Abstract
α-galactosylceramide (αGC) represents a new class of vaccine adjuvants and immunomodulators that stimulate NKT cells to secrete Th1 and Th2 cytokines. Synthetic variants with short or unsaturated acyl chains exhibit a striking Th2 bias in vivo but no evidence of defect in TCR signaling or stimulation of NKT cells in vitro. Using cd1d1fl/fl mice, we demonstrated that distinct antigen-presenting cell-types explained the cytokine bias in vivo. Whereas NKT stimulation by αGC required CD1d expression by DCs, presentation of the Th2 variants was promiscuous and unaffected by DC-specific ablation of CD1d. This DC-independent stimulation failed to activate the feedback loop between DC IL-12 and NK cell IFN-γ explaining the Th2 bias. Conversely, forced presentation of the Th2 variants by DC induced high IL-12. Thus, lipid structural variations that do not alter TCR recognition can activate distinct Th1 or Th2 cellular networks by changing antigen-presenting cell targeting in vivo.
Introduction
NKT cells are CD1d-restricted T cells that express a conserved semi-invariant Vα14(huVα24)-Jα18/Vβ8,7,2(huVβ11) TCR (1, 2). They constitute a separate lineage of innate-like T cells characterized by the expression of the signature transcription factor PLZF, which is induced during thymic development and directs the acquisition of an innate effector program (3, 4). This program includes the ability to produce both Th1 and Th2 cytokines upon primary stimulation and changes in homing and recirculation with permanent downregulation of the lymph node homing receptor CD62L, upregulation of the adhesion/migration receptor CD44, changes in chemokine receptor profile and constitutive activation of the integrin LFA-1 (5).
Most NKT cells recognize microbial lipids characterized by a glycuronosyl group in α-linkage to a ceramide as well as the synthetic analog αGC acC26:0 psC18:0 (termed αGC) (6). These agonist ligands induce the reciprocal activation of NKT cells and CD1d-lipid presenting cells through CD40L/CD40 interactions (7, 8), resulting in the secretion of Th1 and Th2 cytokines and the creation of a rich proinflammatory milieu that can markedly enhance adaptive T and B cell responses. Consequently, NKT ligands have often demonstrated superior adjuvant properties in vivo compared with TLR ligands (9, 10).
Intriguingly, extensive structure-function studies have identified synthetic variants of αGC with a marked Th2 bias and others with a Th1 bias, raising the prospect of their use for cytokine-specific adjuvant therapy as well as immunomodulation of disease (11–13). Based on the assumption that they differentially engaged the NKT cell TCR, the Th2 variants have been referred to as “altered glycolipid ligands” in some reports (11, 14), by analogy to altered peptide ligands with similar cytokine-biasing properties. This hypothesis has received some support for one of the leading ligands, αGC acC24:0 psC9:0 (also called OCH), which was shown to induce a conformational change of the F' pocket of CD1d with corresponding diminution of the binding properties of the TCR (14, 15). OCH failed to induce sufficient c-rel mRNA and CD40L on NKT cells in vivo and elicited diminished IFN-γ but conserved IL-4 from NKT cells (16). Whereas αGC-mediated NKT stimulation indirectly induces NK cells to release a second, long-lasting wave of IFN-γ secretion in vivo (17), OCH conspicuously failed to recruit NK cells. Since the late IFN-γ is dependent of CD40-mediated IL-12 production by DCs, it was concluded that altered TCR signaling impaired both direct and indirect secretion of Th1 cytokines (18).
Other well-studied Th2 variants such as αGC acC20:2 psC18 (termed αGC acC20:2) (12) and αGC acC8:0 psC18 (termed αGC acC8) (13) did not seem to fall into the same category, however, as detailed biophysical and crystallographic studies demonstrated that their interactions with CD1d and the NKT cell TCR were indistinguishable from those of αGC (14, 19, 20). Other properties of these strong agonists might therefore account for their marked Th2 bias. Recently, a unifying set of chemical and cell biological properties was identified for all Th2 ligands, including αGC acC20:2, αGC acC8 and OCH. Their short or unsaturated lipid chains conferred higher solubility in the aqueous environment, which allowed them to directly load CD1d at the cell surface, whereas, in contrast, αGC and other Th1 ligands needed endosomal loading by lipid transfer proteins (20–22). Further, after recycling of CD1d in the lysosomal compartment, the Th2 variants were displaced within seconds by endogenous lipids in a pH-dependent manner, whereas αGC remained stably associated to CD1d (22). As CD1d recycles actively between the cell surface and the lysosome (23), cells pulsed with short and unsaturated lipids lost their ability to stimulate NKT cells in culture faster than those pulsed with αGC (22). In addition, CD1d-αGC complexes were shown to localize preferentially to lipid rafts at the cell surface, whereas the “Th2” variant αGC acC20:2 was largely excluded (20). These common cell biological properties of the Th2 variants suggested several possible mechanisms to explain their functional differences in vivo. For example, the location of CD1d-lipid complexes on membrane lipid rafts might somehow alter NKT cell stimulation towards more IFN-γ production or indirectly favor NK cell recruitment. IFN-γ production might also be selectively impaired upon interruption of TCR signaling due to the fast dissociation of CD1d complexed with short and unsaturated lipid ligands. Here, we directly tested a third possibility, that the Th2 bias of the αGC variants in vivo might be the consequence of different antigen-presenting cells.
