Abstract
Ocular infection with herpes simplex virus (HSV) causes corneal neovascularization (CV), an essential step in the pathogenesis of the blinding immuno-inflammatory lesion, stromal keratitis (SK). The infection results in IL-17A production which contributes to CV in ways that together serve to shift the balance between corneal concentrations of VEGF-A and the soluble receptor molecule sVEGFR-1 which binds to VEGF-A and blocks its function (a so-called VEGF trap). Accordingly, animals lacking responses to IL-17A signaling, either because of IL-17 receptor-A knockout or WT animals that received neutralizing mAb to I L-17A had diminished CV compared to controls. The procedures reduced VEGF-A protein levels, but had no effect on the levels of sVEGFR-1 present. Hence the VEGF trap was strengthened. IL-17A also caused increased CXCL1/KC synthesis, which attracts neutrophils to the inflammatory site. Neutrophils further influenced the extent of CV by acting as an additional source of VEGF-A as well as metalloproteinase (MMP) enzymes that degrade the soluble receptor inhibiting its VEGF blocking activity. Our results indicate that suppressing the expression of IL-17A, or increasing the activity of the VEGF trap, represent useful approaches to inhibit CV and the control of an ocular lesion that is an important cause of human blindness.
Introduction
Optimal vision demands corneal transparency so that light transmission proceeds to the retina without interruption. Several mechanisms are employed to achieve transparency. These include events that suppress tissue damaging inflammatory and immune reactions as well as corneal neovascularization (CV) (1, 2). The control system lacks perfection and breaks down in response to some injurious events, such as herpes simplex virus-1 (HSV) infection of the cornea (3). This can result in corneal blindness, which normally happens after several recrudescences from latent infection in the trigeminal ganglion (4). Stromal Keratitis (SK) is mainly an immunoinflammatory response to infection orchestrated by T lymphocytes (5–7), and is the commonest infectious cause of vision loss in developed countries (8). A prominent feature of SK pathogenesis is the establishment of new blood vessels in the normally avascular cornea, but the mechanisms by which virus infection results in CV are poorly understood (Sarangi P. S., 2010). Several angiogenic molecules may participate in CV with the principal mediator being VEGF-A signaling through the VEGFR-2 receptor (9, 10). Curiously, VEGF-A is synthesized by the normal cornea, but its angiogenic activity is constrained by its binding to an excess of the soluble form of the VEGF receptor 1 (sVEGFR-1) that is produced by corneal epithelial cells (a so called VEGF-A trap) (2, 11, 12). The consequence of HSV ocular infection is a change in the concentration balance between VEGF-A and the soluble receptor (13), and how this might be influenced is the subject of this report.
Previous studies established that HSV infection, either directly or indirectly, caused the increased production of VEGF-A, but at the same time reduced the synthesis sVEGFR-1 (13–15). Additionally, the inflammatory reaction to HSV includes the infiltration of cells that produce enzymes such as MMP-2, -7 and -9 that degrades sVEGFR-1 into inactive fragments (13). Since regulation of VEGF-A levels and molecules that influence its signaling represent valuable approaches to control pathological angiogenesis (10, 16, 17), it is important to understand how the multiple molecules generated in the inflamed cornea after HSV infection impact on VEGF function. The present report focuses on the cytokine IL-17A that is rapidly upregulated in the eye after HSV infection (18). Moreover, past studies demonstrated that fibroblasts and monocytes exposed in vitro to IL-17 may produce VEGF-A (19, 20). Additionally, IL-17 contributed to tumor angiogenesis by causing the proliferation and migration of vascular endothelial cells into tissues (21). Little is currently known about the participation of IL-17 in ocular angiogenesis, particularly how it might impact on the efficiency of the VEGF-A trap.
In the present report, we demonstrate that HSV induced IL-17A expression in the cornea could cause a change in the balance between VEGF-A and sVEGFR-1. Mice lacking IL-17A signaling (IL-17 receptor-A knock-out or IL-17RAKO), or neutralization of IL-17A in WT HSV infected mice, showed reduced CV. IL-17RAKO mice had reduced production of VEGF-A but sVEGFR-1 levels remained unchanged. Additionally, IL-17A induced IL-6 production by corneal stromal fibroblasts, and IL-6, in combination with IL-17A, acted in concert to further up-regulate VEGF-A production. IL-17A also directly enhanced MMP-9 production, which can breakdown sVEGFR-1 into inactive fragments, but had no effect on VEGF-A bioavailability. IL-17A also induced the neutrophil chemoattractant, CXCL1/KC in the cornea, with the recruited neutrophils further affecting the balance between VEGF-A and sVEGFR-1 by providing an additional source of pre-formed VEGF-A as well as sVEGFR-1 degrading MMPs. Taken together our data show that IL-17A plays a central role in regulating ocular angiogenesis and does so, at least in part, by limiting the efficacy of the VEGF-A trap.
Materials and Methods
Mice, Virus and cell lines
IL-17RAKO mice on a C57BL/6 background were obtained from Amgen (Thousand Oaks, CA). C57BL/6 mice were purchased from Harlan Sprague Dawley, Indianapolis, IN. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council. All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)- approved animal facilities. HSV-1 eye infection was performed under anesthesia (avertin), and all efforts were made to minimize animal suffering. HSV 1 RE Tumpey virus was grown in Vero Cell monolayers (ATCC no. CCL81). The virus was concentrated, titrated, and stored in aliquots at −80° C until use. MK/T 1 cell line (Immortalised keratocytes from C57/BL6 mouse corneal stroma) was kindly gifted by Dr. Reza Dana, Schepens Eye Research Institute and Department of Ophthalmology, Boston MA. MK/T-1 cell line was derived from cultures of mouse stromal cells transfected with a human telomerase transcriptase to attain immortalization (22). These cells were fibroblastic in appearance with no cellular transformation in cell culture over several passages. Although, these cells shared similar characteristics of parental cells, they show some structural and morphological alterations such as expression of smooth muscle alpha-actin when stimulated with cytokines such as TGF-B (22).
Corneal HSV Infection and Clinical Scoring
Corneal infections of mice were conducted under deep anaesthesia induced by i.p. injection of avertin (tri-bromomethanol). Mice were scarified on their corneas with 27-gauge needle, and a 3 μl drop containing 1×104 PFU of virus was applied to the eye. The eyes were examined on different days pi for the development and progression of clinical lesion by slit lamp biomicroscope (Kowa Company, Nagoya, Japan). The progression of angiogenesis of individually scored mice was recorded. The severity of angiogenesis was recorded as described previously (23). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.5 mm towards the corneal center. The score of the four quadrants of the eye were then summed to derive the neo vessel index (range 0–16) for each eye at a given time point.
