Abstract
A recent study (Di Lazzaro et al. J Neurophysiol 105: 2150–2156, 2011) describes the findings from a rigorous comparison on the effects of several popular variations of transcranial magnetic stimulation (TMS) protocols. The results demonstrate that excitatory and inhibitory neural networks may be independently modulated based on TMS protocol selection. Moreover, the within-group replication of multiple between-group experiments suggests that independent evaluations of TMS parameters will continue to inform and guide future TMS research.
Keywords: repetitive transcranial magnetic stimulation, theta burst stimulation, paired associative stimulation, neural plasticity
most human in vivo experimental techniques rely on correlational measures of neural activity to infer the function of distinct brain regions. Unfortunately, correlational measures are not able to impart causal inference, thereby limiting their explanatory power. Thus, recent years have seen an exponential increase in the use of transcranial magnetic stimulation (TMS), a noninvasive method to artificially manipulate activity in cortical neural networks, which enables direct causal measures of cortical function and plasticity. However, there is a wide array of TMS parameters that may dramatically affect the neural and behavioral outcomes following stimulation, and it is therefore crucial to delineate these interactions.
While a single TMS pulse may promote neural activity for hundreds of milliseconds (Allen et al. 2007), repetitive TMS (rTMS) can induce changes in the sensitivity of excitatory and inhibitory neural networks that can persist beyond the stimulation period (Hoogendam et al. 2010). Application of high-frequency rTMS (5 Hz or greater) induces increased excitability (Maeda et al. 2000), whereas low-frequency rTMS (1 Hz or less) produces decreased cortical excitability (Chen et al. 1997). However, very low-frequency rTMS (e.g., 0.05 Hz) may yield differential measures of plasticity during paired associative stimulation (PAS) based on pulse timing. For example, if peripheral nerve stimulation is followed by a TMS pulse to the motor cortex at a latency longer than the time required for afferent inputs to reach the cortex (e.g., 25 ms: PAS25), increased cortical excitability is induced following a sufficient number of paired pulses (Stefan et al. 2000). However, if the TMS pulse occurs faster than afferent input to cortex (e.g., 10 ms: PAS10), decreased cortical excitability is observed (Wolters et al. 2003). The outcome is even more complex when successive rTMS pulses are applied. For example, the theta burst stimulation (TBS) method utilizes very high-frequency (e.g., 50 Hz), low-intensity stimulation applied in brief bursts (e.g., 5 Hz) using either continuous (cTBS) or intermittent (iTBS) stimulation. Interestingly, these protocols elicit differential neural effects akin to long-term depression and long-term potentiation, respectively (Hoogendam et al. 2010).
Despite numerous studies documenting the differential effects of various TMS protocols, the focus of this research is typically on excitatory circuits and how stimulation may affect the local region of cortex targeted by TMS. Much less is known regarding the effects of TMS on inhibitory circuits and whether TMS-induced changes translate to distant interconnected regions such as the nonstimulated contralateral cortex. In a recent issue of the Journal of Neurophysiology, Di Lazzaro and colleagues (2011) addressed this gap in knowledge by identifying the most effective rTMS paradigms for modulating excitatory and inhibitory motor networks throughout both local and distant interconnected regions. Furthermore, this research fulfilled the need for a rigorous, head-to-head comparison of predominant rTMS protocols across the same paradigm and participants. It is known that rTMS effects elicit high levels of interindividual variability (Maeda et al. 2000), and previous research on TMS effects often employ a single TMS protocol. Thus, comparing TMS protocols across studies may not be effective due to a lack of internal control via differential paradigms and participant pools. Here, Di Lazzaro and colleagues (2011) utilize a within-subject design and a common experimental paradigm across TMS protocols to establish a proper foundation for the characterization of TMS effects.
Di Lazzaro and colleagues (2011) assessed six different TMS paradigms: 1) 1 Hz rTMS, 2) 5 Hz rTMS, 3) cTBS, 4) iTBS, 5) PAS10, and 6) PAS25. To evaluate the effects of the TMS protocol variants, a battery of outcome measures were assessed: resting motor threshold (RMT), active motor threshold (AMT), motor-evoked potentials (MEP), contralateral silent period (cSP), ipsilateral silent period (iSP), short interval intracortical inhibition (SICI), intracortical facilitation (ICF), and short latency afferent inhibition (SAI). All TMS measures were evaluated bilaterally prior to rTMS, immediately following stimulation, and 30 min after stimulation.