We generated cd1dfl/fl mice and studied the response of mice lacking CD1d selectively on DCs, macrophages or B cells, the main CD1d-expressing cell types. While the presentation of αGC was strictly limited to DC and to a lesser extent macrophages, as previously suggested (24), the stimulation by the Th2 variants was promiscuous and mostly carried out by a variety of non-DC cell types which did not produce IL-12. This DC-independent stimulation failed to induce the IL-12/NK/IFN-γ feedback loop of innate immunity, resulting in a dominant Th2 response. When NKT cells were forced to interact with purified DCs, both αGC and the Th2 variants upregulated CD40L and induced similar quantities of IL-12. Thus, lipid structural variations that do not alter TCR recognition can activate distinct Th1 or Th2 cellular networks by changing antigen-presenting cell targeting in vivo.
Materials and Methods
Mice
Cd19-Cre (B6.129P2(C)-Cd19tm1(cre)Cgn), Lyz2-Cre (B6.129P2-Lyz2tm1(cre)Ifo), Cd4-Cre (B6 Tg(cd4-cre)1Cwi) and C57BL/6J mice were from The Jackson Laboratory. Cd11c-Cre (C57BL/6J-Tg(Itgax-cre,-EGFP)4097Ach/J) mice were obtained from Dr. Alexander Chervonsky at our institution. CD4p-Vα14-Jα18 transgenic mice (25) were maintained in the laboratory. All mice were raised in a specific pathogen free environment at the University of Chicago, and experiments were performed according to a protocol approved the Institutional Animal Care and Use Committee.
Generation of B6.cd1d1fl/fl mice
The mouse cd1 locus includes two cd1d genes, cd1d1 and cd1d2 which are 95% identical. As cd1d2 is a pseudogene in the B6 strain (26), we used homologous recombination to flank exons 2 and 6 of cd1d1 with loxP sites in an ES line of B6 origin. After crossing to B6 mice expressing the Flp recombinase to remove the frt-flanked PGK-neomycin sequence used for in vitro selection of recombined ES cells, the B6.cd1d1fl/fl conditional mutant line was generated.
B6.cd1d1fl/fl mice were crossed to cd19-cre, cd11c-cre and lyz2-cre to obtain cd19 Δ/Δ, cd11c Δ/Δ and lyz2 Δ/Δ mice. Littermate controls included Cre-negative littermates and in some cases Cre-positive cd1d1fl/+ or Cre-positive cd1d1+/+ littermates.
PCR protocol for typing the cd1d1fl/fl and the cre mice
B6.cd1dfl/fl mice were genotyped by PCR (forward primer: 5' -ATG TAT CCA GAG GTT TTA CTT GGT GAA- 3'; reverse primer: 5'- TGG GGT CCA TTC CAG ATA CAA A-3'). Mice with loxP sites generated a 351 bp fragment instead of a 156 bp fragment in wild type mice. Cre-deleter mice were genotyped by PCR with primers (forward primer: 5'- TTA CCG GTC GAT GCA ACG AGT-3'; reverse primer: 5'-TTC CAT GAG TGA ACG AAC CTG G-3') that generated a 400 bp fragment.
Flow Cytometry
Splenic lymphocytes and thymus cells were isolated by mincing and passing through 70 μm nylon cell strainer (Falcon). Hepatic lymphocytes were further purified by running through Percoll gradient (Sigma Aldrich). Fluorochrome labeled monoclonal antibodies against mouse CD1d (1B1), B220 (RA3.6B2), F4/80 (BM8), CD11c (N418), CD11b (M1/70), CD24 (M1/69), CD5 (53-7.3), TCR Vβ7 (TR310), TCR Vβ8.1+8.2 (MR5-2), TCRβ (H57–597) CD4 (GK1.5), CD8α (53–67.5), DX5 (DX5), CD40L (MR1), IL-4 (11B11), IFNγ(XMG1.2), IL-12p40 (C15.6) were purchased form eBioscience or Biolegend. CD1d-PBS57 tetramers were obtained from the NIAID Tetramer Core Facility. For intracellular cytokine staining, cells were stained with cell surface markers first, and were fixed in Cytofix/Cytoperm buffer (BD) for 15 minutes and further stained with antibody against mouse IL-4, IL-12p40 and IFNγ in wash/perm buffer for 1 hour on ice. Samples were analyzed on an LSR ∥ (BD Biosciences) or sorted on a FACSAria (BD Biosciences).
Lipid antigens
αGC (acC26:0 psC18:0) was purchased from Alexis Biochemicals and its variants αGC acC8:0 psC18, αGC acC20:2 psC18 and PBS57 were produced as described previously (6, 13, 27). C-glycoside αGC was obtained from NIH Tetramer Core Facility. All the lipid antigens were dissolved in DMSO and stored at −20 °C at 1 mg/ml.
NKT cell stimulation assays
Fresh NKT cells were purified by sorting as TCRβ+ CD1d-PBS57 tetramer+ cells (spleen and liver) or CD24− tetramer+ (thymus) and cocultured with BMDC pulsed with 200 ng/ml PBS57 for 48 hours. BMDC were obtained after culture of bone marrow cells in medium supplemented with 10% FCS, 10 ng/ml GM-CSF and 10ng/ml IL-4 for 7 days. Alternatively, NKT cells were stimulated with 1 μM ionomycin and 20 ng/ml PMA for 48 hours.
