Abstract
The posterior parietal cortex is situated between visual and motor areas and supports coordinated visually guided behavior. Area LIP in the intraparietal sulcus contains representations of visual space and has been extensively studied in the context of saccades. However, area LIP has not been studied during coordinated movements, so it is not known whether saccadic representations in area LIP are influenced by coordinated behavior. Here, we studied spiking and local field potential (LFP) activity in area LIP while subjects performed coordinated reaches and saccades or saccades alone to remembered target locations to test whether activity in area LIP is influenced by the presence of a coordinated reach. We find that coordination significantly changes the activity of individual neurons in area LIP, increasing or decreasing the firing rate when a reach is made with a saccade compared with when a saccade is made alone. Analyzing spike-field coherence demonstrates that area LIP neurons whose firing rate is suppressed during the coordinated task have activity temporally correlated with nearby LFP activity, which reflects the synaptic activity of populations of neurons. Area LIP neurons whose firing rate increases during the coordinated task do not show significant spike-field coherence. Furthermore, LFP power in area LIP is suppressed and does not increase when a coordinated reach is made with a saccade. These results demonstrate that area LIP neurons display different responses to coordinated reach and saccade movements, and that different spike rate responses are associated with different patterns of correlated activity. The population of neurons whose firing rate is suppressed is coherently active with local populations of LIP neurons. Overall, these results suggest that area LIP plays a role in coordinating visually guided actions through suppression of coherent patterns of saccade-related activity.
Keywords: hand-eye coordination, macaque, sensory-motor, coherence
during natural behavior, saccadic eye movements occur in concert with arm and hand movements (Crawford et al. 2004; Johansson et al. 2001; Land and Hayhoe 2001). The onset and acceleration of hand movements occur at reliable times with respect to the saccade, suggesting common or interrelated control mechanisms (Dean et al. 2011; Helsen et al. 2000; Prablanc and Martin 1992). Situated in the dorsal stream of the visual system, the posterior parietal cortex (PPC) has a well-established role in visually guided behavior (Andersen and Buneo 2002; Bisley and Goldberg 2010; Gottlieb and Snyder 2010) and is critical for the coordination of visual behavior. Damage to the PPC gives rise to sensory-motor deficits such as optic ataxia, the inability to accurately reach to visually presented targets (Battaglia-Mayer et al. 2007; Pisella et al. 2009). Therefore, PPC is a likely site for the coordination of saccades and reaches.
Although the neurophysiological mechanisms of eye and arm movements are predominantly studied in isolation (Carey et al. 2002), many studies have found populations of neurons that are modulated by signals necessary to coordinate eye-hand movements, notably in the frontal eye fields (FEF) (Thura et al. 2011), supplementary eye fields (SEF) (Fujii et al. 2002; Mushiake et al. 1996), supplementary motor areas (Fujii et al. 2002), premotor cortex (Batista et al. 2007; Boussaoud et al. 1998; Jouffrais and Boussaoud 1999; Pesaran et al. 2006, 2010), parietal-occipital cortex (Battaglia-Mayer et al. 2001, 2007; Ferraina et al. 2001), and the superior colliculus (SC) (Lünenburger et al. 2000; Reyes-Puerta et al. 2010; Werner 1993). The lateral bank of the intraparietal sulcus (area LIP) is interconnected with visually guided reaching areas in the medial intraparietal sulcus (Lewis and Van Essen 2000), as well as oculomotor structures in the frontal cortices (Blatt et al. 1990; Medalla and Barbas 2006). Therefore, area LIP is also well-situated to mediate coordinated behavior. Neurons in area LIP have long been studied in the context of intention to make a saccade (Barash et al. 1991; Gnadt and Andersen 1988; Snyder et al. 1997), the allocation of visual-spatial attention (Arcizet et al. 2011; Bisley and Goldberg 2003; Colby et al. 1996; Gottlieb et al. 1998), and the maintenance of an updated representation of visual space (Duhamel et al. 1992; Heiser and Colby 2006). Area LIP also carries spatial cognitive signals related to perceptual and economic decision-making (Churchland et al. 2011; Dorris and Glimcher 2004; Gold and Shadlen 2007; Kiani and Shadlen 2009; Louie and Glimcher 2010; Louie et al. 2011; Platt and Glimcher 1999; Roitman and Shadlen 2002; Seo et al. 2009; Shadlen and Newsome 2001; Sugrue et al. 2004), and the activity of area LIP neurons during a visual attention task is modulated by nonspatially targeted operant limb responses (Oristaglio et al. 2006). However, the activity of area LIP neurons has not been studied during coordinated eye-hand movements.
Along with studies of the activity of individual neurons, there is increasing interest in understanding the neural mechanisms of behavioral processes in terms of the activity of populations of neurons measured using the local field potential (LFP) (Buschman and Miller 2007; Fries et al. 2001; Gregoriou et al. 2009; Hansen and Dragoi 2011; Hwang and Andersen 2011; Khawaja et al. 2009; Pesaran et al. 2002, 2008; Scherberger et al. 2005; Spinks et al. 2008; Wang et al. 2011). LFPs mainly reflect the summation of synaptic potentials generated by neural populations typically within hundreds of micrometers of the recording electrode (Klee et al. 1965; Mitzdorf 1985; Okun et al. 2010; Xing et al. 2009). As a result, analyzing correlations between spiking activity and the LFP may reveal differences in the relationship between the firing of individual neurons and synaptic activity in the local population (Pesaran 2010). Correlations between the spiking activity and the LFP may, for example, reflect differences in the connections between various groups of neurons (Hwang and Andersen 2011). The firing rate of neurons during coordinated movements may also be associated with different patterns of synchronous synaptic activity within local circuits, which is measured by spike-field coherence (SFC). Thus examining the spike-field activity in area LIP may reveal specific relationships between the firing of individual neurons and neural populations that are likely to be important for understanding the neural mechanisms of coordination.
We compare spiking and LFP activity in area LIP during coordinated reach and saccade movements and saccades made alone. We find that the influence of coordinated movements is reflected in both spiking and LFP activity in area LIP. We first examine how performing coordinated movements influences the firing rate of LIP neurons. We then estimate SFC to examine the correlations between individual neurons and the LFP. We find that neurons whose firing rate is suppressed during a coordinated reach and saccade have activity that is temporally coherent with the LFP. Similar to the suppression of coherently active spiking, we find that LFP power is also suppressed during a coordinated reach and saccade. These results are the first evidence that area LIP encodes coordinated eye-hand behaviors, and they demonstrate the presence of a temporally coherent population of neurons whose firing rate is suppressed during coordination. The suppression of coherent patterns of saccade-related activity may play an important role in coordinating saccades with reaches.
METHODS
Experimental Preparation
Two adult male rhesus macaques (Macaca mulatta) participated in this study. A recording chamber was surgically placed over the PPC. For both subjects, a structural MRI-guided stereotaxic instrument (Brainsight, Rogue Research) was used to establish the placement of the chamber. Recording locations were made from area LIP, on the lateral bank of intraparietal sulcus (IPS), 5–7 mm below the cortical surface.
All surgical and animal care procedures were approved by the New York University Animal Care and Use Committee and were performed in accordance with National Institutes of Health guidelines for care and use of laboratory animals.
Behavioral Control
Subjects performed reaches and saccades for liquid rewards. Reaches were performed with the arm contralateral to the recording chamber. The start and end positions of the reach were monitored with an acoustic touch-sensitive screen (ELO Touch Systems). Eye position was constantly monitored with an infrared optical eye tracking system sampling at 120 Hz (ISCAN). Eye positions were digitized at 1 kHz. Visual stimuli were presented on an LCD screen (Dell) placed directly behind the touch screen. The visual stimuli were controlled via custom LabVIEW (National Instruments) software executed on a real-time embedded system (NI PXI-8184, National Instruments).
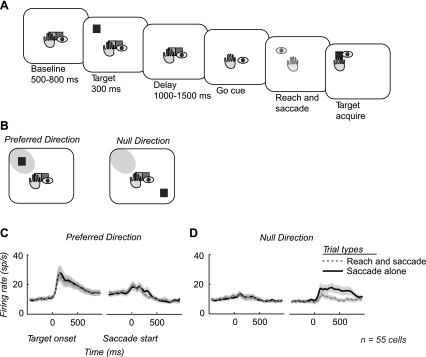
Subjects performed two center-out memory-guided movement tasks: a coordinated reach and saccade task (Fig. 1A) and a saccade-alone task. Although shown in grayscale in Fig. 1, targets consisted of colored squares; each side of the square was 2 × 2° of arc. Initial fixation and touch were represented by a green target and a red target, side by side. The green target indicated the start position for the hand, and the red target indicated the start position for the eye. Initial fixation and touch was always located at the center of the screen. Therefore, initial hand and eye positions were the same for both tasks. The subject fixated while touching the screen for a variable baseline period of 500–800 ms, after which a target would flash 10° in the periphery for 300 ms. Retinal stimulation was the same for both tasks. There were eight possible target locations. Yellow targets indicated a reach and saccade trial, while red targets indicated a saccade-alone trial. After the target flash, subjects maintained fixation and touch for a variable memory period of 1,000–1,500 ms. After the memory period, the central fixation and touch targets would extinguish, cueing the subject to move to the remembered target location. In the reach and saccade task, both central targets would extinguish, cueing a reach and a saccade. In the saccade-alone task, only the red fixation target would extinguish, cueing a saccade alone while the subject continued to touch the center of the screen. The reach and saccade end points were constrained to a window with a 5° radius around the remembered target location. Trials were included for analysis only if the subject responded with a saccade or reach and saccade within 500 ms of the Go cue.
Fig. 1.