Di Lazzaro et al. (2011) show that the RMT was increased most by PAS10 and decreased by PAS25 at the stimulated hemisphere, whereas no changes were observed to the AMT. The MEP from the stimulated hemisphere was observed to be enhanced by iTBS and PAS25 and suppressed by 1 Hz rTMS, cTBS, and PAS10. The cSP was observed to increase at the stimulated hemisphere following 1 Hz rTMS, whereas no changes occurred to the iSP. Finally, a decrease in the SICI at the stimulated hemisphere was demonstrated by PAS10. None of the TMS protocols elicited changes to the ICF or SAI. It is important to note that all significant results were reported for the stimulated hemisphere only. However, iTBS did display a regular decrease of the MEP in the contralateral hemisphere, similar to previous research from the same group (Di Lazzaro et al. 2008).
Together, these results highlight the ability to independently modulate excitatory and inhibitory neural networks through specific manipulations of the TMS protocol. More importantly, although Di Lazzaro et al. (2011) note that a limitation of their study is that only a few commonly used TMS protocols were assessed, their results demonstrate that it is possible to generalize the effects of a TMS protocol manipulation across groups and studies. This is crucial to the field of TMS and opens up the possibility for future TMS study designs to make good use of the compendium of extant TMS data to inform the selection of a TMS protocol, something that would have previously been discouraged due to the perceived interindividual variability of TMS outcomes. In addition to increasing the gravity of previous TMS studies, this also encourages future studies to contribute to the characterization of TMS effects.
To fully appreciate the contribution of this unparalleled exploration into TMS methodology, it is important to consider the limitations of this study by Di Lazzaro et al. (2011). Aside from extraneous factors that are difficult or not possible to control by the researcher, such as initial neural activation state, several modular selections of Di Lazzaro et al. (2011) have the effect of influencing the generalizability of their findings. These include attributes such as the cortical region targeted for stimulation, the specific TMS protocols employed, the selected parameters within each protocol, the tests used to evaluate said protocols, and the poststimulation time required to complete the tests. Although it is unrealistic to thoroughly compare all TMS protocol permutations, the results must be understood as a product of the selected study parameters.
Although the findings of Di Lazzaro et al. (2011) are generally consistent with previous reports on the effects of individual TMS protocols, some aftereffects were not observed as reported in previous research. These included the absence of MEP amplitude enhancement after 5 Hz rTMS, contralateral MEP amplitude suppression following iTBS, as well as modulation of SICI after TBS and 5 Hz rTMS. The authors attribute the discrepancy as either the result of high variability or the limited duration of the effects. The latter was considered more relevant due to the sizeable amount of time required to complete the battery of tests. Furthermore, it was noted that the selection of TMS stimulation parameters may have influenced the results. To address these issues, future studies should take into account several considerations: 1) attempt to limit the poststimulation testing delay by focusing on a subset of tests, 2) acquire neuroimaging data to help characterize interindividual variability of TMS effects, and 3) manipulate TMS stimulation parameters within each TMS protocol.
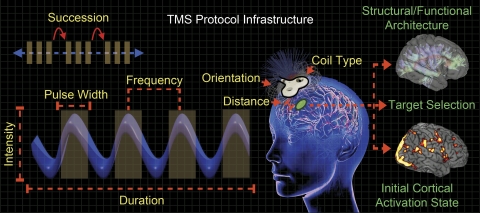
Indeed, there is a great deal of divergent factors that may influence the effects of TMS (Fig. 1). Rothkegel et al. (2010) evaluated the duration of TMS pulses as a contrast to intensity manipulations. Their results suggest longer pulse durations reduce motor thresholds, similar to how more intense TMS pulses induce increased neural responses. Moreover, the effective intensity of TMS is highly contingent on the distance from the TMS coil to an underlying cortical target during stimulation (McConnell et al. 2001), as the strength of the magnetic field decreases exponentially with increasing distance. Therefore, intensity may be adjusted based on the scalp-to-cortex distance, which varies between individuals and across cortical regions. Generally, stimulation protocols dictate that TMS intensity is to be calibrated to an individual participant's motor threshold or phosphene threshold (minimum intensity required to elicit a motor response when applied to motor cortex, or the perception of phosphenes when applied to visual cortex, respectively), yet, coil-to-cortex distance does not correlate with motor threshold (Kozel et al. 2000). Furthermore, motor thresholds do not correlate with phosphene threshold (Stewart et al. 2001), underscoring the variability of TMS effects across individuals and brain regions and highlighting the importance of cortical stimulation target in TMS studies.
Fig. 1.
Factors that influence the effects of transcranial magnetic stimulation (TMS). Mechanical factors (yellow labels) describe TMS parameters that may be adjusted for a desired outcome. Biological factors (green labels) determine where to stimulate and may help guide the selection of specific TMS parameters. All of these factors comprise a TMS protocol, and manipulating any one of them may result in differential outcomes (for details, see text). However, mechanical factors are the most straightforward to address when planning a study, whereas biological factors require a priori knowledge of the neural system to be stimulated.