To obtained fresh in vivo pulsed APC, B6 mice were intraperitoneally injected with lipid (2 μg for αGC or 50 μg for αGC acC8) and their spleens harvested 2 hours later to sort CD11chigh DCs, CD11chigh CD8α+ lymphoid DCs, CD11clo CD11blo F4/80+ red pulp macrophages and B220+ B cells, prior to coculture with the CD1d-αGC responsive NKT hybridoma DN32.D3 overnight and measure of IL-2 release by Cytometric Beads Array Mouse IL-2 Flex Set. In other experiments, CD11c+ DCs were enriched from splenocytes by depletion with a cocktail of anti-CD3, CD11b, F4/80 and B220 beads and subsequent enrichment with anti-CD11c beads using the autoMACS cell separator (Miltenyi Biotec). B cells were enchiched by depletion with a mixture of anti-CD3, CD11b, F4/80 and CD11c beads.
For in vitro stimulation of IL-12p70 production, DCs, macrophages and B cells were sorted as above and cultured with fresh NKT cells sorted as CD5+ cells from Vα14 Tg spleens. In the presence of 1 μg/ml αGC or 2.5 μg/ml αGC acC8 for 48 hours. IL12-p70 released in supernatant was measured with Cytometric Beads Array Mouse IL-12-p70 Flex Set.
Statistical analysis
Different groups were compared using two tailed t test, *p<0.05, **p<0.01, ***p<0.001.
Results
Characterization of cd1d1fl/fl mice
Cd1d1fl/fl mice (Fig. 1A) were produced by homologous recombination in an ES line of B6 origin. Cd1d1fl/fl Cd4-Cre mice (cd4 Δ/Δ) mice showed a 90% reduction of CD1d expression on cortical CD4+CD8+ thymocytes and a corresponding 75 to 86% reduction of NKT cell numbers in the thymus, spleen and liver (Fig. S1A). This result constitutes a direct demonstration that NKT cell development is exquisitely and solely dependent on CD1d expression by DP thymocytes, as previously suggested by bone marrow chimeras and transgenic studies (28–30). Furthermore, as expected in the presence of low concentrations of CD1d ligands, the few residual NKT cells expressed a bias towards expression of Vβ7 (Fig. S1B), which confers the highest affinity for endogenous CD1d ligands when paired with Vα14-Jα18 (31).
Fig. 1. Characterization of cd1d1fl/fl mice.
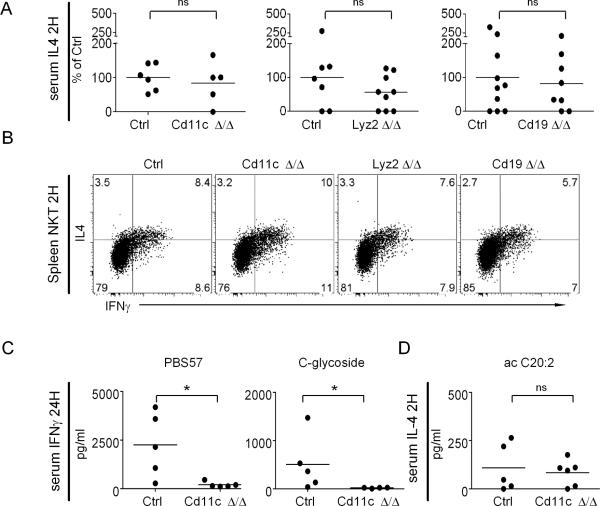
(A) The targeted C57BL/6 cd1d locus (not to scale) contains two loxP sites flanking the exons 2 and 6 of cd1d1 as well as an frt site left over after removal of the frt-neomycin-frt resistance cassette. The cd1d2 gene is a pseudogene in the C57BL/6 strain (26). (B) CD1d expression in splenic DC, macrophage and B cell subsets of WT and Δ/Δ mice gated as indicated. Representative of 2 separate experiments with 2 to 3 mice per groups. (C) CD1d-PBS57 tetramer+ NKT cells among CD24low thymocytes and TCRβ+ spleen and liver cells of control and Δ/Δ mice as indicated. Representative of 2 separate experiments with 2 to 3 mice per groups with mean ± SEM of NKT cell frequency indicated in the FACS plots. (D) Cytokine released by sorted or MACS-enriched CD1d-PBS57 tetramer+ NKT cells at indicated cell numbers from thymus (left) and spleen (right) of WT and Cd11c Δ/Δ co-cultured for 48 hours with 50,000 BMDC pulsed with 200 ng/ml PBS57. Data are from three independent experiments with 2–3 mice pooled per group and are represented as % of the cytokines released by WT NKT cells at 30,000 cells/well. (E) Early serum IL-4 measured in individual WT and Cd11c Δ/Δ mice 90 mn after injection of 1.25μg anti-CD3ε.