Overview task and recordings. A: outline of memory-guided reach and saccade task. Subjects were trained to perform a reach and saccade to a remembered target position. Extinction of the fixation target cued the instructed movement to the remembered target position. This was performed in 8 directions to map the response field of each neuron. B: after response field mapping, the task was limited to a target in the response field, or preferred direction, and the target diametrically opposed, or null direction. The response field is indicated by the shaded gray region in the visual field. A memory-guided saccade-alone task was also performed in the preferred and null directions (not shown). C and D: average firing rates across all recordings from both subjects are shown for both the reach and saccade task (dashed gray) and the saccade-alone task (solid black) in both the preferred and null directions.
Neuronal Recordings
The reach and saccade task was performed in eight directions to map the response properties of each neuron for 80–100 trials. After the response field was identified, the target in the center of the response field was chosen as the preferred direction for that neuron. The target location diametrically opposed to the preferred direction was chosen as the null direction for that neuron (Fig. 1B). Subjects then performed the reach and saccade task and the saccade-alone task in both the preferred and null directions. All four conditions were randomly interleaved so that the monkey could not predict the condition at the start of each trial.
When activity from a neuron was initially isolated, we ran the reach and saccade mapping task. If the activity appeared spatially selective, we proceeded to test the main task conditions. After the experimental recording database was complete, we performed a statistical test to determine spatial selectivity in the responses (see below). Only spatially selective neurons were analyzed for differences between the two tasks. We recorded a total of 204 neurons, of which 55 (27%) were spatially selective during the memory delay period.
To confirm that receptive fields are consistent for saccades made alone and saccades made with reaches, we mapped with both a visually guided reach and saccade task and a visually guided saccade-alone task in 21 neurons that showed spatial selectivity for both tasks (13 from monkey J, 8 from monkey H). The only methodological difference from the memory-guided tasks was the presence of the target throughout the delay period. We found that the majority of cells showed significant tuning for the same direction (9/13 monkey J, 8/8 monkey H; P < 0.05, random permutation). The four cells that did not show tuning for the same target were tuned for targets 45–135° apart and within the same visual hemifield (90 ± 36°, mean ± SD). Therefore, we consider receptive fields to be consistent across tasks.
Recording locations were determined with Brainsight software that allowed us to visualize the cortex under the chamber with a three-dimensional reconstruction of MRI images from each subject. This allowed us to reliably target the lateral bank of the IPS. Specifically, the recording locations in each animal spanned a 5- to 6-mm distance along the IPS whose most medial extent was 6 mm from the parieto-occipital sulcus. Recordings were made within the sulcus 5–7 mm below the cortical surface.
Neural recordings were made with 0.7- to 1.2-MΩ glass-coated tungsten electrodes (Alpha Omega). In each recording session, two to four electrodes were lowered into the IPS. Alpha Omega microdrives were used to lower electrodes. Electrodes were supported by a guide tube and penetrated the dura before entering the brain. The guide tube did not penetrate the dura. Neural activity from each electrode was passed through a head stage (TDT Electronics), amplified (×500–1,000; TDT Electronics), digitized (20 kHz; National Instruments), and continuously streamed to disk. We recorded raw waveforms from each electrode. Neural recordings were referenced to the guide tube, which was in contact with the dura.
Data Analysis
Neural activity.
Spike waveforms were extracted and clustered during the recording sessions to guide the neuron isolations. Spike waveforms were extracted off-line. Spike waveforms were then overclustered and manually merged on a moving 100-ms time window.
Significance in firing rates was determined with a random permutation test. All permutation tests were performed with a minimum of 10,000 randomizations. A neuron was defined as spatially selective if it exhibited significantly higher firing rates (P < 0.05) during the last 500 ms of memory period in the preferred direction compared with the null direction for both the saccade-alone task and the reach and saccade task.
We analyzed spatially selective neural activity in six temporal epochs across the trial. The first three epochs were aligned to the onset of the target. We refer to the 500-ms interval preceding target onset as the baseline epoch. The 500-ms interval that begins with the target flash is the target epoch. The interval 500–1,000 ms after the onset of the target flash is the early memory epoch. The other three epochs were aligned to the start of the saccade. We refer to the 500-ms interval before the saccade begins as the late memory epoch. Because the memory period varied from 1,000 to 1,500 ms, in some trials the early memory and late memory epochs partially overlap. On average, the time between target onset and saccade start was 1,849 ± 357 ms. Therefore, in the majority of trials the epochs are distinct. We refer to the 500-ms interval that begins with the saccade as the movement epoch because it contains the saccade, and on the reach and saccade trials, the reach as well. The 500-ms interval following the movement epoch is the postmovement epoch.
We define three different populations of cells based on their firing rate during the two tasks. Saccade preference cells had significantly higher firing rates for the saccade-alone task compared with the reach and saccade task during at least one of the five trial epochs (the baseline epoch was not included) and did not have significantly higher firing rates for the reach and saccade task in any epoch. Reach and saccade preference cells had a significant increase in firing rate for the reach and saccade task during at least one trial epoch and did not have a significant increase in firing rate for the saccade-alone task in any epoch. It is important to note that we use the term “reach and saccade preference,” rather than “reach preference,” for two reasons. First, all of the cells were tested for spatial selectivity with the saccade-alone task. Therefore, they were all responsive in a saccade-alone condition and not purely reach selective. Second, we do not use a reach-alone task to look for reach-selective cells. As our interest is in the role of area LIP in coordinated hand-eye movements, it was necessary to find cells with saccade-related activity. The remainder of the cells were classified as no preference cells and either did not have significant change in firing rate in any trial epoch or had higher firing rates for the saccade-alone task in some epochs and higher firing rates for the reach and saccade task in other epochs.
Significant differences in each epoch were defined as having a P < 0.01 by a random permutation test. This strict P value criterion allows us to run five parallel permutation tests and still ensure an overall significance of P < 0.05. We performed simulations of the random permutation tests to confirm the overall significance level.
Spike-field coherence analysis.
For each spike-field recording, we calculated the SFC, using multitaper methods with a ±250 ms analysis window, stepped 50 ms between windows with ±5 Hz smoothing aligned either to the target flash or start of the saccade. To assess the interactions between groups of cells and LFPs, we calculated the SFC between the cells in each of three groups and LFPs in area LIP to give a saccade preference SFC, a reach and saccade preference SFC, and a no preference SFC.
Because SFC is influenced by spiking activity recorded on the same electrode as LFP activity (Zanos et al. 2010), we restricted our analysis to the SFC recorded on neighboring electrodes. The minimum spacing between electrodes was 550 μm. The median separation between electrodes was 714 μm. Since we observed that, on average, the SFC magnitude recorded on different electrodes depends on the distance between the electrodes, we tested whether the median distances between electrodes for the saccade preference SFC, reach and saccade preference SFC, and no preference SFC were significantly different. We confirmed that the median of the spike-field distance was not significantly different across groups [saccade preference SFC (694 μm), reach and saccade preference SFC (761 μm), and no preference SFC (745 μm); P > 0.05, rank sum test].
To compare differences in coherence across groups, we calculated the z score for the estimated coherence (Jarvis and Mitra 2001). The z score is given by calculating the expected standard deviation of the coherence given the number of degrees of freedom (ν = number of trials × number of tapers) and transforming the measured coherence so that, under the null hypothesis that the coherence is zero, the transformed coherence is distributed as a normal variate with variance equal to 1. The transformation we used was z = β(q − β), where q = −ν − 2 × log 1 − C, β = 1.25, and C is the coherence. We focused our analysis on the peak of the coherence. To determine the peak, we averaged together all 42 spike-field sessions for both tasks. Coherences that were two standard deviations above the average coherence were considered the peak. The peak ranged from 11 to 31 Hz so we analyzed activity at the center frequency, 20 Hz.
LFP spectrum analysis.
For each LFP recording, we calculated the spectrum, using multitaper methods with a ±250 ms analysis window, stepped 50 ms between windows with ±2.5 Hz smoothing aligned either to the target flash or start of the saccade (Mitra and Pesaran 1999). To assess the strength of task selectivity in LFP activity at each frequency (1–100 Hz), we tested for differences in LFP power between the two tasks, using a random permutation test. We performed this analysis on all LFPs recorded from area LIP and determined the percentage of LFPs that showed a significant difference in power between the two tasks (P < 0.05, permutation test).
We also analyzed LFPs recorded on the same electrode as our three groups of cells (saccade preference, reach and saccade preference, no preference). At each frequency, we determined the range of the percentage of LFPs expected to show a significant difference between the two tasks based on the activity of the entire population (P < 0.05, binomial distribution). This enables us to determine, at each frequency, whether or not the percentage of LFPs in each group differs significantly from the entire population.
Saccade metrics.
To test whether differences in neural activity could be attributed to differences in the eye movements between tasks, we analyzed the saccade metrics. The main sequence, or positive relationship between peak velocity and saccade amplitude, has been shown to be a robust feature of the saccadic system (Bahill 1975) and has been reported previously for coordinated reach and saccade movements (Snyder et al. 2002). We compared peak velocity during the reach and saccade task compared with the saccade-alone task. Following Snyder et al. (2002), for each of the eight directions, we calculated the average peak velocity for each task. First we compared this across all trials, regardless of amplitude. We then restricted the analysis to look at trials with the same amplitude in the following way. For each direction, we found the mean amplitude across both tasks. All trials that fell within a 1° window of that end point were included in the analysis.
We also analyzed spatial differences in saccade end point between the two tasks. To measure accuracy, we calculated the mean saccade end point for each task in each direction. We then calculated the vector from the mean saccade end point to the previously flashed location of the target. Because all trials were memory guided, some inaccuracy is expected. We also analyzed the variance in saccade end points for each target and each task. For each trial, we subtracted the mean end point and then calculated the mean squared error. This gave us a measurement of the spread or precision of saccade end points for a given task and target.