Sub-conjunctival Injection
Sub-conjunctival injections of anti-IL-17 were performed as described previously (24). Briefly, sub-conjunctival injections were done using a 2-cm, 32-gauge needle and syringe (Hamilton) to penetrate the perivascular region of conjunctiva, and the required dose of anti-IL-17 mAb (5 ug in 10ul volume) was delivered into sub-conjunctival space. Control mice received isotype mAb.
Flow Cytometry
Corneas were excised, pooled group wise, and digested with 60 U/ml Liberase for 35 minutes at 37°C in a humidified atmosphere of 5% CO2. After incubation, the corneas were disrupted by grinding with a syringe plunger on a cell strainer and a single cell suspension was made in complete RPMI-1640 medium. Briefly, cell suspension was first blocked with an un-conjugated anti CD32/CD16 mAb for 30 min in FACS buffer. After washing with FACS buffer, samples were incubated with CD45-allophycocyanin (30 F11), CD11b PerCP (M1/79), Ly6G-PE (1A8) and CD31-PE (MEC13.3) (BD Biosciences) for 30 min on ice. Finally, the cells were washed three times and re-suspended in 1% para-formaldehyde. The stained samples were acquired with a FACS Calibur (BD Biosciences) and the data were analyzed using the FlowJo software (Tree Star, Ashland, OR).
MK/T-1 Cell Assay
Immortalized corneal stromal fibroblast (MK/T-1) cells were stimulated in vitro with different concentrations of recombinant IL-17A, IL-6 and IL-1β (R&D Systems) either in the presence or absence of neutralizing anti-IL-17A mAb or anti-IL-6 receptor mAb (BD Biosciences) in DMEM supplemented with 5% FBS for 24 h at 37°C in 5% CO2. After 24 h of stimulation supernatants were collected and stored at −80°C until further use. Supernatants were analyzed for VEGF-A, IL-6 and CXCL1/KC production using sandwich ELISA kit. Cells were collected for gene expression analysis.
Quantitative Real-Time PCR (qRT-PCR)
Cells (from 8 pooled corneas per sample or cultured MK/T-1 cells) were lysed and total mRNA was extracted using TRIzol LS reagent (Invitrogen). Total cDNA was made with 500 ng of RNA using oligo (dT) primer. Quantitative PCR was performed using SYBR Green PCR Master Mix (Applied Biosystem) with iQ5 real-time PCR detection system (Bio-Rad). The expression levels of different molecules were normalized to β-actin using ΔCt calculation. Relative expression between control and experimental groups were calculated using the 2-ΔΔCt formula. The PCR primers used were as follows: β-actin-F5'-TCCGTAAAGAATCTCATGCC-3', R5'-ATCTTCATCCTCCTAGGAGC-3'; VEGF-A-F5'-GTTCATGGATGTCTACCAGCGAAG-3', R5'-GAAGATGTACTCTATCTCGTCGGG-3' sVEGFR-1-F5'-CCTGTACAGAGACATTACCTGG-3', R5'-GACAAGGTTCAGAGTGATGGAG-3'; IL-6-F5'-ATGCTTAGGCATAACGCACTAGGT-3', R5'-CGTGGAAATGAGAAAAGAGTTGTGC-3'; MMP-2-5'-CCGATCTACACCTACACCAAGAAC-3', R5'-CCAGTACCAGTGTCAGTATCAG-3'; MMP-7-F5'-CCTATACTTCAGACTTACCTCGG-3', R5'-CTGTCTCCATGATCTCTCCTTG-3'; MMP-9-F5'-CTCTACAGAGTCTTTGAGTCCG-3', R5'-CCTGTAATGGGCTTCCTCTATG-3'; CXCL1/KC-F5'-GTGTTGCCCTCAGGGCC-3', R5'-GCCTCGCGACCATTCTTG-3'.
Western blot analysis
The supernatants from lysed corneal cells were quantified using BCA protein Assay kit (Thermo scientific). Samples with equal protein concentrations were denatured by boiling in Laemmli buffer. Polypeptides were resolved by SDS PAGE and transferred onto a PVDF membrane. The membrane was blocked with 5% BSA in Tris-buffered saline with Tween20 (20 mM Tris [pH 7.4], 137 mM NaCl, and 0.1% Tween20) overnight at 4°C and probed with specific primary and secondary antibodies. Proteins were detected using chemiluminiscent HRP substrate (Millipore). The membrane was kept in stripping buffer for 10 min and re probed using anti-β-actin antibody. The antibodies used were rat anti-sVEGFR-1 (141515; R&D Systems), mouse anti-β-actin (AC74; Sigma-Aldrich), goat anti rat IgG-HRP (R&D Systems), and donkey anti goat IgG-HRP (Santa Cruz Biotechnology).
Immunofluorescence staining
For immunofluorescence staining, the eyes from naïve uninfected mice were enucleated, and snap frozen in OCT compound. Six micron thick sections were cut, air dried, and fixed in acetone-methanol (1:1) at room −20 °C for 10 min. Sections were blocked with 10% goat serum containing 0.05% Tween20 and 1:200 dilution of Fc block (Clone 2.42G2; BD Biosciences). Rat anti-Ly6G (Clone RB6-8C5; eBioscience) was diluted in 1% BSA containing 0.1% Triton-X and incubated at room temp for 1 hr. After incubation sections were washed several times with PBST and then stained with rabbit anti rat Alexa-488 for 45 min. The corneal sections were repeatedly washed with PBST and mounted with mounting media containing propidium iodide as a nuclear stain (Vector-Laboratories) and visualized under a Immunofluorescence microscope.
ELISA
The pooled corneal samples (4–8 corneas/sample) were homogenized using a tissue homogenizer and supernatant was used for analysis. The concentrations of VEGF-A, sVEGFR-1, CXCXL-1/KC (R&D Systmes) and IL-6 (eBioscience) were measured by sandwich ELISA kits as per manufacturer's instructions.
Statistics
Student's t test was performed to determine statistical significance and data are expressed as mean ± SEM. For some experiments, as mentioned in the figure legend, a one-way ANOVA test was applied.