Cd11c Δ/Δ mice lacked CD1d on splenic DCs, which are CD11chigh, and also on macrophage subsets that express low levels of CD11c such as the F4/80+ red pulp macrophages (Fig. 1B). Lyz2 Δ/Δ mice lacked CD1d on macrophage subsets including the F4/80+ red pulp macrophages but expressed normal levels on CD11chigh DCs. Cd19 Δ/Δ mice lacked CD1d on B cells. In contrast with the cd4 Δ/Δ mice, the cd11c Δ/Δ, lyz2 Δ/Δ, and cd19 Δ/Δ mice exhibited normal development and distribution of NKT cells (Fig. 1C). Furthermore, the intrinsic cytokine secretion properties of NKT cells assessed by stimulation with PBS57-pulsed BMDCs in vitro were preserved, as was the rapid release of IL-4 upon injection of anti-CD3 in vivo, a characteristic properties of NKT cells (32) (Fig. 1D). Thus, the cd1d1fl/fl mouse allowed deletion of CD1d in selective antigen presenting cell subsets without altering the frequencies or functional properties of NKT cells.
Comparison of αGC and αGC acC8 in vivo
The structure and nomenclature of αGC variants used in this study is shown in Fig. S2. The Th2 bias of αGC variants with short acyl chain was previously defined in vitro in whole spleen cell stimulation assays (13). A detailed comparison of their functional properties was performed in a time course study in vivo. Unless otherwise indicated, mice were injected intraperitoneally with doses of αGC and αGC acC8 that were previously shown to elicit similar activation of NKT cells in vitro, 2 μg for αGC and 5 μg for αGC acC8. Fig. 2A showing serum IL-4 and IFN-γ at their respective peaks at 2 hours and 24 hours illustrates the opposite cytokine bias of αGC and αGC acC8. The Th2 bias of αGC acC8 correlated with the lack of detectable IL-12p70 and contrasted with the abundant amount released after αGC injection (Fig. 2B). These findings are superimposable to those previously reported for the other Th2 variants αGC acC20:2 and αGC psC6 (33).
Fig. 2. Comparison of αGC with αGC acC8 in vivo.
B6 mice were injected intraperitoneally with 2μg αGC or 5μg αGC acC8 unless otherwise indicated. (A) Serum IL-4 at 2 h and serum IFNγ at 24 h. (B) Serum IL-12p70 at 6 h. (C) Splenic TCRβ+ CD1d-αGalCer+ NKT cells collected at 1 h, 2 h and 6 h as indicated and directly stained for intracellular IL-4 and IFNγ. Results compiled from 3 separate experiments (D) Spleen cells collected at 6 h, stained with anti-DX5 and anti-IFNγ and displayed after gating on TCRβ−B220−cells. Left, representative FACS plots; right, summary figures showing the frequency of IFNγ secreting cells among NK cells and their MFI for IFNγ. Results compiled from 3 separate experiments. (E) Mice received 5 injections (at 0, 1, 2, 3 and 5 h) of 5μg or 1 injection of 25μg of αGC acC8. Sera were collected at 6h for IL-12p70, 24 h for IFNγ and 2h for IL-4 and were compared with sera of mice receiving 1 injection of 2μg αGC at 0 h. Results compiled from 2 separate experiments.
While the two lipids induced comparable intracellular IL-4 and IFN-γ in splenic NKT cells at 2 hours, the cytokine secretion elicited by acC8 was shifted to earlier time points for acC8 and declined while the cytokines induced by αGC were still increasing between 2 and 6 hours (Fig. 2C). Splenic NK cell production of IFN-γ was markedly inferior for αGC acC8 compared with αGC both in frequency and in mean fluorescence intensity at 6 hours (Fig. 2D).
Repeating the injections of αGC acC8 5 times over a 5-hour period or increasing the dose from 5 to 25 μg resulted in a further increase in IL-4 but completely failed to reverse the defect in IL-12 and IFN-γ, indicating that the Th2 bias was independent of the dose or the duration of exposure to the lipid in vivo (Fig. 2E).
Further kinetic studies indicated that NKT cell stimulation by αGC was simply delayed by 2–3 hours compared with αGC acC8, without significant alterations in the quality of this stimulation. For example, TCR downregulation and the induction of CD25, CD40L and PD-1, all occurred earlier after injection of αGC acC8 than after αGC (Fig. 3). Full expression of PD-1 after αGC as reported by others (34) only occurred at 12 hours, beyond the time frame of this experiment.
Fig. 3. Kinetics of NKT cell activation by αGC and αGC acC8 in vivo.
(A) TCRβ+ CD1d-PBS57+ NKT cells in the spleen at indicated times after injection of lipids. Numbers represent NKT cell frequency ± SEM compiled from 2 experiments with 3 mice per groups. (B) Kinetics of CD40L and CD25 induction on TCRβ+ CD1d-PBS57+ gated NKT cells. (C) Kinetics of PD-1 induction by NKT cells. Results are representative of 2 independent experiments with 3 mice per group.
Differential antigen-presenting cell requirements for αGC and αGC acC8 in vivo
Cd11c Δ/Δ mice showed no detectable serum IL-4 or IFN-γ after injection of αGC, suggesting failure to activate NKT cells (Fig. 4A). This conclusion was confirmed by direct intracellular staining showing absent or massively decreased IL-4 and IFN-γ in splenic and liver NKT cells (Fig. 4B–C) and absent IFN-γ in splenic and liver NK cells (Fig. 4D–E). Thus, despite the broad tissue distribution of CD1d expression in mice, NKT cell stimulation was absolutely dependent on CD1d expression by CD11c-expressing cells.
