2006).
One recent paper has explored the influence of feature-selective attention in area MT explicitly, but in a somewhat different manner than our study. Katzner et al. (2009) trained animals to either report a change in direction of a coherently moving random dot pattern at a cued location or report a change of the dot pattern's color while ignoring possible direction changes at that location. While this study explicitly tested the effects of attention to color versus attention to direction change, the animals were not required to report the feature value specifically. Thus the study differs in one important aspect from the manipulations performed in our study and that of Mirabella et al. (2007). Katzner et al. (2009) reported that attention to either feature resulted in an upregulation of firing rates at the attended location at the population level. They did not report whether there were different neuronal groups, those preferring attention to motion direction versus those preferring attention to color. When averaging our results across the two neuronal groups, we also found that the average population activity for the two attention conditions was basically identical. Nevertheless, we found an approximately equal number of neurons that only showed attentional modulation when attention was directed to motion direction, while others only showed attentional modulation when attention was directed to color. This is somewhat reminiscent of a binocular rivalry study (Logothetis and Schall 1989) in which ∼11% of neurons in area MT showed higher activity when the currently reported percept was in a neuron's preferred direction while ∼11% of MT neurons fired more when the percept was dominated by the nonpreferred stimulus. Thus the average activity across the two populations showed no perception-related modulation in that study (Logothetis and Schall 1989). The dichotomy of neuronal classes could suggest that MT neurons are differentially pooled for subsequent processing to enable adequate rule-dependent behavior, as shown previously for layer 5 MT neurons (Hoffmann et al. 2002). However, such a proposal is also fraught with problems. If there is no straightforward relationship between attentional modulation and stimulus preference, then it could be argued that for some cells “attentional spikes” and “stimulus spikes” would need to be read out differentially coming from the same neuron. While it may not appear immediately obvious how this could be implemented functionally, recent analyses have shown that decoding the locus of attention and the stimulus contrast is possible in V1 cells, provided only a subset of cells is affected by attention (Pooresmaeili et al. 2010). Decoding of the stimulus would then be possible from cells not affected by attention, while decoding the currently attended feature would be possible from the activity of cells affected by attention.
In summary, we found that feature-selective attention can significantly modulate MT neuronal activity. Neurons affected by feature-selective attention broadly fell into one of two classes, namely, those that showed an upmodulation of firing rates when attention was directed to the stimulus motion and those that showed an upmodulation of firing rates when attention was directed to the stimulus color. Surprisingly, the upmodulation occurred earlier for the latter class of neurons. There was little if any evidence that area MT participates in response classification, such that the neuronal activity signaled the upcoming behavioral response rather than the stimulus and/or the attention condition.
GRANTS
The work was supported by the Medical Research Council (MRC) (G0700976), Wellcome Trust, Deutsche Forschungsgemeinschaft (DFG), the Human Frontier Science Program (HFSP), Howard Hughes, and the Hertie Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.C. and A.T. analyzed data; X.C., K.P.H., T.D.A., and A.T. interpreted results of experiments; X.C. prepared figures; X.C. and A.T. drafted manuscript; X.C., K.P.H., T.D.A., and A.T. edited and revised manuscript; X.C., K.P.H., T.D.A., and A.T. approved final version of manuscript; K.P.H. and A.T. conception and design of research; A.T. performed experiments.
REFERENCES
- Beauchamp MS, Cox RW, Deyoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol 78: 516–520, 1997 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300, 1995 [Google Scholar]
- Boynton GM. Attention and visual perception. Curr Opin Neurobiol 15: 465–469, 2005 [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996 [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745–4765, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci 10: 1157–1169, 1993 [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 2: 671–676, 1999 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 11: 2383–2402, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croner LJ, Albright TD. Segmentation by color influences responses of motion-sensitive neurons in the cortical middle temporal visual area. J Neurosci 19: 3935–3951, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkins KR, Albright TD. What happens if it changes color when it moves?: the nature of chromatic input to macaque visual area MT. J Neurosci 14: 4854–4870, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JV, Krug K, Cumming BG, Parker AJ. Perceptually bistable three-dimensional figures evoke high choice probabilities in cortical area MT. J Neurosci 21: 4809–4821, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah M, Stoner GR, Reynolds JH. Stimulus-specific competitive selection in macaque extrastriate visual area V4. Proc Natl Acad Sci USA 104: 4165–4169, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature 443: 85–88, 2006 [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC, Beusmans JM, Carandini M, Zaidi Q, Movshon JA. Chromatic properties of neurons in macaque MT. Vis Neurosci 11: 455–466, 1994 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Oxford, UK: Wiley, 1966 [Google Scholar]