RESULTS
We recorded the activity of 204 neurons in area LIP in two rhesus macaques (121 from monkey H, 83 from monkey J) performing a memory-guided reach and saccade task and a memory-guided saccade-alone task. A total of 55 neurons demonstrated spatially selective responses during the memory delay period (41 from monkey H, 14 from monkey J; P < 0.05, permutation test). We averaged the mean firing rate across all spatially selective neurons for preferred (Fig. 1C) and null (Fig. 1D) directions during the reach and saccade task and the saccade-alone task. In the preferred direction, the mean firing rate shows no significant difference between the two tasks at any time during the trial. Consequently, the population average does not reveal an influence of coordination on neuronal firing in area LIP during the period before movement. Note, however, that in the null direction, postsaccadic activity in the saccade-alone task is significantly suppressed in the reach and saccade task (Fig. 1D).
Firing Rate of Area LIP Neurons Is Modulated by Reaching
Because the properties of the firing rate of individual neurons can vary greatly, effects of reaching on firing rate can be obscured when population activity is averaged. Although the population average did not reveal a systematic effect of reaching on the firing rates before movement, populations of neurons whose activity significantly depends on the presence of a coordinated reach may exist. Therefore, we next tested whether activity in individual neurons significantly increased or decreased when a coordinated reach was made with a saccade.
To analyze individual neurons in the population, we divided the trial into six temporal epochs of duration 500 ms: baseline, target, early memory, late memory, movement, and postmovement. For each trial, we calculated the firing rate over each of the six temporal epochs. To determine significant differences, we compared the firing rates across the two different tasks in the preferred direction, using a random permutation test (see methods).
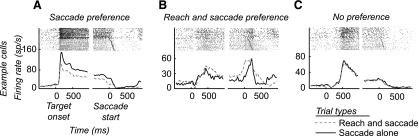
We found that 87% of neurons (48/55) showed significantly higher firing rates during either the coordinated reach and saccade task or the saccade-alone task during at least one epoch of the trial, excluding the baseline epoch. Only 13% of neurons (7/55) showed no significant difference (P > 0.05) between the two tasks during all trial epochs. Since the statistical test we performed compared activity during multiple task epochs, we applied the same tests on permuted data and confirmed that the level of significance for the statistical test was P < 0.05 (see methods). Figure 2 presents the activity in the preferred direction of three example cells: a saccade preference cell (Fig. 2A), a reach and saccade preference cell (Fig. 2B), and a no preference cell (Fig. 2C; see methods). Across the population, 27% of neurons (15/55) were saccade preference cells and 29% of neurons (16/55) were reach and saccade preference cells; 44% (24/55) of neurons were no preference cells, which either showed no significant difference between the two tasks or showed inconsistent differences across the trial epochs. The reach and saccade preference cell appeared to increase firing before the coordinated movement earlier than the saccade made alone. We examined whether this effect was significant across the population and found that, while present in some neurons, the population average response did not reveal this trend across the population. Firing rate differences in the three groups of cells did not show consistent changes locked to the onset of the reach that did not appear to be locked to the saccade. Examining the reach onset symbols on the reach and saccade task rasters reveals that on trials when the reach onset occurs earlier, changes in the response do not occur correspondingly earlier.
Fig. 2.
Responses of LIP neurons during movements to the preferred direction. A: saccade preference cell showing higher firing rates for the saccade-alone task. B: reach and saccade preference cell showing higher firing rates for the reach and saccade task. C: no preference cell showing no significant difference for the reach and saccade task vs. the saccade-alone task.
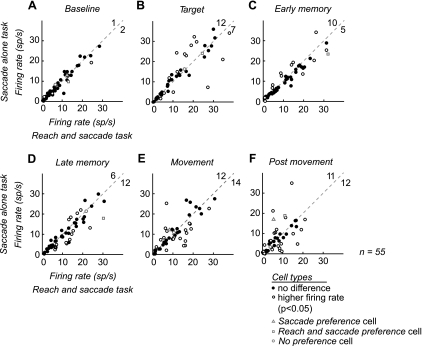
Significant differences in activity between the tasks were present for each epoch after target onset. As expected, there was no significant difference in activity between the two tasks during the baseline period [3/55 (5%); Fig. 3A]. During the target epoch, over a third of the cells showed a significant difference between the two tasks [19/55 (35%)], with nearly twice as many showing higher firing rates for the saccade-alone task than for the reach and saccade task [12/19 (68%) higher for saccade alone, 7/19 (37%) higher for reach and saccade; Fig. 3B]. The trend continued through both of the memory epochs, with a third of cells showing a significant difference between the two tasks [early memory: 15/55 (27%), late memory: 18/55 (33%); Fig. 3, C and D]. During the movement epoch nearly half of the cells [26/55 (47%)] showed a significant difference between the two tasks (Fig. 3E). The number of cells in each population was almost equal, with 12 of 26 (46%) neurons showing higher firing rates for the saccade-alone task and 14 of 26 (54%) neurons showing higher firing rates for the reach and saccade task. Task selectivity persisted well beyond the movements into the postmovement epoch, with 42% neurons (23/55) showing a significant difference between the two tasks, 48% of which increased for the saccade-alone task (14/23) and 52% for the reach and saccade task (Fig. 3F).
Fig. 3.
Scatterplots of cell firing rate during different trial epochs. A–F: average firing rate for each cell during each 500-ms epoch plotted for the saccade-alone task (y-axis) and the reach and saccade task (x-axis) in the preferred direction. Open circles, cells with a significant difference between the 2 tasks. Filled circles, cells without a significant difference between the 2 tasks. Significance was determined with a random permutation test. Three example cells from Fig. 2: saccade preference cell (open gray circle), reach and saccade preference cell (open gray triangle), and no preference cell (open gray square). For visibility, axes are plotted to 50 spikes/s. Therefore, cells with firing rates higher than 50 spikes/s are not shown. The total number of cells showing a significant difference for each task is shown in the top right corner of each plot. This number includes cells not visible on the axes.
The pattern of firing across individual neurons substantially differed from the population average firing rate. The response of individual neurons to a reach and saccade task was heterogeneous: About a third of neurons showed suppressed firing rates, while another third showed increased firing rates.
Spike-Field Coherence Is Greater for Saccade Preference Cells
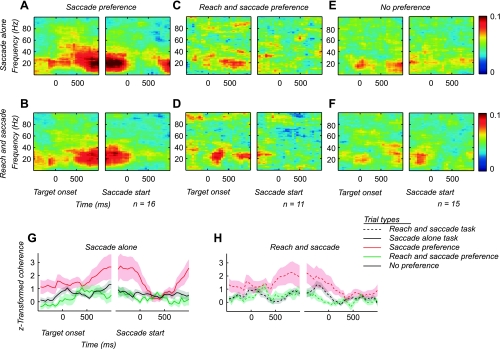
The functional heterogeneity we observe suggests that cells in area LIP receive different inputs or perform different computations on their inputs. LFP activity measures extracellular currents generated by populations of neurons, pooling synaptic inputs in the vicinity of the recording electrode (Pesaran 2010). To test whether functional heterogeneity involves patterns of activity that are temporally coherent, we measured correlations between spiking and LFP activity by estimating the SFC. We compared SFC across the three groups of cells: saccade preference, reach and saccade preference, and no preference. To eliminate the possibility of spiking artifactually influencing the LFP, we analyzed LFPs that were recorded at the same time as cells but on neighboring electrodes within ∼700 μm (see methods).
The saccade preference cells displayed strong SFC for both tasks (Fig. 4, A and B). SFC was less prominent for both the reach and saccade preference cells (Fig. 4, C and D) and the no preference cells (Fig. 4, E and F). In each case we observed a peak in SFC at ∼20 Hz (Fig. 4). SFC at 20 Hz was significantly greater for the saccade preference cells than for the other cell groups through most of the delay period, and this was true for both the saccade-alone task and the reach and saccade task (P < 0.05, 2-sided t-test; Fig. 4. G and H). Coherence was not significantly different between the no preference cells and the reach and saccade preference cells (P = 0.6, 2-sided t-test).
Fig. 4.
Spike-field coherence (SFC) for saccade preference, reach and saccade preference, and no preference cells. A and B: SFC for saccade preference cells during the saccade-alone task (A) and the reach and saccade task (B). C and D: SFC for reach and saccade preference cells during the saccade-alone task (C) and the reach and saccade task (D). E and F: SFC for no preference cells during the saccade-alone task (E) and the reach and saccade task (F). Subpanels are aligned to the onset of the target and the start of the saccade. Color scale indicates coherence magnitude. G and H: z score-transformed coherence at 20 Hz across the trial for the saccade-alone task (G) and the reach and saccade task (H). Subpanels are aligned to the onset of the target and the start of the saccade. For each task, the average z score at 20 Hz is shown for the saccade preference SFC (red), reach and saccade preference SFC (green), and no preference SFC (black). The saccade-alone task is shown by solid lines and the reach and saccade task by dashed lines. The shaded region shows the SE.
Coordination Suppresses Saccade-Related LFP Activity
The SFC analysis suggests that LFP activity in area LIP reflects activity of saccade preference cells. If so, LFP activity should be suppressed during the coordinated reach and saccade task, similar to the firing rate of saccade preference cells. To test this prediction, we analyzed the influence of coordinated reach and saccade movements on LFP activity.