Fig. 4. Antigen presenting cell requirement for αGC in vivo.
All mice were injected with 2μg αGC intraperitoneally. (A) Serum IL-4 at 2 h and serum IFN-γ at 24 h in cd11c Δ/Δ, lyz2 Δ/Δ, cd19 Δ/Δ mice and littermate controls. Data are normalized to the mean of littermate controls. (B) Gated TCRβ+ CD1d-αGalCer+ NKT cells in spleens of WT and Δ/Δ mice were directly stained for intracellular IL-4 and IFNγ at 4 h post-injection. Top, representative FACS plots; bottom, summary figures. (C) Same as B for liver NKT cells collected at 4 h. (D) DX5 and intracellular IFN-γ staining on gated TCRβ− B220− spleen cells at 6 h post-injection. Left, representative FACS plots; right, summary figure. (E) Results as in D for liver NK cells at 6 h. Results are compiled from 2 independent experiments.
Lyz2 Δ/Δ mice exhibited a modest but in some cases significant reduction of cytokines released in the serum or present in the cytosol of NKT and NK cells (Fig. 4A–E). Taken together with the total ablation of NKT cell activation in cd11c Δ/Δ mice, these results suggested that a cell type co-expressing cd11c and lyz2, e.g. a macrophage or DC subset contributed partially to the presentation of αGC and might be preferentially involved in the serum release of IL-4.
Finally, no detectable changes were observed in cd19 Δ/Δ mice (Fig. 4A–E), indicating that presentation by B cells was not significantly involved in NKT or NK cell activation.
Together, these findings established that NT cell stimulation by αGC in vivo was strictly dependent on αGC presentation by DCs and also, to a lesser extent, a subset of CD11c+ lysM+ macrophages or DCs.
In striking contrast with αGC, the Th2 variant αGC acC8 induced equivalent stimulation in controls and in cd11c Δ/Δ mice (Fig. 5A–B). Although αGC acC8 does not induce the late (NK-dependent) IFN-γ in the 24-hour serum, the intracellular IFN-γ produced by NKT cells was similar in cd11c Δ/Δ and littermate controls. Furthermore, lyz2 Δ/Δ and cd19 Δ/Δ mice also showed the same stimulation as littermate controls. Thus, in keeping with the promiscuous ability of αGC acC8 to load CD1d at the cell surface independently of either lysosomal recycling or lipid transfer proteins, there was no dedicated antigen-presenting cell-type required in vivo. Furthermore, the absence of CD1d expression by DC did not alter the pattern of IL-4 and IFN-γ production by NKT cells.
Fig. 5. Antigen presenting cell requirement for αGC acC8 and other variants in vivo.
Δ/Δ mice and their littermate controls were injected with 5 μg αGC acC8 intraperitoneally. (A) Serum IL-4 at 2 h in cd11c Δ/Δ, lyz2 Δ/Δ, cd19 Δ/Δ and littermate controls. Serum IFN-γ at 24 h was undetectable. Data are normalized to the mean of littermate controls. (B) WT and Δ/Δ splenic NKT cells gated as TCRβ+ CD1d-αGalCer+ were stained for intracellular IL-4 and IFN-γ 2 h post injection. Left, representative FACS plots. Representative of 2 independent experiments with 2 to 3 mice per group. (C) Serum IFN-γ at 24 h and (D) serum IL-4 at 2 h post injection of 0.2 μg Th1 variant PBS57, 2 μg Th1 variant C-glycoside or 5 μg Th2 variant αGC acC20:2. IL-4 was indetectable for C-glycoside and IFN-γ was indetectable for αGC acC20:2.
Other Th1 and Th2 αGC variants exhibited patterns similar to αGC and αGC acC8
Other Th1 variants such as αGC C-glycoside (35) and PBS57 (27) exhibited a dependence of CD1d expression by DC like αGC, whereas αGC acC20:2 (12), another well characterized Th2 variant followed the same pattern as αGC acC8 (Fig. 5C–D). These results indicate that the Th1 bias of NKT ligands is tightly associated with their selective presentation by DC.
Forced interaction of NKT cells with αGC acC8-pulsed DCs induced IL-12
Previous studies indicated that the CD8α+ langerin+ subset of DCs was the main cell type producing IL-12 after αGC injection in vivo (24, 36). As IL-12 secretion by DCs required CD40L expression by NKT cells (18, 37, 38) and the Th2 variants potently induced CD40L on NKT cells, one potential explanation for the failure of the Th2 variants to induce IL-12 might be that they were not presented by DCs in vivo, or that the frequency of DC encounters was decreased because of promiscuous presentation by other, more numerous cell types. Direct intracellular staining for IL-12 confirmed that CD8α+ DCs were the main IL-12p40 expressing cell type at 6 hours post injection of αGC (Fig. 6A). However, this experiment clearly established that αGC acC8 also induced IL-12p40, albeit on far fewer DCs than αGC.
Fig. 6. Th2 variant αGC acC8 fails to induce IL12 in vivo.