Results
Lack of IL-17A receptor signaling diminishes severity of HSV induced CV
To study the role of IL-17A receptor signaling effects on HSV induced CV, IL-17RAKO mice were ocularly infected with HSV and the severity of CV was compared with age and sex matched WT infected control mice. At all time points, IL-17RAKO mice showed significantly less CV than WT animals (Fig 1A–B). The onset of visible angiogenic sprouting was delayed, angiogenesis scores were lower and more IL-17RAKO mice had diminished angiogenesis scores compared to their WT counterparts (Fig 1A). Additionally, not only the incidence but also the severity of angiogenesis (scores of ≥8) was reduced in the IL-17RAKO group (2 of 8 eyes at day 21) compared to WT counterparts (9 of 11 eyes at day 21) (Fig 1B). Analysis by FACS of pooled corneal samples from IL-17RAKO and WT mice in each group at day15 pi revealed significantly reduced numbers per cornea of CD31+ cells (a marker for blood vessel endothelium) in IL-17RAKO as compared to WT animals (Fig 1C–D). Although, IL-17RA KO mice showed diminished CV scores as compared to WT animals, the inhibition was not complete since additional angiogenic factors and inducers of VEGF-A such as CpG, IL-6 and IL-1 were still present (3, 15, 25). Collectively, these findings indicate that IL-17A plays a role in HSV induced CV.
Figure 1. IL-17RA KO display diminished HSV induced CV.
To compare the development and progression of CV, WT and IL-17RAKO mice were infected with 1×104 PFU of HSV by corneal scarification. (A) The development of new blood vessels in normally avascular cornea was assessed on day 8, 11, 15 and 21 pi. (B) CV score of individual mice on day 21 pi. (n= 8 to 12 mice per group from two independent experiments; *P<0.03, ***P=0.0003). (C–D) Mice were sacrificed on day 15 pi and corneas were pooled group wise for flow cytometry analysis. (C) Representative FACS plots for corneal CD31+ endothelial cells between WT and IL-17RAKO mice. (D) Bar graphs show reduced total CD31+ endothelial cell number per cornea from IL-17RAKO mice group as compared to WT. (n= 5 for WT and 6 for IL-17RA KO mice. Each sample is representative of two corneas). Data represents means ± SEM.
To further demonstrate the participation of IL-17A, WT HSV infected mice were given neutralizing anti-IL-17A or isotype mAb (5 μg in 10 μl) on day 1, 3 and 5 pi by sub-conjunctival route (Fig 2A). As shown in Fig 2B–C, the neutralizing mAb treatment significantly reduced angiogenesis scores as compared to control isotype mAb treated mice. Consistently, local sub-conjunctival administration of anti-IL17A mAb starting on day 7 followed by day 9, 11 and 13 showed inhibitory effect on the onset as well as severity of CV (Fig 2D–G). Moreover, the frequency of severe angiogenesis (scores of ≥8) was significantly decreased in animals from the anti-IL-17A mAb recipients (1 of 8 eyes) as compared to isotype mAb recipients (7 of 8 eyes) (Fig 2F). Furthermore, corneal samples collected at the end of the experiment revealed reduced total numbers of CD31+ cells per cornea in the IL-17A neutralized group as compared to isotype mAb recipients (Fig 2H–I). Taken together these data indicate that IL-17A signaling affects the extent of CV that follows ocular infection with HSV.
Figure 2. Systemic and local IL-17A neutralization reduces severity of HSV induced Corneal Neovasculariaztion.
WT mice were infected with 1×104 PFU of HSV by corneal scarification. (A) For early IL-17A neutralization, animals were injected sub-conjunctivally with 5 μg in 10 μl/eye of anti-IL-17A or control IgG1 mAb as indicated. (B) The development of new blood vessels in normally avascular cornea was assessed on day 5 and 7 pi. (C) CV score of individual mice on day 15 pi. (n= 16 corneas per group from two independent experiments; *P≤0.037). (D) For late IL-17A neutralization, mice were injected sub-conjunctivally with 5 μg of anti-IL-17A or control IgG1 mAb as indicated. (E) The development of response in normally avascular cornea was assessed on day 8, 11 and 15 pi. (F) CV score of individual mice on day 15 pi. (n= 8 corneas per group from two independent experiments; **P=0.0003). (G) Representative eye photograph of naïve uninfected, local isotype mAb or anti-IL-17A mAb treated mice on day 15 pi. In uninfected mice, cornea is clear and devoid of any blood vessels (left panel). By day 15 pi, new blood vessel development reaches to the center of the cornea in isotype mAb treated mice (middle panel), but only halfway in anti-IL-17A treated mice (right panel) as indicated by white arrows. (H–I) Mice from late IL-17A neutralization groups were sacrificed on day 15 pi and corneas were pooled group wise for flow cytometry analysis. (H) Representative FACS plots for corneal CD31+ endothelial cells between isotype mAb and anti-IL-17A mAb treated mice. (I) Bar graphs show reduced total CD31+ endothelial cell numbers per cornea from anti-IL-17A treated group as compared to isotype treated group. (n= 4 and each sample is representative of two corneas). Data show mean values ± SEM from at least two different independent experiments.
Mechanisms for IL-17A induced CV after HSV Infection
A. IL-17A differentially regulates VEGF-A and sVEGFR-1 expression after HSV infection
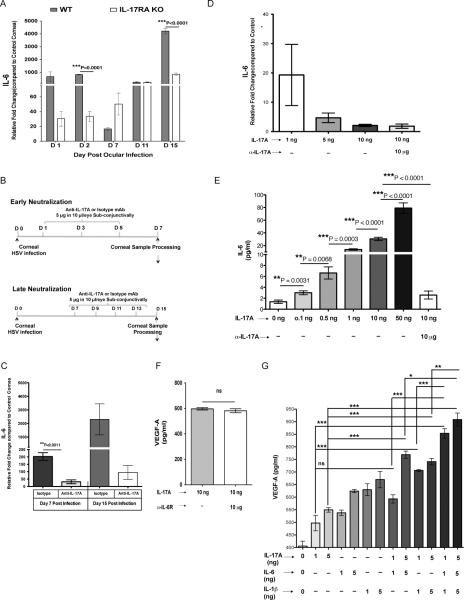
Although, IL-17A could be directly involved in causing CV, a more likely explanation is that the cytokine affects the VEGF-A response and conceivable its regulation by sVEGFR-1. In support of this notion, we could show that WT infected animals markedly upregulated VEGF-A, but not sVEGFR-1 mRNAs over the day 1 to 15 pi observation period compared to uninfected controls (Fig 3A). However, compared to controls, IL-17RAKO animals failed to up-regulate VEGF-A mRNA and their sVEGFR-1 mRNA levels were modestly increased (Fig 3A–B). Similarly, WT infected animals given neutralizing anti-IL-17A mAb failed to up-regulate VEGFA mRNA (Fig 3C–D), with little or no effect on sVEGFR-1 mRNA expression (Data not shown). VEGF-A protein levels were also recorded over the 15 day observation period in WT and IL-17RAKO animals. As is evident in Fig 3E, WT animals expressed readily detectable VEGF-A protein levels, but both IL-17RAKO and WT animals receiving neutralizing anti-IL-17A mAb had reduced VEGF-A protein levels (Fig 3E–F). Additionally, when sVEGFR-1 protein levels were compared in naïve uninfected and WT infected day 7 corneal samples, WT mice showed significantly reduced corneal sVEGFR-1 levels. However, when sVEGFR-1 protein levels were compared between uninfected and day 7 infected IL-17RAKO mice, no significant reduction was observed between these two groups (Fig 3G).