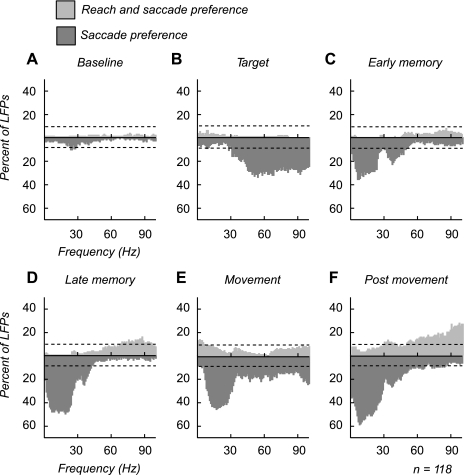
We recorded LFPs at 118 sites in area LIP (82 sites in monkey H, 36 sites in monkey J) and analyzed the activity for significant differences between the two tasks at each frequency (P < 0.05, permutation test). The LFP recording sites were at the same sites where single-unit activity was observed (see above). Figure 5 plots histograms of the percentage of LFPs that showed a significant increase in power for each task at each frequency. LFPs that showed significantly higher power for the reach and saccade task are plotted in light gray, while LFPs that showed a significantly higher power for the saccade-alone task are plotted in dark gray. Unlike the cells, which we found to have fairly even populations with about a third of cells each preferring each of the two tasks, the LFP power was suppressed by the coordinated reach. This suppression is demonstrated by the percentage of LFPs that showed significantly higher power for the saccade-alone task relative to the reach and saccade task.
Fig. 5.
Percentage of local field potentials (LFPs) showing a significant difference in power at each epoch. A–F: histograms indicating % of LFPs that show a significant change in power between the 2 tasks at each frequency during each epoch. Dark gray, LFPs with significantly higher power for the saccade-alone task. Light gray, LFPs with significantly higher power for the reach and saccade task. The dashed line indicates % of LFPs that one would expect to be significant by chance. Therefore, frequencies exceeding the dashed line had a significantly higher percentage of LFPs than chance (P < 0.05, binomial distribution). Significance was determined with a random permutation test (P < 0.05).
We find that two different groups of frequencies are suppressed at different points during the trial. During the target epoch, the suppression was seen at frequencies above 30 Hz. During the remainder of the trial, the suppression was centered on lower frequencies, below 50 Hz. During the baseline, there was not a significant number of LFPs that showed a difference between the two tasks (Fig. 5A). Following target onset, up to 34% of LFPs (at 54 Hz) had significantly higher power for the saccade-alone task compared with the reach and saccade task. Suppression was broadly seen at higher frequencies (16–34% of LFPs at 31–100 Hz; Fig. 5B). During both memory periods, suppression shifted to lower frequencies. The early memory period showed two peaks in the suppression: a larger one from 1–27 Hz that peaked at 28–35% of LFPs (5–21 Hz) and a smaller peak centered around 30–49 Hz (12–24%; Fig. 5C). Low-frequency suppression continued for the rest of the trial, with greater percentages of LFPs in each epoch. In the late memory and movement epochs 40–50% of LFPs were suppressed from 5–24 Hz (Fig. 5D). However, the movement epoch also showed significant suppression at higher frequencies in a smaller percentage of LFPs (15–20%, 31–100 Hz; Fig. 5E). In the postmovement epoch the suppression of LFPs also extended up to 55 Hz. Few LFPs showed any significant increase in power for the reach and saccade task except during the postmovement epoch, when 12–28% of LFPs showed a significant increase in power at higher frequencies (55–100 Hz; Fig. 5F). Therefore, LFP activity is suppressed during the coordinated reach and saccade task, similar to the firing rate of saccade preference cells.
LFP Suppression Reflects Preferential Pooling Across Saccade Preference Cells
If LFP activity simply reflects the activity of neurons near the recording electrode, then the LFP suppression we observe may be due to proximity to saccade preference cells and so would not be present near reach and saccade preference and no preference cells. Alternatively, the LFP suppression may preferentially pool activity related to saccade preference cells. Under this alternative, LFP suppression may not be restricted to recordings near saccade preference cells and may also be present near reach and saccade preference cells and no preference cells.
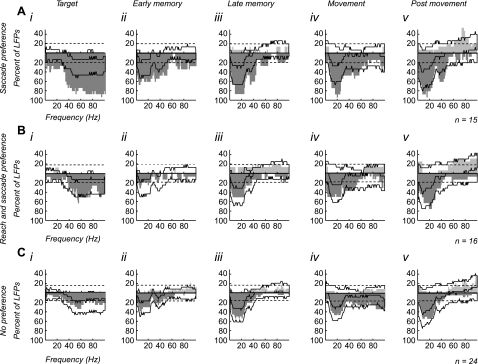
To select between these alternatives, we examined the task selectivity of LFPs divided into three groups according to whether they were recorded on the same electrode as saccade preference cells, reach and saccade preference cells, and no preference cells. Figure 6 presents changes in LFP activity grouped by task preference of cells recorded at that site. The solid black line on each graph indicates the percentage of LFPs one would expect to show a significant difference between the tasks based on the total population. Therefore, frequencies exceeding the solid black line reveal a significantly higher percentage of LFPs than would be expected in the total population (P < 0.05).
Fig. 6.
LFPs recorded simultaneously with cells classified as saccade preference, reach and saccade preference, and no preference: LFPs recorded on the same electrode as saccade preference cells (A), reach and saccade preference cells (B), and no preference cells (C) that showed a significant difference in power between the 2 tasks during each epoch (i–v). Images were generated with the same protocol as Fig. 5. Significance was determined with a random permutation test (P < 0.05). The dark gray histograms indicate LFPs that had significantly higher power for the saccade-alone task. The light gray histograms indicate LFPs that had significantly higher power for the reach and saccade task. The dashed line indicates % of LFPs that one would expect to be significant by chance (P < 0.05, binomial distribution). The solid black line indicates the range of frequencies that are expected to be significant based on the population responses observed in Fig. 5. Frequencies that exceed solid black line are significantly higher percentages than would be expected by chance (P < 0.05, binomial distribution).
The basic pattern of significance is the same across the saccade preference, reach and saccade preference, and no preference groups. No group of cells was more likely to be recorded with LFPs that showed an increase in power for the reach and saccade task. Therefore, LFP activity does not simply reflect the activity of neurons near the recording electrode and instead appears to preferentially pool activity related to saccade preference cells.
More closely examining the results further supports the hypothesis that LFP activity in area LIP preferentially pools activity related to saccade preference cells. For each group of cells, we estimated the percentage of LFPs expected to show a difference between the two tasks, based on the degree to which LFP activity was different between the tasks across the entire population (see methods). This allowed us to determine whether activity in a particular group of recordings was more or less likely to be task selective than expected on average. We found that LFP activity recorded at the same site as saccade preference cells (Fig. 6A) was more likely to show significantly greater power for the saccade-alone task than expected on average (P < 0.05, binomial distribution). This is true at all frequencies for the target epoch (1–100 Hz) and at frequencies up to 55 Hz for the rest of the trial. In most epochs, up to 80–90% of the LFPs had significantly greater power for the saccade-alone task than the reach and saccade task. In contrast, LFP activity recorded at the same site as reach and saccade preference cells (Fig. 6B) was no more likely to have an increase in power for the reach and saccade task than LFP activity in the rest of the population. LFP activity was just as likely to show greater power for the saccade-alone task as LFP activity in the rest of the population. The same was true for LFP activity recorded at the same site as no preference cells (Fig. 6C). Therefore, while LFP activity is suppressed for coordinated reach and saccade movements overall, the suppression influences a greater percentage of LFP sites with saccade preference cells.
The analysis of LFP power suggests that LFP activity in area LIP preferentially represents the activity of saccade preference cells. LFP suppression was not necessarily related to the proximity of the LFPs to saccade preference cells, but it was stronger near saccade preference cells. Taken together, the results of the SFC and LFP power analyses suggest that temporally coherent patterns of saccade-related activity are suppressed by coordinating a reach with a saccade.
Behavioral Differences Between Tasks
To test whether or not differences in neural activity between the reach and saccade task and the saccade-alone task could be attributed to differences in saccadic behavior instead of the coordinated reach, we analyzed the reaction times and saccade metrics for the two tasks. The saccade reaction times for the reach and saccade task (234 ± 35 ms monkey J, 247 ± 48 ms monkey H; mean ± SD) were typically faster than for the saccade-alone task (237 ± 38 ms monkey J, 260 ± 46 ms monkey H; mean ± SD). For reach and saccade trials, the saccade preceded the reach in 94% of all trials (reach reaction times: 338 ± 46 ms monkey J, 345 ± 50 ms monkey H; mean ± SD).
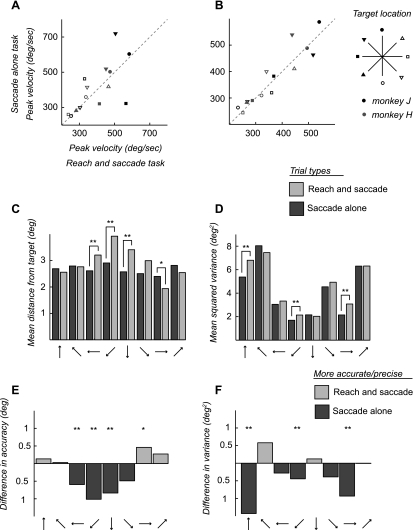
We found no systematic shifts in the saccade metrics between the two tasks. When we compared peak velocities between tasks, there was a slight tendency for the peak velocity to be greater for the saccade-alone task, with 29% of targets having a significantly higher peak velocity (P < 0.05, random permutation) for the saccade-alone task (3/8 monkey H, 1/6 monkey J; Fig. 7A) compared with 21% for the reach and saccade task (2/8 monkey H, 1/6 monkey J). When we constrained trials to those with saccades with amplitudes that fell within a 1° window, we found that this effect persisted, with 29% of targets having higher peak velocity for the saccade-alone task (4/8 monkey H, 0/6 monkey J) and only 14% having higher peak velocities for the reach and saccade task (1/8 monkey H, 1/6 monkey J; Fig. 7B). There was no systematic effect of target location on differences in peak velocity. We also looked for differences in the spatial metrics of the saccade and reach, in terms of precision and accuracy. We measured the accuracy of the saccade and reach by calculating the average distance of the saccade end point from the actual target location. Here we found that saccades made alone were more accurate for the lower targets (below fixation) than saccades made with a reach. This was highly significant for these targets (P < 0.05 and P < 0.01, random permutation test; Fig. 7, C and E). There was also a trend for the saccades made with a reach to be more accurate for the upper targets (above fixation), although this was significant for only one target (Fig. 7, C and E). There were no significant differences between reach accuracy and saccade accuracy for the reach and saccade task (not shown). We also measured the precision or variance of eye movements around each target by subtracting the mean end point and calculating the squared error. For all but two targets, the variance was greater for the reach and saccade task than for the saccade-alone task. While this difference was significant for only three targets, the effect was not limited to the lower targets as in the accuracy analysis. Only two targets had lower variance for the reach and saccade task, but the difference was not significant (Fig. 7, D and F). Reaches tended to be less variable than saccades, but there was no significant difference between the variance for reaches and variance for saccades in the reach and saccade task at any target (not shown).