B6 mice were injected intraperitoneally with 2μg αGC or 5μg αGC acC8. (A) Splenocytes were harvested 6 h later and intracellularly stained for IL-12p40. Top row shows representative FACS plots of gated CD11c+ DC cells co-stained with anti-CD8α. Central row shows gated CD11blo CD11clo cells costained with anti-F4/80. Bottom row shows cells in the lymphoid gate costained for B220. Data representative of 4 experiments with 2 to 3 mice per group. (B) Mice were injected as in A with 2μg αGC or 5μg αGC acC8 and compared with mice injected with the mixture of 2μg αGC and 5μg αGC acC8. Top row shows DC staining for IL-12p40 as in A; bottom row shows staining of TCRβ− B220− splenic cells for DX5+ and intracellular IFN-γ. (C) Serum IL12-p70 at 6 h, IL-4 at 2 h and IFN-γ at 24 h post injection of 2μg αGC, 5μg αGC acC8 or the mixture as indicated.
Furthermore, if the Th2 bias was simply due to a DC-intrinsic defect in presentation of αGC acC8, then injecting mice with a mixture of αGC and αGC acC8 should result in strong IL-12 production and NK cell activation. Fig. 6B–C demonstrated the opposite result, i.e. a strict dominance of the Th2 bias. Thus, the Th2 bias likely resulted from the diversion of NKT cells away from DC towards interactions with other αGC acC8-presenting cell types.
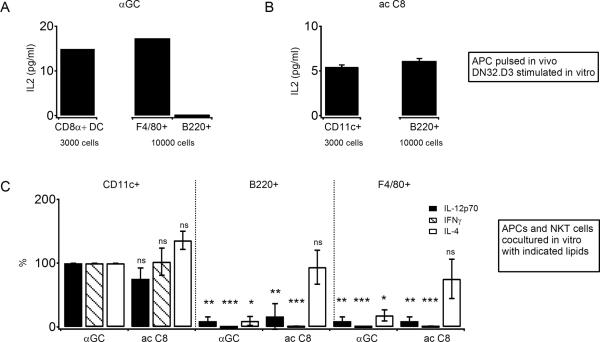
The nature of the preferred APCs for αGC acC8 and αGC was further investigated in vivo and in vitro. Two hours after the intraperitoneal injection of lipid in vivo, different APCs were purified and immediately co-cultured with the αGC-responsive IL-2 producing hybridoma DN32.D3 as a readout of antigen presentation. Whereas both DCs and F4/80 macrophages were effective presenters of αGC, B cells were not (Fig. 7A). In marked contrast, αGC acC8 was efficiently presented by B cells as well as by other cell types (Fig. 7B). In other experiments, CD11c+ DCs, B220+ B cells and F4/80+ macrophages were purified from uninjected mice and incubated in vitro with fresh Vα14 tg NKT cells in the presence of lipid for 48 hours. Notably, when forced to interact exclusively with DCs, αGC acC8 elicited the same amount of IFN-γ and IL-12 as αGC in this culture system, unambiguously demonstrating its intrinsic ability to elicit a Th1 profile (Fig. 7C). αGC acC8 also tended to elicit a little more IL-4 than αGC, but this 1.5 fold increase was not statistically significant. In this NKT-APC coculture assay, highly purified B cells were able to stimulate NKT cells when pulsed with αGalCer acC8 but little stimulation was observed with αGalCer, consistent with a relative defect in CD1d presentation of αGalCer by B cells. Furthermore, αGalCer acC8 induced markedly more IL-4 relative to IFN-γ when presented by B cells than by DCs, consistent with the importance of IL-12 production by DCs for positive feedback on IFN-γ production.
Fig. 7. αGC acC8 can be presented by DCs to induce IL-4, IFN-γ and IL-12p70.
(A) CD11chi CD8α+ DCs, B220+ B cells, and CD11clo CD11blo F4/80+ macrophages were sorted from pooled spleens from 3 B6 mice 2 h after injection of 2 μg αGC and cocultured at indicated numbers with the hybridoma DN32.D3 (50,000 cells/well) overnight and IL-2 released in the supernatant was measured. Error bars represent SEM (B) CD11c+ DCs and B220+ B cells were enriched using autoMACS from the pooled spleens of 3 B6 mice 2 h after injection of 50 μg αGC acC8 and cocultured at indicated numbers with DN32.D3 as in (A). (C) CD11chi DCs, B220+ B cells, CD11clo CD11blo F4/80+ macrophages were sorted from the spleens of uninjected mice and cocultured at 5000 cells per well in the presence of 1 μg/ml αGC or 2.5 μg/ml αGC acC8 for 48 hours with sorted CD5+ cells (50,000 per well) obtained from the spleens of Vα14 Tg mice. IL12-p70, IFN-γ and IL-4 were measured in supernatants. Three separate experiments were performed and data were normalized to the DC+αGC group (100%) in each experiment. Error bars indicate SEM.
Discussion
The generation of mice carrying a conditional allele of cd1d1 allowed a dissection of the role of different CD1d-expressing cells both in the development and in the peripheral activation of NKT cells. The results directly established the essential role of CD1d expression by thymocytes for NKT cell development, as previously suggested by bone marrow radiation chimeras and CD1d transgenic experiments (28–30). They also demonstrated the dispensible role of CD1d expression by DCs, macrophages or B cells for the development of NKT cells and the acquisition of their cytokine profile. The most novel insight derived from these studies was the in vivo demonstration of different antigen-presenting cell requirements for αGalCer and its short and unsaturated acyl variants, which provided a simple and unifying explanation for the Th2 bias of the variants.