Figure 3. IL-17A differentially regulates the corneal VEGF-A and sVEGFR-1 expression.
WT and IL-17RA KO mice were infected with 1×104 PFU of HSV in PBS or mock infected with only PBS by corneal scarification. VEGF-A (A) and sVEGFR-1 (B) mRNA expression were examined and compared between WT and IL-17RAKO mice by quantitative real-time PCR (qRT-PCR). VEGF-A and sVEGFR-1 mRNA levels in mock-infected mice were set to 1 and used for relative fold up-regulation at various days pi (n=4). (C) Local neutralization of IL-17A was carried out using anti-IL-17A mAb by sub-conjunctival injection during early and late stages of HSV infection as indicated. (D) VEGF-A mRNA expression was examined and compared between isotype and anti-IL-17A mAb treated mice by qRT-PCR (n=4). (E) Corneal VEGF-A protein levels were analyzed from WT and IL-17RAKO mice at indicated time points (n=3). (F) Corneal VEGF-A protein levels were analyzed and compared between isotype and anti-IL-17A mAb treated mice at day 7 pi (n=3). (G) Corneal sVEGFR-1 protein levels were analyzed from WT and IL-17RAKO mice at day 7 pi (n=3). MK/T-1 cells (Corneal stromal keratocytes) were stimulated under different concentrations of IL-17A in the presence or absence of anti-IL-17A mAb for 24 hrs. Supernatants were collected for VEGF-A protein estimation by ELISA and cells were collected and pooled for analysis of VEGF-A and sVEGFR-1 mRNA expression by qRTPCR. The mRNA levels in control media were set to 1 and used for relative fold up-regulation at various days pi. Relative fold change in VEGF-A (H) and sVEGFR-1 (I) mRNA expression as compared to control media (n=4). (J) IL-17A stimulated VEGF-A production by MK/T-1 cells in a dose dependent manner (n=15). Data show mean values ± SEM from at least two different independent experiments.
Furthermore, In-vitro experiments were performed using murine corneal stromal fibroblast cell line (MK/T-1 cells) to measure the effects of IL-17A stimulation on the production of VEGF-A and sVEGFR-1. Real-time PCR analysis showed that IL-17A induced up-regulation of VEGF-A mRNA up to 150 fold (Fig 3H) but had no major effect on sVEGFR-1 mRNA expression (Fig 3I). Stimulation of MK/T-1 cells with IL-17A also significantly increased VEGF-A protein levels in a dose dependent manner (Fig 3J). The IL-17A response could be blocked by anti-IL-17A mAb, indicating that the response was IL-17A specific. Taken together these in-vivo and in-vitro experiments demonstrate that IL-17A causes CV by stimulating VEGF-A production, but had little or no effect on the sVEGFR-1. The outcome would be increased bioavailability of VEGF-A to drive CV after HSV infection.
B. IL-17A induces IL-6 expression and in combination expands production of VEGF-A by corneal stromal fibroblasts
Whereas IL-17A promoted VEGF-A expression by the cornea, it was not clear if the effect was direct or indirect, with additional molecules such as IL-6 involved as intermediaries (15, 26–28). To assess the role of IL-17A on IL-6 expression, levels of corneal IL-6 mRNA were compared by real-time PCR between HSV infected IL-17RAKO and WT counterparts at various days pi. Corneal samples from IL-17RAKO mice showed markedly diminished expression of IL-6 compared to WT mice at all tested days pi (Fig 4A). Moreover, local neutralization of IL-17A during the early as well as the late phase of HSV infection resulted in reduced IL-6 mRNA expression on day 7 and day 15 pi (Fig 4B–C). In-vitro experiments also showed that stimulation of corneal fibroblasts with IL-17A caused the up-regulation of IL-6 mRNA and protein (Fig 4D–E). Furthermore, the addition of anti-IL-17A mAb completely blocked IL-6 mRNA and protein production indicating a direct effect of IL-17A on the IL-6 response (Fig 4D–E). These experiments would indicate that IL-17A plays a direct role in inducing IL-6, which itself is known to be an inducer of VEGF-A (15). Although IL-17A could induce IL-6, the latter would not seem to be responsible for VEGF-A production by IL-17A stimulated fibroblasts. Accordingly, VEGF-A expression levels were unaffected by the presence of anti-IL-6R mAb in cultures (Fig 4F). Nevertheless to further determine whether IL-17A stimulation was sufficient to induce maximal amounts of VEGF-A expression, in-vitro experiments were performed to compare the stimulatory effects of IL-17A, as well as with the cytokines IL-6 and IL-1β. As shown in Fig 4G, the addition of IL-17A, IL-β or IL-6 given individually could all promote VEGF-A production by MK/T-1 cells. However, when MK/T-1 cells were stimulated with a combination of IL-17A with IL-6 and/or IL-1β, VEGF-A production by MK/T-1 cells was significantly increased as compared to the response to a single cytokine. Taken together, our experiments indicate that IL-17A may have a direct role in inducing VEGF-A, but the magnitude of VEGF-A induction can be increased by co-exposure to other cytokines such as IL-6 and IL-1β, a likely scenario in corneal stroma after HSV infection.
Figure 4. IL-17A stimulates IL-6 production by corneal stromal fibroblast cells and both the cytokines further stimulate VEGF-A production.