Interestingly, neurons close to their firing threshold are more likely to be affected by a TMS pulse than neurons at rest, which suggests that cortical activation state at the time of stimulation is a preeminent factor in determining the effects of TMS, with the potential to influence the expected outcome of certain stimulation parameters (Pasley et al. 2009). It is plausible that initial cortical activation state may have played a role in a recent study that found a relationship between the motor and phosphene threshold when using a consistent paradigm for each thresholding procedure (Deblieck et al. 2008). This would indicate that the specific cortical region of interest should not solely dictate the appropriate experimental TMS intensity, but also the experimental environment and the participant's behavioral state. However, this hypothesis has not yet been adequately addressed.
Another factor that may influence the effects of TMS is the composition of the underlying structural architecture and functional organization of the stimulated cortical region (Fig. 1) (Pasley et al. 2009). TMS pulses may affect distant neural regions via propagation through anatomical pathways, which may be biased by functional connectivity, where cognitive factors may influence the transmission along the available anatomical infrastructure (Ruff et al. 2009). A primary aim of Di Lazzaro et al. (2011) was to investigate the propensity for the TMS signal to propagate along connected pathways from the stimulation site, effectively influencing distal brain regions. However, as they observed no changes in distant interconnected regions (contrary to previous reports), additional research will be required to delineate how remote TMS effects may be observed and the extent to which functional connectivity biasing may occur.
While consideration of biological factors is crucial, the selection of TMS protocol is the most practical and accessible factor of influence. In this light, experiments like Di Lazzaro et al. (2011), which provide empirical evidence examining the effect of manipulating TMS parameters under generalizable circumstances, are vital. In their investigation, Di Lazzaro et al. (2011) manipulated stimulation frequency and succession. However, it should be noted that there are several flexible elements comprising a TMS protocol (Fig. 1), each of which is important to consider when designing an inductive sequence. These criteria include attributes like the overall duration of stimulation, pulse width, stimulus intensity, coil type, coil-to-cortex distance, as well as magnetic field orientation, each of which has been observed to uniquely influence the ultimate outcome of TMS administration (Chen et al. 1997; McConnell et al. 2001; Rothkegel et al. 2010).
The findings of Di Lazzaro et al. (2011) demonstrate that by using different TMS protocols it is possible to target specific cortical excitatory and inhibitory networks. However, it is important to remain cognizant of the many factors that can potentially determine the effects of TMS. Although Di Lazzaro et al. (2011) assessed some of the most common TMS protocols, there is a vast array of TMS parameters that can be parametrically assessed. Fortunately, the current results replicated previous findings to show that TMS effects may be generalized between different experiments, thereby limiting the necessity of a parametric assessment via a within-subject design. Yet, additional research will be required to verify the supposition that internal controls may not be required to compare TMS protocols across different studies. Furthermore, future research should incorporate neuroimaging data to help understand the basis for interindividual variability of TMS effects. Overall, the data reported by Di Lazzaro et al. (2011) will no doubt serve to inform and guide future researchers in their endeavor to assert causality through the use of TMS.
GRANTS
T. P. Zanto was supported by a postdoctoral fellowship from National Institutes of Health (4F32AG-030249-03).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.T.R. and T.P.Z. interpreted results of experiments; M.T.R. prepared figures; M.T.R. and T.P.Z. drafted manuscript; M.T.R. and T.P.Z. edited and revised manuscript; M.T.R. and T.P.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Timothy Verstynen and Chips McSteely for comments and suggestions.
REFERENCES
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science 317: 1918–1921, 2007 [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403, 1997 [DOI] [PubMed] [Google Scholar]
- Deblieck C, Thompson B, Iacoboni M. Correlation between motor and phosphene thresholds: a transcranial magnetic stimulation study. Hum Brain Mapp 29: 662–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, Ricci V, Bria P, Di Iorio R, de Waure C, Pasqualetti P, Profice P. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol 105: 2150–2156, 2011 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol 586: 3871–3879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 3: 95–118, 2010 [DOI] [PubMed] [Google Scholar]
- Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, Risch SC, George MS. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsych Clin Neurosci 12: 376, 2000 [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan J, Tormos J. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133: 425–430, 2000 [DOI] [PubMed] [Google Scholar]
- McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, George MS. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry 49: 454–459, 2001 [DOI] [PubMed] [Google Scholar]
- Pasley BN, Allen EA, Freeman RD. State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron 62: 291–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkegel H, Sommer M, Paulus W, Lang N. Impact of pulse duration in single pulse TMS. Clin Neurophysiol 121: 1915–1921, 2010 [DOI] [PubMed] [Google Scholar]
- Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: from “virtual lesions” to functional-network accounts of cognition. Cortex 45: 1043–1049, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123.3: 572–584, 2000 [DOI] [PubMed] [Google Scholar]
- Stewart LM, Walsh V, Rothwell JC. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia 39: 415–419, 2001 [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol 89: 2339–2345, 2003 [DOI] [PubMed] [Google Scholar]