Whereas the presentation of αGC and other Th1 variants such as PBS57 and αGC C-Glycoside was entirely restricted to DC/macrophage cell types, presentation by DC/macrophages was negligible compared to other cell types after injection of the Th2 variants αGC acC8 and αGC acC20:2 in vivo. Because only DC and macrophages can produce the IL-12 that is critical for amplification of Th1 responses and the recruitment of NK cells, these findings are sufficient to explain the Th2 bias associated with the main variants studied so far.
The importance of αGC presentation by DC in vivo has been previously suggested based on methods using a DTR-Tg driven by a cd11c promoter (24) or by a langerin promoter (36) to allow DC-specific killing after administration of diphteria toxin. Notably, whereas serum IFN-γ was nearly absent, IL-4 was much less reduced in these experiments, contrasting with the total ablation of both cytokines in the cd11c Δ/Δ mice. The difference may reflect the role of macrophage subsets that were reportedly spared in the DTR models (24) but, due to their intermediate expression of CD11c, had lost CD1d expression in the cd11c Δ/Δ mice. The ablation of serum IFN-γ by mice lacking langerin+ DCs, which largely overlap with the CD8α subset and are located in the marginal zone of the spleen (36), further underscores the specialized role of these IL-12 producers in the recruitment of NK cells. In contrast, NKT cell production of both IL-4 and IFN-γ was preserved after deletion of langerin+ DCs, in keeping with the ability of other DC subsets to load αGC and stimulate NKT cells. In the current study, the total ablation of NKT cell activation in cd11c Δ/Δ mice, as monitored by intracellular cytokines, unambiguously established that DCs, and possibly a small subset of macrophages, constituted the exclusive cell-type mediating αGC induced stimulation in vivo. This selective presentation is at least in part a consequence of two peculiar features of αGC compared with the Th2 variants. Its transport by serum lipoprotein particles and the lipoprotein receptor-mediated uptake by DCs and macrophages (22, 39, 40). Its requirement of lysosomal lipid transfer proteins, which are most active in DCs and macrophages, for CD1d loading (41, 42).
While previous studies of B cell deficient μMT mice suggested enhanced (24) or unmodified (43) stimulation by αGC, the cd19 Δ/Δ mice demonstrated that B cell presentation was not significantly involved for systemic αGC-mediated stimulation. This finding was consistent with the inefficient capture and presentation of αGC by B cells when the lipid was injected alone and with its efficient presentation upon BCR-mediated uptake and endosomal delivery of antigen+lipid coated beads (44).
The uptake and presentation of the Th2 variants in vivo followed drastically different rules. The stimulation was entirely unaffected in cd11c Δ/Δ mice compared with wild type mice, demonstrating that NKT cells did not require professional APC for activation. This finding was fully consistent with previous studies indicating that short or unsaturated αGC variants were rapidly loaded onto CD1d expressed at the cell surface and that lysosomal recycling was not only unnecessary, but in fact was detrimental to sustained presentation (22). Although the removal of CD1d from DCs and macrophages did not impair the stimulation of NKT cells by the Th2 variants, our in vitro and in vivo experiments demonstrated that these cell types could present the Th2 variants efficiently. In fact, CD8α DCs loaded with αGC acC8 or αGC acC20:2 released the same amount of IL-12 as those loaded with αGC in the presence of fresh NKT cells. Conversely, B cells stimulated NKT cells more effectively when loaded with the Th2 variants than with αGC and, consistent with their inability to secrete IL-12, they induced relatively more IL-4 than IFN-γ compared with DCs.
Altogether, the results suggest that the overriding cause of the Th2 bias of the short and unsaturated variants of αGC is their wide ranging and promiscuous presentation by non-IL-12 producing cell-types, which stems from their ability to directly load CD1d at the cell surface. This wide ranging presentation decreases the frequency of NKT cell interactions with CD8α DCs and the associated IL-12 release, while it increases interactions with antigen-presenting cells that are unable to enhance IFN-γ production or recruit NK cells.
Our results do not rule out a contribution of other general mechanisms, however, although these would appear less likely to play a prominent role in the Th2 bias of αGC variants. We did observe that the Th2 variants induced a stimulation that was somewhat less persistent than αGC, but the difference reflected in part a simple time shift of 2 to 3 hours due to the faster loading of these lipids, and the upregulation of CD40L, CD25, PD-1, IL-4 and IFN-γ by NKT cells was comparable for both types of lipids. Furthermore, repeated injections of the short acyl variant did not restore serum IL-12 or IFN-γ. While the Th2 variants elicited less IL-12 production by DCs, this was mostly due to a decrease in the frequency of IL-12-producing DC. It is also possible that the preferential subcellular location of CD1d-αGC on lipid rafts may somehow enhance the recruitment of NK cells, although this effect would have to be independent of the ability to induce CD40L, IL-4 and IFN-γ in NKT cells, or IL-12 in DCs. Furthermore, the injection of a mixture of αGC and αGC acC8, which would lead to preferential loading of DC by αGC and of other antigen-presenting cells by αGC acC8, showed clear dominance of the Th2 variant consistent with the diversion of NKT cells away from IL-12 producing DC.