WT and IL-17RA KO mice were infected with 1×104 PFU of HSV in PBS or mock infected with only PBS by corneal scarification. (A) IL-6 mRNA expression was examined and compared between WT and IL-17RAKO mice by qRT-PCR (n=4). (B) Local neutralization of IL-17A was carried out using anti-IL-17A mAb by sub-conjunctival injection during early and late stages of HSV infection as indicated. (C) IL-6 mRNA expression was examined and compared between isotype and anti-IL-17A mAb treated mice by qRT-PCR. IL-6 mRNA levels in mock-infected mice were set to 1 and used for relative fold up-regulation at various days pi (n=4). (D–E) MK/T-1 cells were stimulated under different concentrations of IL-17A in the presence or absence of anti-IL-17A mAb for 24 hrs. Supernatants were collected for IL-6 protein estimation by ELISA and cells were collected and pooled for analysis of IL-6 mRNA expression by qRT-PCR. (D) Relative fold change in IL-6 mRNA expression as compared to control media (n=4). The mRNA levels in control media were set to 1 and used to relative fold change in mRNA expression. (E) IL-17A stimulated IL-6 production by MK/T-1 cells in a dose dependent manner, n=9. (F) MK/T-1 cells were stimulated with IL-17A (10ng/ml) in the presence or absence of anti-IL-6R mAb for 24 hrs. Supernatants were collected for VEGF-A protein estimation by ELISA (n=6). (G) MK/T-1 cells were stimulated under different concentrations of IL-17A, IL-6 and IL-1β for 24 hrs. Supernatants were collected for VEGF-A protein estimation by ELISA. VEGF-A production by MK/T-1 cells under different concentrations of IL-17A, IL-6 and IL-1β as indicated, n=6–15. One way ANOVA with Bonferroni's multiple comparison test was used to calculate the level of significance. *<0.05, **<0.001, ***<0.0001. Data represents means ± SEM from at least two independent experiments.
C. IL-17A drives increased expression of various MMPs responsible for sVEGFR-1 degradation
It is known that MMPs facilitate the growth of new blood vessels through the extracellular matrix (29) and that some MMPs may differentially breakdown VEGF-A and sVEGFR-1 into non-functional fragments (13, 30). To determine if IL-17A could influence the production of MMP enzymes, corneal samples from HSV infected IL-17RAKO and WT mice were compared for MMP mRNA levels by real-time-PCR analysis. From day 1 through 15 pi, MMP-2, -7 and -9 mRNA levels were lower in samples from IL-17RAKO than WT mice. The differential expression levels ranged from 5 to 10 fold and were evident at all time points tested (Fig 5A–C). Maximal differences in expression levels were noted with MMP-9 on day 11 and 15 pi (Fig 5C).
Figure 5. IL-17A promotes MMP-2, -7 and -9 expression and affects VEGF-A bioavailability through sVEGFR-1 degradation.
WT and IL-17RA KO mice were infected with 1×104 PFU of HSV in PBS or mock infected with only PBS by corneal scarification. MMP-2 (A) MMP-7 (B) and MMP-9 (C) mRNA expression were examined and compared between WT and IL-17RAKO mice by qRT-PCR (n=4). (D–E) Local neutralization of IL-17A was carried out using anti-IL-17A mAb by sub-conjunctival injection during early (D) and late (E) stages of HSV infection as indicated. MMP-2 (left) MMP-7 (middle) and MMP-9 (right) mRNA expression were examined and compared between isotype and anti-IL-17A mAb treated mice by qRT-PCR. MMP-2, -7 and -9 mRNA levels in mock-infected mice were set to 1 and used for relative fold up-regulation at various days pi (n=4). (F) sVEGFR-1 expression was analyzed by WB in corneal samples from isotype and anti-IL-17A mAb treated mice at day 7 pi. β-actin used as loading control. (G) MK/T-1 cells were stimulated under different concentrations of IL-17 in the presence or absence of anti-IL-17A mAb for 24 hrs. Cells were collected and pooled for analysis of MMP-9 mRNA expression by qRT-PCR. Relative fold change in MMP-9 mRNA expression as compared to control media (n=4). The mRNA levels in control media were set to 1 and used to relative fold change in mRNA expression. Data show mean values ± SEM from at least two different independent experiments.
In additional experiments, WT HSV infected mice received neutralizing anti-IL-17A mAb starting from day 1 followed by day 3 and 5 pi (Fig 5D). This resulted into diminished expression of MMP-2 (12 fold), MMP-7 (3 fold) and MMP-9 (25 fold) on day 7 pi, when compared to isotype mAb treated animals (Fig 5D). Even effects of IL-17A neutralization were observed when the treatment was begun on day 7 followed by on day 9, 11 and 13 pi. Levels were reduced 4 fold for MMP-2, 10 fold for MMP-7 and 15 fold for MMP-9 in the anti-IL-17A mAb recipient group as compared to isotype mAb treated mice (Fig 5E). Furthermore, western blot analysis of corneal samples from anti-IL-17A treated mice revealed reduced degradation of sVEGFR-1 as compared isotype mAb treated mice (Fig 5F).
In other experiments, MK/T-1 cells were stimulated with IL-17A for 24 hrs. This resulted in increased MMP-9 mRNA expression in a dose dependent manner, an effect inhibited by neutralizing anti-IL-17A mAb indicating that the increased MMP-9 expression was IL-17A specific (Fig 5G). Collectively, these results indicate that IL-17A could influence the efficacy of the VEGF-A trap by increasing the synthesis of some MMPs that can cause sVEGFR-1 degradation, making more VEGF-A available to induce angiogenesis.
D. IL-17A promotes corneal infiltration of neutrophils through increased CXCL1/KC expression
Neutrophils form a prominent part of the inflammatory response in corneas after HSV infection (3, 31). These cells contribute to the extent of CV by acting as an additional source of VEGF-A, as well as MMP enzymes that can degrade the sVEGFR-1 into fragments that fail to block VEGF-A (13, 32). To measure if IL-17A could participate in the extent of neutrophil recruitment, experiments were done to compare levels of the neutrophil recruiting chemokine CXCL1/KC in WT and animals with blunted signals from IL-17A. Our results show that levels of the CXCL1/KC mRNA and protein in corneal extracts from IL-17RAKO mice were significantly lower than in extracts from WT (Fig 6A–B), or from WT mice that received neutralizing mAb to IL-17A (Fig 6 C–E). The differences were apparent at all time points tested (Fig 6A–E). Further evidence for the role of IL-17A in corneal migration of neutrophils post HSV infection came from comparing the corneal infiltrating neutrophils between HSV infected WT and IL-17RAKO mice. Accordingly, IL-17RAKO mice had significantly reduced total cell numbers of neutrophils when compared to WT counterparts (Fig 6F). Moreover, in-vivo neutralization of IL-17A in WT HSV infected mice showed diminished infiltration of neutrophils as compared to isotype antibody treated animals, further demonstrating the critical role of IL-17A in the migration of these cells to the site of inflammation (Fig 6G–I).