Fig. 7.
Behavioral analysis of saccade metrics. A and B: we compared peak velocities for the saccade-alone task (y-axis) and the reach and saccade task (x-axis). The dashed line indicates equal velocities for both tasks. The peak velocity is shown for each target as well as for each monkey (gray symbols, monkey H; black symbols, monkey J). We first compared all trials (A). Then, to account for differences in saccade amplitude, we compared only trials of the same amplitude, within a 1° window (B). We then compared the mean distance of saccade end points from the actual target location (C) and the spread, or mean squared variance of saccade end points (D), for the saccade-alone task (dark gray) and the reach and saccade task (light gray). We also show the saccade metric data as the difference between tasks (E and F). Directions that were more accurate (E) or precise (F) are shown in light gray for the reach and saccade task and dark gray for the saccade-alone task. *P < 0.05, **P < 0.01 by a random permutation test.
We also tested whether differences in saccade metrics varied across monkeys and recording sessions. For monkey J, differences in accuracy between the tasks were not significant for any target, although they showed the same trend. There were no significant differences between monkeys in variance for any target. We also looked to see whether differences in saccade metrics could be attributed to different recording sessions. We compared trials that were recorded at the same time as saccade preference cells, reach and saccade preference cells, and no preference cells to see whether behavioral differences were stronger when differences in neural activity were recorded. On trials recorded at the same time as saccade preference cells and trials recorded at the same time as reach and saccade preference cells, there were no significant differences in accuracy across trials for either the saccade-alone task (P = 0.09, random permutation test) or the reach and saccade task (P = 0.7, random permutation test). There were no significant differences in variance between these two groups. Accuracy was better for saccade-alone trials during trials recorded at the same time as no preference cells compared with saccade preference cells as well as compared with reach and saccade preference cells (P < 0.01, random permutation test). Again, there were no significant differences in variance between these groups of trials for any target location. Because we saw no difference in the accuracy or variance of saccades between trials recorded at the same time as saccade preference cells and reach and saccade preference cells across targets, differences in the neural activity of individual cells cannot be directly linked to behavioral differences between the tasks.
DISCUSSION
Here we examine the neural mechanisms of coordinated reach and saccade movements that are made together to a single spatial target. We find that eye-hand coordination is selectively encoded by both spiking and LFP activity in area LIP of the PPC. In the spiking activity, eye-hand coordination is encoded by two populations of neurons, defined by their response to the reach and saccade task: saccade preference cells, whose firing rate is suppressed, and reach and saccade preference cells, whose firing rate is increased. Importantly, only the activity of saccade preference cells is significantly coherent with the LFPs in area LIP. SFC for the reach and saccade preference cells and an additional population of no preference cells is significantly weaker. Since all three populations of neurons display spatially selective increases in firing rate, the difference between them is not simply due to differences in the firing rate itself and reflects differences in the temporal patterning of activity observed in different populations of neurons. The SFC suggests that local circuits in area LIP encode coordinated eye-hand movements through suppression of temporally coherent activity. In line with this prediction, the majority of LFPs showed a strong suppression during the reach and saccade task. Few LFPs showed increased power during the reach and saccade task, while up to 60% of LFPs showed suppression. Suppressed LFP power was not only found near saccade preference cells. LFPs recorded on the same electrode as reach and saccade preference cells and no preference cells were just as likely to show suppression in power as the entire population of LFPs. The overall pattern of spike-field responses indicates that suppression of saccade-related activity in area LIP involves temporally coherent patterns of activity and that LFP activity in area LIP preferentially reflects the activity of this temporally coherent population of neurons.
Spike-Field Coherence in Area LIP
Previous work examining SFC in area LIP during memory-guided saccades emphasized the significance of gamma-frequency (25–90 Hz) activity (Pesaran et al. 2002). In contrast, while we observe elevated SFC at up to 90 Hz (Fig. 4, A and B), the SFC we report here is present at lower frequencies, with a peak at ∼20 Hz. Here we discuss important methodological differences that exist between earlier work and the present study that may contribute to the difference in results.
Pesaran et al. (2002) recorded neural activity spiking and LFP activity with 12-μm twisted wire tetrodes. In those data, spiking was clearly observed on some but usually not all channels of the tetrode. To limit the influence of large-amplitude action potentials on LFP calculations, LFP activity was analyzed only on the channel of the tetrode that had the smallest-amplitude action potential. In most cases, there was no appreciable amplitude spiking waveform from the single unit that was the focus of the SFC analysis. Consequently, the SFC estimates were likely from very local sites near the single-unit recording electrode, within ∼50 μm, and the LFP activity was often not appreciably corrupted by single-unit waveforms.
In contrast, our present experiments do not use tetrodes and use multiple single electrodes spaced by at least 550 μm and typically 710 μm (see methods). Since SFC is corrupted by large-amplitude spikes when the LFP is recorded on the same electrode as an isolated single unit, we have focused on presenting SFC on different electrodes.
Several studies have documented how neural correlations decay with electrode separation, in terms of the correlations and coherence between LFP signals (Jia et al. 2011; Leopold et al. 2003; Nauhaus et al. 2009) and the averaged spike-triggered LFP (Nauhaus et al. 2009). The decay in coherence is more pronounced at higher frequencies (Leopold et al. 2003). Consequently the difference in separation between electrode separation in the present study, 650–4,550 μm (714 μm median), and previous work, ∼50 μm, may underlie the differences in the SFC results. Additional work, however, is needed to directly examine this hypothesis.
Suppression of Saccade-Related Activity and the LFP
LFP activity predominantly reflects synchronous synaptic potentials near the recording electrode (Mitzdorf 1985), and recent work indicates that the volume of cortical tissue reflected in LFP activity can be quite local, within 250 μm of the recording electrode (Katzner et al. 2009; Xing et al. 2009). We report changes in LFP power associated with a coordinated reach and saccade across a range of frequencies. Gamma-band (>40 Hz) activity is suppressed after the target flash, while lower beta-band (∼10–40 Hz) activity is suppressed during the later memory period. The overall result is that power is greater before a saccade made alone and suppressed when a coordinated reach is made.
The suppression of LFP power before the coordinated reach is widespread and most pronounced at sites where saccade preference cells are present. The SFC analysis demonstrates that the saccade preference cells are also most correlated with LFP activity. This suggests that suppression in LFP activity preferentially reflects the activity of saccade preference cells, a population of cells whose firing rate is lower before a coordinated reach than before saccades made alone. This interpretation is supported by simulation studies of synaptic inputs to individual neurons and LFP activity that show that SFC can involve synchronous synaptic input to the cell and that synaptic activity can be predominantly reflected in LFP activity (Baker et al. 2003; Zeitler et al. 2006). Since the saccade preference cells display the most significant SFC and reduce their firing rate before coordinated movements compared with saccades made alone, it follows that LFP power is also reduced.
The thread that runs through the spike rate, LFP power, and SFC results is that LFP power reflects the changes in the firing rate of populations of neurons that display temporally coherent activity. In this way, our results are consistent with the hypothesis that LFP power reflects the level of temporally correlated neural signals. It is important to note that the changes in LFP power we observe are not simply due to the firing rate of cells recorded on the same electrode (Fig. 6). Although saccade preference cells were more likely to be recorded near LFPs showing suppression due to the reach, this was not true for our other cell groups. In fact, LFPs recorded on the same electrode as reach and saccade preference cells were no less likely to show suppression due to the coordinated reach than the average population.
Our finding that LFP activity robustly encodes coordination in area LIP indicates that temporally patterned neural activity may underlie coordination. SFC shows that not all area LIP neurons participate in the temporally patterned activity but the neurons that fire coherently reveal a consistent signature of coordination: the suppression of saccade-related activity. Neural coherence, most prominently in the gamma frequency band, has been proposed to underlie a variety of cognitive processes (Fries 2009). In PPC, LFP activity encodes movement plans for saccades (Pesaran et al. 2002) and reaches (Scherberger et al. 2005), decisions (Pesaran et al. 2008), focal attention (Murthy and Fetz 1992), and visual selection (Buschman and Miller 2007), and similar signals are present in human parietal cortex (Van Der Werf et al. 2010). It will be interesting to determine whether the firing rate properties of neurons that reflect factors other than coordination are also preferentially associated with temporally coherent activity patterns in area LIP.
On the basis of our results, we propose that neural coherence in the beta and gamma frequency bands coordinates movements of different effectors by bringing together signals involved in their control. Area LIP neurons that fire coherently encode the spatial location of an intended saccade and, through suppression of firing, whether a coordinated reach is also planned. We are not able to comment on the organization of local SFC in the gamma frequency band because of the distance between single-unit and LFP recording electrodes (see above). However, LFP power in the gamma frequency band appears to increase after coordinated reach and saccade movements compared with saccades made alone (Fig. 6) and may reflect reaching signals arising from the reach-related circuits.