In conclusion, the common physicochemical properties of the Th2 variants, mainly their increased solubility in aqueous compartment, not only underlied their cell biological properties, but in turn dictated their targeting to vastly different sets of antigen-presenting cells in vivo. αGC, which selectively targeted DC and macrophages, is emerging as a choice adjuvant for the crosspriming of CTLs. However, our results predict that the ability of αGC to directly recruit NKT cell help to B cells is likely compromised by inefficient B cell presentation in vivo. By contrast, while the Th2 variants may be inferior at CTL priming they should provide superior recruitment of NKT cells for cognate B cell help and antibody production. In addition, similar differences are likely to govern NKT cell responses to natural endogenous and exogenous lipid ligands differing in lipid length and saturation (45, 46). The cd1d1fl/fl mouse provides therefore a powerful system to dissect the complex cellular interactions involved in NKT cell-mediated adjuvant effects in cellular and humoral immune responses.
Supplementary Material
Acknowledgements
We thank members of the Bendelac laboratory for discussions and the Animal Resource Center, Core Flow Cytometry Facility, DNA Sequencing Facility; the NIAID tetramer facility for CD1d tetramers.
This work was supported by Major State Basic Research Development Program of China (973 program 2012CB825806), National Natural Sciences Foundation of China 31021061, NIH grants AI038339 and AI053725 and by the Digestive Disease Research Core Center P30 DK42086. A.B. is a Howard Hughes Medical Institute Investigator.
REFERENCES
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 3.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savage PB, Teyton L, Bendelac A. Glycolipids for natural killer T cells. Chem Soc Rev. 2006;35:771–779. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)- 12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parekh VV, Wilson MT, Van Kaer L. iNKT-cell responses to glycolipids. Crit Rev Immunol. 2005;25:183–213. doi: 10.1615/critrevimmunol.v25.i3.20. [DOI] [PubMed] [Google Scholar]
- 10.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 12.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, 3rd, Teyton L, Bendelac A, Savage PB. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 14.Wun KS, Cameron G, Patel O, Pang SS, Pellicci DG, Sullivan LC, Keshipeddy S, Young MH, Uldrich AP, Thakur MS, et al. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 2011;34:327–339. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnaud C, Lee D, Donnars O, Park S-H, Beavis A, Koezuka Y, Bendelac A. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. [Cutting Edge] 1999;163:4647–4650. [PubMed] [Google Scholar]
- 18.Oki S, Tomi C, Yamamura T, Miyake S. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int Immunol. 2005;17:1619–1629. doi: 10.1093/intimm/dxh342. [DOI] [PubMed] [Google Scholar]
- 19.Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan BA, Nagarajan NA, Wingender G, Wang J, Scott I, Tsuji M, Franck RW, Porcelli SA, Zajonc DM, Kronenberg M. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J Immunol. 2010;184:141–153. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, Teyton L, Porcelli SA, Savage PB, Bendelac A. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc Natl Acad Sci U S A. 2009;106:10254–10259. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 24.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 25.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S-H, Roark JH, Bendelac A. Tissue specific recognition of mouse CD1 molecules. J. Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- 27.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, 3rd, Ravkov EV, Ibegbu CC, Altman JD, et al. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer MI, Colmone A, Felio K, Xu H, Ma A, Wang CR. A cell-type specific CD1d expression program modulates invariant NKT cell development and function. J Immunol. 2006;176:1421–1430. doi: 10.4049/jimmunol.176.3.1421. [DOI] [PubMed] [Google Scholar]
- 31.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203:1197–1207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimoto T, Paul WE. CD4pos NK1.1pos T cells promptly produced IL-4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 1994;179:1285. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol. 2010;22:68–78. doi: 10.1016/j.smim.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Kaer LV. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrand KJ, Dickgreber N, Stoitzner P, Ronchese F, Petersen TR, Hermans IF. Langerin+ CD8alpha+ dendritic cells are critical for cross-priming and IL-12 production in response to systemic antigens. J Immunol. 2009;183:7732–7742. doi: 10.4049/jimmunol.0902707. [DOI] [PubMed] [Google Scholar]
- 37.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomura M, Yu WG, Ahn HJ, Yamashita M, Yang YF, Ono S, Hamaoka T, Kawano T, Taniguchi M, Koezuka Y, et al. A Novel Function of Valpha14+CD4+NKT Cells: Stimulation of IL-12 Production by Antigen-Presenting Cells in the Innate Immune System. J Immunol. 1999;163:93–101. [PubMed] [Google Scholar]
- 39.Freigang S, Zadorozhny V, McKinney MK, Krebs P, Herro R, Pawlak J, Kain L, Schrantz N, Masuda K, Liu Y, et al. Fatty acid amide hydrolase shapes NKT cell responses by influencing the serum transport of lipid antigen in mice. J Clin Invest. 2010;120:1873–1884. doi: 10.1172/JCI40451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 41.Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 42.Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111:2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, Besra GS, Cerundolo V, Batista FD. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moody DB, Briken V, Cheng TY, Roura-Mir C, Guy MR, Geho DH, Tykocinski ML, Besra GS, Porcelli SA. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat Immunol. 2002;3:435–442. doi: 10.1038/ni780. [DOI] [PubMed] [Google Scholar]
- 46.Bendelac A, Teyton L, Savage PB. Lipid presentation by CD1: the short and the long lipid story. Nat Immunol. 2002;3:421–422. doi: 10.1038/ni0502-421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.