- Hayden BY, Gallant JL. Combined effects of spatial and feature-based attention on responses of V4 neurons. Vision Res 49: 1182–1187, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Gallant JL. Time course of attention reveals different mechanisms for spatial and feature-based attention in area V4. Neuron 47: 637–643, 2005 [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Bremmer F, Thiele A, Distler C. Directional asymmetry of neurons in cortical areas MT and MST projecting to the NOT-DTN in macaques. J Neurophysiol 87: 2113–2123, 2002 [DOI] [PubMed] [Google Scholar]
- Katzner S, Busse L, Treue S. Attention to the color of a moving stimulus modulates motion-signal processing in macaque area MT: evidence for a unified attentional system. Front Syst Neurosci 3: 12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug K. A common neuronal code for perceptual processes in visual cortex? Comparing choice and attentional correlates in V5/MT. Philos Trans R Soc Lond B Biol Sci 359: 929–941, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug K, Cumming BG, Parker AJ. Comparing perceptual signals of single V5/MT neurons in two binocular depth tasks. J Neurophysiol 92: 1586–1596, 2004 [DOI] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cereb Cortex 13: 1334–1343, 2003 [DOI] [PubMed] [Google Scholar]
- Logothetis N, Schall J. Neuronal correlates of subjective visual perception. Science 245: 761–763, 1989 [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Treue S. Feature-based attention in visual cortex. Trends Neurosci 29: 317–322, 2006 [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JHR. Attention to both space and feature modulates neuronal responses in macaque area V4. J Neurophysiol 83: 1751–1755, 2000 [DOI] [PubMed] [Google Scholar]
- McMains SA, Fehd HM, Emmanouil TA, Kastner S. Mechanisms of feature- and space-based attention: response modulation and baseline increases. J Neurophysiol 98: 2110–2121, 2007 [DOI] [PubMed] [Google Scholar]
- Mirabella G, Bertini G, Samengo I, Kilavik BE, Frilli D, Della Libera C, Chelazzi L. Neurons in area V4 of the macaque translate attended visual features into behaviorally relevant categories. Neuron 54: 303–318, 2007 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007 [DOI] [PubMed] [Google Scholar]
- Nienborg H, Cumming BG. Decision-related activity in sensory neurons reflects more than a neuron's causal effect. Nature 459: 89–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzwahl DR, Treue S. Combining spatial and feature-based attention within the receptive field of MT neurons. Vision Res 49: 1188–1193, 2009 [DOI] [PubMed] [Google Scholar]
- Pooresmaeili A, Poort J, Thiele A, Roelfsema PR. Separable codes for attention and luminance contrast in the primary visual cortex. J Neurosci 30: 12701–12711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci 27: 611–647, 2004 [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Alborzian S, Stoner GR. Exogenously cued attention triggers competitive selection of surfaces. Vision Res 43: 59–66, 2003 [DOI] [PubMed] [Google Scholar]
- Riečanský I, Thiele A, Distler C, Hoffmann KP. Chromatic sensitivity of neurones in area MT of the anaesthetised macaque monkey compared to human motion perception. Exp Brain Res 167: 504–525, 2005 [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VAF, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature 395: 376–381, 1998 [DOI] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci 5: 631–632, 2002 [DOI] [PubMed] [Google Scholar]
- Seidemann E, Poirson AB, Wandell BA, Newsome WT. Color signals in area MT of the macaque monkey. Neuron 24: 911–917, 1999 [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. A unified model for perceptual learning. Trends Cogn Sci 9: 329–334, 2005 [DOI] [PubMed] [Google Scholar]
- Thiele A, Distler C, Hoffmann KP. Decision-related activity in the macaque dorsal visual pathway. Eur J Neurosci 11: 2044–2058, 1999a [DOI] [PubMed] [Google Scholar]
- Thiele A, Dobkins KR, Albright TD. The contribution of color to motion processing in macaque middle temporal area. J Neurosci 19: 6571–6587, 1999b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Dobkins KR, Albright TD. Neural correlates of chromatic motion perception. Neuron 32: 351–358, 2001 [DOI] [PubMed] [Google Scholar]
- Thiele A, Hoffmann KP. Neuronal activity in MST and STPp, but not MT changes systematically with stimulus-independent decisions. Neuroreport 7: 971–976, 1996 [DOI] [PubMed] [Google Scholar]
- Thiele A, Hoffmann KP. Neuronal firing rate, inter-neuron correlation and synchrony in area MT are correlated with directional choices during stimulus and reward expectation. Exp Brain Res 188: 559–577, 2008 [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature 382: 539–541, 1996 [DOI] [PubMed] [Google Scholar]
- Vogels R, Spileers W, Orban GA. The response variability of striate cortical neurons in the behaving monkey. Exp Brain Res 77: 432–436, 1989 [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol 13: 187–193, 2003 [DOI] [PubMed] [Google Scholar]