The reach-related inhibitory influence on saccade-related LIP activity we observe could, in principle, be related to sensory proprioceptive input, efferent copy of arm motor commands, or prospective coding of arm movement plans (Nanayakkara and Shadmehr 2003; Ren et al. 2006; Vercher et al. 1996, 2003). Our main observations of suppression are made during the stable hold period after the presentation of the spatial movement cue before the onset of movement. Prospective coding of arm movement plans is a plausible candidate for the reach-related inhibitory influence on saccade-related LIP activity that we observe. Plans are formed and maintained throughout the delay period before the Go cue, and reach plans are associated with changes in beta-frequency LFP activity in the parietal reach region (Scherberger et al. 2005). Beta-frequency activity could also be due to proprioception associated with the arm posture at the central hold target. However, our observation that beta-frequency SFC increases after the spatial cue is delivered is not easily explained by a proprioceptive signal because the arm posture does not change after the spatial cue. A role for efference copy of arm movement commands can be ruled out since the commands are not generated until the Go cue to move, while the changes in beta coherence are present before the Go cue.
Implications of Spike-Field Coherence
The mechanisms of temporally correlated neocortical activity have been widely studied, with many groups proposing that the generation of correlated activity in specific frequency bands critically depends on the balance of activity between excitatory neurons and inhibitory interneurons (Atallah and Scanziani 2009; Brunel and Wang 2003; Kang et al. 2010; Kramer et al. 2008; Traub et al. 1997). The formation of cell assemblies with excitatory and inhibitory interconnections is also thought to underlie the formation of temporally correlated activity across a range of frequency bands (Buzsaki 2006; Harris et al. 2003; Kopell et al. 2011).
In our case, we observe that only certain populations of neurons show correlated activity with the LFP, suggesting the presence of assemblies of cells that fire coherently together. Significant SFC can occur when there are synchronized synaptic inputs to particular neurons and when these synaptic inputs are sufficiently strong to modulate the spiking output of the cell. Simulations have shown that SFC generally underpredicts the strength of synchronous synaptic inputs to a single cell and that the degree of synchronous synaptic input is better revealed by analyzing the spiking of small populations of neurons that share synchronous synaptic inputs (Baker et al. 2003; Zeitler et al. 2006). Therefore, our observation of significant SFC in the activity of individual saccade preference cells indicates that these cells receive strong synchronous synaptic input. In comparison, our results indicate that the degree of synchronous input received by the reach and saccade preference cells and no preference cells is much weaker than that received by the saccade preference cells.
An intriguing implication of the relationship between spike rate suppression and SFC that we observe is that LFP activity in area LIP specifically reflects the action of inhibitory processes that are involved in coordinated behavior. We find that area LIP neurons that fire in a spatially selective manner when a saccade is being planned, and whose activity is correlated with nearby LFP activity, tend to reduce their rate of firing when a coordinated reach is planned with the saccade. One possible mechanism for this relationship is that LFP activity reflects synaptic potentials due to temporally coherent inputs to the nearby cells and these inputs involve the activity of inhibitory interneurons that drive the reduction in firing rate. Further work that develops models of spike-field activity is needed in order to assess the mechanistic implications of the SFC we report and whether the results imply a specific relationship between inhibitory inputs and correlated spike-field activity.
A Role for Area LIP in Hand and Eye Coordination
A growing body of literature points to the hypothesis that neural mechanisms of coordination lie in fronto-parietal networks (Battaglia-Mayer et al. 2007). In SEF and supplementary motor areas (pre-SMA and SMA) populations of neurons have been described that respond preferentially to a saccade-alone task, a reach and saccade task (SEF, pre-SMA, SMA), or a reach-alone task or nonpreferentially to multiple tasks (pre-SMA, SMA) (Fujii et al. 2002; Mushiake et al. 1996). In FEF, neurons do not alter their firing in the presence of reach with a saccade (Mushiake et al. 1996). However, hand position modulates the activity of visual and saccade-related FEF neurons so that the firing rate of these neurons is either increased or suppressed depending on the location of hand relative to a saccade (Thura et al. 2011). The influence of hand position suggests that FEF neurons may compute spatial transformations for coordinating saccades with reaches in a manner similar to that observed in the dorsal premotor cortex (Batista et al. 2007; Boussaoud et al. 1998; Pesaran et al. 2006, 2010). Limb release modulates the response of area LIP neurons signaling visual attention (Oristaglio et al. 2006). Inactivation of area LIP does not affect limb motor planning itself (Balan and Gottlieb 2009), suggesting that limb signals serve a modulatory role in coordinating visual and motor selection. There is also evidence that the effect of eye-hand coordination on the saccadic system extends beyond fronto-parietal networks to include the SC. Reach-related activity in the SC is consistent with control of eye-hand coordination (Lünenburger et al. 2001; Werner 1993), and fixation neurons in the SC are also activated by reaching in a reach and saccade task (Reyes-Puerta et al. 2010).
Unlike previous work on neural mechanisms of eye-hand coordination, our study is the first to examine both single-unit and LFP activity. Our findings indicate that the suppression of temporally coherent saccade-related responses plays an important role in understanding the role that area LIP plays in coordinated behavior. The results suggest that the neuronal mechanism of eye-hand coordination could involve functionally distinct cell assemblies, some of which increase their firing during coordination and some that are suppressed whose properties can be understood in terms of nearby LFP activity.
Area LIP contains connections with several other brain regions that could mediate the coordination of saccadic eye movements with arm movements. Area LIP is predominantly connected with visual areas, and while the precise pattern of connectivity depends on whether the dorsal (LIPd) or ventral (LIPv) subdivisions of area LIP are considered (Andersen et al. 1990; Blatt et al. 1990; Cavada and Goldman-Rakic 1989; Lewis and Van Essen 2000), connections include reach-related cortical regions PO and MIP (Lewis and Van Essen 2000). Indirect pathways between area LIP and reach-related cortical regions may also be important for coordinated reach and saccade movements. Area LIP neurons project to the lateral pulvinar nucleus of the thalamus (Asanuma et al. 1985). Neurons in the lateral pulvinar respond to reach movements and combined look-reach movements (Acuña et al. 1983) and, in turn, project to parietal area 5 and the medial bank of the intraparietal sulcus, also known as PE and PEa, respectively (Acuña et al. 1990; Cappe et al. 2007). Reversible inactivation of the lateral pulvinar results in deficits of visually guided reaching without the presence of a visual field defect or primary motor deficit, further suggesting a potential role for the lateral pulvinar in coordinated visual behavior (Wilke et al. 2010). According to our results, the influence of coordinated reach movements on saccade-related activity in area LIP is mediated by temporally coherent beta-frequency band activity. While beta-frequency band activity in the lateral pulvinar has not yet been reported, the somatomotor circuits of the parietal cortex and motor cortices contain widespread beta-frequency band LFP activity (Donoghue et al. 1998; Kilavik et al. 2011; Murthy and Fetz 1996; Pesaran et al. 2008), suggesting that reach-related modulation of area LIP responses could originate in these circuits and arise in area LIP through direct and indirect projection systems.
Analysis of Saccade Metrics
Saccades show a positive relationship between peak velocity and amplitude called the main sequence (Bahill 1975). Previous reports have found differences in the main sequence of saccades made alone and saccades made with a reach in visually guided tasks (Epelboim et al. 1997; Snyder et al. 2002). The effect of coordination on neural activity in area LIP cannot be explained exclusively by behavioral differences in our tasks. We found no consistent distinctions between the saccade metrics and the two different tasks. These results differ from previous reports that peak saccade velocity is higher when the saccade is accompanied by a reach (Epelboim et al. 1997). The differences with these studies may be attributed to differences in task design. Notably, in our task saccades are memory guided as opposed to visually guided and we study monkeys, not humans. Snyder et al. (2002) studied monkeys but only present data from one animal during memory-guided movements. Differences in training history may also differ between studies, as the animals in this study were not trained to reach to peripheral targets while maintaining central fixation. It is also worth noting that main sequence effects have been shown to deteriorate under certain conditions, including memory guidance (Becker and Fuchs 1969; Smit et al. 1987), darkness (Sharpe et al. 1975), and head fixation (Collewijn et al. 1992). Under all of these conditions, saccades have been shown to be slower and more variable.
We did, however, observe interesting trends in the spatial metrics of the saccade end points. Saccade end points for the reach and saccade task were less accurate than for the saccade-alone task for half of the target locations. These tended to be the lower targets, however. The variance in end point was also greater for the reach and saccade task in all but two directions. This effect was not limited to the lower targets. Variance was never significantly greater for the saccade-alone task at any target location. This result is congruent with data from human subjects that showed greater variance in saccadic end points for reach and saccade movements compared with saccades alone (Lünenburger et al. 2000). It is possible, therefore, that this is a behavioral effect of the suppression of neural activity in area LIP.
Early Influence of Coordination on Area LIP
We find that the effect of the coordinated reach is substantial and present almost immediately after target onset. This suggests that the influence cannot be purely motor and may reflect cognitive factors associated with coordination. One possible explanation for the early differences in task responses could be related to differences in visual attention in response to the target flash. Previous work has shown that visual attention is tightly linked to saccadic eye movements (Kowler et al. 1995). Differences in the allocation of attention associated with different movements may play a role (Baldauf and Deubel 2010; Jonikaitis and Deubel 2011). Improvements in visual attention are present at the target of an impending saccade and sustained during intervening delays. Attentional improvements are also present when a reach is cued to the same location without a saccade, although the improvement is short-lived and is not sustained before delayed manual responses (Deubel and Schneider 2003). Therefore, the modulation of visual attention is potentially a contributing factor to our observations in area LIP. In this context, it is especially interesting that manual signals have also been reported to influence the firing of area LIP neurons in animals engaged in a visual attention task that required a manual operant response (Oristaglio et al. 2006). A substantial fraction of area LIP neurons that signaled the presence of a cue and not a distracter in the response field were also modulated strongly by the limb, left or right, that released the grasp. These results differ from ours in that they report the influence of limb, left or right, in a nonspatial response while we examine the contralateral limb in a spatial response. Taken together, these results and our own clearly demonstrate an influence of skeletomotor signals on the activity of area LIP neurons.