Figure 6. IL-17A promotes neutrophil infiltration in the cornea after HSV infection through CXCL1/KC induction.
WT and IL-17RA KO mice were infected with 1×104 PFU of HSV in PBS or mock infected with only PBS by corneal scarification. (A) CXCL1/KC mRNA expression was examined and compared between WT and IL-17RAKO mice by qRT-PCR. CXCL1/KC mRNA levels in mock-infected mice were set to 1 and used for relative fold up-regulation at various days pi. (B) Corneal CXCL1/KC protein levels were analyzed from WT and IL-17RAKO mice at indicated time points (n=3). (C) Local neutralization of IL-17A was carried out using anti-IL-17A mAb by sub-conjunctival injection during early (left panel) and late (right panel) stages of HSV infection as indicated. (D) CXCL1/KC mRNA expression was examined and compared between isotype and anti-IL-17A mAb treated mice by qRT-PCR. CXCL1/KC mRNA levels in mock-infected mice were set to 1 and used for relative fold up-regulation at various days pi (n=4). (E) Corneal CXCL1/KC protein levels were analyzed and compared between isotype and anti-IL-17A mAb treated mice at day 7 pi (n=4). (F) HSV infected WT and IL-17RAKO Mice were sacrificed on day 1, 2 and 15 pi and corneas were pooled group wise for flow cytometry analysis. Bar graphs show reduced CD45+ CD11b+ Ly6G+ neutrophil numbers per cornea from IL-17RA KO mice group as compared to WT (n=3). (G–H) Mice from local IL-17A neutralization groups were sacrificed on day 15 pi and corneas were pooled group wise for flow cytometry analysis. (G) Representative FACS plots for corneal CD45+ CD11b+ Ly6G+ neutrophils between isotype mAb and anti-IL-17A mAb treated mice. (H) Bar graphs show reduced total CD45+ CD11b+ Ly6G+ neutrophils numbers per cornea from anti-IL-17A treated group as compared to isotype treated group. (n= 4 and each sample is representative of two corneas). (I) Corneal sections from HSV infected corneas of isotype (top) and anti-IL-17A (bottom) mAb treated mice were analyzed by immunofluorescence microscopy with antibody specific for Ly6G (green). Sections were counterstained with propidium idodide (red) for nuclear staining. Bars, 75 μm. (J–L) MK/T-1 cells were stimulated under different concentrations of IL-17A in the presence or absence of anti-IL-17A or anti-IL-6R mAb for 24 hrs. Supernatants were collected for CXCL1/KC protein estimation by ELISA and cells were collected and pooled for analysis of CXCL1/KC mRNA expression by qRT-PCR. The mRNA levels in control media were set to 1 and used for relative fold up-regulation at various days pi. IL-17A stimulated CXCL1/KC production by MK/T-1 cells as analyzed by (J) qRT-PCR (n=4) and (K–L) ELISA (n=6–12). Data show mean values ± SEM from at least two different independent experiments.
Finally in vitro experiments were performed to determine if a corneal cell line stimulated with different concentrations of IL-17A would produce increased amounts of CXCL1/KC chemokine. These experiments revealed an increased in CXCL1/KC expression when stimulated by both IL-17A as well as IL-6 (Fig 6J–K). The effect of IL-17A was inhibited by neutralizing mAb to IL-17A but not anti-IL-6R mAb indicating that the IL-17A induction occurred independently of involvement of IL-6 (Fig 6L).
Collectively, these experiments indicated that IL-17A could influence the function of the VEGF-A trap by elevating the production of a neutrophil recruiting chemokine with the neutrophils providing an additional source of VEGF-A, as well as enzymes that degrade sVEGFR-1 (13).
Discussion
The normal eye employs numerous strategies to maintain the corneal transparency necessary for optimal vision (1). For example, the VEGF-A that is present in normal corneas does not cause vision impairing CV, since it is bound by its soluble receptor forming a so called VEGF-A trap (2). However, infection by HSV can overcome this homeostatic mechanism and CV occurs, an essential step in the pathogenesis of the blinding immune-inflammatory lesion, SK (6). This report shows that the infection results in IL-17A production, which participates in causing CV in several ways. Accordingly, animals lacking responses to IL-17A signaling, either because of IL-17 receptor A knockout or WT animals that received neutralizing mAb to IL-17A, had diminished CV compared to controls. The procedures reduced VEGF-A protein levels, but had no effect on the levels of sVEGFR-1 present. Consequently, the VEGF-A trap could act even more effectively. In addition, the sVEGFR-1 present was less subject to degradation by MMPs in animals without IL-17A signaling, since in normal circumstances IL-17A caused the elevated production of these enzymes. Furthermore, we showed that IL-17A also caused increased CXCL1/KC synthesis, which attracts neutrophils to the inflammatory site. Neutrophils further influence the extent of CV by acting as an additional source of VEGF-A, as well as MMP enzymes that degrade the soluble receptor inhibiting its VEGF-A blocking activity. The MMPs also enhance angiogenesis by facilitating the movement of new blood vessels through the stromal matrix (29, 32). These results demonstrate that IL-17A plays a central role in causing CV and indicate that suppressing the expression of IL-17A could represent a logical approach to achieve anti-angiogenesis and the control of SK. Our overall results are summarized in Fig 7.
Figure 7. Scheme depicting various critical events orchestrated by IL-17A after HSV infection in causing CV.