Postsaccadic Suppression
We also observe suppression associated with movements out of the response field. After the saccade, there is an increase in activity during the saccade-alone task that is suppressed by the addition of the coordinated reach in the reach and saccade task (Fig. 1C). The increased activity after a saccade out of the response field has been thought to be due to a plan to make a return saccade back into the response field (Gnadt and Andersen 1988). Another, potentially related, explanation for this postsaccadic activity is the updating of visual space (Duhamel et al. 1992). Reaching may suppress either of these processes.
Slowing of subsequent saccades by coordinated reaching has been observed in humans (Lünenburger et al. 2000; Neggers and Bekkering 2000, 2001; Prablanc et al. 1986). There are several reasons why slowing of saccade generation could be important for hand-eye coordination. One is that reach accuracy benefits from visual feedback of the hand as it approaches the target (Keele and Posner 1968). Perhaps more telling, reach accuracy benefits when a saccade is made to the target even when both the target and the vision of the hand are occluded (Prablanc et al. 1979; Prablanc and Goodale 1986). This suggests that extraretinal signals from eye position could be important in the accuracy of reaching. In both cases, suppressing saccade-related processes increases the availability of the eye to guide the hand to the target. The present study, however, does not allow a direct test of the hypothesis that suppression of postsaccadic activity is due to reach signals because the experimental design is confounded by differences in hand and eye positions after the saccade. Eye position signals modulate parietal firing according to gain fields (Andersen et al. 1985). Hand position signals, which are present in the superior parietal lobe (Buneo et al. 2002), may also modulate firing in area LIP. Consequently, additional work is needed to more directly test this hypothesis.
GRANTS
This work was supported, in part, by National Science Foundation CAREER Award BCS-0955701, National Institutes of Health (NIH) Grants T32 EY-007136 (M. A. Hagan) and T32 MH-19524 (H. L. Dean), a Fellowship in Brain Circuitry from the Patterson Trust (H. L. Dean), a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (B. Pesaran), a Watson Program Investigator Award from NYSTAR (B. Pesaran), a McKnight Scholar Award (B. Pesaran), and a Sloan Research Fellowship (B. Pesaran).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.H., H.L.D., and B.P. conception and design of research; M.A.H., H.L.D., and B.P. performed experiments; M.A.H., H.L.D., and B.P. analyzed data; M.A.H., H.L.D., and B.P. interpreted results of experiments; M.A.H. and H.L.D. prepared figures; M.A.H., H.L.D., and B.P. drafted manuscript; M.A.H., H.L.D., and B.P. edited and revised manuscript; M.A.H., H.L.D., and B.P. approved final version of manuscript.
REFERENCES
- Acuña C, Cudeiro J, Gonzalez F, Alonso JM, Perez R. Lateral-posterior and pulvinar reaching cells—comparison with parietal area 5a: a study in behaving Macaca nemestrina monkeys. Exp Brain Res 82: 158–166, 1990 [DOI] [PubMed] [Google Scholar]
- Acuña C, Gonzalez F, Dominguez R. Sensorimotor unit activity related to intention in the pulvinar of behaving Cebus Apella monkeys. Exp Brain Res 52: 411–422, 1983 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296: 65–113, 1990 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220, 2002 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science 230: 456–458, 1985 [DOI] [PubMed] [Google Scholar]
- Arcizet F, Mirpour K, Bisley JW. A pure salience response in posterior parietal cortex. Cereb Cortex 21: 2498–2506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma C, Andersen RA, Cowan WM. The thalamic relations of the caudal inferior parietal lobule and the lateral prefrontal cortex in monkeys: divergent cortical projections from cell clusters in the medial pulvinar nucleus. J Comp Neurol 241: 357–381, 1985 [DOI] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62: 566–577, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill A. The main sequence, a tool for studying human eye movements. Math Biosci 24: 191–204, 1975 [Google Scholar]
- Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. Effect of oscillatory activity on corticospinal output. J Neurophysiol 89: 1941–1953, 2003 [DOI] [PubMed] [Google Scholar]
- Balan PF, Gottlieb J. Functional significance of nonspatial information in monkey lateral intraparietal area. J Neurosci 29: 8166–8176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf D, Deubel H. Attentional landscapes in reaching and grasping. Vision Res 50: 999–1013, 2010 [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol 66: 1109–1124, 1991 [DOI] [PubMed] [Google Scholar]
- Batista AP, Santhanam G, Yu BM, Ryu SI, Afshar A, Shenoy KV. Reference frames for reach planning in macaque dorsal premotor cortex. J Neurophysiol 98: 966–983, 2007 [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex 11: 528–544, 2001 [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Mascaro M, Caminiti R. Temporal evolution and strength of neural activity in parietal cortex during eye and hand movements. Cereb Cortex 17: 1350–1363, 2007 [DOI] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Further properties of the human saccadic system: eye movements and correction saccades with and without visual fixation points. Vision Res 9: 1247–1258, 1969 [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science 299: 81–86, 2003 [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol 299: 421–445, 1990 [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Jouffrais C, Bremmer F. Eye position effects on the neuronal activity of dorsal premotor cortex in the macaque monkey. J Neurophysiol 80: 1132–1150, 1998 [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang XJ. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol 90: 415–430, 2003 [DOI] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature 416: 632–636, 2002 [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862, 2007 [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. New York: Oxford Univ. Press, 2006 [Google Scholar]
- Cappe C, Morel A, Rouiller EM. Thalamocortical and the dual pattern of corticothalamic projections of the posterior parietal cortex in macaque monkeys. Neuroscience 146: 1371–1387, 2007 [DOI] [PubMed] [Google Scholar]
- Carey DP, Della Sala S, Ietswaart M. Neuropsychological perspectives on eye-hand coordination in visually-guided reaching. Prog Brain Res 140: 311–327, 2002 [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey. I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol 287: 393–421, 1989 [DOI] [PubMed] [Google Scholar]
- Churchland AK, Kiani R, Chaudhuri R, Wang XJ, Pouget A, Shadlen MN. Variance as a signature of neural computations during decision making. Neuron 69: 818–831, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol 76: 2841–2852, 1996 [DOI] [PubMed] [Google Scholar]
- Collewijn H, Steinman RM, Erkelens CJ, Pizlo Z, van der Steen J. Effect of freeing the head on eye movement characteristics during three-dimensional shifts of gaze and tracking. In: The Head-Neck Sensory Motor System, edited by Berthoz A, Vidal P, Graf W. London: Oxford Univ. Press, 1992, p. 412–418 [Google Scholar]
- Crawford JD, Medendorp WP, Marotta JJ. Spatial transformations for eye-hand coordination. J Neurophysiol 92: 10–19, 2004 [DOI] [PubMed] [Google Scholar]
- Dean H, Marti D, Tsui E, Rinzel J, Pesaran B. Reaction time correlations during eye-hand coordination: behavior and modeling. J Neurosci 31: 2399–2412, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf J, Jensen O, Fries P, Medendorp WP. Neuronal synchronization in human posterior parietal cortex during reach planning. J Neurosci 30: 1402–1412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Delayed saccades, but not delayed manual aiming movements, require visual attention shifts. Ann NY Acad Sci 1004: 289–296, 2003 [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN, Hatsopoulos NG, Gaαl G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J Neurophysiol 79: 159–173, 1998 [DOI] [PubMed] [Google Scholar]
- Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron 44: 365–378, 2004 [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255: 90–92, 1992 [DOI] [PubMed] [Google Scholar]
- Epelboim J, Steinman RM, Kowler E, Pizlo Z, Erkelens CJ, Collewijn H. Gaze-shift dynamics in two kinds of sequential looking tasks. Vision Res 37: 2597–2607, 1997 [DOI] [PubMed] [Google Scholar]
- Ferraina S, Battaglia-Mayer A, Genovesio A, Marconi B, Onorati P, Caminiti R. Early coding of visuomanual coordination during reaching in parietal area PEc. J Neurophysiol 85: 462–467, 2001 [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001 [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224, 2009 [DOI] [PubMed] [Google Scholar]
- Fujii N, Mushiake H, Tanji J. Distribution of eye- and arm-movement-related neuronal activity in the SEF and in the SMA and Pre-SMA of monkeys. J Neurophysiol 87: 2158–2166, 2002 [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216–220, 1988 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007 [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Snyder LH. Spatial and non-spatial functions of the parietal cortex. Curr Opin Neurobiol 20: 731–740, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature 391: 481–484, 1998 [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324: 1207–1210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BJ, Dragoi V. Adaptation-induced synchronization in laminar cortical circuits. Proc Natl Acad Sci USA 108: 10720–10725, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsαki G. Organization of cell assemblies in the hippocampus. Nature 424: 552–556, 2003 [DOI] [PubMed] [Google Scholar]
- Heiser LM, Colby CL. Spatial updating in area LIP is independent of saccade direction. J Neurophysiol 95: 2751–2767, 2006 [DOI] [PubMed] [Google Scholar]