(A) Model proposing the critical role of IL-17A in the deviation of physiological balance between VEGF-A and sVEGFR-1 after HSV infection. (B) Mechanisms by which IL-17A promotes CV after HSV infection. Normally, naïve uninfected cornea constitutively secretes large amounts of sVEGFR-1 and small amounts of VEGF-A. This leads to a perfect physiological balance between these two molecules, sVEGFR-1 counteracting angiogenic properties of VEGF-A. The net result is the absence of any blood vessels in normally uninfected corneas (left panel). However, early after HSV infection there is increased expression of IL-17A in the cornea, which is mainly contributed by early innate cells such as γδ T cells. The increased levels of IL-17A then further contributes to the increased VEGF-A expression by direct stimulation of corneal stromal fibroblast cells as well as indirectly through increased production of IL-6 which in turn in combination with IL-17A further promotes VEGF-A production. Moreover, HSV infection of corneal epithelial cells as well as IL-17A mediated signaling also downregulates sVEGFR-1 expression in the cornea. In addition to reduced expression of sVEGFR-1, IL-17A also promotes expression of MMP-9, which further affects the VEGF-A bioavailability by increased sVEGFR-1 degradation. This free VEGF-A drives the initial angiogenic sprouting early after HSV infection (middle panel). During later stages of SK, when replicating virus is cleared off from the cornea, IL-17A also promotes the CXCL1/KC expression, which promotes the migration of neutrophils in the inflamed cornea. These neutrophils act as an additional source of pre-formed VEGF-A as well as MMPs that contribute to sVEGFR-1 degradation. These events in addition to direct stimulatory effects of IL-17A on VEGF-A, IL-6 and MMP-9 production, further deviates the balance between VEGF-A and sVEGF-1 and leads to the development of CV as observed during late stages of SK (Right Panel).
The cytokine IL-17A has a range of biological activities and is well known to participate in several inflammatory reactions that include SK, as we and others recently reported (18, 33–35). A role for IL-17A in angiogenesis has also been documented especially in tumor systems (19, 21), although whether or not IL-17A itself drives angiogenesis or acts via intermediary molecules is unclear. Thus, the cytokine can induce HUVEC cells, fibroblasts and some cancer cells to produce VEGF-A, as well as some other angiogenic molecules (19, 21, 33), and these could explain the angiogenic effect. In this study, we could also show that corneal fibroblasts exposed to IL-17A upregulated VEGF mRNA and secreted VEGF protein. One report does demonstrate that IL-17A can induce angiogenesis in a rat corneal micropocket assay, consistent, perhaps, with a direct role in angiogenesis (21), although potential intermediaries were not excluded. Accordingly, we feel the balance of evidence favors the notion that IL-17A's participation in angiogenesis is indirect and proceeds by stimulating cells with IL-17 receptors to produce VEGF-A and perhaps other angiogenic factors (36–38). This was shown by in-vitro studies, but these also demonstrated that levels of VEGF-A produced were enhanced when cells were co-stimulated with IL-17A along with other cytokines. Such additive effects were shown when cells were exposed to IL-17A as well as to IL-6 or IL-1β. A similar pattern of results was reported by others using fibroblasts isolated from tumors and from lesions of arthritis (20, 21, 37, 39).
Although our results demonstrate that IL-17A participates in the CV that follows HSV ocular infection, the connection between virus infection and IL-17A production requires explanation. Thus, virus replication is a brief event usually confined to the corneal epithelium but CV response occurs in the underlying stroma and progresses in magnitude beyond the time when the infection has been eliminated (3, 6). Moreover, corneal epithelial cells are an unlikely source of IL-17A and we were unable to demonstrate that such cells produce IL-17A upon infection (unpublished data). More than likely, the initial source of IL-17A were innate cells, which begun invading the stroma 24 to 48 hrs after infection (18). Most of these innate cells were neutrophils, which in some systems were reported to produce IL-17A (40). We failed to confirm this observation, however, in the case of corneal neutrophils. Instead, we could show that γδ T cells in the cornea produced IL-17A based on the observation that depleting such cells reduced IL-17A protein levels in corneal extracts (18). Others have also shown that γδ T cells are spontaneous producers of IL-17A (41–43).
What needs to be explained is the link between the virus infection in the epithelium and the recruitment, and perhaps stimulation, of innate cells to produce IL-17A. Others as well as ourselves have shown that several cytokines and chemokines are produced in the cornea soon after infection (44, 45). Moreover, this induction is mainly the consequence of TLR ligand activity of HSV, rather than being products of infected cells themselves (46, 47). A few cytokines are produced briefly by infected cells, most notably IL-6 (45). Furthermore, IL-6 is known to be a regulator of IL-17A production, particularly in T cells (48, 49), which in the ocular system become the major source of IL-17A production after the initial phase of SK pathogenesis (18). It is also possible that the γδ T cells recruited to the cornea were promoted to rapidly produce IL-17A, as was shown to occur in vitro when such cells were exposed to cytokines such as IL-1β and IL-23 (41). Both of these activating cytokines have increased expression after HSV ocular infection (50, 51) and their action on γδ T cells could help explain how the infection relates to IL-17A production in the initial stages. In later stages, the virus is no longer present and the γδ T cells are almost absent (31, 52), yet commencing IL-17A neutralization at day 7 pi still resulted in significantly less CV. Conceivably, an alternative cellular source of the IL-17A cells could be Th17 cells, but such cells are barely detectable in corneal preparations until at least 15 days pi (18). Further studies are underway to define the cellular origin of IL-17A.
The novel aspect of the present study was to demonstrate in an infectious model of inflammatory disease where pathological angiogenesis is an essential component of pathogenesis, that IL-17A signaling played a major part in causing CV and appeared to act by shifting the balance between VEGF-A and its soluble receptor. This served to make VEGF-A more available with this molecule, rather than IL-17A itself, likely acting as the angiogenic factor. This conclusion was reached because in-vivo and in-vitro experiments both showed that when signaling responses to IL-17A were interrupted, the outcome was a significant reduction in the levels of VEGF-A, but no effect on sVEGFR-1 protein levels. Moreover, in the absence of IL-17A signaling, levels of three MMP enzymes that break down sVEGFR-1 were lower, indicating that IL-17A stimulates MMP production as was shown in-vitro for MMP9 with IL-17A stimulated corneal fibroblasts. Others too have shown that tumor fibroblasts up-regulate some MMPs upon in-vitro stimulation with IL-17A (53). Thus when the IL-17A response is normal, the conditions favor both the production of VEGF-A as well as the breakdown of its soluble receptor both of which will set the stage for CV. Subsequent events also impacted on the balance of VEGF and sVEGFR1 favoring angiogenesis because when neutrophils became a prominent component of the inflammatory response, as occurs in the clinical phase of SK, these cells are an additional source of VEGF as well as MMPs that degrade any sVEGFR-1 (18). We also showed with in-vitro experiments that IL-17A was stimulatory for VEGF-A gene expression in-vitro, but it had the opposite effect on sVEGFR-1 expression. If a similar event also operates in-vivo this could help diminish the function of the VEGF trap. Taken together, the influence of IL-17A on CV is likely to be the consequence of effects on the VEGF-A/sVEGFR-1 balance rather than only acting as an inducer of VEGF-A.