- Helsen WF, Elliott D, Starkes JL, Ricker KL. Coupling of eye, finger, elbow, and shoulder movements during manual aiming. J Mot Behav 32: 241–248, 2000 [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Andersen RA. Effects of visual stimulation on LFPs, spikes, and LFP-spike relations in PRR. J Neurophysiol 105: 1850–1860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput 13: 717–749, 2001 [DOI] [PubMed] [Google Scholar]
- Jia X, Smith MA, Kohn A. Stimulus selectivity and spatial coherence of gamma components of the local field potential. J Neurosci 31: 9390–9403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bδckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci 21: 6917–6932, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonikaitis D, Deubel H. Independent allocation of attention to eye and hand targets in coordinated eye-hand movements. Psychol Sci 22: 339–347, 2011 [DOI] [PubMed] [Google Scholar]
- Jouffrais C, Boussaoud D. Neuronal activity related to eye-hand coordination in the primate premotor cortex. Exp Brain Res 128: 205–209, 1999 [DOI] [PubMed] [Google Scholar]
- Kang K, Shelley M, Henrie JA, Shapley R. LFP spectral peaks in V1 cortex: network resonance and cortico-cortical feedback. J Comput Neurosci 29: 495–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron 61: 35–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele SW, Posner MI. Processing of visual feedback in rapid movements. J Exp Psychol 77: 155–158, 1968 [DOI] [PubMed] [Google Scholar]
- Khawaja FA, Tsui JMG, Pack CC. Pattern motion selectivity of spiking outputs and local field potentials in macaque visual cortex. J Neurosci 29: 13702–13709, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani R, Shadlen MN. Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324: 759–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Ponce-Alvarez A, Trachel R, Confais J, Takerkart S, Riehle A. Context-related frequency modulations of macaque motor cortical LFP beta oscillations. Cereb Cortex (October 20, 2011). doi: 10.1093/cercor/bhr299 [DOI] [PubMed] [Google Scholar]
- Klee MR, Offenloch K, Tigges J. Cross-correlation analysis of electroencephalographic potentials and slow membrane transients. Science 147: 519–521, 1965 [DOI] [PubMed] [Google Scholar]
- Kopell N, Whittington MA, Kramer MA. Neuronal assembly dynamics in the beta1 frequency range permits short-term memory. Proc Natl Acad Sci USA 108: 3779–3784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res 35: 1897–1916, 1995 [DOI] [PubMed] [Google Scholar]
- Kramer MA, Roopun AK, Carracedo LM, Traub RD, Whittington MA, Kopell NJ. Rhythm generation through period concatenation in rat somatosensory cortex. PLoS Comput Biol 4: e1000169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF, Hayhoe M. In what ways do eye movements contribute to everyday activities? Vision Res 41: 3559–3565, 2001 [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex 13: 422–433, 2003 [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428: 112–137, 2000 [DOI] [PubMed] [Google Scholar]
- Louie K, Glimcher PW. Separating value from choice: delay discounting activity in the lateral intraparietal area. J Neurosci 30: 5498–5507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Grattan LE, Glimcher PW. Reward value-based gain control: divisive normalization in parietal cortex. J Neurosci 31: 10627–10639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lünenburger L, Kleiser R, Stuphorn V, Miller LE, Hoffmann KP. A possible role of the superior colliculus in eye-hand coordination. Prog Brain Res 134: 109–125, 2001 [DOI] [PubMed] [Google Scholar]
- Lünenburger L, Kutz DF, Hoffmann KP. Influence of arm movements on saccades in humans. Eur J Neurosci 12: 4107–4116, 2000 [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci 23: 161–179, 2006 [DOI] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J 76: 691–708, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985 [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA 89: 5670–5674, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol 76: 3949–3967, 1996 [DOI] [PubMed] [Google Scholar]
- Mushiake H, Fujii N, Tanji J. Visually guided saccade versus eye-hand reach: contrasting neuronal activity in the cortical supplementary and frontal eye fields. J Neurophysiol 75: 2187–2191, 1996 [DOI] [PubMed] [Google Scholar]
- Nanayakkara T, Shadmehr R. Saccade adaptation in response to altered arm dynamics. J Neurophysiol 90: 4016–4021, 2003 [DOI] [PubMed] [Google Scholar]
- Nauhaus I, Busse L, Carandini M, Ringach DL. Stimulus contrast modulates functional connectivity in visual cortex. Nat Neurosci 12: 70–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Ocular gaze is anchored to the target of an ongoing pointing movement. J Neurophysiol 83: 639–651, 2000 [DOI] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol 86: 961–970, 2001 [DOI] [PubMed] [Google Scholar]
- Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci 30: 4440–4448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oristaglio J, Schneider DM, Balan PF, Gottlieb J. Integration of visuospatial and effector information during symbolically cued limb movements in monkey lateral intraparietal area. J Neurosci 26: 8310–8319, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron 51: 125–134, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature 453: 406–409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. A relative position code for saccades in dorsal premotor cortex. J Neurosci 30: 6527–6537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci 5: 805–811, 2002 [DOI] [PubMed] [Google Scholar]
- Pesaran B. Neural correlations, decisions, actions. Curr Opin Neurobiol 20: 166–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisella L, Sergio L, Blangero A, Torchin H, Vighetto A, Rossetti Y. Optic ataxia and the function of the dorsal stream: contributions to perception and action. Neuropsychologia 47: 3033–3044, 2009 [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature 400: 233–238, 1999 [DOI] [PubMed] [Google Scholar]
- Prablanc C, Echallier J, Komilis E, Jeannerod M. Optimal response of eye and hand motor systems in pointing at a visual target. Biol Cybern 35: 113–124, 1979 [DOI] [PubMed] [Google Scholar]
- Prablanc C, Goodale MA. Visual control of reaching movements without vision of the limb. Exp Brain Res 62: 293–302, 1986 [DOI] [PubMed] [Google Scholar]
- Prablanc C, Martin O. Automatic control during hand reaching at undetected two-dimensional target displacements. J Neurophysiol 67: 455–469, 1992 [DOI] [PubMed] [Google Scholar]
- Prablanc C, Pélisson D, Goodale MA. Visual control of reaching movements without vision of the limb. I. Role of retinal feedback of target position in guiding the hand. Exp Brain Res 62: 293–302, 1986 [DOI] [PubMed] [Google Scholar]
- Ren L, Khan AZ, Blohm G, Henriques DYP, Sergio LE, Crawford JD. Proprioceptive guidance of saccades in eye-hand coordination. J Neurophysiol 96: 1464–1477, 2006 [DOI] [PubMed] [Google Scholar]
- Reyes-Puerta V, Philipp R, Lindner W, Hoffmann KP. Role of the rostral superior colliculus in gaze anchoring during reach movements. J Neurophysiol 103: 3153–3166, 2010 [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberger H, Jarvis MR, Andersen RA. Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron 46: 347–354, 2005 [DOI] [PubMed] [Google Scholar]
- Seo H, Barraclough DJ, Lee D. Lateral intraparietal cortex and reinforcement learning during a mixed-strategy game. J Neurosci 29: 7278–7289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86: 1916–1936, 2001 [DOI] [PubMed] [Google Scholar]
- Sharpe JA, Troost BT, Dell'Osso LF, Daroff RB. Comparative velocities of different types of fast eye movements in man. Invest Ophthalmol 14: 689–692, 1975 [PubMed] [Google Scholar]
- Smit AC, Van Gisbergen JA, Cools AR. A parametric analysis of human saccades in different experimental paradigms. Vision Res 27: 1745–1762, 1987 [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature 386: 167–170, 1997 [DOI] [PubMed] [Google Scholar]
- Snyder LH, Calton JL, Dickinson AR, Lawrence BM. Eye-hand coordination: saccades are faster when accompanied by a coordinated arm movement. J Neurophysiol 87: 2279–2286, 2002 [DOI] [PubMed] [Google Scholar]
- Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J Neurosci 28: 10961–10971, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science 304: 1782–1787, 2004 [DOI] [PubMed] [Google Scholar]
- Thura D, Hadj-Bouziane F, Meunier M, Boussaoud D. Hand modulation of visual, preparatory, and saccadic activity in the monkey frontal eye field. Cereb Cortex 21: 853–864, 2011 [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci 4: 141–150, 1997 [DOI] [PubMed] [Google Scholar]
- Vercher JL, Sarès F, Blouin J, Bourdin C, Gauthier G. Role of sensory information in updating internal models of the effector during arm tracking. Prog Brain Res 142: 203–222, 2003 [DOI] [PubMed] [Google Scholar]
- Vercher JL, Gauthier GM, Guédon O, Blouin J, Cole J, Lamarre Y. Self-moved target eye tracking in control and deafferented subjects: roles of arm motor command and proprioception in arm-eye coordination. J Neurophysiol 76: 1133–1144, 1996 [DOI] [PubMed] [Google Scholar]
- Wang Y, Iliescu BF, Ma J, Josic K, Dragoi V. Adaptive changes in neuronal synchronization in macaque V4. J Neurosci 31: 13204–13213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner W. Neurons in the primate superior colliculus are active before and during arm movements to visual targets. Eur J Neurosci 5: 335–340, 1993 [DOI] [PubMed] [Google Scholar]
- Wilke M, Turchi J, Smith K, Mishkin M, Leopold DA. Pulvinar inactivation disrupts selection of movement plans. J Neurosci 30: 8650–8659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Shapley RM. Spatial spread of the local field potential and its laminar variation in visual cortex. J Neurosci 29: 11540–11549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos TP, Mineault PJ, Pack CC. Removal of spurious correlations between spikes and local field potentials. J Neurophysiol 105: 474–486, 2010 [DOI] [PubMed] [Google Scholar]
- Zeitler M, Fries P, Gielen S. Assessing neuronal coherence with single-unit, multi-unit, and local field potentials. Neural Comput 18: 2256–2281, 2006 [DOI] [PubMed] [Google Scholar]