Finally it is of interest to comment about the therapeutic implications of our observations. Basically, we interpret our studies to show that IL-17A participates in angiogenesis by shifting the balance between the concentrations of VEGF-A and the soluble form of its receptor, which acts to inhibit the angiogenic function of VEGF-A. The idea of using a synthetic VEGF-A trap to control angiogenesis has been advocated for use in a number of situations that include choroidal neovascularization and some tumors (54, 55). In these circumstances the VEGF-A binding molecules used are synthetic derivatives of VEGF-A receptors and have a very high affinity for VEGF-A. In our situation, we are dealing with the IL-17A induced breakdown of a natural VEGF-A trap. Past studies using a mouse model of SK, as well as human corneal samples from HSV infected SK patients have shown that IL-17A is expressed after HSV infection (18, 34, 56). These studies, as well as our present report show that IL-17A promotes CV and is also involved in the pathogenesis of SK. Therefore, we advocate based on the results of this investigation, that the control of angiogenesis induced by HSV infection could be achieved either by blunting the participation or by blocking events that limit the efficacy of the VEGF trap. Blunting IL-17A might be achieved most effectively using nanoparticles encoding siRNA targeting IL-17A gene expression or anti-IL-17A mAb. Facilitating the activity of the VEGF trap could be accomplished using approaches that inhibit MMP enzymes or by boosting the concentration of sVEGFR-1. Both approaches are currently being investigated.
Acknowledgements
We thank Dr. John Dunlop for his assistance with immuno-fluorescence microscopy and Rudragouda Channappanavar, Shalini Sharma and Greg Spencer for invaluable assistance during research and manuscript preparation.
This Study was supported by National Institute of Allergy and Infectious Diseases Grant AI06335 and National Institutes of Health Grant EY 005093
References
- 1.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 2.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas PS, Rouse BT. Early events in HSV keratitis--setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 5.Niemialtowski MG, Rouse BT. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 6.Sarangi PS, R. a., T. B. Herpetic Keratitis. In: Levin LA, Alberts DM, editors. Ocular Disease Mechanisms and Management. Saunders Elsever; Philadelphia, PA: 2010. pp. 91–97. [Google Scholar]
- 7.Hendricks RL, Tumpey TM, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 8.Pepose JD. Herpes simplex virus disease: Anterior segment of the ey. In: Pepose JS, Holland GN, Whlhelmus KR, editors. Ocular Infection and Immunity. Mosby; St. Louis: 1996. p. 27. [Google Scholar]
- 9.Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, Ansari AM, Scaria PV, Woodle MC, Lu P, Rouse BT. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000;80:443–454. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 13.Suryawanshi A, Mulik S, Sharma S, Reddy PB, Sehrawat S, Rouse BT. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J Immunol. 2011;186:3653–3665. doi: 10.4049/jimmunol.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp Eye Res. 2006;82:46–54. doi: 10.1016/j.exer.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 17.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 18.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 20.Ryu S, Lee JH, Kim SI. IL-17 increased the production of vascular endothelial growth factor in rheumatoid arthritis synoviocytes. Clin Rheumatol. 2006;25:16–20. doi: 10.1007/s10067-005-1081-1. [DOI] [PubMed] [Google Scholar]
- 21.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 22.Gendron RL, Liu CY, Paradis H, Adams LC, Kao WW. MK/T-1, an immortalized fibroblast cell line derived using cultures of mouse corneal stroma. Mol Vis. 2001;7:107–113. [PubMed] [Google Scholar]
- 23.Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409. doi: 10.1097/00003226-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002;43:737–743. [PubMed] [Google Scholar]
- 25.Zheng M, Klinman DM, Gierynska M, Rouse BT. DNA containing CpG motifs induces angiogenesis. Proc Natl Acad Sci U S A. 2002;99:8944–8949. doi: 10.1073/pnas.132605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Wang L, Yi TS, Kortylewski M, Pardoll DM, Zeng DF. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. Journal of Experimental Medicine. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- 35.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 36.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honorati MC, Neri S, Cattini L, Facchini A. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis Cartilage. 2006;14:345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 39.Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004;93:39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 44.Tumpey TM, Cheng H, Yan XT, Oakes JE, Lausch RN. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J Leukoc Biol. 1998;63:486–492. doi: 10.1002/jlb.63.4.486. [DOI] [PubMed] [Google Scholar]
- 45.Thomas J, Kanangat S, Rouse BT. Herpes simplex virus replication-induced expression of chemokines and proinflammatory cytokines in the eye: implications in herpetic stromal keratitis. J Interferon Cytokine Res. 1998;18:681–690. doi: 10.1089/jir.1998.18.681. [DOI] [PubMed] [Google Scholar]
- 46.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarangi PP, Kim B, Kurt-Jones E, Rouse BT. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J Virol. 2007;81:11128–11138. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Staats HF, Lausch RN. Cytokine Expression in-Vivo during Murine Herpetic Stromal Keratitis - Effect of Protective Antibody Therapy. Journal of Immunology. 1993;151:277–283. [PubMed] [Google Scholar]
- 51.Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes Infect. 2008;10:302–312. doi: 10.1016/j.micinf.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue T, Inoue Y, Kosaki R, Nishida K, Shimomura Y, Tano Y, Hayashi K. Immunohistological study of infiltrated cells and cytokines in murine herpetic keratitis. Acta Ophthalmol Scand. 2001;79:484–487. doi: 10.1034/j.1600-0420.2001.790511.x. [DOI] [PubMed] [Google Scholar]
- 53.Qiu Z, Dillen C, Hu J, Verbeke H, Struyf S, Van Damme J, Opdenakker G. Interleukin-17 regulates chemokine and gelatinase B expression in fibroblasts to recruit both neutrophils and monocytes. Immunobiology. 2009;214:835–842. doi: 10.1016/j.imbio.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saishin Y, Takahashi K, Lima e Silva R, Hylton D, Rudge JS, Wiegand SJ, Campochiaro PA. VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- 56.Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]